Highlights
-
•
Severe acute disseminated encephalomyelitis led to death in a 4.6 y old child.
-
•
Cerebral toxoplasmosis reactivation may be due to steroid treatment.
-
•
A lack of an appropriate and early screening led to a fatal outcome.
-
•
Accurate methods are required for early detection of Toxoplasma in Lebanese hospitals.
Keywords: Toxoplasma gondii, Acute encephalomyelitis, Reactivation, Cerebral toxoplasmosis, Tachyzoites-bradyzoites
Graphical abstract
Toxoplasmosis is a parasitic disease characterized by a chronic latent phase affecting one third of the worldwide human population. It is tightly controlled by the host immune response. In immunocompromised patients, reactivation of cerebral toxoplasmosis is characterized by severe neurological outcomes that may lead to death. This report describes an apparently immunocompetent 4.6 years old child with severe acute disseminated encephalomyelitis (ADEM). It highlights a peculiar case where medical treatment for ADEM (i.e. steroids) may worsen Toxoplasma infection with ominous consequences. This report highlights the importance to rule out the possibility of such infections in apparently immunocompetent hosts by running the appropriate investigations to prevent lethal complications.
Abstract
Toxoplasma gondii is an opportunistic parasite that infects a broad range of hosts including humans. The chronic latent phase of the disease manifests as intra-neuronal cerebral cysts tightly controlled by the host immune system. In immunocompromised patients, reactivation of cerebral toxoplasmosis can have severe neurological outcomes that may sometimes lead to death. Despite the efficient prophylactic and treatment measures taken against the rare reactivation of cerebral toxoplasmosis, many reports including several recent ones revealed the still occurrence of this spectrum of disease. We present the case of a 4 years-6 months old apparently immunocompetent child whose premortem clinical presentation and investigations were highly consistent with severe acute disseminated encephalomyelitis (ADEM). The patient received all appropriate medications with initial improvement followed by rapid deterioration and death. Postmortem brain autopsy revealed a wide reactivation of cerebral toxoplasmosis. This is a peculiar case presentation as such medical treatment for ADEM (i.e. steroids) may worsen the Toxoplasma infection with ominous consequences. This case highlights the importance to rule out the possibility of such infections in apparently immunocompetent hosts by performing the appropriate investigations to prevent complications.
Introduction
Toxoplasma gondii (T. gondii) is an obligate intracellular parasite that infects all warm-blooded animals, including approximately 30% of the human population worldwide [1]. It exhibits two distinct infectious stages: the tachyzoites responsible for the acute phase of infection, and the bradyzoites which persist in tissue cysts in the brain and skeletal muscles. These cysts can be dormant for the host lifetime under the tight control of the immune system [2]. However, they may become fatal in immunocompromised patients. Despite the efficient prophylactic and treatment measures taken against reactivation of cerebral toxoplasmosis, many reports including several recent ones revealed the continuing occurrence of this spectrum of disease in many immunocompromised patients including transplanted or HIV patients [[4], [5], [6]]. Reactivation of cerebral toxoplasmosis presents with symptoms that are usually neurologic, most frequently consistent with diffuse encephalopathy, meningoencephalitis, cerebral mass lesions, headaches, confusion, poor co-ordination, and seizures. At advanced stages, it could also manifest as respiratory problems, high grade fever, visual disturbance [7,8].
Case
A 4 years-6 months old child presented with a ten-day history of upper respiratory tract illness, vomiting, and headaches. For these symptoms the child was treated with antimicrobials and steroids in a rural hospital. He was brought to our hospital with a combined left sided weakness, more in the upper extremity, and a right face weakness. Outside brain imaging showed multiple right parieto-occipital cerebral white matter and brain stem lesions suggestive of ADEM.
