Graphical Abstract

Keywords: Vitamin D deficiency, Fetuin B, Obesity, Proteomics, Vitamin D receptor
Highlights
-
•
via proteomics, FETUB was identified/confirmed to be higher in VDD obese children.
-
•
VD can directly downregulate hepatocellular FETUB synthesis in vitro.
-
•
VDR silencing in vitro releases FETUB, suggesting VDR is a negative regulator.
-
•
VD-supplementation to juvenile mice for 6 weeks, reduced circulating FETUB in vivo.
-
•
Plasma FETUB levels likely depend on other VD-responsive tissues, with the liver.
Abstract
Vitamin D (VD) deficiency (VDD) correlates to obesity, with VD a recognized mediator of metabolic diseases. From a previous proteomic study identifying adiponectin as a link between VDD and pediatric obesity, herein we analysed another protein (SSP2301) increased with VDD. A focused 2D-electrophoretic analysis identified 4 corresponding plasma proteins, with one predicted to be fetuin B (FETUB). FETUB was studied due to its emerging role in metabolic diseases and cytogenetic location (3q27.3) with adiponectin. Results were confirmed in obese children, where plasma FETUB was higher with VDD. A direct effect by 1α,25-(OH)2D3 on hepatocellular FETUB synthesis was observed, with a time and dose dependent reduction. Further, we demonstrated the VD-receptor (VDR) is key, with FETUB “released” with VDR silencing. Finally, VD supplementation (6weeks) to juvenile mice fed a standard diet, reduced plasma FETUB. Only at 22weeks did liver FETUB correspond to plasma FETUB, highlighting the contribution of other VD-responsive tissues. Overall, FETUB is a key protein linking VDD to pediatric obesity. With an emerging role in metabolic diseases, we demonstrate that VD/VDR directly regulate FETUB.
1. Introduction
Lifestyle-related diseases have reached pandemic levels on a global scale. Vitamin D (VD) deficiency (VDD) is amongst these having a high prevalence in underdeveloped and developed countries, and encompassing both pediatric and adult populations [1,2]. While the historic role of VD is related to skeletal health, in recent years VDD has been linked to numerous chronic conditions such as obesity, autoimmune disease, type 2 diabetes mellitus, dyslipidemia, non-alcoholic fatty liver disease (NAFLD), cardiovascular disease (CVD) and the metabolic syndrome, with an increase in mortality following a J or U-shaped effect [[1], [2], [3], [4], [5], [6]]. Overall, VDD is emerging as a major mediator of metabolic diseases and long-term metabolic outcomes. This is particularly relevant with respect to obese children and their future outcomes.
The vitamin D system encompasses a group of fat soluble prohormones, with the two major forms being ergocalciferol (VD2) and cholecalciferol (VD3). The main source of VD is from endogenous production, whereby UV-B from the sun converts 7-dehydrocholesterol in the skin, while diet-derived VD contributes for 20% [1]. In vivo, VD3 and VD2 are metabolized by the liver to produce 25-dihydroxyvitamin D3 (25-OHD3) or 25-OHD2. These metabolites are then further metabolized by the kidney or extra-renal 1α-hydroxylase (CYP27B1) to produce the bioactive forms 1α,25-(OH)2D3 and 1α,25-(OH)2D2, which function as pleiotropic hormones capable of controlling gene expression and the regulation of proliferation, differentiation, cell survival and metabolism [1]. These pleiotropic actions are principally regulated by the cytosolic/nuclear vitamin D receptor (nVDR) signal-transduction pathways and VD responsive elements (VDRE) found on key genes [7,8]. Rapid non-genomic VD responses occur either via membrane VDR (mVDR), 1,25D3-membrane associated rapid response to steroids receptor (1,25D3-MARRS), or other tyrosine kinase receptors localized in the plasma membrane [7,8]. While data are limited with respect to alternate receptors, the discovery that most tissues and cells express VDR and that VD regulates more than 700 genes, has further highlighted an evident and extensive extra-skeletal and tissue-specific role for VD [9].
With the demonstration that VDD rates closely resemble those of overweight and obesity [10], evidence is accumulating to suggest that there are clear links between obesity and suboptimal VD levels [9,11,12]. For example, an inverse relationship has been repeatedly demonstrated between high body fat and low serum 25-OHD in both adults and children, regardless of the index for body fat used, including BMI [11,[13], [14], [15]], waist circumference [16,17], subcutaneous adipose tissue (sAT [16,18]) and visceral adipose tissue [16,18]. Abnormalities have been observed in adipose tissue (AT)-specific metabolism of VD in adult obese subjects, with the SAT VD metabolic enzymes significantly reduced in adult obese individuals and circulating VD levels increasing by 27% with weight loss [19]. Evidence from a bi-directional Mendelian randomized analysis of 21 adult cohorts (42,024 subjects), suggests that a higher BMI is causal for reduced circulating 25OHD levels [11]. With respects to children, cross-sectional studies have primarily focused on classical cardiometabolic risk factors such as blood pressure, fasting glucose and lipids [20,21], with studies demonstrating a strong association of VDD to hypertension, diabetes and prediabetes [15,[22], [23], [24], [25]]. None of these studies have been able to decipher the metabolic signals that link a poor VD status to obesity [26,27].
To identify potential metabolic signals that could link obesity and its associated complications to VD status, a proteomic approach was previously used to study the proteome-wide plasmatic changes between VDD and VD sufficient (VDS) obese pediatric subjects, where 53 plasmatic proteins were identified to be differentially altered between the two groups [28]. Amongst the top “ten” most significant spots, we identified that the multimeric forms of adiponectin, particularly the high molecular weight (HMW) form, are biomarkers that link VDD and pediatric obesity, with significantly reduced levels observed in VDD subjects.
While an important finding in that adiponectin regulates several important metabolic pathways including fatty acid breakdown and glucose levels [29], it is likely that the molecular links connecting VD status to obesity involve a “concert” of events. Considering the complex nature of VD regulation, we chose in the present investigation to focus on another of the most significant spots previously identified [28]. Herein we observed that SSP2301, in contrast to adiponectin, was significantly upregulated in VDD subjects. With a more focused proteomic analysis we identified this spot as corresponding to fetuin B (FETUB), an emerging cardiovascular and diabetic risk factor, and confirmed that it is significantly upregulated in VDD subjects. Located on the same cytogenetic band as adiponectin, herein we demonstrate that VD acts directly in downregulating FETUB levels both in vitro and in vivo, with the VDR working as a key negative regulator of FETUB synthesis.
2. Methods
2.1. Subjects
Children and adolescents between the age of 5–18 years were retrospectively recruited from an observational study on pediatric obesity, approved by our Local Ethical Committee (Ethics Committee University Hospital “AOU Maggiore della Carità” di Novara; protocol 199/CE; study CE 14/11; www.maggioreosp.novara.it). The protocol was conducted in accordance with the declaration of Helsinki of 1975, as revised in 1983. The purpose of the study was carefully explained, and a written informed consent was obtained from all parents before the evaluations. Eligible subjects for the study had a body mass index (BMI) exceeding the 95th percentile, according to the Italian growth charts [30], were naïve to diet therapy at the time, presented with 25OHD concentrations < 20.0 ng/ml (deficiency; VDD) or > 30 ng/ml (sufficiency; VDS) according to the Endocrine Society Guidelines [31]. Excluded from the study were subjects with intermediate 25OHD concentrations (20.0–30.0 ng/ml), to avoid a possible interference of VD insufficiency. Exclusion criteria also included diabetes mellitus, the use of drugs which could interfere with VD, glucose or lipid metabolism, blood pressure or appetite, as well as endocrine or genetic obesity or a low birth weight. A total of 60 subjects (VDD, n = 31; VDS, n = 29) of our dataset who met all the above criteria, were selected for the proteomic analysis; one subject was later excluded from the VDD group. Following the proteomic results, the study cohort was increased to include a total of 122 subjects (VDD, n = 76; VDS, n = 46) for both ELISA and western immunoblotting analyses. The whole group was also sub-grouped according to the degree of VD status as severe-VDD (≤10 ng/ml) or moderate-VDD (10–20 ng/ml) and VDS [31].
2.2. Anthropometric and biochemical measurements
All the subjects underwent a clinical evaluation according to the Italian growth charts [30]. Pubertal stages were determined by a dedicated group of physicians, using the criteria of Marshall and Tanner [32]. Height was measured to the nearest 0.1 cm using a Harpenden stadiometer. Weight with light clothing to the nearest 0.1 kg by using a manual scale. BMI was calculated as body weight divided by squared height (kg/m2). BMI standard deviation score (BMISDS) was calculated with the LMS method [30]. Waist circumference (WC) to the nearest 0.1 cm was measured at the high point of the iliac crest around the abdomen. Prior to other physical evaluations, the systolic (SBP) and diastolic (DBP) blood pressure were measured using a standard mercury sphygmomanometer. An average of three measurements on the left arm after 15 min at rest in the supine position, were used for analyses. An estimate of UV radiation was calculated as monthly UV radiation (kJ/m2) values obtained from ENEA (Italian National Agency for New Technologies, and Sustainable Economic Development) grids (www.solaritaly.enea.it). We used the mean of the last 3 months (UVR3), according to a previous study [33].
Following a 12 h overnight fast, morning blood samples for proteomic analyses, glucose, insulin, lipids, parathyroid hormone (PTH), alkaline phosphatase (ALP), phosphorus, calcium, 25OHD and FETUB were obtained. Insulin resistance was calculated using the HOMA-IR index. Glucose was expressed in mg/dl (1 mg/dl:0,05551 mMol/liter) and insulin in μIU/ml (1 μIU/ml = 7.175 pmol/l). All routine measurements were performed in the hospital’s analysis laboratory using standardized methods. VD as 25OHD serum concentrations (ng/ml), were assayed by a direct competitive chemiluminescent immunoassay with a CV value of 4% and analytical range of 4–150 ng/ml (Liaison® Test 25OHD total, DiaSorin Inc., Stillwater MN-USA). Parathyroid hormone (PTH) concentrations were assayed by chemiluminescence immunoassay LIAISON®N-TACT®PTH II Assay with an analytical range of 3–1900 pg/ml and CV of 3.4% (DiaSorin Inc). Human total FETUB concentrations (ng/ml) were measured by ELISA according to the manufacturer’s instructions (BioVendor, Brno, Czech Republic), with the intra-assay and inter-assay coefficients of variation <10% and <12%, respectively. The sensitivity of the assay was 36.4 pg/ml.
