Introduction
Catecholamine-secreting tumours are rare, neuroendocrine tumours that arise from chromaffin cells in the adrenal medulla or sympathetic chain.1, 2, 3 They are divided into phaeochromocytomas (intra-adrenal) and paragangliomas (extra-adrenal).1 These lesions emit catecholamines into the circulation triggering hypertension, headaches, diaphoresis, palpitations and tremors.1 Due to the variety of non-specific symptoms, the diagnosis of these lesions can be especially challenging.
Case report
A 77-year-old female was referred to our regional Urology Outpatient Department with an incidental finding of a presumed renal mass in the upper pole of the left kidney. It was initially identified on imaging as part of investigation for biliary colic.
The patient denied haematuria, flank pain or unintentional weight loss. There were no features to suggest any paraneoplastic syndrome. Her medical history included hypertension, chronic obstructive pulmonary disease and a 40-pack year smoking history.
Ultrasound imaging described a mass measuring 63 × 62 × 47mm in dimension interposed between the inferior pole of the spleen and the superior pole of the left kidney. It was suggestive of a possible adrenal lesion.
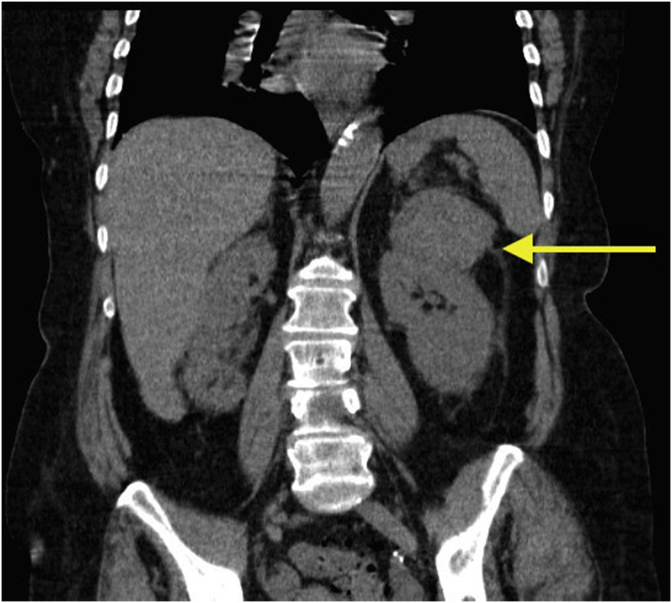
Subsequent contrast computed tomography (CT) abdomen and pelvis characterised the lesion as an exophytic upper pole cortical mass in the left kidney measuring 72 × 60 × 51mm (Fig. 1). It was heterogeneous in enhancement with solid and cystic components, and was reported as a likely renal cell carcinoma (RCC). Bilateral adrenal glands were reported normal. No lymphadenopathy or other solid organ lesions were identified. In addition, the patient underwent a positron emission scan (PET) CT that showed only mild avidity in the left renal mass.
Fig. 1.
Coronal section of a computed tomography (CT) abdomen and pelvis scan depicting a left kidney upper pole mass measuring 72 × 60 × 51mm (indicated by the yellow arrow). Appearances are suggestive of a possible renal cell carcinoma. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Renal function was stable with an estimated glomerular filtration rate (eGFR) was 62 (normal > 59) and creatinine measured 82 μmol/L (normal 45–90 μmol/L). Three urine cytology specimens were negative for urothelial malignancy. Due to the possibility of a primary adrenal mass given the tumour location, investigations were ordered to exclude a possible phaeochromocytoma.
24-h urine normetadrenaline and metadrenaline were secreted at approximately 15 times the upper limit of normal. Normetadrenaline measured 36 μmol/24h (normal = < 2.3 μmol/24h) and metadrenaline measured 11 μmol/24h (normal = < 1.3 μmol/24h). These results were further confirmed on serology with plasma normetadrenaline measuring 22 000pmol/L (normal = < 900pmol/L) and plasma metadrenaline level measuring 3120pmol/L (normal < 500pmol/L); raising the possibility of a catecholamine-producing lesion.
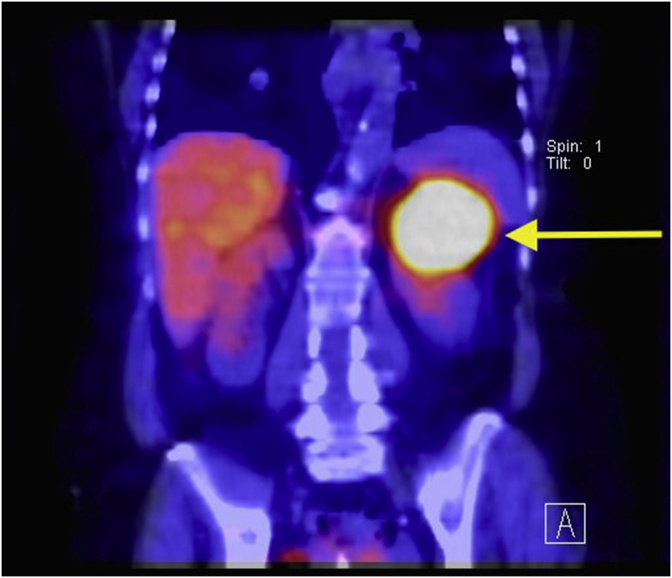
In light of these positive results, whole body MIBG study using 233Mbq 123I-MIBG was ordered. The intensity of the tracer activity corresponded to a large lesion arising in the superior aspect of the left kidney measuring 74 × 59 × 49 mm (Fig. 2). This lesion was separate from the left adrenal gland. No other MIBG avid masses were identified indicative of metastasis. This investigation thus supported the diagnosis of a paraganglioma located in the upper pole of the left kidney.
