Abstract
Osteosarcoma is a rare primary bone tumor, which mainly affects children and adolescents and has a poor prognosis, especially for patients with metastatic disease. A poor therapeutic response to the conventional chemotherapy is observed with the development of lung metastases, highlighting the need for improving the current regimens and the identification of early markers of the recurrent and metastatic disease. Circulating Tumour Cells (CTCs) play a key role in the metastatic process and could be powerful biomarkers of the progressive disease. The present study aimed to isolate CTCs by using a pre-clinical model of human osteosarcoma and to monitor their kinetic of release and their modulation by ifosfamide. CTCs were detectable into the bloodstream before any palpable primary tumors. Ifosfamide increased CTCs count and in contrast decreased the number of lung tumor nodules. On established tumors, ifosfamide slowed down the tumour growth and did not modulate CTC count that could be explained by a release of cancer cells from the primary tumour with reduced properties for inducing lung metastases. This report highlights the biological interest of CTCs in osteosarcoma.
Keywords: Osteosarcoma, Circulating tumor cells, Metastatic process, Biomarker, Recurrent disease
1. Introduction
Osteosarcoma is the most common primary malignant tumour of bone in children and young adults. This neoplasm has its origin in mesenchymal cells committed to the osteoblast differentiation programme [1], [2], [3]. Malignant cells are able to destabilize the accurate osteoblastic/osteoclastic activity balance of bone formation and resorption that in turn facilitates the tumour cell proliferation and formation of tumoral osteoid tissue. Conventional therapy for osteosarcoma patients combines neoadjuvant chemotherapy followed by surgical removal of all detectable disease (including metastases), and postoperative chemotherapy [1], [4], [5]. Current regimens of chemotherapy are based on four drugs: i) high-dose methotrexate (HDMTX) with leucovorin rescue; ii) doxorubicin (adriamycin); iii) cisplatin, and iv) ifosfamide [15]. Unfortunately, a low response rate to chemotherapy is frequently observed leading to the development of metastases preferentially located in the lungs and finally to the patient death.
The cell dissemination process is a very complex mechanism that involves various components of the tumour microenvironment (e.g. blood vessels, immune cells) [7], [8], [9]. Cancer cells that have escaped from the primary tumour and are able to invade the bloodstream to become Circulating Tumour Cells (CTCs), that will be capable to intravasate, survive in the circulation, migrate into the interstitial space and finally establish the tumour growth at a new location [10], [11], [12]. Only a small number of cells will successfully complete all the steps, thus it is important to identify and well characterize which CTCs are able to complete the metastatic process [12].
Numerous studies have underlined the biological value of CTCs that can be used as biomarkers in the follow up of epithelial malignancies such breast and prostate cancers [13]. CTCs, which are collected, isolated and characterized from a non-invasive liquid biopsy, reflect the primary or/and metastatic disease for which a targeted therapy may be given [13], [14] Even if membrane vimentin or bone isoenzyme of alkaline phosphatase appear to be expressed by a maojority of bone sarcomas and could be used for isolating sarcoma CTCs [15], [16], [17], there is no universal marker for the CTC detection in tumours of mesenchymal origin. Consequently, various methods independent of any cell markers have been proposed and are based on size or density discrimination [18], [19], [20]. In light of the few reports available in the literature [15], [16], [17] and the lack of specific marker for isolating sarcoma CTCs, by using a preclinical model of osteosarcoma, the aims of the present study were to: i) isolate CTCs; ii) enumerate CTCs during the tumour growth and iii) determine the effect of ifosfamide treatment on CTC release.
2. Material and methods
2.1. Cell culture
HOS-MNNG human osteosarcoma cell line purchased from the American Type Culture Collection was modified for overexpressing the green fluorescence protein (GFP) [21]. GFP-MNNG/HOS cells were cultured in DMEM (Lonza, Belgium) supplemented with 10% FBS in a humidified 5% CO2/air atmosphere at 37 °C.
2.2. In vivo experiments
For both experiments 6-week-old female athymic nude mice were purchased from Elevages Janvier (France) and Harlan Laboratory (UK) and procedures involving animal handling and care were approved by the Animal Care and Ethics Committee of the French Ethical Committee (CEEA.PdL.06, authorization number: 1280.01) and the Home office in UK [PPL: 70/8967, Establishment license no.: 50/2509]. Mice were acclimatized for at least one week prior to experimental manipulation. Mice were anesthetized by inhalation of Isoflurane/air (1.5%, 1 Lmin−1) before i.m. inoculation of 1.5 × 106 GFP-MNNG/HOS cells resuspended with phosphate-buffered saline (PBS). Osteosarcoma cells were injected in close proximity to the tibia, rapidly leading to tumour growth in the soft tissue with secondary contiguous bone invasion and development of lung metastases [22]. The tumor volumes were monitored twice a week and calculated by measuring two perpendicular diameters using calipers, according to the following formula: V = 0.523 × L × (S)2, in which L and S are, respectively, the largest and the smallest perpendicular diameters. Tumor volumes were monitored until a maximum of 2500 mm3. Blood samples were collected in EDTA tubes by intracardiac puncture in anesthetised animals and mice were euthanized by cervical dislocation. Ifosfamide (Holoxan, Baxter) was prepared extemporaneously, diluting with PBS and administered intraperitoneally at doses of 15 mg/kg or 30 mg/kg per day on cycles of 3 consecutive days. Control mice were injected in the same conditions with an equivalent volume of PBS. The doses used were defined on the most commonly-used dose in the literature [23], [24], [25], [26], on the frequency applicable in clinical practice, and on the recommendations proposed by Reagan–Shaw et al. [27]. Lung metastastatic nodules were macroscopically and manually scored in each animal at the time of necropsy as previously shown [22].
