Abstract
Objective
We hypothesized second trimester serum cortisol would be higher in spontaneous preterm births compared to provider-initiated (previously termed ‘medically indicated’) preterm births.
Study design
We used a nested case-control design with a sample of 993 women with live births. Cortisol was measured from serum samples collected as part of routine prenatal screening. We tested whether mean adjusted cortisol fold-change differed by gestational age at delivery or preterm birth subtype using multivariable linear regression.
Result
An inverse association between cortisol and gestational age category (trend p=0.09) was observed. Among deliveries prior to 37 weeks, the mean adjusted cortisol fold-change values were highest for preterm premature rupture of the membranes (1.10), followed by premature labor (1.03) and provider-initiated preterm birth (1.01), although they did not differ statistically.
Conclusion
Cortisol continues to be of interest as a marker of future preterm birth. Augmentation with additional biomarkers should be explored.
Prematurity affects more than 1 in 10 babies born globally, and is the second leading cause of death in children under 5 years.1 For many years, researchers have examined a range of biomarkers collected in pregnancy that were hoped to reliably predict some of the variance in risk of preterm birth or elucidate underlying biologic pathways. Cortisol is a hormone that is critical in fetal development and organ maturation,2 and it is the end product of the hypothalamic pituitary adrenal (HPA) axis, and thus is stress responsive. Maternal cortisol levels during pregnancy have been studied in saliva, plasma and hair, and thus far have been inconsistently associated with premature delivery.3–7 In a recent study examining cortisol in hair in each trimester, gestational age at delivery was negatively associated with second trimester cortisol, but not first or third.4 Similar findings were reported previously by Sandman and colleagues, where cortisol levels at 15 weeks of gestation predicted preterm birth, but not cortisol measured at later time points.8 A study in 646 women did not find any association between plasma cortisol measured at 24-26 weeks and spontaneous preterm birth.5 Another study found a positive association between hair cortisol (collected at delivery) and gestational age at delivery; however, authors note that the late collection may have primarily measured the strong natural rise in cortisol at the end of pregnancy, obscuring associations.6 Some of the heterogeneity in findings may be due to the timing of sample collection. One hypothesis supporting increased sensitivity to cortisol in the second trimester is that the fetus is relatively protected from maternal psychobiologic environment in the first trimester due to low maternal blood flow to the placenta and a small placental volume.4,9 In the third trimester, the HPA axis displays diminished responsiveness to exogenous corticotropin releasing hormone (CRH) challenge,10 potentially due to significantly higher function of the cortisol metabolizer 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2), or reduced capacity of the maternal adrenal cortex, which is already producing high levels of cortisol in the third trimestser.4,11,12 A review of 15 studies of cortisol and preterm birth concluded that the majority of studies with samples prior to 23 weeks of gestation found relationships between cortisol and preterm birth, while analyses of samples collected later in pregnancy were not as consistent.7
Another area not well documented is whether cortisol is associated with spontaneous versus provider-initiated preterm birth subtypes. Provider-initiated preterm birth (previously referred to as ‘indicated’), accounts for 30-35% of preterm births. It includes delivery due to maternal or fetal factors such as congenital anomalies, maternal medical conditions (e.g., preeclampsia), and, fetal growth restriction.13,14 Spontaneous preterm birth includes premature labor (with intact membranes), accounting for 40-45% of preterm births, and preterm premature rupture of the membranes (PPROM), which accounts for 25-30% of preterm births.
In many cases of both premature labor and PPROM, inflammation or infection is believed to be involved in the underlying pathologic process.13,15–17 Inflammatory activation occurs during healthy parturition. Preterm labor, however, frequently entails early activation of these same processes, often on a larger scale, which at times may result from the bodies response to infection.17–19 Further, there are bi-directional associations between cortisol and inflammation. Pro-inflammatory cytokines IL-1β and TNF-α enhance production of placental CRH, a driver of cortisol, and down-regulate 11βHSD2,17 which could lead to higher cortisol levels. These and other inflammatory cytokines have been shown to be elevated in asymptomatic women who went on to deliver preterm as early as 17-23 weeks of gestation,17,20 and have been described as key mediators of preterm labor.20 Asides from inflammation triggering increased cortisol, biobehavioral models of stress, infection and preterm birth have also postulated associations in the opposite direction. Wadhwa and colleagues hypothesized that maternal chronic stress, through cortisol, may modulate systemic and local immunity to increase susceptibility to intrauterine and fetal infections-inflammatory processes. Among the direct mechanisms, this may occur through inhibition of production and response of lymphocytes to pro-inflammatory cytokines, and suppressing the differentiation of T-cells.18 Finally, further evidence for the association between stress and inflammatory cytokines was reported in the findings that elevated stress was related to IL-6, both in early and late pregnancy, and was also predictive of elevated IL-1β and IL-6 by stimulated lymphocytes in the 3rd trimester.19 Because of the bi-directional association between cortisol and inflammation/infection,21,22,19 we wanted to determine whether cortisol measured in the second trimester predicts the cause of a preterm delivery.
