Abstract
We present a case of a 14-year-old girl, Bacillus Calmette-Guérin (BCG) vaccinated, who presented with vague symptoms of abdominal pain, weight loss, and fatigue. Imaging studies revealed a pelvic mass, later found to be pelvic tuberculosis, a rare diagnosis to consider at this age. The diagnostic approach was difficult, since all investigations pointed strongly to a malignancy, from clinical, imaging (ultrasound and magnetic resonance), laboratory (elevated CA-125), and even macroscopic findings at laparotomy. Histopathology was the first hint (noncaseous granulomata), but the ultimate documentation of Mycobacterium tuberculosis relied on a persistent clinical suspicion, despite contradicting results. Surgical approach could have been mutilating, with irreversible consequences, considering it was a girl with a long reproductive life ahead. Tuberculosis is still a great masquerade, especially the extrapulmonary forms, and although infrequently seen at this age, it should thus be considered in the differential diagnosis of complex pelvic masses in order to avoid surgical iatrogeny/morbidity.
Keywords: Pelvic tuberculosis, Adnexal mass, Ovarian carcinoma, Pelvic MR, Pelvic ultrasound
Introduction
Tuberculosis (TB) may be considered in 2 forms: pulmonary and extrapulmonary (kidneys, bone, central nervous system, gastrointestinal tract, female genital tract, peritoneum, etc). Extrapulmonary TB accounts for 15%-20% of all cases of TB, but among them, female pelvic TB is rare (about 5%). In children and adolescents, extrapulmonary forms are rarely reported in the pelvis, most reports being localized to lymph nodes, bone, and peritoneum [1], [2]. Due to its insidious nature and nonspecific signs and symptoms, radiological investigation plays a crucial role in the early and correct identification of the disease. However, owing to its rarity and the fact that it is restricted to some epidemiologic contexts, imaging findings are easily misdiagnosed as advanced ovarian malignancy or pelvic inflammatory disease [3]. With this case report [4], we aim at promoting recognition and understanding of the imaging findings spectra associated with female pelvic TB and emphasize the need for integration with clinical and laboratory data that are crucial to avoid misdiagnosis, delay of treatment (allowing disease progression), and surgical exploration.
Case report
An African14-year-old girl was admitted to the pediatric disease department for investigation of an adnexal mass.
Five months before admission, she immigrated to Portugal from Angola. One month after being in Portugal, she started feeling fatigue, loss of appetite, daily somnolence, and a persistent dry cough. She was medicated with an antihistaminic with some improvement of the cough. As the symptoms persisted, she had some laboratory tests, which showed microcytic hypochromic anemia that was medicated with iron replacement therapy. Two months later, she went to the emergency department due to aggravating symptoms, fever (of irregular pattern), weight loss (4 kg in 1 month), a low abdominal pain, and pain with inspiration in the right hemithorax. There was no associated past medical or surgical history and no history of recent disease in her family circle. She had no other signs or symptoms, namely, no menstrual disturbances (menarcha at 10 years), no urinary, respiratory (the cough subsided), or gastrointestinal symptoms. On physical examination, a palpable painful hypogastric mass was found, hard on palpation and of irregular contours. A BCG vaccination scar was visible.
