Abstract
The experience of our body as our own (i.e. body ownership) involves integrating different sensory signals according to their contextual relevance (i.e. multisensory integration). Until recently, most studies of multisensory integration and body ownership concerned only vision, touch and proprioception; the role of other modalities, such as the vestibular system and interoception, has been neglected and remains poorly understood. In particular, no study to date has directly explored the combined effect of vestibular and interoceptive signals on body ownership. Here, we investigated for the first time how Galvanic Vestibular Stimulation (left, right, sham), tactile affectivity (a reclassified interoceptive modality manipulated by applying touch at C-tactile optimal versus non-optimal velocities), and their combination, influence proprioceptive and subjective measures of body ownership during a rubber hand illusion paradigm with healthy participants (N = 26). Our results show that vestibular stimulation (left GVS) significantly increased proprioceptive drift towards the rubber hand during mere visual exposure to the rubber hand. Moreover, it also enhanced participants’ proprioceptive drift towards the rubber hand during manipulations of synchronicity and affective touch. These findings suggest that the vestibular system influences multisensory integration, possibly by re-weighting both the two-way relationship between proprioception and vision, as well as the three-way relationship between proprioception, vision and affective touch. We discuss these findings in relation to current predictive coding models of multisensory integration and body ownership.
Keywords: Affective touch, Body ownership, Multisensory integration, Vestibular stimulation, Bodily self
Highlights
-
•
We studied vestibular and affective contributions to body ownership.
-
•
We stimulated the vestibular system in a Rubber Hand paradigm with affective touch.
-
•
Right-hemisphere stimulation increased proprioceptive drift during vision of a RH.
-
•
Applying affective touch further increased proprioceptive drift.
-
•
Affective and vestibular signals may favour vision in multisensory integration.
1. Introduction
The perception of the external world, and our own body, is based on the integration of sensory information conveyed by different modalities (i.e. multisensory integration), each weighted according to their contextual reliability (Fetsch et al., 2011, Stein et al., 2014). For instance, in order to estimate the size of an out-of-reach object, we typically rely upon vision; however, if we are close enough to touch the object, our estimation will result from the integration of visual and tactile information. If there is incongruence between different sensory modalities (e.g. visuo-tactile, Pavani et al., 2000), vision can be weighted more (the so-called ‘visual capture’ effect, Rock and Victor, 1964), or vice versa depending on their contextual relevance (Ernst and Banks, 2002). For example, the precision (i.e. the certainty about sensory representations; Friston et al., 2012) of proprioceptive information (i.e. regarding the position of our body) can be lowered in favour of vision during conflictual situations (Folegatti et al., 2009) and according to the reference plan in space (i.e. vision is more dominant in the horizontal versus in-depth plan, van Beers et al., 2002).
Interestingly, multisensory integration has been linked to bodily consciousness and, specifically, body ownership (i.e. the feeling that our physical body is our own; Gallagher, 2000). Paradigms that generate conflicts between different sensations have been used extensively to explore the role of multisensory integration in body ownership (Tsakiris et al., 2007, Blanke et al., 2015). In the Rubber Hand Illusion (RHI; Botvinick and Cohen, 1998) for example, participants watch a realistic fake hand being stroked in synchrony with their own (unseen) hand (Tsakiris, 2010), giving rise to self-reported feelings of rubber hand ownership and a shift in the perceived location of participants’ real hand towards the rubber hand (i.e. proprioceptive drift). Initially, these two measures were seen as the subjective and ‘objective’ measure of the illusion but it is increasingly understood that subjective feelings of ownership and proprioceptive drift dissociate and may reflect different components of the multisensory integration process (Ehrsson et al., 2004, Ehrsson et al., 2005, Makin et al., 2008, Martinaud et al., 2017, Rohde et al., 2011). More generally, there are now hundreds of studies on the RHI, and related psychophysical or virtual reality adaptations (see Kilteni et al., 2015 for a review), indicating that body ownership is mediated by both bottom-up processes of multisensory integration and top–down expectations (Tsakiris, 2010, Apps and Tsakiris, 2014). In line with this, recent predictive coding (Zeller et al., 2015) and Bayesian causal inference (Samad et al., 2015) models suggest that the successful establishment of the illusion relies on the causal attribution of sensory experiences to a common source (in this case, ‘my body’), according to prior knowledge and the spatio-temporal congruency of these sensations.
Despite this progress, the contribution of certain modalities, such as the vestibular system and interoception to multisensory integration and body ownership have only recently been studied and hence remain poorly understood. First, although the vestibular system's main role is to contribute to the maintenance of balance and posture (Brandt and Dieterich, 1999), there are some indications that vestibular signals play a role in multisensory integration (Bense et al., 2001). The neuroanatomical correlates of the vestibular system remain debated (Fasold et al., 2002, Eulenburg et al., 2012, Lopez et al., 2012b, Lopez, 2016), yet existing evidence suggests an overlap between the cortical areas supporting vestibular sensations (captured by vestibular receptors in the inner ears and conveyed to the central nervous system via the vestibular nerves) and other sensory experiences (such as vision, Brandt et al., 2002; Seemungal et al., 2013; Della-Justina et al., 2015; touch and proprioception, Lackner and DiZio, 2005; Dijkerman and De Haan, 2007), including multimodal areas linked to multisensory integration (e.g. temporoparietal junction, inferior parietal lobule, insula and cingulate cortex, Lopez et al., 2012b; Lopez, 2016). This suggests that vestibular signals may contribute to multisensory integration.
Moreover, recent studies highlight vestibular network contributions to many facets of body representation (Ferrè and Haggard, 2016, Been et al., 2007), from its metric properties (such as shape and size, Lopez et al., 2012c) to body ownership (Lopez, 2015). For example, excitation of the semi-circular canals of the internal ear by insertion of cold or warm water is known to activate contralateral cortical vestibular areas (Caloric vestibular stimulation, CVS) and to modulate spatial cognition (Cappa et al., 1987), bodily awareness (Cappa et al., 1987, Vallar et al., 1993, Bottini et al., 2005) and body ownership (Bisiach et al., 1991) in patients with right hemisphere stroke.
Recently, a less invasive method than CVS (Lopez et al., 2010, Ferrè et al., 2013a, Ferrè et al., 2013b), namely Galvanic Vestibular Stimulation (GVS), has been used to examine the role of vestibular stimulation on multisensory integration and body ownership. GVS involves a small electrical current applied using two electrodes (one anode and one cathode) positioned on the mastoids (Utz et al., 2011a, Utz et al., 2011b). The change in electrical excitability of the vestibular nerves stimulates the vestibular network of the right hemisphere when the anode is on the left mastoid and the cathode on the right (known as LGVS), while the reverse electrode positioning (RGVS) leads to a bilateral activation (Fink et al., 2003, Utz et al., 2010). Most studies on body ownership have focused on the role of LGVS given the assumed right lateralised activation it causes (in right-handed subjects; Dieterich et al., 2003; Eulenburg et al., 2012) and the link of the latter with body representation disorders (Baier and Karnath, 2008, Bisiach et al., 1991, Moro et al., 2016, Zeller et al., 2011). Specifically, Lopez and colleagues (2010) found that LGVS enhances body ownership during the RHI, and influences multisensory integration by promoting visual dominance over proprioception; however, Ferrè et al. (2015), observed a decrease in proprioceptive drift following LGVS, suggesting that LGVS enhances proprioception over vision. Thus, both studies found that stimulation of the right vestibular network influences the balance between proprioceptive and visual information in a hemispheric-specific fashion (Dieterich et al., 2003), but in opposite directions. These conflicting results may be caused by various methodological differences between the two studies (see Discussion for full details); however, taken together, they provide preliminary indications for the role of vestibular signals to the weighting of different sensations during multisensory integration and, hence, to body ownership. The present study aimed to further specify Lopez and colleagues’ findings against those of Ferrè and colleagues by testing two further hypotheses regarding visual capture of proprioception and ownership (VOC; Martinaud et al., 2017), as well as interoception, as explained below.
We administered galvanic vestibular stimulation during a rubber hand illusion task with the hypothesis that LGVS would enhance the RHI, by increasing the weighting of visual signals whilst lowering the precision of proprioceptive ones. During the RHI, the conflict between vision, touch and proprioception is typically solved via a dominance of visual information over proprioceptive one (e.g. see Zeller et al., 2011 and Zeller et al., 2015 for electrophysiological evidence), i.e. what we see can be processed as more reliable than what we feel, resulting in the embodiment of the rubber hand (Folegatti et al., 2009). Hence, when visual information is present and reduces the ambiguity of a conflictual situation, the stimulation of the vestibular system may shift the balance in favour of vision (as in Lopez et al., 2010) rather than proprioception (Ferrè et al., 2015). In order to specifically test this possibility, we included a mere visual capture condition, during which subjects did not receive any touch on either their hand or the rubber hand but were only required to look at the rubber hand (see Crucianelli et al., 2017). However, even though the current study takes into consideration differences in variance at the group level, we could not directly test whether precision is lowered in favour of vision within each of the different trials in each of our subjects (i.e. at the individual level). In order to do so, we would need multiple trials, or some additional signal strength measure (e.g. see Zeller et al., 2015), which were not possible within the current design; hence, we can only speculate, based on previous literature and the current data, that sensory re-weighting of visual and proprioceptive information, with an increase of the former and a concomitant reduction of the latter, may be the mechanism at play should our predictions be confirmed at the group level (see Discussion section for further details on this point).
