Abstract
Key points
Starburst amacrine cells release GABA and ACh.
This study explores the coordinated function of starburst‐mediated cholinergic excitation and GABAergic inhibition to bistratified retinal ganglion cells, predominantly direction‐selective ganglion cells (DSGCs).
In rat retina, under our recording conditions, starbursts were found to provide the major excitatory drive to a sub‐population of ganglion cells whose dendrites co‐stratify with starburst dendrites (putative DSGCs).
In mouse retina, recordings from genetically identified DSGCs at physiological temperatures reveal that ACh inputs dominate the response to small spot‐high contrast light stimuli, with preferential addition of bipolar cell input shifting the balance towards glutamate for larger spot stimuli
In addition, starbursts also appear to gate glutamatergic excitation to DSGCs by postsynaptic and possibly presynaptic inhibitory processes
Abstract
Starburst amacrine cells release both GABA and ACh, allowing them to simultaneously mediate inhibition and excitation. However, the precise pre‐ and postsynaptic targets for ACh and GABA remain under intense investigation. Most previous studies have focused on starburst‐mediated postsynaptic GABAergic inhibition and its role in the formation of directional selectivity in ganglion cells. However, the significance of postsynaptic cholinergic excitation is only beginning to be appreciated. Here, we found that light‐evoked responses measured in bi‐stratified rat ganglion cells with dendrites that co‐fasciculate with ON and OFF starburst dendrites (putative direction‐selective ganglion cells, DSGCs) were abolished by the application of nicotinic receptor antagonists, suggesting ACh could act as the primary source of excitation. Recording from genetically labelled DSGCs in mouse retina at physiological temperatures revealed that cholinergic synaptic inputs dominated the excitation for high contrast stimuli only when the size of the stimulus was small. Canonical glutamatergic inputs mediated by bipolar cells were prominent when GABA/glycine receptors were blocked or when larger spot stimuli were utilized. In mouse DSGCs, bipolar cell excitation could also be unmasked through the activation of mGluR2,3 receptors, which we show suppresses starburst output, suggesting that GABA from starbursts serves to inhibit bipolar cell signals in DSGCs. Taken together, these results suggest that starbursts amplify excitatory signals traversing the retina, endowing DSGCs with the ability to encode fine spatial information without compromising their ability to encode direction.
Keywords: acetylcholine, starburst amacrine cells, feedback inhibition
Key points
Starburst amacrine cells release GABA and ACh.
This study explores the coordinated function of starburst‐mediated cholinergic excitation and GABAergic inhibition to bistratified retinal ganglion cells, predominantly direction‐selective ganglion cells (DSGCs).
In rat retina, under our recording conditions, starbursts were found to provide the major excitatory drive to a sub‐population of ganglion cells whose dendrites co‐stratify with starburst dendrites (putative DSGCs).
In mouse retina, recordings from genetically identified DSGCs at physiological temperatures reveal that ACh inputs dominate the response to small spot‐high contrast light stimuli, with preferential addition of bipolar cell input shifting the balance towards glutamate for larger spot stimuli
In addition, starbursts also appear to gate glutamatergic excitation to DSGCs by postsynaptic and possibly presynaptic inhibitory processes
Introduction
ACh is an excitatory neurotransmitter that often acts as a modulator of glutamate signals in the CNS (Picciotto et al. 2012; Luchicchi et al. 2014). However, recent evidence suggests that ACh can be a primary driver of activity, complementing glutamate (Beierlein, 2014; Sarter et al. 2014). The retina is an ideal system to investigate the complementary nature of these two fast neurotransmitter pathways, as both glutamatergic and cholinergic inputs converge on downstream ganglion cells.
In the retina, ACh is released by a single population of amacrine cells: the GABAergic/cholinergic starburst amacrine cell (Brecha et al. 1988; O'Malley & Masland, 1989; Lee et al. 2010). Anatomical (Keyser et al. 2000; Strang et al. 2003, 2007; Dmitrieva et al. 2007; Liu et al. 2009) and physiological studies (Ariel & Daw, 1982a ; Baldridge, 1996; Strang et al. 2003; Renna et al. 2007) indicate that most ganglion cells express nicotinic receptors. However, light‐evoked synaptic activity in most ganglion cells persists in nicotinic receptor antagonists, indicating that glutamate provides the primary excitation to ganglion cells (Reed et al. 2002; Strang et al. 2015). Even in direction‐selective ganglion cells (DSGCs) whose dendrites co‐stratify with starburst dendrites in two narrow bands in the inner plexiform layer, glutamate is perceived to be the principal source of excitation (Ariel & Daw, 1982b ; Kittila & Massey, 1997; Fried et al. 2005; Lee et al. 2010; Park et al. 2014; Lipin et al. 2015; Poleg‐Polsky & Diamond, 2016). Thus, ACh is thought to play a modulatory role in the retina.
By contrast, recent studies examining DSGCs in mouse and rabbit retina have suggested that under conditions of low stimulus contrast, ACh can be the primary source of excitation (Sethuramanujam et al. 2016; Brombas et al. 2017). Under low contrast conditions, a common set of high sensitivity bipolar cells appear to drive starbursts via AMPA receptors and DSGCs via NMDA receptor‐dominated synapses (Sethuramanujam et al. 2017). As NMDA receptors alone are unable to strongly depolarize ganglion cells, bipolar cell inputs to ganglion cells remain silent in the absence of cholinergic excitation (Sethuramanujam et al. 2016). Thus, DSGCs are completely reliant on ACh for their spiking activity. At higher stimulus contrast, both low and high sensitivity bipolar cell types drive the DSGCs using a combination of AMPA and NMDA receptors, and cholinergic excitation is no longer obligatory for driving spiking responses. However, when patterned stimuli are utilized, irrespective of contrast, cholinergic drive again plays a dominant role (Reed et al. 2004). Thus, multiple mechanisms appear to bias the balance between ACh and glutamate excitation to ganglion cells.
In this study, we first present results from a number of pharmacological experiments carried out in rat retina that revealed two important features of cholinergic signalling pathways: (1) a major role for ACh in driving light‐evoked inputs to bi‐stratified ganglion cells (putative DSGCs), and (2) starburst‐mediated inhibition curbs bipolar cell signals. In the second part of this study, we show that these features are also apparent at more physiological temperatures in genetically identified mouse DSGCs, and the effects were most prominent for high contrast stimuli when responses were probed with small spot sizes. We thus posit a novel role for ACh in the detection of small moving objects.
Methods
Tissue preparation
All procedures were performed in accordance with the US Animal Welfare Act, the National Institute of Health's Guide for the Care and Use of Laboratory Animals and Canadian Council on Animal Care and were approved by the University Animal Care Committee at the State University of New York and University of Victoria's Animal Care Committee. Healthy Sprague Dawley rats (1–2 months old) used in this study were acquired from Harlan Laboratories (Indianapolis, IN, USA), and were housed under 12 hour light–dark cycles with ad libitum feeding. Rats were killed by an overdose of 1–3% isoflurane and decapitated. Mouse experiments were performed on adult Hb9‐EGFP (RRID: MGI_109160) or Hb9 crossed with ChAT‐IRES‐Cre (RRID: MGI_5475195) and Ai32 (RRID:IMSR_JAX:012569; ChR2) animals (Trenholm et al. 2011; Sethuramanujam et al. 2016). The eyeballs were hemisected under infrared light and the posterior eye cup was placed in oxygenated Ringer's solution (95% O2–5% CO2). The retina was detached from the pigment epithelium and flat mounted ganglion side up on a glass coverslip coated with poly‐l‐lysine. All electrophysiological experiments were done under infrared light. Coverslips with a whole mounted retina were transferred to the recording chamber attached to an upright Zeiss Axioskop2 FS fluorescence microscope, equipped with a 40× Achroplan water immersion objective. An infrared sensitive CCD camera (Hamamatsu, Japan) was used to capture the image of the preparation.
The tissue was constantly superfused with oxygenated Ringer's solution containing (in mM): 110 NaCl, 2.5 KCl, 1 CaCl2, 1.6 MgCl2, 10 dextrose and 22 sodium bicarbonate buffered to pH 7.4 using CO2. A gravity‐fed perfusion system was used to maintain a flow rate of ∼1.5 ml/min (2.5 ml/min for mouse recordings).