2.3. Blood processing: enrichment step of blood samples and isolation of circulating tumour cells
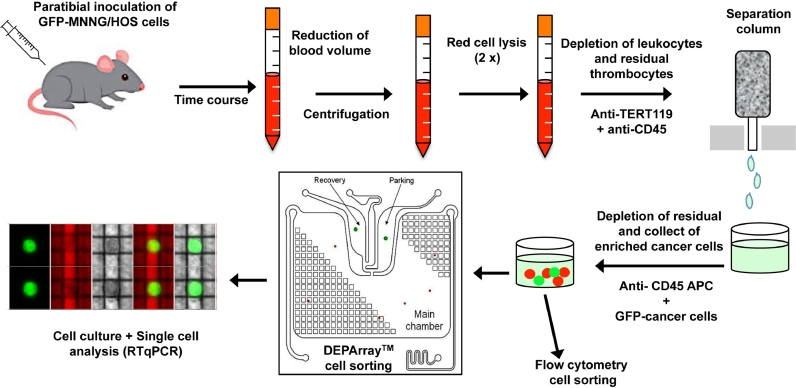
Collection of CTCs from blood combined a pre-enrichment step and an isolation phase. Pre-enrichment was carried out as follow: i) red cell depletion carried out by incubation of blood samples with cell lysis buffer for 10 min at room temperature; ii) cell wash with PBS three times consecutively; iii) leukocytes depletion using double positive selection with CD45- and Tert19-microbeads (Milteny Biotec, Germany) following the manufacturer's protocol. CTCs were the isolated either by fluorescence activated cell sorting (FACS ARIAIII, Becton Dickinson) or by the DEPArray™ platform (Menarini, Silicon Biosystems, Italy) to obtain pure single cells [28] (Fig. 1).
Fig. 1.
CTC capture and characterization workflow. CTCs were isolate from a xenograft murine model of human osteosarcoma cells. Human GFP-MMNG/HOS osteosarcoma cells were inoculated in close contact to the tibia of immunodeficient mice. The kinetic study of CTCs detection into the bloodstream was then carried out. A first pre-enrichment step was performed including successively: i) a reduction of blood volume by centrifugation; ii) two lyses of erythrocytes; iii) an immunomagnetic depletion of leukocytes and residual thrombocytes. In a second step, enriched samples were processed for isolating CTCs by flow cytometry or dielectrophoresis technology (DEPArray™). Isolated cells were enumerated, cultured and singles cells were analysed by RTqPCR.
2.4. RNA extraction and gene expression analysis
Total RNA was extracted using the NucleoSpin® RNA II kit (Macherey-Nagel Gmbh & Co. Kg, Germany). RNA was reverse transcribed using the Maxima H Minus First Strand cDNA Synthesis kit (Thermo Scientific, France) following the manufacturer's instructions. Real-time PCR was performed with a CFX96 real-time PCR detector system (Bio-Rad, France) with SYBR Select master Mix reagents (Life Technologies, France). Primer sequences are detailed in supplementary data 1.
2.5. Proliferation assay
Proliferation was determined by using a WST-1 colorimetric test based on the cleavage of the tetrazolium salt WST-1 by mitochondrial dehydrogenase in viable cell and according the manufacturer's recommendation (Takara, Japan). Two thousands cells per well were seeded into 96 multi-well plates and after 24 h, 48 h and 72 h, cell proliferation was assessed by optical density measurement at 470 nm with Victor 2 1420 (Multilabel Counter, Perkin Elmer).
2.6. Migration and invasion assays
To evaluate migration ability of GFP-MNNG/HOS cells and isolated CTCs, cells were seeded in growth media without serum on the upper side of a Transwell chamber (Falcon) on a porous transparent polyethylene terephthalate membrane (8 µm pore size). The lower chamber of the transwell was filled with growth medium containing 10% FBS. After 24 hours of incubation, cells on the upper side of the filters were mechanically removed and cells migrated to the lower side were fixed (with glutaraldehyde 10%) and stained (with violet crystal 0.1%). Five random fields were taken under light microscope and analysed for each chamber and cells were counted with the ImageJ software (NIH, USA). The same procedure was used for invasion assays seeding cells on the upper side of Matrigel® (Corning, France)-coated transwells (50 ng Matrigel/well).
