Abstract
Data on the clinical importance of newly detected donor specific antibodies (ndDSA) following pediatric heart transplantation is lacking despite mounting evidence of the detrimental effect of de novo DSA in solid organ transplantation. We prospectively tested 237 pediatric heart transplant recipients for ndDSA in the first year post-transplant in order to determine their incidence, pattern and clinical impact. One third of patients developed ndDSA; when present, these were mostly detected within the first 6 weeks after transplant suggesting that memory responses may predominate over true de novo DSA production in this population. In the absence of pre-existing DSA, patients with ndDSA had significantly more acute cellular rejection but not antibody-mediated rejection, and there was no impact on graft and patient survival in the first year post-transplant. Risk factors for ndDSA included common sensitizing events. Given the early detection of the antibody response, memory responses may be more important in the first year following pediatric heart transplantation and patients with a history of a sensitizing event may be at risk even with a negative pre-transplant antibody screen. The impact on late graft and patient outcomes of first year ndDSA is being assessed in an extended cohort of patients.
Introduction
The detection of so-called ‘de novo’ donor specific anti-HLA antibodies (DSA) has been associated with worse outcomes in adult heart, kidney, and lung transplantation.1–11 The pediatric experience is limited to small, single center, predominantly retrospective cohorts, frequently with irregular sampling times, and with varying follow up and outcomes.12–16 The term ‘de novo’ DSA has been used interchangeably with ‘newly detected’ in the literature. Testing to identify an antibody as truly de novo is often not reported or not available due to the lack of assessment of pre-transplant antibody presence or specificity. Arbitrary thresholds for identifying a pre-transplant HLA antibody can also lead to a result that falls below threshold and thus is deemed ‘not present’. When such an antibody ‘reappears’ in later testing, it can be misclassified as de novo when it is truly reflective of a memory response. To avoid inferences about primary versus memory immune responses, we have chosen to use the term newly detected DSA (ndDSA) throughout this study.
No large, prospective multi-institutional studies have established the incidence of ndDSA after pediatric heart transplantation, nor studied their impact on outcomes. The cardiac consortium of the NIAID/NIH-sponsored Clinical Trials in Organ Transplantation in Children (CTOTC) program (www.ctotc.org) was developed to explore the impact of alloantibodies on pre- and post-transplant outcomes in pediatric heart candidates, with a focus on the management of the highly sensitized candidate including those with a positive donor-specific cytotoxicity crossmatch.17,18 Study design, candidate sensitization status, risk factors for sensitization and primary outcomes from CTOTC-04 are described elsewhere in this volume.17,18 CTOTC-04 also provides a unique opportunity to study the clinical importance of ndDSA in the first year after pediatric heart transplantation.
We hypothesized that the presence of ndDSA in the first-year after pediatric heart transplantation would be common in both non-sensitized and sensitized subjects and would be associated with increased rates of both acute cellular (ACR) and antibody-mediated rejection (AMR), but would not affect short term graft and patient survival. The specific aims of this analysis were to: (1) determine the incidence, characteristics, and time course of ndDSA in the first year following pediatric heart transplantation, (2) assess the impact of these ndDSA on acute rejection and graft and patient survival, and 3) analyze risk factors for the presence of these ndDSA.
Material and Methods
Overview of Study Design and Participants
The CTOTC-04 multicenter pediatric heart transplant study prospectively recruited consecutive patients (age <21 yrs) listed for heart transplant at 8 large pediatric centers. All study activities were approved by the Institutional Review Boards at each participating center. The study was monitored by an external Data Safety and Monitoring Board appointed by NIAID. The study is registered at www.clinicaltrials.gov (NCT01005316), where all study objectives and endpoints are listed. Enrollment commenced in February 2011, the last transplant occurred in December 2013, and follow up was completed on December 31, 2014. Immunosuppression was standardized among the 8 clinical sites, and was based on thymoglobulin induction, and maintenance tacrolimus and mycophenolate mofetil, without routine use of maintenance corticosteroids for cytotoxicity crossmatch (CDC-XM) negative subjects. Clinical care was determined at all times by diagnostic testing at the local sites, including routine endomyocardial biopsy (EMB) for rejection surveillance and diagnosis. EMB were graded according the standard criteria of ISHLT.19,20 For this analysis, local site interpretation was used for rejection determination by EMB. Full methods, definitions, clinical protocols and demographic descriptions are published in this issue of the Journal.17
Study Definitions and Alloantibody Core Evaluation
Pre-transplant antibody status was based on the pre-transplant sample closest to transplant, normally obtained within 24 hours prior to transplantation (‘PT0’ sample). Patients were considered as pre-transplant antibody positive or “sensitized” when Core Laboratory sera were positive by Luminex LABScreen® Mixed (One Lambda, Canoga Park, CA) for class I and/or class II, confirmed by the presence of ≥1 HLA antibody at ≥1000 MFI using Luminex LABScreen® Single Antigen (One Lambda, Canoga Park, CA). In a secondary analysis, we retrospectively performed single antigen bead testing on the ‘negative’ samples at the time of transplantation so that all patients ultimately had results based on single antigen bead testing. (Figure 1)
Figure 1. Patient population grouped by pre-transplant (PreTx) antibody (Ab) status and subsequent development of newly detected DSA (ndDSA) categorized into Early and Late.
The pre-transplant antibody negative and pre-transplant antibody positive without DSA groups together formed the pre-transplant non-DSA cohort, n=186 (gray boxes). *Numbers in [square brackets] reflect Luminex LABScreen® Mixed; results in bold font reflect Luminex LABScreen® Single Antigen from the secondary analysis. See Methods: Study Definition and Alloantibody Core Evaluation.
