Abstract
Objective
The aging HIV population has increased comorbidity burden and consequently non-antiretroviral (ARV) medication utilization. Many non-ARV medications have known neurocognitive-adverse effects (“NC-AE medications”). We assessed the cognitive effects of NC-AE medications in HIV+ and HIV− women.
Methods
1558 participants (1037 HIV+; mean age 46) from the Women’s Interagency HIV Study completed a neuropsychological test battery between 2009 and 2011. The total number of NC-AE medications and subgroups (e.g., anticholinergics) were calculated based on self-report. Generalized linear models for non-normal data were used to examine the cognitive burden of medications and factors which exacerbate these effects.
Results
HIV+ women reported taking more NC-AE medications versus HIV− women (p<0.05). NC-AE medication use altogether was not associated with cognitive performance. However, among NC-AE medication subgroups, anticholinergic-acting medications, but not opioids or anxiolytics/anticonvulsants, were negatively associated with performance. HIV-status moderated the association between these NC-AE medication subgroups and performance (p’s<0.05). HIV-serostatus differences (HIV− < HIV+) in global, learning, fluency and motor function were greatest among women taking >1 anticholinergic medications. HIV-serostatus differences in performance on learning and psychomotor speed were also greatest among women taking 1 or more anxiolytics/anticonvulsants and 1 or more opioids, respectively.
Conclusions
HIV+ women have increased cognitive vulnerabilities to anticholinergic, anxiolytic/anticonvulsant, and opioid medications. Potential synergy between these medications and HIV may explain some HIV-related cognitive impairments. It may be important clinically to consider these specific types of medications as a contributor to impaired cognitive performance in HIV+ women and assess the cost/benefit of treatment dosage for underlying conditions.
Keywords: anticholinergic, cognition, HIV, women
INTRODUCTION
Increasing effectiveness and adherence to antiretroviral (ARV) therapies has led to an aging of the HIV population. To date approximately 50% of all individuals with HIV are 50 years or older[1, 2] and about 90% of individuals over 50 years of age have been living with HIV for the majority of their life[3]. Two consequences of aging in the HIV infected population are increases in: 1) prevalence of HIV-associated non-AIDS comorbidities and; 2) prescription and utilization of non-ARV medications to treat these comorbidities. Both consequences may cause detrimental effects on brain structure and function.
One common HIV-associated non-AIDS comorbidity is cognitive impairment which is reported to occur in approximately 30 to 60% of people with HIV at some point during their lifetime[4]. The clinical features of HIV-associated cognitive impairment commonly include alterations in executive function, complex attention, processing speed, learning, and memory[5–9] and these deficits are associated with dysfunction of fronto-striatal networks[10] and altered integrity of hippocampal and prefrontal brain regions[11–13]. Interestingly, the utilization of non-ARV medications with known cognitive adverse effects may in part explain some of the cognitive complications among HIV-infected (HIV+) individuals, particularly among those individuals ≥50 years of age. Many of the non-ARV medications used among HIV+ individuals have known cognitive adverse effects (termed “NC-AE medications”) especially agents with anticholinergic properties[14–18] as well as opioids[19], anxiolytics[20], and anticonvulsants[21]. This is particularly concerning because older adults in general have an increased vulnerability to medication side effects due to the pharmacokinetic and pharmacodynamic changes that occur with aging[22]. For example, with age, metabolism and drug elimination slows, the blood brain barrier changes, and there are age-related deficits in neurotransmission[23]. As a result, many medications with higher side effect burden are not routinely recommended in older patients[24–26].
The primary aim of the present analysis was to examine the potential cognitive burden of NC-AE medications (total number) and to determine whether HIV exacerbates potentially negative effects. We were interested in both the more general, diffuse effects of NC-AE medications[27] as well as the effects of commonly used non-ARV medications with known pharmacodynamic mechanisms including those with anticholinergic properties, opioids, anticonvulsants and anxiolytics. Our overarching hypothesis was that the effects of NC-AE medications would be broad, negative influences on cognitive performance, and that HIV would exacerbate these effects. Furthermore, consistent with previous studies in older adults [16, 18], we expected more specific associations with medications with known anticholinergic properties on measures of learning, memory, and attention.
METHODS
Participants
All participants were enrolled in the Women’s Interagency HIV Study (WIHS), a longitudinal, multisite study (Chicago, Bronx, Brooklyn, Washington DC, San Francisco, Los Angeles) between the time period of 1994 and 2011 (enrollment varied by site). Study methodology, data collection, interviewer training, and retention have been previously reported[28–30].
Participants in this cross-sectional analysis completed a cognitive assessment during the first wave (April 2009 to April 2011) of a comprehensive neuropsychological test battery administered every 2 years in combination with the WIHS semiannual study visits which includes a physical exam, blood draw, medical and psychosocial interviews. Briefly, all active English-speaking WIHS participants completing any of the 4 semiannual WIHS visits (n=1908) were asked to undergo neuropsychological testing and 1595 consented to participate[31]. Of the 1595, we included 1558 (98%) in our analyses. Thirty-seven women were excluded as they were missing non-ARV NC-AE medication data.