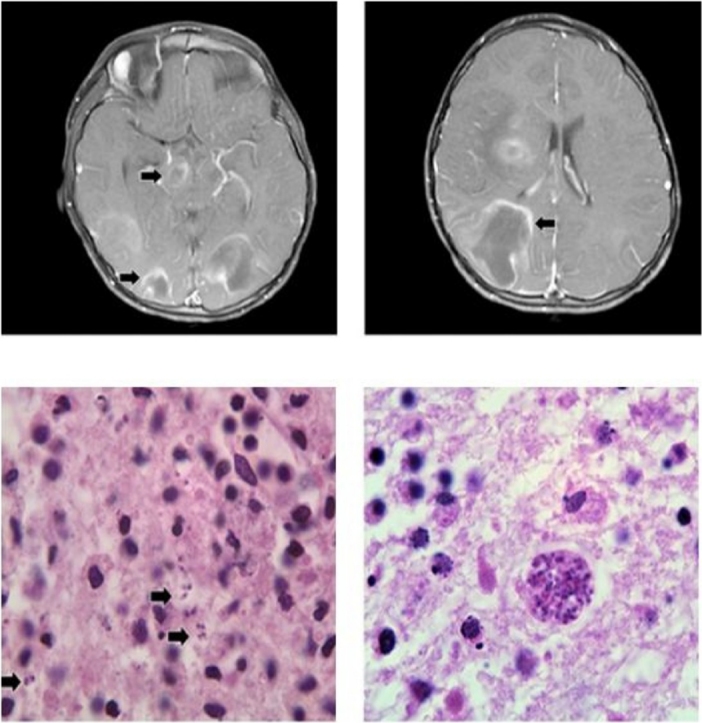
The child had a positive family history of three siblings’ deaths at an early age: one male child was diagnosed with acute disseminated encephalomyelitis and two sisters who died of coagulopathy. The parents were first degree relatives. Two cousins, within similar age range, have been treated for cardiomyopathy. Their younger brother had failure to thrive and hypotonia, moreover, a muscle biopsy suggested mitochondrial dysfunction. Another cousin was followed for seizures and hypotonia. The decedent had a history of milk allergy with small bowel mucosal villous blunting on histological examination. The hospital course was characterized by an initial period of improvement followed by rapid deterioration, coma, and death in the intensive care unit. Upon admission, MRI of the brain with and without intravenous contrast showed multifocal lesions seen within the right frontal, frontoparietal, bilateral occipital regions, and right basal ganglia with extension to the right cerebral peduncle and mid brain. These lesions were centered in the subcortical regions with some involvement of the cortex. They were of high signal on T2-weighted images and were of low signal on T1-weighted images. They showed surrounding edema, few areas of restricted diffusion peripherally, and a rim (open ring) of enhancement post gadolinium administration (Fig. 1A). Findings were suggestive of ADEM or opportunistic infection (mostly in immunocompromised patients).
Fig. 1.
A. Open ring-enhancement on brain MRI (left and right).B. Areas of necrosis with prominent perivascular lymphocytic cuffing. C. Free forms (Tachyzoite) (left) and cyst form (Bradyzoite) (right) of T. gondii organisms in a necrotic background (600x magnification). D. Relative mRNA expression by Syber green PCR of the tachyzoite surface marker SAG-1. E. Relative mRNA expression by Syber green PCR of the bradyzoite surface marker BAG-1 in the autopsy (brain tissue) of the patient.
At autopsy, the child had a normal external appearance compatible with his age. There were no gross malformations. The body weight was 15.5 kg (between 10th and 25th percentiles for age). The body height was 98 cm (between 5th and 10th percentiles for age). The brain was swollen, very soft, and congested. Coronal sections of cerebral hemispheres showed diffuse lesions throughout the white matter, with a soft to liquid consistency. These were observed mainly through the subcortical and central white matter in the right, left cerebral hemispheres (frontal to occipital), and the right basal ganglia. There was a marked compression and collapse of the lateral and third ventricles along with midline shift to the left. Duret hemorrhages (lesion occurring in the central area of brain) were seen mainly on the right side of dorsal upper brainstem, involving midbrain and rostral pons. On microscopic examination, large areas of necrosis with prominent perivascular lymphocytic cuffing were noted mainly within central white matter in the right cerebrum (frontal, parietal, and occipital) and left cerebrum (occipital, Fig. 1B). Microglial nodules were present but no definite additional viral etiology identified. On further microscopic examination, areas with necrosis revealed organisms consistent with Toxoplasma gondii both as free forms (tachyzoites, Fig. 1C) and in cyst (bradyzoites, Fig. 1C). To further confirm the diagnosis, real-time PCR using primers recognizing the specific tachyzoite surface marker [9,10], surface antigen-1 (SAG-1) or the bradyzoite specific heat shock protein antigen-1 (BAG-1) [11,12], confirmed the presence of both forms in the brain, underlying the reactivation of a cerebral toxoplasmosis (Fig. 1D and E respectively).
Discussion
The detection of reactivated toxoplasmosis in a 4 years-6 months old child is considered rare. The positive family history of three siblings’ deaths at an early age, with one diagnosed with ADEM and a cousin with seizures and hypotonia cannot exclude other cases of potential reactivated toxoplasmosis. The transmission of the parasite to these infants may be a routine way through ingestion of contaminated food or water during their early childhood especially that in Lebanon, Toxoplasma incidence is very high compared to other countries [13,14]. However, a vertical placental transmission of the parasite at a late stage of pregnancy cannot be excluded. In light of the lack of information about the mother’s Toxoplasma serology and her medical history during her pregnancy, the last possibility cannot be confirmed. Nonetheless, the history of family myopathies cannot exclude cases of Toxoplasma myocarditis. Factors associated with low socioeconomic and educational levels of the parents can be directly related to the risk of acquiring toxoplasmosis and to the lack of the appropriate screening of their child especially following their siblings’ death.
