Abstract
Introduction
There is mounting evidence supporting the role of tryptophan metabolism via the kynurenine pathway (KP) in the pathogenesis of primary brain tumors. Under normal physiological conditions, the KP is the major catabolic pathway for the essential amino acid tryptophan. However, in cancer cells, the KP becomes dysregulated, depletes local tryptophan, and contributes to an immunosuppressive tumor microenvironment.
Methods
We examined the protein expression levels (in 73 gliomas and 48 meningiomas) of the KP rate-limiting enzymes indoleamine 2,3-dioxygenase (IDO) 1, IDO2, and tryptophan 2,3-dioxygenase (TDO2), as well as, the aryl hydrocarbon receptor (AhR), a carcinogenic transcription factor activated by KP metabolites. In addition, we utilized commercially available small-molecules to pharmacologically modulate IDO1, IDO2, TDO2, and AhR in patient-derived glioma and meningioma cell lines (n = 9 each).
Results
We observed a positive trend between the grade of the tumor and the average immunohistochemical staining score for IDO1, IDO2, and TDO2, with TDO2 displaying the strongest immunostaining. AhR immunostaining was present in all grades of gliomas and meningiomas, with the greatest staining intensity noted in glioblastomas. Immunocytochemical staining showed a positive trend between nuclear localization of AhR and histologic grade in both gliomas and meningiomas, suggesting increased AhR activation with higher tumor grade. Unlike enzyme inhibition, AhR antagonism markedly diminished patient-derived tumor cell viability, regardless of tumor type or grade, following in vitro drug treatments.
Conclusions
Collectively, these results suggest that AhR may offer a novel and robust therapeutic target for a patient population with highly limited treatment options.
Keywords: Aryl hydrocarbon receptor, gliomas, meningiomas, primary patient-derived tumor cells, immunosuppressive kynurenine pathway, tryptophan metabolism
INTRODUCTION
Primary brain tumors, both malignant and non-malignant, occur in adults at an average annual age-adjusted incidence rate of around 40 per 100,000 individuals [1]. Meningiomas and gliomas represent the most common non-malignant and malignant tumor types of the CNS, respectively, with WHO grade IV gliomas (glioblastoma) representing the most common malignancy. Treatment for low-grade gliomas remains controversial; however, recent studies suggest that an earlier, more aggressive personalized treatment nearly doubles the median survival compared to the classic ‘wait-and-see’ approach [2, 3]. The Stupp regimen (maximal surgical resection followed by radiation with concurrent and adjuvant chemotherapy) represents the current standard of care for glioblastoma patients [4]. Low-grade meningiomas are often curable with complete surgical excision, while high-grade and recurrent meningiomas typically undergo resection followed by radiation therapy [5]. To date, there are no FDA-approved chemotherapeutic agents that are effective against meningiomas, with only minimal effects observed using hydroxyurea [6]. Due to the lack of treatment options beyond surgery and radiation, median survival for patients harboring high-grade meningiomas is a meager 2–7 years [7]. Worse yet, despite decades of research and an aggressive treatment regimen, the average survival for glioblastoma patients remains a dismal 15 months [8]. Poor prognosis and lack of effective therapies for primary brain malignancies highlight the urgent need to develop novel therapeutic strategies.
‘Deregulating cellular energetics’ was identified as an emerging hallmark of cancer by Hanahan and Weinberg [9]. Recent studies suggest that altered tryptophan metabolism plays an important role in the pathophysiology of gliomas [10–13]. Likewise, altered levels of tryptophan metabolites have been noted in meningiomas [14]. Tryptophan is an essential amino acid required for protein biosynthesis. Of the tryptophan not incorporated into proteins, more than 95% is metabolized via the kynurenine pathway (KP; Fig. 1) [15]. Under normal physiological conditions, the KP generates various active metabolites and is the main source of the essential metabolic co-factor, nicotinamide adenine dinucleotide (NAD+) [15, 16]. However, in cancer patients, local tryptophan depletion and the overproduction of active metabolites leads to an immunosuppressive tumor microenvironment [17, 18]. The initial and rate-limiting step of the KP, where tryptophan is converted to N-formylkynurenine, is carried out by a trio of enzymes: indoleamine 2,3-dioxygenase 1 (IDO1), indoleamine 2,3-dioxygenase 2 (IDO2), and tryptophan 2,3-dioxygenase (TDO2). N-formylkynurenine is rapidly converted to kynurenine by formamidase. Kynurenine, the central metabolite of the KP, can be further metabolized into several neuroactive metabolites: kynurenic acid (KYNA), 3-hydroxykynurenine, and quinolinic acid.
Figure 1. An abridged version of the kynurenine pathway (KP) of tryptophan metabolism.
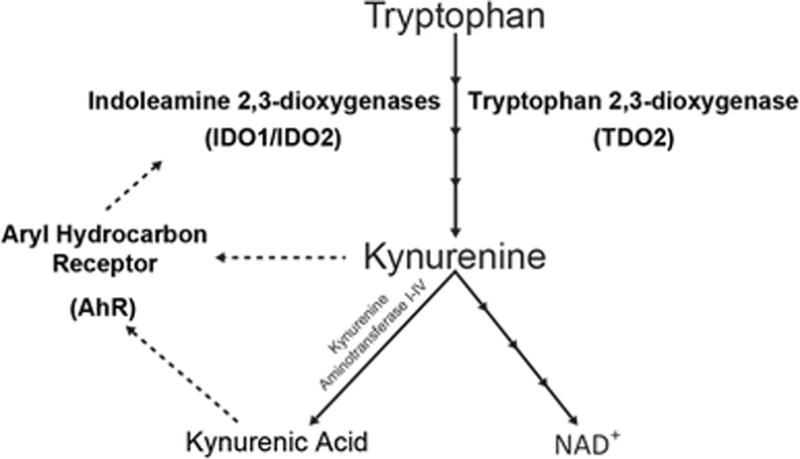
The KP is responsible for majority of the body’s NAD+ production. Kynurenine and kynurenic acid have been discovered to be endogenous ligands of the aryl hydrocarbon receptor (AhR). Solid lines with multiple arrowheads represent a multistep process involving additional enzymes. Dotted lines represent the ability of kynurenine and kynurenic acid to activate the AhR, and for AhR to transcribe indoleamine 2,3-dioxygenase (IDO) 1 and IDO2.