Measures
Medication assessments
At each WIHS visit, participants were asked to recall ARV and non-ARV medications taken currently and since the last visit (~6 months). Medication data was reviewed with specific drugs categorized as NC-AE if there were known adverse cognitive effects[27]. Medication categories[27] were modified to include those medications or drug categories known to adversely impact performance on neuropsychological tests (for drug list see Supplemental Table 1). Additionally, medications reported by participants were categorized by whether they possessed anticholinergic properties according to the Anticholinergic Risk Scale[32]. Our primary exposures of interest were: 1) the total number of NC-AE medications (0, 1, or >1), 2) anticholinergic burden, defined here as the total number of non-ARV medications with anticholinergic properties (0, 1, or >1), 3) taking opioid medications (yes vs. no), and 4) taking anticonvulsant/anxiolytics (yes vs. no) (see Supplemental Table 1). Categorization of each of the primary exposures was based on the data distributions. The focus on anticholinergic, opioid, and anticonvulsant/anxiolytic medications was based on the known adverse effects of these medication categories on cognitive performance and relative high frequency use of these medications in this cohort allowing for subgroup analyses.
Neuropsychological performance
Participants completed a test battery comprised of 8 tests: Hopkins Verbal Learning Test-Revised (HVLT-R), Letter-Number Sequencing, Trail Making (TMT), Stroop Test, Symbol Digit Modalities Test (SDMT), Controlled Oral Word Association Test (COWAT), Category Fluency Test (Animals), and Grooved Pegboard (GPEG). Performance on these tests were used to assess 7 domains: learning (total learning across HVLR-T trials), memory (delayed free recall on HVLT-R), attention/working memory (total correct on LNS control and experimental conditions), psychomotor speed (total correct on SDMT, time to completion on Stroop Trial 2), executive function (time to completion on TMT Part B and Stroop Trial 3), fluency (total correct on COWAT and category fluency), and motor skills (total time to completion for each hand on GPEG). Timed outcome was log transformed to normalize distributions and reverse scored so higher values equated to better performance.
Demographically adjusted T-scores were derived for each outcome and these T-scores were used to create domain scores consistent with previous large-scale HIV cohorts including the WIHS [9, 31, 33–36]. For each domain, a composite T-score was derived by averaging the T-scores for domains with ≥2 outcomes. If only one test in a domain was completed, the T-score for that test was used. A global neuropsychological score was derived for individuals who had T-scores for at least 4 out of 7 cognitive domains.
T-scores were converted into clinical ratings which ranged from 1 to 9 with 1=reflecting above average performance (T-score ≥55), 2=average performance (T-score ≥45 and <55), 3=low average (T-score ≥40 and <45), 4=borderline (used for only domain and global summary ratings not individual test scores), 5=definite mild impairment (T-score ≥35 and <40), 6=mild to moderate impairment (T-score ≥30 and <35), 7=moderate impairment (T-score ≥25 and <30), 8=moderate to severe impairment (T-score ≥20 and <25), and 9=severe impairment (T-score <20)[37]. For each domain rating, a rating was derived based on the test(s) in the domain. If a single test was completed, we used the rating for the domain. If two or more tests in the domain were completed, we averaged the ratings such that if all test ratings are (1-3) or (5-9). If one or more test were scored 1-3 and one or more of the tests was 5 or greater, the domain was scored as the worst test score minus 1 (higher is worse, e.g., a test score of 6 would result in a domain score of 5).
Statistical Analyses
Generalized linear models (GLM) for non-normal data (PROC GENMOD, distribution=Poisson) were performed using SAS version 9.4 (Cary, NC) to examine associations between non-ARV NC-AE medications and cognitive performance. Similar GLM were used to assess whether the association between medication burden and cognitive performance was modulated by HIV-serostatus. In the first set of models, non-ARV NC-AE medication use (0, 1, > 1) was the primary predictor. Models controlled for HIV status, WIHS site, current employment status (employed vs. unemployed), a clinically relevant depressive symptom burden (CES-D≥16), current smoking status (yes vs. no), heavy drinking (≥4 drinks in 1 sitting or ≥7 drinks/week vs. not), marijuana use in the past 6 months (yes vs. no), recent crack, cocaine, and/or heroin use in the past six months (yes vs. no), and HCV RNA. Subsequent models included the interaction between NC-AE medications and HIV-serostatus. Similar sets of models were conducted to include non-ARV medications with anticholinergic properties, opioids, and anxiolytics/anticonvulsants. Results were considered significant at p<0.05 (two-sided). The Benjamini-Hochberg procedure was used to control for the false discovery rate, which was set at 0.10 for each set of analyses.
RESULTS
Table 1 includes demographic, behavioral, clinical, and cognitive characteristics of the HIV+ (n=1037) and HIV− women (n=521) included in the present analysis. Compared to HIV− women, HIV+ women were significantly older (47 vs. 43 years), less likely to be Hispanic, were more likely to have positive HCV serology, and were less likely to be employed and engage in smoking, heavy alcohol, marijuana, or crack, cocaine, and/or heroin use (p’s<0.05). Eighty-three percent of HIV+ women reported combination antiretroviral therapy use with greater than or equal to 95% adherence, 62% were taking low CNS penetrating ARV medications (CNS penetration effectiveness score<8[38]), and 52% of women had an undetectable plasma HIV RNA (below the limits of detection at <48cp/mL).
Table 1.