Electrophysiology
Rat recordings were made from neurons in the ganglion cell layer of retinal wholemounts at room temperature while mice recordings were at 35°C. Green fluorescent protein (GFP) positive Hb9 cells in mouse retina were identified by two‐photon imaging as described previously (Trenholm et al. 2011). The glial end feet were removed using an 8–10 MΩ electrode filled with Ringer's solution to expose the soma of ganglion cells. First, the exposed neurons were sampled for extracellular spike activity by a loose seal (25–50 MΩ) using an 8–10 MΩ electrode filled with Ringer's solution. Based on the extracellular spike recordings, identified cells were then patched for whole cell recordings using a 5–7 MΩ electrode. The pipette internal solution for current clamp experiments contained (in mM): 115 potassium gluconate, 5 KCl, 1 MgCl2, 10 Hepes, 10 EGTA buffered to pH 7.4 with KOH. The internal solution for voltage clamp experiments contained (in mm): 112.5 caesium methanesulfonate, 9.7 KCl, 1 MgCl2, 10 Hepes, 1.5 EGTA buffered to pH 7.4 with CsOH. The cells were visualized with 50–100 μm Alexa‐488 added to the internal solution.
Data were acquired using a Multiclamp 700B Amplifier (Molecular Devices, San Jose, CA, USA). Analog signals were low‐pass filtered at 2 kHz and sampled at 10 kHz with the Digidata 1322A analog‐to‐digital board (Molecular Devices). Clampex 10.1 software (Molecular Devices) was used to control the voltage command outputs, acquire data and trigger stimuli. The currents shown are raw data and were not corrected for electrode junction potential and access resistance. Both the series resistance and the membrane capacitance were constantly monitored by a −20 mV square pulse (50 ms duration) before every light stimulus. Cells in which neither parameter changed during the entire course of the experiment were considered for further analysis. Drug solutions were delivered through a pressure fed Octaflow 2 perfusion system (ALA Scientific Instruments, Farmingdale, NY, USA). Picrotoxin, strychnine, nicotine and hexamethonium chloride were purchased from Sigma‐Aldrich Corp (St Louis, MO, USA). TPMPA [(1,2,5,6‐tetrahydropyridin4yl)methylphosphinic acid], DCG‐IV [(2S,2′R,3′R)‐2‐(2′,3′‐dicarboxycyclopropyl)glycine], dl‐AP4 (dl‐2‐amino‐4‐phosphonopentanoic acid), CNQX (6‐cyano‐7‐nitroquinoxaline‐2,3‐dione), carbachol and d‐tubocurarine chloride were obtained from Tocris Bioscience (Minneapolis, MN, USA).
Light stimulation
For rat recordings, retinas were stimulated by a flash from a green light‐emitting diode (LED, λmax = 520 nm) projected through the objective lens (∼200 μm diameter). The irradiance between 500 and 540 nm of the LED was 2.4 × 104 rod isomerizations per second (R*/rod/s), measured by a RPS900‐R wideband spectroradiometer (International Light Technologies, Peabody, MA, USA). The stimulus contrast was 100% but the effective contrast was likely to be reduced as the stimulus was projected through the objective and focused proximal to the ganglion cell layer. A light stimulus of 1 s was presented every 25 s. Visual stimuli for mouse recordings were produced via a digital light projector (Hitachi Cpx1, refresh rate 75 Hz) that was controlled with custom software. Light stimuli, projected from below the preparation, were focused with a substage condenser onto the photoreceptors, and centred over the soma of the recorded neuron. A 100% positive contrast white stimulus was projected on a dark background with a spot intensity of 20 R*/rod/s. ChR2 (Channelrhodopsin 2) was stimulated using an intense blue LED (λmax = 470 nm; 108 R*/rod/s) as shown previously (Sethuramanujam et al. 2016).
Immunohistochemistry
Wholemount retinas with Alexa‐488 loaded cells were fixed with 4% paraformaldehyde (PFA, pH 7.4) at 4°C for 45–60 min. The immunohistochemistry protocol was a modified version of a previously described procedure (Jain et al. 2012). The fixed tissue was incubated for 1 h in a cocktail containing 3% normal donkey serum, 1% bovine serum albumin and 0.3% Triton X‐100 in phosphate buffered saline (PBS; pH 7.4). The wholemounts were incubated in primary antibodies against choline‐acetyl transferase (ChAT; Santa Cruz Biotechnology, Dallas, Texas, USA) diluted 1:500 for 12 h at 4°C. After washing in PBS for 5 × 5 min, the samples were incubated in donkey anti‐mouse conjugated with Alexa Fluor 594 (1:500) for 1 h at room temperature. The samples were washed again in PBS for 5 × 5 min. The images of immunostained retinal wholemounts were captured on a Zeiss Pascal confocal microscope.
Cluster analysis
Cluster analysis was performed using built‐in functions in IgorPro 6.22 (Wavemetrics, Inc., Lake Oswego, OR, USA) with the following parameters: total dendritic length, dendritic area, asymmetry index and light evoked spike activity. Dendrites of Alexa‐488 loaded cells were reconstructed using the ImageJ Simple neurite tracer program. To estimate the dendritic stratification in the inner plexiform layer (IPL), the relative fluorescence in the Z‐axis was plotted using ImageJ. The profile was compared to the ChAT fluorescence. The peak relative fluorescence was used as the stratification depth in the IPL. Cells were considered bi‐stratified when the relative fluorescence dipped below 50% before rising to a second peak.
The dendritic area was estimated from the area of a polygon encompassing the entire dendritic tree (both ON and OFF). The asymmetry of the dendritic tree was estimated by dividing the area into two sectors (a and b) with the soma as the centre such that Sectorb − Sectora was maximal. Asymmetry index was calculated as (Sectorb − Sectora/Sectorb + Sectora). Symmetric cells will have an index close to 0 while highly asymmetric dendritic arbors will have an index close to 1. The total dendritic length was estimated after skeletonizing the entire tree using in‐built analysis tools in ImageJ.
Co‐fasciculation index
To assess the co‐fasciculation of the ganglion cell dendrites with immunolabelled starburst dendrites, a relatively flat region of the image was considered (16670 ± 1296 μm2). The starburst (red) and ganglion cell dendrites (green) were enhanced in ImageJ. The co‐fasciculation index was calculated in pixels as yellow/(yellow + green), where yellow pixels indicated co‐fasciculating dendrites. The procedure was repeated for the same selected areas after the green channel was rotated by 180° to serve as control. Note that an image with randomly generated pixels would have a co‐fasciculation index close to 0.5.
Data analysis
For analysis involving light evoked synaptic currents, the peak of the EPSC after light onset and offset was used as a measure of the ON and OFF light responses, respectively. Pooled data are expressed as mean ± SEM. Student's t‐test was used to compare values under different conditions and was paired. Differences were considered significant at P ≤ 0.05.
Results
A subpopulation of rat ON‐OFF ganglion cells received endogenous ACh excitation
Initial loose patch extracellular recordings were made from a set of neighbouring rat ganglion cells to determine their spike response to a 200 μm spot stimulus (Fig. 1), at room temperature. The cells were classified as ON‐OFF transient, ON transient, OFF transient, ON sustained and OFF sustained based on their light‐evoked spike activity. In about one‐third of the ON‐OFF neurons studied, the application of a nicotinic ACh receptor antagonist, 100 μM hexamethonium (HEX), eliminated both the ON and the OFF spike activity in response to the light stimulus (Fig. 1 A, B; spikes in control, ON: 6 ± 1, OFF: 5 ± 1, n = 20). The effects of HEX could be washed off and replicated by 50 μM d‐tubocurarine chloride (Fig. 1 B). These cells were designated HEX‐sensitive cells. In the remaining spiking cells, neither HEX nor d‐tubocurarine had a significant effect on spike activity; these cells were designated HEX‐insensitive cells (Fig. 1 A, B; data not shown for 17 ON transient and 4 OFF transient cells). This reveals a subset of ON‐OFF cells in which ACh is critical for light‐evoked spike activity, under these recording conditions.
Figure 1. Acetylcholine‐generated light responses in a subpopulation of rat ganglion cells.