2.7. Statistical analysis
Data analysis was performed using GraphPad Prism (GraphPad Software, Inc). SEM from replicate experiments was calculated as noted in the legends and is shown as error bars. All error bars show SEM for at least triplicate measurement from independent experiments. The mean±SEM was calculated for all groups and compared by non-parametric Mann–Withney Wilconson test. SPSS statistics software (IBM) was used for all statistical analysis. Differences were considered significant at the 95% confidence level (p < 0.05).
3. Results
3.1. CTCs are detectable before the tumour mass in a murine model of osteosarcoma
To validate the methods used and to avoid any bias linked to the mode of cancer cell injection in mice, 2 × 107 GFP-MNNG/HOS cells resuspended in PBS or Matrigel® were inoculated intramuscularly in paraosseous site. After blood collection, the presence of CTCs was analysed by flow cytometry after 24 h and 48 h post inoculation. In all conditions used, no CTCs were observed into the blood demonstrating that CTCs further detected were not associated to a technical artefact such as a leak of cancer cells into the bloodstream immediately after cell inoculation (data not shown).
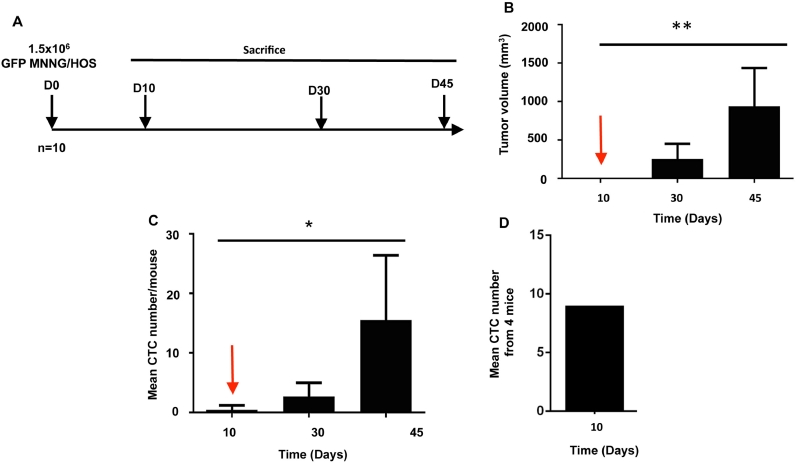
We carried out a kinetic analysis of CTC release into the bloodstream. Thirty mice previously injected with 1.5 × 106 GFP-MNNG/HOS cells, were randomly divided into 3 groups and sacrificed at days 10, 30 and 45 respectively after cell injection (Fig. 2A). For each group, 6 samples were individually processed by flow cytometry cell sorting for CTC isolation and 4 blood samples were pooled for isolating individual CTCs with the DEPArray™. As expected, cells inoculated resulted in the formation of palpable tumours growing in a time dependent manner (Fig. 2B). In parallel of the tumour growth, the number of CTCs has been increasing in a time dependent manner and CTCs were detectable from day 10 post-cell injection, before any palpable tumour mass and lung metastases (Fig. 2C). The early detection of CTCs was confirmed by the two methods used: flow cytometry (Fig. 2C) and DEPArray ™ (Fig. 2D).
Fig. 2.
Monitoring of CTC release into the bloodstream. (A) Summary of the experimental protocole. Human GFP-MMNG/HOS osteosarcoma cells were inoculated in paratibial site of nude mice. Tumor growth monitored (B) twice a week and the CTC dynamic (C,D) in blood were monitored at day 10, 30 and 45 after tumor cell inoculation. CTCs were enumerated by flow cytometry (C) or by DEPArray™ (D). CTCs are precociously detectable in mice before any palpable tumor mass and can be considered an early event associated to the tumor development. D10: day 10; * p < 0.05; ** p < 0.01. n = 5/condition.
3.2. Ifosfamide treatment results in a decrease of the primary tumour volume and an increase of CTCs
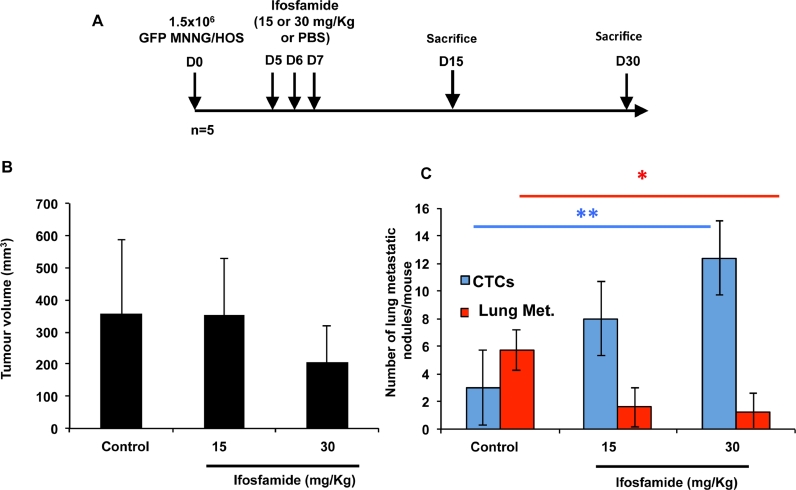
Ifosfamide is one of the conventional drugs used in osteosarcoma. We assessed the effects of ifosfamide and more precisely the number of CTCs, tumour volume and number of metastasis when the drug was administered at early stages of the disease in the murine model (i.e. 10 days after tumor challenge). Fifteen mice, previously injected with GFP-MNNG/HOS cells were divided into 3 groups: group 1, five days after cell inoculation mice received 1 cycle of ifosfamide at a dose of 15 mg/kg (1 cycle = drug administration three consecutive days); group 2, five days after cell inoculation mice received 1 cycle of ifosfamide at a dose of 30 mg/kg; group 3 (control group): mice received equivalent volume of PBS (Fig. 3A). Mice were sacrificed at day 25 and blood samples were collected and individually processed to isolate CTCs by cell sorting. As shown in Fig. 3, there were no statistical significant differences in tumour volumes (Fig. 3B). However, the number of CTCs was higher in treated groups compared to the control group (p < 0.01), and surprisingly, the number of metastases has significantly decreased during the same period of time (p < 0.05) (Fig. 3C).