Overexpression of IDO1 (and to some extent IDO2) has been well established in both primary and metastatic brain tumors [12, 13, 19–22]. TDO2 was suggested to be a predominant rate-limiting enzyme of the KP in gliomas [11]. Moreover, kynurenine was identified as an endogenous ligand of the aryl hydrocarbon receptor (AhR, previously known as the dioxin receptor) [23]. Similarly, KYNA has also been shown to be a potent AhR activator [24]. AhR, a ligand activated transcription factor associated with carcinogenesis, was originally thought to only be activated by exogenous toxins such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [25]. Recently, AhR activity has also been found to increase expression of IDO1 [26] and IDO2 [27]. Increased expression of AhR has been observed in a number of extracranial solid tumors (e.g., lung, cervical, ovarian, and breast cancer) as well as glioblastoma [28, 29]. Furthermore, AhR signaling plays a central role in the generation of the malignant phenotype in glioma cells [30] and is gaining attention as a viable therapeutic target in several cancer types [25, 31, 32]. The discovery that KP metabolites are endogenous ligands of AhR suggests that there may be an intrinsic AhR-activated tumoral component of the KP independent of the immune system.
In this study, we examined the protein expression (using tissue microarrays [TMAs]) and the effects of pharmacological modulation of IDO1, IDO2, TDO2, and AhR (using patient-derived cell lines) in WHO grade II–IV gliomas and WHO grade I–III meningiomas. AhR is a downstream effector of the KP independent of the intact immune system; as such, we hypothesized that AhR antagonism would lead to a greater reduction in cell viability compared to inhibition of the rate-limiting enzymes IDO1, IDO2, and TDO2.
MATERIALS AND METHODS
Reagents
Primary antibodies used were as follows: IDO1 (0.1 mg/mL, cat.# NBP1-87702, Novus Biologicals, Littleton, CO), IDO2 (0.5 mg/mL, cat.# OAAB08672, Aviva Systems Biology, San Diego, CA), TDO2 (0.3 mg/mL, cat.# NBP2-13424, Novus Biologicals), and AhR (0.73 mg/mL, cat.# ab153744, Abcam, Cambridge, MA). All primary antibodies were anti-human rabbit antibodies and were used at a 1:100 dilutions, except IDO2, which was used at a 1:50 dilution. Epacadostat was purchased from Chemietek (cat.# CT-EPAC, Indianapolis, IN), while tenatoprazole, 680C91, and CH223191 were purchased from Sigma (cat.# SML1441, SML0287, and C8124, respectively). To measure cell viability, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Invitrogen, Carlsbad, CA) was used following the manufacturer’s protocol.
Patient-derived cell lines
The Wayne State University Institutional Review Board approved the study and written informed consent was obtained from all patients. Cell lines were generated following our previously established method [33]. Briefly, tumor samples were acquired immediately following microsurgical resection after adequate material was reserved for clinical histopathological analysis. Samples were divided into multiple pieces depending upon the size of the tumor collected, with one piece dissociated into a single cell suspension for in vitro cultures following the manufacturer’s protocol for the gentleMACS Dissociator™ (Miltenyi Biotec, San Diego, CA), in conjunction with the Human Tumor Dissociation Kit (Miltenyi Biotec). Cell count and viability were determined using the acridine orange/propidium iodide staining solution with the Cellometer K2 Image Cytometer (Nexcelom, Lawrence, MA).
Tissue microarray generation
Formalin-fixed paraffin-embedded tissues were used in this study and obtained by the Biobanking and Correlative Sciences Core at Karmanos Cancer Institute. Tissue collection ranged from 2004–2014 and 1998–2012 for gliomas and meningiomas, respectively. Hematoxylin and eosin stained sections of each tumor sample were marked by a pathologist to identify the specific tissue area of each tumor to be used for generating the two TMAs. Areas selected were determined by a pathologist to have the greatest density of tumor cells. The meningioma TMA consisted of the following tumors: WHO grade I (n = 18), grade II (n = 16), and grade III (n = 17). The glioma TMA was comprised of: WHO grade II tumors (n = 6), grade III (n = 15), and grade IV (n = 52). Two cores from each patient tumor were included on each TMA, with control tissues used to ensure proper orientation of the TMA for analysis. TMAs were created using a TMA Master (3DHISTECH, Budapest, Hungary). TMAs were sectioned at 4–5 μm and mounted on slides for analysis.
