Abstract
Purpose
Trabecular Bone Score (TBS) is a software-based method for indirect assessment of trabecular bone structure of the spine, based on analysis of pixels in dual energy x-ray absorptiometry (DXA) images. Few studies describe the use of TBS in patients with primary hyperparathyroidism (PHPT). This study aimed at further describing this relationship, investigating possible correlations between biochemistry, body mass index (BMI), fracture incidence and TBS.
Methods
Cross-sectional study of 195 patients with verified PHPT, surgically (27) or conservatively (168) treated at the Department of Endocrinology, Aalborg University Hospital. TBS was acquired by reanalyzing DXA-images of the included subjects from the outpatient clinic. Biochemical variables were obtained from clinical routine blood samples taken in relation to the DXA-scans. History of fractures and medical history was obtained from radiology reports and medical charts.
Results
Patients with active PHPT had a TBS-score signifying a partly degraded bone structure, whereas surgically treated patients had a normal bone structure as judged by TBS, though the difference in TBS-score was not statistically significant. Use of antiresorptive treatment was negatively associated with BMD but not TBS. No correlations between the biochemical variables and TBS were found. A negative correlation between TBS and BMI in patients with PHPT was present. Patients experiencing a fragility fracture had a significantly lowered TBS, BMD and T-Score.
Conclusion
Biochemistry does not seem to predict bone status in terms of TBS in patients with PHPT. TBS is negatively correlated to BMI, which is also seen in patients not suffering from PHPT. The lack of a predictive value for antiresorptive treatment for TBS may raise concern. TBS appears to have a predictive value when assessing risk of fracture in patients with PHPT.
Mini abstract
This cross-sectional study investigates possible correlations between biochemical variables, body mass index (BMI) and trabecular bone score (TBS) in 195 patients with primary hyperparathyroidism. It finds no correlation between biochemical variables and TBS, but finds a negative correlation between TBS and BMI and a clear association between fracture incidence and low TBS-score.
Keywords: Primary hyperparathyroidism, Trabecular bone score, Body mass index, Osteoporosis, Osteopenia, Biochemical variables
Highlights
-
•
Trabecular Bone Score is not predicted by biochemistry in primary hyperparathyroidism.
-
•
Trabecular Bone Score is negatively correlated to Body Mass Index.
-
•
Low Trabecular Bone Score is associated with fragility fractures in PHPT.
-
•
No correlation found between use of antiresorptives and Trabecular Bone Score.
1. Introduction
Primary Hyperparathyroidism (PHPT) is often an asymptomatic condition at the time of diagnosis (Mosekilde, 2008). Despite lack of symptoms PHPT often leads to bone loss, osteoporosis and increased risk of fractures (Rubin et al., 2008; Valdemarsson et al., 1998; Vestergaard et al., 2000). Hence, to predict and prevent fractures, guidelines suggest close monitoring of bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA)-technology (J.P. et al., 2014). BMD, however, appears to lack sensitivity with regards to predicting fractures in patients with PHPT, and many vertebral fractures (VFx) are diagnosed in patients suffering from osteopenia rather than osteoporosis (De Geronimo et al., 2006; Vignali et al., 2009). Trabecular BMD appears less affected in PHPT as opposed to BMD at cortical sites, such as the distal forearm (Dempster et al., 2007; Silverberg et al., 1989).
Studies utilizing high resolution periphery quantitative computed tomography (HRpQCT) have shown that the microarchitecture in both cortical and trabecular bone is degraded by PHPT (Bandeira et al., 2014; Hansen et al., 2010; ; Stein et al., 2013) which could explain the increased incidence of vertebral fragility fractures in patients with the disease (Khosla et al., 1999). Evaluation of bone microarchitecture seems relevant for refining risk estimation for fractures and for selecting patients for surgical treatment. QCT-technology is limited in its utility by being expensive and lack of distribution. Recently trabecular bone score (TBS)-software applied to DXA-images of the lumbar spine have shown somewhat promising results in fracture risk assessment in both primary osteoporosis and various forms of secondary osteoporosis (Bréban et al., 2012; Leslie et al., 2013b; Pothuaud et al., 2009; Rabier et al., 2010). TBS is a pixel-based grey-level tissue-texture analysis using experimental variograms adapted from 2 D lumbar spine DXA-images that provides a measurement that correlates to the bone microarchitecture (Hans et al., 2011). Thus, TBS does not directly reflect bone microarchitecture, but rather variations in the grey-tones of the DXA-image. TBS has been shown, when compared with μCT in cadaveric bones and HRpQCT in clinical studies, to correlate strongly with various measures of 3D trabecular microarchitecture such as connectivity, density, trabecular number and trabecular spacing, and thus provides an indirect estimate of the bone structure quality.(Hans et al., 2011; Pothuaud et al., 2008; Silva et al., 2013) A low TBS value is associated with a weak bone structure and an increased risk of fragility fractures partly independent of BMD (Harvey et al., 2016; Silva et al., 2014). A few studies, all based on relatively small cohorts, have evaluated TBS in patients with PHPT as a tool in assessing bone quality, fracture risk and improvement in bone structure by medical and surgical treatment of the condition (Cipriani et al., 2017; Eller-Vainicher et al., 2013; Lee et al., 2017; Rolighed et al., 2014; Romagnoli et al., 2013; Silva et al., 2013; Walker et al., 2016). These studies have shown that TBS is reduced in patients with PHPT, that TBS correlates with HRpQCT indices in these patients, and that TBS alone or in combination with lumbar spine-BMD appear more accurate than BMD alone in detecting and predicting VFx. Possible correlations between relevant blood-derived biochemical markers (such as plasma calcium (p‑calcium), plasma parathyroid hormone(p-PTH) and plasma -alkaline phosphatase(p-ALP)) and TBS in PHPT patients, have only been sparsely investigated (Eller-Vainicher et al., 2013; Walker et al., 2016). Such correlations could be valuable, as they would possibly give information about a given patient's bone-microarchitecture from a simple blood sample.
This study contributes to the existing sparse literature in the field with a larger cohort including PHPT patients with active disease as well as surgically treated. We sought to examine further the use of TBS in PHPT, aiming to investigate, by the means of multivariate regression analysis, whether a correlation between commonly used blood analyses and TBS scores could be identified in patients suffering from PHPT. We also studied the association between TBS and incidence of low-energy fractures, and finally to further clarify whether an association could be found between TBS-scores and body mass index (BMI) in patients with PHPT. The latter was a main target for the study, as previous studies have shown conflicting evidence, and a controversial negative correlation between TBS and BMI has been described recently by Donovan Tay et al., despite using newer versions of the TBS-software (Donovan Tay et al., 2018; Hernández et al., 2016; Langsetmo et al., 2016; Leslie et al., 2013a; Romagnoli et al., 2016). Patients suffering from PHPT are generally overweight (Bolland et al., 2005), and knowledge of a BMI correlation to TBS could therefore be of value in interpreting TBS results in clinical as well as research settings.