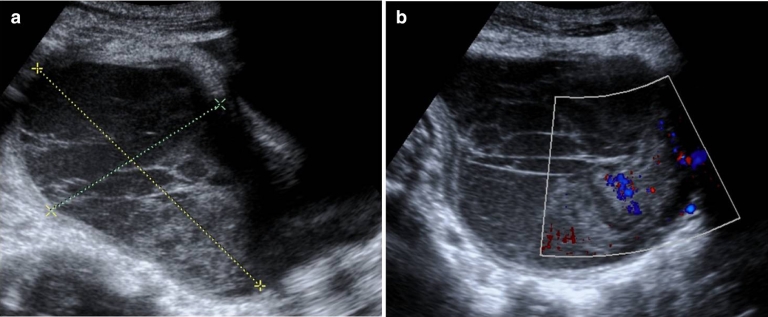
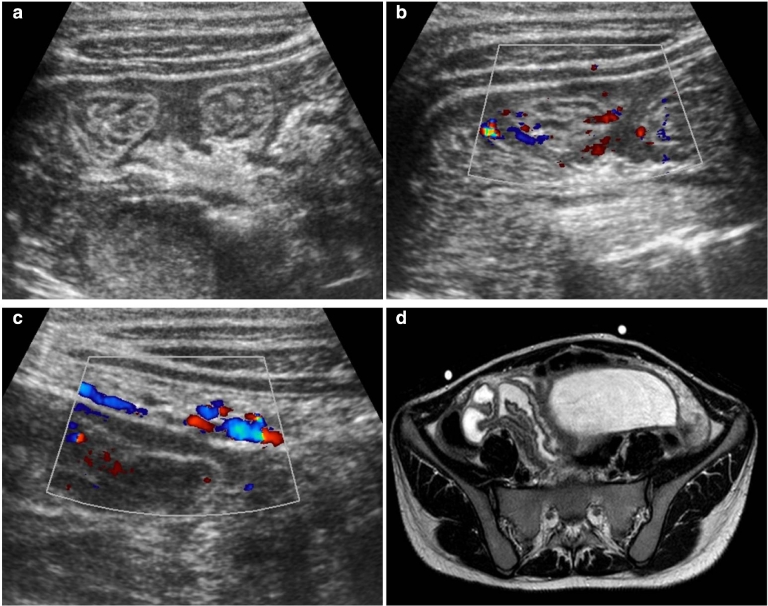
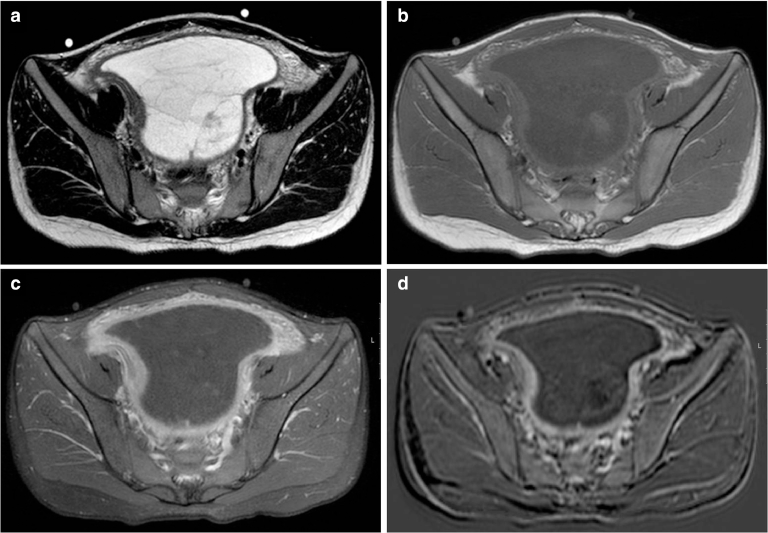
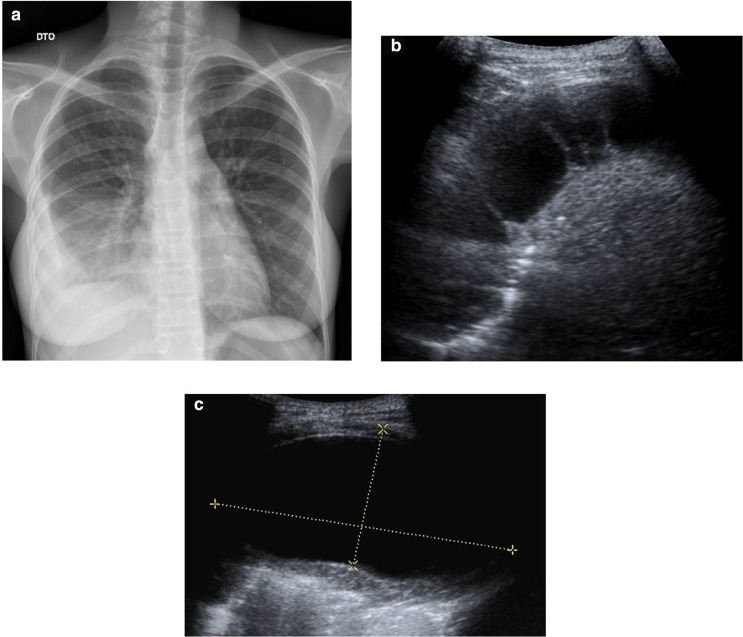
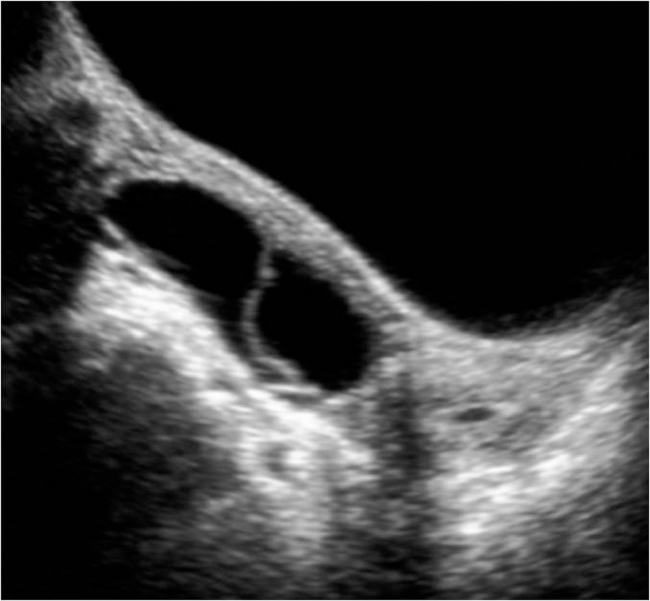
She had an ultrasound (Fig. 1) that showed a large pelvic mass (about 12 × 8 cm), centered in the right adnexal area, predominantly cystic, complex, encapsulated, with multiple thin septa and a solid component that exhibited some vascularity in the Color Doppler study (Fig. 1b), creating a mass effect on the uterus and bladder. The left ovary was heterogeneous and mildly enlarged (not shown). There were associated terminal ileum, peritoneal thickening (Fig. 2), and mild ascitis. Pelvic magnetic resonance (MR; Fig. 3) documented a complex cystic mass, apparently having its epicenter on the right adnexal area, with high signal in T2W sequences and intermediate-to-low signal in T1W sequence, with no saturation on fat-suppressed sequences. The multiple septa were thin, irregular, and had no definite enhancement on post gadolinium sequences. A solid enhancing component was not confirmed. Upon scrolling the sagittal images, the lesion suggested to be contiguous with the uterine horn and terminating at the right ovary, hence a hydropyosalpingitis was neither confirmed nor excluded. There was some distortion of ileal loops that were hard to characterize as they were retracted and adherent close together and in apposition with the mass. Despite this, the lesion seemed to be encapsulated and well defined. Some peritoneal enhancement was noted. No adenopathies were visible in the pelvis or in the lomboaortic chains. Chest radiograph (Fig. 4a) revealed an obliteration of the right