Immunohistochemical staining of tumor tissues
Immunohistochemical (IHC) staining was performed on both the glioma and meningioma TMAs using the rabbit VECTASTAIN® Elite ABC kit (cat.# PK-6101, Vector Labs, Burlingame, CA) and a citrate-based antigen unmasking solution (cat.# H-3300, Vector Labs) following the manufacturer’s protocol for both. Briefly, the unmasking solution was warmed in 55°C water for 40 min. Tissues were rehydrated using xylene, 70%, 90%, and 100% ethanol mixtures. Tissues were then placed in the warmed solution and incubated in a steamer for 20 min. Blocking was performed for 20 min, followed by an hour incubation with the primary antibody at room temperature. Secondary antibody was incubated for 30 min at room temperature. The peroxidase substrate used was Vector ImmPACT® DAB solution (cat.# SK-4105, Vector Labs). Sections were counterstained with hematoxylin and mounted with VectaMount™ (cat.# H-500, Vector Labs). Slides processed with no primary antibody were used as negative controls, while lymph node (IDO1) and liver (IDO2, TDO2, and AhR) were used as positive controls. Staining was scored independently by three researchers on a scale from 0–3 as follows: 0 = no staining; 1 = low staining; 2 = moderate staining; 3 = strong staining. Scores were based on the entirety of each core, taking into account not only intensity of the stain, but also the overall abundance of staining. For each antibody, positive and negative control sections were used to determine the criteria for scores of 0 and 3. Nuclear localization was determined by counting the total number of cells with apparent nuclear staining and dividing by the total number of cells present. Final scores were generated by averaging the three scores. Hematoxylin and eosin staining was performed following a standard protocol [34].
Immunocytochemical staining of patient-derived cell lines
To ensure that the patient-derived cell lines selected to be used for in vitro drug treatments accurately reflected the IHC characteristics of the patient tumor tissue, immunocytochemical (ICC) staining was performed. Cells were seeded onto Millicell® EZ slides (EMD Millipore, Billerica, MA) and fixed with 4% paraformaldehyde for 30 min before proceeding with immunostaining procedures using the rabbit VECTASTAIN® Elite ABC kit (Vector Labs) following the manufacturer’s protocol. The peroxidase substrate used was Vector ImmPACT® DAB solution (Vector Labs). Slides were mounted with VectaMount™ (Vector Labs).
In vitro drug treatment
When cells reached approximately 70% confluency on their flask, they were enzymatically harvested using Accumax (Innovative Cell Technologies, San Diego, CA), counted using the Cellometer K2 Image Cytometer (Nexcelom), as described above, and plated onto 96-well plates at a seeding density of 4,000 cells per well. Cells were allowed to attach and grow overnight in DMEM/F12 media (cat.# MT10090CV, ThermoFisher, Pittsburgh, PA) containing 10% fetal bovine serum (cat.# 26140079, ThermoFisher). Media was removed the next day and replaced with DMEM/F12 media with 10% fetal bovine serum containing small molecule inhibitors for IDO1 (epacadostat, 20 nM), IDO2 (tenatoprazole, 4 μM), TDO2 (680C91, 2 μM), and an AhR antagonist (CH223191, 20 μM). Three cell lines were treated per histologic grade, per tumor type, and experiments were performed in triplicate. All inhibitors were dissolved in DMSO, and DMSO levels never exceeded 1%. Cells were treated for a total of 48 hrs, followed by cell viability measurement via the MTT assay. Plates were read using an Infinite M200 plate reader (Tecan, Mannedorf, Switzerland) and quantified using the Magellan version 6.2 software (Tecan).
Statistical analysis
All statistical analyses were performed using GraphPad Prism version 6.05 for Windows (GraphPad Software, La Jolla, CA). Since the majority of tumors were astrocytic and their values did not appear to be different from oligodendrogliomas, we did not separate these tumor types in the analyses. IHC scores were analyzed using the Kruskal-Wallis test followed by post hoc Mann-Whitney test to compare groups pairwise. MTT data was analyzed using the Wilcoxon signed rank test to determine drug treatment efficacy. ICC AhR nuclear localization scores were analyzed using an unpaired t test. A p < 0.05 was considered statistically significant.
RESULTS
Immunohistochemical and immunocytochemical staining of glioma and meningioma samples
Glioma and meningioma TMA IHC staining showed low abundance of IDO1 as compared to IDO2 and TDO2 (Fig. 2). IDO1 staining had the lowest average scores for both gliomas and meningiomas (0.9 and 0.7, respectively), while TDO2 displayed the highest average scores (1.9 and 2.4, respectively; Fig. 3, Supplemental Table 1). AhR was present in all tumor types and grades, with the most prominent staining in glioblastomas (Fig. 2). In gliomas, staining was highest in glioblastoma (2.7), with modest intensity in grades II and III tumors (0.3 and 0.6, respectively, Fig. 3, Supplemental Table 1). Overall, meningioma AhR staining was modest in grade I and grade III (0.3 and 0.5, respectively) and had the highest staining in grade II tumors (1.1, p < 0.05). Moreover, AhR nuclear localization was noted in a significantly greater proportion of cells in glioblastomas compared to grades II and III gliomas, while meningiomas showed no difference in AhR nuclear staining among grades (Fig. 4A).
Figure 2. Immunohistochemical staining of glioma and meningioma patient tissue microarray sections.
