Abstract
The current epidemic of non-alcoholic fatty liver disease (NAFLD) is reshaping the field of hepatology all around the world. The widespread diffusion of metabolic risk factors such as obesity, type2-diabetes mellitus, and dyslipidemia has led to a worldwide diffusion of NAFLD. In parallel to the increased availability of effective anti-viral agents, NAFLD is rapidly becoming the most common cause of chronic liver disease in Western Countries, and a similar trend is expected in Eastern Countries in the next years. This epidemic and its consequences have prompted experts from all over the word in identifying effective strategies for the diagnosis, management, and treatment of NAFLD. Different scientific societies from Europe, America, and Asia-Pacific regions have proposed guidelines based on the most recent evidence about NAFLD. These guidelines are consistent with the key elements in the management of NAFLD, but still, show significant difference about some critical points. We reviewed the current literature in English language to identify the most recent scientific guidelines about NAFLD with the aim to find and critically analyse the main differences. We distinguished guidelines from 5 different scientific societies whose reputation is worldwide recognised and who are representative of the clinical practice in different geographical regions. Differences were noted in: the definition of NAFLD, the opportunity of NAFLD screening in high-risk patients, the non-invasive test proposed for the diagnosis of NAFLD and the identification of NAFLD patients with advanced fibrosis, in the follow-up protocols and, finally, in the treatment strategy (especially in the proposed pharmacological management). These difference have been discussed in the light of the possible evolution of the scenario of NAFLD in the next years.
Keywords: Non-alcoholic fatty liver disease, Metformin, Liver steatosis, Liver biopsy, Non-invasive diagnosis, Pioglitazone, Clinical guidelines
Core tip: Non-alcoholic fatty liver disease (NAFLD) is becoming the most common cause of chronic liver disease. As such, an increasing number of scientific reports are investing this condition. To translate these evidence into clinical practice, international scientific societies have proposed guidelines for the management of NAFLD. In this review, we will critically analyse both the converging and diverging points in the current clinical guidelines of NAFLD, with a particular focus on the diagnostic and therapeutic aspects.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) includes a spectrum of disorders ranging from the simple fatty liver to non-alcoholic steatohepatitis, with increasing fibrosis leading to cirrhosis[1]. The prevalence of NAFLD is alarmingly growing worldwide in adult and children/adolescent populations, with a bidirectional association between NAFLD and metabolic syndrome[2]. Obesity, insulin resistance, type 2 diabetes mellitus, and dyslipidemia are the most relevant metabolic conditions related to this spectrum of diseases[1,2].
Clinicians and researchers from several scientific Associations worldwide put significant efforts into increasing knowledge and developing high-quality International Guidelines to improve the management of NAFLD patients in clinical practice. Multidisciplinary panels of experts in different continents have performed systematic analysis and review of the literature on specified topics in the last years. These efforts have led to the creation and publication of various Guidelines.
This paper aims to review and compare the most recently published International Guidelines for the diagnosis and the management of NAFLD in adult populations, to critically evaluate similarities and discrepancies. In particular, we tried to analyse some critical questions and challenges for clinicians in real life.
LITERATURE SEARCH
We performed a database search on PubMed selecting papers published between January 2016 and January 2018 in the English language. The following keywords and terms were considered: (1) Fatty liver disease ((“fatty liver”[MeSH Terms] OR (“fatty”[All Fields] AND “liver”[All Fields]) OR “fatty liver”[All Fields]) AND (“disease”[MeSH Terms] OR “disease”[All Fields])) AND guideline (“guideline”[Publication Type] OR “guidelines as topic”[MeSH Terms] OR “guideline”[All Fields]) AND management (“organization and administration”[MeSH Terms] OR (“organization”[All Fields] AND “administration”[All Fields]) OR “organization and administration”[All Fields] OR “management”[All Fields] OR “disease management”[MeSH Terms] OR (“disease”[All Fields] AND “management”[All Fields]) OR “disease management”[All Fields]); (2) Fatty liver disease AND recommendation ((“fatty liver”[MeSH Terms] OR (“fatty”[All Fields] AND “liver”[All Fields]) OR “fatty liver”[All Fields]) AND (“disease”[MeSH Terms] OR “disease”[All Fields])) AND recommendation[All Fields]; (3) Fatty liver disease and position paper ((“fatty liver”[MeSH Terms] OR (“fatty”[All Fields] AND “liver”[All Fields]) OR “fatty liver”[All Fields]) AND (“disease”[MeSH Terms] OR “disease”[All Fields])) AND (position[All Fields] AND (“paper”[MeSH Terms] OR “paper”[All Fields])).
According to this criteria, 119 papers were identified. As a second step, we excluded papers which were not pertinent to any of the following criteria: (1) Clinical Guidelines related to diagnosis and management of NAFLD in the adult population; (2) clinical Guidelines published by Governmental agencies and Scientific Associations.
According to the selection criteria, out of 119 results of PubMed research, 5 Guidelines were finally included in this analysis. These guidelines are strictly focused on the topic of diagnosis and management of NAFLD in adult, excluding pediatric populations and special groups. In detail, the five selected papers included (from the oldest to the newest date of publication): (1) “EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease” by the European Association for the Study of The Liver (EASL), published in 2016[3]; (2) “Nonalcoholic fatty liver disease (NAFLD): Assessment and management” by the National Institute for Health and Care Excellence (NICE), published in 2016[4]; (3)“Asia-Pacific Working Party on Non-Alcoholic Fatty Liver Disease guidelines” published in 2017[5,6]; (4) Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions, published in 2017[7]; (5) “The diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance From the American Association for the Study of Liver Diseases” published in 2018[8].
OPEN QUESTIONS
Definition, classification, and diagnostic criteria of NAFLD
Definition and classification: A definition of NAFLD is reported in all Guidelines (Table 1). The characteristic points of NAFLD definition include (1) the evidence of excessive hepatic fat accumulation in the liver parenchyma (detected by imaging techniques or histology); (2) the absence of other secondary causes of hepatic fat. Out of them, to strictly define NAFLD patients a significant ongoing or recent alcohol consumption have to be excluded in all recommendation[3-8].
Table 1.