Furthermore, we wanted to investigate how the combined effects of vestibular stimulation and vision influence body ownership during the RHI when touch is affective rather than neutral. In order to do so, we administered CT-optimal, affective touch and non CT-optimal, neutral touch during both synchronous and asynchronous conditions of the RHI. C-Tactile (CT) afferents are a specialised, unmyelinated class of fibres innervating the hairy skin of the body (McGlone and Reilly, 2010). They are optimally activated by slow, caress-like tactile stimulation at velocities between 1 and 10 cm/s (McGlone et al., 2014). CT-optimal touch is associated with heightened pleasantness (Löken et al., 2009; Shaikh et al., 2015; Pawling et al., 2017) and has been identified as a type of affective touch (McGlone et al., 2014). CT-optimal touch activates multimodal areas of converging sensory and affective information regarding the state of the body (including posterior insula, Craig, 2002, Craig, 2003; Olausson et al., 2002; McGlone et al., 2012 and cingulate cortex, Case et al., 2017). Moreover, the pleasantness associated with CT-optimal touch is not affected by inhibition of the primary and secondary sensory cortices (Case et al., 2016, Case et al., 2017), thus supporting the notion that the CT-system might play a unique role in conveying affective rather than discriminative aspects of touch. CT-afferents are considered as sharing more characteristics with interoceptive (i.e. related to the sense of the physiological condition of one's own body; Ceunen et al., 2016), rather than exteroceptive, modalities (Björnsdotter et al., 2010), in light of their contribution to the maintenance of our sense of self (Crucianelli et al., 2017).
CT-optimal touch has been found to increase embodiment during the RHI (Crucianelli et al., 2013, Crucianelli et al., 2017, Lloyd et al., 2013, van Stralen et al., 2014). For example, Crucianelli and colleagues (2013) found an increase in subjective measures of embodiment during the RHI using synchronous, CT-optimal touch. Nevertheless, the mechanisms behind such enhancement remain unknown. One possibility is that the pleasantness elicited by CT-optimal touch enhances embodiment because interoception is tightly connected with the feelings of body ownership. However, it is also possible that the ‘seen’ affective touch on the rubber hand, i.e. vicarious affective touch (Morrison et al., 2011a, Morrison et al., 2011b), rather than just the felt affectivity of the touch on participant's own arm, contributes to the effect. Both felt and vicarious CT-optimal touch elicit the same response in the posterior insula, suggesting that the affectivity conveyed by CT-optimal touch is not exclusively anchored to the felt sensation and may also be influenced by vision. Given that the vestibular system may favour visual information by reducing the precision of proprioceptive signals during conflictual situations (as hypothesised above) and owning to the overlap in neural circuits responsible for interoceptive (see Case et al., 2017) and vestibular (see Lopez et al., 2012b) processing, these two modalities may contribute to the bodily self in a combined fashion. Hence, in the current study, we aimed to explore whether vestibular stimulation would shift the balance between vision and interoception by increasing the precision of visual information over signals coming from participant's own body. Specifically, we hypothesised that stimulation of the vestibular system could enhance the effect of the seen affective touch (i.e. vicarious touch) on the rubber hand rather than the felt one on participants’ hand, thus further strengthening embodiment of the rubber hand.
In sum, even though existing research suggests that the vestibular system may contribute to body ownership, little is currently known about the direction of such contribution nor the combined effect of the vestibular system and interoceptive modalities (such as affective touch). In the present study we applied galvanic vestibular stimulation (LGVS, RGVS and sham) during a RHI task in which we manipulated the affectivity of touch (affective, CT-optimal or neutral, non-CT optimal velocity of touch) to i) disambiguate previous findings on the role of the vestibular system in balancing vision, touch and proprioception in relation to body ownership and ii) to investigate the combined contribution of vestibular and interoceptive systems in shaping the experience of our own body. We also devised a condition where no touch was applied and participants simply looked at the rubber hand for 15 s (sufficient to elicit visual capture of the rubber hand, see Martinaud et al., 2017 and Experimental Design below) to test whether visual information alone, with no tactile stimulation, was enough to elicit ownership of the rubber hand (in line with Samad et al., 2015). We expected LGVS (when compared with RGVS) to stimulate a right vestibular network for bodily awareness leading to a stronger visual capture of the rubber hand during synchronous stroking (as in Lopez et al., 2010) and even in the absence of touch. In agreement with Lopez et al. (2010) and Ferrè et al. (2015) findings, we hypothesised that the effects observed in our study would be the result of sensory weighting during multisensory integration, with an increased precision of visual over proprioceptive information: if the effects of GVS during the RHI are due to a separate modulation of vision or proprioception, rather than their integration, we would expect a generic decrease or increase in proprioceptive drift regardless of the synchronicity of the stroking. Finally, we predicted that CT-optimal touch would lead to greater RHI effects compared with non-CT optimal touch (see Crucianelli et al., 2013; Lloyd et al., 2013; van Stralen et al., 2014), and that this effect would be enhanced by LGVS.
2. Materials and methods
2.1. Participants
Twenty-six right-handed healthy participants (13 females, age range: 18–53, M = 29.8 SD = 9.5 years), were recruited via an institutional subject pool. Participants with psychiatric or neurological history (including vestibular disturbances), pregnancy at the time of testing, or metal plaques in their body, were excluded. Participants were asked to refrain from smoking and drinking alcohol during the 24 h before the experiment. The study was approved by an institutional Ethics Committee and all participants gave informed, written consent.
2.2. Experimental design
To examine the effect of vestibular activation on visual capture of proprioception, we applied GVS (LGVS, RGVS and sham) during a visual only condition involving observation of the rubber hand for 15 s, without touching either the participant's or the rubber hand. Then, to investigate the contribution of vestibular and C-tactile systems’ stimulation to body ownership we conducted a rubber hand illusion (RHI) experiment using a 3 (GVS configuration: LGVS, RGVS and sham) × 2 (stroking velocity: slow and fast) × 2 (stroking synchronicity: synchronous and asynchronous) within-subjects design. Stroking velocity was manipulated by administering slow, CT-optimal touch (at 3 cm/s) and fast, non CT-optimal touch (18 cm/s) (see Crucianelli et al., 2013) for 120 s. Stroking synchronicity was manipulated by either stroking the participant's hand and the rubber hand simultaneously (i.e. synchronous conditions) or out of synchrony (i.e. asynchronous conditions). The timings of visual capture and stroking conditions were based on previous research indicating the sufficiency of these durations for eliciting ownership as a result of visual capture (Martinaud et al., 2017) and visuo-tactile stimulation (Lopez et al., 2010) respectively.
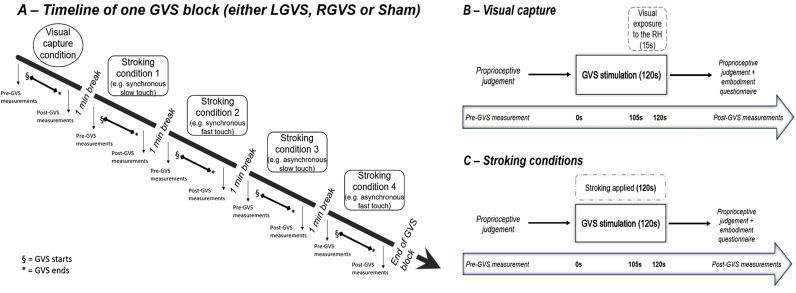
GVS was performed in a block design (LGVS vs. RGVS vs. sham) with the order of GVS blocks counterbalanced across participants. Each GVS block consisted of one visual capture condition and four different stroking conditions: synchronous slow touch, synchronous fast touch, asynchronous slow touch and asynchronous fast touch. Within each GVS block, the pure visual capture condition was always conducted first, and subsequent stroking conditions were randomised for velocity and synchronicity (see Fig. 1.A). As mentioned in the introduction, previous studies (Crucianelli et al., 2013, Crucianelli et al., 2017, Martinaud et al., 2017, Samad et al., 2015) suggested that a brief visual exposure to the rubber hand can elicit feelings of ownership in right-hemisphere stroke patients as well as in healthy subjects. Thus, we wanted to investigate whether healthy participants, when exposed to the rubber hand during right vestibular stimulation, would integrate vision and proprioception in favour of vision, prior to any tactile stimulation (and any related carry-over effects).
Fig. 1.
A) Timeline of one prototypical GVS block. At the beginning of each of the three GVS blocks (either LGVS, RGVS or Sham), participants undertook the visual capture condition, with measures taken before and after stimulation (see B for details of this condition and its measurements). Subsequently, one of the four stroking conditions (see C for further details) was conducted in a randomised order, with measures again taken before and after stimulation. B) Timeline of the visual capture condition. Before the visual capture condition started participants performed a proprioceptive judgement (pre-GVS measurement). Immediately afterwards, the vestibular or sham stimulation commenced for 2 min during which participants sat with their eyes open. During the last 15 s of vestibular or sham stimulation, the experimenter opened the box lid and instructed the participant to look at the rubber hand until told otherwise. After 120 s (total) stimulation the lid was closed and participants immediately performed a second proprioceptive judgement and completed the embodiment questionnaire (post-GVS measurements). C) Timeline of the stroking conditions. Each of the four stroking conditions (synchronous slow touch, synchronous fast touch, asynchronous slow touch and asynchronous fast touch) followed the same structure. Participants made an initial (pre-GVS measurement) proprioceptive judgement, followed immediately by vestibular or sham stimulation and concurrent tactile stimulation (i.e. stroking of both the participant and rubber hand's forearm) for 120 s, during which participants looked continuously at the rubber hand. A second proprioceptive judgement and embodiment questionnaire was completed immediately following completion of the 120 s concurrent vestibular / tactile stimulation (post-GVS measurements).