2. Methods
2.1. Study cohort
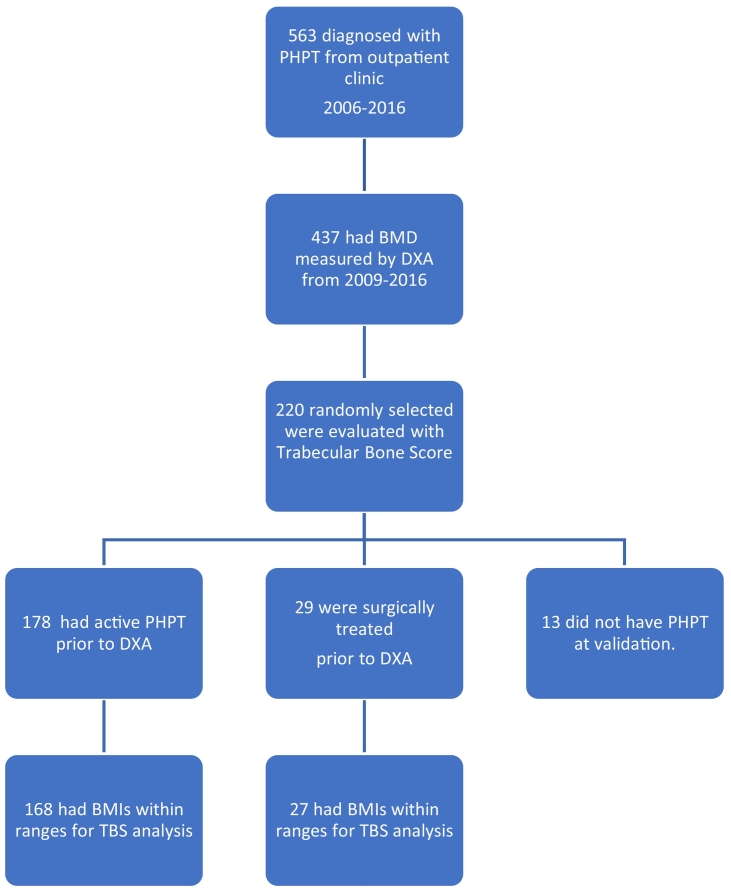
Eligible subjects were patients diagnosed with PHPT and followed in the outpatient clinic of the Department of Endocrinology, Aalborg University Hospital, Denmark, throughout the past 10 years up to the date of TBS analysis. The diagnosis was made according to international guidelines, as repeated elevated p‑calcium and corresponding elevated or inappropriate normal p-PTH, in patients without any relevant differential diagnoses. A power calculation was conducted to ascertain that the available number of subjects would be statistically sufficient to detect relevant differences. Due to lack of previous experience with the usage of TBS at this facility, the power calculation was based on expected difference in BMD between the untreated and surgically treated subgroups. Assuming a 0.75% difference in BMD between the two subgroups, a risk of type 1 errors of 5%, a desired power of 80%, and a standard deviation of 1% of the DXA-measurements of BMD, the number of needed subjects in each subgroup should comprise a minimum of 25 subjects. A sample of 220 subjects of the available cohort was therefore deemed more than sufficient by the authors.
Thus, a random sample of 220 consecutive PHPT patients, who had a DXA-scan performed between 2009 and 2015, were selected for TBS analysis. The diagnosis of PHPT was then adjudicated by the first author by scrutinizing the patients' medical charts, investigating whether relevant differential diagnoses had been ruled out (e.g. correction of possible vitamin D deficiency, familial hypocalciuric hypercalcaemia (FHH), explanatory malignancy or granulomatous disease, iatrogenic hypercalcaemia, or secondary hyperparathyroidism). The patients were divided in two groups consisting of surgically treated patients (“surgically treated subgroup”) or patients with active disease (“active disease subgroup”) prior to the DXA in question (Fig. 1). Misdiagnosed patients were removed from the following analysis. Similarly patients with a BMI lower than 15 or above 37 kg/m2 were removed from the analyses in accordance with the recommendations by The International Society of Clinical Densitometry and the manufacturer's guidelines (Silva et al., 2015).
Fig. 1.
Selection of cohort.
2.2. Data acquisition
TBS was analyzed using TBSiNsight™-software (v. 2.2.0, Medimaps Group SA, Switzerland). All analyses were performed on March 22nd, 2016. In case more DXA-scans were available for the individual subject, the most recent was chosen for analysis. TBS-values for vertebrae L1–L4 of the spine were obtained together with the corresponding BMD (g/cm2) and T-score (lumbar spine). Interpretation of the TBS-value was based on a tertile approach using the thresholds from a recent meta-analysis on TBS in fracture risk prediction by McCloskey et al. (2016), which has been used in a similar fashion in previous studies (Cipriani et al., 2017; Donovan Tay et al., 2018; Silva et al., 2013; Walker et al., 2016). Bone status in terms of TBS-score was thus graded as follows: A score ≥1.310 was considered low risk of fracture, 1.310 > TBS > 1.230 was considered intermediate, and TBS ≤ 1.230 was considered high risk. DXA images used for TBS-analysis were created using either a Hologic Discovery A or a Hologic Horizon A DXA-scanner (both Hologic Inc., MA, USA). For LS-BMD the in vivo precision (CV%) at our facility is 0.90%, for Total Hip 1.00% and for the femoral neck 1.79%. Biochemical variables were measured by the Department of Clinical Biochemistry at Aalborg University Hospital, which is subject to GLP procedures and ISO9000 accredited. Data on biochemical variables were collected in the timespan from September 28th, 2006 to the date of data extraction. Data was extracted on November 25th, 2015 from the LABKA II-system (CSC Denmark A/S), the clinical laboratory information system of the North Denmark Region, Aalborg University Hospital. Biochemical data were matched to the date of the DXA-scans and patients using personal identification numbers and the dates of acquisition for both blood samples and DXA-scans. The blood samples drawn closest to the date of the scan were selected for analysis. Biochemical analyses were performed using a Modular (2006–June 2012) or Cobas-8000 (June 2012–present) analyzer system (both Roche Diagnostics, Switzerland). The following biochemical variables were analyzed and checked for correlation to the TBS-score of vertebrae L1–L4: p-total calcium (norm. 2.20–2.55 mmol/l), p‑calcium ion (norm. 1.18–1.32 mmol/l), p-PTH (norm. 1.3–7.6 pmol/l), p-vitamin-D2 + D3 (norm. 50–160 mmol/l), p-phosphorous (norm. 0.76–1.41 mmol/l), p-creatinine (norm. 45–90 μmol/l), and p-ALP (norm. 35–105 U/l). Additionally, the renal tubular maximum reabsorption rate of phosphate to glomerular filtration rate (TmP/GFR), and the urinary calcium excretion to glomerular filtration rate (U-Ca/GFR) were calculated based on data on fasting excretion of urinary calcium, phosphorous and creatinine from the cohort. Demographic data on age (years), sex(m/f), body weight(kilograms), height(cm) and BMI(kg/m2) were collected from data obtained in relation to the DXA-scans to evaluate the representability of the selected cohort. From scrutinization of the included patients' radiology reports and medical charts further information on the diagnostic workup was obtained. This included, in addition to ruling out differential diagnoses, information about results and performance of parathyroid scintigraphy, ultrasound of the parathyroid glands, incidence of low-energy major osteoporotic fractures (MOF), use of antiresorptive agents or calcimimetics prior to the DXA-procedure, genetic screening for multiple endocrine neoplasia and FHH, previous and later parathyroid surgery (PTX) until 01/03/2018, and timespans from 1st visit in the outpatient clinic and/or previous PTX until the investigated DXA procedure. Fractures were identified through radiology reports provided by on-site radiologists. Fractures were included if they were MOFs i.e. 1st of hip, humerus, forearm or vertebral column (Klop et al., 2015), took place between June 1st 2006 to June 1st 2018, did not involve a history of a high energy trauma, and no previous fracture was described at the same site (fractures at the spine counted once from diagnosis of first vertebral fracture only). At Aalborg University Hospital VFxs are diagnosed following guidelines based on the semiquantitative visual assessment as described by Genant et al. (1993).