2.3. D-Electrophoresis (2-DE) and image analysis
To prepare platelet-free plasma for 2-DE, all samples were centrifuged at 1300 rpm, 4 °C for 10 min followed by a second centrifugation at 2400 rcf 4 °C for 15 min, with storage at −80 °C. Plasma protein concentrations were determined using the DC Protein Assay (BioRad, Hercules, CA). To reduce biological variation in the proteomic analysis, >12 subjects per group were analyzed as recommended [34]. Duplicate 2-DE analyses using 7 cm immobilized pH gradient (IPG) 4–7 strips and 10% SDS-polyacrylamide gels (SDS-PAGE), were performed and analyzed, according to our previous study [28]. Only those spots that showed a statistically significant difference (p < 0.05) between VDD and VDS, were chosen for PDQuest isoelectric point (pI) and molecular weight (MW) estimations and eventual identification.
2.4. Mass spectrometry characterization
From the image analysis, spots of interest were cut and digested in-gel with 20 ug/mL trypsin (Sigma) at 37 °C O/N for liquid chromatography tandem mass spectrometry (LC–MS/MS) analysis. The LC–MS/MS analyses were performed by a micro-LC Eksigent Technologies (Dublin, USA) system that included a micro LC200 Eksigent pump with flow module 5–50 μL, a programmable autosampler CTC PAL with a Peltier unit (1-45 °C). The LC system was interfaced with a 5600+ TripleTOFTM system (AB Sciex, Concord, Canada) equipped with DuoSprayTM Ion Source and CDS (Calibrant Delivery System). The data was acquired with Analyst TF 1.7 (AB SCIEX, Concord, Canada). Files were searched using ProteinPilot software v. 4.2 (ABSciex) with the Paragon algorithm. Protein spots were analyzed with the following parameters taken into consideration: cysteine alkylation, digestion by trypsin, no special factors and False Discovery Rate of 1%. Sequence identification was done using UniProt Swiss-Prot database containing human proteins (version 2015.06.09 reviewed, containing 20,207 sequence entries).
2.5. Animal Model
Juvenile C57BL/6 mice (5weeks; Charles River, Calco, LC) were liberally fed with either a standard diet (STD) or high fat diet (HFD; 60% energy from fat, Mucedola S.r.l, Settimo Milanese, MI, Italy) supplemented with 1000 IU vitamin D3 (10,000 IU/mL; 100 u l/mouse; Abiogen Pharma SpA, Pisa, PI, Italy), or vehicle (STD, n = 7; STDVD, n = 7; HFD, n = 5; HFDVD, n = 5) via gavage three times a week (wk) for up to 6 wks. The dosage of vitamin D3 (VD3) and intermittent administration are based on previous published studies, with a minimal dosage selected for the present investigation [35]. Baseline weights (gm) as well as weight gain (gm) and glucose measurements (mg/dl; caudal vein puncture with Breeze 2 measurements; Bayer, Leverkusen, Germany), were taken in each mouse each week up to the time of sacrifice. A second “long term” group of mice (STD; n = 5; STDVD; n = 5: HFD; n = 5: HFDVD; n = 5) performed the same protocol for up to 22 weeks. At the completion of the protocol, the mice were anesthetized with Avertine (tribromoethanol, 250 mg/kg, Sigma) and sacrificed, with plasma and liver tissue samples collected, snap frozen and stored at −80 °C. All the procedures in mice were approved by the local Ethics Committee for Animal Welfare (IACUC No. 583; IRCCS Oespedale San Raffaelle) and were carried out in compliance with the European and National regulations.
2.6. Sample preparation and western immunoblot
Where described, whole cell lysates and mouse liver tissue samples were prepared using RIPA buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% NP40, 1% sodium deoxycholate, 0.1% SDS, 0.5 mM sodium orthovanadate, 1X SIGMAFAST EDTA free protease inhibitor cocktail [Sigma Aldrich, Saint Louis, MO]) with concentrations determined using the Pierce BCA Protein Assay (Thermo Scientific, Rockford, IL). Independent of the experiments performed, all samples were size-fractionated on 10% SDS-PAGE under reducing conditions and electro-transferred to immuno-blot polyvinylidene difluoride (PVDF) membrane (BioRad). Membranes were incubated with polyclonal anti-FETUB or fibrinogen γ antibodies (SantaCruz, Hercules, CA) and visualized with the appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz). Total protein in all experiments was assessed by Ponceau S staining and used for normalization (Sigma). Immunoreactive proteins were detected using enhanced chemiluminescence (Thermo Scientific, Pierce Biotechnology, Rockford, IL) with image capture performed using either a ChemiDoc Imager or ChemiDoc Touch Imaging System (BioRad). Results were quantified using QuantityOne or Image Lab Version 5.2.1 software, where the values are presented as arbitrary units (AU).
2.7. PNGase F digestions
Representative plasma samples from the two study groups (n = 3) were de-glycosylated using N-glycosidase F (PNGaseF) deglycosylation kit (Sigma) according to the manufacturer’s instructions. Briefly, for each reaction 3 ul of plasma was digested either with or w/o the enzyme (0.5U PNGaseF) at 37 °C O/N. Samples were removed, diluted in SDS/PAGE reducing buffer and FETUB was analyzed by western immunoblot.
2.8. Cell culture and treatments
To address the direct effect of 1α,25-(OH)2D3 on FETUB synthesis and secretion, the human HepG2 and HUH7 hepatocellular carcinoma cell lines were utilized (European Collection of Cell Cultures). Each cell line (HepG2, HUH7) were plated at 0.3 × 106 cells/6 well plate in their maintenance medium (Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 1% penicillin/streptomycin; Sigma) until 60% confluency. Following serum clearance, each of the cell lines was then treated with serum-free medium (SFM) with an equal volume of vehicle (ethanol), or SFM with 10−9 M to 10−7 M 1α,25-(OH)2D3 (Sigma) and left to incubate for up to 48 h. At completion, the conditioned medium was centrifuged at 1000 rpm 4 C, collected and stored at −20 °C prior to western immunoblotting. Likewise, in the time course experiments, aliquots of conditioned medium were removed at the indicated intervals for up to 24 h, centrifuged and stored at −20 °C.
2.9. VDR Silencing
To silence VDR expression in hepatocellular carcinoma cells, five lentiviral vectors expressing diverse shRNA with the gene for puromycin selection, were utilized (505; NM000376.1-923s1c1: 506; NM000376.1-578s1c1: 542; NM000376.1-623s21c1: 543; NM000376.1-1878s21c1: 544; NM000376.1-885s21c1; Sigma). Lentiviral vectors (EB8, EB10) generated inhouse with the promoter for phosphoglycerate kinase (PGK) driving green fluorescent protein (GFP) and puromycin genes, were used as controls. Lentiviral (LV) vectors were generated and expanded [36], with each viral vector tested in both HUH7 and HepG2 cells to optimize transduction and puromycin selection-efficiency. A total of 1 × 105 cells/6 well plate, were infected with a 1/10 dilution of each lentiviral vector for 48 h, at which time the cells were selected using increasing concentrations of puromycin (1–10 ug/ml; Sigma). The LV transduction was assessed by GFP expression in control cells using fluorescence-activated cell sorting (FACS; FACSCalibur BF-FACS3, BD Biosciences, San Jose, CA), with integration assessed by PCR of gDNA using the following primer pairs (shRNA 505 and 506: foward 5′- CAACCTCCCCTTCTACGAGC-3′ and reverse 5′- GGCTAAGATCTACAGCTGCCTTG – 3′: shRNA 542, 543, 544, EB8 and EB10: foward 5′- TTGCTTCCCGTATGGCTTTC-3′ and reverse 5′- GGCTAAGATCTACAGCTGCCTTG – 3′ GAPDH; foward 5′- AACGTGTCAGTGGTGGACCTG-3′and reverse 5′- AGTGGGTGTCGCTGTTGAAGT-3′). All experiments were subsequently performed in HepG2 cells under puromycin selection at 3 mg/ml. Where described, the shVDR HepG2 clones (505, 506, 542, 543 and 544), EB8 and EB10 controls, and HepG2 cells were plated 0.3 × 106 cells/6 well plate and were left to reach 90% confluency, at which time cell lysates were prepared for western immunoblot analysis. Likewise, for the inhibition of endocytosis, the shVDR HepG2 clones (505, 506, 542, 544), EB8 control and HepG2 cells were plated 0.5 × 105 cells/12 well plate in maintenance medium. At 60% confluency, they were treated dose dependently with 1–10 ug/ml of Filipin (Sigma), or 5–10 ug/ml of chlorpromazine hydrochloride (CPZH) with the appropriate excipient for 1hr in serum free medium to block endocytosis. Following 1hr, the inhibitors were replaced with growth medium for a further 24 h, at which time cell lysates were prepared for the analysis of FETUB by western immunoblot.
2.10. Statistical analysis
Data are expressed as mean ± SD, where indicated. Where necessary, skewed variables were logarithmically transformed prior to analyses. Comparisons between groups, treatments and in vitro studies were analyzed by an unpaired Student’s t-test or ANCOVA with age, sex, puberty, log UVR3, BMI (model 1) or WC (model 2) as covariates in patient analyses. Correlations between 25OHD, parathyroid hormone (PTH) or FETUB and clinical and biochemical data were examined using Pearson’s correlation coefficients. A repeated measured ANOVA was used to determine differences in circulating FETUB between severe VDD, VDD and VDS. Statistical significance was assumed for p < 0.05. The statistical analyses were performed with SPSS for Windows version 17.0 (SPSS; Chicago, IL).
3. Results
3.1. Baseline evaluations: VDD and VDS pediatric obese subjects
A clear predominance of VDD in our pediatric obese cohort was evident with 62.3% of all subjects recruited having < 20 ng/ml 25OHD concentrations. As the median 25OHD concentrations were 11.6 ± 2.6 ng/ml for the entire VDD cohort, we used this value to further dichotomize the VDD group as severe- or moderate-VDD for sub-analyses. The clinical and biochemical characteristics of all VDD (n = 76) and VDS (n = 46) pediatric obese subjects included in the study are shown in Table 1. Age and Tanner stages were similar between the VDS and VDD subjects. Baseline evaluations demonstrated that VDD subjects had higher BMI (p < 0.004), BMISDS (p < 0.0001), WC (p < 0.0002), SBP (p < 0.007), DBP (p < 0.03), fasting glucose (p < 0.005) and PTH (p < 0.05) concentrations, as described previously [28].
Table 1.