Demographic, behavioral, clinical, and cognitive characteristics as a function of HIV-serostatus.
| Variable | HIV+ (n=1037) n (%) |
HIV− (n=521) n (%) |
p-value |
|---|---|---|---|
| Age, M (SD) | 46.99 (8.76) | 42.83 (9.97) | <0.001 |
| Years of education | 12.45 (2.99) | 12.50 (2.93) | 0.75 |
| WRAT-3 reading subtest | 92.23 (18.33) | 91.50 (17.50) | 0.45 |
| Race/ethnicity | 0.01 | ||
| Black, non-Hispanic | 664 (64) | 323 (62) | |
| White, non-Hispanic | 142 (14) | 50 (10) | |
| Hispanic | 192 (18) | 126 (24) | |
| Other | 39 (4) | 22 (4) | |
| Annual household income ≤12,000/year | 452 (44) | 232 (44) | 0.93 |
| Currently employed | 370 (36) | 228 (44) | 0.002 |
| Clinically relevant Depressive symptoms† | 322 (31) | 163 (31) | 0.94 |
| Currently smoking | 425 (41) | 243 (47) | 0.03 |
| Recent use | |||
| Heavy alcohol | 145 (14) | 114 (22) | <0.001 |
| Marijuana | 161 (15) | 117 (22) | <0.001 |
| Crack, cocaine, and/or heroin use | 60 (6) | 44 (8) | 0.04 |
| Opioids | 121 (12) | 49 (9) | 0.18 |
| Anti-anxiety | 136 (13) | 38 (7) | <0.001 |
| Anticonvulsants | 59 (6) | 20 (4) | 0.12 |
| Either anti-anxiety or anticonvulsants | 173 (17) | 52 (10) | <0.001 |
| NC-AE medications | <0.001 | ||
| 0 | 632 (61) | 377 (72) | |
| 1 | 223 (22) | 78 (15) | |
| >1 | 182 (18) | 66 (13) | |
| Anticholinergic-acting medications | 0.01 | ||
| 0 | 792 (76) | 431 (83) | |
| 1 | 171 (17) | 66 (13) | |
| >1 | 74 (7) | 24 (4) | |
| Hepatitis C RNA positive | 238 (23) | 66 (13) | <0.001 |
| HIV RNA | |||
| Undetectable (<48cp/ml) | 542 (52) | - | |
| Detectable, median (IQR) | 1207 (14793) | - | |
| ≥10,000cp/ml | 141 (14) | ||
| CD4 count, median (IQR) | - | - | |
| Current | 505 (407) | - | |
| Nadir | 189 (201) | ||
| cART and ≥95% adherence | 667 (83) | - | |
| ART duration (years), median (IQR) | 13.05 (5.40) | - | |
| CPE Exposure | |||
| Low (<8) | 648 (62) | ||
| Medium (8-9) | 224 (22) | ||
| High (>9) | 165 (16) | ||
| Prior AIDS diagnosis | 459 (44) | - | |
| NP test performance | |||
| Clinical Rating Score, mean (SE)║ | |||
| Global | 3.62 (0.13) | 3.39 (0.14) | 0.01 |
| Learning | 2.85 (0.11) | 2.69 (0.12) | 0.07 |
| Memory | 2.71 (0.11) | 2.53 (0.12) | 0.04 |
| Attention/Working Memory | 2.34 (0.11) | 2.17 (0.11) | 0.02 |
| Executive function | 3.10 (0.13) | 2.88 (0.13) | 0.02 |
| Speed | 2.89 (0.12) | 2.72 (0.12) | 0.05 |
| Fluency | 2.67 (0.11) | 2.61 (0.11) | 0.45 |
| Motor | 2.62 (0.12) | 2.62 (0.13) | 0.97 |
Note.
higher is worse, and scores are from models adjusting for ARS, HCV status, depressive symptoms, heavy drinking, smoking, marijuana, crack, cocaine and/or heroin use. SE=standard error. NP=neuropsychological; WRAT-3=Wide Range Achievement Test standard score;
CES-D=Center for Epidemiological Studies Depression scale ≥16 cutoff; current, refers to within the past week; recent, refers to within 6 months of the most recent WIHS visit; heavy alcohol use reflects >7 drinks/week or ≥4 drinks in one sitting; cART=combination antiretroviral therapy; ART = antiretroviral therapy; CPE=CNS Penetration Effectiveness; NC-AE=non-ARV medication with known adverse cognitive effects generally; IQR=interquartile range. Variables reported as n (%) were analyzed with Chi-square tests. Variables reported as M (SD) were analyzed with independent t-tests. Variables reported as median/IQR were analyzed with Wilcoxon-Mann-Whitney test.
HIV+ women reported using more antidepressants, and more non-ARV NC-AE medications including anxiolytics, and medications with known anticholinergic properties versus HIV− women (p’s<0.05). Reported opioid and anticonvulsant use was the same among HIV+ and HIV− women. Among HIV+ women using non-ARV NC-AE medications (n=405), the most common NC-AE medication reported by drug class were: anxiolytics alone (n=46, 11%), opioids alone (n=45, 11%), antihistamines alone (n=31, 8%), antidepressants alone (n=31, 8%), beta-blockers alone (n=23, 6%), and antipsychotics alone (n=21, 5%). In HIV− women who reported using NC-AE medications (n=144), the most common drug classes used were: opioids alone (n=22, 15%), antipsychotics alone (n=14, 10%), antihistamines alone (n=9, 6%), beta-blockers alone (n=8, 5%), and antidepressants alone (n=8, 5%). With respect to cognitive performance, HIV+ women performed lower than HIV− women on global function, memory, attention/working memory, and executive function.
Total non-ARV NC-AE medication burden on cognitive performance
Table 2 presents cognitive performance by NC-AE medication use in the overall sample. Non-ARV NC-AE medication use was not associated with cognitive performance (p’s>0.07) and non-ARV NC-AE medication use did not moderate the association of HIV and performance (p>0.10, Figure 1).
Table 2.