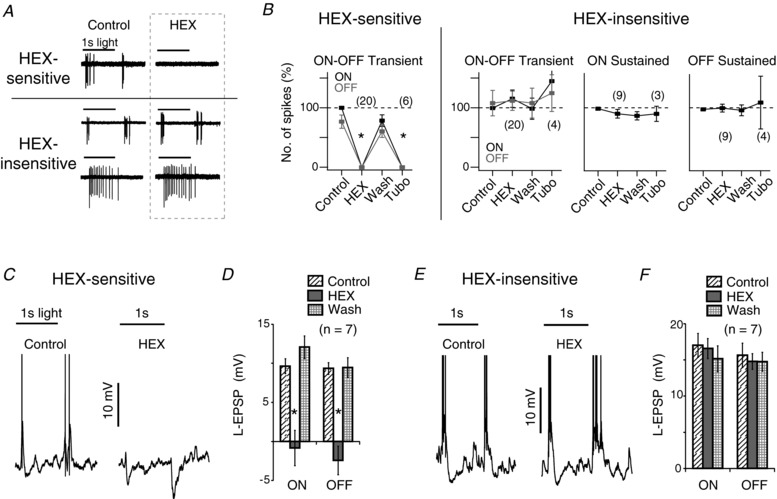
A, the light responses of two ON‐OFF and an ON sustained retinal ganglion cell under control conditions, then in the presence of 100 μM hexamethonium (HEX). The top ON‐OFF cell was designated HEX‐sensitive, as its spiking was blocked completely by HEX (see text for details). The spiking in the lower two cells was unaffected, and designated HEX‐insensitive. The 1s light stimulus, a 200 μm diameter green spot (2.4 × 104 R*/rod/s), is represented by the solid bar above each trace. B, summary of relative number of spikes in HEX‐sensitive and HEX‐insensitive neurons in control, then in the presence of HEX, after recovery, and in the presence of d‐tubocurarine (Tubo) (* P < 0.005). HEX‐sensitive neurons were all ON‐OFF cells (n = 20), while HEX‐insensitive neurons were either ON‐OFF transient (n = 20), ON sustained (n = 9) or OFF sustained (n = 9). C, the L‐EPSP of a HEX‐sensitive neuron recorded in whole cell configuration in control and HEX. D, the average L‐EPSP (spikes not included) of HEX‐sensitive cells (n = 7) in control, HEX and wash (* P < 0.005). E, the L‐EPSPs of a HEX‐insensitive neuron in control and HEX. F, the average L‐EPSP in HEX‐insensitive neurons in control, HEX and wash (n = 7).
This ACh‐dependent spiking could be due to two scenarios: (a) ACh is the primary excitatory drive to HEX‐sensitive neurons in these conditions, i.e. the excitatory postsynaptic potential (EPSP) is predominantly cholinergic, or (b) ACh combines with a sub‐threshold EPSP to generate spikes. We tested these possibilities by performing whole cell recordings. In HEX‐sensitive ganglion cells in which HEX eliminated spike activity, it also completely blocked the EPSPs, often leaving small ON and OFF IPSPs (Fig. 1 C, D; Control: ON EPSP: 9.6 ± 1.0 mV, OFF EPSP: 9.4 ± 0.7 mV; HEX: ON: −0.8 ± 2.2 mV, OFF: −2.4 ± 1.8 mV), indicating that ACh was indeed the primary excitatory drive in these neurons. In ON‐OFF ganglion cells in which HEX did not alter extracellular spike activity (HEX‐insensitive), it did not alter the EPSPs (Fig. 1 E, F). This was not due to a lack of functional nicotinic ACh receptors, as exogenous application of agonists such as nicotine (50 μM) and carbachol (100 μM) produced strong depolarizations with increased baseline spike activity (not illustrated; carbachol: 10.0 ± 1.1 mV mean depolarization, n = 10, P < 0.001; nicotine: 7.5 ± 1.2 mV mean depolarization, n = 13, P < 0.001). Thus, there is a subset of ganglion cells that are driven synaptically by ACh, while there are many other ganglion cells that express nicotinic receptors but do not exhibit ACh‐dependent synaptic responses.
Co‐stratification with cholinergic amacrine cells
Having identified a population of third‐order neurons in rat retina with predominant cholinergic drive, we proceeded to determine an anatomical correlate for HEX sensitivity. Starbursts send processes to two distinct sublamina in the inner plexiform layer of the retina, identified by ChAT antibody staining. Ganglion cells were stained with Alexa‐488 and their processes were localized relative to the ChAT bands using confocal fluorescence microscopy (Fig. 2 A, B). Of the 20 HEX‐sensitive neurons that were identified, 13 were successfully stained and viewed after counterstaining the retina with the ChAT antibody. All 13 cells sent processes that travelled adjacent to the ChAT bands (Fig. 2 C). Conversely, HEX‐insensitive neurons did not stratify adjacent to the ChAT bands (n = 11). The HEX‐insensitive cells between the bands were transient cells while the cells distal and proximal to the bands had sustained responses to light. Further evaluation of the dendritic stratification of the HEX‐sensitive neurons indicated that the co‐fasciculation of its dendrites with starburst dendrites was significantly higher than a random occurrence, providing evidence that these cells receive local cholinergic input (Fig. 2 D, E).
Figure 2. Rat HEX‐sensitive neurons co‐stratify with starburst amacrine cells.
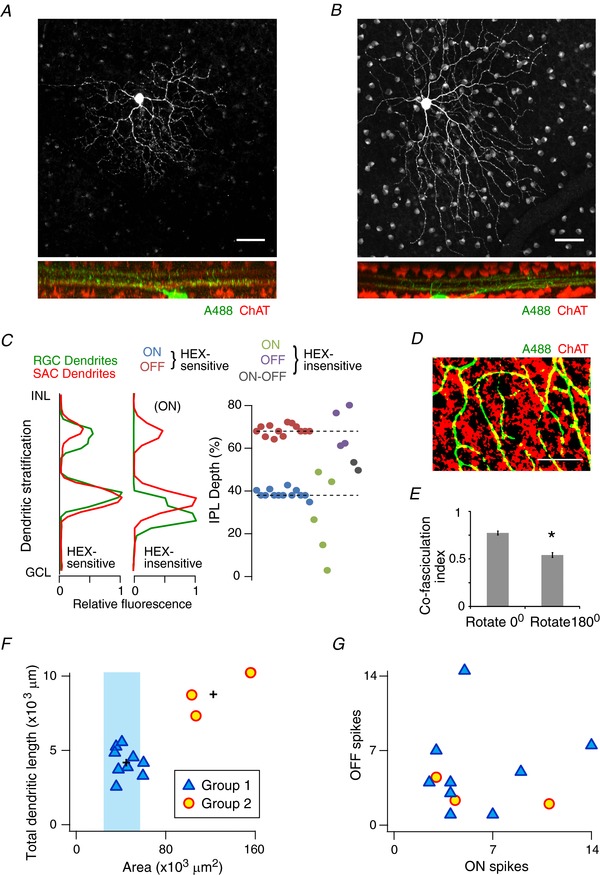
A and B, dendritic stratification of Alexa 488‐stained cells that co‐stratify with starburst dendrites labelled with ChAT antibodies (bottom panel). Scale bar = 50 μm. C, relative fluorescence of starburst dendrites along the depth of the IPL compared to the dendrites of a HEX‐sensitive cell (left) and a HEX‐insensitive neuron (middle). The right panel shows the stratification index (see methods) of 13 HEX‐sensitive cells and 11 HEX‐insensitive cells. The dashed line indicates the average ON (38 ± 1%) and OFF (68 ± 1%) ChAT bands (n = 24). The ON and OFF arbors were not clear for the two ON‐OFF HEX‐insensitive cells and hence are represented by a single value. D, the co‐fasciculation of a HEX‐sensitive neuron's dendrites with the starburst dendrites (scale bar = 50 μm). E, summary of the average co‐fasciculation index for all HEX‐sensitive cells compared to when the index was calculated after rotating the HEX‐sensitive cell's dendrites by 180° (see methods; 0° = 0.77 ± 0.02; 180° = 0.54 ± 0.03; * P < 0.001). F, a plot of the total dendritic length compared to dendritic area in HEX‐sensitive cells. Cluster analysis indicated two groups of HEX‐sensitive cells (n = 12). The (+) symbols indicate the centres of the two groups. The light shaded region shows the dendritic area of DSGC‐like cells observed by Sun et al. (2002). Hence Group 1 cells are likely to be DSGCs. We were unable to reconstruct the complete dendritic tree for one HEX‐sensitive cell, which was excluded from this analysis. G, comparison of the total number of OFF spikes vs. ON spikes evoked by light shown in Fig. 1. The two groups could not be differentiated based on this property.
Next, to explore whether HEX‐sensitive neurons consisted of multiple sub‐types, the dendritic morphology of these neurons was analysed by cluster analysis (Fig. 2 F, G; see Methods). These cells could be segregated into two groups, best visualized by comparing dendritic area and total dendritic length (Fig. 2 F). The majority of neurons (∼70%) were designated as group 1, and had medium dendritic arbors (diameter: 247 ± 9 μm; Fig. 2 A), similar to the DSGCs previously described in the rat retina (Sun et al. 2002, 2011). The group 2 neurons had a larger dendritic arbor (diameter: 393 ± 26 μm; Fig. 2 B), possibly consisting of atypical DSGCs or unidentified neuronal types. Both groups consisted of both symmetric and asymmetric dendritic trees [asymmetric index range (see Methods): group 1: 0.05–0.67; group 2: 0.04–0.52]. Furthermore, we were unable to distinguish the two groups based on their light‐evoked spiking (Fig. 2 G). For the purposes of this study, because all HEX‐sensitive neurons also had similar pharmacological effects in subsequent experiments, data were pooled.