2.3. Statistical analysis
For statistical analysis SPSS 24 (IBM Corp., USA) and STATA 14.1 (StataCorp LP, USA) were used. Standard normal distribution was checked using QQ-plots and histograms. As some of the included patients had surgery performed prior to the included analyses, data were analyzed for treated and active disease subgroups separately. Cut-off level of p-total calcium in the adjudication procedure was 2.55 mmol/l, although results from patients with marginal disease was included if the patients previously had repeated calcium levels above this cutoff. Variables were reported in terms of percentages, means ± SD or medians derived from logarithmic transformation with ranges as applicable and depending on normality. Confidence intervals (95%) were calculated for all reported data. Statistical significance was determined using Chi-square test, Students t or Wilcoxon rank-sum tests depending on the type of data, normality, variance and distribution (F-test). Missing data were left out of analysis, no imputations were made, and number of subjects (n) was reported for each variable. Scatter-plots were used to evaluate possible correlations between TBS for L1–L4 and the various tested biochemical variables. Finally, multivariate regression analyses were computed for the whole verified population using TBS and BMD for L1–L4 as the dependent variables separately, and evaluating subgroup, sex, age, BMI, p‑calcium levels, p-total D2 + D3, p-ALP, p-creatinine, p-PTH, p-phosphorous, use of antiresorptive agents and time since diagnosis as possible predictors. Model assumptions of the distribution of residuals were checked using residual statistics outcomes, histograms and pp-plots.
This paper is written in accordance with the recommendations of the STROBE Statement on the reporting of cross-sectional studies (von Elm et al., 2007).
3. Results
3.1. Demography
A total of 563 patients had been diagnosed with PHPT and followed in the outpatient clinic, Aalborg University Hospital, from 2006 until 2016. Of those, 437 patients had a DXA-scan performed in the timespan of 2009–2015. 220 patients were randomly selected of this cohort for further analysis. Judged from the medical charts 178 had active PHPT at the time of the DXA-scan and 29 were surgically treated (see Fig. 1). When evaluating the validity of the PHPT-diagnosis cohort, 13 were found not to have PHPT and were thus excluded from the study. Of these, one did not meet diagnostic criteria, five had FHH, and seven had secondary hyperparathyroidism (four due to vitamin D deficiency and three due to renal failure). This gives a total diagnostic precision of 207/220 = 94.1% (95% CI: 90.1%; 96.8%).
12 patients were removed from further analysis due to BMIs outside the recommended range for TBS-analysis (10 with active PHPT and 2 surgically treated, all with BMIs above 37.0 kg/m2).
The diagnostic workup included a parathyroid scintigraphy in 75.0% vs. 92.3% (active vs. surg. treated; p < 0.05) of cases, and 65.9% vs 83.3% (p = 0.09) of the scanned patients had one or more adenomas visualized by this procedure. 85.1% vs 84.6% had ultrasound of the neck performed during the investigation, and 65.0% vs. 63.6% of those scanned had visualization of suspected adenomas by this method. Twelve patients (7.1%) in the active disease subgroup had undergone at least one failed parathyroidectomy prior to the DXA-procedure. Eighty-three (49.4%) of the same subgroup had surgery performed in the follow-up period (ending 01/03/2018). Three patients in the surgically treated subgroup had elevated p-PTH above normal range post-PTX, of whom none were hypercalcemic.
Median timespan from 1st visit in the outpatient clinic until time of the included DXA-procedure was 3.77 (95% CI: 2.73; 5.20) months in the active disease subgroup vs 25.2(95% CI: 15.3; 41.5) months (p < 0.001) in the surgically treated subgroup. Median time from surgery until time of DXA-procedure was 14.5 (95% CI: 7.8; 26.9) months in the surgically treated subgroup.
Time from blood-sample to scanning was checked, and did, except for p-ALP, not deviate significantly from the time of scanning; median time for each analysis was: p‑calcium: 9 days, p-PTH: 24 days, p-creatinine: 19.5 days, p-vitamin D: 39 days, p-phosphorous: 15.5 days, p‑calcium ion: 33 days, and p-ALP: 337 days.
In the active disease subgroup 21 patients (12.5%) received antiresorptive therapy prior to the DXA-session. A significantly larger proportion (29.6%; p = 0.02)) of the surgically treated received anti-resorptives at the time of scanning. Two patients in the active disease subgroup were treated with cinacalcet at the time of scanning.
Results on demography are displayed in Table 1. In the active disease fraction subjects were on average 64.1 ± 14.2 years old, 73.8% were women, and they were slightly overweight (BMI: 27.2 kg/m2 (95% CI: 26.6; 27.9)). No significant differences in demographical variables were found when comparing the untreated to the treated subgroup.
Table 1.
Demography and biochemical variables, active disease vs. surgically treated fraction.
| Variable | Surgically treated subgroup |
Active disease subgroup |
||
|---|---|---|---|---|
| Mean ± SD/{Median} | (Ranges) [95% CI] (n) |
Mean ± SD/{Median} | (Ranges) [95% CI] (n) |
|
| Age, years | 60.0 ± 14.3 | (31; 87) [54.3; 65.6] (n = 27) |
64.1 ± 14.2 | (18; 93) [61.9; 66.3] (n = 168) |
| Sex, % women/men | 77.8/22.2 | (n = 27) | 73.8/26.2 | (n = 168) |
| Height, cm | 164.14 ± 10.62 | (147.8; 199.8) [160.0; 168.3] (n = 27) |
165.60 ± 9.71 | (144.6; 194.0) [164.1; 167.1] (n = 168) |
| Weight, kg | {70.0} | (46.5; 148.4) [63.5; 77.1] (n = 27) |
74.6 ± 13.3 | (45.1; 112.3) [72.6; 76.6] (n = 168) |
| BMI, kg/m2 | 26.6 ± 5.1 | (18.4; 36.99) [24.5; 28.6] (n = 27) |
27.2 ± 4.2 | (16.4; 36.8) [26.6; 27.9] (n = 168) |
| P-total calcium, mmol/l | 2.31 ± 0.15~ | (1.78; 2.50) [2.25; 2.37] (n = 27) |
2.74 ± 0.176~ | (2.36; 3.23) [2.72; 2.77] (n = 168) |
| P‑calcium ion, mmol/l | 1.21 ± 0.064~ | (1.00; 1.33) [1.19; 1.24] (n = 26) |
1.46 ± 0.11~ | (1.23; 1.83) [1.44; 1.48] (n = 141) |
| P-PTH, pmol/l | {4.68}~ | (1.7; 46.5) [3.50; 6.26] (n = 26) |
{12.36}~ | (4.71; 100.48) [11.45; 13.34] (n = 162) |
| P-tot-25-D-vitamin, nmol/l | 79.12 ± 34.21⁎ | (17.0; 155.0) [65.00; 93.24] (n = 25) |
65.95 ± 26.67⁎ | (13.0; 148.0) [61.82; 70.1] (n = 163) |
| P-phosphorous, mmol/l | 1.03 ± 0.19~ | (0.59; 1.50) [0.96; 1.11] (n = 26) |
0.79 ± 0.185~ | (0.19; 1.47) [0.764; 0.825] (n = 147) |
| P-creatinine, μmol/l | {73.86} | (54.1; 174.2) [66.22; 82.38] (n = 27) |
{75.16} | (38.86; 179.5) [72.00; 78.47] (n = 168) |
| P-alkaline phosphatase, U/l | 73.41 ± 21.5 | (43.0 ± 117.0) [64.90; 81.91] (n = 27) |
{80.4} | (27.9; 278.7) [76.06; 84.96] (n = 152) |
| U-Ca2+/GFR | {0.040} | (0.015; 0.10) [0.025; 0.066] (n = 11) |
{0.031} | (0.004; 0.104) [0.027; 0.036] (n = 90) |
| TMP/GFR | 0.67 ± 0.16 | (0.46; 0.98) [0.56; 0.78] (n = 11) |
0.61 ± 0.17 | (0.08; 1.39) [0.57; 0.64] (n = 90) |
p < 0.05.