Donor and recipient molecular typing was by low resolution molecular technique including for HLA-C and -DQ. There was variable availability of –DP molecular typing and HLA-DP antibodies were only assessed for DSA status when donor and recipient DP typing were available. For this sub study, all HLA antibody determinations were performed in batched fashion at the Alloantibody Core Laboratory by a single senior technician and all DSA determinations were made by a single senior investigator (AZ, UPMC).
Post-transplant ndDSA were defined as occurring ‘Early’ (≥6 days to <42 days) or ‘Late’ (≥ 42 days). These categories were not mutually exclusive where there were multiple ndDSA. The few patients that had both Early and Late ndDSA (n=7) were counted only in the Early ndDSA group for the purpose of the outcomes analyses (see below). Newly detected DSA were also categorized as transient (present on only one sample throughout the first year) or persistent (present on ≥2 samples during the first year). Both persistent and transient ndDSA were included in this analysis.
Post-transplant samples analyzed for this sub study were collected at scheduled ‘visits’ (day 7, months 1, 3, 6, and 12) and at the time of pre-specified clinical events when outside of a scheduled visit window. These ‘unscheduled visits’ included EMB and clinically-treated rejection episodes. As each scheduled sample collection time in the protocol had a ‘window’ for collection, we calculated the sample time to the nearest day post-transplant (date of sample minus date of transplant), regardless of scheduled visit designation and this exact time post-transplant was used for all analyses, figures and tables.
Statistical Analysis
To mitigate the potential clinical impact of pre-transplant HLA antibody, several groups were identified for analysis (Figure 1): the entire CTOTC-04 cohort that underwent transplantation with an available pre-transplant antibody sample (n=237), pre-transplant antibody negative (non-sensitized, n=108), pre-transplant antibody positive without DSA (n=78), pre-transplant antibody positive with DSA (n=51), and pre-transplant antibody negative combined with pre-transplant antibody positive without DSA (pre-transplant non-DSA cohort, n=186). Pre-transplant sensitization with DSA can have a major impact on post-transplant rejection outcomes, as demonstrated in the CTOTC-04 cohort18, and these DSA often persist post-transplantation. To remove pre-transplant DSA as a confounding factor, rejection outcomes were analyzed using the substantial cohort of 186 subjects who had no evidence of pre-transplant DSA..
Fisher’s exact tests were used for categorical variables and Kruskal-Wallis or Wilcoxon rank-sum test for continuous variables. Statistical significance was set at two-sided α=0.05. Survival curves were estimated using the Kaplan-Meier method and compared with the Wilcoxon test. Logistic regression models were developed to estimate probabilities of developing ndDSA, first for any ndDSA, then for any Early ndDSA and then for any Late ndDSA. Risk factors were identified using a backwards elimination variable selection strategy with α=0.10 threshold for inclusion in the model. Variables included in the model were age at transplant, weight at transplant, diagnosis, race, sex, UNOS status at transplant, prior surgery, prior blood transfusion, ventricular assist device (VAD), extracorporeal membrane oxygenation (ECMO), homograft, prior transplant, and sensitization status at transplant. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Participant Population
From 2/2011 – 12/2013, 240 patients underwent heart transplantation of which 237 (53% males and 44% with congenital heart disease, CHD) had pre-transplant samples for HLA antibody detection.17 Median age at heart transplant was 6 years (1 month - 20 years). Demographic information for the groups considered in this analysis is detailed in Table 1. There were 1599 total post-transplant serum samples collected between 6 and 365 days post-transplant and screened for ndDSA. Nine hundred and sixty-six (60%) samples were collected at scheduled visits and 633 (40%) were collected at other times. Median follow up time post-transplant was 741 days (1–1275 days), though analysis for this study ends at the one year post-transplant visit.
Table 1.
Participant characteristics and detection of newly detected donor specific anti-HLA antibody (ndDSA) within the first year following pediatric heart transplantation (n=237)
| No ndDSA n=157 (66%) |
Early* ndDSA n=61 (26%) |
Late* ndDSA n=19 (8%) |
p-value | |
|---|---|---|---|---|
| Age at HTx (years) | 6.1 (0.1–21.0) | 8.8 (0.4–21.0) | 3.8 (0.3–19.1) | 0.0350 |
| Male sex (%) | 82 (52) | 37 (61) | 7 (37) | 0.1874 |
| Diagnosis CHD (%) | 61 (39) | 37 (61) | 7 (37) | 0.0117 |
| Pre-Tx Ab Status | 0.0300 | |||
| Non-sensitized (n=108) | 79 (50%) | 21 (34%) | 8 (42%) | |
| Sensitized, no DSA (n=78) | 52 (33%) | 18 (30%) | 8 (42%) | |
| Sensitized, DSA (n=51) | 26 (17%) | 22 (36%) | 3 (16%) | |
| Time to ndDSA development (days) | - | 10 (6–39) | 162 (45–355) | - |
| Maximum MFI | - | 5200 (1000–22400) | 3580 (1100–19100) | 0.7992 |
| Class | 0.0654 | |||
| Class I only (%) | 20 (33) | 7 (37) | ||
| Class II only (%) | 12 (20) | 8 (42) | ||
| Class I and Class II (%) | 29 (48) | 4 (21) |
Early (6 to <42 days); Late (>=42 days)
Note: Patients with both early and late ndDSA are included in the ndDSA category.
Note: The median (minimum-maximum) are presented for continuous variables.