costophrenic angle extending upwards along the lateral wall, suggesting a loculated effusion. The corresponding chest ultrasound (Fig. 4b and c) exhibited a pleural effusionat the right lung base, anechogenic, with some thin septa, extending superiorly to the middle lung and causing passive atelectasis of the adjacent lung parenchyma.
Fig. 1.
Ultrasound of the pelvis showing a complex lesion, predominantly cystic, centered in the right adnexal area (a). It appears to be encapsulated with defined margins. There are multiple thin septa and an apparent solid component in a gravity-dependent location, which exhibited some vascularity in Color Doppler study (b).
Fig. 2.
Ultrasound of the right iliac fossa showing associated ileal bowel wall (a) and peritoneal thickening (c) with inflammatory features (Color Doppler signal in b and c). MR image of the corresponding area (d) depicts the thickened ileal bowel loops adjacent to the lesion.
Fig. 3.
MR images of the pelvic lesion. (a) Axial T2-weighted image of the complex lesion, with high signal intensity and some thin septa. (b) Axial T1-weighted image does not show areas of hyperintensity. (c) Axial contrast-enhanced T1-weighted image with fat suppression exhibits prominent enhancement of the lesion's pseudocapsule and peritoneum. (d) Subtraction image does not document any enhancing solid component within the mass.
Fig. 4.
(a) Chest radiograph shows a right loculated pleural effusion. (b, c) Corresponding ultrasound showing a predominantly anechogenic effusion with some thin septa (b). A large pouch of loculated pleural fluid (c), with 96 × 45 mm, shows that the fluid is clear despite the overall complex nature of the effusion.
