Abstract
A brachial plexus root avulsion (BPRA) causes intractable pain in the insensible affected hands. Such pain is partly due to phantom limb pain, which is neuropathic pain occurring after the amputation of a limb and partial or complete deafferentation. Previous studies suggested that the pain was attributable to maladaptive plasticity of the sensorimotor cortex. However, there is little evidence to demonstrate the causal links between the pain and the cortical representation, and how much cortical factors affect the pain. Here, we applied lesioning of the dorsal root entry zone (DREZotomy) and training with a brain–machine interface (BMI) based on real-time magnetoencephalography signals to reconstruct affected hand movements with a robotic hand. The DREZotomy successfully reduced the shooting pain after BPRA, but a part of the pain remained. The BMI training successfully induced some plastic changes in the sensorimotor representation of the phantom hand movements and helped control the remaining pain. When the patient tried to control the robotic hand by moving their phantom hand through association with the representation of the intact hand, this especially decreased the pain while decreasing the classification accuracy of the phantom hand movements. These results strongly suggested that pain after the BPRA was partly attributable to cortical representation of phantom hand movements and that the BMI training controlled the pain by inducing appropriate cortical reorganization. For the treatment of chronic pain, we need to know how to modulate the cortical representation by novel methods.
Keywords: cortical plasticity, magnetoencephalography, neurofeedback, phantom limb pain, robotic hand
Introduction
Chronic pain is not a prolonged acute pain, but is a disease caused by maladapted cortical activities.1,2) Recent neuroimaging studies, for example, have demonstrated that chronification of back pain is predicted by neuroimaging features of resting state functional magnetic resonance imaging (fMRI), not by the intensity of acute pain.3) Similarly, phantom limb pain has been attributed to maladaptive plasticity of the sensorimotor cortex.4–6) Phantom limb pain is an intractable chronic pain7) that frequently occurs in an amputation6) or a partially or completely deafferented body part after severe peripheral nerve injury,8) such as brachial plexus root avulsion (BPRA). Although seminal works have revealed correlations between phantom limb pain and maladaptive cortical reorganization of phantom hand representation,9–11) more recent studies have shown conflicting evidence12,13) and questioned the maladaptive reorganization hypothesis. To clarify the causative link between sensorimotor cortical plasticity and pain, direct experimental manipulation of sensorimotor plasticity is necessary.
In the neurosurgery department of Osaka University hospital, we have used multidisciplinary approaches to treat such intractable pain. To decrease the pain caused by the periphery and central factors, we combined neuromodulations such as lesioning of dorsal root entry zone (DREZotomy),14) spinal cord stimulation,15) repetitive transcranial magnetic stimulation (rTMS),16) electrical motor cortex stimulation (EMCS)17) and brain–machine interface (BMI).18) For example, the DREZotomy is effective for alleviating the shooting pain after the BPRA, but some patients complain of residual continuous pain even after the DREZotomy. We hypothesized that pain after the BPRA partially originated from maladaptive cortical reorganization of the sensorimotor cortex. In some patients, the continuous pain was decreased by EMCS with subdural electrodes17) and rTMS.16) We therefore evaluated how phantom limb pain was modulated by BMI-induced cortical plasticity.
Brain–machine interface is a powerful tool used to induce plastic changes in cortical activities.19–21) BMI can decode neural activity of phantom hand movements and convert the decoded activity into movement of a prosthetic hand.22–28) Moreover, training with the BMI induces plastic changes in cortical activity29,30) and potentially produces changes in associated clinical symptoms.31) Here, we have developed a BMI to precisely decode phantom hand movements using magnetoencephalography (MEG) signals.32–35) We used BMI with patients with phantom limb pain to evaluate the association between pain and the cortical plasticity induced by BMI training.18) We found that BMI training to associate a robotic hand with the intact hand representation reduced pain while decreasing classification accuracy of phantom hand movements. The BMI is a novel neuromodulation to treat intractable chronic pain caused by maladaptive cortical reorganization.
Subjects and Methods
Subjects
From 2000 to 2016, we performed DREZotomy for 24 patients with intractable pain after BPRA in the Neurosurgery Department of Osaka University hospital (Male 22, Female 2, mean age ± SD 46.7 ± 12.6). They had pain in their affected limb for an average of 23.2 ± 11 years. Patients especially experienced shooting pain, a severe pain which attacked frequently at unexpected intervals. We evaluated the pain for 8 of the 21 patients using a Visual Analog Scale (VAS) and the Japanese version of the short-form McGill Pain Questionnaire 2 (SF-MPQ2)36) before and after DREZotomy.