Diagnostic criteria for non-alcoholic fatty liver disease according to the various guidelines
| EASL | NICE | Asia-Pacific | AISF | AASLD | |
| Required criteria | Steatosis in > 5% of hepatocytes by either imaging or histology | Excessive fat in the liver | Hepatic steatosis by either imaging or histology | Hepatic steatosis on either imaging or histology | Evidence of hepatic steatosis either by imaging or histology |
| No other causes of steatosis | No other causes of steatosis | No other causes of steatosis | No other causes of steatosis | No other causes of steatosis | |
| Insulin resistance | No significant alcohol consumption | No significant alcohol consumption | No significant alcohol consumption | No significant alcohol consumption | |
| No coexisting chronic liver disease | |||||
| Alcohol consumption threshold (men) | 30 g/d | 30 g/d | 2 standard drink/d | 30 g/d | 21 standard drink/wk |
| 140 g/wk | 294 g/wk | ||||
| Alcohol consumption threshold (women) | 20 g/d | 20 g/d | 1 standard drink/d | 20 g/d | 14 standard drink/wk |
| 70 g/wk | 196 g/wk |
EASL: European Association for the Study of the Liver; NICE: National Institute for Health and Care Excellence; AISF: Italian Association for the study of the Liver; AASLD: American Association for the Study of Liver Diseases; MRI: Magnetic resonance imaging.
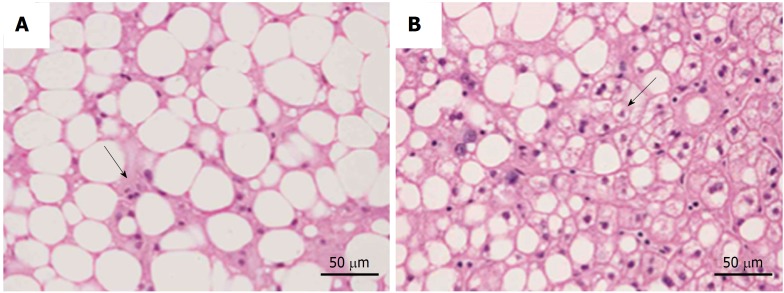
All recommendations identify some different clinical-pathological entities, according to the progression of hepatic histological changes. Simple steatosis and non-alcoholic steatohepatitis (NASH) are defined in all guidelines[3-8]. In detail simple steatosis, also called non-alcoholic fatty liver (NAFL) includes all of the case characterized by steatosis with minimal or absent lobular inflammation. On the contrary, NASH is characterized by hepatocyte ballooning degeneration, diffused lobular inflammation and fibrosis (Figure 1)
Figure 1.
Main difference between non-alcoholic fatty liver and non-alcoholic steatohepatitis. A: Non-alcoholic fatty liver; B: Non-alcoholic steatohepatitis. NAFL is characterized by minimal inflammatory infiltrate without hepatocyte ballooning (arrow). Instead, NASH is associated with lobular inflammatory infiltrate and hepatocyte degeneration (arrow). NAFL: Non-alcoholic fatty liver; NASH: Non-alcoholic steatohepatitis.
Additionally, EASL Asia-Pacific Guidelines and AISF position paper also underline the problem of NAFLD-related HCC, potentially occurring in patients with NAFLD but without cirrhosis[9,10].
Diagnostic criteria: The role of alcohol: The agreement between the different guidelines is not complete when defining the threshold dose of alcohol consumption. As shown in Table 1, EASL[3], NICE, and AISF guidelines[3,4,7] consider as significant an alcohol consumption > 30 g/d in men and > 20 g/d in women. The AASLD guidance[8] indicate the reasonable threshold for significant alcohol consumption > 21 standard drink on average per week in men and > 14 in women. For Asia-Pacific Guidelines[5] a significant alcohol intake was considered > 7 standard alcoholic drinks/week (70 g ethanol) in women and > 14 (140 g) in men.
Who should be screened for NAFLD?
According to the screening programs adopted for other diseases, systematic screening has to be performed for significant health problem with available diagnostic facilities and accepted treatment. Also, there should be recognisable latent or early symptomatic stage, identifiable with sensitive tests. To adequately perform a screening program, the natural history of the disease should be understood, and the economic burden should be suitable.
The international guidelines partially diverge about this topic. This disagreement derives from essential considerations regarding natural history, special groups, diagnosis, and therapy: (1) NAFLD in a common cause of chronic liver disease in general population but cause severe liver disease in a small proportion of affected people[1]; (2)Type II diabetes patients have higher prevalence of NAFLD, NASH and advanced fibrosis[11-13]; (3) There is a current lack of effective drug treatment; (4) Liver biopsy is a procedure with related risks; (6) Few cost-effective analysis are available[14].
All these considerations imply a different approach to screening in NAFLD by the Scientific Societies. Only EASL, NICE Asia-Pacific Guidelines[3-5] recommend screening respectively in particular, “high-risk” groups (Table 2). On the contrary, AASLD guidelines emphasise that, to date, there is no evidence of cost-effectiveness to support a NAFLD screening in adults even if they have several metabolic risk factors, instead suggesting a concept of “vigilance” in these populations[8].
Table 2.
Comparative analysis of the recommendations regarding the screening for non-alcoholic fatty liver disease
| EASL | NICE | Asia-Pacific | AISF | AASLD | |
| Systematic screening | No | No | No | No | No |
| Screening in high-risk groups | Yes | Yes | Yes | Not mentioned | No1 |
| Obesity | Obesity | Obesity | |||
| Metabolic syndrome | Type II Diabetes | Type II Diabetes | |||
| Abnormal liver enzymes | |||||
| Screening modality | Yes liver enzymes | No liver enzymes | No liver enzymes | ||
| Yes ultrasonography | Yes ultrasonography | ||||
| Yes transient elastography |
"Active surveillance" (but not screening) suggested for patients with type II diabetes mellitus. EASL: European Association for the Study of the Liver; NICE: National Institute for Health and Care Excellence; AISF: Italian Association for the Study of the Liver; AASLD: American Association for the Study of Liver Diseases.
Which noninvasive test(s) should be used to diagnose NAFLD?
Worldwide guidelines agree that, whenever NAFLD is suspected, the initial diagnostic workup should include a noninvasive imaging examination to confirm the presence of steatosis and general liver biochemistry[3-8]. Non-invasive assessment should aim first of all to identify NAFLD among patients with metabolic risk factors, and then to monitor disease progression and treatment response, identifying patients with the worst prognosis[3].