Fig. 3.
Opposite effect of one cycle of ifosfamide on the number of lung metastases and CTCs at early stage of osteosarcoma development. (A) Summary of the experimental protocole. Human GFP-MMNG/HOS osteosarcoma cells were inoculated in paratibial site of nude mice. Three days post-inoculation of osteosarcoma cells, mice were treated with ifosfamide at doses of 15 mg/kg or 30 mg/kg i.p. per day, 3 days consecutively. Control mice were injected in the same conditions with an equivalent volume of PBS. Tumor volume (B), number of lung metastases/mouse (lung Met.) (C) and CTCs (C) were enumerated 15 and 30 days after inoculation of cancer cells.
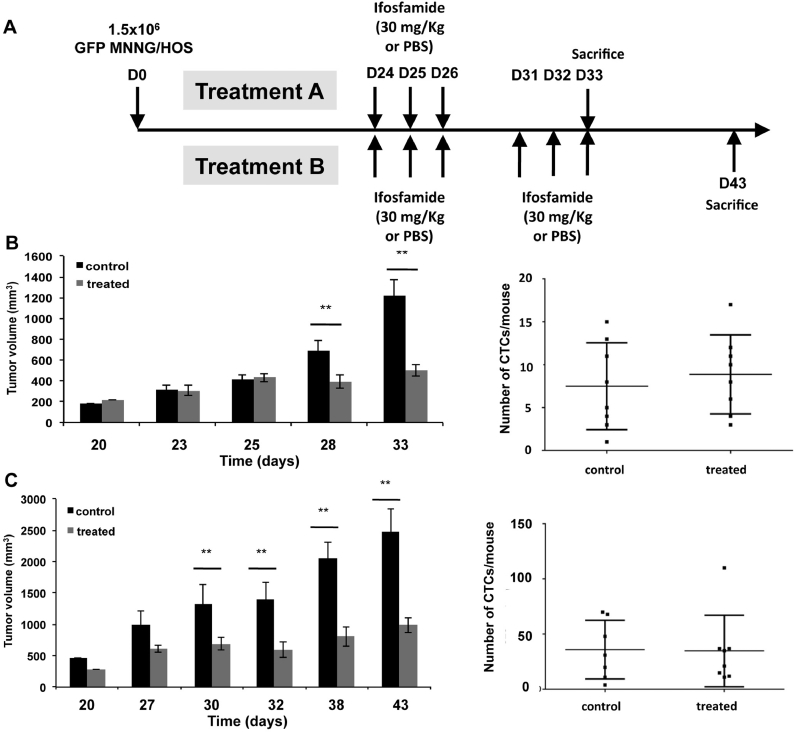
Then, we studied the effect of the administration of multiple ifosfamide doses. Thirty-two mice injected with GFP-MNNG/HOS cells, randomized before treatment in two groups A and B. When the tumor mass was palpable (D24), the mice of group A (n = 8/subgroup) received 1 cycle of ifosfamide at 30 mg/Kg or PBS solution as the control subgroup. In group B, mice were treated with 2 cycles of ifosfamide at 30 mg/kg, at one week interval or PBS as the control group (Fig. 4A). As shown in Fig. 4B, one cycle of ifosfamide reduced significantly the tumor growth (mean tumor volume at D33: 498.3 mm3 for treated mice compared to 1220.5 mm3 for control; p < 0.001) but did not reduced significantly the number of CTCs (Fig. 4B). As expected, in treatment B, treated mice showed a significant decrease in tumor volumes (mean tumor volumes at D45: 663.9 mm3 in treated mice compared to 1454.9 mm3 in control mice; p < 0.01). However two cycles ifosfamide had no impact on CTC number compared to the vehicule group (Fig. 4C). All together, these results revealed the differential effect of ifosfamide on the release of tumor cells according the stage of tumor development.
Fig. 4.
