Introduction
Fibroblast activation protein (FAP) was first described in 1986 by Rettig, et.al.(1), who named it based on its expression by reactive fibroblasts, especially in cancer(2). Independently, Aoyama and Chen(3) identified a serine protease on melanoma cells that they named seprase based on its enzymatic activity(4). By 1997, based on gene sequencing, it was demonstrated that FAP and seprase are the same molecule(5), and now FAP is the widely accepted nomenclature. FAP is a member of the dipeptidyl peptidase (DPP) family, and shares around 50% homology with DPPIV, its closest family member(6). Like other DPP enzymes, FAP has post-proline exopeptidase activity, but FAP is unique in also having gelatinase activity, which allows it to degrade denatured or MMP-cleaved collagen I(7). Structurally, FAP consists of a 6 amino acid cytoplasmic tail, a single 20 amino acid transmembrane domain, and a 734 amino acid extracellular domain(6). This extracellular domain consists of an eight-bladed beta propeller, which acts as a substrate selectivity gate, and an alpha/beta hydrolase domain(8). FAP monomers are not active, but form active homodimers as well as heterodimers with DPPIV(9). Soluble FAP has also been detected in various contexts; this appears to be the dimerized extracellular domains alone(10). FAP is expressed during development, but only rarely in healthy adult tissues(11). However, it is highly upregulated—especially on fibroblasts—at sites of active tissue remodeling, including wound healing, fibrosis, and cancer(12). In the context of cancer, FAP has gained notoriety as a marker of cancer-associated fibroblasts (CAFs), which have a number of pro-tumorigenic functions(12). Moreover, FAP itself has been demonstrated to have pro-tumorigenic activity, both through enzymatic and non-enzymatic means(12–14). In this review, we cover recent advances in FAP expression profiling, molecular function, and targeted therapies in the context of cancer, and pose a number of major questions about FAP that remain to be answered.
Patterns of FAP expression in cancer
FAP expression is typically low to undetectable in most normal adult tissues, but is highly upregulated in a multitude of cancers, including almost all carcinomas. In tumors, various mesenchymal cells express FAP, including mesenchymal stem cells (MSCs), CAFs, sarcoma, and melanoma cells(15–18). FAP expression on epithelial tumor cells has also been reported, but the prevalence and significance of this remains to be established. One difficulty in interpreting reports of FAP expression comes from the fact that, while FAP-specific antibodies do exist, some antibodies on the market lack specificity. Therefore, studies reporting on FAP expression using only immune-based assays should be interpreted based on inclusion of appropriate controls. Below, we summarize recent findings about FAP expression in terms of both tissue and cell type, with a focus on potential prognostic value (Table 1).
Table 1. FAP expression profiling in various tumor types.
Recent studies on patterns of FAP expression are summarized by tissue of cancer origin, cell type observed to express FAP, and methods of detection used. The relevant reagents, mostly antibodies, are reported with the same level of detail available in each study’s material and methods section.
| Cancer | Cell | Method | Reagents | Reference |
|---|---|---|---|---|
| Bone | Tumor | IHC | Mouse antibody (Santa Cruz Biotechnology, Inc.) | 98 |
| Brain | Astrocyte, stem cell, stroma, macs/fibrocyte | ELISA, WB, IF, qRT-PCR | DuoSet ELISA kit (R&D systems), rat monoclonal D8 and D28 (Vitatex), mouse monoclonal F19 | 22 |
| Breast | Fibroblast | IHC, IF, qRT-PCR | NR | 16 |
| Breast | Stroma, tumor | IHC | Rabbit polyclonal antibody ab53066 (Abcam) | 34 |
| Breast | Stroma, tumor | IHC | Polyclonal antibody (Abcam) | 36 |
| Breast | Stroma, tumor | IHC | Polyclonal antibody (Abcam) | 35 |
| Breast | NR | qRT-PCR | 99 | |
| Colorectal | Stroma | IHC | Unspecified antibody (Abcam) | 47 |
| Esophageal | NR | WB, RT-PCR | Rabbit polyclonal antibody N1N3 (GeneTex, Inc.) | 19 |
| Esophageal | Stroma | ELISA, IHC, WB, qRT-PCR | DuoSet ELISA kit (R&D systems), sheep polyclonal antibody AF3715 (R&D) | 25 |
| Esophageal | Stroma | IHC | Unspecified antibody (Abcam) | 54 |
| Gastric | Stroma | IHC, IF | Unspecified antibody (Boster Biological Technology) | 48 |
| Gastric | Mesothelia | IHC | Rabbit polyclonal antibody (Abcam) | 40 |
| Liver | Stroma | IHC | Rabbit polyclonal antibody SAB2900181 (Sigma) | 59 |
| Liver | Stroma | IHC | Rat monoclonal D8 (Vitatex) | 53 |
| Lung | Tumor | IHC | Rabbit polyclonal antibody (Assay Biotech) | 31 |
| Oral | Tumor | IHC, IF | Rabbit polyclonal antibody (LifeSpan BioSciences) | 51 |
| Ovarian | Stroma, tumor | IHC | Rabbit polyclonal antibody LS-A8023 (LifeSpan BioSciences) | 52 |
| Ovarian | Tumor, mesothelia | IHC, ISH | Rabbit polyclonal antibody ab53066 (Abcam) | 39 |
| Pancreatic | Stroma, tumor | ELISA, IHC, IF | DuoSet ELISA kit (R&D systems), polyclonal antibody (LSBio), mouse monoclonal F19 | 20 |
| Pancreatic | Stroma, tumor | IF | Mouse monoclonal F11-24 (Santa Cruz Biotechnology, Inc.) | 21 |
| Pancreatic | Stroma, tumor | IHC | Rabbit polyclonal antibody ab53066 (Abcam) | 37 |
| Pancreatic | Stroma, tumor | IHC, IF | Sheep polyclonal antibody AF3715 (R7D Systems), mouse monoclonal F19 | 38 |
| Parathyroid | NR | RT-PCR, qRT-PCR | 15 | |
| Renal | Stroma | IHC | Rabbit polyclonal antibody ab53066 (Abcam) | 45 |
| Renal | Stroma | IHC | Rabbit polyclonal antibody ab53066 (Abcam) | 46 |
ELISA: enzyme linked immunosorbent assay. IF: immunofluorescence. IHC: immunohistochemistry. ISH: in situ hybridization. NR: not reported. (q)RT-PCR: (quantitative) reverse transcriptase polymerase chain reaction. WB: western blot.
FAP expression in normal tissues
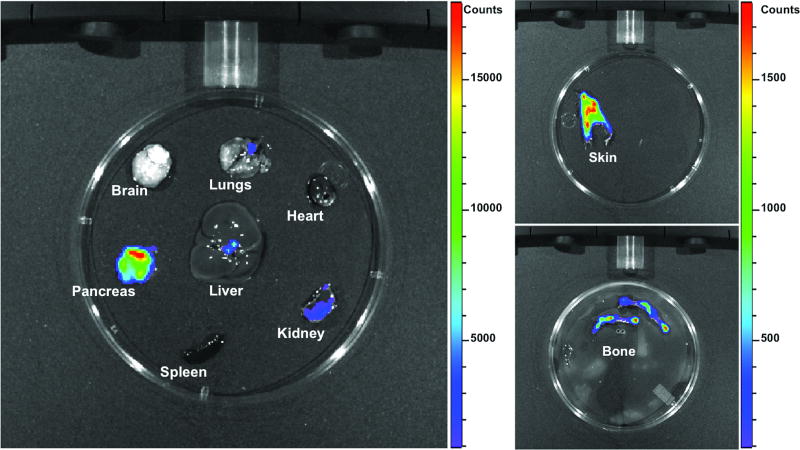
Many human studies rely on using tumor-adjacent tissues for controls. Yet even when these tissues do not show histological evidence of tumor cells, the presence of tumor elsewhere in the same organ can have effects on distal tissue, and thus classifying these samples as “normal” is often misleading. In human tumor-adjacent tissues, FAP was detectable at the RNA level by RT-PCR in the context of esophageal squamous cell carcinoma (ESCC)(19), lung carcinoma(18), and glioma(17). At the protein level, FAP was found in pancreatic ductal adenocarcinoma (PDAC) adjacent tissue(20,21). However, data obtained from non-tumor-bearing subjects suggest that some of these instances of tumor-adjacent FAP expression are not reflective of FAP expression in healthy tissues. For example, protein-level analysis in the brain did not detect FAP expression in samples from non-tumor bearing patients(22). A more systemic approach to FAP expression profiling in mice with extra-chromosomal luciferase under the control of the FAP promoter suggests that low basal levels of FAP expression might be found in many tissues, including muscle, bone marrow, adipose, skin, and pancreas(23). In line with this, using mice expressing luciferase under the control of the endogenous FAP promoter our lab detected expression in healthy adult murine skin, bone, pancreas, and—to a lesser extent—kidney (Fig1). In the pancreas, single cell RNA sequencing revealed that FAP expression is specific to alpha-cells within normal islets(24). Though the cellular source is unknown, FAP can also be detected in the plasma of healthy donors(20,25). These data suggest that, while FAP up-regulation in tumors does provide a potential therapeutic window, its expression in healthy tissues may not be as restricted as previously thought, and must be taken into consideration when evaluating the potential side effects of targeting FAP.
Figure 1. FAP expression in healthy adult mouse.
Genetically engineered mice with luciferase reporter knock-in at both FAP alleles were administered luciferin ten minutes before euthanasia and organs harvested for imaging. Results indicate that FAP is expressed in skin, bone, pancreas, and at very low levels in the kidney. (Data generated by Leslie Hopper and Michele Jacob)
FAP expression by various cell types
FAP expression by fibroblastic stromal cells is well established, but increasing evidence suggests that it may be expressed by additional cell types in the context of the tumor microenvironment (TME). Mature adipocytes undergo rapid de-differentiation when cultured in vitro, and this process was associated with induction of FAP expression(26). In human pancreas, FAP was expressed in glucagon+ alpha cells, often co-localizing with DPPIV expression(27). In glioma, using double immunofluorescence (IF) FAP was seen on mesenchymal stromal cells, but also on astrocytes, neural stem cells, and scattered CD45+ cells(22). These CD45+ cells might be fibrocytes or possibly a macrophage subset, as in murine lung cancer, FAP was expressed by some M2 (CD206+) macrophages(28). This result is consistent with past reports of FAP on macrophages in human breast cancer(29). In reconstructed skin cultures consisting of layered collagen, fibroblasts, melanocytes, and keratinocytes, FAP expression could be induced in melanocytes but not in keratinocytes(30).
Whether epithelial tumor cells express FAP is an area of ongoing research. Using immunohistochemistry (IHC), apparent cytoplasmic FAP stain was observed in lung cancer(31); unfortunately, this study appears to have relied on an antibody that—while marketed as targeting FAP—was actually generated against a peptide fragment of fas-associated phosphatase(32); a protein with no relation to fibroblast activation protein. However, using different antibodies for IHC, FAP has been detected in lung(33), breast(34–36) and pancreatic tumor cells(20,37). Using flow cytometry, FAP was detected on a subset of pancreatic tumor cells which also up-regulate Thy-1 (CD90), perhaps indicative of their undergoing EMT(38). FAP expression has been reported in mesothelial cells in the context of ovarian(39) and gastric cancer(40). In line with these results, certain epithelial tumor cell lines can express FAP, however this expression is often very low-level and/or requires non-physiological levels of induction stimuli(41,42). Pearl, et. al. reported FAP expression on circulating ovarian tumor cells, using a cell isolation method which was based on the ability of circulating cells to migrate through collagen-rich matrix. They were able to detect low levels of FAP mRNA in this cell population, though the protein-level analysis was confounded by their simultaneous, single-color staining for both FAP and CD44. As such, they were able to demonstrate that a population of CD45− circulating cells was able to ingest collagen matrix and express either CD44 or FAP. It would be interesting to demonstrate conclusively if these cells are migrating epithelial tumor cells or, perhaps, are tumor-associated mesenchymal cells traveling with metastasizing cells(43).