Glutamate excitation to HEX‐sensitive neurons is masked by inhibition
While the anatomy predicts the likelihood of neurons receiving cholinergic excitation, it does not explain ACh's dominance over canonical glutamate excitation. The simplest model is that the bipolar cells providing direct AMPA/kainate receptor input to HEX‐sensitive neurons are inactive under the conditions of our experiments. However, our subsequent observations indicate that this interpretation is not correct.
In HEX‐sensitive neurons voltage clamped at −60 mV, a 1 s light stimulus produced transient ON and OFF EPSCs that were largely and reversibly blocked by HEX (Fig. 3 A–C). However, as opposed to the complete elimination of the EPSP, a fraction of the EPSC remained [ON: 24 ± 4%; OFF: 19 ± 5% of the control light‐evoked EPSC (L‐EPSC) remained in HEX]. Importantly, HEX did not significantly alter the light‐evoked IPSCs (L‐IPSCs) when these neurons were held at 0 mV, suggesting that the effects of HEX were largely postsynaptic, direct and restricted to the cholinergic synapse (Fig. 3 D, E; ON: 126 ± 27%; OFF: 123 ± 18% of L‐IPSC remaining in HEX). Furthermore, eliciting light‐evoked synaptic currents at a range of holding potentials indicated that HEX preferentially reduced responses at hyperpolarized potentials (Fig. 3 F). An estimation of the HEX‐sensitive ACh current (Control – HEX) showed an inward rectification typical of ACh receptors (Fig. 3 F inset; Sethuramanujam et al. 2016).
Figure 3. Cholinergic contribution to synaptic responses.
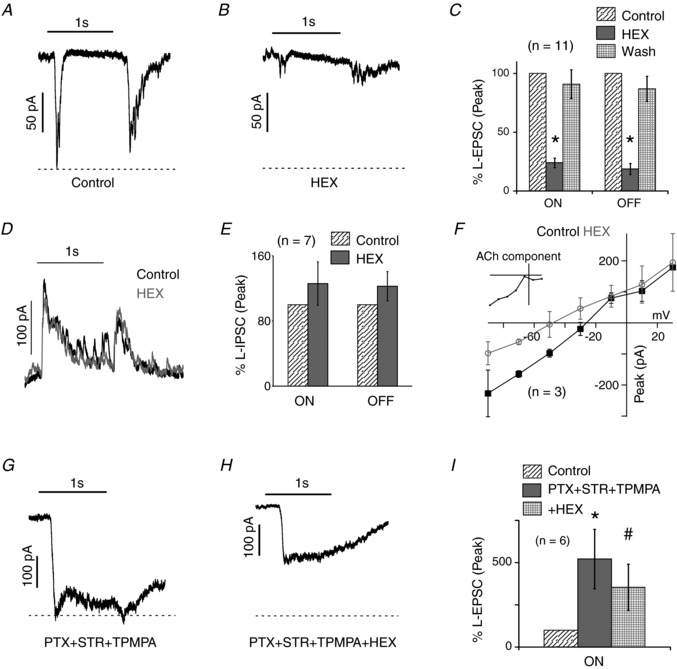
A and B, light evoked excitatory postsynaptic currents (L‐EPSC) of a rat HEX‐sensitive neuron in control and in 100 μM HEX. The light stimulus was a 200 μm diameter green spot with an intensity of 2.4 × 104 R*/rod/s. C, mean relative peak of ON and OFF L‐EPSCs in HEX relative to control and wash (n = 11; * P < 0.001). D, the light‐evoked inhibitory postsynaptic currents (L‐IPSCs) of a HEX‐sensitive neuron in control and in HEX. E, mean relative peak of ON and OFF L‐IPSCs in HEX relative to control (n = 7; ON: P = 0.37; OFF: P = 0.26). F, mean peak amplitude of postsynaptic currents in HEX‐sensitive neurons held at seven different voltages (−90 mV to +30 mV), in control and HEX (n = 3). The inset shows the ACh component estimated as the difference of currents in control and HEX. G and H, the L‐EPSCs of the cell shown in A in PTX + STR + TPMPA, then plus HEX. PTX + STR + TPMPA dramatically increased the L‐EPSCs; note the change in scale. I, peak ON L‐EPSCs in PTX + STR + TPMPA and then plus HEX relative to control (n = 6; * P < 0.005, # P < 0.05).
Interestingly, when inhibition was suppressed by a combination of picrotoxin, TPMPA and strychnine (blockers of GABAA, GABAC and glycine receptors, respectively), then the L‐EPSC was enhanced and the ON response became more prolonged (Fig. 3 G). Under these conditions, HEX suppressed much less of the light‐evoked EPSC (73 ± 7% remained in HEX, Fig. 3 H, I; only the ON response was analysed as prolongation of the ON response confounded effects of HEX on the OFF response). In HEX‐sensitive ganglion cells, blocking inhibition reduced the ACh component of the ON EPSC from 76% to 27% (n = 6; P < 0.001). Moreover, when inhibition was blocked, HEX had little effect on spike activity and EPSPs (n = 2; data not shown), indicating that glutamate now produced a near saturating response. Inhibitory receptor antagonists increased the cholinergic L‐EPSC by ∼2‐fold while increasing the glutamate L‐EPSC by nearly 15‐fold. Hence inhibition produced a disproportionate 7:1 reduction in glutamatergic versus cholinergic current observed at the soma. The simplest explanation is that the direct bipolar cell signals mediated by AMPA/kainate receptors to HEX‐sensitive cells is strongly reduced by inhibition.
Starburst inhibition masks glutamate excitation
Recent physiological studies show that mGluR2 signalling is specific to starbursts in the retina, and reduces the activity of N/P/Q type voltage‐gated calcium channels (Koren et al. 2017), known to be critical for starburst neurotransmitter release (Lee et al. 2010). Immunohistochemistry indicates that the mGluR2 receptor is found exclusively on cholinergic amacrine cells in the inner retina (Koulen et al. 1996; Yoshida et al. 2001), and indeed the activation of mGluR2 reduces directional selectivity of ganglion cells by enhancing their null spiking response (Jensen, 2006). To test if GABA release from starbursts was a major source of inhibition, we blocked starburst output using an mGluR2 receptor agonist. In our experiments, applying an mGluR2,3 agonist (DCG‐IV, 10 μM) to the retina caused a doubling of spike activity of HEX‐sensitive cells in response to light onset and offset (ON: 212 ± 27%; OFF: 208 ± 31%, compared to control; Fig. 4 A–C). DCG‐IV also eliminated most of the suppressive effect of HEX in these cells (ON: 72 ± 19%; OFF: 94 ± 15% remained in HEX, Fig. 4 D–F, compare Fig. 4 F with Fig. 1 B). Together, these results suggest that DCG‐IV increases the ganglion cell's responsiveness to glutamate by blocking starburst GABAergic output.
Figure 4. An mGluR2 agonist reveals glutamate excitation in HEX‐sensitive cells.
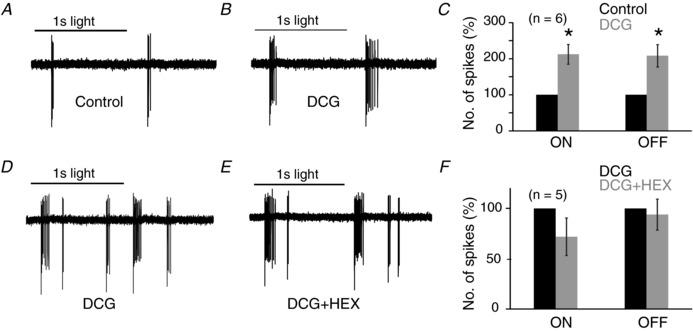
A and B, the light evoked spike activity of a rat HEX‐sensitive neuron in control and 10 μM DCG‐IV (200 μm diameter green spot; 2.4 × 104 R*/rod/s). C, the mean spike count of six cells in DCG‐IV relative to control (* P < 0.05). D and E, the light evoked spike activity of a HEX‐sensitive neuron in DCG‐IV and then DCG‐IV plus HEX. Under control conditions, HEX completely blocked the spiking in these cells (data not shown). F, the mean spike count of five cells in DCG + HEX relative to DCG (ON: P = 0.21, OFF: P = 0.72).