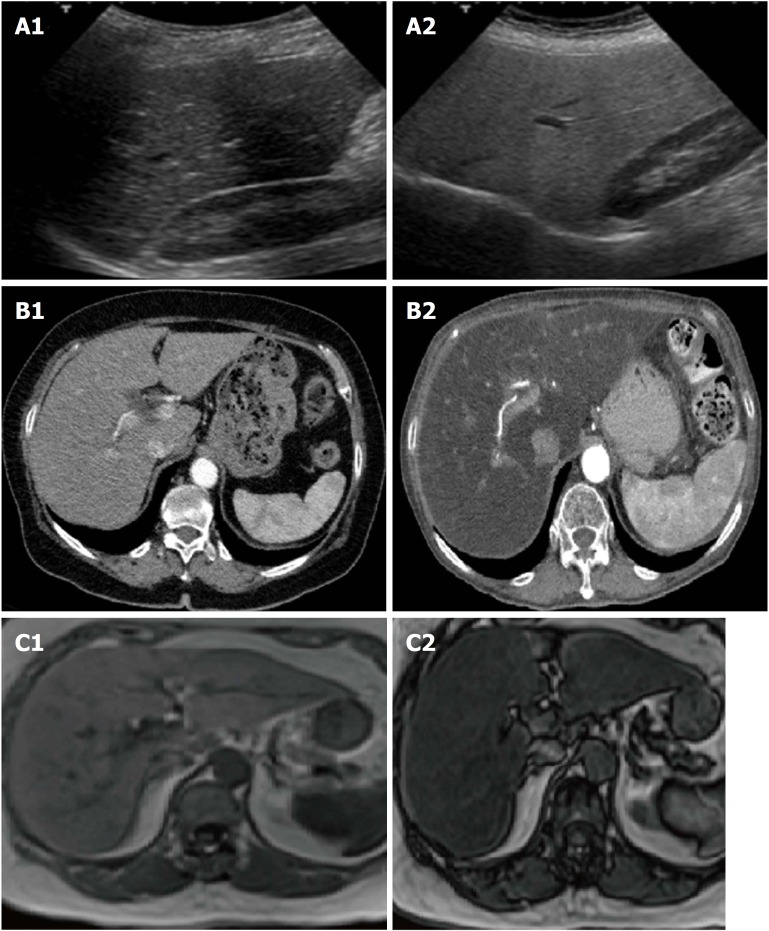
Imaging: There is a consensus for using abdominal ultrasound (US) as the first-line examination to identify liver steatosis in patients with increased liver blood exams or suspected NAFLD, in daily clinical practice (Figure 2). The main advantages of US derive from its broad availability and low cost. However, its sensitivity among morbidly obese patients (BMI > 40 kg/m2) is low, and it may miss the diagnosis when the liver hepatic fat content is < 20%[15,16]. Despite these limitations, EASL and AISF underline how ultrasound can significantly assess moderate and severe steatosis, even if an observer dependency remains[15]. NICE guidelines propose to use liver ultrasound to detect hepatic steatosis for children with metabolic syndrome and type 2 diabetes and to retest it every three years if the first examination is negative[4].
Figure 2.
Aspects of liver steatosis according to the different imaging techniques. In normal ultrasound examination liver parenchyma is isoechoic to the renal parenchyma in normal conditions (A1), becoming hyperechoic in presence of liver steatosis (A2). In comparison to a normal liver (B1), a fatty liver appears hypodense compared to the spleen and to the hepatic veins (B2) in computed tomography scans. Finally, in the setting of a severe steatosis, the magnetic resonance signal has a clear fall from in phase (C1) to out phase sequencings (C2).
On the other hand, magnetic resonance imaging (MRI), either by proton density fat fraction (1H-MRS) or spectroscopy, remains the gold standard to assess and quantify hepatic steatosis, detecting the amount of liver fat as low as 5%-10%, its use in the clinical practice is still limited. In fact, despite its robust accuracy, its limited availability, high costs and a long time of execution, make the procedure not recommended in the daily clinical setting[17]. Asia-Pacific guidelines specify that 1H-MRS is the best option to quantify even moderate changes in liver fat content in clinical trials, considering its high sensitivity compared to histological-proven liver fat reversal. Similarly, EASL guidelines highlight its role, primarily as screening imaging examination for clinical trials and experimental studies[3].
Another imaging technique used to quantify liver fat content is the ultrasonography-based transient elastography (TE) using continuous attenuation parameter (CAP). This promising tool has shown a good sensitivity, measuring simultaneously liver stiffness, potentially evaluating NAFLD severity at the same setting[13]. However, despite its low cost and rapidity of execution, its role in the clinical practice has still to be defined. In fact, EASL guidelines specify that TE has never been compared with hepatic steatosis measured by 1H-MRS and there are limited data about its ability to discriminate different histological patterns[3]. On the other hand, Asia-Pacific guidelines propose CAP as a useful screening tool for NAFLD diagnosis, as well as for demonstrating improvement in hepatic steatosis after lifestyle intervention and body weight reduction[5].
Conventional liver biochemistry: Although NAFLD may present by standard laboratory liver tests, frequently a slight increase of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) or gamma-glutamyl transpeptidase (GammaGT) is observed. However, all the guidelines agree that normal levels of liver enzymes may not exclude NAFLD, being a not sensitive screening test[3-8].
Moreover, laboratory alterations may hide another cause of liver disease, in which steatosis is a coexisting condition. On the other hand, detection of abnormalities of laboratory exams (such as ferritin or autoantibodies) not always reflects the presence of another liver disease, but could be an epiphenomenon of NAFLD with no further clinical significance.
In particular, AASLD guidelines underline that elevate serum ferritin and low titers of autoimmune antibodies (especially antinuclear and anti-smooth muscle antibodies) are common features among NAFLD patients[18,19], and may not automatically indicate the presence of hemochromatosis or autoimmune liver disease[8].
Which is the role of diagnostic and prognostic scores?
Noninvasive predictor biomarkers and scores of steatosis and steatohepatitis: The current absence of a highly specific and sensitive noninvasive marker predicting inflammation and fibrosis is leading to a considerable interest in the identification of new markers of disease progression and to the development of clinical scores of disease severity.