Cognitive performance by NC-AE medication use in The Women’s Interagency HIV Study.
| Total NC-AE medications
|
Generalized linear model†
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Obs | 0 | 1 | >1 | Overall | 1 vs. 0 | >1 vs. 0 | >1 vs. 1 |
| (n=1009) | (n=301) | (n=248) | ||||||
| M (SE) | M (SE) | M (SE) | p-value | p-value | p-value | p-value | ||
| Global NP | 1556 | 3.2 (0.10) | 3.4 (0.13) | 3.5 (0.14) | 0.08 | 0.07 | 0.07 | 0.86 |
| Memory | 1553 | 2.4 (0.09) | 2.4 (0.11) | 2.6 (0.12) | 0.23 | 0.86 | 0.09 | 0.18 |
| Learning | 1553 | 2.5 (0.08) | 2.6 (0.11) | 2.6 (0.12) | 0.16 | 0.11 | 0.13 | 0.95 |
| Attention/WM | 1398 | 2.3 (0.09) | 2.4 (0.11) | 2.2 (0.11) | 0.24 | 0.58 | 0.16 | 0.10 |
| Executive Function | 1540 | 2.8 (0.10) | 2.9 (0.13) | 3.0 (0.13) | 0.07 | 0.04 | 0.11 | 0.59 |
| Speed | 1555 | 2.6 (0.09) | 2.7 (0.11) | 2.8 (0.13) | 0.58 | 0.46 | 0.36 | 0.82 |
| Fluency | 1550 | 2.6 (0.09) | 2.6 (0.11) | 2.6 (0.12) | 0.90 | 0.85 | 0.97 | 0.75 |
| Motor | 1531 | 2.4 (0.09) | 2.6 (0.12) | 2.6 (0.13) | 0.19 | 0.13 | 0.16 | 0.98 |
Note. Bold=significant at p<0.05. WM=working memory; NP=neuropsychological. M = estimated mean T-score; SE = standard error; OR=odds ratio. NC-AE=non-ARV medication with known general adverse cognitive effects.
controlling for HIV− and HCV status, site, current employment status, depressive symptoms, heavy drinking, smoking, marijuana, crack, cocaine and/or heroin use.
after controlling the false discovery rate using the Benjamini-Hochberg procedure the overall association remained significant.
Figure 1.
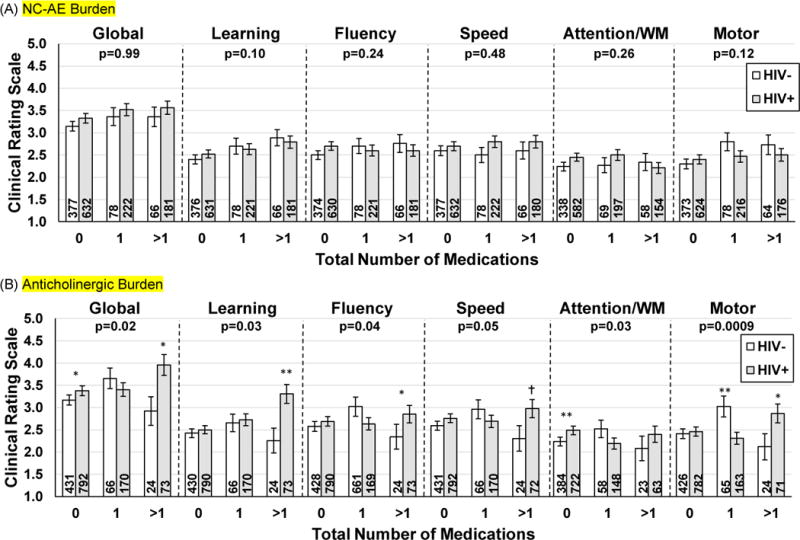
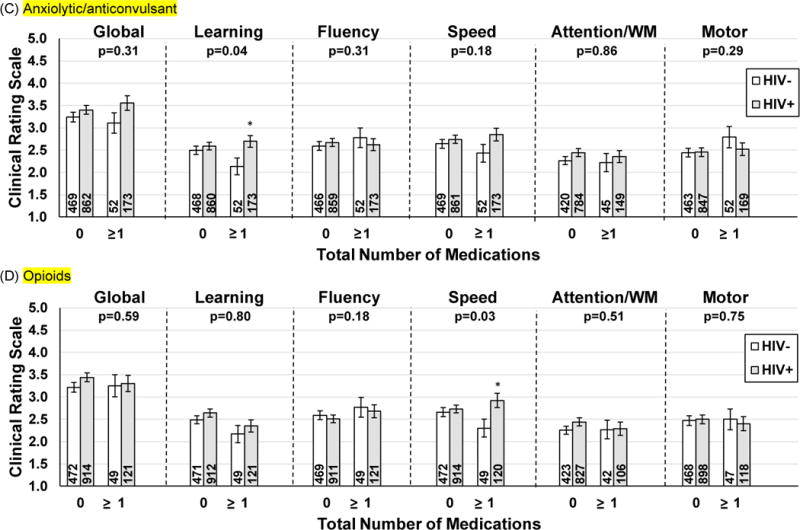
Association of (A) NC-AE, (B) anticholinergic, (C) anxiolytic/anticonvulsant, and (D) opioids use and cognitive performance as a function of HIV-serostatus. Higher Clinical Rating Scale score = worse performance.