To test the extent to which DCG inhibits starburst neurotransmitter release, we utilized mouse retina where ChR2 was specifically expressed in starbursts, using a ChAT‐Cre mouse line (Sethuramanujam et al. 2016). The ChAT‐Cre:ChR2 line was crossed with the Hb9 line to identify DSGCs (Trenholm et al. 2011), a population of ganglion cells known to receive ACh excitation (Fig. 5 A). Once DSGCs were identified, glutamate neurotransmission was blocked using 50 μM dl‐AP4 + 10 μM CNQX (an mGluR6 agonist/NMDA and AMPA receptor antagonists) resulting in complete blockade of spiking (Fig. 5 B). This is because bipolar cell output to starbursts and ganglion cells is blocked. ChR2 stimulation, using an intense blue LED, invariably induced reliable spiking across multiple trials (mean 8 ± 1 spikes; n = 8; Fig. 5 C). Addition of HEX completely blocked spiking in four cells tested, indicating that our stimulus conditions isolated starburst neurotransmitter release. ACh/GABA release from starbursts was directly monitored in voltage clamped Hb9 cells at either −60 mV or 0 mV (Fig. 5 D). A 100 ms intense blue flash elicited robust synaptic currents in Hb9 cells, with GABA currents being ∼3‐fold larger than ACh. Further addition of DCG completely blocked both ACh and GABA currents in six cells tested (Fig. 5 E, F). These results suggest DCG‐IV as a pharmacological tool to block starburst output. Moreover, the finding that DCG‐IV increases spike output in HEX‐sensitive cells could reflect a combined effect of blocking postsynaptic cholinergic/GABAergic inputs to DSGCs, as well a reduction of starburst GABAergic inhibition to bipolar cell terminals (i.e. disinhibition).
Figure 5. mGluR2 receptor activation abolishes starburst neurotransmitter release.
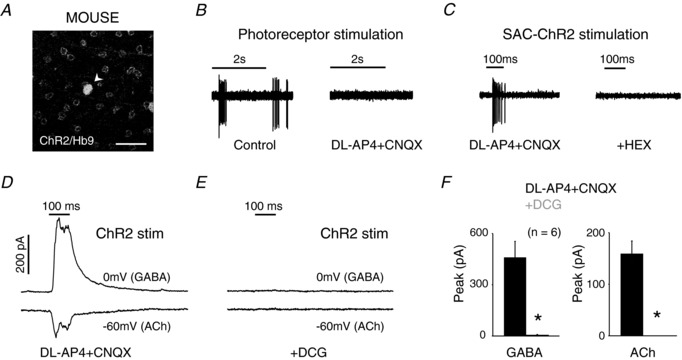
A, YFP/GFP expression in the ganglion cell layer of Hb9 × Chat‐Cre:ChR2 mouse retina. Chat‐positive neurons (starbursts) were YFP labelled and Hb9 neurons (DSGCs) expressed GFP (arrowhead). Scale bar = 50 μm. B, the light‐evoked photoreceptor‐mediated spike responses of an Hb9 cell in Chat‐Cre:ChR2 mouse retina, in control (left) and in the presence of dl‐AP4 + CNQX (right). A 200 μm diameter, white spot of intensity 20 R*/rod/s was used to elicit photoreceptor‐mediated responses. C, the response of the same cell when ChR2 was stimulated using a blue LED (108 R*/rod/s; left; n = 8). The ChR2‐stimulated spiking could be blocked by HEX (right; n = 4). D, the currents generated in a voltage clamped Hb9 neuron held at −60 mV (EPSC; ACh) and 0 mV (IPSC; GABA) in response to ChR2 stimulation in dl‐AP4 + CNQX. E, in the same cell addition of DCG blocked both the ACh and the GABA currents. F, summary of the mean peak GABA currents (left) and ACh currents (right) in the presence and absence of DCG (n = 6; P < 0.005*).
Mouse DSGCs require ACh to signal fine‐scale stimuli under physiological conditions
The experiments described so far were performed in putative DSGCs in rat retina carried out under room temperature. Furthermore, the stimuli were focused through the ganglion cell side, which could have resulted in optical blurring, reducing the effective contrast of the stimulus. Hence, to determine whether ACh excitation was critical for high contrast stimuli in DSGCs, we performed experiments in mouse Hb9‐DSGCs (Trenholm et al. 2011), where the stimulus was focused directly on the photoreceptors via the sub‐condenser. DSGCs were stimulated with varying spot sizes with a fixed high contrast to explore the importance of ACh in this parameter space (Fig. 6). Both ON and OFF spike activity increased with spot size up to 200 μm but decreased for bigger spots (400 μm/200 μm ratio: ON: 0.6 ± 0.1, P < 0.01; OFF: 0.3 ± 0.1, P < 0.001), probably due to wide‐field inhibition (Hoggarth et al. 2015). HEX reduced spiking at all spot sizes, except for OFF responses of the largest spot, with the effect being proportionately greater for smaller spots (Fig. 6 A, B). The average ON and OFF spike count for a 50 μm spot was 10 ± 2 and 5 ± 1 spikes, respectively, and HEX blocked the spiking almost completely (ON: 93 ± 5% blocked; OFF: 92 ± 6%). However, for a 200 μm spot (ON: 29 ± 6 spikes; OFF: 19 ± 3 spikes), HEX reduced the spiking by only 30 ± 8% in ON and 43 ± 10% in OFF. Hence ACh seems to provide a critical component of excitation to generate spike activity for fine scale stimuli.
Figure 6. ACh excitation in mouse DSGCs codes fine‐scale stimuli.
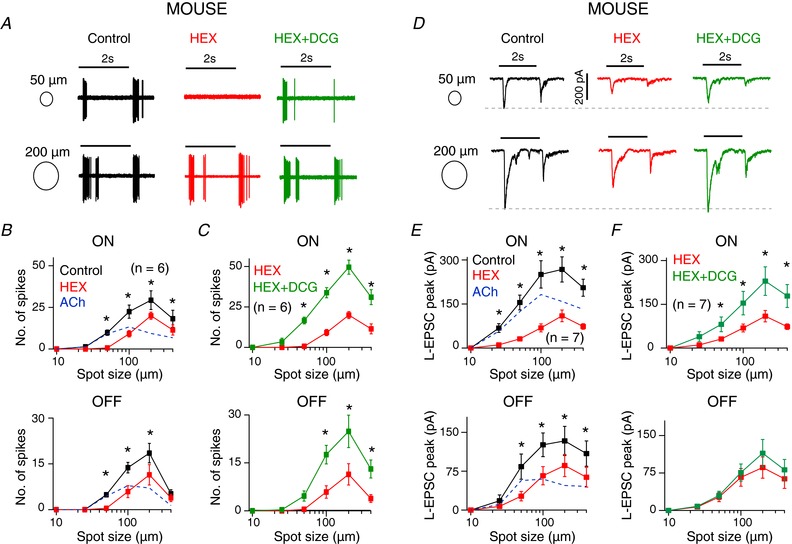
A, the ON and OFF spiking of a GFP+ cell in the Hb9 mouse retina in response to 50 and 200 μm diameter white spots (intensity = 20 R*/rod/s) under control conditions, then in the presence of HEX, and then in HEX + DCG. B, summary of the number of spikes in the ON (top) and OFF (bottom) responses with different spot sizes in control and HEX. Spike activity was significantly reduced in HEX compared to control (n = 6, * P < 0.05). The ACh component was estimated as the difference of spiking in control and HEX (dashed line). C, summary of the average number of spikes in the ON (top) and OFF (bottom) responses with different spot sizes in HEX and HEX + DCG. Spike activity was significantly increased in DCG (n = 6, * P < 0.01). D, the L‐EPSCs of an Hb9 neuron in response to 50 and 200 μm diameter spots of light in control, HEX and HEX + DCG. E and F, summary of the mean peak ON (top) and OFF (bottom) L‐EPSCs to the protocol shown in D (n = 7, * P < 0.05).
Addition of DCG‐IV in the presence of HEX increased spiking at all spot sizes (Fig. 6 A, C), restoring spiking even for stimuli where HEX had completely blocked the response (albeit with variability in OFF spiking). Because HEX was present, this excitation was now due solely to glutamate, corroborating the findings in rat retina that starburst‐mediated inhibition gates DSGC responsiveness to bipolar cell excitation. Importantly, responses decreased progressively when the stimulus size was increased beyond the optimal size (200 μm) indicating surround inhibition was intact under these conditions. This demonstrates that DCG does not block other inhibitory circuits that mediate wide field inhibition (HEX ratio: ON: 0.5 ± 0.1, OFF: 0.3 ± 0.1; DCG + HEX ratio: ON: 0.6 ± 0.1, OFF: 0.5 ± 0.1). To test whether the pharmacological manipulations altered the DSGC inputs, we analysed EPSCs to DSGCs using voltage‐clamp methods (Fig. 6 D–F). We found HEX reduced the EPSCs for small spots disproportionately in ON and OFF responses (Fig. 6 D, E; ON: 50 μm: 21 ± 4% remaining, 200 μm: 42 ± 7%, P < 0.005; OFF: 50 μm: 32 ± 9% remaining, 200 μm: 63 ± 7%, P < 0.005). This indicated that cholinergic transmission selectively mediated responses evoked by smaller light spots. Note that for the rat experiments even a ∼200 μm spot was blocked by HEX. However, as mentioned previously, it is difficult to draw numerical comparisons due to the differences in the recording conditions (temperatures, light intensities, wavelength, contrast).