Note: No p-value was calculated for time to detection of ndDSA because the groups were defined based on this criteria, and hence will be statistically different.
Ab, antibody; CHD, congenital heart disease; HTx, heart transplant; Tx, transplant
Impact of Timing of Samples
To explore any potential bias introduced by varying sampling times (40% at unscheduled visits) on the distribution of Early, Late, and no ndDSA, the analysis was repeated to include only samples obtained at scheduled visits. There was minimal impact, with two patients recategorized from the Late group to the no ndDSA group. Thus, the results presented are based on all 1599 samples.
Frequency, Time Course and Characteristics of Newly Detected DSA
Newly detected DSA were detected in 80 (34%) participants within the first year following heart transplantation; 61 (26%) Early and 19 (8%) Late. (Table 1, Figure 1). Among the pre-transplant DSA negative cohort (n=186, Figure 1), 55 patients (30%) developed ndDSA (39 Early, 16 Late). There were no significant differences in sex between the ndDSA groups though participants with a diagnosis of CHD and of older age were more likely to have Early ndDSA (p=0.01 and p=0.04, respectively). Pre-transplant sensitization was significantly associated with the detection of ndDSA (p=0.03). There was no significant difference in maximum MFI between the Early and Late groups.
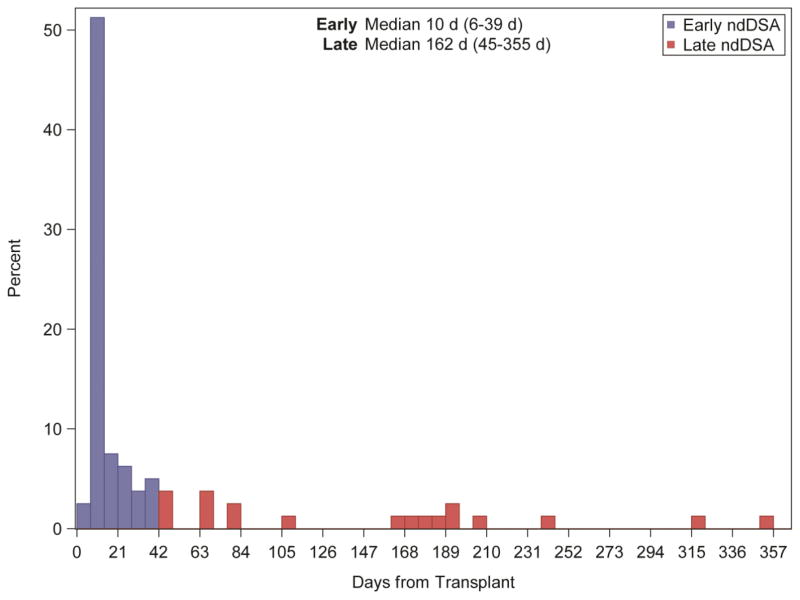
Figure 2 depicts the time course of detection in days from transplant of ndDSA. Each participant is only represented once, at the first time of the detection of ndDSA. Early ndDSA were detected at a median of 10 (6–39) days post-transplant. Late ndDSA were detected at a median of 162 (45–355) days post-transplant. Among the Early group, approximately three-quarters of responses were observed within the first two weeks. We hypothesized that Early ndDSA are memory responses and that in some cases, at least, we would be able to demonstrate retrospectively the presence of the same DSA on pre-transplant samples that had initially been deemed negative using the Luminex LABScreen® (without single antigen bead testing). We therefore retrospectively performed single antigen bead testing on the ‘negative’ samples at the time of transplantation of patients (n=30) with Early ndDSA, searching for pre-transplant evidence of the relevant antibody. In 9 participants (30%), the specific antibody identified as Early ndDSA was indeed present prior to transplantation but below the threshold for detection based on the prospective study definition.
Figure 2.
Time course of newly detected DSA (ndDSA). Time to first ndDSA detection (in days from transplant) on a by subject basis. Each subject (n=80) is only shown once at the first time of detection of a ndDSA.
Twenty-four of the 80 (30%) participants had only transient ndDSA, 27 (34%) had both transient and persistent ndDSA, and 29 (36%) had only persistent ndDSA. A total of 140 ndDSA were detected in 61 patients in the first 6–41 days post-transplant. Of these ndDSA, 89 (64%) were persistent. Forty-three ndDSA were detected in 26 patients between 42 and 365 days post-transplant. Of these, 28 (65%) were persistent. Details of timing of detection and maximum MFI of 28 ndDSA in 24 subjects with only transient ndDSA are summarized in Supplemental Table 1. Median (range) MFI for transient DSA was 1,800 (1,000–19,100) compared to 3,400 (1,000–21,000) at first detection of persistent antibodies (p=0.0012). Median (range) of maximum MFI for persistent antibodies was 4,800 (1,000–22,400).
Table 2 summarizes the HLA class and specificities of the ndDSA response. Newly detected DSA were Class I-only in 27 (34%), Class II-only in 20 (25%), and both Class I and Class II in 33 (41%) patients. Antibodies directed against HLA-A, -B, -DR and -DQ were the most predominant, with HLA-A detected in 38 patients (48%), -B in 31 (39%), -DR in 29 (36%) and -DQ in 37 (46%). Most patients (55%) had more than one ndDSA, with 36% of those showing ≥4 different antibody specificities.
Table 2.