p < 0.001.
3.2. Biochemistry
The mean p-total calcium in the untreated subgroup was 2.74 ± 0.176 mmol/l (Ca2+-ion: 1.46 ± 0.11 mmol/l) vs 2.31 ± 0.15 mmol/l (p < 0.001) (Ca2+-ion: 1.21 ± 0.064 mmol/l) in the treated subgroup. As expected median p-PTH was significantly higher in the active disease cohort (12.36 vs 4.68 pmol/l (p < 0.001)). P-phosphorous was correspondingly lower in active disease patients (0.79 ± 0.185 vs 1.03 ± 0.19 mmol/l (p < 0.001)), and they also had significantly lower p-total 25 vitamin D (65.95 ± 26.67 vs 79.12 ± 34.21 nmol/l (p < 0.05). No significant differences in urinary-Ca2+/GFR, TMP/GFR-ratio, p-creatinine or p-alkaline phosphatase levels were found between the subgroups (See Table 1).
3.3. BMD and TBS-values
There was no significant difference in mean aBMD values for L1–L4 between the two subgroups (0.90 ± 0.15 vs 0.88 ± 0.13 g/cm2 p = 0.57, active vs treated) or T-score (−1.51 ± 1.36 vs −1.63 ± 1.18 (p = 0.68)). There was a trend for mean TBS-scores for L1–L4 being lower in the active disease-group: 1.28 ± 0.10 vs. 1.32 ± 0.12 in the active disease and treated subgroups respectively (p = 0.07), the first being within the partially degraded range, the latter in the normal range (See Table 2).
Table 2.
BMD and TBS, surgically treated vs. active disease fraction.
| Variable | Surgically treated subgroup |
Active disease subgroup |
Significance | ||
|---|---|---|---|---|---|
| Mean ± SD/{Median} | (Ranges) [95% CI] (n) |
Mean ± SD/{Median} | (Ranges) [95% CI] (n) |
||
| TBS-L1–L4 | 1.32 ± 0.12 | (1.02; 1.54) [1.27; 1.37] (n = 27) |
1.28 ± 0.10 | (0.99; 1.54) [1.26; 1.30] (n = 168) |
p = 0.07 |
| BMD-L1–L4, g/cm2 | 0.88 ± 0.13 | (0.66; 1.17) [0.83; 0.94] (n = 27) |
0.90 ± 0.15 | (0.49; 1.42) [0.88; 0.92] (n = 154) |
p = 0.57 |
| T-score, lumbar spine | −1.63 ± 1.18 | (−3.6; 0.7) [−2.13; −1.13] (n = 24) |
−1.51 ± 1.36 | (−5.10; 3.40) [−1,73; −1.30] (n = 151) |
p = 0.68 |
In the active disease subgroup 66 subjects (39.29%) had a TBS-score ≥1.31 indicating a normal bone structure/low fracture risk, 42 subjects (25.00%) had a TBS-score between 1.23 and 1.31, signifying partial degradation/intermediate fracture risk and 60 subjects (35.71%) had a TBS-score ≤1.23 indicating a degraded structure/high fracture risk. Corresponding figures for the treated subgroup were ≥1.31: 15 (55.56%); 1.23 < TBS < 1.31: 6 (22.22%); and ≤1.23: 6 (22.22%).
When excluding the subjects with osteoporosis (T-score < −2.5) the distribution was similar as follows:
Active disease: n = 115, TBS-score ≥ 1.31: 49 (42.60%); 1.23 < TBS < 1.31: 30 (26.10%); and TBS ≤ 1.23: 36 (31.30%).
Surgically treated: n = 15, TBS-score ≥ 1.31: 9 (60.0%); 1.23 < TBS < 1.31: 3 (20.0%); and TBS ≤ 1.23: 3 (20.0%).
3.4. Correlations between TBS and biochemistry (Table 3)
Table 3.
Multivariate Linear Regression, treated and untreated subgroups: TBS/BMD-L1–L4 as dependent variable.
| Predicting variable | TBS L1–L4: Unstandardized B coefficient ± Std. error; (p-value) | BMD L1–L4: Unstandardized B coefficient ± Std. error; (p-value) |
|---|---|---|
| Subgroup | −0.037 ± 0.034; (p = 0.278) | 0.011 ± 0.048 (p = 0.821) |
| Sex | 0.017 ± 0.022; (p = 0.430) | −0.055 ± 0.032 (p = 0.087) |
| Age, years | −0.002 ± 0.001; (p < 0.001⁎) | −0.001 ± 0.001 (p = 0.494) |
| BMI, kg/m2 | −0.005 ± 0.002; (p = 0.006⁎) | 0.013 ± 0.003 (p < 0.001⁎) |
| P-total calcium, mmol/l | −0.041 ± 0.065; (p = 0.525) | −0.024 ± 0.093 (p = 0.796) |
| P-PTH, pmol/l | −0.001 ± 0.001; (p = 0.391) | −0.003 ± 0.002 (p = 0.082) |
| P-Vitamin D (D2 + D3), nmol/l | −2.13 × 10−5 ± 0.000; (p = 0.948) | 0.000 ± 0.000 (p = 0.323) |
| P-Alkaline phosphatase, U/l | 0.000 ± 0.000; (p = 0.303) | −2.22 × 10−5 ± 0.000 (p = 0.954) |
| P-Creatinine, μmol/l | 0.000 ± 0.000; (p = 0.472) | 0.000 ± 0.001 (p = 0.664) |
| P-Phosphorous, mmol/l | −0.100 ± 0.055; (p = 0.071) | −0.062 ± 0.079 (p = 0.431) |
| Antiresorptive therapy | −0.030 ± 0.023; (p = 0.193) | −0.090 ± 0.033 (p < 0.01⁎) |
| Time from diagnosis | 0.000 ± 0.000; (p = 0.451) | 5.78 × 10−5 ± 0.000 (p = 0.794) |
| Model strength: R2 = 0.223 (n = 153) | Model strength: R2 = 0.241 (n = 147) |
p < 0.05.