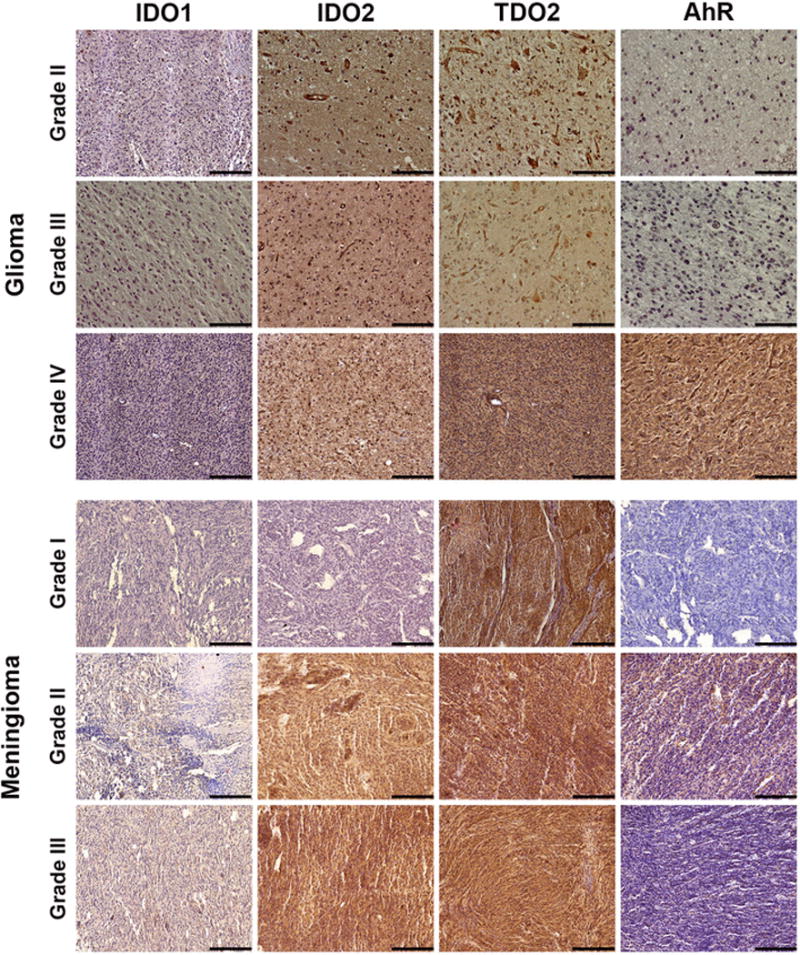
Tissues were stained for the three rate-limiting enzymes of the kynurenine pathway (indoleamine 2,3-dioxygenase (IDO) 1, IDO2, and tryptophan 2,3-dioxygenase 2 [TDO2]), as well as the aryl hydrocarbon receptor (AhR). Regardless of tumor type or histologic grade, IDO1 showed low staining. Conversely, TDO2 showed high staining in all tumor sections. AhR staining was greatest in glioblastomas. Images are representative of the average staining score for the tumor type and antibody used. Images are 20× magnification, with scale bar of 100 μm.
Figure 3. Immunohistochemical staining scores.

Tissue sections from the glioma and meningioma tissue microarrays were scored as described in the Methods section. Averaged scores are represented as a box and whiskers plot using the Tukey method for plotting whiskers and outliers. Mann-Whitney test was used for group comparisons. Glioma tissue staining scores display a general positive trend between staining score and grade; glioblastomas had markedly higher staining that grade II gliomas. Meningioma staining also followed this trend for all rate-limiting enzymes. However, in meningiomas AhR immunostaining was greater in grade II compared to grade III tumors. ** p<0.01, *** p<0.001, and **** p<0.0001.
Figure 4. AhR nuclear staining scores for immunohistochemical and immunocytochemical staining of glioma and meningioma tumor sections and cultured cells. (A).

Immunohistochemical tissue staining of glioma and meningioma tissue microarrays was assessed for the number of cells displaying nuclear localization of AhR. Percentage of cells with nuclear staining are represented as a box and whiskers plot using the Tukey method for plotting whiskers and outliers. Mann-Whitney test was used for group comparisons. Glioma tissue staining scores exhibit a general positive trend between number of cells with nuclear staining score and grade; glioblastomas had a significantly greater number of cells than either grade II or III gliomas. Meningioma staining showed no difference between grades. (B) Immunocytochemical staining of glioma and meningioma cultured patient cells shows no difference in the percentage of cells with AhR nuclear localization in gliomas, however, in meningiomas, we see a significant difference between grades I and III. (C) The overall intensity of AhR nuclear staining was calculated and showed that although there was now difference between glioma grade and the percentage of cells with AhR nuclear localization, the staining intensity of the nuclear AhR significantly increased with tumor grade; glioblastoma cells had the greatest staining intensity. Similarly, in meningiomas we see that grade III cells had the greatest intensity. * p<0.05, ** p<0.005, and **** p<0.0001.
ICC staining displayed similar trends to the IHC for the rate-limiting enzymes (Supplemental Fig. 2): low levels of IDO1 and prominent TDO2 staining. AhR staining in glioma cells showed that although there was no difference across grades for the percentage of cells with nuclear AhR, intensity of the nuclear staining was significantly higher in glioblastoma cells compared to grades II and III cells (Fig. 4B and C). Meningioma cell AhR staining exhibited a significantly higher percentage of cells with nuclear localization in grade III meningioma cells than grade I cells (Fig. 4B). Similar to gliomas, the intensity of the nuclear AhR staining in meningiomas was significantly higher in grade III cells than grade I and II cells (Fig. 4C).
In vitro drug treatments of glioma and meningioma patient-derived cell lines
After confirming the expression of IDO1, IDO2, TDO2, and AhR in our patient-derived cell lines, cells were treated with commercially available small molecule inhibitors or antagonists. Inhibition of IDO1, IDO2, or TDO2 alone lead to no change in cell viability compared to controls (Fig. 5). To further investigate the effect of inhibiting the rate-limiting enzymes, drug combination studies were performed on glioblastomas and grade III meningiomas (Supplemental Fig. 4). No combination of tryptophan-metabolizing enzyme inhibitors, including all three used together, led to a change in cell viability. Only treatment with CH223191, the AhR antagonist, led to a statistically significant decrease in cell viability in both gliomas and meningiomas (p < 0.05), with a >40% reduction in cell viability across all histologic grades and tumor types (Fig. 5).