Characteristics of newly detected DSA (ndDSA)
| Number of patients (n=80) | |
|---|---|
| HLA Class | |
| Class I only | 27 (34%) |
| Class II only | 20 (25%) |
| Class I and II | 33 (41%) |
| HLA Loci* | |
| A | 38 (48%) |
| B | 31 (39%) |
| C | 14 (18%) |
| DP | 2 (3%) |
| DQ | 37 (46%) |
| DR (51/52/53) | 17 (21%) |
| DR (not 51/52/53) | 18 (23%) |
| Number of individual ndDSAs | |
| Exactly one Ab specificity | 36 (45%) |
| More than one Ab specificity | 44 (55%) |
| 2 Abs | 18 |
| 3 Abs | 10 |
| 4 Abs | 6 |
| 5 Abs | 7 |
| 6 Abs | 1 |
| 7 Abs | 0 |
| 8 Abs | 2 |
Ab, antibody
number of subjects with at least one of each HLA loci
Rejection
Table 3 summarizes acute rejection by type (cellular, antibody-mediated, mixed, clinical), whether rejection was associated with hemodynamic compromise (irrespective of type), and the number of events and patients, according to antibody status. Median time to first rejection event (any type) was 28 days (4–362) for patients without ndDSA, 19 days (9–264) for patients with Early ndDSA, and 75 days (8–185) for patients with Late ndDSA.
Table 3.
Frequency of acute rejection in the first year post-transplant*
| No ndDSA, NO pre-Tx DSA (n=131) | No ndDSA, pre-Tx DSA (n=26) | Early ndDSA (n=61) | Late ndDSA (n=19) | |
|---|---|---|---|---|
| Number of Acute Rejection “Events” | ||||
| Cellular | 22 | 16 | 38 | 8 |
| Antibody Mediated | 5 | 17 | 24 | 4 |
| Mixed | 0 | 1 | 5 | 0 |
| Clinical | 10 | 1 | 5 | 1 |
| With Hemodynamic Compromise | 2 | 5 | 4 | 1 |
| Number of Subjects with Acute Rejection “Events” | ||||
| Cellular | 17 (13%) | 9 (35%) | 23 (38%) | 6 (32%) |
| Antibody Mediated | 5 (4%) | 7 (27%) | 15 (25%) | 2 (11%) |
| Mixed | 0 | 1 (4%) | 5 (8%) | 0 |
| Clinical | 9 (7%) | 1 (4%) | 5 (8%) | 1 (5%) |
| With Hemodynamic Compromise | 1 (0.7%) | 3 (12%) | 3 (5%) | 1 (5%) |
Some patients had multiple rejection events of different types and are therefore represented more than once.
Because we previously showed the important impact of pre-transplant DSA on rejection events in CTOTC-0418, we proceeded to analyze rejection outcomes in the cohort of 186 subjects without pre-transplant DSA (see Figure 1). Figure 3 depicts freedom from ACR (Panel A), AMR (Panel B), and rejection with hemodynamic compromise (Panel C) among pre-transplant DSA negative participants. Patients who developed either Early or Late ndDSA had significantly reduced freedom from ACR (p<0.001) but not AMR (p=0.26). Rejection with hemodynamic compromise was too infrequent to draw any definitive associations. A repeat analysis (Supplemental Figure 1) was performed focusing on the subjects with persistent ndDSA which showed similar findings, but with lower freedom from ACR at one year in the Early ndDSA group with persistent antibody (43.5%; CI 24.5–61.2%) compared to the Early ndDSA group with all subjects included in the analysis (57.5%; CI 40.2–71.5%).
Figure 3. Freedom from acute cellular rejection (Panel A), antibody mediated rejection (Panel B) and rejection with hemodynamic compromise (Panel C) in pre-transplant DSA negative patients (n=186) stratified by Early (blue), Late (red) and No (green) newly detected DSA post-transplant.
Estimated using the Kaplan-Meier method and compared with the Wilcoxon test.
Figure 4 shows the temporal relationship between detection of ndDSA and rejection among patients without pre-transplant DSA. Forty-seven percent of patients who developed ndDSA had no rejection events in the first year post-transplant, either prior to, or following, the ndDSA detection. Among 7 patients (13%) with AMR (5 AMR-only and 2 mixed rejection), 5 had ndDSA detected prior to the episode of AMR. Nineteen patients (35%) had ACR without AMR, of whom 16 (84%) had ndDSA detected prior to or at the time of diagnosis of ACR; all of which were Early ndDSA.
Figure 4. Time course of post-transplant newly detected DSA in relation to rejection events.
Subjects without DSA pre-transplant (n=55). The timing of the first ndDSA is represented by a blue star with timing and type of rejection depicted by colored circles.