At late stage of osteosarcoma development, one cycle or two cycles of ifosfamide slow down the tumor volume without any impact on CTC release into the bloodstream. (A) Summary of the experimental protocole. Human GFP-MMNG/HOS osteosarcoma cells were inoculated in paratibial site of nude mice. Three weeks post-inoculation of osteosarcoma cells, mice were treated with one (B) or two cycles (C) of 30 mg/kg/day i.p ifosfamide administered 3 days consecutively. Control mice were injected in the same conditions with an equivalent volume of PBS. Tumor volume and CTCs were measured at the end-point. D0: day 0. ** p < 0.001; *** p < 0.0001. n = 8/group.
3.3. Molecular characterization of isolated CTCs at early stages
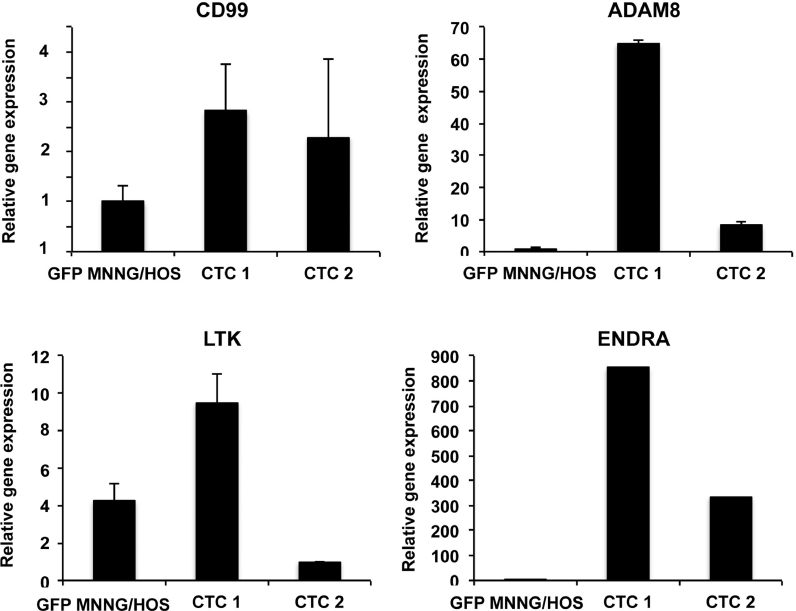
Isolated CTCs exhibited properties of adherence then reflecting indirectly their viability. Single isolated CTCs were cultured and their biological properties and phenotype were compared with the parental cell line. Two cell lines (CTC1, CTC2) were then developed (Fig. 5). Proliferation, migration, invasion assays did not show any significant difference between CTC1, CTC2 and the parental cell line (data not shown). The expression of several markers related to metastatic process (CD99, ADAM8, ENDRA and LTK) was compared between the two clones and the parental cell line. As shown in Fig. 5, ADAM8, LTK and ENDRA were differentially expressed in the three cell lines and in contrast to CD99.
Fig. 5.
Gene expression of CTCs compared to the parental cell line. Isolated CTCs were cultured and used for developing new cell lines. Gene expression profile for CD99, LTK, ENDRA and ADAM8 was analysed by RT-qPCR compared to Hprt1 used as house keeping gene. Relative expression of different genes was obtained with DDCt method.
4. Discussion
Osteosarcoma is the most common bone sarcoma in adolescents. Unfortunately, a poor therapeutic response to the conventional chemotherapy is frequently observed leading to the development of metastatic foci and highlighting the need to improve the current regimens and to identify early biomarkers of the recurrent and/or metastatic disease. The present study analysed the biological value of CTCs into the bloodstream in a preclinical model of osteosarcoma. CTCs were detectable at early stage of the disease before any tumor mass. At early stage of osteosarcoma development, one cycle of ifosfamide (15 or 30 mg/Kg) had any significant effect on the primary tumor growth. However, a high impact on the metastatic process was observed and was characterized by a decreased number of lung metastases, and surprisingly, by an increase in the total number of viable CTCs. At later stage, one or two cycles of ifosfamide had a significant beneficial effect on the growth of primary tumors and no impact on the release of CTCs into the bloodstream.
The biological relevance of CTCs has been already established in epithelial cancers. Indeed, numerous studies demonstrated that metastatic patients with detectable CTCs have a worse prognosis compared to patients with undetectable CTCs [12], [14]. The works focusing on the detection and capture of CTCs in osteosarcoma have been hampered due to the absence of specific markers expressed by mesenchymal cancer cells [15], [18], [19], [20], [21], [22]. However, progressively more and more cues have strengthened the biological interest of CTCs in osteosarcoma. Thus, Satelli et al. observed a higher number of CTCs in metastastic patients compared to patients with localized disease. Using a method based on isolation by size of tumor cells (ISET), Chinen et al. [29] detected CTCs in sarcoma patients. More recently, Li et al. [30] confirmed the detection of CTCs in cryopreserved human peripheral blood mononuclear cells and two studies analysing CTCs by FISH in small cohorts of osteosarcoma patients revealed a potential positive association between CTC count, disease progression and poor prognosis [31], [32]. The present report is the first study investigating the regulation of CTC release after chemotherapy. CTCs were detectable in blood circulation at early stages of the disease (i.e. 10 days after cancer cell inoculation). This finding is in agreement with previous studies in breast [33] and prostate cancer [34]. Overall, these data suggest that CTCs could be used as a non- invasive method to study the early biological events of osteosarcoma development and to monitor the recurrence of the disease in patients.