Clinical and biochemical data for the patient cohort according to vitamin D status.
| Variable | VDS | VDD |
|---|---|---|
| Age (yrs) | 10.8 ± 3.0 | 11.6 ± 2.9 |
| Sex (M/F) | 17/29 | 38/38 |
| Puberty (PP/P) | 16/30 | 22/54 |
| BMI (Kg/m2) | 27.5 ± 4.6 | 29.4 ± 4.2** |
| BMISDS (Kg/m2) | 2.017 ± 0.513 | 2.200 ± 0.438** |
| WC (cm) | 88.7 ± 12.7 | 94.6 ± 11.3** |
| SBP (mmHg) | 123.5 ± 14.5 | 129.8 ± 15.4** |
| DBP (mmHg) | 80.9 ± 9.5 | 84.4 ± 11.1* |
| T-c (mg/dl) | 137.0 ± 26.4 | 144.1 ± 27.3 |
| HDL-c (mg/dl) | 41.0 ± 10.8 | 40.5 ± 9.3 |
| TG (mg/dl) | 77.4 ± 40.1 | 87.6 ± 44.3 |
| Glucose (mg/dl) | 86.3 ± 7.2 | 90.2 ± 7.3** |
| Insulin (mIU/L) | 14.4 ± 8.5 | 18.6 ± 23.0 |
| HOMA-IR | 3.1 ± 1.9 | 3.6 ± 2.3 |
| 25OHD (ng/ml) | 37.9 ± 2.8 | 11.6 ± 2.6*** |
| FETUB (ng/ml) | 89.3 ± 12.7 | 78.9 ± 10.4*** |
| ALP (IU/L) | 501.3 ± 196.2 | 564.0 ± 254.5 |
| Calcium (mg/dl) | 9.1 ± 0.5 | 9.2 ± 0.4 |
| Phosforus (mg/dl) | 4.5 ± 0.5 | 4.7 ± 0.6 |
| PTH (pg/ml) | 15.5 ± 5.1 | 20.2 ± 10.0* |
Data comparing VDS and VDD subjects are expressed as mean ± SD. * p < 0.05; ** p < 0.01; *** p < 0.0001. Abbreviations. PP, pre-pubertal. P, pubertal. ALP, alkaline phosphatases. AU, arbitrary unit. BMI, body mass index. BMISDS, BMI standard deviation score. HDL-c, HDL cholesterol. HOMA-IR, homeostatic model assessment insulin resistance. T-c, total cholesterol. TG, triglycerides. WC, waist circumference.
3.2. Identification of plasma proteins upregulated between VDD and VDS pediatric obese subjects
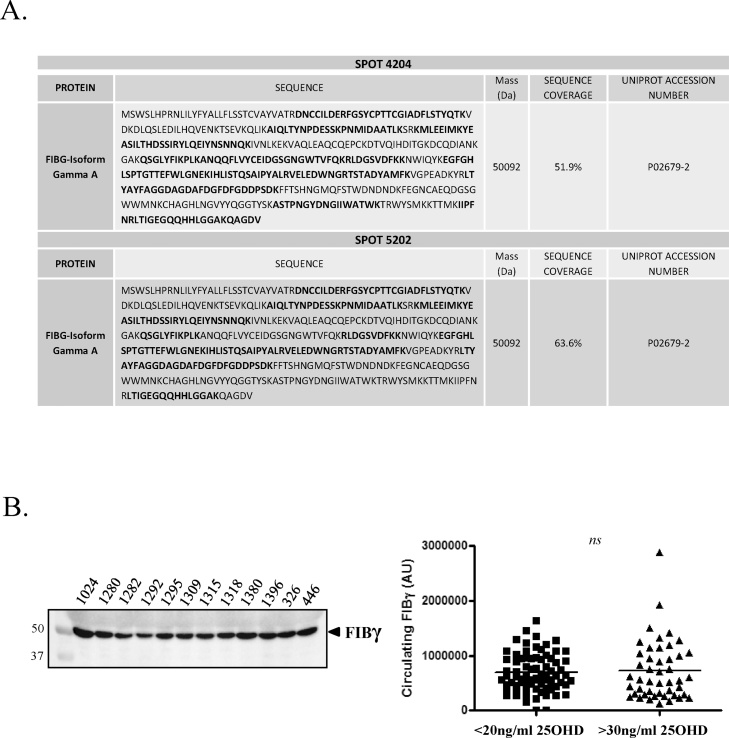
In a previous proteomic analysis (IPG3-10) in plasma from VDD and VDS obese pediatric subjects, amongst the “top ten” protein spots identified, we found that SSP2301 was significantly higher in VDD subjects (p < 0.04; [28];). Localized within a protein cluster of highly abundant plasma proteins, PDQuest estimated SSP2301 to have a MW 55 kDa and a pI5 [28]. Based on these estimates, we sought to investigate this spot by performing a more focused 2DE analysis using IPG 4–7 strips to increase the spot resolution, and increasing our subject cohort to improve the statistical outcome. From a total of 60 subjects (VDD, n = 30; VDS, n = 29) analyzed by 2DE and corrected for Sypro-Ruby background anomalies, 43 “spots” were identified by PDQuest to be differentially expressed between the two groups (p < 0.1), of which 21 were considered significant (p < 0.05; Supplementary Table 1). Of these 21 spots, 4 localized within a 10% range corresponding to the estimated MW and pI of SSP2301, and each was detected at significantly higher circulating levels in VDD subjects (Fig. 1; Table 2). These four spots (SSP 3302, 4202, 4204, 5202) were subsequently analyzed by LC–MS/MS, where SSP 4204 and 5202 where both identified to be Fibrinogen γA/γ’ with a MW = 50.09 kDa (Fig. 2A).
Fig. 1.
Proteomic evaluation highlights four spots significantly higher in the plasma of VDD within the pI and MW range of SSP2301. A 2D-electrophoretic analysis was performed in duplicate for 60 subjects using IPG4–7, with proteins detected by Sypro Ruby staining. Spot/s found to be higher in the plasma of VD deficient (VDD; n = 31) subjects as opposed to VD sensitive (VDS; n = 29) subjects within the pI and MW range of SSP2301, are indicated by the PDQuest identification number SSP3302, SSP4202, SSP4204 and SSP5202. Supportive evidence for the upregulation of SSP3302 and 4202, is shown by 3D images of 2D-gels. Representative gels are shown.
Table 2.
Significantly modulated plasma proteins between VDD and VDS obese pediatric subjects.
| PDQuest ID SSP | MW kDa* | pI* | VDD (n = 31) (AU) | VDS (n = 29) (AU) | P-value |
|---|---|---|---|---|---|
| 3302 | 51 | 5.2 | 432.7 | 207.3 | 0.049 |
| 4202 | 50 | 5.3 | 1542.4 | 1022.2 | 0.016 |
| 4204 | 50 | 5.4 | 9554.3 | 8025.3 | 0.043 |
| 5202 | 50 | 5.6 | 12328.8 | 10871.6 | 0.036 |
PDQuest estimate.
Fig. 2.
LC–MS/MS analysis identifies SSP4202 and SSP5202 to be fibrinogen γ. A. LC–MS/MS analysis of SSP4204 and SSP5202 to be fibrinogen γ. B. Western immunoblot analyses of plasma from VD deficient (VDD; n = 76) and VD sensitive (VDS; n = 46) pediatric obese subjects using an anti-fibrinogen γ antibody.
Obesity-associated hyperfibrinogenemia is frequent in both adults and children, with higher fibrinogen levels increasing the risk of thrombotic events [37,38]. Fibrinogen γ in both adults and children is emerging as an important biomarker for cardiovascular disease (CVD), due to its increased resistance to fibrinolysis [[39], [40], [41]]. On this basis, we chose to investigate the distribution of fibrinogen γ by western immunoblot according to 25OHD levels in the plasma of both VDD (n = 76) and VDS (n = 47) obese pediatric subjects. As demonstrated, no significant difference in total plasma fibrinogen γ levels were observed and nor were they observed with further subdivision of the VDD group, suggesting the differences identified by 2DE relating to VD status could more likely be related to individual fibrinogen γ isoforms and their post translational modifications (Fig. 2B).
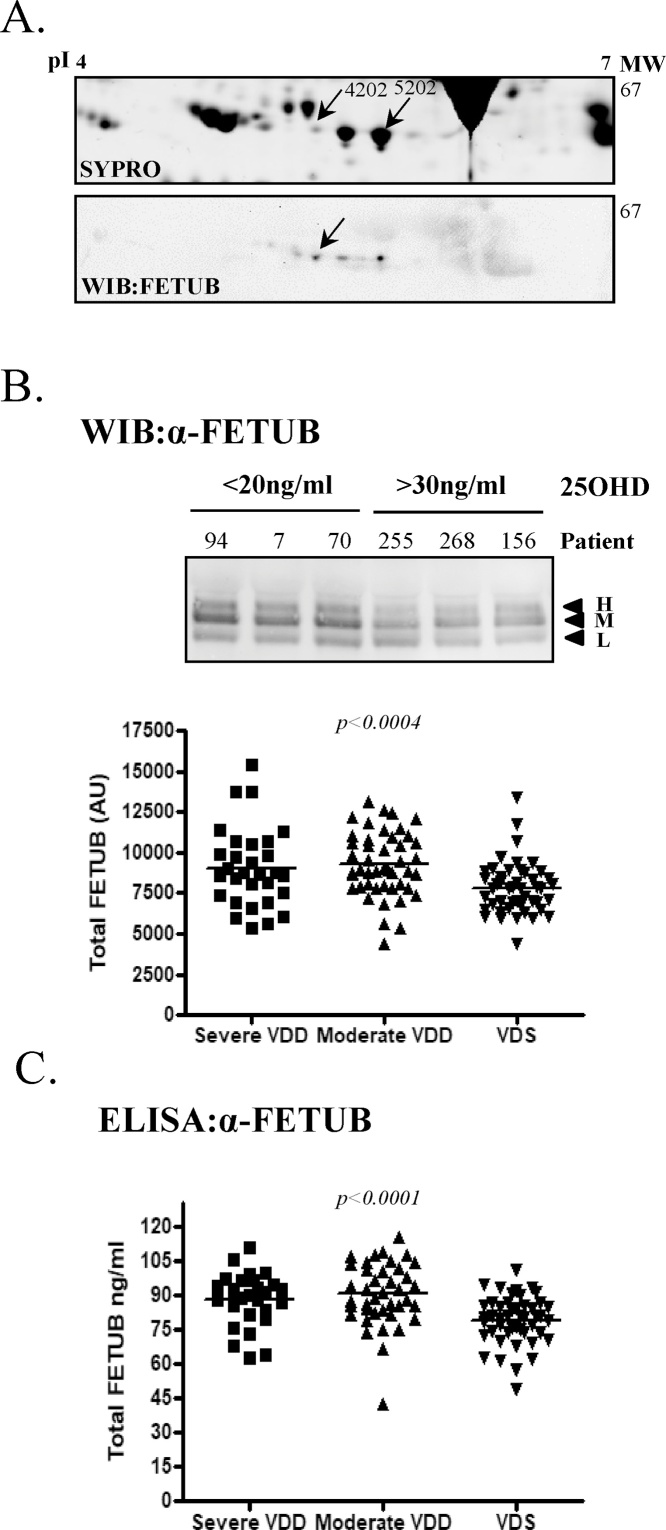
In contrast to SSP4204 and 5202, LC–MS/MS of SSP3302 and 4202 failed to identify any relevant circulating proteins, likely due to low concentrations and keratin interference. To predict the identity of SSP4202 and 3302, gel matching was used as an alternative approach. A 2DE study by Jung et al. [42], comparing the serum from adult patients with acute myocardial infarction and stable angina by PDQuest combined with LC–MS/MS, identified spot SSP4202 to be fetuin B [42]. In addition to FETUB being a circulating protein having a MW/pI corresponding to our range of interest, we chose to investigate whether SSP4202 in the present study could be FETUB due to several important characteristics. Human FETUB is structurally related to fetuin A (FETUA), a protein inhibitor of systemic ectopic calcification and a potent cardiovascular risk factor [43,44]. FETUB itself has a similar role to FETUA, albeit with a lower affinity, and recently it has been shown to be involved in the development of acute myocardial infarction [42,45]. More importantly, FETUB localizes to the identical cytogenetic band where adiponectin is found, 3q27.3 [46]. Adiponectin has recently been demonstrated to be significantly reduced in VDD pediatric obese subjects and to be directly upregulated by VD administration [15,28]. To understand if SSP4202 could be FETUB, 2DE western immunoblots of plasma using IPG4–7 strips and a FETUB-specific antibody were performed, in which 5 isoforms of FETUB were clearly observed (Fig. 3A). When aligned to matched 2DE gels stained for total protein, SSP4202 corresponded to one of these isoforms confirming that SSP4202 was likely a FETUB isoform. To support this finding, additional 2DE western immunoblots of plasma using IPG3-6 and 5–8 strips, further confirmed that SSP4202 is FETUB (Supplementary Fig. 1). While present in the 2DE analyses, the remaining FETUB isoforms identified by 2DE western analyses are most likely masked by the highly abundant fibrinogen γ isoforms, which co-migrate at a similar MW/pI (Fig. 3A).