Graft and Patient Survival
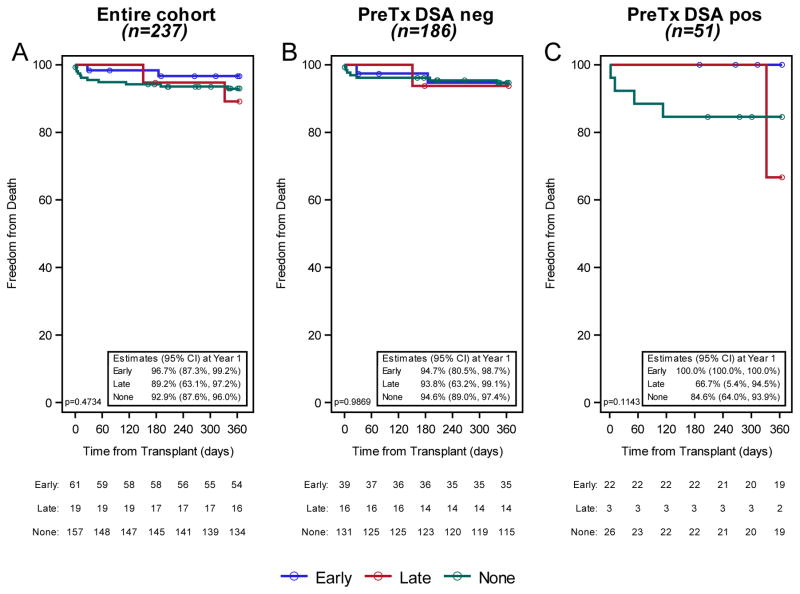
Overall, 21 patients (9%) died (15 in the first year, 4 in the second year, and 2 beyond the second year post-transplant). The presence of ndDSA was not associated with statistically significant differences in first year patient survival regardless of the status by pre-transplant DSA (Figure 5). Two patients underwent retransplantation (one each in the first year and second year post-transplant), both of whom were not sensitized pre-transplant but developed Early ndDSA; the numbers are too small to make any comments beyond reporting this observation.
Figure 5. Patient survival stratified by Early (blue), Late (red), and no (green) newly detected DSA (ndDSA) post-transplant.
Panel A: Entire cohort (n=237), Panel B: Pre-transplant DSA negative (n=186), Panel C: Pre-transplant DSA positive (n=51). Estimated using the Kaplan-Meier method and compared with the Wilcoxon test.
Primary Study Endpoint Stratified by Newly Detected DSA status
There was no difference between the groups for the primary study composite endpoint of death, retransplantation, or rejection with hemodynamic compromise at one year post-transplant. This analysis was repeated focusing on the subjects with persistent ndDSA with similar findings. (Supplemental Figures 2 and 3)
Risk Factors for the Development of Newly Detected DSA
Risk factors for the development of any ndDSA included prior homograft, absence of VAD prior to transplant, and increased age at transplant (Table 4). Risk factors for the development of Early ndDSA were similar, with the addition of prior transplant and CHD diagnosis. Risk factors for the development of Late ndDSA could not be determined due to the small number of events.
Table 4.
Risk Factors for the development of newly detected DSA (ndDSA)
| ANY ndDSA | ||||
|---|---|---|---|---|
| Yes: 80, No: 157 with data available for the model | ||||
|
| ||||
| Parameter | Estimate | P-value | Odds Ratio | 95% Wald Confidence Limit |
| Age at Transplant | 0.0463 | 0.0361 | 1.047 | (1.003, 1.094) |
| VAD (Yes vs. No) | −0.5795 | 0.0041 | 0.314 | (0.142, 0.693) |
| Homograft (Yes vs. No) | 0.5300 | 0.0050 | 2.887 | (1.377, 6.050) |
| EARLY ndDSA | ||||
|---|---|---|---|---|
| Yes: 61, No: 169 with data available for the model | ||||
|
| ||||
| Parameter | Estimate | P-value | Odds Ratio | 95% Wald Confidence Limit |
| Weight at Transplant | 0.2015 | 0.0012 | 1.223 | (1.083, 1.382) |
| Diagnosis (CHD vs. cardiomyopathy) | 0.3345 | 0.0772 | 1.952 | (0.930, 4.099) |
| VAD (Yes vs. No) | −0.5021 | 0.0321 | 0.366 | (0.146, 0.918) |
| Homograft (Yes vs. No) | 0.4739 | 0.0312 | 2.580 | (1.089, 6.110) |
| Prior Transplant (Yes vs. No) | −1.0069 | 0.0725 | 0.133 | (0.015, 1.202) |
per 10 kg increase in weight
Discussion
We have leveraged the detailed pre- and post-transplant data and frequent sample collection from the CTOTC-04 study to provide the first detailed analysis of the incidence, time course, antibody characteristics and clinical impact of newly detected DSA in a large cohort of pediatric heart transplant candidates and recipients. We have also been able to adjust for the confounding impact of the presence of pre-transplant DSA since over 180 patients without pre-transplant antibodies are being followed. Our findings include several new observations. Of note, throughout, we have used the term ‘newly detected’ DSA (ndDSA) rather than ‘de novo’ DSA to avoid any a priori assumptions about the nature of these antibodies.
In our cohort, one third had evidence of ndDSA within the first year post-heart transplant. Reports in the literature cite that ‘de novo’ DSA are common after heart transplantation and associated with poor outcomes.1,10,14 Several studies report de novo HLA antibodies in 25–33% of adult heart transplant recipients.1,3,10,14,21 The pediatric literature is sparser with single center reports with small sample sizes, limited or irregular sampling frequency, and sometimes without clarity as to whether the post-transplant HLA antibodies are even DSA. These reports provide widely varying estimates of de novo DSA from 7% to 48% in the first year post-transplant.12–16 A number of cohorts had longer follow up than our cohort but still the majority of de novo DSA were detected in the first year post-transplant.1,10,14,16 We intend to follow our cohort long-term to establish the incidence of ndDSA beyond one year, and to assess the late impact of these antibodies.
Prior sensitization as a risk for the development of de novo DSA has been noted in other smaller series.10,15 The finding, herein, that VAD use was associated with a lower incidence of ndDSA seems contradictory to the increasing evidence in the literature that VAD use is associated with allosensitization.22–25 One could hypothesize VAD usage is a surrogate for a diagnosis of cardiomyopathy which has a lower risk of developing ndDSA compared to CHD.