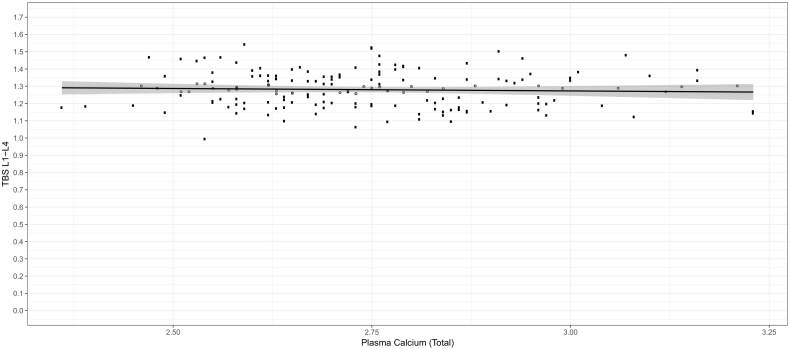
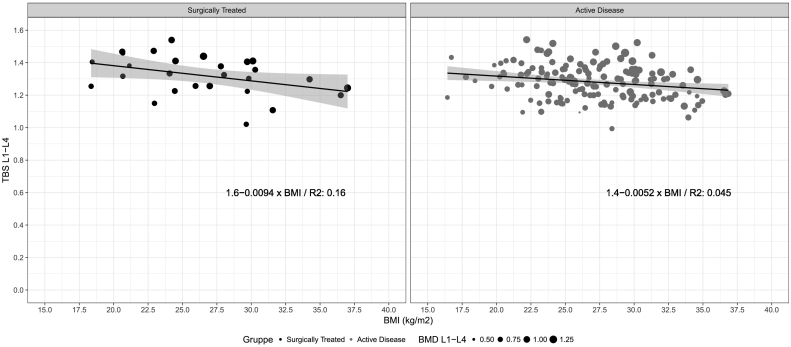
No significant correlations were found between the investigated biochemical variables and TBS for L1-L4 in either patients with active PHPT or in those surgically treated (Fig. 2). Scatterplots for the other investigated variables were similar to that of TBS vs. p-total calcium. Two multivariate regression analyses were performed using TBS and BMD of L1–L4 as the dependent variables, and sex, age, BMI, p-total calcium levels, p-total vitamin D2 + D3, p-ALP, p-creatinine, p-PTH, p-phosphorous, use of antiresorptive drugs and timespan from diagnosis to DXA-procedure as possible predictors (Table 3). Model assumptions were not violated. The explanatory strength of the models as measured by R2 were 0.223 for TBS and 0.241 for BMD. The analyses revealed no significant correlations between TBS or BMD and any of the evaluated biochemical markers. Similarly, there was no association between treatment status (subgroup) or timespan from diagnosis to DXA-procedure and either TBS or BMD. BMI and absence of antiresorptive use was significantly positively correlated with BMD. TBS was significantly negatively associated with age and BMI (age: −0.002 ± 0.001(p < 0.001)), (BMI: −0.005 ± 0.002 (p = 0.006)). The negative correlation between TBS and BMI was confirmed when plotting TBS against corresponding BMI values (Fig. 3).
Fig. 2.
Trabecular Bone Score vs. P-Total Calcium, active disease.
Fig. 3.
Trabecular Bone Score vs. Body Mass Index.
3.5. TBS, BMD and fragility fractures
28 patients were diagnosed with a total of 36 MOFs in the selected timespan. Of these 25 patients (with 32 fractures) were in the active disease subgroup. The fractures in the active disease group were distributed as follows: Hip: 3; Humerus: 3; Forearm: 15, Spine: 11.
TBS-scores were on average significantly lower in the subgroup who experienced fractures (fractures vs no-fractures: 1.23 ± 0.10 vs. 1.29 ± 0.10; p = 0.007). BMD and T-scores followed a similar pattern, although with somewhat lesser statistical significance; BMD: (0.84 ± 0.14 vs. 0.91 ± 0.14; p = 0.029); T-score: (−2.05 ± 1.26 vs. −1.42 ± 1.36; p = 0.045). Data reported here are for the active disease subgroup solely. Data on the total cohort and surgically treated subgroup can be found in the electronically submitted supplementary.
4. Discussion
4.1. TBS and BMD values
This study shows a trend for TBS being improved post-PTX in PHPT-patients, although not statistically significant. Thus, subjects with active, conservatively managed PHPT had a mean TBS within the partially degraded range, whereas surgically treated PHPT patients had a mean score within the normal range tertile.
No significant differences between the subgroups could be found for the corresponding BMDs and BMD-derived T-scores. The level of degradation, based on their TBS, in the active disease subgroup is in line with previously published studies on patients with PHPT (Cipriani et al., 2017; Lee et al., 2017; Rolighed et al., 2014; Silva et al., 2013; Walker et al., 2016). The distribution of patients between the TBS tertiles within each subgroup remained roughly unchanged even when excluding patients with osteoporosis as diagnosed by T-score below −2.5, confirming the independence of TBS from BMD.
Although there was a clear trend, the lack of statistical significance in the TBS-scores between treated and untreated patients, contrasts with what has been reported in some previous studies. Eller-Vainicher et al. (2013) and Rolighed et al. (2014) both reported increasing TBS-values 24 and 6 months after PTX, respectively. Other studies have conflicting results: Cipriani et al. (2017) found no change in TBS 18 months post PTX, and Donovan Tay et al. (2018) found no improvement in either obese or non-obese patients 24 months post-PTX. It has been suggested that this conflicting evidence could be explained by differences in the evaluated cohorts' stages of the disease prior to surgery, and also that it may take longer for TBS to improve compared to BMD (Donovan Tay et al., 2018). The absence of a more pronounced difference in mean TBS-scores between the subgroups reported in this study could alternatively be explained by the difference in timing of the scan. Time from diagnosis to DXA was much longer in the treated group (25 months) than in the patients with active disease (3.7 months). The time that thus has passed since the surgical procedure (median 14.5 months) would at least allow a reasonable catch-up in bone density. Patients offered surgical treatment generally must be expected to have more advanced disease prior to surgery, and may therefore have had a lower T-score, BMD and TBS-score at the time of surgery, the two former known to improve post-PTX (Bollerslev et al., 2007; Rao et al., 2004).
Noteworthy is also the fact that there was no correlation between the use of antiresorptive treatment and TBS-levels in the regression analysis (Table 3), whereas, as expected, a significant impact was found when applying BMD as the dependent variable (−0.088 ± 0.034; (p = 0.01)). The negative correlation found between use of antiresorptive agents and BMD suggests that the patients treated with these drugs have a more deteriorated bone structure than those not offered treatment, which is expectable as they must have fulfilled diagnostic criteria for osteoporosis prior to commencing the treatment. The lack of a predictive value for antiresorptive treatment for TBS found in our analysis, however, may raise concern in a way: Do we treat the right patients suffering from PHPT with bisphosphonates, when we simultaneously find indications that TBS has a predictive value in identifying fractures? Can TBS form the basis for commencing treatment on its own? It is intriguing that a similar correlation could not be found when analysing TBS, especially when considering it is the same image both methods are using for analysis. The lack of association with antiresorptive treatment aligns well with the subdued change post-PTX described above, and it has previously been reported that the effects by antiresorptive drugs on TBS are similar to, although less pronounced than the impact on BMD, though the partial independency of TBS from BMD may be another factor that explains this finding (Harvey et al., 2016). This study finds no correlation between age and BMD, but a significant negative interaction between age and TBS (−0.002 ± 0.001 (p < 0.001)). This is similar to what is reported by Donovan Tay et al. (2018).