Fig. 3.
Protein SSP4202 predicted to be FETUB, with western immunoblot and a FETUB-specific ELISA of plasma samples confirming that FETUB is increased in VDD pediatric obese. A. A western immunoblot of 2DE analyses was performed in human plasma samples using anti-FETUB antibody (n = 4). Each 2DE analysis was also stained for total protein using Sypro Ruby (n = 4). SSP4202 and 5202 are highlighted with the FETUB isoform corresponding to SSP4202 indicated by an arrow. B. A western immunoblot (WIB) analysis under reduced conditions of FETUB in the plasma of representative VDD (<20 ng/ml; n = 76) and VDS (>30 ng/ml; n = 46) subjects. “H” represents a high molecular weight isoform, “M” medium and “L” low molecular weight isoform. Included is a graphical representation of the densitometric analyses of FETUB western immunoblot in severe-VDD, moderate-VDD and VDS subjects. Densitometric results were normalized to plasma protein concentrations. C. Fetuin B-specific ELISA performed on the plasma of severe-VDD (n = 30), moderate-VDD (n = 46) and VDS (n = 47) subjects.
3.3. Validation of higher circulating FETUB in VDD pediatric obese subjects
Several approaches were utilized to investigate if differences exist between plasma FETUB in VDD and VDS obese pediatric subjects. In the first, western immunoblot evaluations of our cohort of VDD (n = 76) and VDS (n = 47) subjects revealed 3 clear FETUB isoforms, which we labelled respectively high-FETUB (H), medium (M) and low (L), according to their MW (Fig. 3B). A densitometric analyses of total FETUB in all subjects, confirmed that FETUB expression was significantly elevated in pediatric obese subjects with VDD (VDD vs VDS; 9196.5 ± 2156 vs 7802.1 ± 1606.7 AU; p < 0.0003). Likewise, when the VDD cohort was further subdivided into severe-VDD (n = 30) and moderate-VDD (n = 46), FETUB levels remained significantly higher in both groups, with respect to the VDS group (p < 0.0004; Fig. 3B). Densitometric analysis of the 3 FETUB isoforms showed that the M- and L-FETUB isoforms were significantly higher in plasma of VDD subjects compared to VDS, while in contrast H-FETUB remained unchanged (Supplementary Fig. 2A). It should be noted that human FETUB has two protein isoforms of 382aa (Fetuin B) and 345aa (Fetuin B beta) generated by alternative splicing and it is a heavily N-linked glycosylated protein. De-glycosylation of plasma FETUB by PNGaseF digestion revealed both FETUB and FETUB beta, establishing that H-FETUB and M-FETUB are glycosylated FETUB (Supplementary Fig. 2B). As H-FETUB is unchanged with respect to 25OHD concentrations, this highlights that VD status may predominantly influence FETUB levels as opposed to the glycosylation status, although this observation requires further investigation.
Due to the significant differences observed by western immunoblot, we chose to investigate total FETUB levels by ELISA in VDD and VDS. This approach confirmed the western immunoblot results, as circulating total FETUB were significantly higher in VDD when compared to VDS (VDD vs VDS; 89.3 ± 12.7 vs 78.9 ± 10.4 ng/ml; p < 0.0001). Likewise, total FETUB circulating concentrations were higher in both severe- VDD and moderate-VDD, when compared to VDS (p < 0.0001; Fig. 3C). When the results were corrected for independent cofounders (age, gender, puberty, UVR3, BMI or WC), FETUB remained higher in VDD subjects according to both model 1 (p < 0.04) and 2 (p < 0.02).
3.4. Circulating FETUB in pediatric obese subjects is associated with 25OHD levels
While total FETUB circulating concentrations are significantly higher in VDD obese pediatric subjects as opposed to VDS, we wanted to understand if these levels were dependent on 25OHD levels, or other principal calcium/phosphate regulating hormones. As described, VDD subjects presented with significantly higher PTH levels with respect to VDS subjects (Table 1). Correlation analyses demonstrated that FETUB positively correlated with alkaline phosphatase (r = 0.246; p < 0.009) and SBP (r = 0.211; p < 0.01), and negatively with 25OHD concentrations (r = −0.359; p < 0.0001), while no correlation was observed to PTH, calcium or phosphorus.
3.5. Vitamin D3 treatment directly and rapidly downregulates FETUB secretion in HepG2 and HUH7 hepatocellular cell lines
The tissue-specific expression of FETUB has been demonstrated to be predominantly but not exclusively expressed in the liver of both humans and mice [45]. To examine if VD can directly regulate FETUB synthesis and secretion, two human hepatocellular carcinoma cell lines, HepG2 and HUH7, were treated for a 48 h period in SFM with or w/o increasing concentrations (10−9–10−7M) of the bioactive form of VD3, 1α,25-(OH)2D3, with the conditioned medium at 48 h. A western immunoblot analysis of FETUB in the conditioned medium, demonstrated a significant decrease in total FETUB secretion with increasing concentrations of 1α,25-(OH)2D3 (n = 4; Fig. 4A). These results were further confirmed in the conditioned medium of both cell lines in time dependent experiments, with 10−7 M 1α,25-(OH)2D3 showing an effect as early as 1hr in HUH7 and 2 h in HepG2 cells (n = 3; Fig. 4B).
Fig. 4.
Total FETUB secretion decreases in hepatocellular carcinoma cell lines treated with 1α,25-(OH)2D3. A. HepG2 and HUH7 hepatocellular carcinoma cells were treated at 60% confluency with increasing concentrations of 1α,25-(OH)2D3 (10–9 to 10–7 M) in serum free medium or serum free medium with vehicle for 48 h. The conditioned medium was analyzed by western immunoblot under reduced conditions and analyzed for FETUB using anti-FETUB antibody. B. HepG2 and HUH7 cells were treated with 10-7M 1α,25-(OH)2D3 or vehicle in serum free medium for up to 24 h. Fetuin B in conditioned medium was analyzed at 1, 2, 3, 5, 7 and 24 h by western immunoblot under reduced conditions using anti-FETUB antibody. The gels in both experiments are representative of n = 4 experiments.
3.6. VDR silencing (shVDR) demonstrates that VDR is a potential negative regulator of FETUB in HepG2 cells
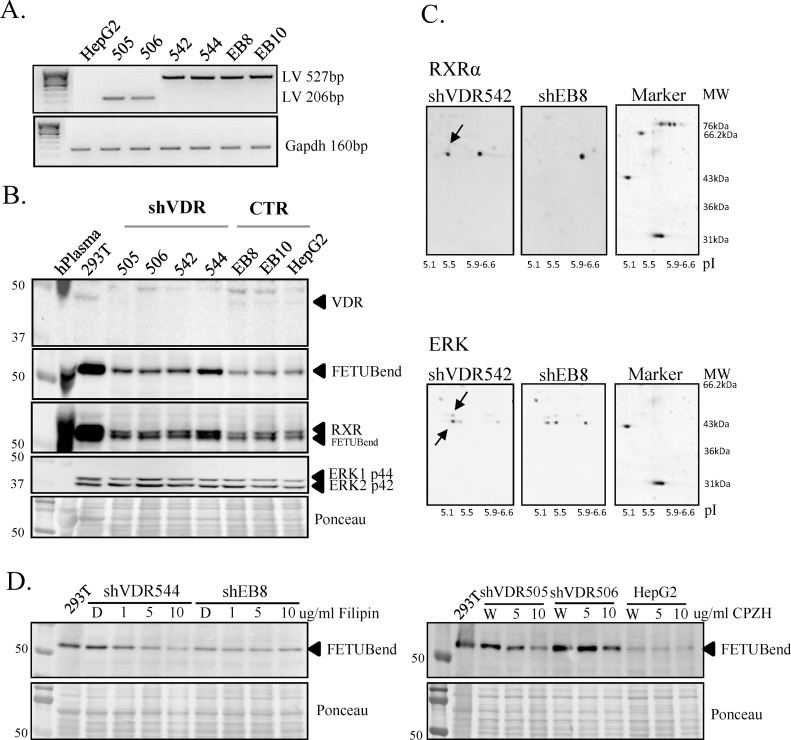
Vitamin D induces its actions primarily via the cytosolic/nuclear vitamin D receptor (VDR) and VD responsive elements (VDRE) found on numerous key genes, with rapid responses occurring via mVDR or 1,25D3-MARRS, both localized in the plasma membrane [7,8]. With a specific and rapid downregulation of FETUB secretion in HepG2 and HUH7 hepatocellular carcinoma cell lines with 1α,25-(OH)2D3 treatments, effects seen as early as 1–2 h, we wanted to perform some preliminary experiments to understand if this direct and rapid effect was coordinated by the VDR and/or other regulatory pathway/s. To assess the role of VDR, the HepG2 expression of VDR was disrupted by shRNA delivery of 5 different lentiviral vectors (505, 506, 542, 543, 544). Following puromycin selection, the integration of the five shVDR and two control vectors (EB8 and EB10) was confirmed by PCR (Fig. 5A). Vitamin D receptor protein expression was demonstrated to be blocked in all five shVDR clones by a VDR-specific western immunoblot, while expression was maintained in the two controls and uninfected HepG2 cells (Fig. 5B). With VDR silencing confirmed, the endogenous synthesis of FETUB was investigated. Interestingly, the FETUB-specific western immunoblot demonstrated a higher endogenous expression of FETUB in the shVDR clones than in the controls and uninfected cells (Fig. 5B). This observation suggests that the VDR could be a central player in the regulation of FETUB production, serving as a potential key negative regulator.
