Abstract
AIM
To observe the effect of herb-partitioned moxibustion (HPM) on expression of colonic cytokines in ulcerative colitis (UC) rats.
METHODS
A UC rat model was established by protein immunization in combination with topical chemical stimulation. Rats in the HPM group (n = 8) received HPM at bilateral Tianshu (ST25) points. The gross injury and pathological scores of the colon were recorded. The expression profile of colonic cytokines was assayed using the protein microarray technique. Specific differential cytokines were selected and verified by ELISA. The corresponding UniProt Accessions of the differentially expressed cytokines were retrieved in the UniProt database. The pathways involved were analyzed with the help of the KEGG PATHWAY database. The DAVID database was used for functional cluster and pathway analysis.
RESULTS
HPM improved colon injuries in UC rats, manifested by accelerated repair of ulcers and alleviation of inflammation, and the gross injury and pathological scores both significantly decreased (P < 0.01). Fold change > 1.3 or < 0.77 was taken as the screening standard. There were 77 down-regulated and 9 up-regulated differentially expressed colonic cytokines in the HPM group compared with the model group, and expression of 20 differed significantly (P < 0.05). Twelve of the 20 significantly differentially expressed cytokines [β-catenin, interleukin-1 receptor 6 (IL-1R6), IL-1β, B7-1, nerve growth factor receptor, AMP-activated protein kinase-α1, neuropilin-2, orexin A, adipocyte differentiation-related protein, IL-2, Fas and FasL] were up-regulated in the model group (n = 3, compared with the normal group) but down-regulated in the HPM group (n = 3, compared with the model group). Functional cluster analysis showed that the differentially expressed colonic cytokines in the HPM group regulated apoptosis and protein phosphorylation. KEGG pathway analysis showed that 52 down-regulated and 7 up-regulated differentially expressed colonic cytokines in the HPM group had pathways. The pathways that interacted between the cytokines and their receptors accounted for the largest proportion (28 of the down-regulated and 5 of the up-regulated cytokines).
CONCLUSION
HPM promotes the repair of colon injuries in UC rats, which is related to the regulation of several abnormally expressed cytokines.
Keywords: Cytokine expression profile, Ulcerative colitis, Protein microarray, Rats, Herb-partitioned moxibustion
Core tip: Herb-partitioned moxibustion (HPM) has been shown to be effective in treating ulcerative colitis (UC) in recent years as a non-drug external therapy. In this study, we observed its effect on the expression profile of cytokines in UC rat colon. By protein functional cluster analysis and KEGG pathway analysis, we can conclude that the effect of HPM in promoting the repair of colon injuries of UC rats is plausibly related to the regulation of multiple abnormally-expressed cytokines, and the regulation of the signal pathways interacting between the cytokines and their receptors may be its significant immunological mechanism.
INTRODUCTION
As two major types of inflammatory bowel disease (IBD), ulcerative colitis (UC) and Crohn’s disease (CD) are both mainly characterized by chronic nonspecific inflammation of the colon. The incidence of UC is higher than that of CD, and it is increasing annually, with an incidence of 24.3/100000 in Europe and America, and 2.22/100000 in Guangzhou, which is the highest in China[1-3]. As a commonly encountered digestive disorder, UC is often recurrent and persistent, manifested by abdominal pain, diarrhea and bloody or purulent stools, and has an adverse effect on quality of life. Long-term medical treatment may also incur a high cost[4-8]. Immune dysfunction in the intestinal lining is recognized as the biological mechanism in the development of UC. In patients with UC, a large number of immunocytes (T cells, B cells, macrophages and dendritic cells) and cytokines [proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-6, IL-12, IL-17, IL-21, IL-23, and integrin; anti-inflammatory cytokines such as IL-10, transforming growth factor (TGF)-β and IL-35] are abnormally expressed in the colon[9]. The imbalance of cytokines modulated by activated immunocytes should be the initial factor that causes diffuse superficial inflammatory injuries in UC[10,11]. Regulation of the activity of immune cells and expression of cytokines is beneficial to healing the colonic lining and alleviation of inflammation in UC patients[12,13].
During recent years, acupuncture-moxibustion at Qihai (CV6) and bilateral Tianshu (ST25) has been shown to significantly improve symptoms, quality of life and immune homeostasis in the colon of UC patients[14-16]. Acupuncture-moxibustion is characterized by moderate therapeutic effect, content treatment perception and rapid action, and is especially effective in mitigating abdominal pain and diarrhea in UC patients[17]. Compared with oral medications, acupuncture-moxibustion, as a nondrug external therapy, can also promote colonic blood circulation and intestinal peristalsis, heal damaged colonic lining, regulate colonic immune reactions, enhance self-healing and reduce relapse[18]. Although the efficacy of acupuncture-moxibustion in treating UC in both the short- and long-term is well recognized, their mechanism of action is still vague. Therefore, it is important to discover how acupuncture-moxibustion works in treating UC, and this will boost its application in the treatment of UC.
Protein microarray is an important tool in profiling the protein expression in IBD and screening new drugs. This technique is known for its high throughput, high sensitivity and high precision, and thoroughly and quantitatively analyzes the genre, number and correlation of proteins, by which complicated network and possible regulatory mechanisms are investigated[19-21]. Protein microarray has been gradually applied to research into traditional Chinese medicine (TCM), such as pharmacodynamics and toxicology of Chinese medications[22-24], yet it has rarely been used in the study of acupuncture-moxibustion therapy.
The present study established experimental UC rats and adopted the protein microarray technique to investigate the effect of herb-partitioned moxibustion (HPM) on the expression profile of colonic cytokines, to select those most associated with the therapeutic action of HPM. We used the UniProt and KEGG PATHWAY databases, as well as DAVID pathway and functional cluster analysis to study the immunopathogenesis of experimental UC and its treatment with HPM from the angle of cytokine expression profile.
MATERIALS AND METHODS
Experimental animals
Twenty-seven male Sprague-Dawley rats weighing 160 ± 20 g were provided by the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine (SCXK (Hu) 2007-0005). The rats were housed for 3 d under controlled conditions (22 ± 2 °C, relative humidity 64%) before the experiment started. All procedures for animal experiments were conducted in accordance with the International Guiding Principles for Biomedical Research Involving Animals recommended by the World Health Organization and were approved by the Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine. All rats were randomly divided into three groups as normal group, model group and HPM group, with nine rats per group.
Reagents and equipment
The following reagents and equipment were used: Freund’s adjuvant (Sigma, St Louis, MO, United States), refined moxa wool (Nanyang Hanyi Moxa Co. Ltd., He’nan, China), Fugui formula 1 (Huaji Pharmaceutical Co. Ltd., Shanghai, China), hematoxylin and eosin (HE) staining kit (Nanjing Jiancheng Technology Co. Ltd., Nanjing, China), self-made cone-like copper moxa-cone mold, self-made columnar copper herbal cake mold, biotin label-based rat antibody array (90 rat proteins, RayBiotech, Norcross, GA, United States), ELISA kits for rat Fas/TNFRSF6, FasL/TNFSF6, IL-1R6 (IL-1Rrp2) and IL-1β (Bio-Techne, Minneapolis, MN, United States), phosphatase/protease inhibitor cocktail (Roche, Basel, Switzerland), RIPA lysis buffer, phenylmethanesulfonyl fluoride (PMSF) (Beyotime, Jiangsu, China), microplate reader (Thermo Fisher Scientific, Waltham, MA, United States), scanner (Qinghua Ziguang, Beijing, China), ScanAlyze image analysis software (Stanford University, CA, United States), pathological analysis system (Leica, Wetzlar, Germany), and light microscope and imaging system (Olympus, Tokyo, Japan).
Rat model of UC
The rat model of UC was established using immunization plus topical stimulation with formalin[25,26]. After modeling, each group contributed one rat for histopathological observation to verify the success of the UC model.