Next, to test the effects of starburst GABA on bipolar cell output, we examined the effects of DCG on EPSCs measured in DSGCs. Interestingly, we found that effects of DCG were different in the ON and OFF responses. Addition of DCG (in HEX) increased the ON EPSC (50 μm EPSC in DCG = 255 ± 45% compared to HEX alone; P < 0.05) but not the OFF EPSC (50 μm EPSC = 119 ± 19% compared to HEX; P = 0.37 alone; Fig. 6 D, F). These voltage‐clamp findings would normally indicate starburst‐mediated inhibition of ON bipolar cell terminals, but potential incomplete space‐clamp of DSGCs makes this conclusion tentative (see Discussion).
Finally, we tested the effects of HEX on moving spots of different sizes. As with static spots, we found that HEX blocks spiking to small diameter spots even for moving stimuli (Fig. 7). The neurons were more sensitive and robust to spots moving in the preferred direction (direction eliciting maximum response) compared to static spots as they started responding to ∼ 20 μm spots (17 ± 5 spikes). However, HEX completely blocked the spiking at these spot sizes (Fig. 7). And similar to static spots, HEX disproportionately affected small spots (dotted line in Fig. 7 B). Therefore, starburst ACh appears critical for mediating responses to small spot stimuli, regardless of whether they were moving or static.
Figure 7. ACh excitation is critical for fine‐scale motion detection in mouse DSGCs.
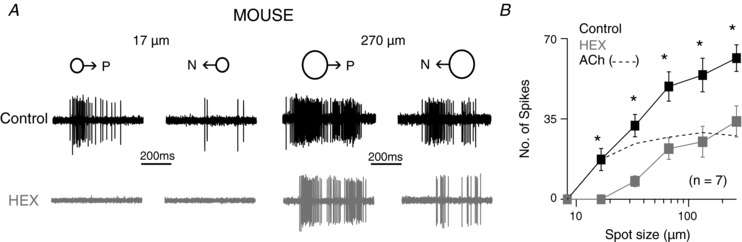
A, the spike response of an Hb9 neuron to 17 and 270 μm diameter moving white spots in the preferred and null direction (1 mm/s; intensity = 20 R*/rod/s), under control conditions and in HEX. B, the average number of spikes of the preferred direction responses in the conditions shown in A (n = 7; * P < 0.005). The ACh component was estimated as the difference of spiking in control and HEX (dashed line).
Discussion
These results indicate that starburst amacrine cells provide local excitation exclusively to neurons co‐fasciculating with their dendrites, predominantly DSGCs. Furthermore, ACh provides the primary drive in these neurons when coding fine scale stimuli, both stationary and moving. This appears to be mediated by feedforward excitation and inhibition of DSGCs along with possible presynaptic inhibition to bipolar cells.
Cholinergic transmission in the inner retina
Single cell staining coupled with exogenous cholinergic agonist application indicates that a wide variety of retinal ganglion cells express functional nicotinic ACh receptors, confirming previous reports (Masland & Ames, 1976; Ariel & Daw, 1982a ; Baldridge, 1996; Kittila & Massey, 1997; Strang et al. 2003; Renna et al. 2007). In rat retina, we found that light‐evoked responses in only a subpopulation of ON‐OFF cells were sensitive to HEX, suggesting that only these cells were activated by endogenous release of ACh. Reconstruction of cell morphology showed that the dendrites of HEX‐sensitive cells co‐fasciculate with those of starbursts, providing an anatomical correlate for the physiological findings. Most of these HEX‐sensitive rat ganglion cells (>70%, Group 1) fit the RGD2 bi‐stratified subtype classified by Sun et al. (2002), a class with morphological similarities to rat DSGCs (Sun et al. 2011). It is interesting to note that a small fraction of the rat HEX‐sensitive neurons (Group 2) had dramatically larger dendritic fields compared to previously described DSGCs. They are atypical DSGCs or an unknown neuronal type.
By contrast, a recent study using elegant trans‐synaptic viral tracing methods combined with optogenetic stimulation of starbursts in mouse retina revealed functional cholinergic inputs to a diverse set of ganglion cells including ON and OFF alpha, OFF DSGCs in addition to ON‐OFF DSGCs (Beier et al. 2013). More perplexingly, nicotinic ACh receptors are also expressed by a variety of HEX‐insensitive neurons, those that do not co‐stratify with starbursts and are not activated during synaptic transmission. It is possible that the limited stimuli we used did not stimulate cholinergic inputs to other ganglion cells optimally, and thus our study underestimates the number of HEX‐sensitive ganglion cells in the retina. For instance, our experiments did not reveal the ON DSGC population, which is a well‐established target for ACh excitation (Brombas et al. 2017). A second, although not mutually exclusive possibility is that nicotinic ACh receptors are activated through paracrine mechanisms, which do not evoke fast currents that we evaluated here. In fact paracrine transmission is the normal mode of cholinergic transmission in other parts of the brain (Picciotto et al. 2012).
Information coding by ACh
In mouse retina we posit that under certain stimulation conditions, ACh is the primary driver of activity in ganglion cells rather than simply a neuromodulator, consistent with recent studies (Sethuramanujam et al. 2016; Brombas et al. 2017). Here we reveal an important role for cholinergic inputs in conveying high‐spatial‐frequency information, while previous reports stressed their role in conveying high‐sensitivity information. While both small spots and low‐contrast stimuli produce comparable non‐optimal responses in DSGCs, they probably drive responses via different mechanisms. Large (>200 μm) low‐contrast stimuli are expected to weakly stimulate many high‐sensitivity bipolar cells, but not low‐sensitivity bipolar cells (Poleg‐Polsky & Diamond, 2016; Sethuramanujam et al. 2016). On the other hand, small (∼30–50 μm) high‐contrast stimuli are expected to strongly stimulate fewer bipolar cells, but both high‐ and low‐sensitivity bipolar cells. Importantly, probing the DSGC's receptive field with small spots activates local regions of the DSGC's receptive field, distinct from large low‐contrast spot stimuli (Trenholm et al. 2014).
Anatomical studies indicate that a majority of the bipolar cell inputs to ON starbursts are shared with DSGCs (Helmstaedter et al. 2013; illustrated by BP5‐I in Fig. 8). However, these common bipolar cells, which have high sensitivity, drive DSGCs predominantly via silent NMDA receptors but drive starbursts via AMPA receptors (Sethuramanujam et al. 2017). Thus under low‐contrast conditions, where AMPA receptor‐mediated inputs from low‐sensitivity bipolar cells (BP5‐II in Fig. 8) are inactive, spiking responses in DSGCs are contingent on ACh and NMDA receptors (Sethuramanujam et al. 2016). Under high‐contrast conditions, where both high‐ and low‐sensitivity bipolar cells would be activated, both ACh and AMPA receptor‐mediated activity at DSGCs are similar (Sethuramanujam et al. 2016). However, for small spot stimuli, the greater convergence of bipolar inputs to starbursts – relative to DSGCs – would probably make starburst‐cholinergic inputs dominate DSGC excitation (Fig. 8). Specifically, the ON starbursts receive AMPA excitation from type 7 bipolar cells (BP7 in Fig. 8; Helmstaedter et al. 2013) and BP5‐I, while, DSGCs receive AMPA excitation only from BP5‐II. In addition, it is possible non‐linearities in the starburst dendrites further amplify its output (Taylor & Smith, 2012).
Figure 8. Schematic illustrating the HEX‐sensitive/DS cell circuitry.