FAP expression and prognostic value in tumors
While individual reports of FAP’s prognostic value vary from study to study, across a wide range of human cancers FAP is reported to correlate to higher tumor grade and worse overall survival. A recent meta-analysis reported that across a range of IHC-based studies (many of which are discussed individually below) in various tumor types, the most consistent results associated high FAP expression with increased lymph node metastasis and poor overall survival(44). In breast invasive ductal carcinoma (IDC), FAP associated to higher grade, and was reported to correspond with inflammatory-type stroma(34) and adipose-type stroma(36), though correlations between FAP expression and more classical subtypes (e.g. hormone receptor positivity) were not replicable between studies. FAP expression was higher in invasive lobular carcinoma (ILC) than in invasive carcinoma of no special type(35). Interestingly, FAP expression appeared to correspond to malignancy of breast phyllodes tumors, whose rare transition from benign to malignant has been difficult to predict(16). In colorectal cancer (CRC), high FAP expression associated with worse overall survival(45–47). FAP expression in primary CRC also correlated to grade, as well as the sarcomatoid phenotype, which itself putatively relates to high levels of epithelial to mesenchymal transition (EMT)(46). FAP+ primary tumors yielded more lymph node metastasis, which themselves then expressed FAP(46). High FAP also corresponded to a shift in immune cell populations within the tumor: reduced CD3+ but increased CD11b+ cells(47). In gastric cancer, high FAP expression correlated to higher grade, lymph node and peritoneal invasion, and worse overall survival(40,48,49). In glioma, FAP expression associated to higher grade and the mesenchymal subtype(22). In intrahepatic cholangiocarcinoma (ICC), high FAP expression correlated to high CCL2 and STAT3 expression, along with reduced overall survival and increased probability of reccurrance(50). In oral squamous cell carcinoma (OSCC) high FAP expression was reported to associate with higher tumor stage, lymph node metastasis, and reduced overall survival(51). In ovarian cancer, high FAP associated to platinum chemotherapeutic resistance and probability of recurrence(52) as well as higher stage, lymph node metastasis, and reduced survival(39). In pancreatic ductal adenocarcinoma (PDAC), high FAP expression correlated to higher grade/stage and reduced survival(21,37,38).
Conversely, in hepatocellular carcinoma (HCC), FAP expression did not significantly correlate to grade, stage, or survival(53). Tumor-associated FAP was not found to be a robust predictor of overall survival in ESCC unless used in conjunction with other stromal markers(54). Interestingly, another study in ESCC found that low FAP levels in plasma robustly associated with higher tumor stage and low plasma HDL(25). Levels of circulating FAP did not serve as a robust biomarker for CRC unless combined with other markers(55). Collectively, these data indicate that, given the appropriate context and detection method, FAP may serve as a valuable prognostic marker across diverse human tumor types.
Molecular biology of FAP
While more groups are recognizing the usefulness of FAP as a marker for CAFs, the basic biology of FAP is understudied. Since FAP can and does play active pro-tumorigenic roles, understanding this basic biology might be crucial in exploiting FAP as a therapeutic target. Below we address recent advances in knowledge about the induction of FAP expression, its cellular localization, and what enzymatic and non-enzymatic roles it might play in cancer.
Transcriptional regulation
Multiple groups have demonstrated up-regulation of FAP expression driven by various tumor-derived factors (Fig 2). Fibroblasts and MSCs co-cultured directly with tumor cell lines show marked up-regulation of FAP(56,57), but no-contact co-culture with adenoma explants could also induce FAP expression by bone-marrow MSCs (BM-MSCs)(15), as could conditioned media from myeloma cells(57,58). Additionally, conditioned media from liver cancer cells enhanced FAP expression on umbilical cord MSCs(59) and hepatic stellate cells(60), the latter in a STAT3- dependent manner. Various factors may be implicated in tumor-mediated up-regulation of FAP. High doses of TNFα yielded increases in ERK1/2 phosphorylation along with modest increases in FAP expression in breast cancer cell lines(42), though similar doses did not yield significant up-regulation of FAP by BM-MSCs. BM-MSCs instead up-regulated FAP in response to IL-1β and TGFβ(61). Treatment of melanoma-bearing mice with an inhibitor of the A2BR adenosine receptor reduced the number of FAP+ cells in a transplant model of melanoma, while an A2BR agonist increased FAP+ cells. These cells showed enhanced ERK1/2 phosphorylation and expression of FGF2 and CXCL12. Interestingly, hypoxia induced the expression of CD73, which produces extracellular adenosine, indicating that hypoxia in the TME might enhance signaling through A2BR and thus induce FAP expression(62). Treatment of a human fibroblast line with progranulin enhanced FAP expression, as did co-culture with progranulin-expressing colon cancer cells. When progranulin was silenced in the tumor cells, FAP was not up-regulated(63). Luo et. al. reported that stimulation of primary fibroblasts with estrogen caused phosphorylation of ERK1/2 and upregulation of both TGFβ and FAP(64). Jia et. al. investigated estrogen signaling in the context of prostate cancer and discovered opposing activity of the classic estrogen receptor alpha (ERα) and an alternate receptor, GPR30. They noted that CAFs expressed higher levels of GPR30 and lower ERα than normal fibroblasts, and using both receptor overexpression and silencing studies demonstrated that FAP expression is promoted by GPR30 but inhibited by ERα(65).
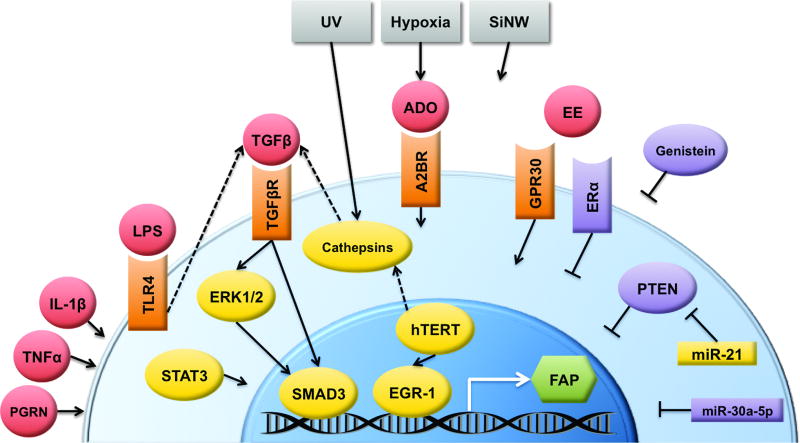
Figure 2. Potential regulatory networks of FAP transcription.
Multiple environmental and soluble factors have been observed to alter FAP expression, though detailed mechanistic pathways for many of them are unknown. The best characterized is TGFβ activation of SMAD3, which binds directly to the FAP promoter.
A2BR: adenosine 2B receptor. ADO: adenosine. EE: estrogens. EGR-1: early growth response protein 1. ERα: estrogen receptor alpha. ERK1/2: extracellular signal-related kinases 1 and 2. FAP: fibroblast activation protein. GPR30: g-protein coupled receptor 30. hTERT: human telomerase reverse transcriptase. IL-1β: interleukin 1 beta. LPS: lipopolysaccharide. miR: microRNA. PGRN: progranulin. PTEN: phosphatase and tensin homolog. SiNW: silicon nanowires. SMAD3: mothers against decapentaplegic homolog 3. STAT3: Signal transducer and activator of transcription 3. TGFβ: transforming growth factor beta. TGFβR: TGFβ receptor. TLR4: toll-like receptor 4. TNFα: tumor necrosis factor alpha. UV: ultraviolet radiation.
Malignant ascites-derived exosomes caused a modest increase in FAP expression by a mesothelial cell line, which was abrogated in the presence of a TGFβ inhibitor(66). Indeed, TGFβ seems to be at the center of most signaling pathways that have been implicated in FAP expression (Fig2). LPS signaling through TLR4 induced TGFβ expression by primary fibroblasts, which corresponded to high FAP expression(67). UV radiation induced FAP expression by up-regulating cathepsins which in turn induce TGFβ(30). hTERT induced both cathepsin D and EGR-1, resulting in increased FAP(68). This result has interesting implications for the use of cell lines in studying FAP expression, since any which have been immortalized by hTERT may show FAP up-regulation as an artifact of this process. The studies mentioned above demonstrate some link between FAP and various signaling pathways, though they do not demonstrate any direct action on the FAP promoter, meaning that the observed upregulation of FAP might be downstream of some master program of fibroblast activation. In contrast, promoter analysis has revealed direct roles for EGR-1(69) and SMAD3(70)—downstream of TGFβ signaling—in inducing FAP transcription. Promoter bioanalysis has also identified putative binding sites for AP-1, c/EBP and Ets proteins, and an E-box, though the functionality of these sequences is yet to be validated(70).
Less is known about what factors suppress FAP expression, either acutely or as part of basal regulation keeping FAP expression low in normal tissues (Fig2). As mentioned above, estrogen signaling through ERα specifically was observed to inhibit FAP expression, and ERα levels are higher in normal fibroblasts than in CAFs(65). Genistein—an isoflavone with multiple mechanisms of action on cells—was observed to reduce FAP expression on gastric cancer cell lines, through mechanisms unknown(71). Yet it is interesting to note that genistein is capable of acting as an agonist to various estrogen receptors(72,73). In the context of breast tumors, knockdown of PTEN induced FAP expression, indicating that PTEN is a FAP repressor. In the same study, miR-21 was demonstrated to be a negative regulator of PTEN, and thus a FAP inducer(16). Recently, miR-30a-5p was reported to target FAP mRNA directly, in OSCC cell lines(74). During their expression profiling of FAP in glioblastoma, Busek et. al. noted that the correlation between FAP mRNA levels (by RT-PCR) and protein levels (by ELISA) had an r value of only 0.32, and cases with detectable RNA were negative for protein; these observations together may be indicative of post-transcriptional regulation of FAP(22).
One highly understudied question surrounding the regulation of FAP expression is what roles biomechanical stimuli might play. Ha et. al. report that FAP expression was low to undetectable in fibroblasts cultured on 2D silicon surfaces, but reversibly up-regulated in culture on 3D silicon nanowire arrays. Levels of FAP were inversely proportional to wire length(75). What exact property of these arrays is responsible for the enhanced FAP expression has not been fully elucidated, though there are many intriguing possibilities. The 3D arrays induce formation of a larger number of filopodia, which is associated with higher adhesive force and expression of FAK(76). Arrays comprised of longer nanowires have lower spring constant than shorter wires, and this associates with reduced cell adhesion and expression of both FAK and alpha 2 integrin(77). Whether this—or some other—mechanosignaling pathway lies upstream of FAP expression is a very interesting question. Wäster, et. al. noted that extended in vitro culture of melanocytes caused both loss of FAP expression and acquisition of a senescent phenotype(30); our lab has had similar results with primary fibroblasts, which rapidly down-regulated FAP expression in monolayer culture on plastic (unpublished data). These results could indicate that FAP is highly sensitive to substratum mechanics.
Cellular localization
Active FAP resides in dimeric form on the plasma membrane(6). Wonganu and Berger identified a conserved region of the transmembrane domain that is necessary for dimerization. This sequence consists of 3 small, polar residues spaced four residues apart. If these residues are mutated, FAP monomers no longer associate, which also results in reduced enzymatic activity and intracellular accumulation of the mutant FAP, possibly indicating that FAP dimerization and cellular localization are linked(78). A rare human genetic mutation of FAP demonstrated that a single-nucleotide polymorphism in the beta-propeller domain also inhibited appropriate protein trafficking, resulting in an accumulation of mutant FAP in the endoplasmic reticulum. Mutant FAP was then degraded by the proteasome(79). Multiple groups have suggested that, once at the plasma membrane, FAP localizes to invadopodia(42,80,81). Knopf et. al. used a combination of mass spectrometry-based screening, co-immunoprecipitation (co-IP), and immunofluorescence (IF) to identify multiple FAP interaction partners, including caveolin-1 and stomatin which are known to localize to lipid rafts(81).
Secreted FAP can be found at varying levels in circulation, and appears to result from shedding of cell-surface FAP at invadopodia, but despite the fact that this has been known for some time, the exact mediators of FAP shedding are still unknown(80). This process might be very useful to understand, given that in more than one tumor type, levels of circulating FAP were inversely proportional to levels of tumor-associated FAP(20,25), suggesting that FAP activity within tumors might be at least partially regulated by cell-level retention. In support of this hypothesis, induction of FAP expression in fibroblasts did not always correlate to high levels of FAP shed into the supernatant(25). Up-regulation of FAP expression in response to various stimuli can be transient, and certain culture methods caused loss of FAP expression from previously expressing cells(26,30), indicating that FAP was undergoing turnover, though, again, the mechanisms controlling this phenomenon are unknown. Using western blotting, various groups observed protein bands which react with anti-FAP antibodies and yet have a smaller molecular mass than full length monomers; whether these are non-specific artifacts or indicate the presence of proteolytic fragments of FAP is unclear(19,78).