Figure 5. In vitro drug treatment of patient-derived glioma and meningioma cell lines.
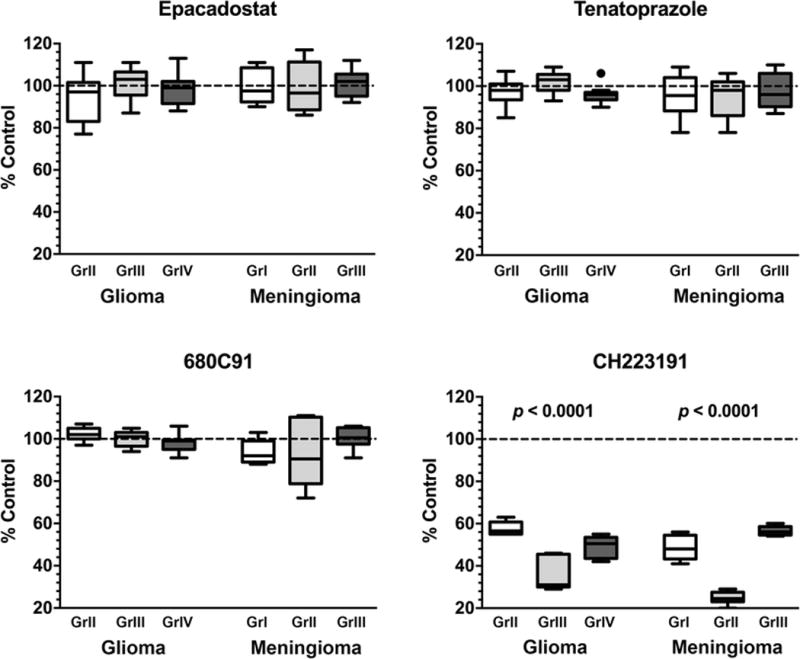
Cultured patient-derived cell lines of each tumor type and grade (n = 3) were treated in triplicate for 48 hrs with the listed drugs at the following concentrations: epacadostat (IDO1 inhibitor) = 20 nM, tenatoprazole (IDO2 inhibitor) = 4 μM, 680C91 (TDO2 inhibitor) = 2 μM, and CH223191 (AhR antagonist) = 20 μM. After treatment, cell viability was measured via MTT assay and represented as percent control and plotted as a box and whiskers plot using the Tukey method. Wilcoxon signed rank test was used to determine drug treatment differences. Inhibition of the rate-limiting enzymes of the kynurenine pathway did not result in a significant decrease in tumor cell viability in all grades and tumor types. However, blockade of AhR activity by the CH223191 antagonist led to a statistically significant loss of cell viability in all tumors.
DISCUSSION
Due to advances in diagnostics and therapeutics, overall primary cancer death rates have dropped by nearly 20% over the last 65 years [35]. In stark contrast, the death rate for brain tumor patients has remained relatively consistent since 1975 at approximately 4.5 per 100,000 people [35]. There is a clear lack of targeted therapeutic options available for glioma and meningioma patients, which is reflected in the essentially unchanged survival over the past 40 years. Tryptophan metabolism via the KP may offer such a target, as altered levels of the KP have been observed in both gliomas and meningiomas [10–14], as well as a number of other solid tumors [36–41]. Beyond immunosuppression [42], there appears to be an intrinsic tumoral component of the KP utilizing AhR signaling activated by the KP metabolites kynurenine and KYNA [11, 23, 24]. The data presented in this study support AhR as a suitable therapeutic target for a diverse brain tumor patient population, based on protein expression and the efficacy of targeting AhR across various tumor types and histologic grades in vitro.
Our IHC data suggest that for both gliomas and meningiomas, TDO2 is the predominant rate-limiting enzyme of the KP. These data are concordant with previous findings in gliomas [10, 11] and confirm our demonstration of high TDO2 expression in meningiomas [43]. Although TDO2 showed the highest immunostaining in both tumor types, inhibition of TDO2 led to no change in cell viability via MTT assay in any tumor type or histologic grade. The same held true for inhibition of IDO1 and IDO2, and the combined inhibition of the 3 rate-limiting KP enzymes. These results however were not surprising, as they support the notion that the anti-tumoral effects of KP inhibition are connected to immune system activation – a mechanism not present in an in vitro system. Furthermore, while the inhibition of all three rate-limiting enzymes should abrogate the conversion of tryptophan into new kynurenine, there may be additional unknown endogenous AhR ligands that continue to activate AhR, despite the drug treatment’s prevention of new kynurenine formation. However, further studies would need to be performed to validate this hypothesis. In contrast to enzyme inhibition, antagonism of AhR led to a statistically significant decrease in viability via MTT assay in all tumor types and grades.
Previously, AhR was linked with the generation of a malignant phenotype in glioma cells [30]. Our immunostaining data corroborate these findings with greater numbers of cells or more intense nuclear AhR staining in glioblastoma tumors and cells compared to the lower grade gliomas. Similar findings were observed in grade III meningiomas compared to the lower grades, suggesting greater AhR activation in the highest grades in both types of malignant tumors. The canonical (genomic) pathway of AhR activation is ligand-bound AhR translocating from the cytosol to the nucleus, a mechanism that has been recently confirmed [44]. Alternatively, a non-genomic pathway for AhR activation has also been proposed [45, 46]; however, discovery of this pathway is relatively recent and remains incompletely understood.