The outcome measures, collected before (Proprioceptive drift) and after each stroking condition (Proprioceptive drift and Embodiment Questionnaire), were as follow:
-
a)
Proprioceptive drift: the perceived shift of the participant's hand towards the rubber hand, measured in centimetres. Proprioceptive drift was calculated by subtracting a post-GVS estimate of the left hand's location from a pre-stimulation estimate (Fig. 1.A), with positive values meaning that participants perceived their hands as being closer to the rubber hand.
-
b)
Embodiment Questionnaire: a short version of the questionnaire from Longo et al. (2008), see Supplementary materials) was used to measure: feelings of ownership of the rubber hand, perceived location of participants’ hand and affective aspects of the experience. One of the questions, relating to touch, was administered only in the stroking conditions. The order of the questions was randomised in each condition and responses were given on a 7-point Likert scale, ranging from −3 (‘Strongly disagree’) to +3 (‘Strongly agree’). These ratings were used to obtain a composite embodiment score for each condition by calculating the average score obtained from the ownership and location questions (these individual aspects, as well as affective ones, were also analysed separately; see Supplementary materials, 2.2, 2.3).
2.3. Experimental setup and materials
2.3.1. Galvanic vestibular stimulation
A bipolar stimulation with fixed intensity (1 mA) and duration (2 min per condition) was applied to all participants using a direct current stimulator (NeuroConn DC-stimulator, neuroCare Group GmbH, München, Germany). The total amount of stimulation per GVS block was 10 min, with breaks of 20 min between blocks to minimise possible after-effects (Utz et al., 2010). Therefore, the entire experiment involved 30 min (including Sham) of non-continuous stimulation.
Two 3 × 3 cm carbon rubber electrodes were inserted into sponges, previously soaked in a saline solution, and then fixed either on the participants’ mastoid bones (LGVS and RGVS) or neck (Sham) using a rubber band. During left-anodal/right-cathodal stimulation (i.e. LGVS), the anode was placed on the left mastoid process and the cathode on the right. During left-cathodal/right-anodal GVS (i.e. RGVS), the anode was on the right and the cathode on the left. In the Sham stimulation, both electrodes were placed on the nape (~5 cm below the end of the mastoid processes) with the anode and the cathode randomly positioned either on the left or the right side of the neck.
2.3.2. Rubber hand illusion
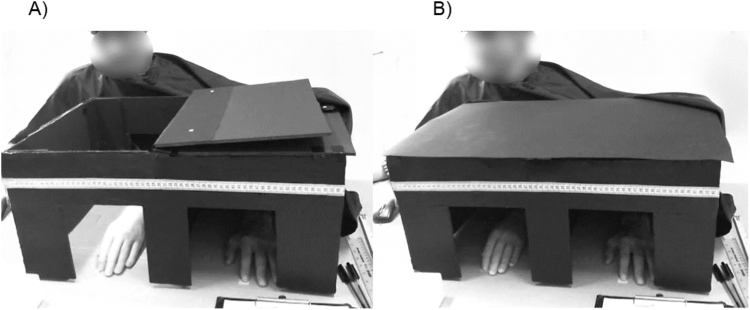
A black wooden box (62 cm × 43 cm × 26 cm), was positioned on a table 10 cm from the participant's torso. The box was divided in two equal halves by a piece of black cardboard. Two spots were marked on the table with tape, one for the left rubber hand's index finger and one for the participant's left index finger (distance between the rubber hand and the participant's hand = 30 cm; Fig. 2). On the upper side of the box, visible to the experimenter only, there was a measuring tape, used to record proprioceptive drift. In order to reduce the influence of external visual cues, participants wore a black cloth covering their shoulders and arms.
Fig. 2.
Participants sat in front of the table, facing the box, and were asked to insert their left hand into the left compartment of the box, while the rubber hand was positioned in the compartment on the right, aligned with participant's left shoulder. Both the participant's and rubber hand's left index fingers were located on the corresponding marked spots. A) While the box was open, participants were asked to look inside and observe the rubber hand. In particular, during the stroking conditions, participants were instructed to follow the brush while it was stroking the rubber hand's forearm. B) Before and after each condition, with the box closed and covered by a black carton, participants had to perform a proprioceptive judgement (see Section 2.4).
Stroking was administered to the participant's and rubber hand using two identical make-up brushes (Natural hair Blush Brush, N°7, The Boots Company) by an experimenter who was extensively trained to deliver touches in a uniform manner, controlling for speed and pressure (as in Crucianelli et al., 2013 and Krahé et al., 2016; see also Triscoli et al., 2013 for discussions regarding the advantages and limitations of administering pleasant touch using human versus mechanical methods).
2.4. Experimental procedure
2.4.1. Main task
After removing any jewellery from the participant's left arm/hand, the experimenter aligned the participant's left shoulder and index finger with the rubber hand, which was hidden from the participant's view inside the box. Participants were instructed to avoid moving their left arm during each condition. The experimenter sat opposite the participant.
Each GVS block consisted of five conditions (Fig. 1.A), one visual capture and four stroking conditions. Each condition commenced with a proprioceptive judgement (Lloyd et al., 2013): the experimenter passed the tip of a pen along the top of the closed box (~1 cm/s), starting randomly from the left or the right-hand side of the box. Participants were instructed to verbally stop the experimenter when the pen reached the point vertically in line with the perceived position of the participant's left index finger. The experimenter then recorded the actual position and the perceived position, obtaining a pre-GVS measurement of proprioceptive drift (actual finger position minus perceived finger position).
Immediately after the proprioceptive judgement, the experimenter started the GVS. During the first condition (visual capture, Fig. 1.B), participants were instructed to relax for the first 1 min and 45 s of stimulation, after which the experimenter opened the box, revealing the rubber hand. Participants were then asked to look continuously at the rubber hand for the last 15 s of stimulation. Once the stimulation ended, the lid was closed and the participant performed a second proprioceptive judgement and completed the embodiment questionnaire (post-GVS measurements).
After the visual capture condition, there was a one-minute break, during which participants removed their arm from the box and two adjacent areas, each measuring 9 × 4 cm, were drawn with a washable marker on the participant's left dorsal forearm, in order to control for pressure and habituation (see Crucianelli et al., 2013). Subsequently, one of the four stroking conditions commenced. Each stroking condition started with a pre-GVS proprioceptive judgement (Fig. 1.C), followed by 2 min of simultaneous vestibular and tactile stimulation. Touch was always administered proximally to distally and each stroke was followed by a 1 s break. In the synchronous slow, CT-optimal touch condition, the participant's forearm was touched in synchrony with the rubber hand's forearm at 3 cm/s for 2 min (i.e. single touch = 3 s). In the synchronous fast, non CT-optimal touch, the stroking was synchronous, at 18 cm/s (i.e. single touch = 0.5 s). During the asynchronous touch conditions, the timing of touches delivered to the real and rubber hand was offset, such that while the experimenter was stroking the rubber hand, the participant's forearm was not being touched and vice-versa. During each stroking condition, participants were instructed to continuously look at the rubber hand. After the vestibular-tactile stimulation ended, the post-GVS proprioceptive judgement was obtained and participants answered the embodiment questionnaire (post-GVS measurements). The next stroking condition began after a one-minute break, during which participants were instructed to move their left arm, in order to reduce any discomfort and possible cumulative effects of the illusion.
At the end of the experiment, after having removed the GVS electrodes, participants were asked to report any physical sensation associated with the vestibular stimulation (see section 2.7., Supplementary materials).
2.4.2. Pleasantness ratings
As an additional control task, at the end of the 3 GVS blocks (without any vestibular stimulation applied) participants were asked to verbally rate on a scale from 0 “Not at all pleasant” to 100 “Extremely pleasant”, the pleasantness of touches delivered at 3 cm/s and 18 cm/s, in order to check that they perceived slow touch as more pleasant than fast touch, in line with the findings reported in previous studies (Löken et al., 2009, Crucianelli et al., 2013, Crucianelli et al., 2017). The results confirmed that participants perceived CT-optimal touch as more pleasant than non CT-optimal touch (see Supplementary Materials, Section 2.5.).
2.5. Data analysis
To examine hemispheric specific effects of GVS on multisensory integration, compared with a generic effect of vestibular stimulation (Ferrè et al., 2015), we conducted planned comparisons of (i) left vs. right vestibular network activation (i.e. LGVS vs. RGVS), to test the hypothesis that LGVS is specifically linked with a right-hemisphere network for bodily awareness, and also (ii) we compared this hemispheric-specific hypothesis with a generic arousal due to the stimulation of the vestibular nerves, irrespective of the polarity (i.e. comparing (LGVS+RGSV)/2 vs. Sham). These analyses were conducted using t-tests (visual capture conditions) and repeated measures ANOVA (stroking conditions) on all the outcome measures (proprioceptive drift and embodiment questionnaire; see Supplementary Materials for additional analyses on the latter). For the stroking conditions, we used repeated measures ANOVA in order to analyse the possible interactions between type of stimulation, stroking synchronicity and stroking velocity, according to our initial hypotheses.
In addition, we ran a trend analysis to examine the possibility of a cumulative bias in baseline proprioceptive judgements due to vestibular carry-over effects over time within each GVS block. In order to do so, we analysed linear trends in pre-stimulation proprioceptive judgements of the stroking conditions in the order they were administered per participant. These trends were analysed using a repeated measures ANOVA with polynomial contrasts aimed at comparing the trends in each of the three GVS blocks. The results are reported in full in the Supplementary Materials (see section 2.5.2.).
Data were analysed using the IBM Statistical Package for Social Sciences (SPSS) for Windows, version 23 (Armonk, NY).