The frequency and morbidity associated with preterm deliveries warrants a continued research effort to identify early prenatal biomarkers that may signal a woman’s increased risk of any subtype of this outcome. Using a nested case-control design with a sample of 993 live births in the state of California, we aimed to test two hypotheses. First, we hypothesized an inverse association between gestational age at delivery and second trimester serum cortisol levels. Second, among deliveries prior to 37 weeks, we hypothesized that due to unmeasured maternal stress, inflammation or infection, maternal serum cortisol would be the higher among spontaneous preterm births (both premature labor and PPROM) compared with provider-initiated preterm birth. Given the heterogeneity in causes of PPROM and premature labor, we chose to analyze these indications individually. Also, given the racial and ethnic disparities observed in both preterm birth23,24 and cortisol profiles,25 we further tested both of these aims taking into account race/ethnicity.
Methods
Source of the sample
From a cohort of 757,853 singleton live births in the state of California between 2009-2010, a nested case-control study of 993 live births was created. All women had a first trimester ultrasound and had a second trimester serum marker test done as part of routine prenatal screening for aneuploidies and neural tube defects done by the California Genetic Disease Screening Program (n = 241 000). Candidate cases and controls all had a record of a 15 to 20 week second trimester serum sample that was banked by the California Biobank Program after it was used for routine screening (n = 77 604) and had detailed demographic and obstetric information in a linked hospital discharge birth cohort database maintained by the California Office of Statewide Health Planning and Development (OSHPD) (n = 61 339). A number of previous studies have leveraged data and screening results for women in this and other California cohorts.26,27 The final source set for this study included 4 025 singletons with births before 37 weeks, and 56 081 with births on or after 37 completed weeks through 44 weeks. From this set, 500 terms births and 500 preterm births were selected with over-sampling for births before 32 completed weeks gestation (n = 200). This sample included 196 women with deliveries before 32 weeks, 300 with deliveries between 32 and 36 weeks, and 497 with deliveries at or after 37 weeks, all with available serum samples. Serum samples for the remaining seven women were unavailable due to insufficient volume of sample or due to an inability to locate the specimen by the California Biobank Program.28 Methods and protocols for the study were approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California.
Cortisol
Sample metabolic profiling was performed by Metabolon, a commercial supplier of global metabolomic data.29 Samples were split into four extracts of approximately 25 ug; one for chromatography/mass spectrometry (LC/MS) in positive ionization mode, one for chromatography/mass spectrometry (LC/MS) in negative ionization mode, one for gas chromatography/mass spectrometry (GC/MS), and one aliquot was retained. LC/MS was performed on a Waters ACQUITY UPLC and a Thermo-Finnigan LTQ mass spectrometer, which consists of an electrospray ionization (ESI) source and linear ion-trap (LIT) mass analyzer. The sample extracts were analyzed under acidic positive and basic negative ion optimized conditions. Sample extracts designated for GC/MS analysis were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. Peaks were identified using Metabolon’s proprietary peak integration software. Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Metabolon maintains a library based on authenticated standards that contains the retention time/index (RI), mass to charge ratio (m/z), and chromatographic data (including MS/MS spectral data) on all molecules present in the library. Data normalization was performed to correct variation resulting from instrument inter-day tuning differences.
In the data normalization process, a median normalization (to a value of 1.0) was performed to correct for variation from inter-day/inter-batch tuning differences. The fold change was calculated as the ratio of each observation scaled value to the median of 1.0. Cortisol values were slightly right (positive) skewed (skewness=0.93, Kurtosis=1.4, Shapiro-Wilk W=0.93, p<0.001). Transformation of the values by taking the square root reduced the right skew (skewness=0.24, Kurtosis=0.57, Shapiro-Wilk W=0.99, p<0.001). However, results were unchanged when estimated with the square root of the fold change; thus, for ease of interpretation, all results are shown as the untransformed values.