4.2. TBS and biochemistry
Fig. 2 shows, and the multivariate regression model confirms that no significant correlations of clinical importance were found between any of the investigated biochemical parameters and TBS, in either surgically treated patients or patients with active disease. This is in accordance with previously published poster-abstracts (E., 2012, 2010; Manzanares, 2014), and as briefly stated by Eller-Vainicher et al. (2013) and Walker et al. (2016)).
P-vitamin D levels were statistically lower in the subgroup with active disease, which is expectable as patients with PHPT have an increased turnover of vitamin D (Rolighed et al., 2014). We found no association between vitamin D levels and TBS scores. This corresponds well with the results reported by two recent cross-sectional studies by Lee et al. (2017) and Walker et al. (2016). None of those studies found differences in TBS-levels when comparing vitamin D sufficient and vitamin D insufficient patients with PHPT. Our results also align with the lack of effect on TBS by vitamin D-treatment in patients with PHPT found by Rolighed et al. (2014). The difference between the treated and active disease groups in terms of p-phosphorous and p-vitamin D confirm that the surgically treated patients are indeed cured, as they differ significantly on these parameters which in addition to p-calcium and PTH are affected in PHPT. Three patients in the surgically treated subgroup had elevated p-PTH, one had years after PTX developed renal impairment, and two had suspected hungry bones syndrome (Brasier and Nussbaum, 1988; Lee et al., 2006).
4.3. TBS and BMI
This study is among the first ones to report a negative correlation between TBS and BMI in patients with PHPT irrespective of surgical status. The association remains negative when removing the 12 patients having BMI-values outside the working ranges recommended by TBSiNsight (15 to 37 (McCloskey et al., 2016)). This confirms the association described between TBS and weight by Walker et al. and confirms and underlines the associations found between TBS and BMI by Donovan Tay et al. (2018) and Walker et al. (2016).
This controversial negative correlation between TBS and BMI has been previously reported in various studies on other diseases than PHPT (Hernández et al., 2016; Langsetmo et al., 2016; Romagnoli et al., 2016; Shin et al., 2017). It is interesting since BMD is generally known to increase with increased bodyweight (Evans et al., 2015; Leslie et al., 2013a), which was also the case in this study (0.013 ± 0.003; (p < 0.001)). A recent publication by Mazzetti et al. (2017) demonstrated a significant difference in the relationship between TBS and BMI depending on the densitometer used (the manufacturer). The study concluded that a negative correlation was apparent when using densitometers from Hologic, but not from GE Lunar. This can be due to differences in how the densitometers accommodate soft tissues, e.g. interposed fat tissue, which must be adjusted for in the TBS-processing (Mazzetti et al., 2017). Langsetmo et al. show in their study in older men that the correlation between lumbar spine volumetric BMD and TBS is attenuated by increasing BMI, potentially indicating a waning association between TBS and incident fractures in patients with higher levels of BMI (Langsetmo et al., 2016). We used DXA-images derived from densitometers produced by Hologic Inc. Thus, our results are in keeping with those described by Mazzetti et al., and indicate that the described relationship between TBS and BMI can also be present in cohorts with PHPT. This correlation, being a result of either an actual weakened bone structure, or attenuated precision of TBS due to noise from the interposed tissue, may be important if TBS is used in clinical assessment, as it could potentially lead to an overestimation of the fracture risk in patients. This could be of particular importance when evaluating patients suffering from PHPT, keeping in mind that PHPT is usually associated with an elevated BMI (Bolland et al., 2005), a finding also observed in the patient cohorts in this study.
4.4. TBS, BMD and fragility fractures
This study finds TBS, when used in a tertile approach, to be at least comparable with BMD in predicting fragility fractures in patients with PHPT. TBS was significantly lower in patients enduring a fragility fracture throughout the study-period compared to those who did not. As also shown by the level of significance, this difference appears more pronounced for TBS than that for LS-BMD and corresponding T-score, which was in the osteopenic range. These results are in line with previous findings reported elsewhere (Eller-Vainicher et al., 2013; Romagnoli et al., 2013).
5. Strengths and limitations
A strength of this study is the relatively large sample of patients. This gives the study high power, which is relevant when a negative result is reported. The inclusion of both surgically treated patients and patients with active PHPT is another advantage, as it allows for direct comparison and indirect studying of the effect of PTX. The subgroups are demographically well matched, and the subgroups do, although they differ in size, both live up to the number dictated by the power calculation. The rather large variations of age in the patients within each subgroup gives a real life picture, and although this diversity in the cohort can influence the results, age was included in the regression analysis, and thereby adjusted for when evaluating other parameters. The inclusion of patients treated with antiresorptive agents can potentially affect the TBS analysis; however excluding this subgroup would also impose different limitations to the study, as these would likely be the patients with the most advanced stage of the disease. The method of data acquisition, being based on a diagnosis-classification system (ICD-10) can, when combined with scrutinizing the medical charts, give a valuable impression of the patients' medical history. The diagnostic precision appears to be high even before scrutinizing the medical charts, and so data validity can be assumed high. However, the study also has limitations. The difference in time from diagnosis to the DXA (27 months in the treated group vs 2.5 months for patients with active disease), and the time that has passed since the surgical procedure to the DXA procedure for patients in the treated subgroup, complicates the interpretation when comparing TBS and BMD values directly for the two subgroups, but is of less importance in the evaluation of possible correlations with biochemical markers and demographic variables.
Therefore, we cannot provide time-defined estimations of development in e.g. BMD. The median timespan from scanning procedure to blood sampling was adequately short for most variables. However, for p-ALP the timespan was significantly longer, which can possibly be attributed to the mean-values being in the normal level in both subgroups (Table 1), and that this test is performed on a rarer basis than the other tests. The impact of this time-difference on the results seems therefore to be limited. The cross-sectional design and the lack of a healthy control group impose limitations on evaluation of causality but is adequate for fulfilment of the aims in this project by allowing identification of possible correlations between variables.
6. Conclusion
Patients with PHPT have on average a partly degraded bone structure when assessed by TBS, but the score seems to improve post PTX. This study found no clinically relevant correlations between p-total calcium, p-Ca2+-ion, p-PTH, p-ALP, p- total-25- vitamin D and TBS irrespective of surgical status of the patient. The lack of a predictive value of antiresorptive treatment for TBS may raise concern. TBS is negatively associated with increasing BMI in PHPT-patients even when removing the subgroup of patients with BMI >37 kg/m2. TBS is significantly lowered in PHPT-patients who suffer fragility fractures. These associations, however, warrants further study.
Transparency document
Transparency document
Acknowledgments
Acknowledgements
The authors would like to acknowledge medimapsgroup.com for providing the free TBSiNsight-software for the analysis. We would also like to thank Line Rosengreen Kaldahl for help with performing the TBS-analyses.
Funding
This study was funded by Aalborg University and Aalborg University Hospital.
Declarations of interest
None.
Ethical approval
For this type of study formal consent is not required. The project has been notified to the Danish Data Protection Agency.
Footnotes
The Transparency document associated with this article can be found, in online version.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2018.08.001.
Contributor Information
Julius Simoni Leere, Email: j.leere@rn.dk.
Christian Kruse, Email: c.kruse@rn.dk.
Maciej Robaczyk, Email: mgr@rn.dk.
Jesper Karmisholt, Email: jsk@rn.dk.