FAP substrates
FAP’s dual enzymatic activity gives it a range of putative substrates (Fig 3). Several reported substrates are shared with DPPIV, namely neuropeptides NPY, BNP, substance P, and PYY, which were all identified using an in vitro assay(82). Using ex vivo assays with addition of candidate substrates directly to FAP-containing plasma, Wong et. al. validated NPY as a physiological substrate of FAP(83). BNP, substance P, and PYY were far more efficiently cleaved by other proteases in the human plasma, indicating that physiologically, FAP is not likely responsible for cleavage of these factors(83). In the context of prostate cancer, FAP was able to cleave perlecan only after initial cleavage events by MMP7; this subsequent FAP activity generated unique fragments relative to MMP7 digestion alone(84). This result is similar to FAP activity on fibrillar collagens, which is dependent on initial cleavage by MMP collagenases(85,86). FAP cleavage of collagen I was shown to enhance macrophage adhesion in vitro, an effect which is independent of integrin binding but at least partially mediated by the macrophage scavenger receptor SR-A(87). Given that some tumor-associated macrophages express FAP, it is intriguing to speculate that FAP expression by macrophages might promote their recruitment to and/or retention by the TME. Taking a more global approach, Koczorowska, et. al. used a peptide library screen to identify a panel of putative FAP substrates, including ADAM15, IL-6, Serine Protease 23, Testican 1, and TGFβ1, though each of these candidates need to be validated as bona fide FAP substrates based on evidence of direct FAP activity on their proteolytic processing(88).
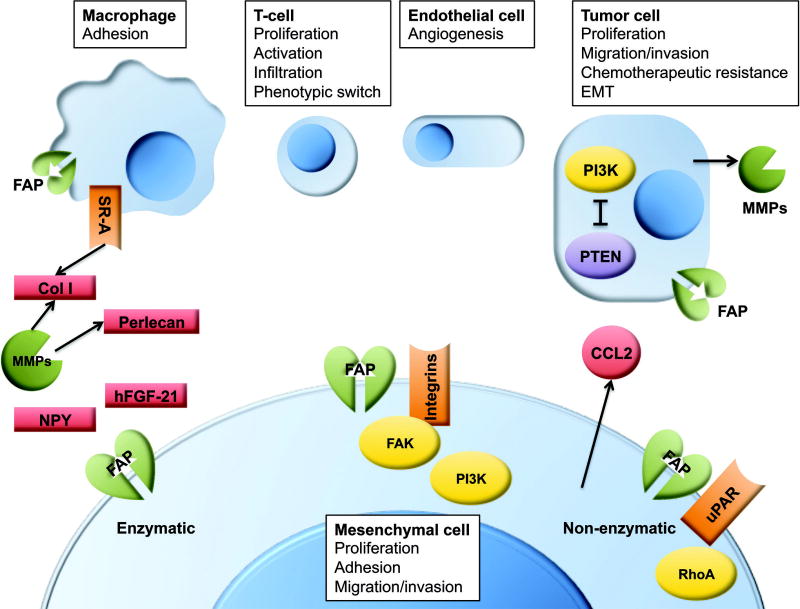
Figure 3. Selected mechanisms of action for FAP in the TME.
Shown are some of the best-elucidated pathways by which FAP promotes tumor growth. FAP can be expressed by macrophages, tumor cells, and tumor-promoting mesenchymal stromal cells, and it exerts effects though both enzymatic and non-enzymatic means.
CCL2: (MCP1) c-c motif chemokine ligand 2. Col I: type I collagen. FAK: focal adhesion kinase. FAP: fibroblast activation protein. hFGF-21: human fibroblast growth factor 21. MMPs: matrix metalloproteinases. NPY: neuropeptide Y. PI3K: phosphoinositide 3-kinase. PTEN: phosphatase and tensin homolog. RhoA: ras homolog gene family member A. SR-A: (MSR1) class A macrophage scavenger receptor. uPAR: urokinase plasminogen activator receptor.
Three groups recently investigated FAP cleavage of FGF21, which—in primates—contains a canonical gly-pro DPP cleavage site at the c-terminus. Given that FGF21 plays important roles in responding to metabolic stress, it is possible that FAP-mediated inactivation of FGF21 could promote systemic programs of obesity and inflammation that accelerate cancer growth(89). Ex vivo assays of FAP activity on human or monkey plasma revealed that FAP is able to cleave and inactivate FGF21, though murine FGF21 contains a gly to glu substitution which renders it resistant to FAP activity(90–92). Additionally, administration of FAP inhibitor to monkeys resulted in an increase of intact FGF21 in circulation(91). FAP is also capable of cleaving the n-terminus of FGF21, an event required for FGF21 activation, but depletion studies in human plasma demonstrated that n-terminal cleavage can occur in the absence of FAP. Conversely, FAP is indispensible for the c-terminal, inactivating cleavage event(92). These results suggest that FAP might play a role in systemic metabolic regulation, which can greatly influence tumor risk and progression.
Functional roles for FAP in cancer
Taken together, recent published results demonstrate that FAP can directly enhance proliferation, migration, and invasion of cells by which it is expressed (Fig 3). Co-culture with FAP+ cells can also promote proliferation, activation, and invasion of additional cell types including tumor, endothelial, and immune cells. One meta-analysis of TCGA gastric cancer data revealed that cases with high FAP expression also display enrichment of gene pathways for immune regulation, angiogenesis, cell migration, and cell differentiation and growth(49).
Direct roles of FAP on fibroblasts
Partial silencing of FAP in primary fibroblasts using siRNA resulted in modest reduction of both proliferation and production of the ECM molecules collagen I, fibronectin, and laminin(64). siFAP also inhibited UV-induced fibroblast invasion through basement membrane extract(30). Stable overexpression of FAP in a CAF line resulted in significant changes to the CAF secretome, including upregulation of proliferative, inflammatory, and ECM remodeling factors. Functionally, co-culture of these FAP+ CAFs with endothelial cells enhanced angiogenesis as measured by sprouting(88). This is consistent with prior results from FAP-null mice, that showed reduced vascularization in lung tumors(93).
Direct roles of FAP on mesenchymal tumor cells
Stable transfection of FAP in HT1080 fibrosarcoma cells resulted in increased adhesion to and migration on fibronectin and collagens I and IV; this could be reversed through antibody blocking of FAP, which itself resulted in internalization and loss of FAP from the cell surface. FAP+ cells expressed higher levels of integrin-signaling pathway components ILK, p-FAK, and Rac-1, and were also more sensitive to integrin blocking, displaying a greater loss of adhesion and migration than FAP− HT1080 cells given the same treatment. Treatment with Src kinase II or PI3K inhibitors reduced adhesion and migration of only the FAP+ cells, indicating that FAP plays a role in cell-ECM interactions through classical integrin signaling pathways(94). Tansi, et. al. reported that, when injected with matrigel plugs into mice, FAP+ HT1080 cells formed smaller primary tumors than the parental line, but the FAP+ cells showed more evidence of invasion through the plugs and the mice displayed enhanced lung metastasis(95). Somewhat conversely, Baird et. al. reported that FAP+ HT1080 cells had enhanced primary tumor growth relative to the parental line(96). However, these two studies may not be directly comparable due to differences in the overexpression lines, mice, and injection techniques used, making it difficult to conclude which is the more physiologically relevant result. In the second study, FAP+ cells were more sensitive to doxyrubicin (but not cisplatin), as measured by the MTT assay, but did not show up-regulation of classical markers of apoptosis or necrosis; instead, FAP+ cells treated with doxyrubicin were found to have increased mitochondrial membrane permeability, lysosomal permeability, and oxidative stress(96). FAPhi melanoma lines were more invasive in vitro; either TGFβ inhibition or direct silencing of FAP with shRNA reduced this invasiveness(97). In a separate study with FAP expressing melanoma cells in a zebrafish model, treatment with an anti-FAP antibody reduced invasive spread of tumor cells(30). Osteosarcoma lines with FAP knockdown showed reduced in vitro proliferation, migration, and adhesion to as well as invasion through matrigel(98).
Direct roles of FAP on epithelial tumor cells
In gastric cancer, tumor cell lines with more endogenous FAP expression proliferated faster, and siFAP treatment reduced this proliferation(71). Forced expression of FAP in MCF7 breast cancer cells, was able to enhance proliferation, but reduced both adhesion and migration, though FAP expression in the MDA-MB-231 line did not show comparable effects, suggesting that the impact of overexpression may be context dependent(99). siFAP treated OSCC cells displayed reduced colony formation, migration and invasion through matrigel(74). Similarly, shFAP treatment of OSCC lines resulted in reduced proliferation and migration with enhanced adhesion to fibronectin. This corresponded to enhanced primary tumor growth and experimental metastasis in a xenograft model. In a separate study, shFAP-treated OSCC cells showed decreased levels of cell-cycle promoters and markers of EMT with increased levels of MMP2 and 9, all indicative of an overall shift in cellular phenotype. Silencing of FAP also resulted in increased PTEN, associated with decreased PI3K, AKT, and ERK signaling. Silencing of PTEN was able to reverse the shFAP-induced reduction of proliferation, migration, and invasion, while transfection with constitutively active PTEN enhanced the phenotypes, suggesting that a PTEN/FAP axis can mediate tumorigenic cell behaviors(51).
Indirect effects of FAP on tumor cells
Recombinant FAP added to the culture media of ovarian cancer cells allowed enhanced survival in the presence of cisplatin, through mechanisms unknown(52). Conditioned media (CM) from CAFs promoted the in vitro proliferation and migration of gastric cancer cells, as well as survival of cancer cells when treated with chemotherapeutic agents. All of these effects were partially abrogated when the CAFs were treated with shFAP. Co-injection of cancer cells with FAP+ CAFs enhanced tumor growth while reducing T-cell infiltration; again, treatment of CAFs with shFAP reduced these tumor-promoting effects. shFAP CAFs allowed for improved T-cell function and better overall survival of mice relative to FAP+ CAFs(48). Transwell co-culture of lung cancer cells with FAP+ CAFs resulted in increased cancer cell proliferation, migration, and invasion through matrigel. Treatment of the CAFs with an anti-FAP antibody that results in FAP internalization had no effect on tumor cell proliferation, caused a slight reduction of migration, and significantly reduced invasion. This would suggest that FAP expression by CAFs can alter their secretome in such a way as to promote tumor cell invasion, but that FAP might be dispensable in CAF promotion of migration or proliferation(100), a result somewhat at odds with the prior study cited. This difference may be based on tumor context; e.g. gastric vs. lung cancer, but highlights the importance of understanding mechanistic pathways by which FAP expression on CAFs can alter tumor cell behavior. In direct co-culture with pancreatic tumor cells, FAP+ 3T3 fibroblasts—relative to parental FAP− 3T3 cells— enhanced tumor cell invasion through matrigel and progression through cell cycle, which was associated with increased phosphorylation of Rb in the tumor cells(37). Different CAF lines subcultured from the same tumor can display variable endogenous levels of FAP; in a transplant model of colon cancer, FAPhi CAFs—relative to FAPlo CAFs from the same primary source—inhibited the efficacy of anti-PD1 therapy and altered the immune cell profile in tumors, causing reduced numbers and functionality of T-cells but increased myeloid-derived suppressor cells (MDSCs). FAPhi CAFs also expressed more of the myeloid cell chemotactic factor CCL2, and inhibition of CCL2 abrogated their enhanced tumor-promoting effects. Inhibition of FAP with the DPP inhibitor linagliptin also abrogated these effects, but since linagliptin also inhibits DPPIV, we cannot conclude that FAP alone was directly responsible(47).
Indirect effects of FAP on immune cells
Co-culture with BM-MSCs, which endogenously express FAP, promoted survival of myeloma cell lines treated with bortezomib, an effect that was reduced by siFAP. In the presence of FAP+ BM-MSCs, bortezomib-treated tumor cells had enhanced expression of β-catenin, inhibition of which resulted in tumor cell apoptosis. Treatment of tumor cells with various combinations of putative BM-MSC-derived soluble factors was unable to replicate the drug-resistance phenotype, which could indicate a role for cell-contact(57). Co-culture of T-cells with BM-MSCs suppressed CD4+ T-cell proliferation and promoted senescence. BM-MSCs from healthy donors caused a shift in T-cell phenotype towards Tregs, while BM-MSCs from multiple myeloma (MM) patients induced a Th17 phenotype along with increased phosphorylation of AKT. Treatment of the MM BM-MSCs with the DPP inhibitor PT100 was able to restore T-cell proliferation and cause a shift back to Treg phenotype along with reduced p-AKT, indicating that FAP expression by stromal cells can have a profound effect on the immune compartment of tumors(58).