3. Results
Three participants were excluded from the analyses: one was excluded because he failed to follow instructions and another participant was more than 2.5 SD away from the group mean in proprioceptive measures across most LGVS and Sham conditions (8/10). Additionally, in order to create a homogeneous sample in terms of pleasantness ratings of touch, we excluded another participant who was more than 2.5 SD away from the group mean in the pleasantness ratings task (when rating the 3 cm/s velocity on both palm and forearm).
Hence, the final sample consisted of 23 participants (12 females; age range = 18–53 years, mean = 30.04, SD = 9.77).
3.1. Proprioceptive drift
3.1.1. Hemispheric specific effects
3.1.1.1. Visual capture conditions
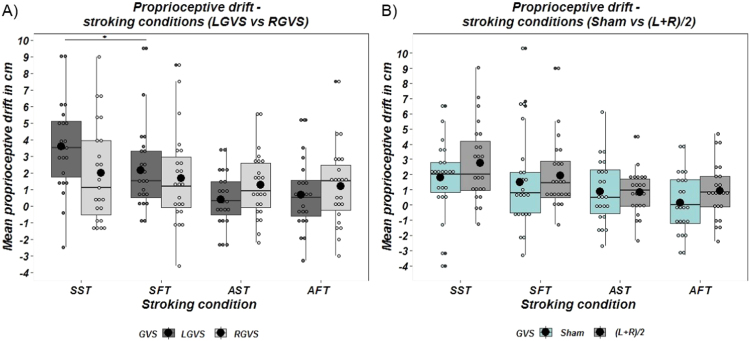
A paired sample t-test was used to compare proprioceptive drift after observation of the rubber hand without tactile stimulation following LGVS versus RGVS. As predicted, LGVS led to greater proprioceptive drift than RGVS (LGVS: M = 2.60 cm, SD = 2.50; RGVS: M = 0.17 cm, SD = 2.30; t22 = 3.601, p = .002, d=1.08,) after visual exposure to the rubber hand (see Fig. 3.A below).
Fig. 3.
GVS effects during Visual Capture on Proprioceptive drift. A) Mean values of the proprioceptive drift measured in cm in the LGVS and in the RGVS; B) Mean values of the proprioceptive drift measured in cm in (LGVS+RGVS)/2 and Sham obtained during visual capture conditions. *= p < 0.01; Solid line=median; Black dot= mean; Whiskers: upper whisker = min(max(x), Q_3 + 1.5* IQR); lower whisker = max(min(x), Q_1 – 1.5* IQR).
3.1.1.2. Stroking conditions
A 2 (stimulation: LGVS and RGVS) × 2 (stroking synchronicity: synchronous and asynchronous) × 2 (stroking velocities: slow and fast) repeated measures ANOVA revealed a main effect of Synchronicity (F(1,22)= 19.426, p < .001, ηp2 = 0.469), but no main effect of Stimulation (F(1,22)= 0.271, p = 0.608, ηp2 = 0.012) or Velocity (F(1,22)= 2.688, p = 0.115, ηp2 = 0.109). As hypothesised, synchronous stroking led to greater proprioceptive drifts. The interaction between Stimulation and Synchronicity was significant (F(1,22)= 9.149, p = 0.006, ηp2 = 0.294), as was the 3-way interaction between Stimulation, Synchronicity and Velocity of stroking (F(1,22)= 5.260, p = 0.032, ηp2 = 0.193). No other interactions were significant (Stimulation*Velocity, F(1,22)= 0.663, p = 0.424, ηp2 = 0.029; Synchrony*Velocity, F(1,22)= 3.254, p = 0.085, ηp2 = 0.129) (Fig. 4.A).
Fig. 4.
A) Mean values of the proprioceptive drift measured in cm in the LGVS and in the RGVS; B) Mean values of the proprioceptive drift measured in cm in (LGVS+RGVS)/2 and Sham obtained during the stroking conditions of the rubber hand illusion. SST= synchronous slow touch; SFT= synchronous fast touch; AST= asynchronous slow touch; AFT= asynchronous fast touch. * = p < 0.01; Solid line=median; Black dot= mean; Whiskers: upper whisker = min(max(x), Q_3 + 1.5* IQR); lower whisker = max(min(x), Q_1 – 1.5* IQR).
To explore the nature of the above, significant, interactions, we conducted two additional 2 (stroking synchronicity) × 2 (stroking velocity), repeated-measures ANOVAs on LGVS and RGVS separately. For LGVS, a main effect of Synchronicity (F(1,22)= 23.345, p < 0.001, ηp2 = 0.515) indicated that synchronous conditions resulted in an increase in proprioceptive drift. There was no main effect of Velocity (F(1,22)= 3.467, p = 0.076, ηp2 = 0.136), but there was a significant Synchronicity*Velocity interaction (F(1,22)= 10.052, p = 0.004, ηp2 = 0.314). This was further analysed using post-hoc paired sample t-tests (Bonferroni corrected, α = 0.025), to compare slow and fast touch during synchronous conditions, and separately, during asynchronous conditions. As expected, synchronous slow touch led to significantly higher proprioceptive drift than synchronous fast touch (synchronous slow touch: M = 3.57 cm, SD = 2.70; synchronous fast touch: M = 2.16 cm, SD = 2.42; t22 = 3.131, p = 0.005, d = 0.94), while the difference between the two asynchronous conditions was not significant (asynchronous slow touch: M = 0.40 cm, SD = 1.64; asynchronous fast touch: M = 0.68 cm, SD = 2.12; t22 =−0.807, p = 0.428, d = 0.24).
Conversely, for RGVS there were no main effects or interactions (Synchronicity: F(1,22)= 2.327, p = 0.141, ηp2 = 0.096; Velocity: F(1,22)= 0.335, p = 0.569, ηp2 = 0.015; Synchronicity*Velocity: F(1,22)= 0.087, p = 0.770, ηp2 = 0.004).
3.1.2. Non-hemispheric specific effects
3.1.2.1. Visual capture conditions
As expected, we did not find a generic effect of GVS on proprioceptive drift after visual exposure to the rubber hand; a paired sample t-test comparing the overall effect of stimulation (LGVS+RGVS/2) with Sham was not significant (LGVS+RGVS/2: M=1.39 cm, SD=1.78; Sham: M=0.92 cm, SD=2.58; t22 =0.749, p = 0.462, d=0.22; Fig. 3.B).
3.1.2.2. Stroking conditions
A 2 (stimulation: (LGVS+RGVS/2) and Sham) × 2 (stroking synchronicity: synchronous and asynchronous) × 2 (stroking velocities: slow and fast) repeated measures ANOVA revealed a main effect of Stimulation (F(1,22)= 4.862, p = 0.038, ηp2 = 0.181) and main effect of Synchronicity (F(1,22)= 16.744, p < 0.001, ηp2 = 0.432), indicating that the average of the two GVS stimulations, as well as synchronous conditions, increased proprioceptive drift. The main effect of Velocity was also approaching significance (F(1,22)= 4.183, p = 0.053, ηp2 = 0.160), with slow touch leading to marginally greater proprioceptive drift than fast touch. None of the interactions were significant (Stimulation*Synchronicity, F(1,22)= 0.438 p = 0.515, ηp2 = 0.020; Stimulation*Velocity (F(1,22)= 0.058, p = 0.812, ηp2 = 0.003; Synchronicity*Velocity, F(1,22)= 0.296, p = 0.592, ηp2 = 0.013; Stimulation*Synchronicity*Velocity, F(1,22)= 0.931, p = 0.345, ηp2 = 0.041) (Fig. 3.B).
3.2. Embodiment questionnaire
3.2.1. Hemispheric specific effects
3.2.1.1. Visual capture conditions
A paired sample t-test comparing LGVS and RGVS did not indicate a significant difference between the two vestibular stimulations in subjective aspects of embodiment following mere visual exposure to the rubber hand (t22 =−0.064, p = 0.950, d=0.01) (Table 1).
Table 1.
Descriptive statistics of embodiment questionnaire's average values in the different conditions.
| LGVS | RGVS | Sham | (L+R)/2 | |
|---|---|---|---|---|
| Conditions | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Visual Capture | − 0.61 (1.60) | − 0.60 (1.54) | − 0.45 (1.65) | − 0.60 (1.46) |
| Synchronous Slow Touch | 0.59 (1.40) | 0.38 (1.45) | 0.85 (1.35) | 0.48 (1.33) |
| Synchronous Fast Touch | 0.94 (1.38) | 0.59 (1.42) | 0.90 (1.37) | 0.76 (1.36) |
| Asynchronous Slow Touch | − 0.63 (1.46) | − 0.65 (1.38) | − 0.46 (1.71) | − 0.64 (1.35) |
| Asynchronous Fast Touch | − 0.40 (1.49) | − 0.65 (1.35) | − 0.17 (1.47) | 0.53 (1.30) |
3.2.1.2. Stroking conditions
A 2 (stimulation: LGVS and RGVS) × 2 (stroking synchronicity: synchronous and asynchronous) × 2 (stroking velocities: slow and fast) repeated measures ANOVA revealed a main effect of Synchronicity (F(1,22)= 30.502, p < 0.001, ηp2 = 0.581), indicating that synchronous conditions led to greater embodiment. There was no main effect of Stimulation (F(1,22)= 1.787, p = 0.195, ηp2 = 0.075) or Velocity of stroking (F(1,22)= 2.851, p = 0.105, ηp2 = 0.115) and none of the interactions were significant (Stimulation*Synchronicity, F(1,22)= 0.970 p = 0.335, ηp2 = 0.042; Stimulation*Velocity, F(1,22)= 1.422, p = 0.246, ηp2 = 0.061; Synchronicity*Velocity, F(1,22)= 1.524, p = 0.230, ηp2 = 0.065; Stimulation*Synchronicity*Velocity, F(1,22)= 0.119, p = 0.734, ηp2 = 0.005).