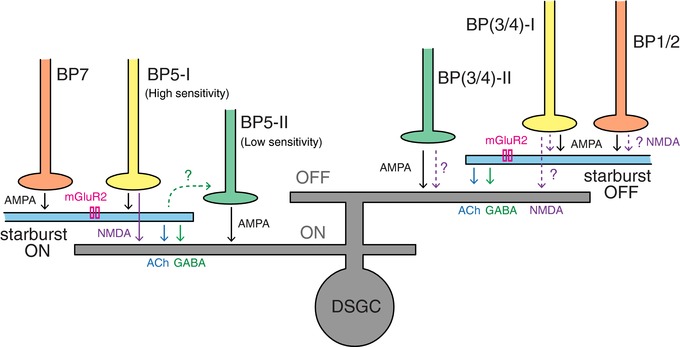
The ON starbursts and ON dendrites of DSGCs receive depolarizing glutamate excitation (i.e. AMPA receptor activity) from distinct bipolar cells (Poleg‐Polsky & Diamond, 2016; Sethuramanujam et al. 2017). BP5‐I, a bipolar cell with high‐contrast sensitivity, probably provides AMPA receptor‐mediated excitation to ON starbursts while providing NMDA receptor‐mediated excitation to ON dendrites of DSGCs (Sethuramanujam et al. 2017). BP5‐II, a low‐sensitivity bipolar cell, provides AMPA receptor‐mediated excitation exclusively to DSGC ON dendrites. Furthermore, BP7 provides exclusive AMPA receptor‐mediated inputs to starbursts (Helmstaedter et al. 2013). In the OFF pathway, BP1/2 and BP(3/4)‐I provide excitation to starbursts via AMPA and NMDA receptors (Fransen & Borghuis, 2017). BP(3/4)‐I also provides excitation to DSGCs (Helmstaedter et al. 2013), probably via NMDA receptors to ensure robust direction computation (Sethuramanujam et al. 2017). BP(3/4)‐II provides exclusive excitation to DSGCs via AMPA receptors and possibly via NMDA receptors. The OFF NMDA receptor pathways require further confirmation and hence are indicated by a dashed line. Both starbursts provide ACh excitation and GABA inhibition to DSGCs. Starbursts express mGluR2 receptors, which upon activation reduce ACh/GABA release. Furthermore, the ON starbursts seem to provide GABA inhibition to BP5‐II, a circuit feature absent in the OFF pathway.
Interestingly, ACh transmission is not only more sensitive to a smaller spot but is also selective for it. This is evident in the reduction of the relative cholinergic contribution to the EPSC when the diameter of the stimulus is increased above 100 μm (Fig. 6 E). One possible reason for the decreased cholinergic output is that starbursts reciprocally inhibit each other (Ding et al. 2016). The optimal distance for starburst–starburst inhibition is estimated to be ∼100 μm in the mouse, based on anatomy (Ding et al. 2016). Indeed, blocking GABA inhibition increases the cholinergic input (Fried et al. 2005), even in optogenetic studies where only starburst cells are activated (S Sethuramanujam & GB Awatramani, unpublished data). Hence, it is likely that spot sizes larger than 100 μm begin to engage starburst–starburst interactions and reduce ACh output, generating size selectivity.
Presynaptic modulation of bipolar cell output by starbursts
Here, we found that blocking starburst cell output with DCG‐IV leads to an increase in the glutamate‐evoked spike activity as well as the ON EPSC measured in DSGCs. This modulation may be mediated by a combination of post‐ and presynaptic mechanisms. While postsynaptic starburst cell‐mediated inhibition is well documented, whether starburst cells also provide feedback inhibition to bipolar cells is a hotly debated issue. Optogenetic stimulation of starbursts did not elicit inhibitory currents in a large proportion of the observed type 5 bipolar cells; a small fraction of the cells had weak starburst‐mediated inhibitory currents (Chen et al. 2014). However, it is unclear whether the study examined all subtypes of type 5 bipolar cells. When optical techniques were used to monitor Ca2+ signals in bipolar cell terminals/or bipolar cell glutamate release, no evidence for presynaptic inhibition from starburst cells was found; however, it should be noted that these studies were looking for a directional inhibition rather than a direct silencing of these cells (Yonehara et al. 2013; Park et al. 2014). Conversely, electrophysiological studies indicated that starburst cells provide directional inhibition to bipolar cells, i.e. larger inhibition for null direction stimuli (for reviews see Vaney et al. 2012; Percival et al. 2017). Computational studies point out that the large inhibitory conductance may prevent the ability to obtain adequate space‐clamp of the DSGC dendrites, and thus produce an artefactual directionality in the EPSCs (Schachter et al. 2010; Poleg‐Polsky & Diamond, 2011). However, the strength of this directionality in the EPSC was not correlated with the strength of directional inhibition, arguing against voltage‐clamp artefacts being the sole source of the observed directionality (Percival et al. 2017). Here, we found that DCG enhances the ON EPSC to a larger degree compared to the OFF response (Fig. 6 F). Importantly, even when the absolute amplitude of the EPSC in HEX was comparable (50 μm spot: ON = 31 ± 7 pA; OFF = 27 ± 9 pA), DCG had dramatically different effects (ON = 255 ± 45% compared to HEX; OFF = 119 ± 19%). If the length constant was increased by DCG's removal of inhibition, one might expect a greater enhancement of the electrotonically isolated OFF dendrites. Furthermore, the ∼150% EPSC increase observed for responses evoked by 50 μm spots is too large to be explained solely by clamp errors (50%; Poleg‐Polsky & Diamond, 2011). It is important to note that we used static spots in these experiments, which are expected to evoke weak IPSCs compared to spots moving in the DSGC's null direction. While this diminishes the chance of voltage‐clamp artefacts, the results do not unequivocally establish presynaptic inhibition of bipolar cells.
In view of the recent anatomical studies which failed to reveal starburst cell synapses onto bipolar cell terminals in mouse retina (Ding et al. 2016), we posit that GABA feedback may be mediated via ‘spill over’ to bipolar cell terminals (Ichinose & Lukasiewicz, 2002). Interestingly, spillover mechanisms are more prominent at room temperature (due to the temperature sensitivity of the GABA uptake mechanisms; Mitchell & Silver, 2000) and may account for the relatively stronger apparent feedback inhibition observed in rat DSGCs performed at lower temperatures. Furthermore, studies have suggested that stimulating starbursts using channelrhodopsin was found to evoke slow inhibitory currents in type 5 bipolar cells (Chen et al. 2014), albeit weakly in a small fraction of cells, consistent with such spillover mechanisms. Alternatively, the apparent feedback inhibition could also occur at the level of the unconventional vGlut3‐positive amacrine cells, which were recently shown to drive glutamate synapses at DSGCs (Lee et al. 2014). In conclusion, while the results indicate that starbursts gate glutamate signals to DSGCs, future investigations are necessary to dissect its precise synaptic mechanisms.
Additional information
Competing interests
None of the authors have any conflicts of interest.
Author contributions
The studies on rat retina were performed at the University at Buffalo, New York, USA. The studies on mouse retina were performed at the University of Victoria, British Columbia, Canada. S.S. contributed to the design, acquisition, analysis, interpretation and drafting of this work. M.M.S. and G.B.A. contributed to the design, interpretation and drafting of this work.
Funding
This work was supported by grants from the National Eye Institute (EY05725) to M.M.S. and grants from Foundation Fighting Blindness (Canada), Brain Canada, and Canadian Institutes for Health Research (CIHR MOP 142301) awarded to G.B.A.
Biography
Santhosh Sethuramanujam received his PhD from the University at Buffalo, studying how different sources of excitation and inhibition help process visual information in distinct retinal microcircuits. This work sparked his interest in understanding the computational role of mixed GABAergic/cholinergic starburst amacrine cells, found in the heart of the direction‐selective circuit. Continuing as a post‐doctoral fellow at the University of Victoria (Canada), recently he has defined a novel interaction between GABAergic/cholinergic starbursts and ‘silent’ NMDA receptors, crucial for mediating retinal direction selectivity. His future investigations will be directed towards understanding the spatial dynamics of cholinergic transmission using two‐photon imaging techniques.