HPM
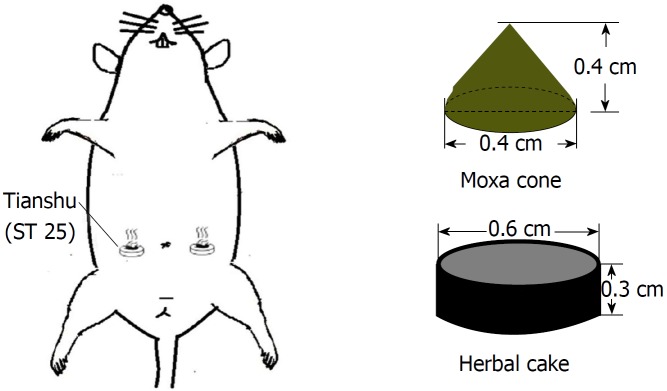
Rats in the HPM group were treated with HPM at bilateral Tianshu (ST25) points (Figure 1) by using a rat fixator. An herbal cake was placed on each point with a moxa cone on the top. The moxa cone was ignited, with two cones for each point as one session. The intervention was conducted once every other day, for four sessions and 8 d in total. Rats in the model group did not receive any interventions except the same grasping and fixing as in the HPM group. Rats in the normal group did not receive any modeling operation or interventions except for the same grasping and fixing.
Figure 1.
Illustration of herb-partitioned moxibustion. An herbal cake was placed on Tianshu (ST25) point with a moxa cone on the top to ignite. This point is located at the lower 1/3 between the xiphoid process and the midpoint of the pubic symphysis, 0.5 cm away.
Each moxa cone was made of 90 mg refined moxa wool by a copper mold, 0.4 cm in diameter and 0.4 cm in height. Guifu formula 1 was used to make herbal cakes, consisting of Radix aconiti laterails preparata, Cortex cinnamon, Radix salvia miltiorrhizae, Flos carthami and Radix aucklandiae (ratio: 10:2:3:3:1). The herbal powder was mixed with yellow rice wine immediately before HPM treatment and then made into cakes of the same size using a specific mold (0.6 cm in diameter and 0.3 cm thickness).
Colon sample preparation
After the treatment, the rats were euthanized by an intraperitoneal injection of pentobarbital sodium (150 mg/kg). Colons (6-8 cm, cut at 2 cm away from the anus) were collected and opened longitudinally to observe and score gross injuries under a microscope. The scoring standard is shown in Supplementary Table 1. The colons were then cut into 2 pieces. The proximal part was stored at -80 °C and the distal part was fixed in 10% neutral-buffered formalin.
Morphological observation of the colon
After fixation in 10% neutral-buffered formalin overnight, colons were dehydrated, embedded in paraffin, sliced (3-5 μm) and baked (60 °C) using the Leica pathological analysis system, followed by dewaxing and dehydration by dimethylbenzene and graded ethanol. The specimens were stained with hematoxylin for 10 min, differentiated by 1% HCl and ethanol, stained blue by 1% ammonia solution, stained by 0.5% eosin for 3 min, dehydrated through 70%, 85%, 95% and 100% ethanol, made transparent by dimethylbenzene, and sealed in neutral resin. Finally, the colon tissues were observed under a light microscope and scored. The scoring standard is shown in Supplementary Table 2.
Total protein extraction in the colon
Three colon samples were selected from each of the three groups to extract total protein. Lysis buffer containing protease inhibitor was added at 1 mL/250 mg (1 mL RIPA was mixed with 5 μL protease inhibitor solution, 5 μL PMSF and 5 μL phosphatase/protease inhibitor cocktail), and homogenized at a low speed (3000 rpm) for complete lysis of colon tissues. The colonic lysate was centrifuged at 14000 rpm for 15 min. The supernatant was collected to determine the concentration of total protein by bicinchoninic acid method.
Cytokine detection in colon
The protein chips were put into the reaction chamber of the chemiluminescent protein microarray kit from RayBiotech. A total of 2 mL blocking buffer was added into the reaction chamber, interacting for 30 min at room temperature. The protein chips were incubated with 1 mL sample supernatant (content of total proteins 50-500 μg), streptavidin antibody and horseradish-peroxidase-labeled anti-streptavidin antibody in sequence for 2 h at each step. After thorough washing, 500 μL chemiluminescent solution was added to the reaction chamber, which was incubated for 2 min at room temperature. The solution was then removed, and the protein chips were taken for imaging. Ninety kinds of cytokines are detailed in Supplementary Table 3.
Image and data analysis
The images were scanned and saved in .tiff format. ScanAlyze was used to transform the images into data that were then input into Microsoft Excel for normalization and analysis. Normalized result = [Original value - Blank (average)]/Positive (average). First, the protein abundance of rat colonic cytokines in each group was described. Second, intergroup fold change in normalized protein abundance was calculated. Fold change > 1.3 or < 0.77 were taken as the standard to define upregulation and downregulation of a cytokine, respectively.
UniProt accession and KEGG pathway analysis
Accessions of the differentially expressed cytokines were retrieved in the UniProt database to understand their protein structure and physicochemical properties. The corresponding pathways of the cytokines were sought in the KEGG PATHWAY database and then underwent functional cluster and pathway analysis with DAVID.
Validation of differentially expressed proteins in the colon
ELISA was used to verify the specific differentially expressed colonic cytokines. The appropriate amount of diluent samples, standard products and 10 μL avidin and 50 μL enzyme-labeled reagent were added to a 96-well dish and incubated at 37 °C for 30 min. When the above solutions were washed off, 50 μL chromogenic reagents A and B were added successively and mixed. The dish was kept at 37 °C for 15 min in the dark to allow developing. Finally, 50 μL stop solution was added to terminate the reaction. A microplate reader from Thermo Fisher was used to determine OD450. The protein concentration was positively correlated with the OD value. The standard curve was drawn to reflect the cytokine concentration.
Statistical analysis
SPSS version 18.0 (IBM, Armonk, NY, United States) was used for statistical analysis. Measurement data including body weight, gross colon injury score, histopathological score, and expression of the 90 cytokines and differentially expressed cytokines, which completely or substantially conformed to a normal distribution and homogeneity of variance, were expressed as means ± SD. Body weight, gross colon injury score, histopathological score and expression of colonic differentially expressed cytokines were analyzed by one-way ANOVA followed by least significant difference test. An independent t-test was used for the between-group comparison of the expression of the 90 cytokines. P < 0.05 was considered to indicate statistical significance.
RESULTS
General condition
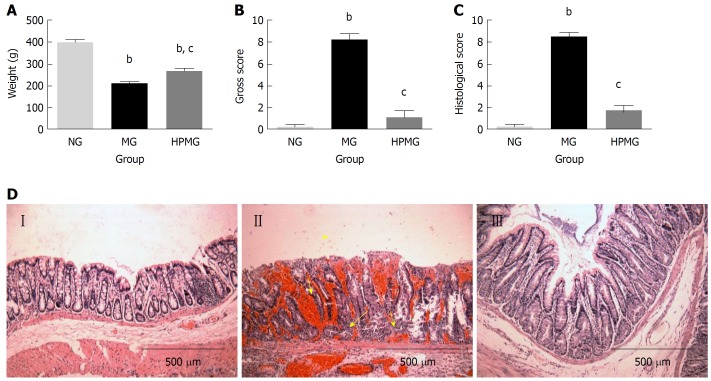
Rats in the normal group were vigorous and had normal intake of food, defecation, ruddy anus and steady increase of body weight. On the contrary, rats in the model group were weak and irritable, with reduced food intake, soft or loose stool sometimes with blood or pus, stained anus, and slower increase of body weight (P < 0.01). Compared with the model group, rats in the HPM group showed improvements in dieting, stool pattern and spiritual state, and body weight increased more significantly (P < 0.01) (Figure 2A).