Gestational age at delivery, preterm birth subtype
Gestational age at delivery was categorized into <32 weeks (births at or prior to 32 weeks 0 days) 32-36 weeks (births between 32 weeks 0 days and 36 weeks 6 days), 37-38 weeks (births at 37 weeks 0 days and 38 weeks 6 days), and >38 weeks (39 weeks 0 days and later). The first two categories are reflective of “very preterm birth” and “late preterm births.” Separating 37-38 weeks (“early term”) from >38 weeks accounts for the maturational heterogeneity that continues to occur through late gestation.30 For deliveries prior to 37 weeks, preterm birth subtype was defined as spontaneous preterm birth which included “premature labor” or “PPROM” analyzed separately, as coded in hospital discharge records by ICD-9 CM diagnosis. As described elsewhere,26 mothers delivering before 37 weeks receiving tocolytics based on hospital discharge records were also coded as having had a spontaneous preterm birth with premature labor. Preterm babies without a spontaneous preterm birth were considered to have a provider-initiated preterm birth if there was no spontaneous preterm birth and if there was a code for cesarean delivery or induction present in the hospital discharge file. If none of the codes for spontaneous or provider-initiated preterm birth were present in the hospital discharge files the baby was coded as having preterm birth with an unknown subtype.
Covariates
We relied on hypothesized causal models operationalized as directed acyclic graphs to select covariates a priori for multivariable adjustment from hospital discharge records and birth records. In final models, we included race/ethnicity, maternal pre-pregnancy weight, Medi-Cal recipient status, and sex of the infant in models as potential confounders. Maternal pre-pregnancy weight was missing in 30 samples (3%). We relied on single imputation by regressing weight on race/ethnicity, age, education and parity in order to calculate each woman’s predicted weight and used these values in instances of missing responses.
Statistical analyses
We first described the maternal and pregnancy characteristics by their frequency or mean values, both for the full sample and stratified by gestational age at delivery.
Gestational age at delivery and cortisol fold-change
To evaluate cortisol levels by gestational age category, we performed multivariable linear regression, with gestational age category as the predictor (with >38 weeks reference) and cortisol concentration as the outcome. We then abstracted the multivariable adjusted mean cortisol levels for each gestational age category from this model to present in a figure. Finally, we again performed multivariable linear regression with a continuous indicator variable for gestational age category to test for trend.
Gestational age at delivery and cortisol by race
To examine potential effect measure modification by race/ethnicity, the multivariable linear regression model was then stratified by each race/ethnicity, testing the association between gestational age category and cortisol level for each strata.
Preterm birth indication and cortisol fold-change
To estimate the association between cortisol and indication of preterm birth, we restricted the sample to those with deliveries prior to 37 weeks. We regressed cortisol on preterm birth subtype in multivariable linear regression, with provider-initiated deliveries serving as the reference group for PPROM and premature labor. Because very preterm birth (<32 weeks completed gestation) may have different underlying etiology than preterm deliveries between 32-36 weeks of gestation,26,31 we chose to further stratify the model by very preterm/preterm births.
Preterm birth indication and cortisol fold-change by race/ethnicity
To examine heterogeneity by race/ethnicity, we stratified the multivariable model by race/ethnicity, and again regressed cortisol level on the categorical indication for preterm birth variable, with provider-initiated deliveries serving as the reference. We then abstracted each adjusted mean cortisol value for bar graphs in the figure.
All analyses were performed in SAS 9.4. A significance level of 0.05 was used for all analyses.
Results
Of the 991 births with complete data, 196 (19.8%) were delivered at less than 32 weeks of gestation, 300 (30.2%) were delivered between 32 and 36 weeks gestation, 165 (16.6%) were delivered between 37 and 38 weeks gestation, and 330 (33.3%) were delivered after 38 weeks gestation (Table 1). On average, maternal serum samples were collected at 16.5 weeks of gestation (range 15-20). Cortisol was not associated significantly with gestational age at sample collection (p=0.13). The median time lapse between second trimester serum collection and delivery was 20 weeks (range 4-26 weeks). Among births prior to 37 weeks, the median time lapse was 17 weeks (range 4-21 weeks).
Table 1.
Maternal and pregnancy characteristics of 991 women with live, singleton births in a nested case-control sample from California in the years 2009-2010.