To assess the presence of steatosis, EASL, Asia-Pacific, and Italian guidelines mention the Fatty Liver Index (FLI)[20] and the NAFLD liver fat score[21]. Both of these scores are easily calculated using common blood exams and simple clinical information. In detail, FLI is calculated from serum triglyceride, body mass index, waist circumference, and gamma-glutamyltransferase[20], while NAFLD liver fat score is calculated evaluating the presence/absence of metabolic syndrome and type 2 diabetes, fasting serum insulin, and aminotransferases[21].They have been validated in a cohort of severely obese patients and the general population, reliably predicting the presence of steatosis, but not its severity[22]. On the contrary, the AASLD guidelines underline that only inflammation and fibrosis dictate the prognosis of NAFLD patients and, consequently, highlight the lack of evidence of the usefulness of quantifying hepatic steatosis in the routine clinical setting. Instead, AASLD guidelines underline that the simultaneous presence of several metabolic diseases is the most potent predictor of hepatic inflammation and adverse outcome in patients with NAFLD.
The cytokeratin-18 fragment is currently the most studied biomarker to assess the presence of inflammation. Its circulating levels have been largely investigated as a signal of hepatocellular apoptotic activity and therefore as a characteristic feature of NASH[23]. Its role is addressed both by Asia-Pacific and EASL guidelines, which agree that the current evidence does not support its use in clinical practice and that more studies are needed[3,5]. In particular, Asia-Pacific guidelines highlight how increased levels of cytokeratin-18 have good predictive value for NASH vs normal livers but do not differentiate NASH vs simple steatosis[24,25]. On the other hand, EASL guidelines specify that has been demonstrated that cytokeratin-18 serum levels decrease parallel with histological improvement, but its predictive value is not better than ALT in identifying histological responders[26].
To conclude, guidelines agree that noninvasive tests for detecting NASH and distinguishing it from simple steatosis are not currently available and that liver biopsy remains necessary to detect hepatocyte ballooning and lobular inflammation[3-8].
Noninvasive assessment of advanced fibrosis: Liver fibrosis is considered the leading prognostic factor among patients with NAFLD because of its strong correlation with survival rate and liver-related outcomes[27]. Therefore, NAFLD patients with advanced fibrosis need a closer monitoring and a rigorous adherence to treatment. However, to date, no methods easily performed in daily clinical practice and with a high predictive value for differentiating grades of liver fibrosis have been identified.
Different tools have been investigated at this purpose, including noninvasive scores (NAFLD fibrosis score, Fibrosis 4 calculator, AST/ALT ratio index), serum biomarkers (ELF panel, Fibrometer, Fibrotest, Hepascore) and imaging techniques, such as transient elastography, magnetic resonance elastography (MRE) and shear wave elastography[28].
According to the NICE guideline, the enhanced liver fibrosis (ELF) blood test has shown the best cost-effectiveness in identifying patients with advanced fibrosis stages[29] and therefore should be offered to all patients with an incidental diagnosis of NAFLD[4]. On the other hand, EASL and Italian guidelines suggest the use of NAFLD fibrosis score (NFS) and Fibrosis 4 calculator (FIB-4) as noninvasive scores to identify patients with different risk of advanced fibrosis[3,7]. These two scores have been validated in various ethnically NAFLD patients, predicting liver and cardiovascular-related mortality[30]. Moreover AASLD guidelines highlight that in a recent study both NFS and FIB-4 have shown the best predictive value for advanced fibrosis among histological proven NAFLD patients in comparison with other scores[28]. EASL guidelines underline that NFS has a stronger negative predictive value for advanced fibrosis than the corresponding positive predictive value[30]. Hence, it should be used for excluding the presence of advanced fibrosis better than stratifying NAFLD patients on different fibrosis stages[3].
Transient elastography has been recently approved by US Food and Drug Administration to investigate adult and pediatric patients with liver disease. Its cut-off value for advanced fibrosis for adults with NAFLD has been established to 9.9 KpA with 95% sensitivity and 77% specificity[31]. In particular, elastography score has been shown to have good diagnostic accuracy for the presence of clinically significant fibrosis, with an AUROC of 0.93 (95%CI: 0.89-0.096) for advanced fibrosis (≥ F3) and cirrhosis, and with a negative predictive value of 90% in ruling out cirrhosis when using a cut-off of 7.9 kPa. However, the ability in differentiating between F2 and F3 fibrosis seems less robust. Because of this high rate of false-positive results, EASL and Asia-Pacific guidelines point out that its low specificity limits its use in daily practice in diagnosing advanced grade of fibrosis and cirrhosis, as well as by a high failure rate[5]. Moreover considering the unreliable results among patients with high BMI and thoracic fold thickness, EASL guidelines highlight that it should not be used alone as first-line detection tool to identify advanced fibrosis or cirrhosis[3]. In this setting, its poor performance can be improved by using M or XL-probe, increasing the success rate[32,33].
American guidelines underline the vital role of magnetic resonance elastography (MRE) in identifying different degrees of fibrosis in patients with NAFLD, performing better than transient elastography for recognising intermediate stage of fibrosis, but showing a same predictive value for advanced fibrosis stages[34]. Therefore AASLD guidelines conclude that MRE and transient elastography are both useful tools for identifying NAFLD patients with advanced liver fibrosis.
On the other hand, shear wave elastography, in the same way as transient elastography, seems to be inappropriate to discriminate between intermediate stages of fibrosis and provide reliable results only in 73% of patients with BMI ≥ 30 kg/m2[35].
Which are the best diagnostic algorithms and follow-up strategies?
The optimal strategy for stratifying NAFLD patients and follow disease progression has not been yet established. According to EASL and Italian guidelines, the combination of noninvasive scores (NFS and FIB-4) and transient elastography should be used to identify patients at low risk of advanced liver disease and for clinical decision-making. Moreover, this combination may instead identify patients who should undergo a liver biopsy to confirm advanced fibrosis, and in whom a more intensive approach is needed)[3,7]. Noninvasive serum scores should be calculated for every patient with NAFLD to exclude the presence of significant fibrosis. If it cannot be ruled out, then transient elastography should be performed. Hence, if advanced fibrosis is suspected, liver biopsy should be performed for final diagnosis[3,7]. Moreover, a clinical, laboratory and instrumental follow-up for noninvasive monitoring of fibrosis, is suggested every two years for NAFLD patients with normal liver enzymes and low risk of advanced fibrosis. Patients with evidence of NASH or fibrosis should be screened annually and those with cirrhosis every six months, to perform HCC surveillance[3,7].
Similarly, the AASLD guidelines consider NFS, FIB-4, transient elastography, and MRE as the first-line examination to detect patients with advanced fibrosis[8]. Differently from the EASL guidance, however, no diagnostic algorithms or follow up strategies are provided.