(A) NC-AE Burden
(B) Anticholinergic Burden
(C) Anxiolytic/anticonvulsant
(D) Opioids
Note. WM=working memory. p=is the p-value for the interaction between HIV-status and total number of medications. Numbers in the bars represent the sample size. NC-AE=non-ARV medication with known general adverse cognitive effects. Executive function and memory as there were no interactions between total meds and HIV-serostatus on these two domains.**p<0.01; *p<0.05; †p=0.05
Total anticholinergic burden on cognitive performance
Table 3 presents cognitive performance by non-ARV medications with anticholinergic properties in the overall sample. Total anticholinergic burden was negatively associated with learning and executive function. Regarding executive function, women taking at least two medications with anticholinergic properties demonstrated lower performance than women taking no medications, potentially indicating a threshold effect of anticholinergic burden on these domains. There were incremental increases on learning impairment with anticholinergic burden (0 > 1 medication > more than 1 medication).
Table 3.
Cognitive performance by anticholinergic burden.
| Total Anticholinergic Burden
|
Generalized linear model†
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Obs | 0 | 1 | >1 | Overall | 1 vs. 0 | >1 vs. 0 | >1 vs. 1 |
| (n=1223) | (n=237) | (n=98) | ||||||
| M (SE) | M (SE) | M (SE) | p-value | p-value | p-value | p-value | ||
| Global NP | 1556 | 3.3 (0.10) | 3.4 (0.14) | 3.6 (0.20) | 0.07 | 0.21 | 0.03 | 0.26 |
| Memory | 1553 | 2.4 (0.08) | 2.6 (0.12) | 2.6 (0.17) | 0.22 | 0.18 | 0.17 | 0.69 |
| Learning | 1553 | 2.4 (0.08) | 2.7 (0.12) | 3.0 (0.18) | <0.001* | 0.03 | 0.0003 | 0.06 |
| Attention/WM | 1398 | 2.4 (0.09) | 2.2 (0.11) | 2.3 (0.17) | 0.40 | 0.21 | 0.50 | 0.86 |
| Executive Function | 1540 | 2.8 (0.09) | 2.8 (0.13) | 3.3 (0.20) | 0.02 | 0.69 | 0.0003 | 0.02 |
| Speed | 1555 | 2.7 (0.09) | 2.8 (0.12) | 2.8 (0.17) | 0.78 | 0.63 | 0.56 | 0.83 |
| Fluency | 1550 | 2.6 (0.09) | 2.7 (0.12) | 2.7 (0.16) | 0.84 | 0.57 | 0.84 | 0.87 |
| Motor | 1531 | 2.4 (0.09) | 2.5 (0.13) | 2.7 (0.18) | 0.34 | 0.57 | 0.15 | 0.35 |
Note. Bold=significant at p<0.05. WM=working memory; NP=neuropsychological. M = estimated mean T-score; SE = standard error; OR=odds ratio.
controlling for HIV− and HCV status, site, current employment status, depressive symptoms, heavy drinking, smoking, marijuana, crack, cocaine and/or heroin use.
after controlling the false discovery rate using the Benjamini-Hochberg procedure the overall association remained significant.
In models including the two-way interaction between total anticholinergic burden and HIV-status, the interaction was significant for global function, learning, attention/working memory, fluency, and motor function (p’s<0.05); trend for speed (p=0.05) (Figure 1). Follow-up analyses indicated that the magnitude of performance differences between HIV+ and HIV− women on global function, learning, fluency, and speed depended on the reported number of medications with anticholinergic properties. For these four domains, the greatest performance differences between HIV+ and HIV− women were among women taking two or more medications with anticholinergic properties (p<0.05). Differences in performance on attention and working memory tasks were only observed between HIV+ and HIV− women who were not taking medications with anticholinergic properties (p=0.002). Motor function performance differed between HIV+ and HIV− women among women taking one or more medications. Among women taking one medication, HIV− women demonstrated lower performance than HIV+ women (p=0.002). However, among women taking more than 1 medication, HIV+ women demonstrated lower performance than HIV− women (p=0.04).
To ensure that the pattern of medication effects on cognitive outcomes was not driven by uncontrolled viremia in a subset of HIV+ women, we re-conducted the analyses only among HIV+ women with suppressed plasma HIV RNA (n=542) and HIV− women. The same results were observed for global function, learning, attention/working memory, speed, and motor function. We also examined whether there were any differences in other HIV-related clinical characteristics including the prevalence of different ARV regimens (Supplemental Table 2) that could account for the pattern of associations. The only measured HIV-associated factor that differed among HIV+ women taking none, one, or more than one medication with anticholinergic properties was having a previous AIDS diagnosis. Re-analysis of these data indicated incremental increases in impairment for global, learning, and for motor function specifically among women taking two or more medications with anticholinergic activity (HIV+ with previous AIDS diagnosis > HIV+ without previous AIDS diagnosis > HIV−, p’s<0.05).
Opioid and/or Anxiolytic/anticonvulsant use on cognitive performance
Overall, neither opioid nor anxiolytic/anticonvulsant use was significantly associated with cognitive performance (p >0.05 after controlling for the false discovery rate)(Supplemental Table 3). However, both opioid and anxiolytic/anticonvulsants use moderated the association between HIV status and cognitive functioning. Opioid use moderated the association between HIV status and processing speed (p=0.03) whereas anxiolytic/anticonvulsants moderated the association between HIV status and learning performance (p=0.04; Figure 1). Specifically, among women taking anxiolytic/anticonvulsants, HIV+ women performed worse on learning compared to HIV− women (p=0.01). Similarly, among women taking opioids, HIV+ women performed worse on psychomotor speed compared to HIV− women (p=0.01). There were no differences between HIV+ and HIV− women not taking these medications for either outcome (p’s>0.27). To test if these interactions were driven by uncontrolled viremia, we re-conducted these analyses among the subset of HIV+ women with suppressed plasma HIV RNA and HIV− women. Among the latter group of women, the interaction between anxiolytic/anticonvulsants medications and HIV-serostatus on learning and between opioid use and HIV-serostatus on psychomotor speed were not significant (p’s>0.46).