Non-enzymatic effects
While the experiments above suggest that the presence of FAP can enhance various tumorigenic processes, it is not always clear whether this is based on FAP’s enzymatic activity (Fig 3). MCF-7 breast cancer cells transfected to express FAP displayed enhanced growth, adhesion, and migration, and this was true even if the FAP construct contained an S624A mutation that ablates enzymatic activity. Wild-type and mutant FAP caused comparable increases in p-PI3K, p-AKT, and MMP9 expression, suggesting that, at least in this context, FAP might have effects on intrinsic cell signaling independent of its enzymatic activity(101). In BM-MSC, shFAP caused reduced migration through activation of RhoA; but inhibition of FAP with two unique peptidase inhibitors did not replicate the RhoA activation and loss of migratory ability(61). As above, Yang et. al. found that FAP+ CAFs—relative to FAP− CAFS—express more CCL2, as well as CXCL2, CXCL12, and IL6. In co-injection experiments they saw that only FAP+ CAFs were able to enhance tumor growth relative to tumor cells alone, and that this associated with increased tumoral MDSCs. Transplants into CCR2-null mice resulted in none of these phenotypes, indicating that CCL2 is the primary mediator of these effects. Upstream, CCL2 was induced by p-STAT3, silencing of uPAR in the CAFs reduced both STAT3 phosphorylation and CCL2 secretion, and uPAR was able to co-IP with FAP. Yet inhibition of FAP with PT100 does not reduce levels of p-STAT3 or CCL2 secretion, which suggests that association of FAP with uPAR induced pro-tumorigenic effects in a non-enzymatic manner(50). Clues to FAP’s non-enzymatic roles in cancer may be discerned from discovering other proteins that it associates with. Knopf et. al. demonstrated that FAP interacts with DPPIV, erlin-2, adenosine deaminase (ADA), stomatin, prohibitin-2, thy-1, and caveolin-1. FAP association with ADA was dependent on DPPIV, but coIP experiments suggest direct binding of FAP to erlin-2, stomatin, and caveolin-1(81). As yet unknown is what the functional consequences of these associations might be.
FAP-targeted therapies
Since FAP is a useful marker of CAFs, many potential FAP-targeted therapies have the ultimate goal of depleting FAP+ cells. Promising preclinical approaches have included various FAP-targeting vaccines and immunotherapies(102–104). Although the anti-tumorigenic effects of deleting FAP+ cells was validated using transgenic mice with diphtheria toxin receptor in FAP+ cells, these studies also revealed that total ablation of FAP+ cells results in impaired hematopoiesis and development of cachexia(23), indicating that there is a window of efficacy in terms of stromal cell depletion but dosing must be designed to avoid these potential side effects. For targeting FAP at the molecular level, various small molecule inhibitors have been employed, but one difficulty in designing FAP-specific inhibitors is the close homology that the active site shares with other DPP family members as well as prolyl oligopeptidase (PREP).
FAP+ cell depletion
Various vaccination modalities have been employed to target FAP+ cells in preclinical models, including DNA vaccines(105–108), adenoviral vectors(109), peptide immunization(110), and whole-cell vaccines(111,112). In addition, various immunotoxins(113–115), antibodies(116,117), FAP-targeting liposomes(118–121), and FAP-directed chimeric antigen receptor T-cells(122–124) can also deplete FAP+ cells and thus provide therapeutic benefit. From this plethora of treatments, certain patterns emerge. Functionally, FAP+ cells play important roles in immunosuppression(111,113,115,123), especially of CD8+ T-cells(109,112), as well as promoting desmoplasia(105,111,124). In terms of therapeutic efficacy, FAP-directed treatments often combine effectively with chemotherapy(108,114), tumor antigen vaccines(109,115), or antibody treatments(116,117). This could be an important consideration, since prior single-agent human trials, such as of the anti-FAP antibody sibrotuzumab, have shown limited efficacy(125).
FAP-activatable prodrugs
Another avenue of FAP-mediated therapy is the use of prodrugs which can be activated by FAP cleavage in the TME, thus increasing the dose which can be administered without systemic toxicity(126,127). Additional safety can be built in by requiring iterative activation events, as with an emitine-based prodrug which requires dual self-cleavage and FAP cleavage events in order to become active(128). Heightened efficacy can be achieved by linking multiple drugs with a FAP-cleavable linker, as with a construct where doxycycline and light-activatable phthalocyanine inactivate each other until separated by FAP-mediated cleavage(129). More sophisticated delivery methods designed to increase bioavailability to tumors include loading chemotherapeutics into nanoparticle carriers; in this case FAP can be used to activate the drugs(130) or to help dissemble the carrier to mediate drug release and/or uptake(131,132). Similar concepts can be applied to imaging techniques for cancer detection by using FAP-cleavable reporter constructs(133,134).
Enzymatic inhibition
Some of the small-molecules most commonly used as FAP inhibitors in research are PT-100 (Val-boro-Pro, talabostat) and linagliptin, but these inhibitors can also act on DPPIV. Despite promising preclinical studies, clinical trials of PT-100 have shown minimal efficacy, even in combination with chemotherapy(135–137), and the reasons for this disconnect are not fully known. More recently, In a murine transplant model of colon cancer, chemotherapeutic treatment with oxaliplatin increased CAF markers (FAP, vimentin) in tumor while reducing overall tumor volume and enhancing overall survival. Combination treatment with oxaliplatin and PT-100 reduced CAF markers as well as further reducing tumor growth and enhancing survival. Within tumors, the combined treatment promoted apoptosis, restricted accumulation of dendritic cells and macrophages, and reduced vascularization(138). The success of PT-100 in this specific model could be due to the fact that the chemotherapeutic appeared to first enhance the desmoplastic response thus expanding the tumor region that could be targeted by FAP inhibition. To extrapolate to human cases, FAP inhibition may be most useful for those patients with highly desmoplastic tumors.
Similarly, linagliptin was used in conjunction with anti-PD1 for treatment of murine gastric cancer transplant tumors. This combinatorial approach showed enhanced survival relative to either treatment alone, along with reduced tumor volume, reduced collagen accumulation, and an enhanced ratio of CD8+ T-cells to Tregs in both tumor and lymph node(48). A pseudopeptide inhibitor of both FAP and PREP was able to inhibit xenograft growth of lung and colon cancers, causing enhanced apoptosis, reduced vascularization and accumulation of thick collagen fibers within the tumors(139). While all these results are encouraging in terms of clinical benefit, since none of the inhibitors used are entirely specific to FAP, it cannot be determined whether inhibition of FAP is their sole mechanism of action. One approach to demonstrating the necessity of FAP activity to any phenotype is comparing a dual-specific inhibitor to a DPPIV specific inhibitor; using this approach in lung cancer demonstrated that inhibition of FAP reduced growth of murine lung and colon cancers(93).
Designing highly specific FAP inhibitors is not impossible, however, as a recent study by Jansen, et. al. examined around 60 structurally related small molecules for inhibition of FAP, PREP, DPPIV, DPP9, and DPP2 and discovered several structural components that can yield highly specific FAP inhibitors with low in vivo toxicity which may be good candidates for future preclinical studies(140). Since there is some indication that FAP and DPPIV may play opposing roles in some cancer contexts(71), it will be interesting to see if FAP-specific inhibitors are more efficacious than broader spectrum DPP inhibitors. In terms of delivery and integration into combinatorial therapies, conjugation of both a FAP inhibitor and a reporter fluorophore to a polymer carrier yielded tumor-specific fluorescent signal; this concept could be extrapolated to combined administration of FAP inhibitors with chemotheraputics(141).
Conclusions
The majority of recent studies relating to FAP in cancer fall into two categories: those that observe up-regulation of FAP in response to some stimuli and use this as a surrogate marker for pro-tumorigenic stroma, and those that describe therapeutic methods of depleting tumor stroma, using FAP as the target. Many of these papers are scientifically fascinating and clinically promising, but the fact remains that our understanding of FAP’s basic biology is far outstripped by our use of it as a molecular marker. A better understanding of FAP would allow for the design of even more physiologically relevant studies, which might in turn enhance the—thus far rather low—efficacy of FAP-targeted therapies in the clinic. Major consensus of recent results reveals that FAP expression is of prognostic value in multiple tumor types, and that FAP itself promotes pro-tumorigenic functions of various cell types, including the proliferation and motility of mesenchymal, tumor, and immune cells. In this review we have asked, at what levels might FAP be expressed in normal, healthy and/or tumor adjacent tissue? Answering this question would allow us to better delineate therapeutic windows of any FAP-targeted therapy and indicate the importance of tumor-targeted delivery of FAP-targeted therapies. We have also asked what cell types FAP is truly expressed on in vivo, and what role mechanosignaling plays in the regulation of FAP, understanding which would allow us to design better model systems for additional studies of FAP. Knowing what signaling pathways FAP interacts with, including which functional roles of FAP rely on enzymatic vs. non-enzymatic activity would expand our repertoire of FAP-related targets. These open questions create the possibility of much exciting research about FAP yet to come.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare
References
- 1.Rettig WJ, Chesa PG, Beresford HR, Feickert H-J, Jennings MT, Cohen J, et al. Differential Expression of Cell Surface Antigens and Glial Fibrillary Acidic Protein in Human Astrocytoma Subsets. [cited 2017 Aug 28];Cancer Res [Internet] 1986 46(12 Part 1) Available from: http://cancerres.aacrjournals.org/content/46/12_Part_1/6406.full-text.pdf. [PubMed] [Google Scholar]
- 2.Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Ozer HL, Schwab M, et al. Regulation and Heteromeric Structure of the Fibroblast Activation Protein in Normal and Transformed Cells of Mesenchymal and Neuroectodermal Origin1. [cited 2017 Aug 28];CANCER Res [Internet] 1993 53:3327–35. Available from: http://cancerres.aacrjournals.org/content/canres/53/14/3327.full.pdf. [PubMed] [Google Scholar]
- 3.Aoyama A, Chen WT. A 170-kDa membrane-bound protease is associated with the expression of invasiveness by human malignant melanoma cells. [cited 2017 Oct 2];Proc Natl Acad Sci U S A [Internet] 1990 Nov;87(21):8296–300. doi: 10.1073/pnas.87.21.8296. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2172980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monsky WL, Lin CY, Aoyama A, Kelly T, Akiyama SK, Mueller SC, et al. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. [cited 2017 Oct 2];Cancer Res [Internet] 1994 Nov 1;54(21):5702–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7923219. [PubMed] [Google Scholar]
- 5.Goldstein LA, Ghersi G, Piñeiro-Sánchez ML, Salamone M, Yeh Y, Flessate D, et al. Molecular cloning of seprase: a serine integral membrane protease from human melanoma. [cited 2017 Oct 2];Biochim Biophys Acta [Internet] 1997 Jul 10;1361(1):11–9. doi: 10.1016/s0925-4439(97)00032-x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9247085. [DOI] [PubMed] [Google Scholar]
- 6.Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. [cited 2017 Oct 2];Proc Natl Acad Sci U S A [Internet] 1994 Jun 7;91(12):5657–61. doi: 10.1073/pnas.91.12.5657. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7911242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast Activation Protein, a Dual Specificity Serine Protease Expressed in Reactive Human Tumor Stromal Fibroblasts. [cited 2017 Oct 3];J Biol Chem [Internet] 1999 Dec 17;274(51):36505–12. doi: 10.1074/jbc.274.51.36505. Available from: http://www.jbc.org/lookup/doi/10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 8.Aertgeerts K, Levin I, Shi L, Snell GP, Jennings A, Prasad GS, et al. J Biol Chem [Internet] 20. Vol. 280. American Society for Biochemistry and Molecular Biology; 2005. May 20, [cited 2017 Oct 3]. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha; pp. 19441–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15809306. [DOI] [PubMed] [Google Scholar]