The majority of these ndDSA were detected early (i.e. within 6 weeks) of transplant and are thus most consistent with memory response as opposed to a primary immune response. In a significant minority of those with Early ndDSA, we were able to retrospectively demonstrate that the same antibody had been present at very low strength pre-transplant, confirming the memory response. We suspect that we would have been able to demonstrate this in more patients if there were more historical samples from nearer the time of sensitizing events, such as prior surgery for congenital heart disease. Of note, in this population, early responses predominated with only 19 subjects demonstrating ndDSA beyond 6 weeks, and 11 after 3 months from transplantation. This suggests we should exercise caution in assuming that pediatric heart candidates with negative alloantibody ‘screen’ are not at risk for early memory responses and subsequent graft injury from rejection. A case can be made for performing single antigen bead testing on all candidates with prior history of sensitizing events.
Though most of the studies in the literature report a preponderance of Class II DSA post-transplant 1,12,16 we observed a preponderance of Class I, or Class I with Class II in the first year. These differences may reflect differing lengths of follow-up, as Class II antibodies may be more frequent during longer-term follow-up, or possibly different distributions of original diagnoses and prior interventions. Anti-DQ and -DR antibodies made up the significant majority of Class II DSA, but Class I HLA-A and –B were detected in roughly similar proportions and with similar frequency to DQ antibodies. Time to development of ndDSA was not associated with peak MFI.
The persistence of ndDSA likely has an impact on outcome.26 Nearly all Early ndDSA in this cohort were no longer detected beyond 3 months post-transplant. This contrasts with adult heart data in which most de novo DSA (84%) were persistent and associated with poor survival compared to transient DSA.1 In the 3 pediatric patients reported by Irving with persistent DSA, all experienced graft loss.10 However, prior reports were generally associated with much longer follow up than the current cohort. We observed persistent ndDSA in 70% of our cohort. Overall clinical outcomes were similar whether we examined all subjects together or only those with persistent ndDSA, the latter, however, having lower freedom from ACR.
Rejection has been implicated in the kidney literature as a stimulus for the development of de novo DSA.7,10,27,28 In an adult cardiac population, rejection occurred predominantly in the first year post-transplant but de novo DSA production was noted on an ongoing basis every year post-transplant.1 Late de novo DSA were associated with the number of biopsy-proven rejection episodes in the first year and the authors concluded that this was supportive for rejection as a stimulus for antibody production. The retrospective nature of many studies and the annual or irregular sampling for DSA compromises the ability to discern the true temporal relationship between DSA development and rejection episodes.1,10 Chen observed the majority of antibody detection prior to, or concurrent, with an episode of acute cellular rejection in a small cohort of pediatric heart transplant recipients.16 We also observed that ndDSA were usually detected either prior to, or concurrent with, episodes of rejection in our prospectively followed patient cohort. Though speculative, this does lead to the consideration that the association of ndDSA with rejection is more likely to be causative as opposed to a secondary epiphenomenon. This presumed causal relationship does not preclude the potential for tissue damage induced by DSA leading to further release of soluble allo- (and auto) antigens with subsequent enhanced sensitization.
In the current study, ndDSA were associated with increased risk of ACR but not AMR. By contrast, we previously showed in this same cohort that pre-transplant DSA and CDC-XM status impact both ACR and AMR.18 The lack of association of ndDSA with AMR was contrary to our initial hypothesis; most of the AMR episodes observed in this study were associated with pre-formed DSA.18 Of note, Clerkin reported a cohort of 221 adult heart transplant recipients of whom 31% had DSA (24% de novo DSA) and found no correlation between presence of DSA (pre-existing or de novo) and concurrent pathologic changes of AMR on biopsy.3 Although in our study ndDSA were associated with increased ACR, these findings are insufficient at this time to provide recommendations on how to treat asymptomatic pediatric heart recipients who develop ndDSA. Randomized clinical trials will be required to address this critical question, though it is unlikely that they can be adequately powered in a pediatric population.
Interestingly, there is a sizeable proportion of our cohort who did not develop AMR or ACR despite the development of ndDSA. Thus, even though ndDSA is a risk factor for the development of ACR, much more must be learned before we can accurately predict which patients and which antibody profiles are likely to lead to acute graft injury. Ongoing studies within CTOTC-04 are exploring which patient and antibody characteristics (e.g. Ig subtype, class, complement fixing ability, concentration, strength) may better predict who is most at risk of adverse outcomes and thus who should be treated more aggressively. Of note, neither pre-transplant DSA, nor post-transplant ndDSA, impacted one year graft and patient survival nor the primary composite endpoint (death, retransplant or rejection with hemodynamic compromise). Longer-term follow-up of an expanded cohort of patients within CTOTC will establish the long-term impact of ndDSA in pediatric heart candidates. There is certainly evidence from many organs that de novo DSA development is associated with subsequent development of graft vasculopathy, graft dysfunction and ultimately graft loss.1–10,29 Cardiac allograft vasculopathy (CAV) after heart transplantation has been associated with de novo DSA, particularly class II.3,12,14,30 Within CTOTC, a Coronary Angiography Core is currently being utilized during long-term follow-up of this cohort.