Peter Vestergaard, Email: p.vestergaard@rn.dk.
Appendix A. Supplementary data
Data tables on TBS and BMD by fracture status for the total cohort and subgroups.
Data on medical history by subgroup.
P-PTH vs p-calcium vs TBS by subgroup.
References
- Bandeira F., Cusano N.E., Silva B.C., Cassibba S., Almeida C.B., Machado V.C.C., Bilezikian J.P. Bone disease in primary hyperparathyroidism. Arq. Bras. Endocrinol. Metabol. 2014;58:553–561. doi: 10.1590/0004-2730000003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland M.J., Grey A.B., Gamble G.D., Reid I.R. Association between primary hyperparathyroidism and increased body weight: a meta-analysis. J. Clin. Endocrinol. Metab. 2005;90(3):1525–1530. doi: 10.1210/jc.2004-1891. [DOI] [PubMed] [Google Scholar]
- Bollerslev J., Jansson S., Mollerup C., Nordenstrom J., Lundgren E., Tørring O. Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: a prospective, randomized trial. J. Clin. Endocrinol. Metab. 2007;92(5):1687–1692. doi: 10.1210/jc.2006-1836. [DOI] [PubMed] [Google Scholar]
- Brasier A.R., Nussbaum S.R. Hungry bone syndrome: clinical and biochemical predictors of its occurence after parathyroid surgery. Am. J. Med. 1988;84(4):654–660. doi: 10.1016/0002-9343(88)90100-3. [DOI] [PubMed] [Google Scholar]
- Bréban S., Briot K., Kolta S., Paternotte S., Ghazi M., Fechtenbaum J., Roux C. Identification of rheumatoid arthritis patients with vertebral fractures using bone mineral density and trabecular bone score. J. Clin. Densitom. 2012;15:260–266. doi: 10.1016/j.jocd.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Cipriani C., Abraham A., Silva B.C., Cusano N.E., Rubin M.R., McMahon D.J., Zhang C., Hans D., Silverberg S.J., Bilezikian J.P. Skeletal changes after restoration of the euparathyroid state in patients with hypoparathyroidism and primary hyperparathyroidism. Endocrine. 2017;55:591–598. doi: 10.1007/s12020-016-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geronimo S., Romagnoli E., Diacinti D., D'Erasmo E., Minisola S. The risk of fractures in postmenopausal women with primary hyperparathyroidism. Eur. J. Endocrinol. 2006;155:415–420. doi: 10.1530/eje.1.02225. [DOI] [PubMed] [Google Scholar]
- Dempster D.W., Müller R., Zhou H., Kohler T., Shane E., Parisien M., Silverberg S.J., Bilezikian J.P. Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone. 2007;41:19–24. doi: 10.1016/j.bone.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan Tay Y.-K., Cusano N.E., Rubin M.R., Williams J., Omeragic B., Bilezikian J.P. Trabecular bone score in obese and non-obese subjects with primary hyperparathyroidism before and after parathyroidectomy. J. Clin. Endocrinol. Metab. 2018 doi: 10.1210/jc.2017-02169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E. M. 2010. Molecular Mechanism of the ACVR1R206H Mutation of Fibrodysplasia Ossificans Progressiva; pp. 82–135. [Google Scholar]
- E. M. European Congress on Osteoporosis & Osteoarthritis (IOF-ECCEO12) Osteoporos. Int. 2012;23:85–386. [PubMed] [Google Scholar]
- Eller-Vainicher C., Filopanti M., Palmieri S., Ulivieri F.M., Morelli V., Zhukouskaya V.V., Cairoli E., Pino R., Naccarato A., Verga U., Scillitani A., Beck-Peccoz P., Chiodini I. Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur. J. Endocrinol. 2013;169:155–162. doi: 10.1530/EJE-13-0305. [DOI] [PubMed] [Google Scholar]
- von Elm E., Altman D., Egger M., Pocock S., Gøtzsche P.C., Vandenbroucke J., Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Evans A.L., Paggiosi M.A., Eastell R., Walsh J.S. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J. Bone Miner. Res. 2015;30:920–928. doi: 10.1002/jbmr.2407. [DOI] [PubMed] [Google Scholar]
- Genant H., Wu C., van Kuijk C., Nevitt M. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- Hans D., Barthe N., Boutroy S., Pothuaud L., Winzenrieth R., Krieg M.A. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J. Clin. Densitom. 2011;14:302–312. doi: 10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Hansen S., Jensen J.E.B., Rasmussen L., Hauge E.M., Brixen K. Effects on bone geometry, density, and microarchitecture in the distal radius but not the tibia in women with primary hyperparathyroidism: a case-control study using HR-pQCT. J. Bone Miner. Res. 2010;25:1941–1947. doi: 10.1002/jbmr.98. [DOI] [PubMed] [Google Scholar]
- Harvey N.C., Glüer C.C., Binkley E.V., McCloskey E.V., Brandi M.L., Cooper C., Kendler D., Lamy O., Laslop A., Camargos B.M., Reginster J.Y., Rizzoli R., Kanis J.A. Europe PMC funders group Trabecular Bone Score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2016:216–224. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández J.L., López-Mejías R., Blanco R., Pina T., Ruiz S., Sierra I., Ubilla B., Mijares V., González-López M.A., Armesto S., Corrales A., Pons E., Fuentevilla P., González-Vela C., González-Gay M. Association of Trabecular Bone Score with inflammation and adiposity in patients with psoriasis: effect of adalimumab therapy. J. Osteoporos. 2016;2016 doi: 10.1155/2016/5747852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J.P. B., B M.L., R. E., S S.J., U. R., C. M. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J. Clin. Endocrinol. Metab. 2014;99(10):3561–3569. doi: 10.1210/jc.2014-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S., Melton L.J., Wermers R.A., Crowson C.S., O'Fallon W.M., Riggs B.L. Primary hyperparathyroidism and the risk of fracture: a population-based study. J. Bone Miner. Res. 1999;14:1700–1707. doi: 10.1359/jbmr.1999.14.10.1700. [DOI] [PubMed] [Google Scholar]
- Klop C., Welsing P.M.J., Leufkens H.G.M., Elders P.J.M., Overbeek J.A., Van Den Bergh J.P., Bijlsma J.W.J., De Vries F. The epidemiology of hip and major osteoporotic fractures in a Dutch population of community-dwelling elderly: implications for the Dutch FRAX ® Algorithm. PLoS One. 2015;10:1–10. doi: 10.1371/journal.pone.0143800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsetmo L., Vo T.N., Ensrud K.E., Taylor B.C., Cawthon P.M., Schwartz A.V., Bauer D.C., Orwoll E.S., Lane N.E., Barrett-Connor E., Schousboe J.T. The association between trabecular bone score and lumbar spine volumetric BMD is attenuated among older men with high body mass index. J. Bone Miner. Res. 2016;31:1820–1826. doi: 10.1002/jbmr.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.-T., Sheu W.H.-H., Tu S.-T., Kuo S.-W., Pei D. Bisphosphonate pretreatment attenuates hungry bone syndrome postoperatively in subjects with primary hyperparathyroidism. J. Bone Miner. Metab. 2006;24:255–258. doi: 10.1007/s00774-005-0680-x. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Kim J.H., Hong A.R., Kim S.W., Shin C.S. 2017. Skeletal Effects of Vitamin D Deficiency among Patients with Primary Hyperparathyroidism; pp. 1667–1674. [DOI] [PubMed] [Google Scholar]