Figure 2.
General condition, morphological observation and injury score of colon tissues in each group. A: Weight change of rats in each group; B: Gross injury score of rat colon in each group; C: Histological score of rat colon in each group; D: Histological and morphological structure under microscope by hematoxylin-eosin staining (Amplification × 100). NG (I): Normal group, MG (II): Model group; HPMG (III): Herb-partitioned moxibustion group. Data are presented as the mean ± SD, n = 8 per group. bP < 0.01 (vs normal group); cP < 0.01 (vs model group).
Gross colon injury score
Compared with the normal group, colons in the model group were harder and darker, with thickened intestinal walls, less smooth lining with significant congestion and scattered ulcers covered by discharge, and the gross injury score was significantly higher (P < 0.01). Compared with the model group, colons in the HPM group showed notable improvement in colon injuries, presenting with soft pink intestines, slightly thickened intestinal walls, smooth and complete lining without visible ulcers, and the gross injury score was significantly lower (P < 0.01) (Figure 2B).
Morphological changes in the colon
Compared with the normal group, the model group had a significantly higher histopathological score for colon injuries (P < 0.01) and presented with disconnected colon lining, colonic gland necrosis or missing, disorganized crypts, atrophy in some colonic glands, and infiltration of the lamina propria and submucosa by many inflammatory cells such as neutrophils, eosinophils and mononuclear cells. Compared with the model group, rats in the HPM group showed significant improvements in colon injuries, manifested by substantially complete lining without noticeable ulcers but obvious growth of well-structured colonic glands, coupled with inflammatory polyps, alleviated inflammation in the lamina propria and submucosa, and a significantly lower histopathological score (P < 0.01), (Figure 2C and D).
Analysis of cytokine expression profiles
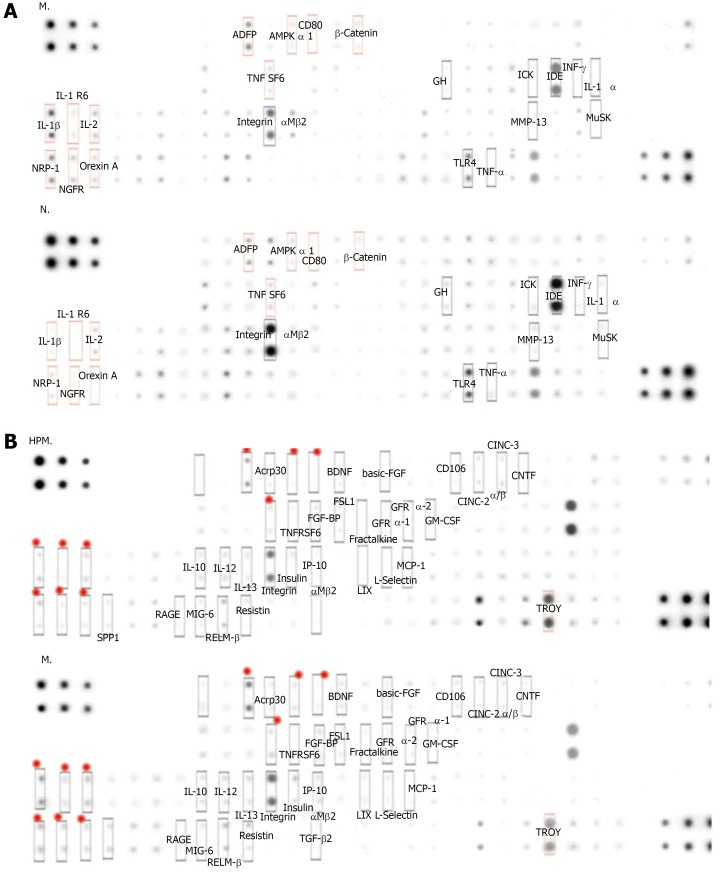
Compared with the normal group, 30 cytokines in colon tissues (n = 3) showed > 1.3 fold change in the model group (Table 1 and Figure 3A), among which 22 cytokines were significantly differentially expressed (P < 0.05); 34 cytokines showed < 0.77 fold change (Table 2, Figure 3A, and Figure 4), and 4 of them were significantly differentially expressed (P < 0.05).
Table 1.
Cytokines associated with the development of ulcerative colitis (up-regulated)
| Cytokine (up) | FC (M/N) | P value | Cytokine (up) | FC (M/N) | P value |
| FGF-BP | 1.362082 | 0.02 | FADD | 2.012878 | 0.15 |
| IL-5 | 1.383255 | 0.12 | B7-1 | 2.121888 | < 0.00 |
| MIG-6 | 1.502947 | 0.01 | Fas | 2.133077 | 0.03 |
| IL-10 | 1.538953 | 0.04 | E-Selectin | 2.193775 | 0.35 |
| Fractalkine | 1.562316 | 0.01 | SPP1 | 2.286921 | 0.02 |
| PDGF-AA | 1.621171 | 0.05 | IL-2 | 2.298734 | 0.01 |
| β-Catenin | 1.63583 | < 0.000 | Activin A | 2.328823 | 0.01 |
| FSL1 | 1.645565 | 0.004 | IL-3 | 2.356267 | 0.08 |
| AMPKα1 | 1.747397 | 0.001 | ADFP | 2.429592 | < 0.00 |
| RAGE | 1.778351 | 0.02 | Fas Ligand | 2.476364 | 0.02 |
| BDNF | 1.800268 | < 0.00 | Neuropilin-2 | 2.742889 | 0.02 |
| IC-1 | 1.812053 | 0.39 | NGFR | 3.519845 | < 0.00 |
| Insulin | 1.917783 | 0.03 | IL-1Rrp2/IL-1R6 | 3.668512 | < 0.00 |
| ACTH | 1.919645 | 0.15 | Orexin A | 4.768017 | 0.01 |
| PK1 | 1.956145 | 0.08 | IL-1β | 5.017418 | < 0.000 |
FC: Fold change; M/N: Average of normalized result in the model group/average of normalized result in the normal group.
Figure 3.
Protein abundances of colonic cytokines in each group. A: MG vs NG in comparing the change in mean protein abundance, and the cytokines with an obvious change are shown in the figure; B: HPMG vs MG in comparing the change in mean protein abundance, and the cytokines with an obvious change are shown in the figure. The significantly down-regulated proteins are marked in red (the focus of this study). NG: Normal group; MG: Model group; HPMG: Herb-partitioned moxibustion group.
Table 2.
Cytokines associated with the development of ulcerative colitis (down-regulated)
| Cytokine (down) | FC (M/N) | P value | Cytokine (down) | FC (M/N) | P value |
| MuSK | 0.025485 | 0.26 | GM-CSF | 0.558641 | 0.01 |
| MMP-13 | 0.191098 | 0.50 | TRAIL | 0.561294 | 0.73 |
| ICK | 0.206558 | 0.99 | GH | 0.583295 | 0.11 |
| IFN-γ | 0.210272 | 0.60 | TLR4 | 0.605775 | 0.09 |
| TNF-α | 0.232403 | 0.54 | CCR4 | 0.614899 | 0.15 |
| IL-1α | 0.283395 | 0.64 | VEGF | 0.630412 | 0.62 |
| MIP-3α | 0.297499 | 0.82 | TIMP-2 | 0.636711 | 0.48 |
| EGFR | 0.362763 | 0.82 | TGF-β1 | 0.644985 | 0.19 |
| MMP-2 | 0.366125 | 0.94 | CD106 | 0.6635 | 0.04 |
| MIF | 0.441963 | 0.52 | ICAM-1 | 0.701176 | 0.3 |
| MIP-1α | 0.446228 | 0.69 | Thrombospondin | 0.708378 | 0.09 |
| Ubiquitin | 0.485159 | 0.59 | IDE | 0.711151 | 0.27 |
| TIMP-3 | 0.489656 | 0.65 | β-NGF | 0.727731 | 0.13 |
| MIP-2 | 0.513305 | 0.68 | IC-3 | 0.748519 | 0.66 |
| TIE-2 | 0.528072 | 0.81 | MDC | 0.749424 | 0.13 |
| TGF-β3 | 0.550782 | 0.08 | VEGF-C | 0.768934 | 0.42 |
| Growth hormone R | 0.553239 | 0.50 | CNTF | 0.769193 | 0.02 |
FC: Fold change; M/N: Average of normalized result in the model group/average of normalized result in the normal group.