Fig. 5.
VDR silencing (shVDR) in HepG2 cells demonstrates that VDR is a potential negative regulator of FETUB. A. Representative PCR performed on gDNA from shVDR clones, viral controls (EB8 and EB10) and HepG2 cells (-ve control) using backbone specific primers to demonstrate successful integration. Concentration and gDNA quality were assessed a Gapdh PCR. Integration PCRs were performed for each independent shRNA delivery (n = 3). B. Western immunoblot analyses under reduced conditions in 20ug of whole cell lysates from shVDR clones (shVDR) and controls (CTR). Antibodies specific for VDR, FETUB, RXRα and ERK were utilized to analyze their endogenous expression, with results normalized to total protein. Gels are representative of n = 3 experiments for 3 independent viral deliveries performed. C. Representative western immunoblot analyses for RXRα and ERK of 2DE with 100ug of whole cell lysates from shVDR clones (shVDR542) and controls (EB8). Arrows highlight pI shifts observed for RXRα and ERK1 p44 and ERK2 p42. D. Representative western immunoblot analyses of endogenous FETUB (FETUBendog) under reduced conditions in 20ug of whole cell lysates from shVDR clones and controls (shEB8 or HepG2) following a 1hr treatment with increasing concentrations of filipin (1–10 ug/ml) or CPZH (5-10ug/ml), followed by 24 h in maintenance medium. Results are representative of 4 independent shVDR clones and are normalized to total protein (n = 3).
Based on these unexpected results, we opted to perform a preliminary evaluation of the potential molecular players involved in FETUB upregulation in the absence of VDR. According to the traditional “genomic” model, the VD/ nVDR complex dimerizes with the retinoid X receptor (RXR) which binds to VDRE in VD-responsive genes, recruits either coactivators or corepressors which in turn modulate gene expression. Fetuin B lacks a classic VDRE, however, the examination of RXR in VDR-silenced HepG2 cells, demonstrated that RXRα parallels that of endogenous FETUB expression, with higher levels in shVDR cells, when compared to controls (Fig. 5B). Furthermore, a 2DE-western immunoblot demonstrated a significant reduction in the pI of RXRα in shVDR HepG2 cells, suggesting the likely phosphorylation of RXRα in the absence of VDR (Fig. 5C). It is well accepted that acidic phosphate groups cause the pI to decrease and therefore proteins to shift to acidic pI region, depending on the number and site of phosphorylation [47]. A central player in the “non-genomic” VD actions or crosstalk between VD activated membrane receptors and gene regulation, is the mitogen-activated protein kinase (MAPK) pathway. No differences in the levels of extracellular signal–regulated kinase (ERK) 1 or 2 was observed (Fig. 5C), however, both ERKs had a reduced pI in 2DE western immunoblot of shVDR HepG2 cells, suggesting an increase in the phosphorylation and hence activity of ERK. The increased activity of ERK in VDR silenced HepG2 cells highlights the involvement of other potential membrane receptors in FETUB upregulation. As such we investigated what would happen to FETUB synthesis should endocytosis be inhibited in shVDR cells and controls in growth conditions. With the inhibition of endocytosis and therefore membrane receptor/s internalization with two independent inhibitors, we observed a specific downregulation of FETUB endogenous expression in shVDR HepG2 cells, highlighting the co-involvement of other membrane receptors in the regulation of FETUB (Fig. 5D). These preliminary results suggest that VD/VDR are key negative regulators of FETUB synthesis and that the observed upregulation of FETUB in the absence of VDR may be the result of non-genomic events involving other receptor-mediated regulatory mechanisms which are independent of the traditional “genomic” model.
3.7. Vitamin D3 administration to mice fed STD or HFD downregulates FETUB protein expression
To understand if the vitamin D3-specific downregulation of FETUB observed in vitro in two independent liver cell lines could be replicated in vivo, two groups of mice subjected to a standard (STD) or high fat diet (HFD), were supplemented with 1000 IU vitamin D3 (STDVD; HFDVD) or placebo (STD; HFD), for up to 6 weeks. Two diet models were selected based on previous contradictory findings with respect to FETUB, particularly with reference to a HFD. While hFETUB has been demonstrated to be significantly elevated in patients with liver steatosis [48] and hFETUA has been shown to be upregulated in pediatric obesity and fatty liver disease [49], a proteomic study identified that both rat FETUB (rFETUB) and rFETUA were downregulated in the plasma of rats fed a HFD and prone to obesity [50]. We were therefore interested in understanding the effects of vitamin-D3 on FETUB in mice, particularly with respects to diet.
With regards to %Δ weight gain over the 6week period, mice subjected to a HFD gained more weight than STD, with the differences becoming significant at 4 weeks (Fig. 6A). Mice fed a STD with vitamin D3 supplementation paralleled those fed a STD, while those fed a HFD with vitamin D3 gained weight faster than mice fed exclusively a HFD, with HFD mice catching up at 5 weeks. Finally, STDVD versus HFDVD mice remained significantly different from the outset of the experiment to its completion, with HFDVD mice gaining almost 50% more weight than STDVD mice (Fig. 6A). With respect to glycaemia, no significant differences were observed in glucose levels between the 4 groups over the duration of the experiment. At 6 weeks, circulating mFETUB levels were analyzed by western immunoblot in the plasma from the 4 groups. In contrast to hFETUB, a single dominant mFETUB isoform was detected (Fig. 6B). A densitometric analysis of this isoform demonstrated that there was no difference in FETUB levels according to the type of ad libitum diet, however a difference was observed with regards to vitamin-D3 supplementation. At 6 weeks a significant decrease in circulating mFETUB in STDVD mice with respect to STD mice (p < 0.05) was observed, while in HFD mice there was a modest yet insignificant trend (Fig. 6B). These results support the in vitro findings in human liver cells.
Fig. 6.
Vitamin D administration for 6 weeks downregulates murine circulating FETUB in a diet dependent fashion, with a delayed response observed in liver tissue FETUB expression. A. Graphical representation of the %Δ weight gain (gm) measured weekly with respect to baseline in STD, STDVD, HFD and HFDVD mice after 6 weeks (T6) of supplementation with VD. Data are expressed as mean ± SEM. B. Western immunoblot analysis of FETUB in the plasma of representative STD (n = 7), STDVD (n = 7), HFD (n = 5) and HFDVD (n = 5) mice following 6 weeks of supplementation. Data are expressed as mean ± SEM. C. Representative western immunoblot analysis of FETUB protein expression in 20ug of liver cell lysates from STD (n = 5), STDVD (n = 5), HFD (n = 5) and HFDVD (n = 5) mice after 22 weeks of supplementation. Results are normalized to total protein and are expressed as mean ± SEM.
We then chose to focus on the source of circulating mFETUB. Being that FETUB is predominantly synthesized by the liver [45] and we observed a direct VD effect in two human liver cell lines on FETUB synthesis, we examined the endogenous FETUB levels in mouse livers to understand if the demonstrated circulating levels at 6 weeks were dependent on the liver production of FETUB. Unexpectedly, we saw no significant difference in endogenous mFETUB levels regardless of the type of diet or vitamin-D3 supplementation (Supplementary Fig. 3A). In contrast to mFETUB, we observed that mFETUA showed significantly higher levels in the HFD model supporting the findings of Reinehr and Roth, [49], however, these levels were exclusively dependent on the type of diet and independent of vitamin-D3 supplementation (Supplementary Fig. 3B).
To understand if the lack of association at 6weeks of treatment between mFETUB circulating levels and the liver production of FETUB could be time dependent, a second group of mice administered a STD or HFD supplemented with or without VD3 for up to 22weeks, were investigated. In contrast to mice treated for 6 weeks, at 22 weeks an effect of both diet and VD supplementation on liver-specific endogenous FETUB production was observed by western immunoblot analysis (Fig. 6C). Mice fed exclusively a HFD showed near significantly higher FETUB levels than those fed a STD (p < 0.057), while VD supplementation was associated with significantly reduced FETUB expression in both diet models with respect to placebo treated mice (Fig. 6C). Although an age-related effect cannot be ruled out, these results suggest that while the liver-specific production contributes to circulating FETUB levels, it likely that the early effect observed at 6weeks comes from other VD-responsive tissues.
4. Discussion
Amongst lifestyle-related diseases, pediatric obesity and VDD are raising significant concerns in many countries, particularly with regards to their long-term metabolic outcomes and eventual impact on national healthcare systems. While it is must be recognized that genotype, lifestyle and behavioral factors such as diet and the levels of physical activity play critical roles in these epidemics, there is evidence indicating that VD may contribute to the regulation of weight gain, particularly when associated to energy-restricted diets [26,51,52]. Despite the clear associations found between body weight composition and VD levels [9,[11], [12], [13], [14], [15], [16], [17], [18]], studies to date have not been able to decipher the causal metabolic signals that link a poor VD status to obesity and its complications [26,27]. In a previous investigation we used a proteomic approach to identify potential circulating biomarkers that could provide a link between VDD and pediatric obesity [28]. This study highlighted the multimeric forms of adiponectin as a molecular link and demonstrated a direct VD regulation of adiponectin synthesis. Considering the complex nature of VD regulation, we chose in the present investigation to focus on another of the top “ten” spots identified [28]. Spot SSP2301, in contrast to adiponectin, was found to be significantly upregulated in VDD subjects.
To investigate SSP2301 we used an increased cohort of pediatric obese patients and a more focused 2D-electrophoretic analysis based on predicted pI and MW values by PDQuest for SSP2301. In our increased cohort of pediatric obese subjects, we observed a clear dominance of VDD with respect to VDS, with almost 29% of these VDD subjects classified with severe VDD. The incongruent dataset used in this study is a limitation, however as the median 25OHD concentration in the VDD group was borderline with severe VDD, we opted to perform a large part of our analyses between VDD and VDS. In agreement with previous surveys in children, the VDD children enrolled were more obese, more insulin resistant and showed higher fasting glucose, SBP and DBPthan their VDS counterparts [28,[53], [54], [55]]. The 2DE analysis of the plasma from these subjects highlighted four protein spots which were significantly elevated in VDD subjects and corresponded to the estimated pI and MW for SSP2301. Two of these spots were predicted to be Fibrinogen γ and one to be FETUB. While plasma analyses confirmed that FETUB concentrations were significantly higher in the plasma from severe- and moderate-VDD subjects and independent of PTH concentrations, no relationship could be demonstrated for fibrinogen γ. A possible explanation for the incongruent fibrinogen γ results is that VD status could be correlated to the individual fibrinogen γ isoforms, as opposed to total circulating levels. Fibrinogen γ has two alternative splice variants and is N-glycosylated [56,57]. The alternatively spliced variants have been shown to be differentially regulated by inflammatory responses [58], while the glycosylation of fibrinogen has important consequences on the structure of clots [57], suggesting that the influence of VD status could be related to the post translational modifications of fibrinogen γ. An alternative explanation is that as fibrinogen γ is a highly abundant plasma protein with a similar pI and MW to FETUB, it could be masking the identification of the FETUB isoforms by LC–MS/MS. Fetuin B like fibrinogen γ, has multiple isoforms in circulation as the result of alternative splicing and N-linked glycosylation [59]. While we can conclude that the total plasma concentration of FETUB correlated to VD concentrations and were independent of PTH in obese pediatric subjects, further experimentation is required to understand the relationship to fibrinogen γ.