DISCUSSION
To our knowledge, this is the first study to examine the general effects of non-ARV NC-AE medications as well as the effects of commonly used non-ARV classes of medications such as anticholinergics, opiates, anticonvulsants and anxiolytics with known neurocognitive effects on cognitive performance in a sample of HIV+ and HIV− women. While we demonstrated that non-ARV NC-AE medications are more commonly used among HIV+ compared to HIV− women, differential usage of these non-ARV does not appear to explain greater cognitive impairment among HIV+ compared to HIV− women. However, greater use of non-ARV medications with anticholinergic properties as well as anxiolytics/anticonvulsants and opioids among HIV+ versus HIV− women may in part explain some of the greater cognitive impairment among HIV+ compared to HIV− women particularly on global function, learning, fluency, psychomotor speed, and motor function.
In the present study, there were no overall influences of non-ARV NC-AE drugs on cognitive performance in women irrespective of HIV-serostatus. Typically, there are generalized latency effects, as well as sedative effects, of many medications[39] that can result from influences on a number of neurotransmitter systems. The accumulated influence of these latency and sedative properties are likely reasons for the lower global cognitive function. However, non-ARV NC-AE burden was not associated with any cognitive outcome across all women.
Of the drug categories further examined, anticholinergic-acting medications may in part explain our previous findings of a greater persistence of impairment observed in the learning domain and the gradual decline in motor function over time in HIV+ compared to HIV− women[8]. Among women taking one anticholinergic-acting drug, HIV− women showed lower performance on motor function than HIV+ women. Detailed medication review in the each group revealed that the percentage of individuals taking anti-dopaminergic medications (i.e. antipsychotics) was higher in HIV− women than HIV+ women (36% vs. 25%) within women taking one anticholinergic-acting medication. As motor performance is also robustly influenced by medications with anti-dopaminergic properties[16], similar anticholinergic exposure but higher anti-dopaminergic exposure in HIV− women compared to HIV+ women may in part explain these findings on motor function. Additionally, on domains where we have not previously seen a HIV-serostatus difference over time such as fluency and psychomotor speed[8], anticholinergic-acting medications may have synergistic effects with HIV to yield performance differences. The general[15–17] and more specific adverse cognitive effects[16, 18] of medications with anticholinergic properties are thought to result from suppression of cholinergic system by direct blockade of muscarinic acetylcholine receptors in the brain. Specific cognitive effects appear to depend on selectivity of the medication for one of the five muscarinic receptor subtypes (M1-M5)[40]. Transgenic mouse models lacking the M1 receptor have impairments in learning, memory, and attention. Similarly, in humans, M1-selective antagonism decreases similar cognitive abilities[41].
One possible explanation for medications with anticholinergic properties exacerbating HIV-serostatus differences on cognitive performance globally is that neurotoxic viral proteins may have additive or interactive effects with these medications. Effects on memory may be due to the neurotoxic effects of HIV viral proteins on brain regions important for learning such as the hippocampus[42, 43]. Envelope glycoprotein, gp120, is one example that may be important in HIV-induced cognitive impairment[44]. It has been suggested that gp120-mediated cognitive impairment may, in part, result from impairing cholinergic function[44]. It was also shown that memory impairment induced by gp120 could be reversed by hippocampal cholinergic stimulation in mice[44]. Another neurotoxic viral protein that may contribute to adverse cognitive outcomes is Tat [45]. Tat protein selectively enhances acetylcholine release from human and rodent cortical synaptosomes[46]. The selectivity of Tat on the release of acetylcholine but not other neurotransmitters (i.e. dopamine, glutamate, aspartate, GABA, serine, norepinephrine) suggests that cholinergic neurons may be uniquely sensitive to HIV Tat proteins. These findings, taken together with this present work suggest there may be an additive adverse consequence of HIV neurotoxicity and anticholinergic medication burden. Although gp120 and Tat protein may be factors that contributes to these effects, there could be drug-drug interactions (e.g., non-ARV drug by non-ARV drug; non-ARV drug by ARV drugs; non-ARV drugs by illicit drug use such as crack/cocaine[47]), as well as pharmacodynamics effects such anticholinergic-induced inflammation (e.g., increases interleukin-1β expression[48]) that have not been accounted for in this study. Medication-induced inflammation compounded with HIV-induced inflammation[49] could adversely impact global cognitive performance.
Although HIV+ women reported using more anticonvulsants than HIV− women, unexpectedly anticonvulsant medications were not negatively associated with cognitive performance in women. While using these medications were associated with higher impairment among HIV+ versus HIV− women on learning, these medications were not differentially related to cognitive performance by HIV-serostatus when accounting for uncontrolled viremia among the HIV+ women. Anticonvulsant medications (e.g. phenytoin, carbamazepine, valproate, etc.) are typically associated with cognitive side effects[50, 51]. The adverse effects of anticonvulsant medications on cognition are thought to be related to their influence on glutamate transmission in the brain[52]. Although the extent and type of cognitive adverse effects may vary among anticonvulsants, many induce impairments in attention, memory and mental speed[50, 51]. The lack of association in our analyses after accounting for uncontrolled viremia is likely due to the relatively higher utilization of some newer anticonvulsant drugs (i.e. lamotrigine and pregabalin) which may not adversely influence cognition to a similar degree as older medications[53, 54].