Although the underlying mechanisms are not fully understood, it is generally accepted that one consequence of aging is a decline in immune function [47]. The median age at presentation for patients with glioblastomas and malignant meningiomas is around 65 years [1]. Given this, along with the fact that cytotoxic agents can further suppress immune functions, brain tumor patients would likely benefit from a therapeutic target that works via non-immunologically mediated pathways such as AhR antagonism. Moreover, a recent study found that some IDO1 inhibitors used in clinical trials are in fact AhR agonists themselves [48], potentially limiting the effectiveness of these inhibitors in brain tumor patients. Nevertheless, the findings do not exclude that IDO1, IDO2, TDO2, nor IDO/TDO pan inhibitors could be effective in vivo by remobilizing the immune system.
Taken together, our work demonstrates that: (i) AhR expression is present in the two most common brain tumor types; (ii) AhR nuclear staining increases in intensity with histologic grade in gliomas and meningiomas; and (iii) AhR antagonism (but not inhibition of the KP rate-limiting enzymes) significantly reduces cell viability in gliomas and meningiomas regardless of tumor grade. Although our study was limited to an in vitro model, the promising results of AhR antagonism in patient-derived tumor cells demonstrates that AhR may prove to be an effective target for patients that are in dire need of more efficacious therapeutic options. As newer, more potent AhR antagonists are developed, additional studies will clearly be needed to assess the efficacy of targeting AhR in vivo.
Supplementary Material
Supplemental Figure 1. Immunohistochemical staining of positive controls. Immunostaining for the stated antibody were performed on the following tissues: lymph node (IDO1) and liver (IDO2, TDO2, and AhR). Images are 20× magnification, with scale bar of 100 μm.
Supplemental Figure 2. Immunocytochemical staining of patient-derived cells. Cultured patient-derived glioma and meningioma cells were plated onto 8-well chamber slides. When cells were at a sufficient density, cells were fixed in formalin for 30 min. Immunocytochemical staining was performed as described in the Methods section. Cells were stained for the three rate-limiting enzymes of the kynurenine pathway (indoleamine 2,3-dioxygenase (IDO) 1, IDO2, and tryptophan 2,3-dioxygenase 2 (TDO2)), as well as the aryl hydrocarbon receptor (AhR). IDO1 showed lower staining in gliomas than in meningiomas. IDO2 and TDO2 showed staining in all tumor sections, with TDO2 having the greatest staining intensity. Unlike in the tissue staining, cellular staining of AhR was greatest in the highest grade of each tumor type. Furthermore, cellular staining showed that higher grade tumors also had abundant nuclear staining of AhR. Images are representative of the average staining score for the cell type and antibody used and are 20× magnification, with scale bar of 100 μm.
Supplemental Figure 3. Representative primary antibody immunocytochemical negative controls are shown for each of the tumor types and histological grades. Images are 20× magnification, with scale bar of 100 μm.
Supplemental Figure 4. Drug combination study of high-grade gliomas and meningiomas. Cultured patient-derived grade IV glioblastoma (n = 3) and grade III meningioma cell lines (n = 3) were treated in triplicate for 48 hrs with the listed drug combinations. Drugs used were as follows: IDO1 – epacadostat (20 nM); IDO2 – tenatoprazole (4 μM); and TDO2 – 680C91 (2 μM). After treatment, cell viability was measured via MTT assay. Cell viability is represented as percent control and plotted with a box and whiskers plot using the Tukey method. No combination of the rate-limiting enzyme inhibitors produced a significant loss of cell viability.
Supplemental Table 1. Average immunohistochemical scores of all tumor types and histologic grades.
Acknowledgments
The study was supported, in part, by the following grants: 1F31CA210682-01A1 (A.R.G.) from the National Cancer Institute; 4T32CA009531-30 (A.R.G.) from the National Cancer Institute; R25GM058905 (A.R.G.) from the National Institute of General Medical Sciences; R01CA123451 (C.J and S.M.) from the National Cancer Institute; the Fund for Medical Research and Education, Wayne State University School of Medicine (S.M.); a Strategic Research Initiative Grant from Karmanos Cancer Institute (S.M.); and The Office of the Vice President for Research, Wayne State University (S.M.). The Biobanking and Correlative Sciences Core is supported, in part, by the National Institute of Health Center Grant P30CA022453 to the Karmanos Cancer Institute at Wayne State University. We wish to thank the patients who graciously donated their tumor tissue for this study.