Figure 4.
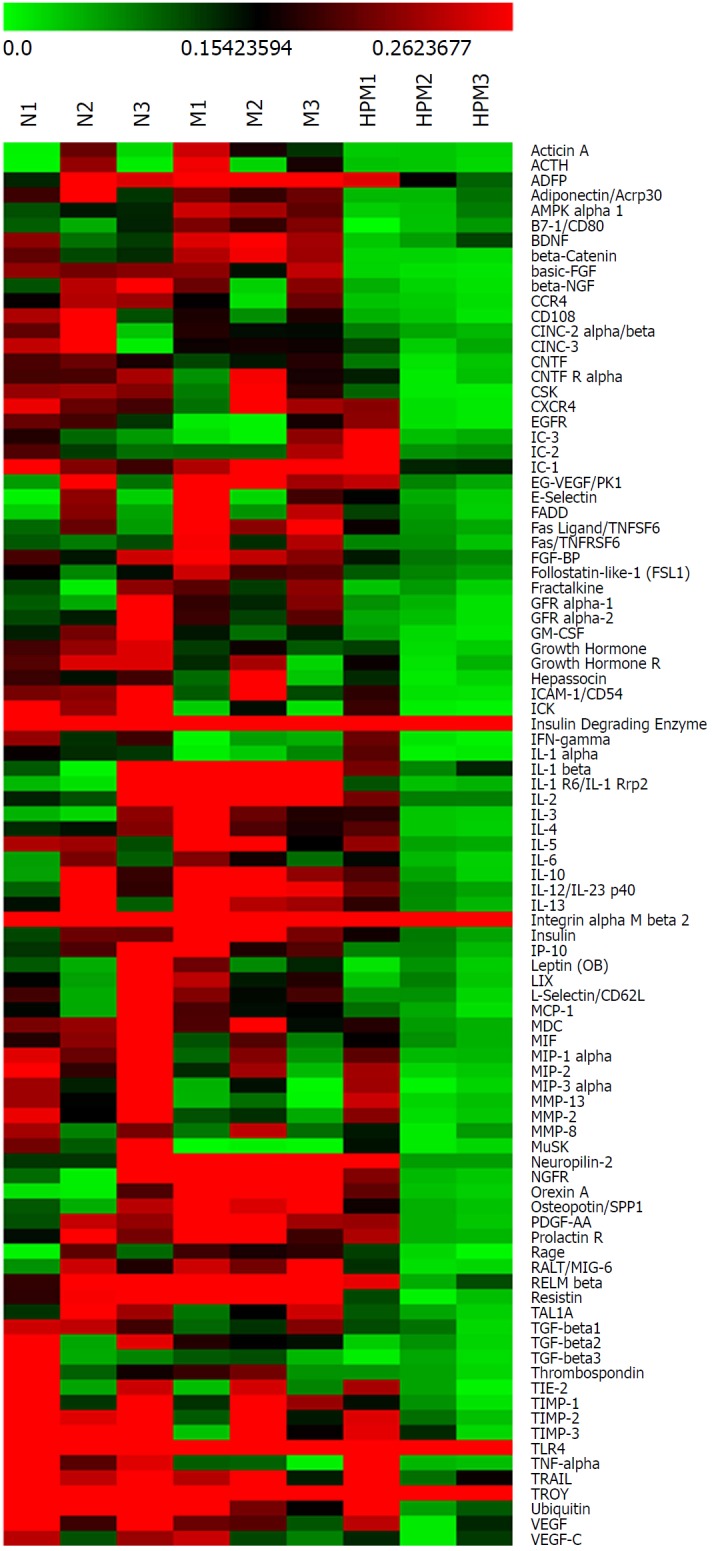
Clustering heatmap of colonic cytokines in each group. Red stands for those higher than the average density (marked in dark); green for those lower than the average density. n = 3 rats per group. N: Normal group; M: Model group; HPM: Herb-partitioned moxibustion group.
Compared with the model group, 77 colonic cytokines showed < 0.77 fold change in the HPM group (Table 3, Figure 3B, and Figure 4), and 14 of them were significantly differentially expressed (P < 0.05); 9 cytokines showed > 1.3 fold change (Table 4 and Figure 3B), and 6 of them were significantly differentially expressed (P < 0.05).
Table 3.
Cytokines associated with the action of herb-partitioned moxibustion (down-regulated)
| Cytokine (down) | FC (H/M) | P value | Cytokine (down) | FC (H/M) | P value |
| basic-FGF | 0.0978 | 0.70 | MCP-1 | 0.322376 | 0.71 |
| β-Catenin | 0.099918 | 0.03 | PDGF-AA | 0.329641 | 0.13 |
| IL-1Rrp2/IL-1R6 | 0.157933 | 0.03 | TGF-β3 | 0.335626 | 0.50 |
| Resistin | 0.165169 | 0.45 | FADD | 0.338264 | 0.33 |
| B7-1/CD80 | 0.165526 | 0.02 | Prolactin R | 0.352073 | 0.57 |
| Activin A | 0.169884 | 0.23 | IL-10 | 0.353484 | 0.23 |
| IL-1β | 0.181118 | 0.01 | IL-3 | 0.354765 | 0.15 |
| NGFR | 0.205767 | 0.02 | FSL1 | 0.374421 | 0.06 |
| β-NGF | 0.206263 | 0.52 | PK1 | 0.374728 | 0.22 |
| ACTH | 0.206715 | 0.50 | CINC-2 α/β | 0.375162 | 0.76 |
| AMPKα1 | 0.218363 | 0.01 | FGF-BP | 0.378172 | 0.21 |
| CSK | 0.220766 | 0.54 | ICAM-1 | 0.379167 | 0.45 |
| GFRα-2 | 0.222922 | 0.65 | TAL1A | 0.379464 | 0.52 |
| CCR4 | 0.229987 | 0.26 | CNTF Rα | 0.380412 | 0.57 |
| MIG-6 | 0.234297 | 0.22 | IL-13 | 0.381048 | 0.52 |
| Neuropilin-2 | 0.23433 | 0.04 | IL-12 | 0.395678 | 0.55 |
| Fractalkine | 0.238117 | 0.39 | CXCR4 | 0.397749 | 0.92 |
| CD106 | 0.238859 | 0.32 | IL-4 | 0.400025 | 0.30 |
| GFRα-1 | 0.248202 | 0.66 | CINC-3 | 0.404805 | 0.89 |
| Orexin A | 0.256555 | 0.01 | MDC | 0.417278 | 0.50 |
| GM-CSF | 0.259198 | 0.16 | IL-5 | 0.41803 | 0.33 |
| TGF-β2 | 0.261203 | 0.58 | E-Selectin | 0.424327 | 0.43 |
| LIX | 0.270135 | 0.91 | Hepassocin | 0.428314 | 0.61 |
| BDNF | 0.270917 | 0.06 | GH | 0.441903 | 0.02 |
| RAGE | 0.27422 | 0.24 | Integrin αMβ2 | 0.458492 | 0.03 |
| Leptin (OB) | 0.276614 | 0.79 | IL-6 | 0.47843 | 0.60 |
| SPP1 | 0.278682 | 0.07 | TGF-β1 | 0.511556 | 0.12 |
| TIMP-1 | 0.280948 | 0.88 | MMP-8 | 0.525606 | 0.65 |
| L-Selectin | 0.283725 | 0.91 | GH R | 0.581019 | 0.17 |
| Fas | 0.289612 | 0.06 | TIMP-2 | 0.581976 | 0.31 |
| RELMβ | 0.290633 | 0.47 | VEGF-C | 0.610166 | 0.54 |
| ADFP | 0.290674 | 0.01 | IC-1 | 0.639561 | 0.12 |
| Acrp30 | 0.291541 | 0.81 | IDE | 0.678174 | 0.01 |
| IP-10 | 0.295663 | 0.88 | TIMP-3 | 0.69365 | 0.16 |
| IL-2 | 0.298121 | 0.03 | MIP-2 | 0.713848 | 0.13 |
| Insulin | 0.305481 | 0.09 | MIF | 0.725496 | 0.18 |
| CNTF | 0.310717 | 0.10 | Ubiquitin | 0.73656 | 0.09 |
| Fas Ligand | 0.319733 | < 0.05 | MIP-1α | 0.761068 | 0.09 |
| Thrombospondin | 0.322261 | 0.55 | VEGF | 0.765926 | 0.13 |
FC: Fold change; H/M: Average of normalized result in the herb-partitioned moxibustion group/average of normalized result in the model group.