Fetuin B as a potential link between obesity and VDD, can be supported by several very important characteristics. Human FETUB is structurally related to fetuin A (FETUA), both of which are members of the cystatin superfamily comprising of structurally related protease inhibitors [60]. Fetuins have diverse functions including the regulation of osteogenesis, mineralization and systemic inflammation [45,61,62]. Circulating FETUA levels are increased in NAFLD independent of obesity [63], while it also considered an independent risk factor for type 2 diabetes mellitus in adults and children [64], and a potent cardiovascular risk factor [44]. Only now is data emerging on the importance of FETUB, with the levels of FETUB increased in liver steatosis and type 2 diabetes mellitus [48], patients with chronic obstructive pulmonary disease [65], coronary artery disease [66], as well as the demonstration of its functional involvement in the development of acute myocardial infarction and [42]. At the cellular level, treatment with FETUB in hepatoyctes and myocytes in vitro results in insulin resistance, supported by the observation of glucose intolerance following FETUB administration to lean mice [48]. Another interesting observation is that human FETUB is found on chromosome 3q27.3, the identical cytogenetic band in which adiponectin is located [46]. As described, adiponectin has been demonstrated to be significantly reduced in VDD pediatric obese subjects and to be directly upregulated by VD administration [15,28]. These findings also highlight that 3q27.3 could be an important regulatory region for VD actions.
To investigate whether VD has a direct effect on FETUB synthesis, we used hepatocellular carcinoma cell lines as it has been demonstrated that FETUB is predominantly expressed by the liver in humans and mice [45]. Both the nVDR and mVDR are expressed in the liver and regulate VD actions [67], with a differential expression profile observed according to the liver cell type [68]. While the highest VDR expression has been observed in bilary epithelial cells, kupffer and other non-parenchymal cells, a modest expression has been seen in unstimulated hepatocytes and in hepatocellular carcinoma cell lines [68,69]. While it remains to be understood the effect of VD on other liver cell types, in the present study we demonstrated that VD could act directly with the secretion of FETUB inhibited with 1α,25-(OH)2D3 supplementation. The direct regulation of FETUB by 1α,25-(OH)2D3 is an important finding not only from the perspective of VDD and pediatric obesity, but in light that the VD axis is now receiving much attention in liver pathophysiology. A high prevalence of VDD is seen in both adult and pediatric patients with liver diseases, including hepatitis C virus (HCV) and HBV, NAFLD, autoimmune liver diseases, liver transplantation, liver fibrosis, hepatocellular carcinoma and acute liver injury [70]. As described, the levels of FETUB are significantly increased in liver steatosis, with FETUB administration in lean mice causing glucose intolerance [48]. While a role for FETUB in other liver diseases remains to be defined, in the present study we have demonstrated that FETUB can be directly downregulated by VD supplementation at the cellular level.
The pleiotropic actions of VD are achieved via an orchestra of events involving both genomic and non-genomic pathways which lead to the activation or repression of target genes [7,8]. Activation of 1α,25-(OH)2D3-dependent target genes, such as p21 [71], occurs via a ligand-dependent association of the VDR with coactivator proteins and heterodimerization with RXR, with binding to VDREs [7,8]. The VDR is also central to 1α,25-(OH)2D3-dependent repression of target genes, however, the mechanisms are quite distinct from the 1α,25-(OH)2D3 mediated activation. Target genes repressed by 1α,25-(OH)2D3 such as parathyroid hormone [72]. Interleukin-2 (IL2 [73]) and granulocyte-macrophage colony-stimulating factor (GM-CSF [74]), have a “negative” VDRE (nVDRE) which have a different sequence organization and can operate independently of RXR. With 1α,25-(OH)2D3-specific repression of FETUB demonstrated in HepG2 and HUH7 cells, we investigated whether the VDR could be central to this regulation by silencing its expression. Interestingly, we observed that silencing of VDR under normal growth conditions appeared to release FETUB synthesis with significantly higher endogenous and secreted levels observed in shVDR cells. This observed FETUB upregulation was paralleled by an increase in expression and phosphorylation of RXR, and the phosphorylation of ERK. While a more comprehensive investigation is required to understand the molecular players involved in the VD regulation of FETUB, our observations suggest that VDR is a central player. To our knowledge there is no demonstration of classic VDREs in hFETUB promoter, suggesting that 1α,25-(OH)2D3-mediated repression of FETUB may involve nVDREs, or alternate mechanisms. Examples of 1α,25-(OH)2D3-mediated repression include GM-CSF gene, where VD-repression involves a two-hit mechanism; VDR outcompeting NFAT1 at a novel nVDRE which in turn stabilizes a Jun-Fos heterodimer to an adjacent AP-1 site through a direct interaction between VDR and c-Jun [74]. It cannot be excluded that the negative regulation of FETUB by VDR involves epigenetic mechanisms or micro RNAs [75]. While the mechanism of 1α,25-(OH)2D3-repression of FETUB remains to be established, the present study has highlighted that VDR is likely key to the molecular architecture co-involved in the negative regulation of FETUB.
Our preliminary results provide evidence the potential involvement of RXR and ERK as molecular players in the regulation of FETUB. Without further investigation, we are unable to make specific conclusions, however, in hepatocellular tissue and cell lines it is known that activated Ras-mitogen activated protein kinase (MAPK) pathway phosphorylates RXRα [76]. Phosphorylated RXRα resists ubiquitination and proteasome-mediated degradation, which under normal conditions occurs following its release from heterodimers [76]. What we could be seeing in our shVDR cell model is that in the absence of VDR, the increased phosphorylation of RXR potentially linked an increased Erk phosphorylation by alternate receptor/signalling pathways, leads to the accumulation RXR, due to its resistance to ubiquitination and degradation. While for some tissues and cell lines phosphorylated RXR is inactive, alternatively in other cell systems phosphorylated RXR is involved gene docking and regulation [7]. For example, the farnesoid X receptor (FXR), a member of the nuclear receptor family, directly regulates FETUB in human hepatocytes, and it does so in collaboration with RXR [59]. In fact, in the present study we observed that with the inhibition of endocytosis and subsequent internalization of membrane receptors, there was a suppression of the shVDR-specific upregulation of FETUB, highlighting the importance of other receptor mediated signalling pathways and a clear crosstalk in the regulation of FETUB.
Despite numerous studies providing evidence of increased circulating FETUB levels in several disease states, it remains that there are only a limited number of studies investigating the clinical significance of elevated FETUB. As described, Jung et al. [42], demonstrated that FETUB is involvement in the development of acute myocardial infarction, while others [48] observed that treatment with FETUB in primary hepatoyctes and myocytes in vitro caused insulin resistance, with FETUB administration causing the development of glucose intolerance in lean mice. Further, in obese mice with increased FETUB levels [50], a 72% reduction of circulating FETUB using adenoviral administration of shFETUB, resulted in an overall improvement in whole-body glucose metabolism, independent of an effect body mass [48]. Collectively, these results highlight the importance of reducing circulating FETUB levels to improve clinical outcomes. The scope of our in vivo model was to investigate whether VD3-supplementation in juvenile mice could reduce FETUB circulating levels in two ad libitum diet-dependent models. At 6 weeks vitamin-D3 supplementation, we could demonstrate that juvenile STD mice had reduced circulating FETUB levels, while HFD mice were unresponsive. Despite reduced plasma levels in STD mice, however, we surprisingly observed no effect on the endogenous production of liver-specific FETUB, with a clear effect eventually observed only by extending vitamin-D3 supplementation to 22 weeks. While an age effect cannot be ruled out at 22 weeks, these results suggest that while the liver-specific production contributes to circulating FETUB levels, it is likely that the effect observed at 6 weeks comes from other VD-responsive tissues. In support of this observation, Meex et al. [48], also observed decreased FETUB expression in white adipose tissue and heart with adenoviral-shFETUB, although they emphasize that liver is the most likely primary source of circulating FETUB. With respects to clinical outcome, no effect on glucose levels at 6 weeks with vitamin-D3 supplementation were observed, however, a negative effect on HFD mice was demonstrated with a dramatic increase %Δ weight gain with respect to placebo. 125(OH)2D3 has been shown to inhibit adipogenesis in mouse 3T3-L1 preadipocytes [77,78], but it has also been shown to enhance adipogenesis in primary mouse preadipocytes and human subcutaneous preadipocytes during maturation and lipid accumulation [79]. While there is an increase in adipogenesis by VD, this is thought to be a healthy remodeling of AT, with the prevention oxidative stress and increased glucose metabolism observed in HFD-mice supplemented with VD3 [79,80]. Other tissues such as adipose tissue was not investigated in the present investigation for FETUB expression. The large discrepancy in body mass with vitamin-D3 supplementation could be an important issue with respects to circulating FETUB levels.