- 9.Ghersi G, Zhao Q, Salamone M, Yeh Y, Zucker S, Chen W-T. The Protease Complex Consisting of Dipeptidyl Peptidase IV and Seprase Plays a Role in the Migration and Invasion of Human Endothelial Cells in Collagenous Matrices. [cited 2017 Oct 3];Cancer Res [Internet] 2006 May 1;66(9):4652–61. doi: 10.1158/0008-5472.CAN-05-1245. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16651416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KN, Jackson KW, Christiansen VJ, Lee CS, Chun J-G, McKee PA. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. [cited 2017 Oct 3];Blood [Internet] 2006 Feb 15;107(4):1397–404. doi: 10.1182/blood-2005-08-3452. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16223769. [DOI] [PubMed] [Google Scholar]
- 11.Niedermeyer J, Garin-Chesa P, Kriz M, Hilberg F, Mueller E, Bamberger U, et al. Expression of the fibroblast activation protein during mouse embryo development. [cited 2017 Oct 3];Int J Dev Biol [Internet] 2001 Apr;45(2):445–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11330865. [PubMed] [Google Scholar]
- 12.M Jacob LC, E P. Fibroblast Activation Protein in Remodeling Tissues. Curr Mol Med [Internet] 2012;12:1–24. doi: 10.2174/156652412803833607. Available from: http://linkinghub.elsevier.com/retrieve/pii/B9780123943088000030. [DOI] [PubMed] [Google Scholar]
- 13.Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. [cited 2017 Oct 3];PROTEOMICS - Clin Appl [Internet] 2014 Jun 8;(5–6):454–63. doi: 10.1002/prca.201300095. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24470260. [DOI] [PubMed]
- 14.Zi F, He J, He D, Li Y, Yang L, Cai Z. Fibroblast activation protein ?? in tumor microenvironment: Recent progression and implications (Review) Mol Med Rep. 2015;11(5):3203–11. doi: 10.3892/mmr.2015.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdelli C, Avagliano L, Creo P, Guarnieri V, Scillitani A, Vicentini L, et al. Tumour-associated fibroblasts contribute to neoangiogenesis in human parathyroid neoplasia. [cited 2017 Aug 29];Endocr Relat Cancer [Internet]. BioScientifica. 2015 Feb 1;22(1):87–98. doi: 10.1530/ERC-14-0161. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25515730. [DOI] [PubMed] [Google Scholar]
- 16.Gong C, Nie Y, Qu S, Liao J-Y, Cui X, Yao H, et al. miR-21 Induces Myofibroblast Differentiation and Promotes the Malignant Progression of Breast Phyllodes Tumors. [cited 2017 Aug 29];Cancer Res [Internet] 2014 74(16) doi: 10.1158/0008-5472.CAN-14-0125. Available from: http://cancerres.aacrjournals.org/content/74/16/4341.long. [DOI] [PubMed] [Google Scholar]
- 17.Krepela E, Busek P, Hilser M, Vanickova Z, Sedo A. [cited 2017 Aug 30];Species-specific real-time RT-PCR analysis of expression of stromal cell genes in a tumor xenotransplantation model in mice. 2017 doi: 10.1016/j.bbrc.2017.07.061. Available from: http://ac.els-cdn.com/S0006291X17314031/1-s2.0-S0006291X17314031-main.pdf?_tid=79d1019e-8d94-11e7-a27e-00000aab0f6c&acdnat=1504105629_542e1c7080b0448d8ea101b397be54cf. [DOI] [PubMed]
- 18.Tyulkina DV, Pleshkan VV, Alekseenko IV, Kopantseva MR, Sverdlov ED. Dokl Biochem Biophys [Internet] 1. Vol. 470. Pleiades Publishing; 2016. Sep 6, [cited 2017 Aug 30]. Expression of the FAP gene in non-fibroblast human cell lines. Development of cancer-associated fibroblast models; pp. 319–21. Available from: http://link.springer.com/10.1134/S1607672916050033. [DOI] [PubMed] [Google Scholar]
- 19.Augoff K, Hryniewicz-Jankowska A, Tabola R, Czapla L, Szelachowski P, Wierzbicki J, et al. Upregulated expression and activation of membrane-associated proteases in esophageal squamous cell carcinoma. [cited 2017 Aug 29];Oncol Rep [Internet] 2014 Jun;31(6):2820–6. doi: 10.3892/or.2014.3162. Available from: https://www.spandidos-publications.com/ [DOI] [PubMed] [Google Scholar]
- 20.Busek P, Vanickova Z, Hrabal P, Brabec M, Fric P, Zavoral M, et al. Increased tissue and circulating levels of dipeptidyl peptidase-IV enzymatic activity in patients with pancreatic ductal adenocarcinoma. [cited 2017 Aug 29];Pancreatology [Internet] 2016 Sep;16(5):829–38. doi: 10.1016/j.pan.2016.06.001. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27320722. [DOI] [PubMed] [Google Scholar]
- 21.Patsouras D, Papaxoinis K, Kostakis A, Safioleas MC, Lazaris AC, Nicolopoulou-Stamati P. Fibroblast activation protein and its prognostic significance in correlation with vascular endothelial growth factor in pancreatic adenocarcinoma. [cited 2017 Sep 16];Mol Med Rep [Internet] 2015 Jun;11(6):4585–90. doi: 10.3892/mmr.2015.3259. Available from: https://www.spandidos-publications.com/ [DOI] [PubMed] [Google Scholar]
- 22.Busek P, Balaziova E, Matrasova I, Hilser M, Tomas R, Syrucek M, et al. Tumor Biol [Internet] 10. Vol. 37. Springer; Netherlands: 2016. Oct 4, [cited 2017 Aug 30]. Fibroblast activation protein alpha is expressed by transformed and stromal cells and is associated with mesenchymal features in glioblastoma; pp. 13961–71. Available from: http://link.springer.com/10.1007/s13277-016-5274-9. [DOI] [PubMed] [Google Scholar]
- 23.Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med [Internet] 2013;210(6):1137–51. doi: 10.1084/jem.20122344. Available from: http://jem.rupress.org/content/210/6/1137.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackermann AM, Wang Z, Schug J, Naji A, Kaestner KH. Mol Metab [Internet] 3. Vol. 5. Elsevier; 2016. Mar, [cited 2017 Oct 30]. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes; pp. 233–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26977395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Y, Xing S, Xu B, Liu W, Zhang G. Evaluation of the circulating level of fibroblast activation protein α for diagnosis of esophageal squamous cell carcinoma. [cited 2017 Sep 16];Oncotarget [Internet]. Impact Journals, LLC. 2017 May 2;8(18):30050–62. doi: 10.18632/oncotarget.16274. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28415791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lessard J, Pelletier M, Biertho L, Biron S, Marceau S, Hould F-S, et al. PLoS One [Internet] 3. Vol. 10. Public Library of Science; 2015. [cited 2017 Aug 30]. Characterization of dedifferentiating human mature adipocytes from the visceral and subcutaneous fat compartments: fibroblast-activation protein alpha and dipeptidyl peptidase 4 as major components of matrix remodeling; p. e0122065. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25816202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busek P, Hrabal P, Fric P, Sedo A. Histochem Cell Biol [Internet] 5. Vol. 143. Springer Berlin Heidelberg; 2015. May 2, [cited 2017 Aug 30]. Co-expression of the homologous proteases fibroblast activation protein and dipeptidyl peptidase-IV in the adult human Langerhans islets; pp. 497–504. Available from: http://link.springer.com/10.1007/s00418-014-1292-0. [DOI] [PubMed] [Google Scholar]
- 28.Arnold JN, Magiera L, Kraman M, Fearon DT. Cancer Immunol Res [Internet] 2. Vol. 2. Europe PMC Funders; 2014. Feb, [cited 2017 Aug 30]. Tumoral immune suppression by macrophages expressing fibroblast activation protein-α and heme oxygenase-1; pp. 121–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24778275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tchou J, Zhang PJ, Bi Y, Satija C, Marjumdar R, Stephen TL, et al. Hum Pathol [Internet] 11. Vol. 44. Elsevier Inc.; 2013. Fibroblast activation protein expression by stromal cells and tumor-associated macrophages in human breast cancer; pp. 2549–57. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wäster P, Orfanidis K, Eriksson I, Rosdahl I, Seifert O, Öllinger K. Br J Cancer [Internet] 4. Vol. 117. Nature Publishing Group; 2017. Aug 8, [cited 2017 Aug 31]. UV radiation promotes melanoma dissemination mediated by the sequential reaction axis of cathepsins–TGF-β1–FAP-α; pp. 535–44. Available from: http://www.nature.com/doifinder/10.1038/bjc.2017.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du H, Chen D, Zhou Y, Han Z, Che G. Fibroblast phenotypes in different lung diseases. [cited 2017 Aug 29];J Cardiothorac Surg [Internet]. BioMed Central. 2014 Sep 5;9:147. doi: 10.1186/s13019-014-0147-z. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25189096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanov VN, Lopez Bergami P, Maulit G, Sato T-A, Sassoon D, Ronai Z. Mol Cell Biol [Internet] 10. Vol. 23. American Society for Microbiology; 2003. May 15, [cited 2017 Oct 24]. FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface; pp. 3623–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12724420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science (80-) [Internet] 2010;330(6005):827–30. doi: 10.1126/science.1195300. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21051638%5Cn http://www.sciencemag.org/content/330/6005/827%5Cn http://www.sciencemag.org/content/330/6005/827.full.pdf. [DOI] [PubMed] [Google Scholar]
- 34.Park SY, Kim HM, Koo JS. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. [cited 2017 Aug 29];Breast Cancer Res Treat [Internet] 2015 Feb 10;149(3):727–41. doi: 10.1007/s10549-015-3291-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25667103. [DOI] [PubMed] [Google Scholar]
- 35.Park CK, Jung WH, Koo JS. Breast Cancer Res Treat [Internet] 1. Vol. 159. Springer; US: 2016. Aug 28, [cited 2017 Aug 29]. Expression of cancer-associated fibroblast-related proteins differs between invasive lobular carcinoma and invasive ductal carcinoma; pp. 55–69. Available from: http://link.springer.com/10.1007/s10549-016-3929-2. [DOI] [PubMed] [Google Scholar]
- 36.Yang Jung Y, Kyung Lee Y, Seung Koo J. [cited 2017 Aug 29];Expression of cancer-associated fibroblast-related proteins in adipose stroma of breast cancer. doi: 10.1007/s13277-015-3594-9. Available from: https://link.springer.com/content/pdf/10.1007%2Fs13277-015-3594-9.pdf. [DOI] [PubMed]
- 37.Kawase T, Yasui Y, Nishina S, Hara Y, Yanatori I, Tomiyama Y, et al. Fibroblast activation protein-α-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. [cited 2017 Aug 29];BMC Gastroenterol [Internet]. BioMed Central. 2015 Sep 2;15:109. doi: 10.1186/s12876-015-0340-0. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26330349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo A, Li C-P, Buza EL, Blomberg R, Govindaraju P, Avery D, et al. JCI Insight [Internet] 19. Vol. 2. American Society for Clinical Investigation; 2017. Oct 5, [cited 2017 Oct 30]. Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. Available from: https://insight.jci.org/articles/view/92232#sd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Xu L, Wang X, Sun B, Ding J. Oncol Lett [Internet] 1. Vol. 10. Spandidos Publications; 2015. Jul, [cited 2017 Sep 16]. Expression levels of seprase/FAPα and DPPIV/CD26 in epithelial ovarian carcinoma; pp. 34–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26170973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miao Z-F, Zhao T-T, Wang Z-N, Miao F, Xu Y-Y, Mao X-Y, et al. Tumor Biol [Internet] 6. Vol. 35. Springer; Netherlands: 2014. Jun 11, [cited 2017 Sep 16]. Tumor-associated mesothelial cells are negative prognostic factors in gastric cancer and promote peritoneal dissemination of adherent gastric cancer cells by chemotaxis; pp. 6105–11. Available from: http://link.springer.com/10.1007/s13277-014-1808-1. [DOI] [PubMed] [Google Scholar]
- 41.Kahounová Z, Kurfürstová D, Bouchal J, Kharaishvili G, Navrátil J, Remšík J, et al. The fibroblast surface markers FAP, anti-fibroblast, and FSP are expressed by cells of epithelial origin and may be altered during epithelial-to-mesenchymal transition. [cited 2017 Aug 30];Cytom Part A [Internet] 2017 Apr; doi: 10.1002/cyto.a.23101. Available from: http://doi.wiley.com/10.1002/cyto.a.23101. [DOI] [PubMed]