| Full sample n=991 |
<32 weeks n=196 (19.8%) |
32-36 weeks n=300 (30.2%) |
37-38 weeks n=165 (16.6%) |
39-40 weeks n= 330 (33.3%) |
|
|---|---|---|---|---|---|
| Race/ethnicity | |||||
| Non-Hispanic White | 324 (32.7) | 65 (33.2) | 93 (31.0) | 41 (24.9) | 125 (37.9) |
| Hispanic | 483 (48.7) | 100 (51.0) | 142 (47.3) | 87 (52.7) | 154 (46.7) |
| Asian | 97 (9.8) | 15 (7.7) | 36 (12.0) | 23 (13.9) | 23 (7.0) |
| Black | 21 (2.1) | 5 (2.6) | 9 (3.0) | 2 (1.2) | 5 (1.5) |
| Other/not given | 66 (6.7) | 11 (5.6) | 20 (6.7) | 12 (7.2) | 23 (7.0) |
| Maternal age | |||||
| <18 years | 11 (1.1) | 4 (2.0) | 3 (1.0) | 1 (0.6) | 3 (0.9) |
| 18-34 years | 698 (70.4) | 139 (70.9) | 204 (68.0) | 117 (70.9) | 238 (72.1) |
| >34 years | 282 (28.5) | 53 (27.0) | 93 (31.0) | 47 (28.5) | 89 (27.0) |
| Medi-Cal recipient | |||||
| Yes | 410 (41.4) | 97 (49.5) | 122 (40.7) | 72 (43.6) | 119 (36.1) |
| Infant sex | |||||
| Male | 519 (52.4) | 113 (57.7) | 156 (52.0) | 95 (57.6) | 155 (46.9) |
| Preterm birth subtype | |||||
| Provider-initiated | 87 (17.5) | 20 (10.2) | 67 (22.3) | n/a | n/a |
| Premature labor | 235 (47.4) | 99 (50.5) | 136 (45.3) | n/a | n/a |
| PPROM | 157 (31.7) | 74 (37.8) | 83 (27.7) | n/a | n/a |
| Unknown | 17 (3.4) | 3 (1.5) | 14 (4.7) | n/a | n/a |
| Maternal weight (lbs) (mean, SD) | 147.6 (36.5) | 148.8 (41.2) | 149.4 (38.3) | 145.2 (33.2) | 146.5 (33.3) |
| Fold-change of cortisol (mean, SD)a | 1.1 (0.4) | 1.1 (0.4) | 1.1 (0.4) | 1.0 (0.4) | 1.0 (0.4) |
| Gestational age (weeks) at sample collection (mean, SD) | 16.5 (1.1) | 16.4 (1.0) | 16.5 (1.1) | 16.7 (1.1) | 16.5 (1.2) |
Fold change is the ratio of the observed cortisol concentration to the median of 1.0.
Cortisol fold-change levels and gestational age
The multivariable adjusted mean cortisol value decreased with increasing categorical length of gestation (p for trend=0.09), however, in the full sample, the mean cortisol levels for each category of gestational length did not statistically differ from each other (Figure 1). When effects were stratified by race/ethnicity (Table 2), the same pattern of decreasing cortisol with increasing gestational age category was only observed among non-Hispanic White women. Among non-Hispanic White mothers, cortisol was significantly higher among women who gave birth at <32 weeks gestation relative to those who delivered after 38 weeks of gestation (standardized beta coefficient=0.14, 95% CI 0.01, 0.27). There were no other differences in cortisol levels by category of gestational age at delivery for any other race/ethnicity groups.
Figure 1.
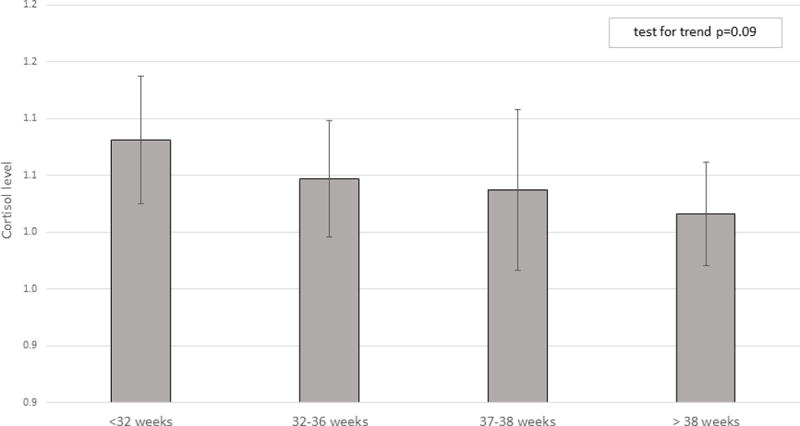
Adjusted mean cortisol by gestational age at delivery. Models adjusted for race/ethnicity, maternal weight, Medi-Cal recipient, and sex of the infant.
Table 2.