Fig. 2.
233Mbq 123I-MIBG SPECT CT coronal section of the abdomen scanned at 4 hours post MIBG injection illustrating high MIBG avidity of the left upper kidney lesion measuring 74 × 59 × 49 mm (indicated by the yellow arrow). This lesion corresponds the lesion identified on CT scan and confirms a renal paraganglioma. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Discussion
Of all catecholamine-secreting tumours, 15% of are paragangliomas, 5–10% are multiple and 10% are malignant.2 The majority of paragangliomas occur in the retroperitoneum (85%),2 and paragangliomas are more likely to be malignant (40%) than phaeochromocytomas (10%).3 Malignant paragangliomas are characterised by the presence of distant sites of metastasis.3 Sympathetic paragangliomas of the kidney are extremely rare and the mechanism by which they develop in the kidney is unknown.4 The may occur in the hilum (32%), upper pole (26%) or lower pole (42%) of the kidney.5
Functional studies using iodine labeled metaiodobenxylguinidine (123I-MIBG) are performed to localise catecholamine-secreting lesions.1 123I-MIBG is a noradrenaline analogue that is stored within intracellular storage granules of the presynaptic adrenergic neurons.6 In this case, MIBG scintigraphy was highly avid in the left upper pole of the kidney (Fig. 2).
This patient's journey emphasizes the diagnostic challenge associated with diagnosing renal tumours. She was largely asymptomatic from this lesion, however in retrospect had difficult to treat hypertensive disease and was prescribed multiple anti-hypertensive medications prior to diagnosis. Many features initially favoured the diagnosis of RCC including the patient's age, smoking status and RCC being the most common cause of malignant renal tumours. The CT scan appearance of the lesion was described as likely RCC, however there are currently no criteria to distinguish RCC from phaeochromocytoma on CT imaging.6
The current mainstay of treatment for paragangliomas is complete resection, in this case a total left nephrectomy would be recommended. Careful perioperative management is required with preoperative treatment aimed at adrenergic blockage with alpha and beta blocking agents to avoid intraoperative complications such as lethal hypertensive crisis and arrhythmias with tumour handling.1 Removing catecholamine-secreting tumours is an exceedingly high-risk procedure and should be performed at a centre with a specialized operating team.
This patient, however, declined operative treatment and planned for conservative management. She is currently prescribed phenoxybenzamine 20–30mg daily under endocrine specialist guidance and has been referred to palliative care services.
Conclusion
This patient's renal mass was identified, characterised and diagnosed by different imaging modalities. Renal paraganglioma is a rare condition, and the patient may have been misdiagnosed with RCC had further investigation and MIBG scintigraphy not been performed. Thus, careful consideration is required when evaluating renal masses.
General urology
Declarations of interest
None.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eucr.2018.08.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Carty SY W. Paragangliomas: epidemiology, clinical presentation, diagnosis, and histology: UpToDate. 2018. https://www.uptodate.com/contents/paragangliomas-epidemiology-clinical-presentation-diagnosis-and-histology [updated 15 August 2017. Available from:
- 2.Disick G.I., Palese M.A. Extra-adrenal pheochromocytoma: diagnosis and management. Curr Urol Rep. 2007;8(1):83–88. doi: 10.1007/s11934-007-0025-5. [DOI] [PubMed] [Google Scholar]
- 3.Yehia Z.A., Sayyid R.K., Haydar A.A. Renal hilar paraganglioma: a case report. World J Radiol. 2014;6(1):15–17. doi: 10.4329/wjr.v6.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti C. Urology pertinent neuroendocrine tumors: focusing on renal pelvis, bladder, prostate located sympathetic functional paragangliomas. Geka Chiryo. 2016;37(2):55–60. doi: 10.11138/gchir/2016.37.2.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes W.S., Davidson A.J., Grimley P.M., Hartman D.S. Extraadrenal retroperitoneal paraganglioma: clinical, pathologic, and CT findings. AJR Am J Roentgenol. 1990;155(6):1247–1250. doi: 10.2214/ajr.155.6.2173385. [DOI] [PubMed] [Google Scholar]
- 6.Wiseman G.A., Pacak K., O'Dorisio M.S. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. J Nucl Med. 2009;50(9):1448–1454. doi: 10.2967/jnumed.108.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.