Laboratory investigations elicited a normal leucogram and C-reactive protein (CRP), persistence of a microcytic hypochromic anemia (9.1 g/dL) and an elevated CA-125 (576 U/mL, normal values <35 U/mL).
As an ovarian malignant tumor was suspected, she was submitted to an exploratory laparotomy with the intent of an eventual resection. A large right adnexal whitish mass was found, occupying the small pelvis, with an uninterrupted capsule, from which adhesions emanated to the bladder and ileon bowel walls, preventing it from resection. There was extensive perilesional inflammatory reaction, adherent to the peritoneal fat and lateral wall of the pelvic region, and multiple lesions with morphology suggestive of implants to the wall of the ileal loops in contact with the mass and in the peritoneum adjacent to the mass.
The biopsy of the lesion revealed numerous granulomata with giant cells but no caseous necrosis and no evidence of neoplastic cells. Further laboratory testing revealed an elevated seric ADA (83 U/L, normal ranges being 4.8-23 U/L). As the results of the initial mass sample testing were nonspecific, she was submitted to an ultrasound guided fine-needle aspiration of the cystic lesion and a diagnostic thoracocentesis (also ultrasound guided).
The tuberculin skin testing (TST/Mantoux) was marginally positive (13 mm).
Interferon-gamma release assay (IGRA) QuantiFERON testing was negative but further testing with IGRA T-SPOT was positive, substantiating the clinical suspicion of tuberculosis.
Pleural liquid analysis revealed a very elevated LDH (1083 U/L, 5 times the normal seric value) and elevated ADA (188 U/L, normal being < 45 U/L), with normal leucocyte count. No BK was identified either by PCR or cultural exam.
The mass aspirate was PCR positive for Mycobacterium tuberculosis (MTB). Almost 1 month later, cultural examination became positive for M. tuberculosis.
She started antibacillary therapy with progressive symptomatic and overall improvement. After 8 months on combined antibacillary therapy, ultrasonography (Fig. 5) exhibited a residual complex cystic lesion in the right adnexal area, with some distortion of normal ovarian morphology. CA-125 returned to normal values.
Fig. 5.
Pelvic ultrasound, 8 months after antibacillary therapy showing almost complete regression of the lesion, with a residual 65 mm biloculated lesion.
Discussion
Differential diagnosis of adnexal masses is based on their imaging appearance (cystic vs solid, enhancement, fat and blood products content, among others) further supported by ancillary findings suggesting local or distant spread of a malignant process and tumor markers. Neoplastic etiologies (either benign or malignant) are first considered given the ominous prognosis usually associated with malignant lesions. Although diagnostic “decision trees” do include infectious causes (usually in the context of inflammatory pelvic disease), tuberculosis is a forgotten “infection” not only because extrapulmonary, pelvic tuberculosis is rare (about 1.5% of TB affected females; Ref. 5) and related to specific epidemiologic contexts, but also because it rarely presents in a recognizable imaging pattern. Pelvic tuberculosis has been reported in several case reports coming from the developing countries. The striking feature in the case we present is the fact that it presented in a child (although post pubertal, but the majority of literature reports come from women at childbearing age) and all investigations had no definite suggestion of a diagnosis: the imaging features either in ultrasound and MR were hard to categorize into a most probable diagnosis, although a very high suspicion of malignancy was present (eventually a cystadenoma borderline); laboratory tests had difficulty unmasking the culprit, all together with an elevated CA-125, a misleading sum of events that led to an unnecessary laparotomy because of the reasonable suspicion of an ovarian malignancy. The result could have been more devastating if hysterectomy and salpingo-oophorectomy had been undertaken, as it has occurred in many others cases reported [7]. This is one further argument stressing the importance of this case promoting awareness in considering tuberculosis as part of the diagnostic workup of adnexal masses, especially in patients with a long potential fertile/reproductive life ahead. As presentation is often so insidious and nonspecific (majority presenting with abdominal pain, infertility, or abnormal menstrual cycles/bleeding), the diagnosis is highly supported in imaging findings, but they can