- Leslie W.D., Krieg M.-A., Hans D. Clinical factors associated with trabecular bone score. J. Clin. Densitom. 2013;16:374–379. doi: 10.1016/j.jocd.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Leslie W.D., Aubry-Rozier B., Lamy O., Hans D. TBS (trabecular bone score) and diabetes-related fracture risk. J. Clin. Endocrinol. Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- Manzanares World congress on osteoporosis, osteoarthritis and musculoskeletal diseases (WCO-IOF-ESCEO 2014): poster abstracts. Osteoporos. Int. 2014;25:159–440. doi: 10.1007/s00198-018-4465-1. [DOI] [PubMed] [Google Scholar]
- Mazzetti G., Berger C., Leslie W.D., Hans D., Langsetmo L., Hanley D.A., Kovacs C.S., Prior J.C., Kaiser S.M., Davison K.S., Josse R., Papaioannou A., Adachi J.R., Goltzman D., Morin S.N. Densitometer-specific differences in the correlation between body mass index and lumbar spine trabecular bone score. J. Clin. Densitom. 2017;20:233–238. doi: 10.1016/j.jocd.2016.11.003. [DOI] [PubMed] [Google Scholar]
- McCloskey E.V., Odén A., Harvey N.C., Leslie W.D., Hans D., Johansson H., Barkmann R., Boutroy S., Brown J., Chapurlat R., Elders P.J.M., Fujita Y., Glüer C.C., Goltzman D., Iki M., Karlsson M., Kindmark A., Kotowicz M., Kurumatani N., Kwok T., Lamy O., Leung J., Lippuner K., Ljunggren Ö., Lorentzon M., Mellström D., Merlijn T., Oei L., Ohlsson C., Pasco J.A., Rivadeneira F., Rosengren B., Sornay-Rendu E., Szulc P., Tamaki J., Kanis J.A. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Miner. Res. 2016;31:940–948. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- Mosekilde L. Primary hyperparathyroidism and the skeleton. Clin. Endocrinol. 2008;69:1–19. doi: 10.1111/j.1365-2265.2007.03162.x. [DOI] [PubMed] [Google Scholar]
- Pothuaud L., Carceller P., Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42:775–787. doi: 10.1016/j.bone.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Pothuaud L., Barthe N., Krieg M.A., Mehsen N., Carceller P., Hans D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, Case-Control Study. J. Clin. Densitom. 2009;12:170–176. doi: 10.1016/j.jocd.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Rabier B., Héraud A., Grand-Lenoir C., Winzenrieth R., Hans D. A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): Analysing the odds of vertebral fracture. Bone. 2010;46:176–181. doi: 10.1016/j.bone.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Rao D., Phillips E., Divine G., Talpos G. Randomized controlled trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2004;89(11):5415–5422. doi: 10.1210/jc.2004-0028. [DOI] [PubMed] [Google Scholar]
- Rolighed L., Rejnmark L., Sikjaer T., Heickendorff L., Vestergaard P., Mosekilde L., Christiansen P. Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J. Clin. Endocrinol. Metab. 2014;99(3):1072–1080. doi: 10.1210/jc.2013-3978. [DOI] [PubMed] [Google Scholar]
- Romagnoli E., Cipriani C., Nofroni I., Castro C., Angelozzi M., Scarpiello A., Pepe J., Diacinti D., Piemonte S., Carnevale V., Minisola S. “Trabecular Bone Score” (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone. 2013;53:154–159. doi: 10.1016/j.bone.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Romagnoli E., Lubrano C., Carnevale V., Costantini D., Nieddu L., Morano S., Migliaccio S., Gnessi L., Lenzi A. Assessment of trabecular bone score (TBS) in overweight/obese men: effect of metabolic and anthropometric factors. Endocrine. 2016;54:342–347. doi: 10.1007/s12020-016-0857-1. [DOI] [PubMed] [Google Scholar]
- Rubin M.R., Bilezikian J.P., McMahon D.J., Jacobs T., Shane E., Siris E., Udesky J., Silverberg S.J. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J. Clin. Endocrinol. Metab. 2008;93:3462–3470. doi: 10.1210/jc.2007-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.H., Gong H.S., Lee K.J., Baek G.H. Older age and higher body mass index are associated with a more degraded trabecular bone score compared to bone mineral density. J. Clin. Densitom. 2017;1–6 doi: 10.1016/j.jocd.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Silva B.C., Boutroy S., Zhang C., McMahon D.J., Zhou B., Wang J., Udesky J., Cremers S., Sarquis M., Guo X.-D.E., Hans D., Bilezikian J.P. Trabecular bone score (TBS)–a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2013;98:1963–1970. doi: 10.1210/jc.2012-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva B.C., Leslie W.D., Resch H., Lamy O., Lesnyak O., Binkley N., McCloskey E.V., Kanis J.A., Bilezikian J.P. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 2014;29:518–530. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- Silva B.C., Broy S.B., Boutroy S., Schousboe J.T., Shepherd J.A., Leslie W.D. 10 - ISCD position - fracture risk prediction by non-BMD DXA measures: the 2015ISCD official positions part 2: trabecular bone score. J. Clin. Densitom. 2015;18:309–330. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Silverberg, S.J., Shane, E., de la Cruz, L., Dempster, D.W., Feldman, F., Seldin, D., Jacobs, T., Siris, E.S., Cafferty, M., Parisien, M.V., Bilezikian, J.P., 1989. Skeletal disease in primary hyperparathyroidism. J. Bone Miner. Res. 4, 283–91. DOI: 10.1002/jbmr.5650040302. [DOI] [PubMed]
- Stein E.M., Silva B.C., Boutroy S., Zhou B., Wang J., Udesky J., Zhang C., McMahon D.J., Romano M., Dworakowski E., Costa A.G., Cusano N., Irani D., Cremers S., Shane E., Guo X.E., Bilezikian J.P. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J. Bone Miner. Res. 2013;28:1029–1040. doi: 10.1002/jbmr.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdemarsson S., Lindergard B., Tibblin S., Bergenfelz A. Increased biochemical markers of bone formation and resorption in primary hyperparathyroidism with special reference to patients with mild disease. J. Intern. Med. 1998;243:115–122. doi: 10.1046/j.1365-2796.1998.00241.x. [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Mollerup C.L., Frokjaer V.G., Christiansen P., Blichert-Toft M., Mosekilde L. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ. 2000;321:598–602. doi: 10.1136/bmj.321.7261.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali E., Viccica G., Diacinti D., Cetani F., Cianferotti L., Ambrogini E., Banti C., Del Fiacco R., Bilezikian J.P., Pinchera A., Marcocci C. Morphometric vertebral fractures in postmenopausal women with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2009;94:2306–2312. doi: 10.1210/jc.2008-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.D., Saeed I., Lee J.A., Zhang C., Hans D., Lang T., Silverberg S.J. Effect of concomitant vitamin D deficiency or insufficiency on lumbar spine volumetric bone mineral density and trabecular bone score in primary hyperparathyroidism. Osteoporos. Int. 2016;27:3063–3071. doi: 10.1007/s00198-016-3637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document
Data tables on TBS and BMD by fracture status for the total cohort and subgroups.
Data on medical history by subgroup.
P-PTH vs p-calcium vs TBS by subgroup.