The definite diagnosis of reactivation of cerebral toxoplasmosis was performed in an in-house optimized molecular method. More sensitive and accurate methods of detection of this parasite at the early onset of the reactivation occurrence should be implemented in all hospitals in Lebanon, to be able to take the appropriate treatment measures at early stages of reactivation.
Ethics statements
- Ethics approval and consent to participate: This report does not include “living individuals” and the IRB determined that this proposed activity is not a “human research” as defined by DHHS and USFDA regulations and the need for approval was waived.
- Consent for publication
This case report doesn’t contain any individual person’s details, images or videos, and the presented case is not alive. However, a consent for publication was retrospectively obtained.
Conflict of interest statement
None of the authors have any competing interests.
Financial support
The molecular analysis was supported by the American University of Beirut Faculty of Medicine Medical Practice Plan and the Centre National de Recherche Scientifique Libanais (CNRS-L) granted to Dr Hiba El Hajj.
Authors’ contributions
SB: Reading the autopsy and taking microscopic pictures, writing of manuscript and reporting to IK. REH: Performance of real time PCR experiments, writing of manuscript and reporting to IK and to HEH. IK and HEH: Planning of the study and writing of manuscript. All authors have read and approved the manuscript.
Acknowledgements
Authors are thankful to the American University of Beirut core facilities and to its medical Center for providing access to the microscopes and Real Time PCR machine.
Contributor Information
Sami Bannoura, Email: sb92@aub.edu.lb.
Rana El Hajj, Email: rh164@aub.edu.lb.
Ibrahim Khalifeh, Email: ik08@aub.edu.lb.
Hiba El Hajj, Email: he21@aub.edu.lb.
References
- 1.Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey J.P. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28:1019–1024. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 4.Basavaraju A. Toxoplasmosis in HIV infection: an overview. Trop Parasitol. 2016;6:129–135. doi: 10.4103/2229-5070.190817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapuis A., Chabrot C., Mirand A., Poirier P., Nourrisson C. Encephalitis caused by an unusual human herpes virus type 6 and Toxoplasma gondii co-infection in a cord blood transplant recipient. Int J Infect Dis. 2016;46:79–81. doi: 10.1016/j.ijid.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Kodym P., Maly M., Beran O., Jilich D., Rozsypal H., Machala L. Incidence, immunological and clinical characteristics of reactivation of latent Toxoplasma gondii infection in HIV-infected patients. Epidemiol Infect. 2015;143:600–607. doi: 10.1017/S0950268814001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luft B.J., Remington J.S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 8.Prandota J. The importance of toxoplasma gondii infection in diseases presenting with headaches. Headaches and aseptic meningitis may be manifestations of the Jarisch-Herxheimer reaction. Int J Neurosci. 2009;119:2144–2182. doi: 10.3109/00207450903149217. [DOI] [PubMed] [Google Scholar]
- 9.Nagel S.D., Boothroyd J.C. The major surface antigen, P30, of Toxoplasma gondii is anchored by a glycolipid. J Biol Chem. 1989;264:5569–5574. [PubMed] [Google Scholar]
- 10.Cleary M.D., Singh U., Blader I.J., Brewer J.L., Boothroyd J.C. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot Cell. 2002;1:329–340. doi: 10.1128/EC.1.3.329-340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohne W., Gross U., Ferguson D.J., Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 12.Parmley S.F., Weiss L.M., Yang S. Cloning of a bradyzoite-specific gene of Toxoplasma gondii encoding a cytoplasmic antigen. Mol Biochem Parasitol. 1995;73:253–257. doi: 10.1016/0166-6851(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 13.Bouhamdan S.F., Bitar L.K., Saghir H.J., Bayan A., Araj G.F. Seroprevalence of Toxoplasma antibodies among individuals tested at hospitals and private laboratories in Beirut. J Med Liban. 2010;58:8–11. [PubMed] [Google Scholar]
- 14.Nahouli H., El Arnaout N., Chalhoub E., Anastadiadis E., El Hajj H. Seroprevalence of Anti-Toxoplasma gondii antibodies among Lebanese pregnant women. Vector Borne Zoonotic Dis. 2017;17:785–790. doi: 10.1089/vbz.2016.2092. [DOI] [PubMed] [Google Scholar]