3.2.2. Non-hemispheric specific effects
3.2.2.1. Visual capture conditions
A paired sample t-test comparing the average of the two stimulations ((LGVS+RGVS)/2) with Sham was not significant (t22 =−0.714, p = .483, d=−0.21); hence, we did not find generic arousal effects due to GVS on subjective embodiment following visual exposure to the rubber hand.
3.2.2.2. Stroking conditions
A 2 (stimulation: (LGVS+RGVS)/2 and Sham) x 2 (stroking synchronicity: synchronous and asynchronous) x 2 (stroking velocities: slow and fast) repeated measures ANOVA revealed main effects of Synchronicity (F(1,22)= 26.753, p < 0.001, ηp2 = 0.549), Stimulation (F(1,22)= 6.296, p = 0.020, ηp2 = 0.223) and Velocity (F(1,22)= 4.581, p = 0.044, ηp2 = 0.172). No significant interactions were found (Stimulation*Synchronicity, F(1,22)= 0.007 p = 0.935, ηp2 = 0.000; Stimulation*Velocity, F(1,22)= 0.031, p = 0.863, ηp2 = 0.001; Synchronicity*Velocity, F(1,22)= 0.065, p = 0.801, ηp2 = 0.003; Stimulation*Synchrony*Velocity, F(1,22)= 2.103, p = 0.161, ηp2 = 0.087). This analysis suggested that each of the factors independently contributed to a higher level of subjective embodiment (see Table 1 below). As expected, synchronous conditions significantly increased the subjective experience of the rubber hand illusion. Furthermore, the overall level of embodiment was higher during Sham stimulation, indicating that GVS decreased participants’ subjective feelings of ownership towards the rubber hand. Finally, fast touch led to a slightly higher subjective embodiment than slow touch.
3.3. Control analysis – cumulative effects of GVS
We checked for the presence of a cumulative bias in participants’ proprioceptive judgements due to carry-over effects of the stimulation within each GVS block by conducting a repeated measures ANOVA with polynomial contrasts on the pre—stimulation proprioceptive judgements. The results confirmed that the changes in participants’ proprioceptive judgements were increasing over time regardless of the type of stimulation, thus suggesting the absence of a specific effect of LGVS on deteriorating proprioception in comparison with RGVS and Sham and excluding the possibility that carry-over effects could explain away our abovementioned findings (see section 2.5.2., Supplementary Materials).
4. Discussion
We administered GVS to healthy participants during a rubber hand illusion task, in order to explore the influence of vestibular stimulation on body ownership during multisensory integration. We found that participants showed a significantly greater perceived hand displacement towards the rubber hand during LGVS, but not RGVS, even in the absence of any tactile stimulus (i.e. during pure ‘visual capture’) and beyond any general (i.e. not lateralised) GVS effect. Furthermore, when participants’ forearms were stroked synchronously with the rubber hand, proprioceptive drifts were greater during LGVS in comparison with RGVS conditions. Lastly, during LGVS synchronous conditions only, slow, CT-optimal touch led to greater proprioceptive drifts in comparison to fast, non CT-optimal touch.
Our findings corroborate the hypothesis that the vestibular system plays a modulatory role in multisensory integration: when there is a conflict between proprioception and vision, the right vestibular network may solve the ambiguity by increasing the relative weighting of visual signals over proprioceptive ones. This interpretation is consistent with the idea that the vestibular system actively contributes to the formation of an updated representation of our body in space according to bodily and environmental changes (Pfeiffer et al., 2014). It has been showed that LGVS induces a disruption of the normal egocentric (i.e. based on the perceiver) reference frame when performing an allocentric (i.e. based on the external environment) judgement (Fink et al., 2003). A recent study (Harris and Hoover, 2015) found that GVS disrupted the natural self-advantage (i.e. greater accuracy in 1st person perspective) when detecting delays in virtually reproduced self-generated fingers movements, with no difference in participants’ performance when the stimuli were presented from a 3rd person perspective. Our results could be due to a vestibular-induced disruption of the normal body representation, based on an egocentric reference frame: a perturbation of body-centred multisensory processing might lead to an increased weighting of visual cues with a concomitant reduction of proprioceptive ones, thus favouring a proprioceptive displacement towards an external object (i.e. the rubber hand). Within a predictive coding framework, Zeller and colleagues (2015) suggested that the occurrence of the RHI may be the result of lowering the precision (i.e. the certainty about sensory representations) of somatosensory signals to allow a top-down resolution of sensory ambiguity. In the current study, this may have translated into an increased ‘visual capture’ of the rubber hand, in absence of any tactile stimulation (in line with the findings reported by Samad et al., 2015 in healthy subjects and Martinaud et al., 2017 in right-hemisphere stroke patients), as well as of the touch delivered to the rubber hand, during synchronous conditions only. Nevertheless, as mentioned in the introduction, our design did not allow us to directly test changes in variances associated with the proprioceptive drifts at the individual level but only at the group one. Hence, we can only speculate that this may be the mechanism at play here. Further studies are needed in order to clearly define the degree to which the increased weighting of visual information is accompanied by a decrease in precision of proprioceptive one (e.g. as suggested by Zeller et al., 2015). Importantly, the enhanced visual capture of the seen touch observed in the present study occurs solely when there is temporal congruency (synchronous stroking conditions), a factor deemed necessary to allow multisensory integration and ownership (Costantini et al., 2016). Moreover, our trend analysis of proprioceptive judgements demonstrates that these effects are observed over and above a generic, vestibular-induced progressive bias in participants’ proprioceptive ability over time (see Supplementary Materials, section 2.5.2. for details on this). Thus, these findings corroborate the hypothesis that the vestibular system acts on multisensory integration rather than at the unimodal level (as suggested by Ferrè et al., 2015).
This ‘visual capture’ of proprioception and touch is also consistent with the observed increase in proprioceptive drifts following synchronous slow, CT-optimal touch compared to fast touch (with no difference between asynchronous conditions) during LGVS conditions only. That is, the proprioceptive displacement towards a rubber hand was enhanced by synchronous, affective touch only during a right vestibular network stimulation. The specific contribution of the CT-system in our study may hence relate to the affectivity conveyed by the touch seen on the rubber hand, rather than only by the one felt. Observing the administration of CT-optimal touch on another person's skin leads to the activation of cortical areas involved in affective processing (“vicarious touch”, Morrison et al., 2011a). In addition, two recent studies found that inhibition of the somatosensory cortices does not influence the perceived pleasantness of CT-optimal touch, highlighting the possible anatomical and cognitive dissociation between affective and discriminative aspects of CT-optimal touch (Case et al., 2016, Case et al., 2017). In the current study, the stimulation of the right vestibular network, may have promoted a visual capture of the seen affective touch, by rebalancing multisensory weighting in favour of vision while lowering the precision of felt sensations, in line with the hypothesised top-down modulation discussed above. Future studies are needed to shed light on the role of vicarious affective touch in multisensory integration as well as on the precise contribution of each sensory modality, with paradigms investigating changes in variances at the individual level.
It might be argued that our results could be explained in terms of a generic, vestibular-dependent shift in space towards the left side. It has been shown that LGVS induces a leftward shift on the lateral plane in right hemisphere stroke patients (Utz et al., 2011a, Utz et al., 2011b, Wilkinson et al., 2014) and healthy subjects (Ferrè et al., 2013a, Ferrè et al., 2013b). Accordingly, during rotational vestibular stimulation, peri-personal space is remapped in a direction-specific fashion and sensory congruency further expands the boundaries of subjects’ peri-personal space (Pfeiffer et al., 2018). However, in a separate control condition devised within a follow-up experiment (see section 2.8 of the Supplementary Materials) we did not find that galvanic vestibular stimulation induces shifts on the lateral plane per se (i.e. when no rubber hand is present). Differences in the types of stimulation used (rotational vs galvanic) as well as duration of stimulation (as further discussed below in relation to Ferrè and colleagues’ findings) may partially account for the contradicting results. However, further research should investigate the effects of GVS on multisensory integration when there is a stimulus in view (e.g. a rubber hand) but such stimulus is displaced in depth rather than laterally.
Our results also corroborate the hypothesis of a hemispheric specific vestibular network for bodily awareness (see also Ferrè et al., 2015). However, since LGVS stimulates mainly areas in the right-hemisphere, it is possible that the mechanisms observed in the current study are limited to the left hand, and may not represent a generalised effect on bodily awareness. On the other hand, given that the right hemisphere is dominant for vestibular processing (Dieterich et al., 2003, Eulenburg et al., 2012) and generally considered crucial for body representation in both healthy subjects and clinical population (Cappa et al., 1987, Bisiach et al., 1991, Naito et al., 2005, Tsakiris et al., 2007, Tsakiris, 2010), it is reasonable to assume that if the hand used for the illusion was the right rather than the left, the results would point in the same direction. Accordingly, previous studies showed that when the RHI is performed on the right hand, areas of the fronto-parietal network are activated bilaterally, i.e. the activation occurs in the right as well as the left hemisphere (Ehrsson et al., 2004, Gentile et al., 2013). Furthermore, evidence from anosognosia for hemiplegia suggests that lesions in the right fronto-parietal network may lead to a generalised bodily awareness impairment, affecting the paralysed as well as the healthy limb (Preston et al., 2010). Further studies should investigate this specific hypothesis regarding laterality.