- 42.Wolczyk D, Zaremba-Czogalla M, Hryniewicz-Jankowska A, Tabola R, Grabowski K, Sikorski AF, et al. Cell Oncol (Dordr) [Internet] 4. Vol. 39. Springer; 2016. Aug, [cited 2017 Aug 31]. TNF-α promotes breast cancer cell migration and enhances the concentration of membrane-associated proteases in lipid rafts; pp. 353–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27042827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearl ML, Dong H, Tulley S, Zhao Q, Golightly M, Zucker S, et al. Gynecol Oncol [Internet] 2. Vol. 137. NIH Public Access; 2015. May, [cited 2017 Aug 30]. Treatment monitoring of patients with epithelial ovarian cancer using invasive circulating tumor cells (iCTCs) pp. 229–38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25769657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu F, Qi L, Liu B, Liu J, Zhang H, Che D, et al. PLoS One [Internet] 3. Vol. 10. Public Library of Science; 2015. [cited 2017 Sep 16]. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis; p. e0116683. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25775399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López JI, Errarte P, Erramuzpe A, Guarch R, Cortés JM, Angulo JC, et al. Fibroblast activation protein predicts prognosis in clear cell renal cell carcinoma. [cited 2017 Sep 16];Hum Pathol [Internet] 2016 Aug;54:100–5. doi: 10.1016/j.humpath.2016.03.009. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0046817716300247. [DOI] [PubMed] [Google Scholar]
- 46.Errarte P, Guarch R, Pulido R, Blanco L, Nunes-Xavier CE, Beitia M, et al. PLoS One [Internet] 12. Vol. 11. Public Library of Science; 2016. [cited 2017 Sep 16]. The Expression of Fibroblast Activation Protein in Clear Cell Renal Cell Carcinomas Is Associated with Synchronous Lymph Node Metastases; p. e0169105. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28033421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Qiu X, Wang X, He J. FAP positive fibroblasts induce immune checkpoint blockade resistance in colorectal cancer via promoting immunosuppression. [cited 2017 Aug 25];Biochem Biophys Res Commun [Internet] 2017 487:8–14. doi: 10.1016/j.bbrc.2017.03.039. Available from: http://ac.els-cdn.com/S0006291X17304965/1-s2.0-S0006291X17304965-main.pdf?_tid=37ba96e2-89ae-11e7-ad0d-00000aab0f27&acdnat=1503676881_cf62ae0155062dc532540052be201851. [DOI] [PubMed] [Google Scholar]
- 48.Wen X, He X, Jiao F, Wang C, Sun Y, Ren X, et al. Fibroblast Activation Protein-α-Positive Fibroblasts Promote Gastric Cancer Progression and Resistance to Immune Checkpoint Blockade. [cited 2017 Aug 25];Oncol Res Featur Preclin Clin Cancer Ther [Internet] 2017 Apr 14;25(4):629–40. doi: 10.3727/096504016X14768383625385. Available from: http://www.ingentaconnect.com/content/10.3727/096504016X14768383625385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu M, Qian C, Hu Z, Fei B, Zhou H. Biomarkers in Tumor Microenvironment? Upregulation of Fibroblast Activation Protein-α Correlates with Gastric Cancer Progression and Poor Prognosis. [cited 2017 Sep 16];Omi A J Integr Biol [Internet] 2017 Jan;21(1):38–44. doi: 10.1089/omi.2016.0159. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28206814. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, et al. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3–CCL2 Signaling. [cited 2017 Sep 6];Cancer Res [Internet] 2016 76(14) doi: 10.1158/0008-5472.CAN-15-2973. Available from: http://cancerres.aacrjournals.org/content/76/14/4124.long. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y, et al. Cell Death Dis [Internet] 4. Vol. 5. Nature Publishing Group; 2014. Apr 10, [cited 2017 Sep 6]. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma; p. e1155. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24722280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mhawech-Fauceglia P, Yan L, Sharifian M, Ren X, Liu S, Kim G, et al. Cancer Microenviron [Internet] 1. Vol. 8. Springer; 2015. Apr, [cited 2017 Sep 16]. Stromal Expression of Fibroblast Activation Protein Alpha (FAP) Predicts Platinum Resistance and Shorter Recurrence in patients with Epithelial Ovarian Cancer; pp. 23–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25331442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim GJ, Rhee H, Yoo JE, Ko JE, Lee JS, Kim H, et al. PLoS One [Internet] 8. Vol. 9. Public Library of Science; 2014. [cited 2017 Sep 16]. Increased expression of CCN2, epithelial membrane antigen, and fibroblast activation protein in hepatocellular carcinoma with fibrous stroma showing aggressive behavior; p. e105094. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25126747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ha SY, Yeo S-Y, Xuan Y, Kim S-H. PLoS One [Internet] 6. Vol. 9. Public Library of Science; 2014. [cited 2017 Sep 16]. The prognostic significance of cancer-associated fibroblasts in esophageal squamous cell carcinoma; p. e99955. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24945657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Werner S, Krause F, Rolny V, Strobl M, Morgenstern D, Datz C, et al. Evaluation of a 5-Marker Blood Test for Colorectal Cancer Early Detection in a Colorectal Cancer Screening Setting. [cited 2017 Sep 16];Clin Cancer Res [Internet] 2016 22(7) doi: 10.1158/1078-0432.CCR-15-1268. Available from: http://clincancerres.aacrjournals.org/content/22/7/1725.long. [DOI] [PubMed] [Google Scholar]
- 56.Park J-I, Lee J, Kwon J-L, Park H-B, Lee S-Y, Kim J-Y, et al. Transl Oncol [Internet] 1. Vol. 9. Neoplasia Press; 2016. Feb, [cited 2017 Aug 31]. Scaffold-Free Coculture Spheroids of Human Colonic Adenocarcinoma Cells and Normal Colonic Fibroblasts Promote Tumorigenicity in Nude Mice; pp. 79–88. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26947885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zi F-M, He J-S, Li Y, Wu C, Wu W-J, Yang Y, et al. Cancer Biol Ther [Internet] 10. Vol. 15. Taylor & Francis; 2014. Oct, [cited 2017 Sep 6]. Fibroblast activation protein protects bortezomib-induced apoptosis in multiple myeloma cells through β-catenin signaling pathway; pp. 1413–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25046247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Wang Y, Xu J, Luo T, Deng J, Hu Y, et al. MM-BMSCs induce naïve CD4+ T lymphocytes dysfunction through fibroblast activation protein α. [cited 2017 Sep 11];Oncotarget [Internet]. Impact Journals. 2017 Aug 7;8(32):52614–28. doi: 10.18632/oncotarget.17538. Available from: http://www.oncotarget.com/fulltext/17538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J, Miao Y, Chang Y, Zhang F, Wang Y, Zheng S. Am J Transl Res [Internet] 8. Vol. 8. e-Century Publishing Corporation; 2016. [cited 2017 Aug 31]. Condition medium of HepG-2 cells induces the transdifferentiation of human umbilical cord mesenchymal stem cells into cancerous mesenchymal stem cells; pp. 3429–38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27648133. [PMC free article] [PubMed] [Google Scholar]
- 60.Cui Y, Sun S, Ren K, Quan M, Song Z, Zou H, et al. Reversal of liver cancer-associated stellate cell-induced stem-like characteristics in SMMC-7721 cells by 8-bromo-7-methoxychrysin via inhibiting STAT3 activation. [cited 2017 Aug 31];Oncol Rep [Internet] 2016 May;35(5):2952–62. doi: 10.3892/or.2016.4637. Available from: https://www.spandidos-publications.com/ [DOI] [PubMed] [Google Scholar]
- 61.Chung K-M, Hsu S-C, Chu Y-R, Lin M-Y, Jiaang W-T, Chen R-H, et al. PLoS One [Internet] 2. Vol. 9. Public Library of Science; 2014. [cited 2017 Sep 4]. Fibroblast activation protein (FAP) is essential for the migration of bone marrow mesenchymal stem cells through RhoA activation; p. e88772. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24551161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorrentino C, Miele L, Porta A, Pinto A, Morello S. Activation of the A2B adenosine receptor in B16 melanomas induces CXCL12 expression in FAP-positive tumor stromal cells, enhancing tumor progression. [cited 2017 Aug 31];Oncotarget [Internet]. Impact Journals, LLC. 2016 Sep 27;7(39):64274–88. doi: 10.18632/oncotarget.11729. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27590504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Yang D, Tian J, Gao A, Shen Y, Ren X, et al. Tumor necrosis factor receptor 2/AKT and ERK signaling pathways contribute to the switch from fibroblasts to CAFs by progranulin in microenvironment of colorectal cancer. [cited 2017 Aug 31];Oncotarget [Internet]. Impact Journals, LLC. 2017 Apr 18;8(16):26323–33. doi: 10.18632/oncotarget.15461. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28412748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo N, Guan Q, Zheng L, Qu X, Dai H, Cheng Z. Transl Res [Internet] 3. Vol. 163. Elsevier; 2014. Mar 1, [cited 2017 Aug 31]. Estrogen-mediated activation of fibroblasts and its effects on the fibroid cell proliferation; pp. 232–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24316382. [DOI] [PubMed] [Google Scholar]
- 65.Jia B, Gao Y, Li M, Shi J, Peng Y, Du X, et al. Endocrinology [Internet] 8. Vol. 157. Oxford University Press; 2016. Aug 1, [cited 2017 Aug 31]. GPR30 Promotes Prostate Stromal Cell Activation via Suppression of ERα Expression and Its Downstream Signaling Pathway; pp. 3023–35. Available from: https://academic.oup.com/endo/article-lookup/doi/10.1210/en.2016-1035. [DOI] [PubMed] [Google Scholar]
- 66.Wei M, Yang T, Chen X, Wu Y, Deng X, He W, et al. Malignant ascites-derived exosomes promote proliferation and induce carcinoma-associated fibroblasts transition in peritoneal mesothelial cells. [cited 2017 Aug 31];Oncotarget [Internet]. Impact Journals, LLC. 2017 Jun 27;8(26):42262–71. doi: 10.18632/oncotarget.15040. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28178689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo J, Zheng L, Chen L, Luo N, Yang W, Qu X, et al. Int J Clin Exp Pathol [Internet] 9. Vol. 8. e-Century Publishing Corporation; 2015. [cited 2017 Aug 31]. Lipopolysaccharide activated TLR4/NF-κB signaling pathway of fibroblasts from uterine fibroids; pp. 10014–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26617709. [PMC free article] [PubMed] [Google Scholar]
- 68.Park Y-J, Kim EK, Bae JY, Moon S, Kim J. Human telomerase reverse transcriptase (hTERT) promotes cancer invasion by modulating cathepsin D via early growth response (EGR)-1. [cited 2017 Aug 31];Cancer Lett [Internet] 2016 Jan;370(2):222–31. doi: 10.1016/j.canlet.2015.10.021. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0304383515006527. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Valianou M, Cheng JD. Front Biosci (Elite Ed) [Internet] Vol. 2. NIH Public Access; 2010. Jun 1, [cited 2017 Nov 20]. Identification and characterization of the promoter of fibroblast activation protein; pp. 1154–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20515787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tulley S, Chen W-T. J Biol Chem [Internet] 22. Vol. 289. American Society for Biochemistry and Molecular Biology; 2014. May 30, [cited 2017 Aug 30]. Transcriptional regulation of seprase in invasive melanoma cells by transforming growth factor-β signaling; pp. 15280–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24727589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong Q, Shi W, Li L, Wu X, Ma H. Anal Chem [Internet] 16. Vol. 88. American Chemical Society; 2016. Aug 16, [cited 2017 Sep 6]. Ultrasensitive Fluorescent Probes Reveal an Adverse Action of Dipeptide Peptidase IV and Fibroblast Activation Protein during Proliferation of Cancer Cells; pp. 8309–14. Available from: http://pubs.acs.org/doi/abs/10.1021/acs.analchem.6b02231. [DOI] [PubMed] [Google Scholar]
- 72.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. [cited 2017 Oct 25];Endocrinology [Internet] 1998 Oct;139(10):4252–63. doi: 10.1210/endo.139.10.6216. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9751507. [DOI] [PubMed] [Google Scholar]
- 73.Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. [cited 2017 Oct 25];Mol Cell Endocrinol [Internet] 2014 May 25;389(1–2):71–83. doi: 10.1016/j.mce.2014.02.002. NIH Public Access; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24530924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruan P, Tan A, Tao Z. Low expression of miR-30a-5p induced the proliferation and invasion of oral cancer via promoting the expression of FAP. [cited 2017 Oct 16];Biosci Rep [Internet] 2017 Oct 12;:BSR20171027. doi: 10.1042/BSR20171027. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29026005. [DOI] [PMC free article] [PubMed]
- 75.Ha Q, Yang G, Ao Z, Han D, Niu F, Wang S, et al. Nanoscale [Internet] 14. Vol. 6. The Royal Society of Chemistry; 2014. Jun 26, [cited 2017 Aug 31]. Rapid fibroblast activation in mammalian cells induced by silicon nanowire arrays; p. 8318. Available from: http://xlink.rsc.org/?DOI=c4nr01415d. [DOI] [PubMed] [Google Scholar]