Vitamin D repletion has been suggested as a promising approach for the prevention of obesity and the development of its associated complications. Despite the discovery of VD at the beginning of the last century and extensive investigations, the “concert” of metabolic events that link obesity with VD levels remain largely unknown. In the present study, we used a proteomic approach to study the global plasmatic changes between VD deficient and normal obese pediatric subjects identifying circulating FETUB as a novel plasmatic biomarker that could provide a mechanistic link between VDD and pediatric obesity, with a direct effect of 1α,25-(OH)2D3 on FETUB synthesis and secretion in both in vitro and in vivo models. While the mechanism of VD control over FETUB remains to be clearly defined, the silencing of the VDR, highlights that the VDR central to the negative regulation of FETUB by VD, and thus important therapeutic target for the direct modulation of FETUB.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to acknowledge the help of Dr. Ester Borroni for the control lentiviral vectors and Elena Ferrari for technical assistance with regards to the animal model. The shVDR experiments were supported by Horizon 2020 n° HEMACURE_667421_(AF) and in part by ERC_261178_(AF).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jsbmb.2018.04.009.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Holick M.F. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017;18(2):153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 3.Cimini F.A., Barchetta I., Carotti S., Bertoccini L., Baroni M.G., Vespasiani-Gentilucci U., Cavallo M.G. Morini S relationship between adipose tissue dysfunction, vitamin D deficiency and the pathogenesis of non-alcoholic fatty liver disease. World J. Gastroenterol. 2017;23(19):3407–3417. doi: 10.3748/wjg.v23.i19.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grübler M.R., März W., Pilz S., Grammer T.B., Trummer C., Müllner C., Schwetz V., Pandis M., Verheyen N., Tomaschitz A., Fiordelisi A., Laudisio D., Cipolletta E., Iaccarino G. Vitamin-D concentrations, cardiovascular risk and events - a review of epidemiological evidence. Rev. Endocr. Metab. Disord. 2017;18(2):259–272. doi: 10.1007/s11154-017-9417-0. [DOI] [PubMed] [Google Scholar]
- 5.Pilz S., Grübler M., Gaksch M., Schwetz V., Trummer C., Hartaigh B.Ó, Verheyen N., Tomaschitz A., März W. Vitamin D and mortality. Anticancer Res. 2016;36(3):1379–1387. [PubMed] [Google Scholar]
- 6.Muscogiuri G., Annweiler C., Duval G., Karras S., Tirabassi G., Salvio G., Balercia G., Kimball S., Kotsa K., Mascitelli L., Bhattoa H.P., Colao A. Vitamin D and cardiovascular disease: from atherosclerosis to myocardial infarction and stroke. Int. J. Cardiol. 2017;230:577–584. doi: 10.1016/j.ijcard.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 7.Haussler M.R., Jurutka P.W., Mizwicki M., Norman A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)₂vitamin D₃: genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25(4):543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Hii C.S., Ferrante A. The non-genomic actions of vitamin D. Nutrients. 2016;8(3):135. doi: 10.3390/nu8030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pannu P.K., Calton E.K., Soares M.J. Calcium and vitamin D in obesity and related chronic disease. Adv. Food Nutr. Res. 2016;77:57–100. doi: 10.1016/bs.afnr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Pereira-Santos M., Costa P.R., Assis A.M., Santos C.A., Santos D.B. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes. Rev. 2015;16(4):341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 11.Vimaleswaran K.S., Berry D.J., Lu C., Tikkanen E., Pilz S., Hiraki L.T., Cooper J.D., Dastani Z., Li R., Houston D.K., Wood A.R., Michaëlsson K., Vandenput L., Zgaga L., Yerges-Armstrong L.M., McCarthy M.I., Dupuis J., Kaakinen M., Kleber M.E., Jameson K., Arden N., Raitakari O., Viikari J., Lohman K.K., Ferrucci L., Melhus H., Ingelsson E., Byberg L., Lind L., Lorentzon M., Salomaa V., Campbell H., Dunlop M., Mitchell B.D., Herzig K.H., Pouta A., Hartikainen A.L., Genetic Investigation of Anthropometric Traits-GIANT Consortium, Streeten E.A., Theodoratou E., Jula A., Wareham N.J., Ohlsson C., Frayling T.M., Kritchevsky S.B., Spector T.D., Richards J.B., Lehtimäki T., Ouwehand W.H., Kraft P., Cooper C., März W., Power C., Loos R.J., Wang T.J., Järvelin M.R., Whittaker J.C., Hingorani A.D., Hyppönen E. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10(2):e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barchetta I., De Bernardinis M., Capoccia D., Baroni M.G., Fontana M., Fraioli A., Morini S., Leonetti F., Cavallo M.G. Hypovitaminosis D is independently associated with metabolic syndrome in obese patients. PLoS One. 2013;8:e68689. doi: 10.1371/journal.pone.0068689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellan M., Guzzaloni G., Rinaldi M., Merlotti E., Ferrari C., Tagliaferri A., Pirisi M., Aimaretti G., Scacchi M., Marzullo P. Altered glucose metabolism rather than naive type 2 diabetes mellitus (T2DM) is related to vitamin D status in severe obesity. Cardiovasc. Diabetol. 2014;13:57. doi: 10.1186/1475-2840-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cediel G., Corvalán C., López de Romaña D., Mericq V., Uauy R. Prepubertal adiposity, vitamin D Status, and insulin resistance. Pediatrics. 2016;138(1) doi: 10.1542/peds.2016-0076. pii:e20160076. [DOI] [PubMed] [Google Scholar]
- 15.Mai S., Walker G.E., Vietti R., Cattaldo S., Mele C., Priano L., Mauro A., Bona G., Aimaretti G., Scacchi M., Marzullo P. Acute vitamin D₃ supplementation in severe obesity: evaluation of multimeric adiponectin. Nutrients. 2017;9(5) doi: 10.3390/nu9050459. pii: E459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng S., Massaro J.M., Fox C.S., Larson M.G., Keyes M.J., McCabe E.L., Robins S.J., O’Donnell C.J., Hoffmann U., Jacques P.F., Booth S.L., Vasan R.S., Wolf M., Wang T.J. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham heart study. Diabetes. 2010;59(1):242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald K., Godziuk K., Yap J., LaFrance R., Ansarian M., Haqq A., Mager D.R. Vitamin D Status, cardiometabolic, liver and mental health status in obese youth attending a pediatric weight management Centre. J. Pediatr. Gastroenterol. Nutr. 2017;65(4):462–466. doi: 10.1097/MPG.0000000000001598. [DOI] [PubMed] [Google Scholar]
- 18.Rajakumar K., de las Heras J., Chen T.C., Lee S., Holick M.F., Arslanian S.A. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J. Clin. Endocrinol. Metab. 2011;96(5):1560–1567. doi: 10.1210/jc.2010-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wamberg L., Christiansen T., Paulsen S.K., Fisker S., Rask P., Rejnmark L., Richelsen B., Pedersen S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue -- the effect of obesity and diet-induced weight loss. Int. J. Obes. (Lond.) 2013;37(5):651–657. doi: 10.1038/ijo.2012.112. [DOI] [PubMed] [Google Scholar]
- 20.Atabek M.E., Eklioglu B.S., Akyürek N., Alp H. Association between vitamin D level and cardiovascular risk in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2014;27(7-8):661–666. doi: 10.1515/jpem-2013-0379. [DOI] [PubMed] [Google Scholar]
- 21.Kelishadi R., Ardalan G., Motlagh M.E., Shariatinejad K., Heshmat R., Poursafa P., Fakhri M., Tajadini M., Taslimi M. National report on the association of serum vitamin D with cardiometabolic risk factors in the pediatric population of the Middle East and North Africa (MENA): the CASPIAN-III study. Nutrition. 2014;30(1):33–38. doi: 10.1016/j.nut.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Kao K.T., Abidi N., Ranasinha S., Brown J., Rodda C., McCallum Z., Zacharin M., Simm P.J., Magnussen C.G., Sabin M.A. Low vitamin D is associated with hypertension in paediatric obesity. J. Paediatr. Child Health. 2015;51(12):1207–1213. doi: 10.1111/jpc.12935. [DOI] [PubMed] [Google Scholar]
- 23.Ekbom K., Marcus C. Vitamin D deficiency is associated with prediabetes in obese Swedish children. Acta Paediatr. 2016;105(10):1192–1197. doi: 10.1111/apa.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savastio S., Cadario F., Genoni G., Bellomo G., Bagnati M., Secco G., Picchi R., Giglione E., Bona G. Vitamin D deficiency and glycemic Status in children and adolescents with type 1 diabetes mellitus. PLoS One. 2016;11(9):e0162554. doi: 10.1371/journal.pone.0162554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gul A., Ozer S., Yılmaz R., Sonmezgoz E., Kasap T., Takcı S., Demir O. Association between vitamin D levels and cardiovascular risk factors in obese children and adolescents. Nutr. Hosp. 2017;34(2):323–329. doi: 10.20960/nh.412. [DOI] [PubMed] [Google Scholar]
- 26.Song Q., Sergeev I.N. Calcium and vitamin D in obesity. Nutr. Res. Rev. 2012;25(1):130–141. doi: 10.1017/S0954422412000029. [DOI] [PubMed] [Google Scholar]
- 27.Pelczyńska M., Grzelak T., Walczak M., Czyżewska K. Hypovitaminosis D and adipose tissue - cause and effect relationships in obesity. Ann. Agric. Environ. Med. 2016;23(3):403–409. doi: 10.5604/12321966.1219177. [DOI] [PubMed] [Google Scholar]
- 28.Walker G.E., Ricotti R., Roccio M., Moia S., Bellone S., Prodam F., Bona G. Pediatric obesity and vitamin D deficiency: a proteomic approach identifies multimeric adiponectin as a key link between these conditions. PLoS One. 2014;9(1):e83685. doi: 10.1371/journal.pone.0083685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Díez J.J., Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003;148(3):293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 30.Cacciari E., Milani S., Balsamo A., Spada E., Bona G., Cavallo L., Cerutti F., Gargantini L., Greggio N., Tonini G., Cicognani A. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr) J. Endocrinol. Invest. 2006;29:581–593. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- 31.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Hassan Murad M., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 32.Tanner J.M., Whitehouse R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis. Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prodam F., Zanetta S., Ricotti R., Marolda A., Giglione E., Monzani A., Walker G.E., Rampone S., Castagno M., Bellone S., Petri A., Aimaretti G., Bona G. Influence of ultraviolet radiation on the association between 25-hydroxy vitamin D levels and cardiovascular risk factors in obesity. J. Pediatr. 2016;171:83–89. doi: 10.1016/j.jpeds.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Mischak H., Allmaier G., Apweiler R., Attwood T., Baumann M. Recommendations for biomarker identification and qualification in clinical proteomics. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3001249. 46ps. [DOI] [PubMed] [Google Scholar]