The Asia-Pacific guidelines also agree that combined use of serum tests and imaging tools may offer more reliable information than using either method alone[6]. However, they do not specify which noninvasive test is best.
According to the NICE guidelines, every patient with an incidental finding of NAFLD should be screened for advanced fibrosis by ELF blood test. If negative, it should be repeated every three years for adults and two years for children. Moreover, children and young people with type 2 diabetes mellitus or metabolic syndrome, but without steatosis at ultrasound examination, should be reevaluated every three years[4].
Who should undergo liver biopsy?
To date, liver biopsy is the gold standard for diagnosing NASH and staging liver fibrosis, despite several limitations such as sampling error, variability in interpretation by pathologists, high cost and patient discomfort[36]. The “NAFLD Activity Score” (NAS)[37] and the “Steatosis Activity Fibrosis” (SAF) scoring system[38] are recommended to assess disease activity[8].
Except for the NICE guidelines (which do not provide specific indications about which patients should undergo liver biopsy), all of the remaining guidelines substantially agree that confirmatory liver biopsy should not be performed in every NAFLD patients. Instead, it should be reserved for the following two situations: (1) Uncertain diagnosis; (2) suspect of NAFLD-related advanced liver disease.
The AASLD guidelines suggest to perform liver biopsy in patients with metabolic syndrome who are at increased risk of liver inflammation, or when NFS, FIB-4 or liver stiffness measured by transient elastography or MRE suggest the presence of advanced liver fibrosis. In that case, patients would benefit the most from diagnosis, obtaining crucial prognostic information[8].
Similarly, EASL and Italian guidelines recommend performing a liver biopsy when both serum and imaging noninvasive tools show a medium/high risk of advanced liver disease, with the aim to confirm the presence of advanced liver fibrosis. Furthermore, they underline that in selected NAFLD patients at high risk of disease progression, the repetition of liver biopsy should be considered case-by-case every five years[3,7]. On the other hand, the Asia-Pacific guidelines recommend biopsy only when a competing aetiology of chronic liver disease cannot be excluded just by laboratory exams and personal anamnesis, or results of noninvasive tests are inconclusive[5,6].
How to treat NAFLD?
Lifestyle changes: Lifestyle modification consisting of diet, exercise, and weight loss has been advocated to treat patients with NAFLD in all guidelines (Tables 3 and 4). Indeed, weight loss has been reported as a keystone element in improving the histology features of NASH[39,40].
Table 3.
Comparison of recommendations about non-invasive evaluation of fibrosis and follow up strategies
| EASL | NICE | Asia-Pacific | AISF | AASLD | |
| Non-invasive | NFS and FIB-4 upon diagnosis. If inconclusive, perform transient elastography | ELF blood test | Combination of serum tests and imaging tools (no specification about the preferred tests) | NFS + FIB-4 upon diagnosis. If inconclusive, perform transient elastography | NFS, FIB-4 and transient elastography (or MRE) upon diagnosis |
| Follow up | Negative markers > reassess every 2 yr; Fibrosis or abnormal liver enzymes > reassess every year; Cirrhosis-> surveillance every 6 mo | Negative ELF test, > reassess every 3 yr; Positive ELF test > liver biopsy | No information provided | Negative markers > reassess every 2 yr; Fibrosis or abnormal liver enzymes > reassess every year; Cirrhosis > surveillance every 6 mo | No information provided |
EASL: European Association for the Study of the Liver; NICE: National Institute for Health and Care Excellence; AISF: Italian Association for the study of the Liver; AASLD: American Association for the Study of Liver Diseases; NFS: NAFLD fibrosis score; FIB-4: Fibrosis-4; ELF: Enhanced Liver Fibrosis; MRE: Magnetic resonance elastography.
Table 4.
Guidance statements about lifestyle interventions
| EASL | NICE | Asia-Pacific | AISF | AASLD | |
| Dietary restrictions | 500-1000 kcal deficit; weight loss of 500-1000 g/wk with a 7%-10% total weight loss | Main recommendations on diet of NICE’s obesity and preventing excess weight gain guidelines | 500-1000 kcal deficit | 1200-1600 kcal/d; fat-low (< 30% of total calories); carbohydrate-low (< 50% of total calories) | 500-1000 kcal deficit |
| Physical activity | Aerobic and resistance training (150-200 min/wk in 3-5 sessions) | Main recommendation of on physical activity of NICE’s obesity and preventing excess weight gain guidelines | Aerobic and resistance training | Aerobic and resistance training | Aerobic and resistance training (> 150 min/wk) |
| Gold standard diet | Low-to-moderate fat and moderate-to-high carbohydrate intake | No specific suggestions | All, excluding very low-calorie diets | Mediterranean diet | No specific suggestions |
| Low-carbohydrate ketogenic diets or high-protein | |||||
| Mediterranean diet |
EASL: European Association for the Study of the Liver; NICE: National Institute for Health and Care Excellence; AISF: Italian Association for the Study of the Liver; AASLD: American Association for the Study of Liver Diseases.
According to the AISF position paper[7], the best therapeutic approach is an adequate lifestyle change focused on weight loss and achieved by physical activity (aerobic activities and resistance training) and healthy diet. In particular, an energy restriction obtained with a low calorie (1200-1600 kcal/d), low fat (less than 10% of saturated fatty acid), low carbohydrate diet (< 50% of total kcal) is suggested. A Mediterranean diet is recommended as the most effective dietary option to induce a weight loss together with beneficial effects on all cardio-metabolic risk factors associated with NAFLD[7].
The Asia-Pacific guidelines agree with a lifestyle intervention strategy for the treatment of NAFLD, focusing the attention on the timing of weight loss that should be gradual because of the deleterious effect of crash diets on NASH. Very low-calorie diets are considered unsustainable, and any specific regimen is preferred over the others[6].
Also, the EASL[3], NICE[4], and AASLD[8] guidelines recommend structured programmes aimed at lifestyle changes towards a healthy diet and habitual physical activity. According to all of these guidelines, a 7%-10% weight loss is the target of most lifestyle interventions.