be misleading as granulomata spreading throughout the peritoneal surfaces and omenta may appear as enhancing nodular thickening, suggesting carcinomatosis [3]. Furthermore, the complex adhesions elicited by the inflammatory response distort the anatomical planes and surrounding structures, hampering the Radiologist's task. In regard to pelvic tuberculosis, salpingitis is the most frequent form [6] and indeed several features (in MR) in our case could point to that, although severe pelvic anatomy distortion prevented us from understanding the nature/origin of the mass (a tubo-ovarian mass being the most correct label). At ultrasound, tubo-ovarian masses do produce a very complex appearance with wall thickening, and pseudosolid areas and Doppler interrogation may suggest a solid neoplastic component. The imaging criteria in which Radiologists rely upon to suspect a malignant process when presented with a pelvic mass are well represented in pelvic tuberculosis, which is why the definitive diagnosis relies on histopathology analysis of biopsy specimens. However, tuberculosis can be overlooked if tuberculoid granulomata are minimally or noncaseating as observed in our case, so a high index of suspicion must be held to account and persisted on. Some features could have reinforced an underlying chronic ongoing infection, such as the persistence of microcytic anemia. Culture and identification of MTB is still a lengthy process to yield results (2-8 weeks, and with a wide range of sensibility reported, from 20% to 80%), limiting its clinical utility in an acute setting. IGRA was particularly helpful overcoming the interference of BCG vaccination and further supporting the diagnosis. A great confounding factor is still CA-125, whose increased levels are readily attributed to a malignant ovarian process, and this report also serves to emphasize the fact that elevated levels of CA-125 can be produced by inflammation of epithelial cells (peritoneum, pleura and pericardium) explaining why it can be increased in varied situations such as infections, tuberculosis, endometriosis, Meigs syndrome, menstruation, pericarditis, pneumonia, and acute pancreatitis [3], [6]. Normalization of CA-125 levels has been associated with the response to anti-TB therapy [4], which we also verified in our case.
Retrospectively analyzing, the elicited clinical history suggests a primary pulmonary focus (substantiated by a very elevated ADA in pleural fluid), probably spread by hematogeneous route to the pelvis, where it had its major expression.
Thus, pelvic tuberculosis remains very deceitful in all approaches, either by clinical, imaging, histopathology, or laboratory tests. In order to find it, it should be thought of first, and it will require a comprehensive and integrated data analysis to reach a consistent diagnosis. It presents as one of a kind but it actually is kind of a lot (of presentations).
Overall, this case reminds us that proficient Radiologists should look at patients as a whole, integrating clinical and laboratory data with imaging features in order to avoid disconnect care.
Informed consent: Informed consent was obtained from the parents of the child included in this case report.
Reference
- 1.Lotfian F, Bolursaz MR, Khalilzadeh S, Baghaie N, Hassanzad M. Features of adolescents tuberculosis at a referral TB's Hospital in Tehran, Iran. Mediterr J Hematol Infect Dis. 2016;8(1) doi: 10.4084/MJHID.2016.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baghaie N, Khalilzade S, Boloursaz MR, Khodayari AA, Velayati AA. Extra pulmonary tuberculosis in children: two years study. Acta Med Iran. 2010;48(4):239–243. [PubMed] [Google Scholar]
- 3.Sah SK, Shi X, Du S, Li X, Li CH, Shah S. CT findings and analysis for misdiagnosis of female pelvic tuberculosis. Radiol Inf Dis. 2017;4:19–25. [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2(5):38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oge T, Ozalp SS, Yalcin OT, Kabukcuoglu S, Kebapci M, Arik D. Peritoneal tuberculosis mimicking ovarian cancer. Eur J Obst Gyn Reprod Biol. 2012;162:105–108. doi: 10.1016/j.ejogrb.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Delgado RE, Fernandes DS, Ahouagi AC, Almeida LJ. Peritoneal tuberculosis mimicking advanced ovarian carcinoma with peritoneal carcinomatosis. Brazilian National Cancer Institute, Rio de Janeiro - Single Center. JSM Surg Oncol Res. 2017;2(2):1016. [Google Scholar]
- 7.Gatongi DK, Gitau G, Kay V, Ngwenya S, Lafong C, Hasan A. Female genital tuberculosis. Obstet Gynaecol. 2005;7:75–79. [Google Scholar]