Ifosfamide belongs to the four major alkylating agents used in the treatment of osteosarcoma including also methotrexate, cisplatin and doxorubucin [35]. In the present report, we observed an opposite effect of ifosfamide on the CTC and lung metastasis formation at early stage of the tumor development. Indeed, ifosfamide decreased the number of lung nodules whereas CTC count increased on the period of time and may be considered as a new biological tool for monitoring therapeutic response. On established tumors, ifosfamide whatever the dose regimen, had a therapeutic benefit as demonstrated by a slow down of tumor progression after drug administration. The differential effects of ifosfamide on tumor volume between early and late stages of tumor development could be explained by the vasculature network formed in established tumors leading to a higher bioavailability of ifosfamide close to the tumor tissues. After inoculation, cancer cells establish cell contact with their microenvironment and progressively proliferate and elaborate an osteoid matrix [5], [7], [8]. The fragile interface between cancer cells and their environment at early stage could explain the significant release of CTCs after ifosfamide treatment not observed on later stages. CTC count was higher in large tumors than at the early stage of the disease and was not modulated by ifosfamide revealing a potential “equilibrium” or “saturating” state in the metastatic process in osteosarcoma. However, CTCs detectable after ifosfamide treatment appear unable to induce the formation of lung metastases. This increase could be explained by a release of phenotypically altered cancer cells from the primary tumour induced by chemotherapy and with reduced properties for inducing lung metastases. CTC extravasation is a complex mechanism mixing physical properties of cancer cells (e.g. lower deformability) and biological aspects such as genetic properties and specific addressing molecules that lead to the reciprocal recognition of lymphatic/blood vessel walls of distant organs by CTCs. Therefore, CTC heterogeneity and phenotypic specificities drive their extravasation into the metastatic site and the formation of metastatic nodules [6], [36].
CTCs play a key role in the metastatic process and could be powerful biomarkers of the progressive disease or/and therapeutic response [37]. The present work analysed for the first time the effects of a conventional chemotherapeutic agent in a pre-clinical model of osteosarcoma. Overall, our study strengthens the potential interest to enumerate and characterize CTCs in osteosarcoma. Osteosarcoma are highly prone to induce lung metastases, which more frequently occur within 3 years following the diagnosis and which have a major impact on the overall survival rate. Based on the present results, CTCs appear detectable before any tumor mass and may be considered as an early event of the metastatic dissemination and/or may reflect the recurrent disease. To assess the biological value of CTCs in osteosarcoma recurrence, murine models could be used. The enumeration of CTCs in a murine model based on patient-derived sarcoma xenografts or murine-tumor allograft surgically implanted into hindlimbs, subsequently amputated followed by an observational period of time for development of metastasis could be an option [38], [39], [40]. The number of CTCs may reflect the therapeutic response suggesting a strong clinical interest in the follow up of osteosarcoma patients. The lack of ifosfamide-treated CTCs to implant in the lung should be assessed by inoculating treated CTCs in non-treated immunodeficient mice and determining their ability to establish lung metastases. Of course, the “clinical applications” of the present work should be taken in consideration with caution, in particular, since ifosfamide shows a paradoxal effect in patients with a therapeutic response on established soft-tissue/para-osteal tumours but not on sub-clinical disease. The present study should be considered as a proof-of-conecpt for isolating CTCs in osteosarcoma in absence of universal biomarkers. The next step of the study will be to determine the biological and clinical value of CTC in osteosarcoma and the isolation/enumeration/characterization of CTCs in a large series of patients (e.g. metastastic versus non-metastatic disease; kinetic of CTC release during chimiotherapy) will be then required.
Acknowledgments
Acknowledgment
The works was performed at Inserm (University of Nantes) and at the University of Sheffield (INSERM). We thank the Cytometry Facility Cytocell for expert technical assistance (SFR Bonamy, University of Nantes, FR).
Funding
This study was written as a part of a research project which was funded by the Bone Cancer Research Trust (UK, research project number 144681) and the Seventh Framework Programme ([FP7) under grant agreement no. 264817 – BONE-NET.
Conflict of interest
Authors have no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.07.002.