Table 4.
Cytokines associated with the action of herb-partitioned moxibustion (up-regulated)
| Cytokine (up) | FC (H/M) | P value | Cytokine (up) | FC (H/M) | P value |
| EGFR | 1.335106 | 0.13 | TNF-α | 1.937065 | 0.04 |
| TROY | 1.34044 | 0.31 | IFN-γ | 2.000647 | 0.01 |
| TLR4 | 1.511846 | 0.04 | MMP-13 | 2.19335 | 0.04 |
| IC-3 | 1.664604 | 0.74 | MuSK | 12.24563 | 0.02 |
| IL-1α | 1.845212 | 0.01 |
FC: Fold change; H/M: Average of normalized result in the herb-partitioned moxibustion group/average of normalized result in the model group.
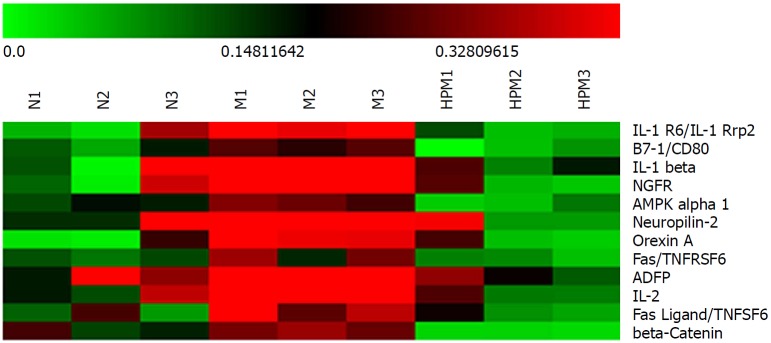
Further analysis discovered that 12 cytokines that were upregulated (> 1.3 fold change) in the model group compared with those in the normal group were downregulated (< 0.77 fold change) after HPM intervention (Figure 5): e.g., β-catenin, IL-1 receptor 6 (IL-1R6), B7-1, IL-1β, nerve growth factor receptor, AMP-activated protein kinase-α1, neuropilin-2, orexin A, adipocyte differentiation-related protein, IL-2, Fas and FasL. However, no cytokines that were downregulated (< 0.77 fold change) in the model group compared with those in the normal group were upregulated (> 1.3 fold change) after the intervention with HPM.
Figure 5.
Clustering heatmap of the specific differential cytokines in each group. Red stands for those higher than the average density (marked in dark); green for those lower than the average density. n = 3 rats per group. N: Normal group; M: Model group; HPM: Herb-partitioned moxibustion group.
Functional cluster of the differentially expressed cytokines
Compared with the normal group, the differentially expressed cytokines in the model group regulated apoptosis, protein phosphorylation and modification, proliferation of white blood cells, lymphocytes and mononuclear cells, migration of white blood cells and vascular endothelial cells, activation of chemokines, and modulation of transcription factors of nuclear factor-κB and angiogenesis (Supplementary Tables 4 and 5). Compared with the model group, the differentially expressed cytokines in the HPM group regulated apoptosis, protein phosphorylation, cell migration, protein metabolism, activation and chemotaxis of neutrophils, activation of lymphocytes and transcription factors, migration of vascular endothelial cells, and secretion of cytokines (Supplementary Tables 6 and 7).
KEGG pathway analysis
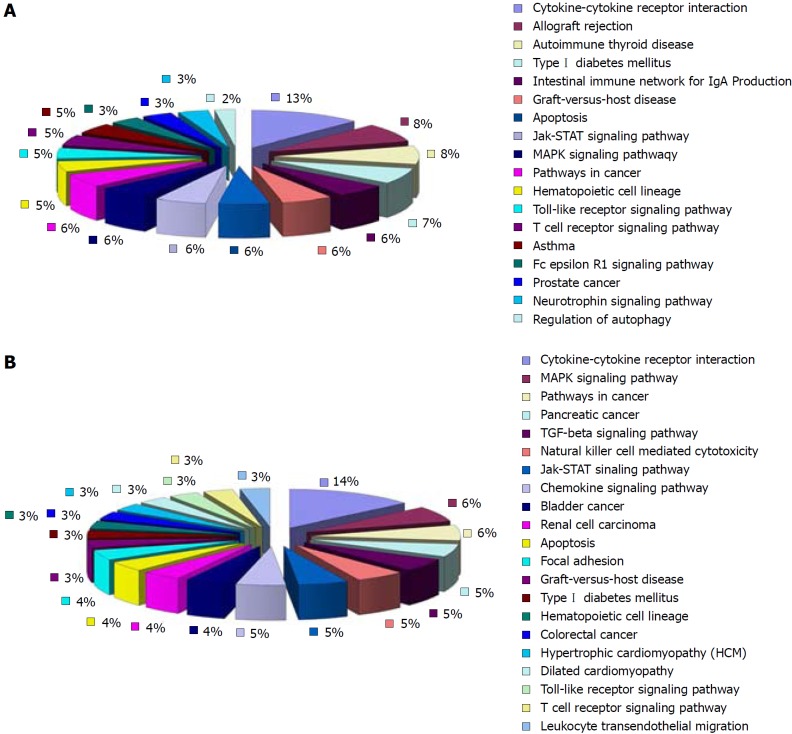
Compared with the normal group, 18 of the up-regulated differentially expressed cytokines in the model group involved 18 signaling pathways. Eleven of them were pathways that interacted between cytokines and their receptors. The rest were pathways mainly involved in allotransplantation reactions, autoimmune thyroid disorders, type 1 diabetes, network of intestinal immunoglobulins, apoptosis, mitogen-activated protein kinase (MAPK) signaling pathway, graft vs host reaction, Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway and Toll-like receptor (TLR) signaling pathway (Figure 6). Twenty-seven of the down-regulated differentially expressed cytokines in the model group involved 21 signaling pathways. Thirteen of them were pathways for the interaction between cytokines and their receptors. The rest were pathways mainly involved in the MAPK signaling pathway, TGF-β signaling pathway, natural killer (NK)-cell-mediated cytotoxicity, JAK/STAT signaling pathway, and apoptosis (Figure 6).
Figure 6.
KEGG pathway analysis of the cytokines related to the development of ulcerative colitis. A: Eleven of all the major signaling pathways of the up-regulated cytokines in the model group (compared with the normal group) are involved in the interaction between cytokines and their receptors (36.7%); B: Of the major signaling pathways of the down-regulated cytokines in the model group (compared with the normal group), 13 pathways regulate the interaction between cytokines and their receptors (39.4%) (Supplementary Tables 4 and 5).