- 76.Qi S, Yi C, Ji S, Fong C-C, Yang M. [cited 2017 Oct 20];Cell Adhesion and Spreading Behavior on Vertically Aligned Silicon Nanowire Arrays. doi: 10.1021/am800027d. Available from: http://pubs.acs.org/doi/pdf/10.1021/am800027d. [DOI] [PubMed]
- 77.Kuo S-W, Lin H-I, Hui J, Ho-Chun, Shih Y-RV, Chen H-F, et al. Regulation of the fate of human mesenchymal stem cells by mechanical and stereo-topographical cues provided by silicon nanowires. [cited 2017 Oct 20];Biomaterials [Internet] 2012 33:5013–22. doi: 10.1016/j.biomaterials.2012.03.080. Available from: https://ac.els-cdn.com/S0142961212003936/1-s2.0-S0142961212003936-main.pdf?_tid=a2fbcfe6-b5de-11e7-8d1c-00000aacb35e&acdnat=1508535530_85f62f339f9808a716e0a198b7d8ec50. [DOI] [PubMed] [Google Scholar]
- 78.Wonganu B, Berger BW. A specific, transmembrane interface regulates fibroblast activation protein (FAP) homodimerization, trafficking and exopeptidase activity. [cited 2017 Sep 4];Biochim Biophys Acta - Biomembr [Internet] 2016 Aug;1858(8):1876–82. doi: 10.1016/j.bbamem.2016.05.001. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0005273616301432. [DOI] [PubMed] [Google Scholar]
- 79.Osborne B, Yao T-W, Wang XM, Chen Y, Kotan LD, Nadvi NA, et al. A rare variant in human fibroblast activation protein associated with ER stress, loss of enzymatic function and loss of cell surface localisation. [cited 2017 Sep 4];Biochim Biophys Acta - Proteins Proteomics [Internet] 2014 1844(7):1248–59. doi: 10.1016/j.bbapap.2014.03.015. Available from: http://www.sciencedirect.com/science/article/pii/S1570963914000855. [DOI] [PubMed] [Google Scholar]
- 80.Mueller SC, Ghersi G, Akiyama SK, Sang QX, Howard L, Pineiro-Sanchez M, et al. A novel protease-docking function of integrin at invadopodia. [cited 2017 Oct 24];J Biol Chem [Internet] 1999 Aug 27;274(35):24947–52. doi: 10.1074/jbc.274.35.24947. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10455171. [DOI] [PubMed] [Google Scholar]
- 81.Knopf JD, Tholen S, Koczorowska MM, De Wever O, Biniossek ML, Schilling O. The stromal cell-surface protease fibroblast activation protein-α localizes to lipid rafts and is recruited to invadopodia. [cited 2017 Sep 4];Biochim Biophys Acta - Mol Cell Res [Internet] 2015 Oct;1853(10):2515–25. doi: 10.1016/j.bbamcr.2015.07.013. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0167488915002505. [DOI] [PubMed] [Google Scholar]
- 82.Keane FM, Nadvi NA, Yao T-W, Gorrell MD. FEBS J [Internet] 8. Vol. 278. Blackwell Publishing Ltd; 2011. Apr 1, [cited 2017 Sep 5]. Neuropeptide Y, B-type natriuretic peptide, substance P and peptide YY are novel substrates of fibroblast activation protein-α; pp. 1316–32. Available from: http://doi.wiley.com/10.1111/j.1742-4658.2011.08051.x. [DOI] [PubMed] [Google Scholar]
- 83.Wong PF, Gall MG, Bachovchin WW, McCaughan GW, Keane FM, Gorrell MD. Neuropeptide Y is a physiological substrate of fibroblast activation protein: Enzyme kinetics in blood plasma and expression of Y2R and Y5R in human liver cirrhosis and hepatocellular carcinoma. [cited 2017 Sep 5];Peptides [Internet] 2016 Jan;75:80–95. doi: 10.1016/j.peptides.2015.11.004. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0196978115300048. [DOI] [PubMed] [Google Scholar]
- 84.Grindel BJ, Martinez JR, Pennington CL, Muldoon M, Stave J, Chung LW, et al. Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. [cited 2017 Sep 5];Matrix Biol [Internet] 2014 Jun;36:64–76. doi: 10.1016/j.matbio.2014.04.005. NIH Public Access; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan M-H, Zhu Q, Li H-H, Ra H-J, Majumdar S, Gulick DL, et al. Fibroblast Activation Protein (FAP) Accelerates Collagen Degradation and Clearance from Lung in Mice. J Biol Chem [Internet] 2015;291(15):8070–89. doi: 10.1074/jbc.M115.701433. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26663085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Christiansen VJ, Jackson KW, Lee KN, McKee PA. Effect of fibroblast activation protein and α2-antiplasmin cleaving enzyme on collagen Types I, III, and IV. [cited 2017 Oct 25];Arch Biochem Biophys [Internet] 2007 Jan 15;457(2):177–86. doi: 10.1016/j.abb.2006.11.006. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17174263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazur A, Holthoff E, Vadali S, Kelly T, Post SR. PLoS One [Internet] 3. Vol. 11. Public Library of Science; 2016. [cited 2017 Sep 5]. Cleavage of Type I Collagen by Fibroblast Activation Protein-α Enhances Class A Scavenger Receptor Mediated Macrophage Adhesion; p. e0150287. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26934296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koczorowska MM, Tholen S, Bucher F, Lutz L, Kizhakkedathu JN, De Wever O, et al. Mol Oncol [Internet] 1. Vol. 10. Wiley-Blackwell; 2016. Jan, [cited 2017 Sep 5]. Fibroblast activation protein-α, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations; pp. 40–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26304112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luo Y, Ye S, Chen X, Gong F, Lu W, Li X. Rush to the fire: FGF21 extinguishes metabolic stress, metaflammation and tissue damage. [cited 2017 Oct 30];Cytokine Growth Factor Rev [Internet] 2017 Aug 26; doi: 10.1016/j.cytogfr.2017.08.001. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28887067. [DOI] [PubMed]
- 90.Coppage AL, Heard KR, DiMare MT, Liu Y, Wu W, Lai JH, et al. PLoS One [Internet] 3. Vol. 11. Public Library of Science; 2016. [cited 2017 Sep 5]. Human FGF-21 Is a Substrate of Fibroblast Activation Protein; p. e0151269. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26962859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dunshee DR, Bainbridge TW, Kljavin NM, Zavala-Solorio J, Schroeder AC, Chan R, et al. Fibroblast Activation Protein Cleaves and Inactivates Fibroblast Growth Factor 21. J Biol Chem [Internet] 2016;291(11):5986–96. doi: 10.1074/jbc.M115.710582. Available from: http://www.jbc.org/lookup/doi/10.1074/jbc.M115.710582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhen EY, Jin Z, Ackermann BL, Thomas MK, Gutierrez JA. Biochem J [Internet] 5. Vol. 473. Portland Press Ltd; 2016. Mar 1, [cited 2017 Sep 5]. Circulating FGF21 proteolytic processing mediated by fibroblast activation protein; pp. 605–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26635356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santos A, Jung J, Aziz N, Kissil J. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin [Internet] 2009;119(12):3613–25. doi: 10.1172/JCI38988. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2786791/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baird SK, Allan L, Renner C, Scott FE, Scott AM. Clin Exp Metastasis [Internet] 5. Vol. 32. Springer Netherlands; 2015. Jun 21, [cited 2017 Sep 4]. Fibroblast activation protein increases metastatic potential of fibrosarcoma line HT1080 through upregulation of integrin-mediated signaling pathways; pp. 507–16. Available from: http://link.springer.com/10.1007/s10585-015-9723-4. [DOI] [PubMed] [Google Scholar]
- 95.Tansi FL, Rüger R, Böhm C, Kontermann RE, Teichgraeber UK, Fahr A, et al. Data Br [Internet] Vol. 9. Elsevier; 2016. Dec, [cited 2017 Sep 6]. Dataset on FAP-induced emergence of spontaneous metastases and on the preparation of activatable FAP-targeting immunoliposomes to detect the metastases; pp. 143–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27642620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baird SK, Rigopoulos A, Cao D, Allan L, Renner C, Scott FE, et al. Apoptosis [Internet] 11. Vol. 20. Springer US; 2015. Nov 5, [cited 2017 Sep 6]. Integral membrane protease fibroblast activation protein sensitizes fibrosarcoma to chemotherapy and alters cell death mechanisms; pp. 1483–98. Available from: http://link.springer.com/10.1007/s10495-015-1166-5. [DOI] [PubMed] [Google Scholar]
- 97.Tulley S, Chen W-T. J Biol Chem [Internet] 22. Vol. 289. American Society for Biochemistry and Molecular Biology; 2014. May 30, [cited 2017 Aug 31]. Transcriptional regulation of seprase in invasive melanoma cells by transforming growth factor-β signaling; pp. 15280–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24727589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding L, Ye L, Xu J, Jiang WG. Oncol Lett [Internet] 3. Vol. 7. Spandidos Publications; 2014. Mar, [cited 2017 Sep 6]. Impact of fibroblast activation protein on osteosarcoma cell lines in vitro; pp. 699–704. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24520291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia J, Martin TA, Ye L, Jiang WG. FAP-α (Fibroblast activation protein-α) is involved in the control of human breast cancer cell line growth and motility via the FAK pathway. [cited 2017 Sep 6];BMC Cell Biol [Internet]. BioMed Central. 2014 May 21;15:16. doi: 10.1186/1471-2121-15-16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24885257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teichgräber V, Monasterio C, Chaitanya K, Boger R, Gordon K, Dieterle T, et al. Specific inhibition of fibroblast activation protein (FAP)-alpha prevents tumor progression in vitro. [cited 2017 Sep 6];Adv Med Sci [Internet] 2015 Sep;60(2):264–72. doi: 10.1016/j.advms.2015.04.006. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1896112615000279. [DOI] [PubMed] [Google Scholar]
- 101.Lv B, Xie F, Zhao P, Ma X, Jiang WG, Yu J, et al. Cancer Genomics Proteomics [Internet] 3. Vol. 13. International Institute of Anticancer Research; 2016. May 1, [cited 2017 Sep 4]. Promotion of Cellular Growth and Motility Is Independent of Enzymatic Activity of Fibroblast Activation Protein-α; pp. 201–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27107062. [PubMed] [Google Scholar]
- 102.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor Immunotherapy Targeting Fibroblast Activation Protein, a Product Expressed in Tumor-Associated Fibroblasts. [cited 2017 Oct 26];Cancer Res [Internet] 2005 Dec 1;65(23):11156–63. doi: 10.1158/0008-5472.CAN-05-2805. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16322266. [DOI] [PubMed] [Google Scholar]
- 103.Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116(7):1955–62. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ostermann E, Garin-Chesa P, Heider KH, Kalat M, Lamche H, Puri C, et al. Effective Immunoconjugate Therapy in Cancer Models Targeting a Serine Protease of Tumor Fibroblasts. [cited 2017 Oct 26];Clin Cancer Res [Internet] 2008 Jul 15;14(14):4584–92. doi: 10.1158/1078-0432.CCR-07-5211. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18628473. [DOI] [PubMed] [Google Scholar]
- 105.Xia Q, Zhang F-F, Geng F, Liu C-L, Xu P, Lu Z-Z, et al. Cancer Immunol Immunother [Internet] 5. Vol. 65. Springer; Berlin Heidelberg: 2016. May 28, [cited 2017 Sep 19]. Anti-tumor effects of DNA vaccine targeting human fibroblast activation protein α by producing specific immune responses and altering tumor microenvironment in the 4T1 murine breast cancer model; pp. 613–24. Available from: http://link.springer.com/10.1007/s00262-016-1827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xia Q, Geng F, Zhang FF, Liu CL, Xu P, Lu ZZ, et al. Vaccine [Internet] 38. Vol. 34. Elsevier; 2016. Aug 31, [cited 2017 Sep 19]. Enhancement of fibroblast activation protein α-based vaccines and adenovirus boost immunity by cyclophosphamide through inhibiting IL-10 expression in 4T1 tumor bearing mice; pp. 4526–35. Available from: http://www.sciencedirect.com/science/article/pii/S0264410X16306521. [DOI] [PubMed] [Google Scholar]
- 107.Xia Q, Zhang FF, Geng F, Liu CL, Wang YQ, Xu P, et al. Cell Immunol [Internet] Vol. 310. Academic Press; 2016. Dec 1, [cited 2017 Sep 19]. Improvement of anti-tumor immunity of fibroblast activation protein α based vaccines by combination with cyclophosphamide in a murine model of breast cancer; pp. 89–98. Available from: http://www.sciencedirect.com/science/article/pii/S0008874916300788. [DOI] [PubMed] [Google Scholar]