Appendix. Supplementary materials
References
- 1.Brown H.K., Schiavonne K., Gouin F., Heymann M.F., Heymann D. Biology of bone sarcomas and new therapeutic developments. Calcif. Tissue Int. 2018;102:174–195. doi: 10.1007/s00223-017-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohseny A.B., Szuhai K., Romeo S., Buddingh A.P., Briaire-de Bruijn I., de Jong D., van Pel M., Cleton-Jansen A.M., Hogendoorn P.C. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J. Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- 3.Mutsaers A.J., Walkley C.R. Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone. 2014;62:56–63. doi: 10.1016/j.bone.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Ségaliny A.I., Tellez-Gabriel M., Heymann M.F., Heymann D. Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J. Bone Oncol. 2015;4:1–12. doi: 10.1016/j.jbo.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heymann M.F., Brown H.K., Heymann D. Drugs in early clinical development for the treatment of osteosarcoma. Expert Opin. Investig. Drugs. 2016;25:1265–1280. doi: 10.1080/13543784.2016.1237503. [DOI] [PubMed] [Google Scholar]
- 6.Luetke A., Meyers P.A., Lewis I., Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat. Rev. 2014;40:523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Alfranca A., Martinez-Cruzado L., Tornin J., Abarrategi A., Amaral T., de Alava E., Menendez P., Garcia-Castro J., Rodriguez R. Bone microenvironment signals in osteosarcoma development. Cell Mol. Life Sci. 2015;72:3097–3113. doi: 10.1007/s00018-015-1918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heymann M.F., Lezot F., Heymann D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cellular Immunol. 2018 doi: 10.1016/j.cellimm.2017.10.011. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q., Zhang H., Jiang X., Qian C., Liu Z., Luo D. Factors involved in cancer metastasis: a better understanding to seed and soil hypothesis. Mol. Cancer. 2017;16:176. doi: 10.1186/s12943-017-0742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang S.P., Cabrera R.M., Segall J.E. Am. J. Physiol. Cell. Physiol. 2016;311:C1–C14. doi: 10.1152/ajpcell.00238.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jie X.X., Zhang X.Y., Xu C.J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: mechanisms and clinical applications. Oncotarget. 2017;8:81558–81571. doi: 10.18632/oncotarget.18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantel K., Speicher M.R. The biology of circulating tumor cells. Oncogene. 2016;35:1216–1224. doi: 10.1038/onc.2015.192. [DOI] [PubMed] [Google Scholar]
- 13.Alix-Panabières C., Pantel K. Cancer Discov. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 14.Pantel K., Alix-Panabières C. Liquid biopsy: potential and challenges. Mol. Oncol. 2016;10:371–373. doi: 10.1016/j.molonc.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satelli A., Mitra A., Cutrera J.J., Devarie M., Xia X., Ingram D.R., Dibra D., Somaiah N., Torres K.E., Ravi V., Ludwig J.A., Kleinerman E.S., Li S. Universal marker and detection tool for human sarcoma circulating tumor cells. Cancer Res. 2014;74:1645–1650. doi: 10.1158/0008-5472.CAN-13-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruland Ø.S., Høifødt H., Saeter G., Smeland S., Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin. Cancer Res. 2005;11:4666–4673. doi: 10.1158/1078-0432.CCR-05-0165. [DOI] [PubMed] [Google Scholar]
- 17.Bruland Ø.S., Høifødt H., Hall K.S., Smeland S., Fodstad Ø. Bone marrow micrometastases studied by an immunomagnetic isolation procedure in extremity localized non-metastatic osteosarcoma patients. Cancer Treat. Res. 2009;152:509–515. doi: 10.1007/978-1-4419-0284-9_30. [DOI] [PubMed] [Google Scholar]
- 18.Chang L., Asatrian G., Dry S.M., James A.W. Circulating tumor cells in sarcomas: a brief review. Med. Oncol. 2015;32:430. doi: 10.1007/s12032-014-0430-9. [DOI] [PubMed] [Google Scholar]
- 19.Tellez-Gabriel M., Brown H.K., Young R., Heymann M.F., Heymann D. The challenges of detecting circulating tumor cells in sarcoma. Front. Oncol. 2016;6:202. doi: 10.3389/fonc.2016.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriel M.T., Calleja L.R., Chalopin A., Ory B., Heymann D. Circulating tumor cells: a review of non-EpCAM-based approaches for cell enrichment and isolation. Clin. Chem. 2016;62:571–581. doi: 10.1373/clinchem.2015.249706. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez Calleja L., Jacques C., Lamoureux F., Baud'huin M., Tellez Gabriel M., Quillard T., Sahay D., Perrot P., Amiaud J., Charrier C., Brion R., Lecanda F., Verrecchia F., Heymann D., Ellissen L.W., Ory B. ΔNp63α silences a miRNA program to aberrantly initiate a wound-healing program that promotes TGFβ-induced metastasis. Cancer Res. 2016;76:3236–3251. doi: 10.1158/0008-5472.CAN-15-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ségaliny A.I., Mohamadi A., Dizier B., Lokajczyk A., Brion R., Lanel R., Amiaud J., Charrier C., Boisson-Vidal C., Heymann D. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int. J. Cancer. 2015;137:73–85. doi: 10.1002/ijc.29376. [DOI] [PubMed] [Google Scholar]