Pharmacological treatment: (1) Who to treat: According to the EASL guidelines[3], pharmacological therapy should be reserved for: Progressive NASH (bridging fibrosis and cirrhosis); early-stage NASH at high risk for disease progression (age > 50 years, metabolic syndrome, diabetes mellitus or increased ALT)[41]; active NASH with high necroinflammatory activities[42]. Similarly, in the AASLD and Asia-Pacific guidelines, a pharmacological approach is recommended only for patients with NASH and fibrosis[8]. In the NICE guidance, just people with an advanced liver fibrosis (ELF test > 10.51) are proposed for pharmacological treatment[4]. In the AISF position paper, drug therapy is suggested for patients who are at high risk for disease progression[7]. (2) Pharmacologic treatment: Currently, no drugs have been approved for the treatment of NASH by the US Food and Drug Administration or by the European Medicines Agency. All guidelines acknowledge that any medicines prescribed explicitly for NAFLD should be considered as an off-label treatment and that the decision should be discussed with the patient, carefully balancing the benefits and the safety. However, the guidelines are widely discordant about possibly helpful drugs (Table 5).
Table 5.
Recommendations about pharmacological treatment of non-alcoholic fatty liver disease
| EASL | NICE | ASIA-PACIFIC | AISF | AASLD | |
| Metformin | Insufficient evidence | Not beneficial | Not beneficial | Not mentioned | Not beneficial |
| Vitamin E | Insufficient evidence | Consider use regardless of diabetes | Not beneficial | Insufficient evidence | Consider use in non-diabetic, biopsy-proven NASH |
| PPAR-gamma agonists | Consider use in selected diabetic patients | Consider pioglitazone in adults regardless of diabetes | Insufficient evidence in Asian | Insufficient evidence, potentially useful | Pioglitazone indicated in biopsy-proven NASH (regardless of diabetes) |
| PUFA | Not beneficial | Insufficient evidence | Not beneficial | Not mentioned | Not beneficial |
| Pentoxifylline | Insufficient evidence | Not mentioned | Not beneficial | Not mentioned | Not mentioned |
| GLP-1 analogues | Insufficient evidence, potentially useful | Insufficient evidence | Insufficient evidence in Asian patients | Insufficient evidence, potentially useful | Insufficient evidence |
| UDCA | Not beneficial | Not beneficial | Not mentioned | Not mentioned | Not beneficial |
| Obetycolic acid | Scarce evidence | Not mentioned | waiting for ongoing RCT results | Waiting for ongoing RCT results | Insufficient evidence |
| Silymarin | Not mentioned | Not mentioned | insufficient evidence, potentially useful | Not mentioned | Not mentioned |
| Statins | Safe but not beneficial | Safe but not beneficial | Safe but not beneficial | Safe but not beneficial | Safe but not beneficial |
EASL: European Association for the Study of the Liver; NICE: National Institute for Health and Care Excellence; AISF: Italian Association for the Study of the Liver; AASLD: American Association for the Study of Liver Diseases; PPAR: Peroxisome proliferator-activated receptors; PUFA: Poly-unsaturated fatty acids; GLP-1: Glucagon-like peptide-1.
Metformin: Due to the evidence of its limited efficacy in improving the histological features of NAFLD[43-45], metformin is not recommended by any guidelines to specifically treat NAFLD[3-8].
Pioglitazone: Pioglitazone, a thiazolidinedione, is a peroxisome proliferator-activated receptor (PPAR) gamma agonist with insulin-sensitising effects. Treatment with pioglitazone improves insulin sensitivity, aminotransferases, steatosis, inflammation, and ballooning in patients with NASH and prediabetes or T2DM[46]. The PIVENS trial (a large multicenter RCT) compared low dose pioglitazone (30 mg/d) vs vitamin E (800 UI/d) vs placebo for two years in patients without overt diabetes. Pioglitazone improved all histological features (except for fibrosis) and achieved resolution of NASH more often than placebo[47]. The histological benefit occurred together with ALT improvement and partial correction of insulin resistance. The main side effects of glitazones are weight gain[48-51], and bone fractures in women[52]. The use of pioglitazone for the treatment of NAFLD is endorsed both by the NICE and AASLD guidelines, with significant limitations. In the first case, pioglitazone should be prescribed only in second and third level centres, after a careful evaluation[4]. In the latter case, pioglitazone is reserved for patients with biopsy-proven NASH[8]. The EASL guidelines are more cautious, generically suggesting to consider pioglitazone for the treatment of diabetes in patients with a concurrent NAFLD[3]. Even the Asia-Pacific and the Italian guidelines acknowledge the potential benefits of pioglitazone, however, suggest that more evidence should be available before a firm recommendation can be made[6,7].
Vitamin E: Vitamin E is an anti-oxidant and has been investigated to treat NASH. In the PIVENS trial, vitamin E at a dose of 800 IU/d of α-tocopherol for 96 wk was associated with a decrease in serum aminotransferases and histological improvement in steatosis, inflammation, and ballooning and resolution of steatohepatitis in adults with NASH[47]. Long-term safety of vitamin E is under dispute, with two different meta-analyses leading to conflicting results when analysing the all-cause mortality in patients treated with t doses of > 800 IU/d[51,52]. Similarly to pioglitazone, vitamin E is recommended by the NICE and AASLD guidelines (limited to biopsy-proven NASH in the latter case)[4,8]. EASL and AISF guidelines call for more evidence before any recommendation[3,7], while Asia-Pacific guidelines advice against the use of vitamin E which is described as not beneficial by the current evidence[6].
Glucagon-like peptide-1 (GLP-1) analogues: Incretin-mimetics, acting on the glucose-insulin interplay have shown favourable results in pre-marketing studies on liver enzymes[53]. Also, in a published randomised, placebo-controlled trial consisting of 52 patients with biopsy-proven NASH, liraglutide administered subcutaneously once-daily for 48 wk was associated with greater resolution of NASH and less progression of fibrosis[54]. Both the AASLD and NICE recommendations state that there is still too few evidence to support the use of GLP-1 analogues to specifically treat liver disease in patients with NAFLD[4,8]. The remaining guidelines also agree on this point, however also state that further evidence may prove the efficacy of these drugs. In particular, the APASL guidelines consider some more elements in their recommendations. On the one hand, GLP-1 agonists appeared to reduce glycated haemoglobin more efficiently in Asian patients with type 2 diabetes mellitus[55]. On the other hand, there has been no study on Asian NASH patients, even if the pharmacokinetics of GLP-1 agonists do not appear to differ between Asian and non-Asian patients according to preliminary evidence[56,57].