While the role of proprioception, vision and touch in processing information coming from different body parts is well established, the same is not necessarily true for the vestibular system, which has been predominantly considered responsible for the perception of our body as a whole in space. However, as suggested by Ferrè and Haggard (2016), the vestibular system contributes to processing of bodily signals at different hierarchical levels (i.e. somatosensation, somatoperception and somatorepresentation), including processing of different body parts in space (somatoperception) as well as their attribution to the self (somatorepresentation). Hence, as for every other sensory modality, the vestibular system appears to influence body representation at different levels of the hierarchical multisensory processing, including the ones related to specific body parts (e.g. hand's shape and size, Lopez et al., 2012a, Lopez et al., 2012b). However, no study to date has investigated whether the re-weighting of visual and proprioceptive information following vestibular stimulation, and observed in the current and previous studies in relation to specific body parts, also extends to whole-body illusions. Future studies could address this question.
The comparison between sham and the average of LGVS and RGVS indicated a generic, non-task specific effect of stimulation on proprioceptive drift, during stroking conditions, suggesting that, beyond the polarity specific effects, GVS might also induce a generic decrease in participants’ proprioceptive ability. As suggested by Schmidt et al. (2013), GVS might lead to a decrease in participants’ proprioceptive ability during an arm positioning task. Hence, vestibular stimulation might have an additional effect on multisensory integration by modulating proprioceptive displacement of participants’ hand in relation to the rubber hand (determined as a differential between pre and post proprioceptive judgements) on the top of the rebalancing suggested above. Further research should disentangle the differential effects of vestibular stimulation on basic proprioception and multisensory integration.
By contrast, our vestibular manipulations did not reveal any specific effects on subjective ratings of body ownership. The dissociation between subjective and behavioural measures of the RHI has been confirmed by previous studies (Abdulkarim and Ehrsson, 2016, Makin et al., 2008, Rohde et al., 2011). In our case, the change in proprioceptive drift was not accompanied by a similar change in felt ownership. This was true also for the visual capture conditions: additional analyses on questionnaire values (see Supplementary Materials, Section 2.4) revealed that visual capture conditions did not differ significantly from asynchronous conditions and were significantly lower than the synchronous conditions in all the different GVS configurations (LGVS, RGVS, Sham and L+R/2), thus suggesting that this condition did not lead to the embodiment of the rubber hand. These findings on visual capture are different than similar ones reported by Samad and colleagues (2015): in their study, participants felt ownership of the rubber hand after visual exposure (in absence of stroking) as measured via questionnaires as well as changes in skin conductance. On the contrary, Rohde et al. (2011), reported a significant proprioceptive drift following a no stroking condition in absence of feelings of ownership of the rubber hand (it has to be noted, however, that they did not use a standardised questionnaire but only recorded, anecdotally, differences in ownership following no stroking, synchronous and asynchronous conditions). These differences between the studies will need to be explored in future research, ideally with larger samples to examine whether they relate to individual differences or differences between the experimental designs or set-up of the studies. One such difference may relate to the distance between the real and the rubber hand. Specifically, as discussed in detail below, the distance between real and rubber hand has been shown to play different roles in embodiment and in proprioceptive displacement: whilst greater distances may favour proprioceptive displacement (Preston, 2013), the same may not be true for felt ownership (Samad et al., 2015). Samad and colleagues’ computational model predicts that feelings of ownership during the RHI should fail to occur when the rubber and real hands are further than 30 cm apart. Even though feelings of ownership still occur following synchronous stroking when the real hand and the rubber hand are positioned 35 cm apart (Preston, 2013), the same may not be true when tactile stimulation is absent and does not contribute to multisensory processing. Thus, it may be possible that increasing the distance between real and rubber hand during a mere visual exposure to the latter is enough to abolish the occurrence of feelings of ownership. Further studies should specifically address this possibility.
Furthermore, it is possible that the aforementioned disruption of the egocentric reference frame induced by LGVS did not translate into corresponding feelings of embodiment towards an external object in our setup. This might be due to the fact that the proprioceptive drift is a measure of perceived position in space and it is hence influenced by the relative weighting of vision and proprioception induced by the vestibular activation. On the other hand, as mentioned above, feelings of embodiment might involve additional, higher-order processes that are not affected by the stimulation (Tsakiris, 2010, Martinaud et al., 2017). In the current study, the comparison between sham and the average of the other two GVS configurations indicated that feelings of ownership of the rubber hand were higher during sham, suggesting that GVS possibly reduces subjective (explicit) experiences of body ownership as measured via self-report questionnaires. These findings contrast with Lopez and colleagues (2010), who found that LGVS increased felt ownership towards the rubber hand. We also found that fast touch led to greater subjective embodiment overall, compared with slow touch, irrespective of the synchronicity and type of stimulation (in contrast with Crucianelli et al., 2013 and Lloyd et al., 2013). However, fast stroking did not itself lead to particularly strong embodiment, with scores not above 1 on average (see Ehrsson et al., 2004; Petkova and Ehrsson, 2009; Kalckert and Ehrsson, 2012 for discussions regarding the use of a minimum score for embodiment). These unexpected findings may be explained, at least in part, by variability between studies in the measures (i.e. embodiment questions) used to assess the illusion, and further research is needed to examine these effects on subjective embodiment.
Methodological differences between the current set-up and previous studies may also account for the negative finding on enhancement of the proprioceptive displacement by slow, CT-optimal touch during Sham conditions. In van Stralen and colleagues (2014) the velocities used to administer the stroking were 0.3 cm/s (non CT-optimal), 3 cm/s (CT-optimal) and 30 cm/s (CT-optimal). It may be possible that using markedly different velocities as control conditions (30 cm/s rather than 18 cm/s) would result in differences in behavioural and subjective measures of the RHI. However, the same velocities (3 cm/s and 30 cm/s) have been implemented in Lloyd et al., 2014, but without replication of van Stralen's findings. Our results are in line with the negative findings reported by Crucianelli et al., 2013 as well as Lloyd et al., 2014, i.e. that CT-optimal touch does not enhance proprioceptive displacement (in the absence of vestibular stimulation). However, as mentioned above, we did not replicate their positive findings on enhancement of feelings of embodiment following slow, CT-optimal stroking. In the current study, we used the same velocities (3 cm/s and 18 cm/s) used in previous studies from our group (Crucianelli et al., 2013, Crucianelli et al., 2017); however, we did not use the exact same paradigm in recording embodiment, i.e. we did not have a pre-measurement of embodiment following visual capture as a baseline for each condition. In addition, all these studies involve manual touch and it is possible that there are some experimenter effects, either due to the way the touch is administered or due to other factors such as gender (Gazzola et al., 2012) or attractiveness (unpublished data from our group). Future or meta-analytic studies should investigate the influence of such factors on the various measures of body ownership.
Our findings are in line with the positive trend for LGVS to increase proprioceptive drift reported by Lopez et al. (2010), but contradict findings by Ferrè and colleagues (2015). One possible explanation for the variability in findings is a difference in the GVS protocols used across studies. While Ferrè and colleagues used brief GVS pulses (around 4.5 s), both Lopez et al. and our study implemented longer stimulation windows (1 min in Lopez et al. and 2 min in our study). Interestingly, fMRI studies (Bense et al., 2001, Della-Justina et al., 2015) have shown that longer GVS pulses (of 27.5 and 21 s respectively) caused a decrease in activation of somatosensory areas, thus suggesting a possible inhibition of proprioceptive processing during vestibular stimulation. Stimulation periods of 4.5 s may hence fail to elicit the somatosensory inhibition mentioned above, which might be responsible for the dominance of visual aspects of multisensory processing over proprioceptive ones during longer stimulation windows. We did not directly address this possibility in the current study, and further research is needed.
Finally, several studies have also examined the effects of hand position on body ownership during the RHI (Zopf et al., 2010, Preston, 2013, Kalckert and Ehrsson, 2014). In Ferrè and colleagues, the distance between the rubber and real hands was approximately 20 cm, whereas in Lopez and colleagues the hands were 24.5 cm apart. In the current study, the rubber hand and the real hand were positioned approximately 30 cm apart, both on the left of the participant's midline. Preston (2013) found that similar spatial arrangements (i.e. rubber hand and real hand at approximately 35 cm distance) to our own produced greater differences in proprioceptive drift in synchronous relative to asynchronous conditions of the RHI when compared against other spatial configurations. Existing studies in healthy and clinical populations suggest that GVS affects spatial cognition (see Utz et al., 2010 for a review). Thus, it may be that right-hemisphere vestibular stimulation is especially effective in modulating multisensory integration when the hands are further apart, and closer to left extra-personal space, where the fake hand is less readily incorporated into the body representation. Future research could investigate this possibility.
5. Conclusions
Our study confirmed the suggestion that visual dominance over proprioception is the preferred way through which multisensory conflict is resolved in ambiguous situations. Furthermore, we highlighted the specificity of a right vestibular network for bodily awareness: our findings support the hypothesis of a lateralisation of bodily-related stimuli processing. We also showed that right vestibular stimulation during synchronous tactile stroking modulates multisensory integration such that synchronous touch is more ‘captured’ by vision. Lastly, our results suggest the importance of affective processing in multisensory integration during sensory conflicting situations: slow, affective touch may reduce sensory ambiguity by promoting a visual capture of the seen affective touch.
Conflict of interest
None declared.
Acknowledgements
This work was supported by the European Research Council (ERC) Starting Grant ERC-2012-STG GA313755 (to A.F.) and a University of Hertfordshire studentship (to S.P.).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.neuropsychologia.2018.06.020.