Limitations
Although this is a large, prospective study of pediatric heart recipients, the patient population remains too small for robust subgroup analyses, particularly for late ndDSA. This limitation is being addressed within the larger cohort in our long-term CTOTC-09 study. The prevalence of ndDSA may be underestimated, since it was not always possible to determine if HLA-DP antibodies were donor-specific, given the incomplete typing for DP. Nonetheless, DP antibodies appear rare in this population (of the 54 patients with donors with DP typing, there were 2 with preformed DSAs and one that developed a DP DSA post-transplant; data not reported), which is in keeping with a previously reported large cohort of pediatric renal transplant recipients.31
In addition, we are not able to prove or disprove passive antibody transfer as a source of ndDSA early after transplantation. If early antibodies were the result of pre-transplant transfusion, we would have detected them during SAB testing at transplant. If they were transferred peri-operatively, we might expect to see evidence of ‘self-reactivity’ in the early post-transplant alloantibody profiles. We are also not aware of data that suggests that passive transfer of HLA antibody via blood products in this clinical setting causes early ACR, yet ndDSA was associated with ACR in this study. We also acknowledge that any cut- off time point for ‘early’ versus ‘late’ ndDSA is somewhat arbitrary, though we believe that a six week time point is well accepted in the field as reflective of the time frame to observe a memory response, whereas for newly developed antibody (true de novo), the minimal time is generally considered to be greater than 6 weeks. However, to avoid making inferences about the nature of the antibody response (‘memory’, ‘de novo’, ‘passive’) in any specific patient, we have chosen to consistently use the term ‘newly detected’ (nd) when reporting our results. Of note, if a cut-off of three months were used to define the Late group (as has been performed in some studies), there would have been only 11 subjects in the Late group.
Finally, in this initial report, follow-up is limited to the first year post-transplantation; therefore we are not in a position at this time to evaluate the impact of first-year ndDSA on late outcomes. More detailed antibody characterization (e.g. IgG subtype, complement fixing ability, and titer) were not part of this analysis, yet may be of greater predictive value than MFI of DSA for clinical outcomes. To overcome these limitations, we continue to study an expanded cohort of patients within our long-term CTOTC-09 study (which includes these CTOTC-04 subjects). This follow-up study includes echocardiographic assessment of graft function and angiographic assessment of graft coronary vasculopathy using core laboratories for independent evaluation of the impact of DSA on graft outcomes.
Conclusion
In this large, multicenter prospective cohort, most pediatric heart transplant recipients did not develop ndDSA within the first year after transplantation. When present, these were mostly detected within the first 6 weeks after transplant suggesting that memory responses may predominate over true de novo DSA production in this population. Patients with neither pre-transplant nor ndDSA had the highest freedom from all forms of rejection. Newly detected DSA were associated with increased risk of acute cellular but not antibody mediated rejection. The impact on late graft and patient outcomes of first year newly detected DSA are being assessed in an extended cohort of patients within the CTOTC consortium.
Supplementary Material
Estimated using the Kaplan-Meier method and compared with the Wilcoxon test.
Estimated using the Kaplan-Meier method and compared with the Wilcoxon test.
Estimated using the Kaplan-Meier method and compared with the Wilcoxon test.
Table S1: Timing and MFI of 28 ndDSA in 24 subjects with only transient ndDSA.
Acknowledgments
This research was performed as a project of the Clinical Trials in Organ Transplantation in Children, a collaborative clinical research project headquartered at the National Institute of Allergy and Infectious Diseases. The work was supported by Grant U01AI077867 “Alloantibodies in Cardiac Transplantation - Intervention, Outcomes and Mechanisms” from the Division of Allergy, Immunology and Transplantation of the National Institutes of Health.
Abbreviations
- ACR
acute cellular rejection
- AMR
antibody mediated rejection
- CDC-XM
donor-specific cytotoxicity crossmatch
- CHD
congenital heart disease
- cPRA
calculated panel reactive antibody
- CTOTC
Clinical Trials in Organ Transplantation in Children
- DSA
donor specific antibody
- EMB
endomyocardial biopsy
- ISHLT
International Society for Heart and Lung Transplantation
- MFI
median fluorescence intensity
- ndDSA
newly detected donor specific antibodies
- NIAID/NIH
National Institute of Allergy and Infectious Diseases, National Institutes of Health
- PRA
panel reactive antibody
- VAD
ventricular assist device
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Smith JD, Banner NR, Hamour IM, et al. De Novo HLA specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11:312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee PC, Zhu L, Terasaki PI, Everly MJ. HLA-specific antibodies developed in the first year post-transplant are predictive of chronic rejection and renal graft loss. Transplantation. 2009;88:568–574. doi: 10.1097/TP.0b013e3181b11b72. [DOI] [PubMed] [Google Scholar]
- 3.Clerkin K, Farr M, Restaino S, et al. Donor specific anti-HLA antibodies with antibody mediated rejection and long term outcomes following heart transplantation. J Heart Lung Transplant. 2017;36:540–545. doi: 10.1016/j.healun.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas M, Mirocha J, Reinsmoen NL, et al. Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int. 2017;91:729–737. doi: 10.1016/j.kint.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015;15:2921–30. doi: 10.1111/ajt.13347. [DOI] [PubMed] [Google Scholar]
- 6.Everley MJ, Rebellato LM, Haisch CE, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95:410–7. doi: 10.1097/TP.0b013e31827d62e3. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JE, Gralla J, Cagle L, Goldberg R, Chan L, Wiseman AC. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation. 2011;91:1103–9. doi: 10.1097/TP.0b013e3182139da1. [DOI] [PubMed] [Google Scholar]