Statins: Historically, the use of statins in patients with chronic liver diseases has been considered as potentially troublesome due to the risk of hepatotoxicity. At the same time, a considerable portion of NAFLD patients usually receives statins because of their multiple cardiovascular risk factors. Consequently, the primary concern of the guidelines is the safety of statins. In this regard, a recent review underlined the safety of statin and their efficacy in reducing the associated cardiovascular morbidity in patients with NAFLD, including those with slightly elevated alanine transaminases (up to 3 × reference upper limit)[58]. All of the guidelines agree about the safety of prescribing statins (or continuing an ongoing statin therapy) in patients with NAFLD, even with compensated cirrhosis. However, routine prescription of a statin is not recommended in patients with decompensated cirrhosis and acute liver failure[59,60].
Silymarin: Silymarinis a complex mixture of six major flavonolignans (silybins A and B, isosilybins A and B, silychristin, and silydianin), as well as other minor polyphenolic compounds[61].In a randomised, double-blinded, placebo-controlled study on patients with biopsy-proven NASH, silymarin dosage of 700 mg three times daily for 48 wk resulted in a significantly higher percentage of fibrosis reduction compared with placebo (22.4% vs 6.0%, P = 0.023)[62]. The dosage was safe and well tolerated[62]. Silymarin is mentioned as a potentially useful treatment for NASH in Asia-Pacific guidelines only. However, optimal dose and duration still require further studies before a full recommendation[6].
Bariatric surgery: In patients unresponsive to lifestyle changes and pharmacotherapy, bariatric surgery is an option for reducing weight and metabolic complications, with stable results in the long-term[63]. Bariatric surgery can also improve liver histology, both regarding steatosis and ballooning[64,65] and fibrosis[65]. However, the presence of established cirrhosis is associated with peri-operative risks. In particular, in the analysis performed from the Nationwide Inpatient Sample (1998-2007), mortality was higher in patients with compensated cirrhosis (0.9%) and much higher in those with decompensated cirrhosis (16.3%)[66]. No robust data on the comparative effects of different bariatric procedures on liver fat are available in the literature.
Based on the evidence as mentioned earlier, the EASL guidelines consider bariatric surgery an option in patients unresponsive to lifestyle changes and pharmacotherapy, for reducing weight and metabolic complications[3]. Guidance statements by the AASLD also consider a role of foregut bariatric surgery in otherwise eligible obese individuals with NAFLD or NASH[8].
The Asia-Pacific recommendation limits the role of bariatric surgery only to patients with class II obesity (BMI > 32.5 kg/m2 in Asians and 35 kg/m2 in Caucasians)[6]. AISF and NICE guidelines do not mention bariatric surgery.
Liver transplantation: NASH is becoming the most common indication to liver transplantation in Western Countries[67]. Because of the high prevalence of obesity, sarcopenia, cardiovascular disease and chronic kidney disease among patients with NASH, there is a higher frequency of post-transplant complications and increased graft loss[68,69]. Because of the risk of prolonged ventilation, poor wound healing, higher rate of primary graft non-function, and increased infectious complications, patients with severe obesity (BMI > 40 kg/m2) may even be considered unfit for liver transplantation, unless efforts are made preoperatively to reduce body weight with individualized plans of lifestyle modifications[70].
AISF and NICE guidance do not mention liver transplantation. All of the remaining guidelines agree that liver transplantation is an acceptable procedure in NASH patients with an end-stage liver disease, with the same indications adopted for other etiologies of liver disease[3-8].
CONCLUSION
The comparative analysis of the most recent international guidelines for the management of NAFLD showed some common orientation between the different recommendations, as well as diverging points. The most notable differences involved: the identification of the alcohol threshold defining NAFLD, the screening strategies in high-risk populations, the preferred non-invasive biomarkers for the assessment of advanced fibrosis, and the pharmacological treatment. These differences should not be necessarily seen as a limitation, but rather an expression of the geographical differences in genetic predisposition to NAFLD, lifestyle habits, healthcare systems. Arguably, the similarity in the recommendations could greatly help in ensuring homogenous management of NALFD all over the world, with favourable repercussions both in clinical practice and in clinical trials. In the next years, we might see a trend toward more homogenous guidelines thanks to the increasing body of evidence. In particular, the advancements in the imaging technologies could lead to new and widely accepted noninvasive methods to assess advanced liver fibrosis. Moreover, some clinical trials are investigating potentially effective drugs. If positive, the currently diverging pharmacological recommendations may reach a higher concordance. NAFLD is becoming a leading field of research in hepatology: new evidence is destined to change the current landscape of knowledge, prompting greater benefits to the patients as well as changes in the recommendations for clinical practice.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: To the best of our knowledge, no conflict of interest exists.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 10, 2018
First decision: May 24, 2018
Article in press: June 25, 2018
P- Reviewer: Jamali B, Lee HC, Jamali R, Yoshioka K S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
Contributor Information
Simona Leoni, Department of Medical and Surgical Sciences (DIMEC), Division of Internal Medicine, University of Bologna, Bologna 40136, Italy. simona.leoni@aosp.bo.it.
Francesco Tovoli, Department of Medical and Surgical Sciences (DIMEC), Division of Internal Medicine, University of Bologna, Bologna 40136, Italy.
Lucia Napoli, Department of Medical and Surgical Sciences (DIMEC), Division of Internal Medicine, University of Bologna, Bologna 40136, Italy.
Ilaria Serio, Department of Medical and Surgical Sciences (DIMEC), Division of Internal Medicine, University of Bologna, Bologna 40136, Italy.
Silvia Ferri, Department of Medical and Surgical Sciences (DIMEC), Division of Internal Medicine, University of Bologna, Bologna 40136, Italy.
Luigi Bolondi, Department of Medical and Surgical Sciences (DIMEC), Division of Internal Medicine, University of Bologna, Bologna 40136, Italy.