Edited by: Ole Paulsen & William Taylor
References
- Ariel M & Daw NW (1982a). Effects of cholinergic drugs on receptive field properties of rabbit retinal ganglion cells. J Physiol 324, 135–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel M & Daw NW (1982b). Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J Physiol 324, 161–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge WH (1996). Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. J Neurosci 16, 5060–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Borghuis BG, El‐Danaf RN, Huberman AD, Demb JB & Cepko CL (2013). Transsynaptic tracing with vesicular stomatitis virus reveals novel retinal circuitry. J Neurosci 33, 35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M (2014). Synaptic mechanisms underlying cholinergic control of thalamic reticular nucleus neurons. J Physiol 592, 4137–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha N, Johnson D, Peichl L & Wassle H (1988). Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and gamma‐aminobutyrate immunoreactivity. Proc Natl Acad Sci U S A 85, 6187–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brombas A, Kalita‐de Croft S, Cooper‐Williams EJ & Williams SR (2017). Dendro‐dendritic cholinergic excitation controls dendritic spike initiation in retinal ganglion cells. Nat Commun 8, 15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lee S, Park SJ, Looger LL & Zhou ZJ (2014). Receptive field properties of bipolar cell axon terminals in direction‐selective sublaminas of the mouse retina. J Neurophysiol 112, 1950–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Smith RG, Poleg‐Polsky A, Diamond JS & Briggman KL (2016). Species‐specific wiring for direction selectivity in the mammalian retina. Nature 535, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva NA, Strang CE & Keyser KT (2007). Expression of alpha 7 nicotinic acetylcholine receptors by bipolar, amacrine, and ganglion cells of the rabbit retina. J Histochem Cytochem 55, 461–476. [DOI] [PubMed] [Google Scholar]
- Fransen JW & Borghuis BG (2017). Temporally diverse excitation generates direction‐selective responses in ON‐ and OFF‐type retinal starburst amacrine cells. Cell Rep 18, 1356–1365. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA & Werblin FS (2005). Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron 46, 117–127. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS & Denk W (2013). Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174. [DOI] [PubMed] [Google Scholar]
- Hoggarth A, McLaughlin AJ, Ronellenfitch K, Trenholm S, Vasandani R, Sethuramanujam S, Schwab D, Briggman KL & Awatramani GB (2015). Specific wiring of distinct amacrine cells in the directionally selective retinal circuit permits independent coding of direction and size. Neuron 86, 276–291. [DOI] [PubMed] [Google Scholar]
- Ichinose T & Lukasiewicz PD (2002). GABA transporters regulate inhibition in the retina by limiting GABAC receptor activation. J Neurosci 22, 3285–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V, Ravindran E & Dhingra NK (2012). Differential expression of Brn3 transcription factors in intrinsically photosensitive retinal ganglion cells in mouse. J Comp Neurol 520, 742–755. [DOI] [PubMed] [Google Scholar]
- Jensen RJ (2006). Activation of group II metabotropic glutamate receptors reduces directional selectivity in retinal ganglion cells. Brain Res 1122, 86–92. [DOI] [PubMed] [Google Scholar]
- Keyser KT, MacNeil MA, Dmitrieva N, Wang F, Masland RH & Lindstrom JM (2000). Amacrine, ganglion, and displaced amacrine cells in the rabbit retina express nicotinic acetylcholine receptors. Visual Neurosci 17, 743–752. [DOI] [PubMed] [Google Scholar]
- Kittila CA & Massey SC (1997). Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol 77, 675–689. [DOI] [PubMed] [Google Scholar]
- Koren D, Grove JCR & Wei W (2017). Cross‐compartmental modulation of dendritic signals for retinal direction selectivity. Neuron 95, 914–927, e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Malitschek B, Kuhn R, Wassle H & Brandstatter JH (1996). Group II and group III metabotropic glutamate receptors in the rat retina: distributions and developmental expression patterns. Eur J Neurosci 8, 2177–2187. [DOI] [PubMed] [Google Scholar]
- Lee S, Chen L, Chen M, Ye M, Seal RP & Zhou ZJ (2014). An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron 84, 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim K & Zhou ZJ (2010). Role of ACh‐GABA cotransmission in detecting image motion and motion direction. Neuron 68, 1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipin MY, Taylor WR & Smith RG (2015). Inhibitory input to the direction‐selective ganglion cell is saturated at low contrast. J Neurophysiol 114, 927–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McGlinn AM, Fernandes A, Milam AH, Strang CE, Andison ME, Lindstrom JM, Keyser KT & Stone RA (2009). Nicotinic acetylcholine receptor subunits in rhesus monkey retina. Invest Ophthalmol Vis Sci 50, 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchicchi A, Bloem B, Viana JN, Mansvelder HD & Role LW (2014). Illuminating the role of cholinergic signaling in circuits of attention and emotionally salient behaviors. Front Synaptic Neurosci 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH & Ames A, 3rd (1976). Responses to acetylcholine of ganglion cells in an isolated mammalian retina. J Neurophysiol 39, 1220–1235. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ & Silver RA (2000). GABA spillover from single inhibitory axons suppresses low‐frequency excitatory transmission at the cerebellar glomerulus. J Neurosci 20, 8651–8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley DM & Masland RH (1989). Co‐release of acetylcholine and gamma‐aminobutyric acid by a retinal neuron. Proc Natl Acad Sci U S A 86, 3414–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Kim IJ, Looger LL, Demb JB & Borghuis BG (2014). Excitatory synaptic inputs to mouse on‐off direction‐selective retinal ganglion cells lack direction tuning. J Neurosci 34, 3976–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival KA, Venkataramani S, Smith RG & Taylor WR (2017). Directional excitatory input to direction‐selective ganglion cells in the rabbit retina. J Comp Neurol 10.1002/cne.24207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ & Mineur YS (2012). Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg‐Polsky A & Diamond JS (2011). Imperfect space clamp permits electrotonic interactions between inhibitory and excitatory synaptic conductances, distorting voltage clamp recordings. PLoS One 6, e19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg‐Polsky A & Diamond JS (2016). Retinal circuitry balances contrast tuning of excitation and inhibition to enable reliable computation of direction selectivity. J Neurosci 36, 5861–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BT, Amthor FR & Keyser KT (2002). Rabbit retinal ganglion cell responses mediated by alpha‐bungarotoxin‐sensitive nicotinic acetylcholine receptors. Vis Neurosci 19, 427–438. [DOI] [PubMed] [Google Scholar]
- Reed BT, Keyser KT & Amthor FR (2004). MLA‐sensitive cholinergic receptors involved in the detection of complex moving stimuli in retina. Vis Neurosci 21, 861–872. [DOI] [PubMed] [Google Scholar]
- Renna JM, Strang CE, Amthor FR & Keyser KT (2007). Strychnine, but not PMBA, inhibits neuronal nicotinic acetylcholine receptors expressed by rabbit retinal ganglion cells. Vis Neurosci 24, 503–511. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H & Berry AS (2014). Deterministic functions of cortical acetylcholine. Eur J Neurosci 39, 1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter MJ, Oesch N, Smith RG & Taylor WR (2010). Dendritic spikes amplify the synaptic signal to enhance detection of motion in a simulation of the direction‐selective ganglion cell. PLoS Comput Biol 6, e1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuramanujam S, McLaughlin AJ, deRosenroll G, Hoggarth A, Schwab DJ & Awatramani GB (2016). A central role for mixed acetylcholine/GABA transmission in direction coding in the retina. Neuron 90, 1243–1256. [DOI] [PubMed] [Google Scholar]
- Sethuramanujam S, Yao X, deRosenroll G, Briggman KL, Field GD & Awatramani GB (2017). “Silent” NMDA synapses enhance motion sensitivity in a mature retinal circuit. Neuron 96, 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang CE, Amthor FR & Keyser KT (2003). Rabbit retinal ganglion cell responses to nicotine can be mediated by beta2‐containing nicotinic acetylcholine receptors. Vis Neurosci 20, 651–662. [DOI] [PubMed] [Google Scholar]
- Strang CE, Long Y, Gavrikov KE, Amthor FR & Keyser KT (2015). Nicotinic and muscarinic acetylcholine receptors shape ganglion cell response properties. J Neurophysiol 113, 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang CE, Renna JM, Amthor FR & Keyser KT (2007). Nicotinic acetylcholine receptor expression by directionally selective ganglion cells. Vis Neurosci 24, 523–533. [DOI] [PubMed] [Google Scholar]
- Sun L, Han X & He S (2011). Direction‐selective circuitry in rat retina develops independently of GABAergic, cholinergic and action potential activity. PLoS One 6, e19477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li N & He S (2002). Large‐scale morophological survey of rat retinal ganglion cells. Vis Neurosci 19, 483–493. [DOI] [PubMed] [Google Scholar]
- Taylor WR & Smith RG (2012). The role of starburst amacrine cells in visual signal processing. Vis Neurosci 29, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, Johnson K, Li X, Smith RG & Awatramani GB (2011). Parallel mechanisms encode direction in the retina. Neuron 71, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, McLaughlin AJ, Schwab DJ, Turner MH, Smith RG, Rieke F & Awatramani GB (2014). Nonlinear dendritic integration of electrical and chemical synaptic inputs drives fine‐scale correlations. Nat Neurosci 17, 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Sivyer B & Taylor WR (2012). Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat Rev Neurosci 13, 194–208. [DOI] [PubMed] [Google Scholar]
- Yonehara K, Farrow K, Ghanem A, Hillier D, Balint K, Teixeira M, Juttner J, Noda M, Neve RL, Conzelmann KK & Roska B (2013). The first stage of cardinal direction selectivity is localized to the dendrites of retinal ganglion cells. Neuron 79, 1078–1085. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I & Nakanishi S (2001). A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron 30, 771–780. [DOI] [PubMed] [Google Scholar]