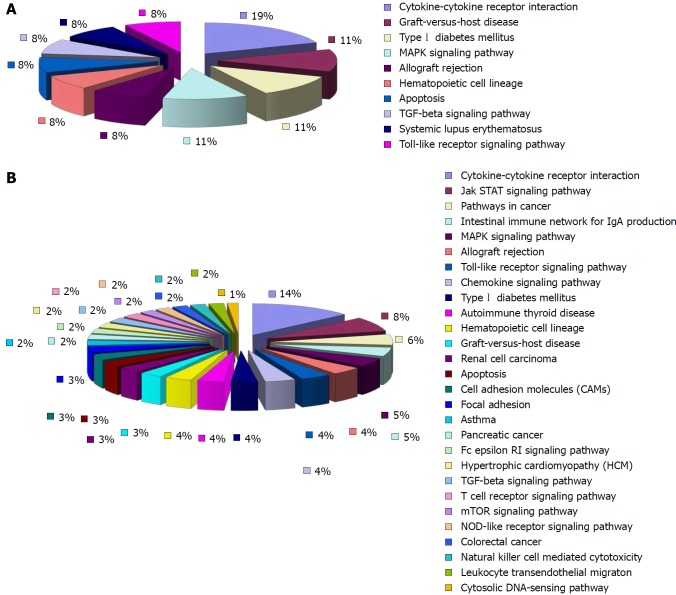
Compared with the model group, 52 of the 77 down-regulated differentially expressed cytokines in the HPM group were involved in 28 signaling pathways. The rest were involved in the JAK/STAT signaling pathway, cancer pathways, network of immunoglobulin A, MAPK signaling pathway, immunological rejection, TLR signaling pathway, chemokine signaling pathway, apoptosis, intercellular adhesion, graft vs host reaction, FcεRI and TGF-β signaling pathways, T-cell receptor pathways, mammalian target of rapamycin signaling pathway, NOD-like receptors and NK-cell-mediated cytotoxicity (Figure 7). There were ten pathways associated with the up-regulated cytokines that were differentially expressed in the HPM group compared with the model group (seven of the nine differentially expressed cytokines involved ten pathways). Five of them were for interactions between cytokines and receptors. The rest were mainly involved in the MAPK signaling pathway, graft vs host reaction, transplantation rejection, apoptosis, and TGF-β and TLR signaling pathways (Figure 7).
Figure 7.
KEGG pathway analysis of the cytokines related to the action of herb-partitioned moxibustion. A: Among the major signaling pathways related to the up-regulated cytokines in the herb-partitioned moxibustion group (compared with the model group), five pathways were involved in the interaction between cytokines and their receptors (55.6%); B: Of the major signaling pathways related to the down-regulated cytokines in the herb-partitioned moxibustion group (compared with the model group), 27 pathways were involved in the interaction between cytokines and their receptors (38%) (Supplementary Tables 6 and 7).
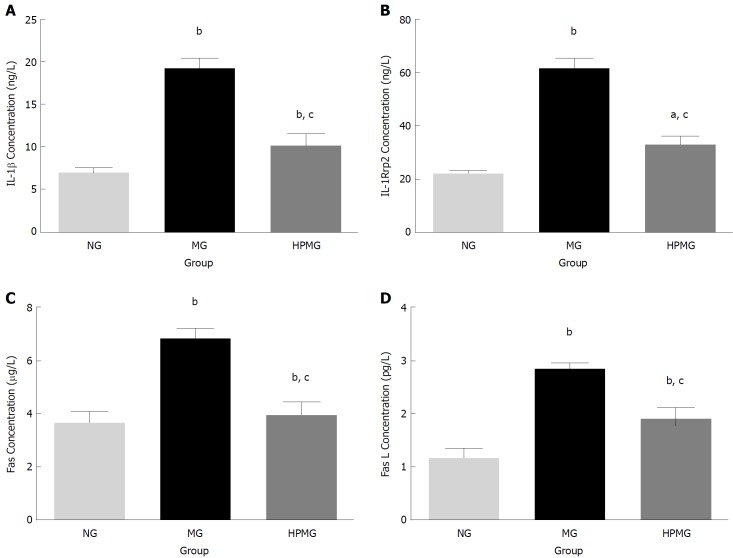
Verification of specific differential cytokines IL-1β, IL-1R6, Fas and FasL
Based on the protein microarray and functional cluster and pathway analysis, specific differential cytokines IL-1β, IL-1R6, Fas and FasL were selected for further verification by ELISA. Compared with the normal group, there were significant increases in expression of IL-1β, IL-1R6, Fas and FasL in the colon tissues of model group rats (P < 0.01, Figure 8). Compared with the model group, expression of IL-1β, IL-1R6, Fas and FasL decreased significantly in the HPM group (P < 0.01, Figure 8).
Figure 8.
Validation of the specific differential cytokines. A: Expression of IL-1β protein in colon; B: Expression of IL-1Rrp2/IL-1R6 protein in colon; C: Expression of Fas protein in colon; D: Expression of FasL protein in colon. Data are presented as the mean ± SD, n = 8 rats per group. bP < 0.01, aP < 0.05, (vs normal group); cP < 0.01 (vs model group). NG: Normal group; MG: Model group; HPMG: Herb-partitioned moxibustion group.
DISCUSSION
Although the development and application of oral medications or biological agents for UC have progressed, the treatment results have differed individually, not to mention that adverse effects have often been reported. Some research has proposed the use of more than two drugs together as a multitarget treatment to boost the efficacy, but more research is needed[27]. Conventional acupuncture-moxibustion therapy can produce a multisystem, multitarget and multidirectional effect, without causing notable adverse reactions. For all these merits, it is recommended as an effective therapeutic approach for UC. Establishment of the mechanism of action of HPM in treating UC could provide important evidence to promote its application in treating IBD. The present study was a pilot study that used high-throughput protein microarray to elucidate the mechanism of action of HPM in the treatment of UC. Here, 86 differentially expressed cytokines in the colon were triggered by HPM. They mostly regulated apoptosis, protein phosphorylation, lymphocyte activation and chemotaxis, protein metabolism, and activation of transcription factors. This indicates that the effect of HPM in promoting the repair of colon injuries in UC rats is possibly linked to the effective regulation of several abnormal cytokines. According to KEGG pathway analysis, 38 pathways were related to these differentially expressed cytokines. The high number suggests a plausible relationship between the immunological action of HPM and the regulation of cytokines, their receptors and the involved pathways in treating UC.
Despite the unclear picture of the pathogenesis of UC, it is widely accepted that chronic nonspecific inflammation in the colon is its cardinal feature. A large number of immunocompetent cells presenting with excessive chemotaxis and abnormally expressed cytokines have been found in the colon of UC patients. Cytokines and their receptors, as well as the factors regulating signaling pathways, mainly serve to regulate the molecular network of colonic epithelial cells, immunocytes in lamina propria, and bacteria. Therefore, abnormal cytokines are possibly a crucial factor in causing immune disorders and colon injuries in UC[28,29]. Cytokines form a complicated network, interacting with immunocytes both as cause and effect, but their mechanism of action is still unclear. According to previous studies, the cytokines related to UC are comparatively precise, mainly focused on inflammatory factors, anti-inflammatory factors, chemokines and lymphocyte-activating factors, with a limited quantity for measurement. The present study has made up for the previous studies in genre and quantity of cytokines by examining important factors involved in apoptosis, cell proliferation, signaling pathway regulation, protein modification, tumor, and intestinal immune network. Besides the focus on inflammation, this study also used UniProt and KEGG pathway databases to further explore the possible pathogenesis of UC, providing new insights and foundations for UC and clinical intervention studies in the future.