Appendix A. Supplementary material
Supplementary material
References
- Abdulkarim Z., Ehrsson H.H. No causal link between changes in hand position sense and feeling of limb ownership in the rubber hand illusion. Atten. Percept. Psychophys. 2016;78(2):707–720. doi: 10.3758/s13414-015-1016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps M.A., Tsakiris M. The free-energy self: a predictive coding account of self-recognition. Neurosci. Biobehav. Rev. 2014;41:85–97. doi: 10.1016/j.neubiorev.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B., Karnath H.O. Tight link between our sense of limb ownership and self-awareness of actions. Stroke. 2008;39(2):486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Been G., Ngo T.T., Miller S.M., Fitzgerald P.B. The use of tDCS and CVS as methods of non-invasive brain stimulation. Brain Res. Rev. 2007;56:346–361. doi: 10.1016/j.brainresrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Bense S., Stephan T., Yousry T.A., Brandt T., Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI) J. Neurophysiol. 2001;85(2):886–899. doi: 10.1152/jn.2001.85.2.886. [DOI] [PubMed] [Google Scholar]
- Bisiach E., Rusconi M.L., Vallar G. Remission of somatoparaphrenic delusion through vestibular stimulation. Neuropsychologia. 1991;29(10):1029–1031. doi: 10.1016/0028-3932(91)90066-h. [DOI] [PubMed] [Google Scholar]
- Björnsdotter M., Morrison I., Olausson H. Feeling good. On the role of C fiber mediated touch in interoception. Exp. Brain Res. 2010;207:149–155. doi: 10.1007/s00221-010-2408-y. [DOI] [PubMed] [Google Scholar]
- Blanke O., Slater M., Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88:145–166. doi: 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Bottini G., Paulesu E., Gandola M., Loffredo S., Scarpa P., Sterzi R., Santilli I., Defanti C.A., Scialfa G., Fazio F., Vallar G. Left caloric vestibular stimulation ameliorates right hemianesthesia. Neurology. 2005;65(8):1278–1283. doi: 10.1212/01.wnl.0000182398.14088.e8. [DOI] [PubMed] [Google Scholar]
- Botvinick M., Cohen J. Rubber hands' feel' touch that eyes see. Nature. 1998;391(6669):756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Brandt T., Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Ann. N. Y. Acad. Sci. 1999;871:293–312. doi: 10.1111/j.1749-6632.1999.tb09193.x. [DOI] [PubMed] [Google Scholar]
- Brandt T., Glasauer S., Stephan T., Bense S., Yousry T.A., Deutschländer A., Dieterich M. Visual‐vestibular and visuovisual cortical interaction. Ann. N. Y. Acad. Sci. 2002;956(1):230–241. doi: 10.1111/j.1749-6632.2002.tb02822.x. [DOI] [PubMed] [Google Scholar]
- Cappa S., Sterzi R., Vallar G., Bisiach E. Remission of hemineglect and anosognosia during vestibular stimulation. Neuropsychologia. 1987;25:775–782. doi: 10.1016/0028-3932(87)90115-1. [DOI] [PubMed] [Google Scholar]
- Case L.K., Laubacher C.M., Olausson H., Wang B., Spagnolo P.A., Bushnell M.C. Encoding of touch intensity but not pleasantness in human primary somatosensory cortex. J. Neurosci. 2016;36:5850–5860. doi: 10.1523/JNEUROSCI.1130-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case L.K., Laubacher C.M., Richards E.A., Spagnolo P.A., Olausson H., Bushnell M.C. Inhibitory rTMS of secondary somatosensory cortex reduces intensity but not pleasantness of gentle touch. Neurosci. Lett. 2017;653:84–91. doi: 10.1016/j.neulet.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceunen E., Vlaeyen J.W.S., van Diest I. On the origin of interoception. Front. Psychol. 2016;7:743. doi: 10.3389/fpsyg.2016.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini M., Robinson J., Migliorati D., Donno B., Ferri F., Northoff G. Temporal limits on rubber hand illusion reflect individuals' temporal resolution in multisensory perception. Cognition. 2016;157:39–48. doi: 10.1016/j.cognition.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig A.D. Interoception. The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Crucianelli L., Krahe C., Jenkinson P.M., Fotopoulou A.K. Interoceptive ingredients of body ownership: affective touch and cardiac awareness in the rubber hand illusion. Cortex. 2017 doi: 10.1016/j.cortex.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Crucianelli L., Metcalf N.K., Fotopoulou A., Jenkinson P.M. Bodily pleasure matters. Velocity of touch modulates body ownership during the rubber hand illusion. Front. Psychol. 2013:4. doi: 10.3389/fpsyg.2013.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Justina H.M., Gamba H.R., Lukasova K., Nucci-da-Silva M.P., Winkler A.M., Amaro E. Interaction of brain areas of visual and vestibular simultaneous activity with fMRI. Exp. Brain Res. 2015;233:237–252. doi: 10.1007/s00221-014-4107-6. [DOI] [PubMed] [Google Scholar]
- Dieterich M., Bense S., Lutz S., Drzezga A., Stephan T., Bartenstein P., Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb. Cortex. 2003;13(9):994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Dijkerman H.C., De Haan E.H. Somatosensory processes subserving perception and action. Behav. Brain Sci. 2007;30(02):189–201. doi: 10.1017/S0140525X07001392. [DOI] [PubMed] [Google Scholar]
- Ehrsson H.H., Holmes N.P., Passingham R.E. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J. Neurosci. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson H.H., Spence C., Passingham R.E. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305(5685):875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Ernst M.O., Banks M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415:429–433. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- Eulenburg P. zu, Caspers S., Roski C., Eickhoff S.B. Meta-analytical definition and functional connectivity of the human vestibular cortex. NeuroImage. 2012;60:162–169. doi: 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Fasold O., von Brevern M., Kuhberg M., Ploner C.J., Villringer A., Lempert T., Wenzel R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17(3):1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Ferrè E.R., Day B.L., Bottini G., Haggard P. How the vestibular system interacts with somatosensory perception: a sham-controlled study with galvanic vestibular stimulation. Neurosci. Lett. 2013;550:35–40. doi: 10.1016/j.neulet.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrè E.R., Haggard P. The vestibular body: vestibular contributions to bodily representations. Cogn. Neuropsychol. 2016;33:67–81. doi: 10.1080/02643294.2016.1168390. [DOI] [PubMed] [Google Scholar]
- Ferrè E.R., Berlot E., Haggard P. Vestibular contributions to a right-hemisphere network for bodily awareness. Combining galvanic vestibular stimulation and the “Rubber Hand Illusion”. Neuropsychologia. 2015;69:140–147. doi: 10.1016/j.neuropsychologia.2015.01.032. [DOI] [PubMed] [Google Scholar]
- Ferrè E.R., Longo M.R., Fiori F., Haggard P. Vestibular modulation of spatial perception. Front. Hum. Neurosci. 2013:7. doi: 10.3389/fnhum.2013.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetsch C.R., Pouget A., DeAngelis G.C., Angelaki D.E. Neural correlates of reliability-based cue weighting during multisensory integration. Nat. Neurosci. 2011;15:146–154. doi: 10.1038/nn.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G.R., Marshall J.C., Weiss P.H., Stephan T., Grefkes C., Shah N.J., Zilles K., Dieterich M. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame. An fMRI study with clinical implications. NeuroImage. 2003;20:1505–1517. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Folegatti A., Vignemont F., de, Pavani F., Rossetti Y., Farnè A., Harris J. Losing one's hand. Visual-proprioceptive conflict affects touch perception. PLoS One. 2009;4:e6920. doi: 10.1371/journal.pone.0006920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Shiner T., FitzGerald T., Galea J.M., Adams R., Brown H., Dolan R.J., Moran R., Stephan K.E., Bestmann S. Dopamine, affordance and active inference. PLoS Comput. Biol. 2012;8(1):e1002327. doi: 10.1371/journal.pcbi.1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conceptions of the self. Implications for cognitive science. Trends Cogn. Sci. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gazzola, V., Spezio, M.L., Etzel, J.A., Castelli, F., Adolphs, R., Keysers, C., 2012. Primary somatosensory cortex discriminates affective significance in social touch. Proc. Natl. Acad. Sci. USA, 109(25), E1657-E1666. [DOI] [PMC free article] [PubMed]
- Gentile G., Guterstam A., Brozzoli C., Ehrsson H.H. Disintegration of multisensory signals from the real hand reduces default limb self-attribution: an fMRI study. J. Neurosci. 2013;33(33):13350–13366. doi: 10.1523/JNEUROSCI.1363-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L.R., Hoover A.E.N. Disrupting vestibular activity disrupts body ownership. Multisens. Res. 2015;28:581–590. doi: 10.1163/22134808-00002472. [DOI] [PubMed] [Google Scholar]
- Kalckert A., Ehrsson H.H. Moving a rubber hand that feels like your own: a dissociation of ownership and agency. Front. Hum. Neurosci. 2012:6. doi: 10.3389/fnhum.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckert A., Ehrsson H.H. The spatial distance rule in the moving and classical rubber hand illusions. Conscious. Cogn. 2014;30:118–132. doi: 10.1016/j.concog.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Kilteni K., Maselli A., Kording K.P., Slater M. Over my fake body: body ownership illusions for studying the multisensory basis of own-body perception. Front. Hum. Neurosci. 2015;9:141. doi: 10.3389/fnhum.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahé C., Drabek M.M., Paloyelis Y., Fotopoulou A. Affective touch and attachment style modulate pain: a laser-evoked potentials study. Philos. Trans. R. Soc. B. 2016;371(1708):20160009. doi: 10.1098/rstb.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner J.R., DiZio P. Vestibular, proprioceptive, and haptic contributions to spatial orientation. Annu. Rev. Psychol. 2005;56:115–147. doi: 10.1146/annurev.psych.55.090902.142023. [DOI] [PubMed] [Google Scholar]