Multivariable beta estimates for association between cortisol fold increase and gestational age at delivery
| White (n=324) | Hispanic (n=483) | Asian (n=97) | Black (n=21) | Other (n=66) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | β estimate | 95% CI | n | β estimate | 95% CI | n | β estimate | 95% CI | n | β estimate | 95% CI | n | β estimate | 95% CI | |
| <32 weeks | 65 | 0.14 | 0.01, 0.27 | 100 | 0.06 | −0.04, 0.15 | 15 | −0.13 | −0.41, 0.15 | 5 | 0.48 | −0.04, 1.00 | 11 | −0.04 | −0.32, 0.24 |
| 32-36 weeks | 93 | 0.02 | −0.09, 0.14 | 142 | 0.04 | −0.04, 0.13 | 36 | −0.05 | −0.28, 0.18 | 9 | 0.03 | −0.46, 0.52 | 20 | 0.13 | −0.11, 0.36 |
| 37-38 weeks | 41 | −0.10 | −0.25, 0.05 | 87 | 0.09 | −0.01, 0.19 | 23 | −0.04 | −0.28, 0.21 | 2 | 0.58 | −0.07, 1.23 | 12 | −0.03 | −0.30, 0.25 |
| > 38 weeks | 125 | reference | 154 | reference | 23 | reference | 5 | reference | 23 | reference | |||||
Model adjusted for race/ethnicity, maternal weight, medi-Cal recipient, and sex of infant. Sample size reflective of complete case analysis.
Preterm birth subtype
In births prior to 37 weeks of gestation, cortisol fold-change values were generally highest for PPROM deliveries, followed by premature labor and provider-initiated preterm births; however, standardized beta coefficients did not statistically differ from the reference group (Table 3). When stratified by early (<32 weeks) and late (32-36 weeks) preterm birth, cortisol levels in provider-initiated deliveries were not statistically different than in the other two categories. Cortisol values by preterm birth subtypes for births prior to 37 weeks differed by race/ethnicity (Figure 2). Cortisol level in women with provider-initiated preterm births were significantly lower than those in women with PPROM in both non-Hispanic White and Black women, and significantly lower than Black women with premature labor. Cortisol did not differ by preterm birth subtype for any other race/ethnicity groups.
Table 3.
Multivariable beta estimates for association between cortisol and type of preterm birth (provider initiated, premature labor, PPROM).
| All deliveries prior to 37 weeks | Stratified sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n=479 | <32 weeks or less (n=193) | 32-36 weeks (n=286) | |||||||
| n | β estimate | 95% CI | n | β estimate | 95% CI | n | β estimate | 95% CI | |
| Provider-initiated | 87 | reference | 20 | reference | 67 | reference | |||
| Premature labor | 235 | 0.02 | −0.08, 0.13 | 99 | −0.05 | −0.26, 0.15 | 136 | 0.06 | −0.06, 0.18 |
| PPROM | 157 | 0.10 | −0.01, 0.21 | 74 | 0.13 | −0.07, 0.34 | 83 | 0.04 | −0.08, 0.17 |
Adjusted for race/ethnicity, maternal weight, Medi-Cal recipient, and sex of infant. Sample size reflective of complete case analysis.
Figure 2.
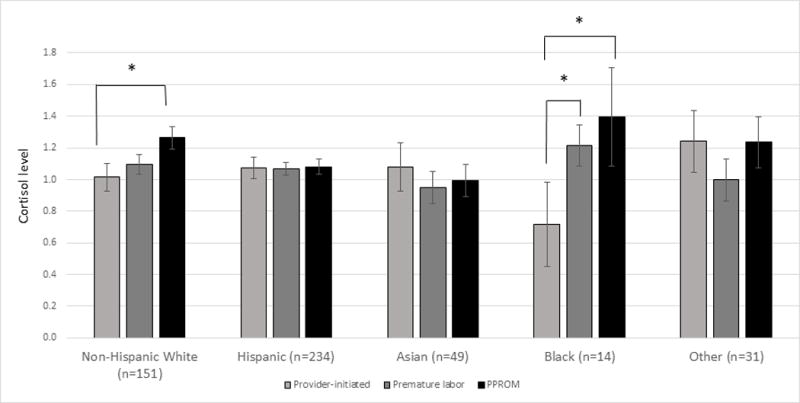
Adjusted mean cortisol levels by indication and race/ethnicity in births prior to 37 weeks of gestation. Models adjusted for maternal weight, Medi-Cal recipient, and sex of infant. Sample sizes reflective of complete case analysis. * indicates p<0.05.
Discussion
Cortisol has been an inconsistent predictor of preterm birth in past research and our findings are no exception. We observed a marginally significant trend (p<0.10) for lower plasma cortisol with increasing gestational age, although the only statistically significant difference was observed between the earliest and latest gestational age subgroups among non-Hispanic White women. When we limited births to deliveries prior to 37 weeks, cortisol levels appeared higher in both PPROM and premature labor indications compared to provider-initiated deliveries; however, these differences were not statistically significant. In additional analyses stratified by race/ethnicity, cortisol levels were significantly higher in PPROM compared to provider-initiated deliveries among non-Hispanic White and Black women. This finding was not observed for Hispanics, Asians, or other race/ethnicities. Of note, exploratory analyses by race/ethnicity relied on small samples sizes, thus effect estimates may be unstable and would benefit from replication in larger samples.