References
- 1.De Minicis S, Day C, Svegliati-Baroni G. From NAFLD to NASH and HCC: pathogenetic mechanisms and therapeutic insights. Curr Pharm Des. 2013;19:5239–5249. [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence (UK) Non-Alcoholic Fatty Liver Disease: Assessment and Management. Available from: http//www.niceorg.uk\guidance\ng49. [PubMed]
- 5.Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, Fan J, Goh KL, Hamaguchi M, Hashimoto E, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 6.Chitturi S, Wong VW, Chan WK, Wong GL, Wong SK, Sollano J, Ni YH, Liu CJ, Lin YC, Lesmana LA, et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: Management and special groups. J Gastroenterol Hepatol. 2018;33:86–98. doi: 10.1111/jgh.13856. [DOI] [PubMed] [Google Scholar]
- 7.Italian Association for the Study of the Liver (AISF) AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig Liver Dis. 2017;49:471–483. doi: 10.1016/j.dld.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 9.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 10.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 11.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, Webb A, Hecht J, Cusi K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100:2231–2238. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, Leebeek FW, Hofman A, Stricker BH, Castera L, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology. 2016;63:138–147. doi: 10.1002/hep.27981. [DOI] [PubMed] [Google Scholar]
- 13.Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–1368. doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 14.Klebanoff MJ, Corey KE, Chhatwal J, Kaplan LM, Chung RT, Hur C. Bariatric surgery for nonalcoholic steatohepatitis: A clinical and cost-effectiveness analysis. Hepatology. 2017;65:1156–1164. doi: 10.1002/hep.28958. [DOI] [PubMed] [Google Scholar]
- 15.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 16.Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–1122. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 17.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 18.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G, et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905–912. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Vuppalanchi R, Gould RJ, Wilson LA, Unalp-Arida A, Cummings OW, Chalasani N, Kowdley KV; Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hepatol Int. 2012;6:379–385. doi: 10.1007/s12072-011-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstråle M, Groop L, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V; LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209–1222. doi: 10.1111/apt.12963. [DOI] [PubMed] [Google Scholar]
- 23.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen J, Chan HL, Wong GL, Chan AW, Choi PC, Chan HY, Chim AM, Yeung DK, Yu J, Chu WC, et al. Assessment of non-alcoholic fatty liver disease using serum total cell death and apoptosis markers. Aliment Pharmacol Ther. 2012;36:1057–1066. doi: 10.1111/apt.12091. [DOI] [PubMed] [Google Scholar]
- 25.Chan WK, Sthaneshwar P, Nik Mustapha NR, Mahadeva S. Limited utility of plasma M30 in discriminating non-alcoholic steatohepatitis from steatosis--a comparison with routine biochemical markers. PLoS One. 2014;9:e105903. doi: 10.1371/journal.pone.0105903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuppalanchi R, Jain AK, Deppe R, Yates K, Comerford M, Masuoka HC, Neuschwander-Tetri BA, Loomba R, Brunt EM, Kleiner DE, et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2121–2130.e1-2. doi: 10.1016/j.cgh.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 28.Kaswala DH, Lai M, Afdhal NH. Fibrosis Assessment in Nonalcoholic Fatty Liver Disease (NAFLD) in 2016. Dig Dis Sci. 2016;61:1356–1364. doi: 10.1007/s10620-016-4079-4. [DOI] [PubMed] [Google Scholar]
- 29.Fagan KJ, Pretorius CJ, Horsfall LU, Irvine KM, Wilgen U, Choi K, Fletcher LM, Tate J, Melino M, Nusrat S, et al. ELF score ≥9.8 indicates advanced hepatic fibrosis and is influenced by age, steatosis and histological activity. Liver Int. 2015;35:1673–1681. doi: 10.1111/liv.12760. [DOI] [PubMed] [Google Scholar]
- 30.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, Kaye P, Burt AD, Ryder SD, Aithal GP, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–460. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 31.Tapper EB, Challies T, Nasser I, Afdhal NH, Lai M. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients With Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2016;111:677–684. doi: 10.1038/ajg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, Choi PC, Merrouche W, Chu SH, Pesque S, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–1871. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 33.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 34.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637.e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 35.Cheah MC, McCullough AJ, Goh GB. Current Modalities of Fibrosis Assessment in Non-alcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2017;5:261–271. doi: 10.14218/JCTH.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 37.Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 38.Bedossa P; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–575. doi: 10.1002/hep.27173. [DOI] [PubMed] [Google Scholar]
- 39.Haufe S, Engeli S, Kast P, Böhnke J, Utz W, Haas V, Hermsdorf M, Mähler A, Wiesner S, Birkenfeld AL, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. 2011;53:1504–1514. doi: 10.1002/hep.24242. [DOI] [PubMed] [Google Scholar]
- 40.Asrih M, Jornayvaz FR. Diets and nonalcoholic fatty liver disease: the good and the bad. Clin Nutr. 2014;33:186–190. doi: 10.1016/j.clnu.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L; American Association for the Study of Liver Diseases; United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392–1405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 44.Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 45.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Therap Adv Gastroenterol. 2009;2:157–163. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 47.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 49.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 50.Yau H, Rivera K, Lomonaco R, Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr Diab Rep. 2013;13:329–341. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 51.Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 52.Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4:158–170. doi: 10.2174/1874609811104020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 55.Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900–909. doi: 10.1111/dom.12293. [DOI] [PubMed] [Google Scholar]
- 56.Cui YM, Guo XH, Zhang DM, Tham LS, Tang CC, Mace K, Linnebjerg H. Pharmacokinetics, safety, and tolerability of single- and multiple-dose exenatide once weekly in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2013;5:127–135. doi: 10.1111/1753-0407.12020. [DOI] [PubMed] [Google Scholar]
- 57.Ingwersen SH, Petri KC, Tandon N, Yoon KH, Chen L, Vora J, Yang W. Liraglutide pharmacokinetics and dose-exposure response in Asian subjects with Type 2 diabetes from China, India and South Korea. Diabetes Res Clin Pract. 2015;108:113–119. doi: 10.1016/j.diabres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Cohen DE, Anania FA, Chalasani N; National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C–81C. [Google Scholar]
- 59.Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59:1958–1965. doi: 10.1007/s10620-014-3179-2. [DOI] [PubMed] [Google Scholar]
- 60.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 61.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 62.Wah Kheong C, Nik Mustapha NR, Mahadeva S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:1940–1949. doi: 10.1016/j.cgh.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–540. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 65.Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, Raverdy V, Leteurtre E, Dharancy S, Louvet A, et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015;149:379–388; quiz e15-16. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 66.Bower G, Toma T, Harling L, Jiao LR, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: a Systematic Review of Liver Biochemistry and Histology. Obes Surg. 2015;25:2280–2289. doi: 10.1007/s11695-015-1691-x. [DOI] [PubMed] [Google Scholar]
- 67.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 68.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 69.Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 70.Hakeem AR, Cockbain AJ, Raza SS, Pollard SG, Toogood GJ, Attia MA, Ahmad N, Hidalgo EL, Prasad KR, Menon KV. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl. 2013;19:551–562. doi: 10.1002/lt.23618. [DOI] [PubMed] [Google Scholar]