- 8.LePavec J, Suberbielle C, Lamrani L, et al. De novo donor specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. 2016;35:1067–1077. doi: 10.1016/j.healun.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Safavi S, Robinson D, Soresi S, et al. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014;33:1273–1281. doi: 10.1016/j.healun.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Ho EK, Vlad G, Colovai AI, et al. Alloantibodies in heart transplantation. Hum Immunol. 2009;70:825–829. doi: 10.1016/j.humimm.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Mangiola M, Marrari M, Feingold B, Zeevi A. Significance of anti-HLA antibodies on adult and pediatric heart allograft outcomes. Front Immunol. 2017;8:1–10. doi: 10.3389/fimmu.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irving C, Carter V, Parry G, Hasan A, Kirk R. Donor specific HLA antibodies in pediatric cardiac transplant recipients are associated with poor graft survival. Pediatr Transplantation. 2011;15:193–197. doi: 10.1111/j.1399-3046.2010.01446.x. [DOI] [PubMed] [Google Scholar]
- 13.Irving C, Carter V, Gennery A, et al. Effect of persistent versus transient donor specific HLA antibodies on graft outcomes in pediatric cardiac transplantation. J Heart Lung Transplant. 2015;34:1310–1317. doi: 10.1016/j.healun.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Statsny P, Lavingia B, Fixler DE, Yancy CW, Ring WS. Antibodies against donor human leukocyte antigens and the outcome of cardiac allografts in adults and children. Transplantation. 2007;84:738–745. doi: 10.1097/01.tp.0000281918.51138.3f. [DOI] [PubMed] [Google Scholar]
- 15.Tran A, Fixler D, Huang R, et al. Donor specific HLA alloantibodies: impact on cardiac allograft vasculopathy, rejection and survival after pediatric heart transplantation. J Heart Lung Transplant. 2016;35:87–91. doi: 10.1016/j.healun.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Chen CK, Manlhiot C, Conway J, Allain-Rooney T, McCrindle BW, Dipchand AI. Development and impact of de novo HLA antibodies in pediatric heart recipients. Am J Transplant. 2015;15:2215–2222. doi: 10.1111/ajt.13259. [DOI] [PubMed] [Google Scholar]
- 17.Zuckerman W, Zeevi A, Mason K, et al. Study rationale, design and pre-transplant alloantibody status: A first report of the Clinical Trials in Organ Transplantation in Children-04 (CTOTC-04) study in pediatric heart transplantation. Am J Transplant. 2017 doi: 10.1111/ajt.14695. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webber S, Mason K, Zeevi A, et al. Pediatric heart transplantation across a positive donor-specific cytotoxicity cross-match: first year results from the CTOTC-04 multi-institutional study. Am J Transplant. 2017 doi: 10.1111/ajt.14876. (submitted) [DOI] [PubMed] [Google Scholar]
- 19.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Berry GJ, Burke MM, Anderson C, et al. ISHLT Consensus Statement: International Society for Heart and Lung Transplantation 2013 working formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2013;32:1147–1162. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Tambur A, Pamboukian S, Constanzo MR, et al. The presence of HLA directed antibodies after heart transplantation is associated with poor allograft outcome. Transplantation. 2005;80:1019–1025. doi: 10.1097/01.tp.0000180564.14050.49. [DOI] [PubMed] [Google Scholar]
- 22.Askar M, Hsich E, Reville P, et al. HLA and MICA allosensitization patterns among patients supported by ventricular assist devices. J Heart Lung Transplant. 2013;32:1241–8. doi: 10.1016/j.healun.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Schall C, Smith D, et al. HLA sensitization in pediatric pre-transplant cardiac patients supported by mechanical assist devices: the utility of Luminex. J Heart Lung Transplant. 2009;28:123–9. doi: 10.1016/j.healun.2008.11.908. [DOI] [PubMed] [Google Scholar]
- 24.Alba AC, Tinckam K, Foroutan F, et al. Factors associated with anti-human leukocyte antigen antibodies in patients supported with continuous-flow devices and effect on probability of transplant and post-transplant outcomes. J Heart Lung Transplant. 2015;34:685–92. doi: 10.1016/j.healun.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Arnaoutakis GJ, George TJ, Kilic A, et al. Effect of sensitization in US heart transplant recipients bridged with a ventricular assist device: update in a modern cohort. J Thorac Cardiovasc Surg. 2011;142:1236–45. doi: 10.1016/j.jtcvs.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeevi A, Lunz J, Feingold B, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32:98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chemouny JM, Suberbielle C, Rabant M, et al. De novo donor-specific human leukocyte antigen antibodies in nonsensitized kidney transplant recipients After T cell-mediated rejection. Transplantation. 2015;99:965–72. doi: 10.1097/TP.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 28.Moreso F, Carrera M, Goma M, et al. Early subclinical rejection as a risk factor for late chronic humoral rejection. Transplantation. 2012;93:41–6. doi: 10.1097/TP.0b013e31823bb647. [DOI] [PubMed] [Google Scholar]
- 29.Morrell M, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014;33:1288–1294. doi: 10.1016/j.healun.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Xydas S, Yang JK, Burke EM, et al. Utility of post-transplant anti- HLA antibody measurements in pediatric cardiac transplant recipients. J Heart Lung Transplant. 2005;24:1289–96. doi: 10.1016/j.healun.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Kim JJ, Balasubramanian R, Michaelides G, et al. The clinical spectrum of de novo donor-specific antibodies in pediatric renal transplant recipients. Am J Transplant. 2014;14:2350–2358. doi: 10.1111/ajt.12859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimated using the Kaplan-Meier method and compared with the Wilcoxon test.
Estimated using the Kaplan-Meier method and compared with the Wilcoxon test.
Estimated using the Kaplan-Meier method and compared with the Wilcoxon test.
Table S1: Timing and MFI of 28 ndDSA in 24 subjects with only transient ndDSA.