FUNDING
The study was supported, in part, by the following grants: 1F31CA210682-01A1 (A.R.G.) from the National Cancer Institute; 4T32CA009531-30 (A.R.G.) from the National Cancer Institute; R25GM058905 (A.R.G.) from the National Institute of General Medical Sciences; R01CA123451 (C.J and S.M.) from the National Cancer Institute; the Fund for Medical Research and Education, Wayne State University School of Medicine (S.M.); a Strategic Research Initiative Grant from Karmanos Cancer Institute (S.M.); and The Office of the Vice President for Research, Wayne State University (S.M.). The Biobanking and Correlative Sciences Core is supported, in part, by the National Institute of Health Center Grant P30CA022453 to the Karmanos Cancer Institute at Wayne State University.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delgado-Lopez PD, Corrales-Garcia EM, Martino J, Lastra-Aras E, Duenas-Polo MT. Diffuse low-grade glioma: a review on the new molecular classification, natural history and current management strategies. Clin Transl Oncol. 2017 doi: 10.1007/s12094-017-1631-4. [DOI] [PubMed] [Google Scholar]
- 3.Mittal S, Szlaczky MC, Barger GR. Low-grade gliomas in adults. Curr Treat Options Neurol. 2008;10:271–284. doi: 10.1007/s11940-008-0030-0. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Norden AD, Drappatz J, Wen PY. Advances in meningioma therapy. Curr Neurol Neurosci Rep. 2009;9:231–240. doi: 10.1007/s11910-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain MC. Hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma. J Neurooncol. 2012;107:315–321. doi: 10.1007/s11060-011-0741-z. [DOI] [PubMed] [Google Scholar]
- 7.McNeill KA. Epidemiology of Brain Tumors. Neurol Clin. 2016;34:981–998. doi: 10.1016/j.ncl.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Xie Q, Mittal S, Berens ME. Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro Oncol. 2014;16:1575–1584. doi: 10.1093/neuonc/nou147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Adams S, Teo C, McDonald KL, Zinger A, Bustamante S, Lim CK, Sundaram G, Braidy N, Brew BJ, Guillemin GJ. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS One. 2014;9:e112945. doi: 10.1371/journal.pone.0112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 12.Batista CE, Juhász C, Muzik O, Kupsky WJ, Barger G, Chugani HT, Mittal S, Sood S, Chakraborty PK, Chugani DC. Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol. 2009;11:460–466. doi: 10.1007/s11307-009-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guastella AR, Michelhaugh SK, Klinger NV, Kupsky WJ, Polin LA, Muzik O, Juhász C, Mittal S. Tryptophan PET Imaging of the Kynurenine Pathway in Patient-Derived Xenograft Models of Glioblastoma. Mol Imaging. 2016;15 doi: 10.1177/1536012116644881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talari NK, Panigrahi M, Madigubba S, Challa S, Phanithi PB. Altered tryptophan metabolism in human meningioma. J Neurooncol. 2016;130:69–77. doi: 10.1007/s11060-016-2225-7. [DOI] [PubMed] [Google Scholar]
- 15.Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol. 1991;294:345–358. doi: 10.1007/978-1-4684-5952-4_32. [DOI] [PubMed] [Google Scholar]
- 16.Badawy AA. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int J Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 19.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 20.Alkonyi B, Mittal S, Zitron I, Chugani DC, Kupsky WJ, Muzik O, Chugani HT, Sood S, Juhász C. Increased tryptophan transport in epileptogenic dysembryoplastic neuroepithelial tumors. J Neurooncol. 2012;107:365–372. doi: 10.1007/s11060-011-0750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juhász C, Nahleh Z, Zitron I, Chugani DC, Janabi MZ, Bandyopadhyay S, Ali-Fehmi R, Mangner TJ, Chakraborty PK, Mittal S, Muzik O. Tryptophan metabolism in breast cancers: molecular imaging and immunohistochemistry studies. Nucl Med Biol. 2012;39:926–932. doi: 10.1016/j.nucmedbio.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zitron IM, Kamson DO, Kiousis S, Juhász C, Mittal S. In vivo metabolism of tryptophan in meningiomas is mediated by indoleamine 2,3-dioxygenase 1. Cancer Biol Ther. 2013;14:333–339. doi: 10.4161/cbt.23624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safe S, Lee SO, Jin UH. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci. 2013;135:1–16. doi: 10.1093/toxsci/kft128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, Ott M, Ochs K, Lutz C, Liu X, Anastasov N, Lehmann I, Hofer T, von Deimling A, Wick W, Platten M. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–1051. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 29.Johnson J, Ascierto ML, Mittal S, Newsome D, Kang L, Briggs M, Tanner K, Marincola FM, Berens ME, Vande Woude GF, Xie Q. Genomic profiling of a Hepatocyte growth factor-dependent signature for MET-targeted therapy in glioblastoma. J Transl Med. 2015;13:306. doi: 10.1186/s12967-015-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gramatzki D, Pantazis G, Schittenhelm J, Tabatabai G, Kohle C, Wick W, Schwarz M, Weller M, Tritschler I. Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28:2593–2605. doi: 10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 31.Safe S, Cheng Y, Jin UH. The Aryl Hydrocarbon Receptor (AhR) as a Drug Target for Cancer Chemotherapy. Curr Opin Toxicol. 2017;2:24–29. doi: 10.1016/j.cotox.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolluri SK, Jin UH, Safe S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch Toxicol. 2017;91:2497–2513. doi: 10.1007/s00204-017-1981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michelhaugh SK, Guastella AR, Varadarajan K, Klinger NV, Parajuli P, Ahmad A, Sethi S, Aboukameel A, Kiousis S, Zitron IM, Ebrahim SA, Polin LA, Sarkar FH, Bollig-Fischer A, Mittal S. Development of patient-derived xenograft models from a spontaneously immortal low-grade meningioma cell line, KCI-MENG1. J Transl Med. 2015;13:227. doi: 10.1186/s12967-015-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008:pdb prot4986. doi: 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 35.Howlader NNA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2014. National Cancer Institute; Bethesda, MD: SEER data submission, posted to the SEER web site, April 2017. based on November 2016. https://seer.cancer.gov/csr/1975_2014/. [Google Scholar]