- Lloyd D.M., Gillis V., Lewis E., Farrell M.J., Morrison I. Pleasant touch moderates the subjective but not objective aspects of body perception. Front. Behav. Neurosci. 2013:7. doi: 10.3389/fnbeh.2013.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Longo M.R., Schüür F., Kammers M.P., Tsakiris M., Haggard P. What is embodiment? A psychometric approach. Cognition. 2008;107:978–998. doi: 10.1016/j.cognition.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Lopez C. Making sense of the body. The role of vestibular signals. Multisens. Res. 2015;28:525–557. doi: 10.1163/22134808-00002490. [DOI] [PubMed] [Google Scholar]
- Lopez C. The vestibular system. Curr. Opin. Neurol. 2016;29:74–83. doi: 10.1097/WCO.0000000000000286. [DOI] [PubMed] [Google Scholar]
- Lopez C., Blanke O., Mast F.W. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience. 2012;212:159–179. doi: 10.1016/j.neuroscience.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Lopez C., Lenggenhager B., Blanke O. How vestibular stimulation interacts with illusory hand ownership. Conscious. Cogn. 2010;19:33–47. doi: 10.1016/j.concog.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Lopez C., Schreyer H.-M., Preuss N., Mast F.W. Vestibular stimulation modifies the body schema. Neuropsychologia. 2012;50:1830–1837. doi: 10.1016/j.neuropsychologia.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Makin T.R., Holmes N.P., Ehrsson H.H. On the other hand: dummy hands and peripersonal space. Behav. Brain Res. 2008;191:1–10. doi: 10.1016/j.bbr.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Martinaud O., Besharati S., Jenkinson P.M., Fotopoulou A. Ownership illusions in patients with body delusions: different neural profiles of visual capture and disownership. Cortex. 2017;87:174–185. doi: 10.1016/j.cortex.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone F., Olausson H., Boyle J.A., Jones‐Gotman M., Dancer C., Guest S., Essick G. Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. Eur. J. Neurosci. 2012;35(11):1782–1788. doi: 10.1111/j.1460-9568.2012.08092.x. [DOI] [PubMed] [Google Scholar]
- McGlone F., Reilly D. The cutaneous sensory system. Neurosci. Biobehav. Rev. 2010;34:148–159. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- McGlone F., Wessberg J., Olausson H. Discriminative and affective touch. Sensing and feeling. Neuron. 2014;82:737–755. doi: 10.1016/j.neuron.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Moro V., Pernigo S., Tsakiris M., Avesani R., Edelstyn N.M., Jenkinson P.M., Fotopoulou A. Motor versus body awareness: voxel-based lesion analysis in anosognosia for hemiplegia and somatoparaphrenia following right hemisphere stroke. Cortex. 2016;83:62–77. doi: 10.1016/j.cortex.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Morrison I., Bjornsdotter M., Olausson H. Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J. Neurosci. 2011;31:9554–9562. doi: 10.1523/JNEUROSCI.0397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I., Löken L.S., Minde J., Wessberg J., Perini I., Nennesmo I., Olausson H. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain. 2011;134:1116–1126. doi: 10.1093/brain/awr011. [DOI] [PubMed] [Google Scholar]
- Naito E., Roland P.E., Grefkes C., Choi H.J., Eickhoff S., Geyer S., Zilles K., Ehrsson H.H. Dominance of the right hemisphere and role of area 2 in human kinesthesia. J. Neurophysiol. 2005;93(2):1020–1034. doi: 10.1152/jn.00637.2004. [DOI] [PubMed] [Google Scholar]
- Olausson H., Lamarre Y., Backlund H., Morin C., Wallin B.G., Starck G., Ekholm S., Strigo I., Worsley K., Vallbo Å.B., Bushnell M.C. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Pavani F., Spence C., Driver J. Visual capture of touch: out-of-the-body experiences with rubber gloves. Psychol. Sci. 2000;11(5):353–359. doi: 10.1111/1467-9280.00270. [DOI] [PubMed] [Google Scholar]
- Pawling R., Cannon P.R., McGlone F.P., Walker S.C. C-tactile afferent stimulating touch carries a positive affective value. PLoS One. 2017;12:e0173457. doi: 10.1371/journal.pone.0173457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova V.I., Ehrsson H.H. When right feels left: referral of touch and ownership between the hands. PLoS One. 2009;4(9):e6933. doi: 10.1371/journal.pone.0006933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer C., Serino A., Blanke O. The vestibular system. A spatial reference for bodily self-consciousness. Front. Integr. Neurosci. 2014;8:219. doi: 10.3389/fnint.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer C., Noel J.P., Serino A., Blanke O. Vestibular modulation of peripersonal space boundaries. Eur. J. Neurosci. 2018;47(7):800–811. doi: 10.1111/ejn.13872. [DOI] [PubMed] [Google Scholar]
- Preston C., Jenkinson P.M., Newport R. Anosognosia for hemiplegia as a global deficit in motor awareness: evidence from the non-paralysed limb. Neuropsychologia. 2010;48(12):3443–3450. doi: 10.1016/j.neuropsychologia.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Preston C. The role of distance from the body and distance from the real hand in ownership and disownership during the rubber hand illusion. Acta Psychol. 2013;142:177–183. doi: 10.1016/j.actpsy.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Rock I., Victor J. Vision and touch: an experimentally created conflict between the two senses. Science. 1964;143:594–596. doi: 10.1126/science.143.3606.594. [DOI] [PubMed] [Google Scholar]
- Rohde M., Di Luca M., Ernst M.O. The Rubber Hand Illusion: feeling of ownership and proprioceptive drift do not go hand in hand. PLoS One. 2011;6:e21659. doi: 10.1371/journal.pone.0021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad M., Chung A.J., Shams L. Perception of body ownership is driven by Bayesian sensory inference. PLoS One. 2015;10(2):e0117178. doi: 10.1371/journal.pone.0117178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L., Artinger F., Stumpf O., Kerkhoff G. Differential effects of galvanic vestibular stimulation on arm position sense in right-vs. left-handers. Neuropsychologia. 2013;51(5):893–899. doi: 10.1016/j.neuropsychologia.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Shaikh S., Nagi S.S., McGlone F., Mahns D.A., Bensmaia S.J. Psychophysical investigations into the role of low-threshold C fibres in non-painful affective processing and pain modulation. PLoS One. 2015;10:e0138299. doi: 10.1371/journal.pone.0138299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B.E., Stanford T.R., Rowland B.A. Development of multisensory integration from the perspective of the individual neuron. Nat. Rev. Neurosci. 2014;15:520–535. doi: 10.1038/nrn3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triscoli C., Olausson H., Sailer U., Ignell H., Croy I. CT-optimized skin stroking delivered by hand or robot is comparable. Front. Behav. Neurosci. 2013:7. doi: 10.3389/fnbeh.2013.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Hesse M.D., Boy C., Haggard P., Fink G.R. Neural signatures of body ownership. A sensory network for bodily self-consciousness. Cereb. Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Utz K.S., Dimova V., Oppenländer K., Kerkhoff G. Electrified minds. Transcranial direct current stimulation (tDCS) and Galvanic Vestibular Stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—A review of current data and future implications. Neuropsychologia. 2010;48:2789–2810. doi: 10.1016/j.neuropsychologia.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Utz K.S., Keller I., Kardinal M., Kerkhoff G. Galvanic vestibular stimulation reduces the pathological rightward line bisection error in neglect—a sham stimulation-controlled study. Neuropsychologia. 2011;49(5):1219–1225. doi: 10.1016/j.neuropsychologia.2011.02.046. [DOI] [PubMed] [Google Scholar]
- Utz K.S., Korluss K., Schmidt L., Rosenthal A., Oppenländer K., Keller I., Kerkhoff G. Minor adverse effects of galvanic vestibular stimulation in persons with stroke and healthy individuals. Brain Inj. 2011;25(11):1058–1069. doi: 10.3109/02699052.2011.607789. [DOI] [PubMed] [Google Scholar]
- Vallar G., Bottini G., Rusconi M.L., Sterzi R. Exploring somatosensory hemineglect by vestibular stimulation. Brain. 1993;116:71–86. doi: 10.1093/brain/116.1.71. [DOI] [PubMed] [Google Scholar]
- van Beers R.J., Wolpert D.M., Haggard P. When feeling is more important than seeing in sensorimotor adaptation. Curr. Biol. 2002;12:834–837. doi: 10.1016/s0960-9822(02)00836-9. [DOI] [PubMed] [Google Scholar]
- van Stralen H.E., van Zandvoort M.J., Hoppenbrouwers S.S., Vissers L.M., Kappelle L.J., Dijkerman H.C. Affective touch modulates the rubber hand illusion. Cognition. 2014;131:147–158. doi: 10.1016/j.cognition.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Wilkinson D., Zubko O., Sakel M., Coulton S., Higgins T., Pullicino P. Galvanic vestibular stimulation in hemi-spatial neglect. Front. Integr. Neurosci. 2014:8. doi: 10.3389/fnint.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller D., Gross C., Bartsch A., Johansen-Berg H., Classen J. Ventral premotor cortex may be required for dynamic changes in the feeling of limb ownership: a lesion study. J. Neurosci. 2011;31(13):4852–4857. doi: 10.1523/JNEUROSCI.5154-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller D., Litvak V., Friston K.J., Classen J. Sensory processing and the rubber hand illusion—an evoked potentials study. J. Cogn. Neurosci. 2015;27(3):573–582. doi: 10.1162/jocn_a_00705. [DOI] [PubMed] [Google Scholar]
- Zopf R., Savage G., Williams M.A. Crossmodal congruency measures of lateral distance effects on the rubber hand illusion. Neuropsychologia. 2010;48:713–725. doi: 10.1016/j.neuropsychologia.2009.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material