HIV+ and HIV− women reported similar rates of opioid use and in our study sample we did not identify associations between these drugs and cognitive performance. We did find that opioid use was associated with worse psychomotor speed among HIV+ versus HIV− women. However, after accounting for uncontrolled viremia this opioid use was not differentially related to performance on psychomotor speed by HIV-serostatus. These findings were also unexpected as opioid receptors are involved with pain as well as other central neuro-modulatory systems including cognition, and are generally considered to have cognitive impairing effects[55]. Although there is no consensus on differential effects of opioids by cognitive domain, most previous studies have shown that opioid use is associated with decreased psychomotor speed[55, 56]. Opioids are commonly used for the short-term management of pain, often prescribed to take ‘as-needed’. The doses and long- versus short-term use of opioids in our study sample were not consistently available. Thus, the lack of association observed in our study may be partially related to inconsistent, or ‘as needed’ use, which may be less likely to induce notable cognitive adverse effects than higher dose or longer-term exposures.
Limitations of the present study include the cross-sectional study design. Longitudinal studies are underway to examine the longer-term impact of non-ARV medications with adverse cognitive effects on cognitive performance among HIV+ and HIV− women as they age. In addition, medication dose or duration of use was not available and therefore could not be examined or accounted for in the present study. Medication history interviews (e.g., names, dose, times per day, route, reason) and adherence assessments, even when conducted by trained research or medical professionals, are prone to recall bias in the absence of objective pill counts or pharmacy refill data. This may result in observed attenuation of drug-outcome relationships when there is inaccurate reporting of adherence, omission of medications, or reporting medications not actually taken.
In conclusion, HIV+ women appear to take a greater number of non-ARV NC-AE medications as well as non-ARVs with anticholinergic properties as well as anxiolytics and anticonvulsants compared to HIV− women. Despite these differences, non-ARV NC-AE medications appear to have no general effects on global or domain specific cognitive performance among HIV+ and HIV− women. However, HIV+ women may have increased cognitive vulnerabilities to anticholinergic medications. Potential synergy between anticholinergic medications and HIV may explain some HIV-related cognitive impairments. It may be important clinically to consider anticholinergic medication use in HIV+ women.
Supplementary Material
Acknowledgments
We would like to thank the WIHS participants and the Neurocognitive Working Group of the WIHS.
Study Funding: Dr. Rubin’s effort was supported by Grant Number 1K01MH098798-01 from the National Institute of Mental Health (NIMH). Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Footnotes
Conflicts of Interest: None of the authors report any conflicts of interest.
References
- 1.HIV Among People Aged 50 and Over. Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 2.Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV infection and older Americans: the public health perspective. Am J Public Health. 2012;102(8):1516–1526. doi: 10.2105/AJPH.2012.300844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valcour VG. HIV, aging, and cognition: emerging issues. Topics in antiviral medicine. 2013;21(3):119–123. [PMC free article] [PubMed] [Google Scholar]
- 4.Grant I. Neurocognitive disturbances in HIV. Int Rev Psychiatry. 2008;20(1):33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- 5.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacktor N, Skolasky RL, Cox C, Selnes O, Becker JT, Cohen B, et al. Longitudinal psychomotor speed performance in human immunodeficiency virus-seropositive individuals: impact of age and serostatus. J Neurovirol. 2010;16(5):335–341. doi: 10.3109/13550284.2010.504249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrman-Lay AM, Paul RH, Heaps-Woodruff J, Baker LM, Usher C, Ances BM. Human immunodeficiency virus has similar effects on brain volumetrics and cognition in males and females. J Neurovirol. 2016;22(1):93–103. doi: 10.1007/s13365-015-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin LH, Springer G, Maki PM, Anastos K, Gustafson D, Villacres M, Waldrop-Valverde D, Vance D, Bolivar H, Valcour V. Cognitive trajectories over 4 years among HIV+ women wiht optimal viral supression; Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, WA. 2017. [Google Scholar]
- 9.Cysique LA, Heaton RK, Kamminga J, Lane T, Gates TM, Moore DM, et al. HIV-associated neurocognitive disorder in Australia: a case of a high-functioning and optimally treated cohort and implications for international neuroHIV research. J Neurovirol. 2014;20(3):258–268. doi: 10.1007/s13365-014-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS. 2014;28(6):803–811. doi: 10.1097/QAD.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 11.Castelo JM, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Wang Q, Ding H, Shang H. Association of Hippocampal Magnetic Resonance Imaging With Learning and Memory Deficits in HIV-1-Seropositive Patients. J Acquir Immune Defic Syndr. 2015;70(4):436–443. doi: 10.1097/QAI.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 13.Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, et al. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72(19):1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell NL, Boustani MA, Lane KA, Gao S, Hendrie H, Khan BA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–159. doi: 10.1212/WNL.0b013e3181e7f2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. doi: 10.1186/s12877-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eum S, Hill SK, Rubin LH, Carnahan RM, Reilly JL, Ivleva EI, et al. Cognitive burden of anticholinergic medications in psychotic disorders. Schizophr Res. 2017 doi: 10.1016/j.schres.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: A systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209–220. doi: 10.1111/bcp.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papenberg G, Backman L, Fratiglioni L, Laukka EJ, Fastbom J, Johnell K. Anticholinergic drug use is associated with episodic memory decline in older adults without dementia. Neurobiol Aging. 2017;55:27–32. doi: 10.1016/j.neurobiolaging.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31(5):1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hindmarch I. Cognitive toxicity of pharmacotherapeutic agents used in social anxiety disorder. International journal of clinical practice. 2009;63(7):1085–1094. doi: 10.1111/j.1742-1241.2009.02085.x. [DOI] [PubMed] [Google Scholar]