The underlying causes of preterm delivery are assumed to be multifactorial.32 Our hypotheses that maternal cortisol levels may predict the timing of preterm birth and subtype relied on two rationales. First, premature labor may be an early activation of the normal labor process, which is influenced by fetal cortisol levels that stimulate a change in the progesterone/estrogen ratio at the time of parturition.13 Both maternal plasma and amniotic fluid cortisol have been shown to gradually increases from the 8th week of gestation to the 36th week, with a steep rise through the onset of labor.33 Fetal cortisol concentrations of maternal origin are 10-fold lower than maternal levels, maintained through the expression of 11βHSD2 in the placenta.34 However, elevated maternal plasma cortisol, which is further stimulated through the HPA axis by placental CRH, may lead to elevated cortisol exposure to the fetus, particularly in gestations with attenuated expression of 11βHSD2. Many factors have been associated with attenuated 11βHSD2 activity in humans and animal models, including stress, nutritional factors, environmental exposures, and elevated cortisol itself.35–38 The resulting elevated fetal cortisol early in pregnancy may prematurely prompt mechanisms for parturition typically delayed until later in pregnancy. Our results showing an inverse marginal effect of maternal cortisol concentration with increasing gestational age support this thinking. Additionally, the cortisol concentrations, which were collected at a standard time in pregnancy, were measured an average of 17 weeks prior to preterm deliveries, reducing the likelihood that we were observing cortisol spikes associated with imminent labor or delivery. Interestingly, our samples collected in the second trimester correspond with a reported “sensitive period” with respect to timing of cortisol elevation and preterm delivery; however, without measures at other time points, we could not formally test this hypothesis.
The second rationale informing our hypothesis concerned preterm birth subtype. Many risk factors for spontaneous preterm birth– both for premature labor and PPROM – result in inflammation in the form of maternal systemic or local infections, tobacco use, stress, and immune disorders.13 As previously discussed, there is bi-directional cross-talk between inflammatory markers and cortisol. We therefore hypothesized that cortisol would be higher among both types of spontaneous preterm births compared to provider-initiated preterm births. Our results in the full sample of births prior to 37 weeks, and among non-Hispanic White women and Black women, supported this hypothesis; however, we did not observe this pattern among women of other race/ethnicity groups. It should be noted, however, that we did not have complete information on the reasons for provider-initiated preterm delivery, which may have also been inflammatory in nature and would be expected to diminish the contrast with spontaneous or PPROM deliveries. Thus, future studies might be able to obtain such information and fine tune these analyses.
The underlying rationale for assessing the role of cortisol and preterm birth in previous studies is due to a large literature reporting an association between prenatal anxiety, depression or stress and the increased risk of preterm birth (reviewed in 201539). However, cortisol may be elevated for other reasons than psychological stress, including inflammation, obesity, physical inactivity, age, smoking, alcohol intake, and medical conditions.40–42 Thus, while it is important to understand the contribution from stress and the intact HPA axis, we chose to focus only on cortisol as opposed to upstream psychosocial predictors. By evaluating cortisol as a biomarker, particularly as it relates to preterm birth subtypes, we may be able to identify women at risk of preterm birth, as well as generate additional hypotheses to test in relation to the underlying mechanisms.
Although many studies have attempted to characterize the role of cortisol in preterm delivery, our study has unique strengths that add to the literature. The nested case-control study design allowed for a sufficient sample of very premature deliveries for analysis, which enabled us to study the differences with late preterm births. Additionally, cortisol was obtained from non-targeted metabolomics, which allow for reliable identification of small molecule biomarkers. Further, this is one of the few studies that has considered the role of cortisol in the indication for preterm delivery, which aims to elucidate underlying mechanisms and provide a biomarker that can be considered in routine clinical care. Our findings must be considered in light of the limitations. Our serum samples were collected throughout the day as part of routine prenatal screening, and are subject to variability of the natural circadian rhythm. Although we do not expect systematic bias in the time of sample collection, the fluctuations based on the timing of sample collection may attenuate results. Additionally, our sample had very few Black women, who are 49% more likely to deliver preterm compared with all other women.43 We were unable to adequately characterize cortisol in this high-risk group, and strongly support further investigation with larger samples. Lastly, we would have benefited from measures of CRH and placental 11βHSD2 to inspect the interplay with cortisol, as well as repeated measures, which were not available as we relied upon second trimester banked serum.