We entrained nine BPRA patients (all males; mean age, 52.1 years; range, 38–60 years) who all experienced pain in their phantom limb (Table 1). Some of the patients had experience of DREZotomy. Four patients are the same patients in the above mentioned DREZotomy group. In performing this study, we adhered to the Declaration of Helsinki and acted in accordance with protocols approved by the Ethics Committee of Osaka University Clinical Trial Center (no. 12107, UMIN000010180). All patients were informed of the purpose and possible consequences of this study, and written informed consent was obtained.
Table 1.
Clinical profiles of patients
| Patient ID | Age (years)/Sex | Diagnosis | JART FSIQ/VIQ/PIQ | Disease duration (years) | Mirror therapy |
|---|---|---|---|---|---|
| 1 | 50/M | Right BPRA of C6-8 | 100/100/90 | 34 | Effective only for a short period |
| 2 | 51/M | Left BPRA of C5-Th1 | 96/96/96 | 6 | Not effective |
| 3 | 58/M | Right BPRA of C6-Th2 | 112/114/108 | 40 | No experience |
| 4 | 49/M | Right BPRA of C7-Th1 | 102/102/101 | 29 | No experience |
| 5 | 56/M | Right BPRA of C7-8 | 114/116/110 | 38 | Not effective |
| 6 | 51/M | Right BPRA of C6-Th1 | 110/112/107 | 11 | No experience |
| 7 | 56/M | Left BPRA of C7-Th1 | 83/82/87 | 13 | Not effective |
| 8 | 38/M | Right BPRA of C6-8 | 85/84/89 | 21 | No experience |
| 9 | 60/M | Right BPRA of C6-8 | 114/116/110 | 20 | No experience |
BPRA: brachial plexus root avulsion, FSIQ: full-scale intelligence quotient, JART: Japanese adult reading test, M: male, PIQ: performance intelligence quotient, VIQ: verbal intelligence quotient.
MEG recording
We used a 160-channel whole-head MEG (MEGvision NEO; Yokogawa Electric Corporation, Musashino, Tokyo) to record cortical activities related to patient phantom hand movements. Signals were sampled at 1000 Hz with an online low-pass filter at 200 Hz and acquired online by FPGA DAQ boards (PXI-7854R; National Instruments, Austin, TX, USA) after passing through an optical isolation circuit. The signals for the 84 selected channels were used for offline analysis and online control of the prosthesis.
Experimental design
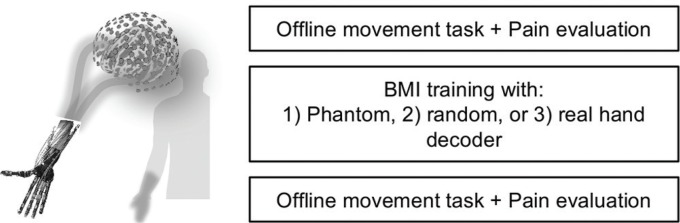
All patients participated in a crossover trial consisting of three experiments on different days. In each experiment, all patients performed the movement task before and after the BMI training (Fig. 1). In the first offline task, each patient attempted to move his or her phantom hand or intact hand (grasping and opening) at specified times24) while the MEG signals of the selected channels were recorded. After the offline task, pain was evaluated with a VAS. The acquired MEG signals were used to construct the decoder to control the robotic hand; the patient then used the trained decoder to control the robotic hand during the BMI training.35) Before the BMI training, each patient was instructed to control the robotic hand by moving his phantom hand while watching the movement of the robotic hand on a monitor.
Fig. 1.
BMI training and experimental design. (Left) Patients were instructed to control the robotic hand by moving their phantom hands. Three types of decoders were used to control the robotic hand based on MEG signals acquired online. (Right) The experimental design. For the BMI training, we used three types of decoders: phantom decoder, random decoder and real hand decoder. Before the experiment with the real hand decoder, the patients also performed an offline movement task with their intact hand after the task with their phantom hand.
The experiment was performed three times with different decoders, with an interval of at least 2 weeks between experiments. The order of the phantom decoder and random decoder experiments was randomly assigned to the patients to balance group sizes, and then the experiment with the real hand decoder was performed.