- 21.Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. 2011;4(6):385–407. doi: 10.1177/1756285611417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging. 1993;3(4):335–348. doi: 10.2165/00002512-199303040-00004. [DOI] [PubMed] [Google Scholar]
- 24.Griebling TL. Re: American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Urol. 2016;195(3):667–668. doi: 10.1016/j.juro.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 25.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 26.Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effects. Clinical interventions in aging. 2008;3(2):273–278. doi: 10.2147/cia.s1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radtke KK, Bacchetti P, Anastos K, Merenstein D, Crystal H, Karim R, et al. Use of nonantiretroviral medications that may impact neurocognition: patterns and predictors in a large, long-term HIV cohort study. JAIDS. doi: 10.1097/QAI.0000000000001658. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 30.Hessol NA, Weber KM, Holman S, Robison E, Goparaju L, Alden CB, et al. Retention and attendance of women enrolled in a large prospective study of HIV-1 in the United States. J Womens Health (Larchmt) 2009;18(10):1627–1637. doi: 10.1089/jwh.2008.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, et al. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology. 2015;84(3):231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–513. doi: 10.1001/archinternmed.2007.106. [DOI] [PubMed] [Google Scholar]
- 33.Rubin LH, Pyra M, Cook JA, Weber KM, Cohen MH, Martin E, et al. Post-traumatic stress is associated with verbal learning, memory, and psychomotor speed in HIV-infected and HIV-uninfected women. J Neurovirol. 2016;22(2):159–169. doi: 10.1007/s13365-015-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin LH, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, et al. The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. J Neurovirol. 2015;21(4):422–432. doi: 10.1007/s13365-015-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86(4):334–340. doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 37.Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26(6):894–908. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain Cogn. 2008;68(3):415–435. doi: 10.1016/j.bandc.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- 41.Katz IR, Sands LP, Bilker W, DiFilippo S, Boyce A, D’Angelo K. Identification of medications that cause cognitive impairment in older people: the case of oxybutynin chloride. J Am Geriatr Soc. 1998;46(1):8–13. doi: 10.1111/j.1532-5415.1998.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 42.Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, et al. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117(1):43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- 43.Carey AN, Liu X, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, et al. Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:49–54. doi: 10.1016/j.pnpbp.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farr SA, Banks WA, Uezu K, Freed EO, Kumar VB, Morley JE. Mechanisms of HIV type 1-induced cognitive impairment: evidence for hippocampal cholinergic involvement with overstimulation of the VIPergic system by the viral coat protein core. AIDS Res Hum Retroviruses. 2002;18(16):1189–1195. doi: 10.1089/08892220260387931. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotoxicity research. 2009;16(3):205–220. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- 46.Feligioni M, Raiteri L, Pattarini R, Grilli M, Bruzzone S, Cavazzani P, et al. The human immunodeficiency virus-1 protein Tat and its discrete fragments evoke selective release of acetylcholine from human and rat cerebrocortical terminals through species-specific mechanisms. J Neurosci. 2003;23(17):6810–6818. doi: 10.1523/JNEUROSCI.23-17-06810.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wayman WN, Chen L, Persons AL, Napier TC. Cortical consequences of HIV-1 Tat exposure in rats are enhanced by chronic cocaine. Current HIV research. 2015;13(1):80–87. doi: 10.2174/0929867322666150311164504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshiyama Y, Kojima A, Itoh K, Uchiyama T, Arai K. Anticholinergics boost the pathological process of neurodegeneration with increased inflammation in a tauopathy mouse model. Neurobiol Dis. 2012;45(1):329–336. doi: 10.1016/j.nbd.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Walsh JG, Reinke SN, Mamik MK, McKenzie BA, Maingat F, Branton WG, et al. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology. 2014;11:35. doi: 10.1186/1742-4690-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aldenkamp AP. Effects of antiepileptic drugs on cognition. Epilepsia. 2001;42(Suppl 1):46–49. doi: 10.1046/j.1528-1157.2001.00516.x. discussion 50-41. [DOI] [PubMed] [Google Scholar]
- 51.Mula M, Trimble MR. Antiepileptic drug-induced cognitive adverse effects: potential mechanisms and contributing factors. CNS drugs. 2009;23(2):121–137. doi: 10.2165/00023210-200923020-00003. [DOI] [PubMed] [Google Scholar]
- 52.Ortinski P, Meador KJ. Cognitive side effects of antiepileptic drugs. Epilepsy & behavior : E&B. 2004;5(Suppl 1):S60–65. doi: 10.1016/j.yebeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Javed A, Cohen B, Detyniecki K, Hirsch LJ, Legge A, Chen B, et al. Rates and predictors of patient-reported cognitive side effects of antiepileptic drugs: An extended follow-up. Seizure. 2015;29:34–40. doi: 10.1016/j.seizure.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Aldenkamp AP, Baker G. A Systematic Review of the Effects of Lamotrigine on Cognitive Function and Quality of Life. Epilepsy & behavior : E&B. 2001;2(2):85–91. doi: 10.1006/ebeh.2001.0168. [DOI] [PubMed] [Google Scholar]
- 55.Chapman SL, Byas-Smith MG, Reed BA. Effects of intermediate- and long-term use of opioids on cognition in patients with chronic pain. Clin J Pain. 2002;18(4 Suppl):S83–90. doi: 10.1097/00002508-200207001-00010. [DOI] [PubMed] [Google Scholar]
- 56.Ersek M, Cherrier MM, Overman SS, Irving GA. The cognitive effects of opioids. Pain Manag Nurs. 2004;5(2):75–93. doi: 10.1016/j.pmn.2003.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