Preterm birth and the sequelae of prematurity can have sizable adverse effects on future health. In searching for early predictors of preterm birth, researchers aim both to identify those at increased risk, and to understand underlying pathways of this multifactorial outcome. Our findings that support the role of cortisol as a biomarker for both timing and indication of delivery could be refined by models that incorporate in other biomarkers and multiple time points for trajectory analysis. Ultimately, the refinement of these predictive markers may allow for early identification and intervention of this intractable outcome.
Acknowledgments
This work was supported by NIH/NHLBI grants (RC2 HL101748, RO1 HD-57192, and R01 HD-52953), the Bill and Melinda Gates Millennium grants (OPP52256 and RSDP 5K12 HD-00849-23), March of Dimes grants (6-FY11-261 and FY10-180) and the California Preterm Birth Initiative at the UCSF Benioff Children’s Hospital funded by Marc and Lynne Benioff. Data from the California Prenatal Screening Program was obtained through the California Biobank Program (Screening Information System request no. 476). Data were obtained with an agreement that the California Department of Public Health is not responsible for the results or conclusions drawn by the authors of this publication.
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose for this work.
References
- 1.Howson CP, Kinney MV, McDougall L, Lawn JE. Born Toon Soon: Preterm birth matters. Reprod Health. 2013;10:S1–S1. doi: 10.1186/1742-4755-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Himes KP, Simhan HN. Plasma corticotropin-releasing hormone and cortisol concentrations and perceived stress among pregnant women with preterm and term birth. Am J Perinatol. 2011;28:443–448. doi: 10.1055/s-0030-1270119. [DOI] [PubMed] [Google Scholar]
- 4.HOFFMAN MC, MAZZONI SE, WAGNER BD, LAUDENSLAGER ML, ROSS RG. Measures of Maternal Stress and Mood in Relation to Preterm Birth. Obstet Gynecol. 2016;127:545–552. doi: 10.1097/AOG.0000000000001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer MS, Lydon J, Goulet L, Kahn S, Dahhou M, Platt RW, et al. Maternal stress/distress, hormonal pathways and spontaneous preterm birth. Paediatr Perinat Epidemiol. 2013;27:237–246. doi: 10.1111/ppe.12042. [DOI] [PubMed] [Google Scholar]
- 6.Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, et al. Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169:1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- 7.Giurgescu C. Are Maternal Cortisol Levels Related to Preterm Birth? J Obstet Gynecol Neonatal Nurs. 2009;38:377–390. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Burton GJ, Jaunaiux E. Maternal vascularisation of the human placenta: Does the embryo develop in a hypoxic environment? Gynecol Obstet Fertil. 2001;29:503–508. doi: 10.1016/s1297-9589(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 10.SCHULTE HM, WEISNER D, ALLOLIO B. the Corticotrophin Releasing Hormone Test in Late Pregnancy: Lack of Adrenocorticotrophin and Cortisol Response. Clin Endocrinol (Oxf) 1990;33:99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 11.Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; What needs to be done. Best Pract Res Clin Obstet Gynaecol. 2014;28:25–35. doi: 10.1016/j.bpobgyn.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31:285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy UM, Ko C-W, Raju TNK, Willinger M. Delivery indications at late-preterm gestations and infant mortality rates in the United States. Pediatrics. 2009;124:234–40. doi: 10.1542/peds.2008-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Romero R, Miller D, Silva P, Panaitescu B, Theis KR, et al. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am J Reprod Immunol. 2018:e12820. doi: 10.1111/aji.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science (80-) 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keelan JA. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol. 2018;125:89–99. doi: 10.1016/j.jri.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. 2001;15:17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 19.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Vrachnis N, Karavolos S, Iliodromiti Z, Sifakis S, Siristatidis C, Mastorakos G, et al. Impact of mediators present in amniotic fluid on preterm labour. In Vivo (Brooklyn) 2012;26:799–812. [PubMed] [Google Scholar]