- 108.Xia Q, Geng F, Zhang F-F, Liu C-L, Xu P, Lu Z-Z, et al. Cyclophosphamide enhances anti-tumor effects of a fibroblast activation protein α-based DNA vaccine in tumor-bearing mice with murine breast carcinoma. [cited 2017 Sep 20];Immunopharmacol Immunotoxicol [Internet] 2017 Jan 2;39(1):37–44. doi: 10.1080/08923973.2016.1269337. Available from: https://www.tandfonline.com/doi/full/10.1080/08923973.2016.1269337. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Ertl HCJ. Depletion of FAP+ cells reduces immunosuppressive cells and improves metabolism and functions CD8+T cells within tumors. [cited 2017 Sep 19];Oncotarget [Internet]. Impact Journals, LLC. 2016 Apr 26;7(17):23282–99. doi: 10.18632/oncotarget.7818. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26943036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang G-M, Xie W-Y, Wang H-S, Du J, Wu B-P, Xu W, et al. Curcumin combined with FAPαc vaccine elicits effective antitumor response by targeting indolamine-2,3-dioxygenase and inhibiting EMT induced by TNF-α in melanoma. [cited 2017 Sep 19];Oncotarget [Internet]. Impact Journals, LLC. 2015 Sep 22;6(28):25932–42. doi: 10.18632/oncotarget.4577. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26305550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meng M, Wang W, Yan J, Tan J, Liao L, Shi J, et al. Tumor Biol [Internet] 8. Vol. 37. Springer; Netherlands; 2016. Aug 3, [cited 2017 Sep 19]. Immunization of stromal cell targeting fibroblast activation protein providing immunotherapy to breast cancer mouse model; pp. 10317–27. Available from: http://link.springer.com/10.1007/s13277-016-4825-4. [DOI] [PubMed] [Google Scholar]
- 112.Chen M, Xiang R, Wen Y, Xu G, Wang C, Luo S, et al. Sci Rep [Internet] Vol. 5. Nature Publishing Group; 2015. Sep 23, [cited 2017 Sep 19]. A whole-cell tumor vaccine modified to express fibroblast activation protein induces antitumor immunity against both tumor cells and cancer-associated fibroblasts; p. 14421. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26394925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhen Z, Tang W, Wang M, Zhou S, Wang H, Wu Z, et al. Nano Lett [Internet] 2. Vol. 17. American Chemical Society; 2017. Feb 8, [cited 2017 Sep 19]. Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy To Enhance Cytotoxic T Cell Infiltration and Tumor Control; pp. 862–9. Available from: http://pubs.acs.org/doi/abs/10.1021/acs.nanolett.6b04150. [DOI] [PubMed] [Google Scholar]
- 114.Fang J, Xiao L, Joo K-I, Liu Y, Zhang C, Liu S, et al. Int J cancer [Internet] 4. Vol. 138. NIH Public Access; 2016. Feb 15, [cited 2017 Sep 19]. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice; pp. 1013–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26334777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fang J, Hu B, Li S, Zhang C, Liu Y, Wang P. Mol Ther oncolytics [Internet] Vol. 3. American Society of Gene & Cell Therapy; 2016. [cited 2017 Sep 19]. A multi-antigen vaccine in combination with an immunotoxin targeting tumor-associated fibroblast for treating murine melanoma; p. 16007. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27119119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang T, Wang H, Chen NG, Frentzen A, Minev B, Szalay AA. Mol Ther oncolytics [Internet] Vol. 2. American Society of Gene & Cell Therapy; 2015. [cited 2017 Sep 19]. Expression of anti-VEGF antibody together with anti-EGFR or anti-FAP enhances tumor regression as a result of vaccinia virotherapy; p. 15003. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brünker P, Wartha K, Friess T, Grau-Richards S, Waldhauer I, Koller CF, et al. Mol Cancer Ther [Internet] 5. Vol. 15. American Association for Cancer Research; 2016. May 1, [cited 2017 Sep 26]. RG7386, a Novel Tetravalent FAP-DR5 Antibody, Effectively Triggers FAP-Dependent, Avidity-Driven DR5 Hyperclustering and Tumor Cell Apoptosis; pp. 946–57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27037412. [DOI] [PubMed] [Google Scholar]
- 118.Rüger R, Tansi FL, Rabenhold M, Steiniger F, Kontermann RE, Fahr A, et al. In vivo near-infrared fluorescence imaging of FAP-expressing tumors with activatable FAP-targeted, single-chain Fv-immunoliposomes. [cited 2017 Sep 26];J Control Release [Internet] 2014 186:1–10. doi: 10.1016/j.jconrel.2014.04.050. Available from: https://ac.els-cdn.com/S0168365914002831/1-s2.0-S0168365914002831-main.pdf?_tid=38a91ec6-a2cb-11e7-a30a-00000aacb362&acdnat=1506438117_fc3bdc59cdca28879ece931311ac8901. [DOI] [PubMed] [Google Scholar]
- 119.Rabenhold M, Steiniger F, Fahr A, Kontermann RE, Rüger R. Bispecific single-chain diabody-immunoliposomes targeting endoglin (CD105) and fibroblast activation protein (FAP) simultaneously. [cited 2017 Sep 26];J Control Release [Internet] 2015 201:56–67. doi: 10.1016/j.jconrel.2015.01.022. Available from: https://ac.els-cdn.com/S0168365915000620/1-s2.0-S0168365915000620-main.pdf?_tid=6871ce9c-a2ca-11e7-8d58-00000aacb35f&acdnat=1506437769_30e86a2d2e937acf19e3217a55102acd. [DOI] [PubMed] [Google Scholar]
- 120.Tansi FL, Rüger R, Ohm CB€, Kontermann RE, Teichgraeber UK, Fahr A, et al. [cited 2017 Sep 26];Potential of activatable FAP-targeting immunoliposomes in intraoperative imaging of spontaneous metastases. 2016 doi: 10.1016/j.biomaterials.2016.02.028. Available from: https://ac.els-cdn.com/S0142961216001423/1-s2.0-S0142961216001423-main.pdf?_tid=a34441fe-a2c9-11e7-a61d-00000aacb361&acdnat=1506437437_6429503039d2b63dc5fb3b670a82c1eb. [DOI] [PubMed]
- 121.Tansi FL, Rüger R, Böhm C, Steiniger F, Kontermann RE, Teichgraeber UK, et al. Activatable bispecific liposomes bearing fibroblast activation protein directed single chain fragment/Trastuzumab deliver encapsulated cargo into the nuclei of tumor cells and the tumor microenvironment simultaneously. [cited 2017 Sep 25];Acta Biomater [Internet] 2017 54:281–93. doi: 10.1016/j.actbio.2017.03.033. Available from: [DOI] [PubMed] [Google Scholar]
- 122.Kakarla S, Chow KKH, Mata M, Shaffer DR, Song X-T, Wu M-F, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther [Internet] 2013;21(8):1611–20. doi: 10.1038/mt.2013.110. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res [Internet] 2014;2(2):154–66. doi: 10.1158/2326-6066.CIR-13-0027. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24778279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lo A, Wang L-CS, Scholler J, Monslow J, Avery D, Newick K, et al. Cancer Res [Internet] 14. Vol. 75. NIH Public Access; 2015. Jul 15, [cited 2017 Sep 19]. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells; pp. 2800–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25979873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hofheinz R-D, al-Batran S-E, Hartmann F, Hartung G, Jäger D, Renner C, et al. Stromal Antigen Targeting by a Humanised Monoclonal Antibody: An Early Phase II Trial of Sibrotuzumab in Patients with Metastatic Colorectal Cancer. [cited 2017 Oct 26];Oncol Res Treat [Internet] 2003 Mar 14;26(1):44–8. doi: 10.1159/000069863. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12624517. [DOI] [PubMed] [Google Scholar]
- 126.Deng L-J, Wang L-H, Peng C-K, Li Y-B, Huang M-H, Chen M-F, et al. J Med Chem [Internet] 13. Vol. 60. American Chemical Society; 2017. Jul 13, [cited 2017 Sep 25]. Fibroblast Activation Protein α Activated Tripeptide Bufadienolide Antitumor Prodrug with Reduced Cardiotoxicity; pp. 5320–33. Available from: http://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.6b01755. [DOI] [PubMed] [Google Scholar]
- 127.Brennen WN, Rosen DM, Chaux A, Netto GJ, Isaacs JT, Denmeade SR. Prostate [Internet] 13. Vol. 74. NIH Public Access; 2014. Sep, [cited 2017 Sep 26]. Pharmacokinetics and toxicology of a fibroblast activation protein (FAP)-activated prodrug in murine xenograft models of human cancer; pp. 1308–19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25053236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Akinboye ES, Brennen WN, Rosen DM, Bakare O, Denmeade SR. Iterative design of emetine-based prodrug targeting fibroblast activation protein (FAP) and dipeptidyl peptidase IV DPPIV using a tandem enzymatic activation strategy. [cited 2017 Sep 26];Prostate [Internet] 2016 Jun;76(8):703–14. doi: 10.1002/pros.23162. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26835873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ke MR, Chen SF, Peng XH, Zheng QF, Zheng BY, Yeh CK, et al. A tumor-targeted activatable phthalocyanine-tetrapeptide-doxorubicin conjugate for synergistic chemo-photodynamic therapy. Eur J Med Chem. 2017 doi: 10.1016/j.ejmech.2016.12.056. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Y, Zhang X, Liu H, Cai S, Wu B. Int J Nanomedicine [Internet] Vol. 10. Dove Press; 2015. [cited 2017 Sep 26]. Mixed nanomicelles as potential carriers for systemic delivery of Z-GP-Dox, an FAPα-based doxorubicin prodrug: formulation and pharmacokinetic evaluation; pp. 1625–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25759584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ji T, Zhao Y, Ding Y, Wang J, Zhao R, Lang J, et al. Angew Chem Int Ed Engl [Internet] 3. Vol. 55. Wiley-Blackwell; 2016. Jan 18, [cited 2017 Sep 26]. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation; pp. 1050–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26283097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim M-G, Shon Y, Kim J, Oh Y-K. J Natl Cancer Inst [Internet] 1. Vol. 109. Oxford University Press; 2017. Jan 11, [cited 2017 Sep 26]. Selective Activation of Anticancer Chemotherapy by Cancer-Associated Fibroblasts in the Tumor Microenvironment; p. djw186. Available from: https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/djw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng X, Wang Q, Liao Y, Zhou X, Wang Y, Liu W, et al. Int J Nanomedicine [Internet] Vol. 12. Dove Press; 2017. [cited 2017 Sep 25]. A synthetic urinary probe-coated nanoparticles sensitive to fibroblast activation protein α for solid tumor diagnosis; pp. 5359–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28794628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bainbridge TW, Dunshee DR, Kljavin NM, Skelton NJ, Sonoda J, Ernst JA. Sci Rep [Internet] 1. Vol. 7. Nature Publishing Group; 2017. Dec 2, [cited 2017 Oct 5]. Selective Homogeneous Assay for Circulating Endopeptidase Fibroblast Activation Protein (FAP) p. 12524. Available from: http://www.nature.com/articles/s41598-017-12900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Narra K, Mullins SR, Lee H-O, Strzemkowski-Brun B, Magalong K, Christiansen VJ, et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. [cited 2017 Oct 26];Cancer Biol Ther [Internet] 2007 Nov;6(11):1691–9. doi: 10.4161/cbt.6.11.4874. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18032930. [DOI] [PubMed] [Google Scholar]
- 136.Eager RM, Cunningham CC, Senzer N, Richards DA, Raju RN, Jones B, et al. Phase II Trial of Talabostat and Docetaxel in Advanced Non-small Cell Lung Cancer. [cited 2017 Oct 26];Clin Oncol [Internet] 2009 Aug;21(6):464–72. doi: 10.1016/j.clon.2009.04.007. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19501491. [DOI] [PubMed] [Google Scholar]
- 137.Eager RM, Cunningham CC, Senzer NN, Stephenson J, Anthony SP, O’Day SJ, et al. Phase II assessment of talabostat and cisplatin in second-line stage IV melanoma. [cited 2017 Oct 26];BMC Cancer [Internet] 2009 Dec 30;9(1):263. doi: 10.1186/1471-2407-9-263. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19643020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li M, Li M, Yin T, Shi H, Wen Y, Zhang B, et al. Mol Med Rep [Internet] 3. Vol. 13. Spandidos Publications; 2016. Mar, [cited 2017 Sep 26]. Targeting of cancer-associated fibroblasts enhances the efficacy of cancer chemotherapy by regulating the tumor microenvironment; pp. 2476–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26846566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jackson KW, Christiansen VJ, Yadav VR, Silasi-Mansat R, Lupu F, Awasthi V, et al. Neoplasia [Internet] 1. Vol. 17. Neoplasia Press; 2015. Jan, [cited 2017 Sep 26]. Suppression of tumor growth in mice by rationally designed pseudopeptide inhibitors of fibroblast activation protein and prolyl oligopeptidase; pp. 43–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25622898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jansen K, Heirbaut L, Verkerk R, Cheng JD, Joossens J, Cos P, et al. J Med Chem [Internet] 7. Vol. 57. American Chemical Society; 2014. Apr 10, [cited 2017 Sep 26]. Extended Structure–Activity Relationship and Pharmacokinetic Investigation of (4-Quinolinoyl)glycyl-2-cyanopyrrolidine Inhibitors of Fibroblast Activation Protein (FAP) pp. 3053–74. Available from: http://pubs.acs.org/doi/abs/10.1021/jm500031w. [DOI] [PubMed] [Google Scholar]
- 141.Dvořáková P, Bušek P, Knedlík T, Schimer J, Etrych T, Kostka L, et al. J Med Chem [Internet] American Chemical Society; 2017. Sep 27, [cited 2017 Sep 29]. Inhibitor-Decorated Polymer Conjugates Targeting Fibroblast Activation Protein. acs.jmedchem.7b00767. Available from: http://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.7b00767. [DOI] [PubMed] [Google Scholar]