- 23.Sommer K., Peters S.O., Robins I.H., Raap M., Wiedemann G.J., Remmert S., Sieg P., Bittner C., Feyerabend T. A preclinical model for experimental chemotherapy of human head and neck cancer. Int. J. Oncol. 2001;18:1145–1149. doi: 10.3892/ijo.18.6.1145. [DOI] [PubMed] [Google Scholar]
- 24.Odri G.A., Dumoucel S., Picarda G., Battaglia S., Lamoureux F., Corradini N., Rousseau J., Tirode F., Laud K., Delattre O., Gouin F., Heymann D., Redini F. Zoledronic acid as a new adjuvant therapeutic strategy for Ewing's sarcoma patients. Cancer Res. 2010;70:7610–7619. doi: 10.1158/0008-5472.CAN-09-4272. [DOI] [PubMed] [Google Scholar]
- 25.Hanly L., Figueredo R., Rieder M.J., Koropatnick J., Koren G. The effects of N-acetylcysteine on ifosfamide efficacy in a mouse xenograft model. Anticancer Res. 2012;32:3791–3798. [PubMed] [Google Scholar]
- 26.Gobin B., Baudhuin M.B., Lamoureux F., Ory B., Charrier C., Lanel R., Battaglia S., Redini F., Lezot F., Blanchard F., Heymann D. BYL719, a new α-specific PI3K inhibitor: single administration and in combination with conventional chemotherapy for the treatment of osteosarcoma, Int. J. Cancer. 2015;136:784–796. doi: 10.1002/ijc.29040. [DOI] [PubMed] [Google Scholar]
- 27.Regan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisted. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 28.Tellez-Gabriel M., Charrier C., Brounais-Le Royer B., Mullard M., Brown H.K., Verrecchia F., Heymann D. Analysis of gap junctional intercellular communications using a dielectrophoresis-based microchip. Eur. J. Cell Biol. 2017;96:110–118. doi: 10.1016/j.ejcb.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Chinen L.T., Mello C.A., Abdallah E.A., Ocea L.M., Buim M.E., Breve N.M., Junior Gasparini J.L., Fanelli M.F., Paterlini-Bréchot P. Isolation, detection, and immunomorphological characterization of circulating tumor cells (CTCs) from patients with different types of sarcoma using isolation by size of tumor cells: a window on sarcoma-cell invasion. OncoTargets Ther. 2014;7:1609–1617. doi: 10.2147/OTT.S62349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H., Meng Q.H., Noh H., Batth I.S., Somaiah N., Torres K.E., Xia X., Wang R., Li S. Detection of circulating tumor cells from cryopreserved human sarcoma peripheral blood mononuclear cells. Cancer Lett. 2017;406:216–223. doi: 10.1016/j.canlet.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.H. Zhang, P. Gao, X. Xiao, M. Heger, L. Geng, B. Fan, Y. Yuan, C. Huang, G. Chen, Y. Liu, Y. Hu, X. Yu, S. Wu, L. Wang, Z.A. Wang, A liquid biopsy-based method for the detection and quantification of circulating tumor cells in surgical osteosarcoma patients, Int. J. Oncol. In Press. [DOI] [PMC free article] [PubMed]
- 32.Zhang H.Q., Li M.H., Wang Z., Lan P.H., Lu Y.J., Chen G.J., Wang L. Detection and clinical significance of circulating tumor cells in osteosarcoma using immunofluorescence combined with in situ hybridization. Zhonghua Zhong Liu Za Zhi. 2017;339:485–489. doi: 10.3760/cma.j.issn.0253-3766.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Yu M., Stott S., Toner M., Maheswaran S., Haber D.A. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 2011;192:373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.StottL S.L., Lee R.J., Nagrath S., Yu M., Miyamoto D.T., Ulkus L., Inserra E.J., Ulman M., Springer S., Nakamura Z., Moore A.L., Tsukrov D.I., Kempner M.E., Dahl D.M., Wu C.L., Iafrate A.J., Smith M.R., Tompkins R.G., Sequist L.V., Toner M., Haber D.A., Maheswaran S. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3000403. 25ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jürgens H., Treuner J., Winkler K., Göbel U. Ifosfamide in pediatric malignancies. Semin. Oncol. 1989;16:46–50. [PubMed] [Google Scholar]
- 36.Micalizzi D.S., Maheswaran S., Haber D.A. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31:1827–1840. doi: 10.1101/gad.305805.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L., Dicker D.T., Matthew E., El-Deiry W.S., Alpaugh R.K. Circulating tumor cells: silent predictors of metastasis. F1000Res. 2017;6:1445. doi: 10.12688/f1000research.11313.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstein S.D., Hayashi M., Albert C.M., Jackson K.W., Loeb D.M. An orthotopic xenograft model with survival hindlimb amputation allows investigation of the effect of tumor microenvironment on sarcoma metastasis. Clin. Exp. Metastasis. 2015;32:703–715. doi: 10.1007/s10585-015-9738-x. [DOI] [PubMed] [Google Scholar]
- 39.Aanstoos M.E., Regan D.P., Rose R.J., Chubb L.S., Ehrhart N.P. Do mesenchymal stromal cells influence microscopic residual or metastatic osteosarcoma in a murine model? Clin. Orthop. Relat. Res. 2016;474:707–715. doi: 10.1007/s11999-015-4362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maloney C., Edelman M.C., Kallis M.P., Soffer S.Z., Symons M., Steinberg B.M. Intratibial injection causes direct pulmonary seeding of osteosarcoma cells and is not a spontaneous model of metastasis: a mouse osteosarcoma model. Clin. Orthop. Relat. Res. 2018;476:1514–1522. doi: 10.1007/s11999.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.