- 36.Fotopoulou C, Sehouli J, Pschowski R, S VONH, Domanska G, Braicu EI, Fusch G, Reinke P, Schefold JC. Systemic changes of tryptophan catabolites via the indoleamine-2,3-dioxygenase pathway in primary cervical cancer. Anticancer Res. 2011;31:2629–2635. [PubMed] [Google Scholar]
- 37.Trott JF, Kim J, Abu Aboud O, Wettersten H, Stewart B, Berryhill G, Uzal F, Hovey RC, Chen CH, Anderson K, Graef A, Sarver AL, Modiano JF, Weiss RH. Inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget. 2016;7:66540–66557. doi: 10.18632/oncotarget.11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santhanam S, Alvarado DM, Ciorba MA. Therapeutic targeting of inflammation and tryptophan metabolism in colon and gastrointestinal cancer. Transl Res. 2016;167:67–79. doi: 10.1016/j.trsl.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heng B, Lim CK, Lovejoy DB, Bessede A, Gluch L, Guillemin GJ. Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget. 2016;7:6506–6520. doi: 10.18632/oncotarget.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, Nemkov TG, D’Alessandro A, Hansen KC, Richer JK. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75:4651–4664. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chuang SC, Fanidi A, Ueland PM, Relton C, Midttun O, Vollset SE, Gunter MJ, Seckl MJ, Travis RC, Wareham N, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, Bueno-de-Mesquita HB, Boeing H, Wientzek A, Kuehn T, Kaaks R, Tumino R, Agnoli C, Palli D, Naccarati A, Aicua EA, Sanchez MJ, Quiros JR, Chirlaque MD, Agudo A, Johansson M, Grankvist K, Boutron-Ruault MC, Clavel-Chapelon F, Fagherazzi G, Weiderpass E, Riboli E, Brennan PJ, Vineis P, Johansson M. Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2014;23:461–468. doi: 10.1158/1055-9965.EPI-13-0770. [DOI] [PubMed] [Google Scholar]
- 42.Mandi Y, Vecsei L. The kynurenine system and immunoregulation. J Neural Transm (Vienna) 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 43.Bosnyák E, Kamson DO, Guastella AR, Varadarajan K, Robinette NL, Kupsky WJ, Muzik O, Michelhaugh SK, Mittal S, Juhász C. Molecular imaging correlates of tryptophan metabolism via the kynurenine pathway in human meningiomas. Neuro Oncol. 2015;17:1284–1292. doi: 10.1093/neuonc/nov098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuji N, Fukuda K, Nagata Y, Okada H, Haga A, Hatakeyama S, Yoshida S, Okamoto T, Hosaka M, Sekine K, Ohtaka K, Yamamoto S, Otaka M, Grave E, Itoh H. The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio. 2014;4:796–803. doi: 10.1016/j.fob.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai CF, Hsieh TH, Lee JN, Hsu CY, Wang YC, Lai FJ, Kuo KK, Wu HL, Tsai EM, Kuo PL. Benzyl butyl phthalate induces migration, invasion, and angiogenesis of Huh7 hepatocellular carcinoma cells through nongenomic AhR/G-protein signaling. BMC Cancer. 2014;14:556. doi: 10.1186/1471-2407-14-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin UH, Kim SB, Safe S. Omeprazole Inhibits Pancreatic Cancer Cell Invasion through a Nongenomic Aryl Hydrocarbon Receptor Pathway. Chem Res Toxicol. 2015;28:907–918. doi: 10.1021/tx5005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moyer BJ, Rojas IY, Murray IA, Lee S, Hazlett HF, Perdew GH, Tomlinson CR. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors activate the aryl hydrocarbon receptor. Toxicol Appl Pharmacol. 2017;323:74–80. doi: 10.1016/j.taap.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Immunohistochemical staining of positive controls. Immunostaining for the stated antibody were performed on the following tissues: lymph node (IDO1) and liver (IDO2, TDO2, and AhR). Images are 20× magnification, with scale bar of 100 μm.
Supplemental Figure 2. Immunocytochemical staining of patient-derived cells. Cultured patient-derived glioma and meningioma cells were plated onto 8-well chamber slides. When cells were at a sufficient density, cells were fixed in formalin for 30 min. Immunocytochemical staining was performed as described in the Methods section. Cells were stained for the three rate-limiting enzymes of the kynurenine pathway (indoleamine 2,3-dioxygenase (IDO) 1, IDO2, and tryptophan 2,3-dioxygenase 2 (TDO2)), as well as the aryl hydrocarbon receptor (AhR). IDO1 showed lower staining in gliomas than in meningiomas. IDO2 and TDO2 showed staining in all tumor sections, with TDO2 having the greatest staining intensity. Unlike in the tissue staining, cellular staining of AhR was greatest in the highest grade of each tumor type. Furthermore, cellular staining showed that higher grade tumors also had abundant nuclear staining of AhR. Images are representative of the average staining score for the cell type and antibody used and are 20× magnification, with scale bar of 100 μm.
Supplemental Figure 3. Representative primary antibody immunocytochemical negative controls are shown for each of the tumor types and histological grades. Images are 20× magnification, with scale bar of 100 μm.
Supplemental Figure 4. Drug combination study of high-grade gliomas and meningiomas. Cultured patient-derived grade IV glioblastoma (n = 3) and grade III meningioma cell lines (n = 3) were treated in triplicate for 48 hrs with the listed drug combinations. Drugs used were as follows: IDO1 – epacadostat (20 nM); IDO2 – tenatoprazole (4 μM); and TDO2 – 680C91 (2 μM). After treatment, cell viability was measured via MTT assay. Cell viability is represented as percent control and plotted with a box and whiskers plot using the Tukey method. No combination of the rate-limiting enzyme inhibitors produced a significant loss of cell viability.
Supplemental Table 1. Average immunohistochemical scores of all tumor types and histologic grades.