- 35.Swami S., Krishnan A.V., Wang J.Y., Jensen K., Horst R., Albertelli M.A., Feldman D. Dietary vitamin D₃ and 1,25-dihydroxyvitamin D₃ (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153(6):2576–2587. doi: 10.1210/en.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Follenzi A., Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–464. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- 37.Ernst E., Resch K.L. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann. Intern. Med. 1993;118(12):956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 38.Balagopal P.B., de Ferranti S.D., Cook S., Daniels S.R., Gidding S.S., Hayman L.L., McCrindle B.W., Mietus-Snyder M.L., Steinberger J., American Heart Association Committee on Atherosclerosis Hypertension and Obesity in Youth of the Council on Cardiovascular Disease in the Young, Council on Nutrition, Physical Activity and Metabolism, Council on Epidemiology and Prevention Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American heart association. Circulation. 2011;123(23):2749–2769. doi: 10.1161/CIR.0b013e31821c7c64. [DOI] [PubMed] [Google Scholar]
- 39.Falls L.A., Farrell D.H. Resistance of gammaA/gamma’ fibrin clots to fibrinolysis. J. Biol. Chem. 1997;272(22):14251–14256. doi: 10.1074/jbc.272.22.14251. [DOI] [PubMed] [Google Scholar]
- 40.Lovely R.S., Falls L.A., Al-Mondhiry H.A., Chambers C.E., Sexton G.J., Ni H., Farrell D.H. Association of gammaA/gamma’ fibrinogen levels and coronary artery disease. Thromb. Haemost. 2002;88(1):26–31. [PubMed] [Google Scholar]
- 41.Lovely R., Hossain J., Ramsey J.P., Komakula V., George D., Farrell D.H., Balagopal P.B. Obesity-related increased γ’ fibrinogen concentration in children and its reduction by a physical activity-based lifestyle intervention: a randomized controlled study. J. Pediatr. 2013;163:333–338. doi: 10.1016/j.jpeds.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Jung S.H., Won K.J., Lee K.P., Kim H.J., Seo E.H., Lee H.M., Park E.S., Lee S.H., Kim B. The serum protein fetuin-B is involved in the development of acute myocardial infarction. Clin. Sci. (Lond.) 2015;129(1):27–38. doi: 10.1042/CS20140462. [DOI] [PubMed] [Google Scholar]
- 43.Schafer C., Heiss A., Schwarz A., Westenfeld R., Ketteler M., Floege J., Muller-Esterl W., Schinke T., Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Invest. 2003;112(3):357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiore C.E., Celotta G., Politi G.G., Di Pino L., Castelli Z., Mangiafico R.A., Signorelli S.S., Pennisi P. Association of high alpha2-Heremans-Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis. 2007;195(1):110–115. doi: 10.1016/j.atherosclerosis.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 45.Denecke B., Gräber S., Schäfer C., Heiss A., Wöltje M., Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem. J. 2003;376(Pt 1):135–145. doi: 10.1042/BJ20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rankinen T., Zuberi A., Chagnon Y.C., Weisnagel S.J., Argyropoulos G., Walts B., Pérusse L., Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14(4):529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 47.Gauci S., van Breukelen B., Lemeer S.M., Krijgsveld J., Heck A.J. A versatile peptide pI calculator for phosphorylated and N-terminal acetylated peptides experimentally tested using peptide isoelectric focusing. Proteomics. 2008;8(23-24):4898–4906. doi: 10.1002/pmic.200800295. [DOI] [PubMed] [Google Scholar]
- 48.Meex R.C., Hoy A.J., Morris A., Brown R.D., Lo J.C., Burke M., Goode R.J., Kingwell B.A., Kraakman M.J., Febbraio M.A., Greve J.W., Rensen S.S., Molloy M.P., Lancaster G.I., Bruce C.R., Watt M.J. Fetuin B Is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab. 2015;22(6):1078–1089. doi: 10.1016/j.cmet.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Reinehr T., Roth C.L. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J. Clin. Endocrinol. Metab. 2008;93:4479–4485. doi: 10.1210/jc.2008-1505. [DOI] [PubMed] [Google Scholar]
- 50.Choi J.-W., Wang X., Joo J.-I., Dong H.-K., Oh T.-S., Choi D.-K., Yun J.-W. Plasma proteome analysis in diet-induced obesity-prone and obesity-resistant rats. Proteomics. 2010;10(24):4386–4400. doi: 10.1002/pmic.201000391. [DOI] [PubMed] [Google Scholar]
- 51.Farhangi M.A., Mesgari-Abbasi M., Hajiluian G., Nameni G., Shahabi P. Adipose tissue inflammation and oxidative stress: the ameliorative effects of vitamin D. Inflammation. 2017 doi: 10.1007/s10753-017-0610-9. [DOI] [PubMed] [Google Scholar]
- 52.Gomaa A.M., El-Aziz E.A. Vitamin D reduces high-fat diet induced weight gain and C-reactive protein, increases interleukin-10, and reduces CD86 and caspase-3. Pathophysiology. 2017;24(1):31–37. doi: 10.1016/j.pathophys.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Reis J.P., von Mühlen D., Miller E.R., 3rd, Michos E.D., Appel L.J. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124:e371–e379. doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly A., Brooks L.J., Dougherty S., Carlow D.C., Zemel B.S. A cross-sectional study of vitamin D and insulin resistance in children. Arch. Dis. Child. 2011;96(5):447–452. doi: 10.1136/adc.2010.187591. [DOI] [PubMed] [Google Scholar]
- 55.Olson M.L., Maalouf Naim M., Oden Jon D., White Perrin C., Hutchison Michele R. Vitamin D. Deficiency in obese children and its relationship to glucose Homeostasis. J Clin. Endocrinol. Metab. 2012;97(1):279–285. doi: 10.1210/jc.2011-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fornace A.J., Jr, Cummings D.E., Comeau C.M., Kant J.A., Crabtree G.R. Structure of the human gamma-fibrinogen gene. Alternate mRNA splicing near the 3’ end of the gene produces gamma A and gamma B forms of gamma-fibrinogen. J. Biol. Chem. 1984;259(20):12826–12830. [PubMed] [Google Scholar]
- 57.Nagel T., Meyer B. Simultaneous characterization of sequence polymorphisms, glycosylation and phosphorylation of fibrinogen in a direct analysis by LC-MS. Biochim. Biophys. Acta. 2014;1844(12):2284–2289. doi: 10.1016/j.bbapap.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 58.Rein-Smith C.M., Anderson N.W., Farrell D.H. Differential regulation of fibrinogen γ chain splice isoforms by interleukin-6. Thromb. Res. 2013;131(1):89–93. doi: 10.1016/j.thromres.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murakami T., Walczak R., Caron S., Duhem C., Vidal V., Darteil R., Staels B. The farnesoid X receptor induces fetuin-B gene expression in human hepatocytes. Biochem. J. 2007;407(3):461–469. doi: 10.1042/BJ20070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee C., Bongcam-Rudloff E., Sollner C., Jahnen-Dechent W., Claesson-Welsh L. Type 3 cystatins; fetuins, kininogen and histidine-rich glycoprotein. Front Biosci. (Landmark Ed.) 2009;14:2911–2922. doi: 10.2741/3422. [DOI] [PubMed] [Google Scholar]
- 61.Szweras M., Liu D., Partridge E.A., Pawling J., Sukhu B., Clokie C., Jahnen-Dechent W., Tenenbaum H.C., Swallow C.J., Grynpas M.D., Dennis J.W. Alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J. Biol. Chem. 2002;277(22):19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 62.Ombrellino M., Wang H., Yang H., Zhang M., Vishnubhakat J., Frazier A., Scher L.A., Friedman S.G., Tracey K.J. Fetuin, a negative acute phase protein, attenuates TNF synthesis and the innate inflammatory response to carrageenan. Shock. 2001;15(3):181–185. doi: 10.1097/00024382-200115030-00004. [DOI] [PubMed] [Google Scholar]
- 63.Stefan N., Hennige A.M., Staiger H., Machann J., Schick F., Kröber S.M., Machicao F., Fritsche A., Häring H.U. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29(4):853–857. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 64.Stefan N., Häring H.U., Schulze M.B. Association of fetuin-A level and diabetes risk. JAMA. 2008;300(19):2247. doi: 10.1001/jama.2008.614. [DOI] [PubMed] [Google Scholar]
- 65.Diao W.Q., Shen N., Du YP Liu B.B., Sun X.Y., Xu M., He B. Fetuin-B (FETUB): a plasma biomarker candidate related to the severity of lung function in COPD. Sci. Rep. 2016;6:30045. doi: 10.1038/srep30045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu K., Wang Y., Shu P., Zhou Q., Zhu J., Zhou W., Du C., Xu C., Liu X., Tang L. Increased serum levels of fetuin B in patients with coronary artery disease. Endocrine. 2017;doi doi: 10.1007/s12020-017-1387-1. [DOI] [PubMed] [Google Scholar]
- 67.Norman A.W. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147(12):5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 68.Gascon-Barré M., Demers C., Mirshahi A., Néron S., Zalzal S., Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37(5):1034–1042. doi: 10.1053/jhep.2003.50176. [DOI] [PubMed] [Google Scholar]
- 69.Moya M., Gómez-Lechón M.J., Castell J.V., Jover R. Enhanced steatosis by nuclear receptor ligands: a study in cultured human hepatocytes and hepatoma cells with a characterized nuclear receptor expression profile. Chem. Biol. Interact. 2010;184(3):376–387. doi: 10.1016/j.cbi.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Elangovan H., Chahal S., Gunton J.E. Vitamin D in liver disease: current evidence and potential directions. Biochim. Biophys. Acta. 2017;1863(4):907–916. doi: 10.1016/j.bbadis.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Liu M., Lee M.H., Cohen M., Bommakanti M., Freedman L.P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10(2):142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 72.Demay M.B., Kiernan M.S., DeLuca H.F., Kronenberg H.M. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U. S. A. 1992;89(17):8097–8101. doi: 10.1073/pnas.89.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alroy I., Towers T.L., Freedman L.P. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol. Cell. Biol. 1995;15(10):5789–5799. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Towers T.L., Staeva T.P., Freedman L.P. A two-hit mechanism for vitamin D3-mediated transcriptional repression of the granulocyte-macrophage colony-stimulating factor gene: vitamin d receptor competes for DNA binding with NFAT1 and stabilizes c-Jun. Mol. Cell. Biol. 1999;19(6):4191–4199. doi: 10.1128/mcb.19.6.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zenata O., Vrzal R. Fine tuning of vitamin D receptor (VDR) activity by post-transcriptional and post-translational modifications. Oncotarget. 2017;8(21):35390–35402. doi: 10.18632/oncotarget.15697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimizu M., Takai K., Moriwaki H. Strategy and mechanism for the prevention of hepatocellular carcinoma: phosphorylated retinoid X receptor alpha is a critical target for hepatocellular carcinoma chemoprevention. Cancer Sci. 2009;100(3):369–374. doi: 10.1111/j.1349-7006.2008.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blumberg J.M., Tzameli I., Astapova I., Lam F.S., Flier J.S., Hollenberg A.N. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J. Biol. Chem. 2006;281(16):11205–11213. doi: 10.1074/jbc.M510343200. [DOI] [PubMed] [Google Scholar]
- 78.Kong J., Li Y.C. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am. J. Physiol. Endocrinol. Metab. 2006;290(5):E916–E924. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- 79.Nimitphong H., Holick M.F., Fried S.K., Lee M.J. 25-hydroxyvitamin D₃ and 1,25-dihydroxyvitamin D₃ promote the differentiation of human subcutaneous preadipocytes. PLoS One. 2012;7(12):e52171. doi: 10.1371/journal.pone.0052171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manna P., Achari A.E., Jain S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017;615:22–34. doi: 10.1016/j.abb.2017.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.