- 21.DeSantis AS, DiezRoux AV, Hajat A, Aiello AE, Golden SH, Jenny NS, et al. Associations of salivary cortisol levels with inflammatory markers: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2012;37:1009–1018. doi: 10.1016/j.psyneuen.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeager MP, Pioli PA, Guyre PM. Cortisol Exerts Bi-Phasic Regulation of Inflammation in Humans. Dose-Response. 2011;9:332–347. doi: 10.2203/dose-response.10-013.Yeager. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MENON R, DUNLOP AL, KRAMER MR, FORTUNATO SJ, HOGUE CJ. An overview of racial disparities in preterm birth rates: caused by infection or inflammatory response? Acta Obstet Gynecol Scand. 2011;90:1325–1331. doi: 10.1111/j.1600-0412.2011.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Vaznaugh EV, Braveman PA, Egerter S, Marchi KS, Heck K, Curtis M. Latina Birth Outcomes in California: Not so Paradoxical. Matern Child Health J. 2016;20:1849–1860. doi: 10.1007/s10995-016-1988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon CD, Adam EK, Holl JL, Wolfe KA, Grobman WA, Borders AEB. Prenatal Stress and the Cortisol Awakening Response in African-American and Caucasian Women in the Third Trimester of Pregnancy. Matern Child Health J. 2016;20:2142–2149. doi: 10.1007/s10995-016-2060-7. [DOI] [PubMed] [Google Scholar]
- 26.Jelliffe-Pawlowski LL, Baer RJ, Blumenfeld YJ, Ryckman KK, O’Brodovich HM, Gould JB, et al. Maternal characteristics and mid-pregnancy serum biomarkers as risk factors for subtypes of preterm birth. BJOG. 2015;122:1484–1493. doi: 10.1111/1471-0528.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallenstein M, Jelliffe-Pawlowski LL, Yang W, Carmichael S, Stevenson D, Ryckman K, et al. Inflammatory biomarkers and spontaneous preterm birth among obese women. J Matern Fetal Neonatal Med. 2016;29:3317–22. doi: 10.3109/14767058.2015.1124083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Califonia Biobank Program. https://www.cdph.ca.gov/Programs/CFH/DGDS/Pages/cbp/default.aspx.
- 29.Ryckman KK, Jelliffe-pawlowski L, Bedell B, Holt T, Bell LN, Shaw GM, et al. Global Metabolic Profiling Implicates Altered Fatty Acid and Amino Acid Metabolism in Preterm Birth. 2017 unpublished. [Google Scholar]
- 30.Spong C. Defining ‘term’ pregnancy: Recommendations from the defining ‘term’ pregnancy workgroup. JAMA. 2013;309:2445–2446. doi: 10.1001/jama.2013.6235. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro-Mendoza CK, Lackritz EM. Epidemiology of late and moderate preterm birth. Semin Fetal Neonatal Med. 2012;17:120–125. doi: 10.1016/j.siny.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behrman R, Butler A, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): 2007. [DOI] [PubMed] [Google Scholar]
- 33.Chaim W, Mazor M. The relationship between hormones and human parturition. Arch Gynecol Obstet. 1998;262:43–51. doi: 10.1007/s004040050226. [DOI] [PubMed] [Google Scholar]
- 34.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20:439–450. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 35.Clarke KA, Ward JW, Forhead AJ, Giussani DA, Fowden AL. Regulation of 11 beta-hydroxysteroid dehydrogenase type 2 activity in ovine placenta by fetal cortisol. J Endocrinol. 2002;172:527–34. doi: 10.1677/joe.0.1720527. [DOI] [PubMed] [Google Scholar]
- 36.Seth S, Lewis AJ, Saffery R, Lappas M, Galbally M. Maternal Prenatal Mental Health and Placental 11β-HSD2 Gene Expression: Initial Findings from the Mercy Pregnancy and Emotional Wellbeing Study. Int J Mol Sci. 2015;16:27482–27496. doi: 10.3390/ijms161126034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shang Y, Jia Y, Sun Q, Shi W, Li R, Wang S, et al. Sexually dimorphic effects of maternal dietary protein restriction on fetal growth and placental expression of 11β-HSD2 in the pig. Anim Reprod Sci. 2015;160:40–48. doi: 10.1016/j.anireprosci.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Mikelson C, Kovach MJ, Troisi J, Symes S, Adair D, Miller RK, et al. Placental 11β-Hydroxysteroid dehydrogenase type 2 expression: Correlations with birth weight and placental metal concentrations. Placenta. 2015;36:1212–1217. doi: 10.1016/j.placenta.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth : A systematic review. Women and Birth. 2015;28:179–193. doi: 10.1016/j.wombi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Feller S, Vigl M, Bergmann MM, Boeing H, Kirschbaum C, Stalder T. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology. 2014;39:132–140. doi: 10.1016/j.psyneuen.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Melin EO, Thunander M, Landin-Olsson M, Hillman M, Thulesius HO. Depression, smoking, physical inactivity and season independently associated with midnight salivary cortisol in type 1 diabetes. BMC Endocr Disord. 2014;14:75. doi: 10.1186/1472-6823-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology. 2015;62:301–318. doi: 10.1016/j.psyneuen.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Premature Birth Report Cards. March of Dimes. 2017;2017:1–4. [Google Scholar]