HPM integrates moxibustion and Chinese herbal medicine and produces both pharmaceutical effects on the skin and physical thermal effects. It has been shown that HPM can exert definite effects in treating mild-to-moderate UC, by boosting colon healing and relief of inflammation; thus it is well accepted by patients[17]. Compared with oral medication and surgery, HPM is simple to operate, gentle in action, consistent in efficacy and extensive in regulation. It can significantly improve quality of life and restore intestinal function[30-32]. More and more cytokine antibodies have been approved for treatment of IBD, such as anti-TNF (e.g., infliximab, etanercept and adalimumab) and anti-IL-12/23 p40 (e.g., ustekinumab), indicating that regulation of cytokines is practical and essential in treating colitis[33]. How does HPM exert its effect on UC? Is it also related to the regulation of cytokines, and what else is involved? In this study, protein microarrays revealed that 12 cytokines upregulated (fold change > 1.3) in the model group compared with the normal group were downregulated (fold change < 0.77) after HPM intervention. These cytokines are involved in the modulation of inflammation, apoptosis, adhesion, activation of co-stimulatory signals, metabolism, and nervous regulation. The pathway analysis also suggested an association between the effect of HPM in healing colon injuries of UC and the downregulation of several abnormally expressed cytokines. Hence, regulation of signaling pathways of cytokines and their receptors is possibly an important immunological mechanism. So far, we have seen that HPM has several regulatory effects on colonic cytokines. However, it remains a question whether this non-drug therapy can compare with oral or venous application of antibodies, immunosuppressive agents, steroidal and anti-inflammatory drugs.
We showed that HPM significantly downregulated the expression of IL-1β, IL-1R6, Fas and FasL, which agreed with previous studies[16,34-40]. We demonstrated that inflammatory infiltration and apoptosis in the colonic epithelium, controlled by cytokines and their network, are crucial factors in the pathogenesis of UC. We also discovered that the proinflammatory factor IL-1β was significantly upregulated, while the immunoregulatory factor TGF-β was down-regulated in UC. This caused an imbalance between proinflammatory and anti-inflammatory factors, which also agreed with previous studies. However, in our study, the proinflammatory factors TNF-α and IFN-γ were downregulated, although without significance, which differed from the previous results. The UC modeling method could have accounted for this difference. IL-1β originates from the innate intestinal immune system, while TNF-α and IFN-γ are produced by T helper cells of the acquired immune system. Our study used immunological and chemical stimulation to establish the UC model, which may have a different mechanism of injury from that induced by bacteria[41,42]. By using protein microarray, we systematically discussed the pathogenesis of UC and possible action mechanism of HPM and hope to inspire more studies to further verify the findings.
ARTICLE HIGHLIGHTS
Research background
The imbalance of cytokines modulated by activated immunocytes has been verified as an initial factor inducing inflammatory injuries in ulcerative colitis (UC). Herb-partitioned moxibustion (HPM), a non-drug external therapy, produces valid efficacy in treating UC, but its potential mechanism is still unclear.
Research motivation
The action mechanism of HPM was explored by using high throughout analysis of cytokine expression profiles in the colon and their network effects in our research.
Research objectives
By identifying the key cytokines in the action of HPM and analyzing their signal pathways, our research aimed to provide research ideas and crucial targets for further elaboration of the anti-inflammation mechanism of HPM in treating UC.
Research methods
A UC rat model was established by protein immunization in combination with topical chemical stimulation. Rats in the HPM group received HPM at bilateral Tianshu (ST25) points. The expression profile of colonic cytokines was assayed using the protein microarray technique.
Research results
Seventy-seven down-regulated and nine up-regulated differentially-expressed colonic cytokines were found in the HPM group. Functional cluster analysis showed that the differentially-expressed colonic cytokines in the HPM group regulated apoptosis and protein phosphorylation. KEGG pathway analysis showed that the pathways interacting between the cytokines and their receptors accounted for the largest proportion (28 of 52 down-regulated cytokines and 5 of 7 up-regulated cytokines).
Research conclusions
HPM promotes the repair of colon injuries in UC rats, which is related to the regulation of several abnormally-expressed cytokines.
Research perspectives
By functional cluster and KEGG pathway analyses, this study selected specific differential cytokines in HPM treatment of UC, which can provide potential targets for targeted therapy in the future and also research ideas for further elaboration of signal pathways in the action of HPM for UC. The results showed that the signal pathways interacting between cytokines and their receptors were closely related to the action of HPM, and the MAPK signaling pathway and JAK/STAT signaling pathway were possibly involved in the anti-inflammation process of HPM for colitis. A focus on this field may help better understand the mechanism of moxibustion in treating UC.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine.
Conflict-of-interest statement: The authors declare that there is no conflict of interest regarding the publication of this paper.
Data sharing statement: No additional data are available.
The ARRIVE guidelines statement: The ARRIVE Guidelines have been adopted.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 9, 2018
First decision: June 15, 2018
Article in press: June 30, 2018
P- Reviewer: Kukongviriyapan V, Weinhausel A S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
Contributor Information
Dan Zhang, Laboratory of Acupuncture-moxibustion and Immunology, Shanghai Research Institute of Acupuncture and Meridian, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China.
Yan-Bo Ren, Department of Integrated Traditional Chinese Medicine and Western Medicine, North Branch of Huashan Hospital, Fudan University, Shanghai 201907, China.
Kai Wei, Key Laboratory of Systems Biomedicine (Ministry of Education), Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong University, Shanghai 200240, China.
Jue Hong, Laboratory of Acupuncture-moxibustion and Immunology, Shanghai Research Institute of Acupuncture and Meridian, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China.
Yan-Ting Yang, Yueyang Clinical Medicine School, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Li-Jie Wu, Yueyang Clinical Medicine School, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Ji Zhang, Yueyang Clinical Medicine School, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Zheng Shi, Laboratory of Acupuncture-moxibustion and Immunology, Shanghai Research Institute of Acupuncture and Meridian, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China.
Huan-Gan Wu, Laboratory of Acupuncture-moxibustion and Immunology, Shanghai Research Institute of Acupuncture and Meridian, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China.
Xiao-Peng Ma, Laboratory of Acupuncture-moxibustion and Immunology, Shanghai Research Institute of Acupuncture and Meridian, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China; Yueyang Clinical Medicine School, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. pengpengma@163.com.
References
- 1.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111–119. doi: 10.5217/ir.2016.14.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Włodarczyk M, Sobolewska-Włodarczyk A, Stec-Michalska K, Fichna J, Wiśniewska-Jarosińska M. The influence of family pattern abnormalities in the early stages of life on the course of inflammatory bowel diseases. Pharmacol Rep. 2016;68:852–858. doi: 10.1016/j.pharep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Bernklev T, Jahnsen J, Schulz T, Sauar J, Lygren I, Henriksen M, Stray N, Kjellevold Ø, Aadland E, Vatn M, Moum B. Course of disease, drug treatment and health-related quality of life in patients with inflammatory bowel disease 5 years after initial diagnosis. Eur J Gastroenterol Hepatol. 2005;17:1037–1045. doi: 10.1097/00042737-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Zeitz J, Ak M, Müller-Mottet S, Scharl S, Biedermann L, Fournier N, Frei P, Pittet V, Scharl M, Fried M, et al. Pain in IBD Patients: Very Frequent and Frequently Insufficiently Taken into Account. PLoS One. 2016;11:e0156666. doi: 10.1371/journal.pone.0156666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casellas F, López-Vivancos J, Casado A, Malagelada JR. Factors affecting health related quality of life of patients with inflammatory bowel disease. Qual Life Res. 2002;11:775–781. doi: 10.1023/a:1020841601110. [DOI] [PubMed] [Google Scholar]
- 8.Burkhalter H, Stucki-Thür P, David B, Lorenz S, Biotti B, Rogler G, Pittet V. Assessment of inflammatory bowel disease patient’s needs and problems from a nursing perspective. Digestion. 2015;91:128–141. doi: 10.1159/000371654. [DOI] [PubMed] [Google Scholar]
- 9.Ince MN, Elliott DE. Immunologic and molecular mechanisms in inflammatory bowel disease. Surg Clin North Am. 2007;87:681–696. doi: 10.1016/j.suc.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 10.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 11.Loddo I, Romano C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front Immunol. 2015;6:551. doi: 10.3389/fimmu.2015.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorino G, Bonovas S, Cicerone C, Allocca M, Furfaro F, Correale C, Danese S. The safety of biological pharmacotherapy for the treatment of ulcerative colitis. Expert Opin Drug Saf. 2017;16:437–443. doi: 10.1080/14740338.2017.1298743. [DOI] [PubMed] [Google Scholar]
- 13.Katsanos KH, Papadakis KA. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver. 2017;11:455–463. doi: 10.5009/gnl16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joos S, Wildau N, Kohnen R, Szecsenyi J, Schuppan D, Willich SN, Hahn EG, Brinkhaus B. Acupuncture and moxibustion in the treatment of ulcerative colitis: a randomized controlled study. Scand J Gastroenterol. 2006;41:1056–1063. doi: 10.1080/00365520600580688. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Chen H, Hua X, Shi Z, Zhang L, Chen J. Clinical therapeutic effect of drug-separated moxibustion on chronic diarrhea and its immunologic mechanisms. J Tradit Chin Med. 1997;17:253–258. [PubMed] [Google Scholar]
- 16.Zhou EH, Liu HR, Wu HG, Shi Z, Zhang W, Zhu Y, Shi DR, Zhou S. Down-regulation of protein and mRNA expression of IL-8 and ICAM-1 in colon tissue of ulcerative colitis patients by partition-herb moxibustion. Dig Dis Sci. 2009;54:2198–2206. doi: 10.1007/s10620-008-0620-4. [DOI] [PubMed] [Google Scholar]
- 17.Ji J, Huang Y, Wang XF, Ma Z, Wu HG, Im H, Liu HR, Wu LY, Li J. Review of Clinical Studies of the Treatment of Ulcerative Colitis Using Acupuncture and Moxibustion. Gastroenterol Res Pract. 2016;2016:9248589. doi: 10.1155/2016/9248589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji J, Lu Y, Liu H, Feng H, Zhang F, Wu L, Cui Y, Wu H. Acupuncture and moxibustion for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:158352. doi: 10.1155/2013/158352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mourad FH, Yau Y, Wasinger VC, Leong RW. Proteomics in Inflammatory Bowel Disease: Approach Using Animal Models. Dig Dis Sci. 2017;62:2266–2276. doi: 10.1007/s10620-017-4673-0. [DOI] [PubMed] [Google Scholar]
- 20.Chan PP, Wasinger VC, Leong RW. Current application of proteomics in biomarker discovery for inflammatory bowel disease. World J Gastrointest Pathophysiol. 2016;7:27–37. doi: 10.4291/wjgp.v7.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yau Y, Leong RW, Zeng M, Wasinger VC. Proteomics and metabolomics in inflammatory bowel disease. J Gastroenterol Hepatol. 2013;28:1076–1086. doi: 10.1111/jgh.12193. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Hou Q, Zhou ZZ, Li MR, Zhong LZ, Deng XD, Zhu ZY, Cheng ZY, Zhu J, Xiang CL, et al. Comparative Proteomic Analysis of the Effect of the Four-Herb Chinese Medicine ANBP on Promoting Mouse Skin Wound Healing. Int J Low Extrem Wounds. 2017;16:154–162. doi: 10.1177/1534734617720623. [DOI] [PubMed] [Google Scholar]
- 23.Teschke R, Eickhoff A. Herbal hepatotoxicity in traditional and modern medicine: actual key issues and new encouraging steps. Front Pharmacol. 2015;6:72. doi: 10.3389/fphar.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang H, Wang K, Zhang J. Transcriptome and proteome analyses of drug interactions with natural products. Curr Drug Metab. 2008;9:1038–1048. doi: 10.2174/138920008786927802. [DOI] [PubMed] [Google Scholar]
- 25.Xu SY, Bian RL, Chen X. Pharmacology Experiment Methodology. Beijing, China: People’s Medical Publishing House; 2002. p. 1335. [Google Scholar]
- 26.Wang X, Liu Y, Dong H, Wu L, Feng X, Zhou Z, Zhao C, Liu H, Wu H. Herb-Partitioned Moxibustion Regulates the TLR2/NF-κB Signaling Pathway in a Rat Model of Ulcerative Colitis. Evid Based Complement Alternat Med. 2015;2015:949065. doi: 10.1155/2015/949065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Sario A, Bendia E, Schiadà L, Sassaroli P, Benedetti A. Biologic Drugs in Crohn’s Disease and Ulcerative Colitis: Safety Profile. Curr Drug Saf. 2016;11:55–61. doi: 10.2174/157488631101160212171757. [DOI] [PubMed] [Google Scholar]
- 28.Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–323. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 29.Beck PL, Wallace JL. Cytokines in inflammatory bowel disease. Mediat of Inflammat. 1997;6:95–103. doi: 10.1080/09629359791785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, He T, Xu Q, Li Z, Liu Y, Li F, Yang BF, Liu CZ. Acupuncture and regulation of gastrointestinal function. World J Gastroenterol. 2015;21:8304–8313. doi: 10.3748/wjg.v21.i27.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi T. Effect and mechanism of acupuncture on gastrointestinal diseases. Int Rev Neurobiol. 2013;111:273–294. doi: 10.1016/B978-0-12-411545-3.00014-6. [DOI] [PubMed] [Google Scholar]
- 32.Cheifetz AS, Gianotti R, Luber R, Gibson PR. Complementary and Alternative Medicines Used by Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:415–429.e15. doi: 10.1053/j.gastro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol. 2015;12:537–545. doi: 10.1038/nrgastro.2015.135. [DOI] [PubMed] [Google Scholar]
- 34.De Santis S, Kunde D, Galleggiante V, Liso M, Scandiffio L, Serino G, Pinto A, Campiglia P, Sorrentino R, Cavalcanti E, et al. TNFα deficiency results in increased IL-1β in an early onset of spontaneous murine colitis. Cell Death Dis. 2017;8:e2993. doi: 10.1038/cddis.2017.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonseca-Camarillo G, Yamamoto-Furusho JK. Immunoregulatory Pathways Involved in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2188–2193. doi: 10.1097/MIB.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 36.Yao J, Cao X, Zhang R, Li YX, Xu ZL, Zhang DG, Wang LS, Wang JY. Protective Effect of Baicalin Against Experimental Colitis via Suppression of Oxidant Stress and Apoptosis. Pharmacogn Mag. 2016;12:225–234. doi: 10.4103/0973-1296.186342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu HG, Liu HR, Tan LY, Gong YJ, Shi Y, Zhao TP, Yi Y, Yang Y. Electroacupuncture and moxibustion promote neutrophil apoptosis and improve ulcerative colitis in rats. Dig Dis Sci. 2007;52:379–384. doi: 10.1007/s10620-006-9561-y. [DOI] [PubMed] [Google Scholar]
- 40.Ma TM, Xu N, Ma XD, Bai ZH, Tao X, Yan HC. Moxibustion regulates inflammatory mediators and colonic mucosal barrier in ulcerative colitis rats. World J Gastroenterol. 2016;22:2566–2575. doi: 10.3748/wjg.v22.i8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh UP, Singh NP, Murphy EA, Price RL, Fayad R, Nagarkatti M, Nagarkatti PS. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–49. doi: 10.1016/j.cyto.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan Q, Zhang J. Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediators Inflamm. 2017;2017:4810258. doi: 10.1155/2017/4810258. [DOI] [PMC free article] [PubMed] [Google Scholar]