Cortical current estimation by VBMEG
The Freesurfer software (http://surfer.nmr.mgh.harvard.edu/)37) was used to construct a polygonal model of the cortical surface based on structural MRI (T1-weighted; Signa HDxt Excite 3.0T; GE Healthcare UK Ltd., Buckinghamshire, UK). At the beginning of each experiment, the 3-dimensional facial surface and 50 points on the scalp of each participant (FastSCAN Cobra; Polhemus, Colchester, VT, USA) were scanned to align MEG data with individual MRI data. Three-dimensional facial surface data were superimposed on the anatomical facial surface provided by the MRI data. The positions of five marker coils before each recording were used to estimate cortical current with VBMEG (ATR Neural Information Analysis Laboratories, Kyoto).38,39) The hyperparameters m0 and γ0 were set to 100 and 10, respectively. The inverse filter was estimated by using MEG signals in all trials from 0 to 1 s in the offline task, with the baseline of the current variance estimated from the signals from −1.5 to −0.5 s. The filter was then applied to sensor signals in each trial to calculate cortical currents.
Classification of movement types in the offline task
The estimated cortical currents on the sensorimotor cortex were converted to z-scores by using the mean and standard deviation of the currents. Using the z-scored cortical currents, the two types of phantom hand movements were classified using a support vector machine,35) producing a nested 10-fold cross-validation.32) All decoding analyses were performed with the MATLAB R2013a using a radial basis function kernel support vector machine.
Results
DREZotomy significantly reduced intermittent pain
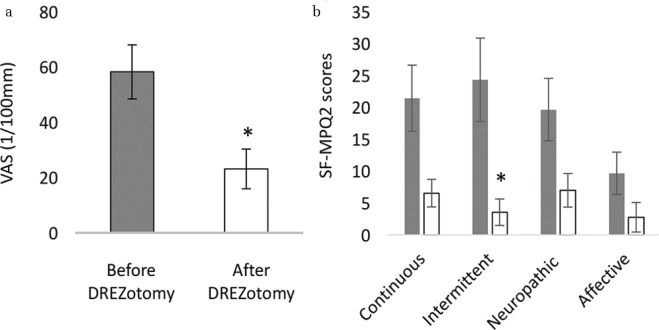
Among eight patients with pain after BPRA, the pain was significantly decreased after the DREZotomy (Fig. 2a P, < 0.05, Student’s t-test). Moreover, the scores for intermittent pain significantly decreased among four types of pain in SF-MPQ2 (Fig. 2b P, < 0.05, Student’s t-test, uncorrected). For the patients with shooting pain, the DREZotomy was effective in decreasing their pain significantly, although continuous pain remained for some patients.
Fig. 2.
Alteration of pain after DREZotomy. Pain was significantly reduced after the DREZotomy in VAS (a) and SF-MPQ2 scores (b). Gray: pain before surgery; White; pain after surgery.
BMI training with a robotic hand
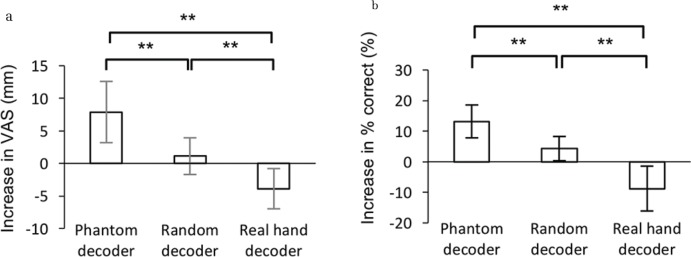
Among the nine patients with phantom limb pain, VAS scores changed significantly after the BMI training, depending on the decoder type (n = 9 each, P = 0.0006, one-way ANOVA) (Fig. 3a). After the training with the real hand decoder, VAS scores decreased significantly compared to those of the random decoder and the phantom decoder (n = 9, P = 0.009 and 0.003, respectively, paired Student’s t-test, Bonferroni corrected). In contrast, VAS scores increased significantly after training with the phantom decoder compared to the random decoder (n = 9, P = 0.006, paired Student’s t-test, Bonferroni corrected).
Fig. 3.
Alteration in pain and classification accuracies among the three experiments. (a) The averaged differences in VAS scores (post – pre) are shown with the 95% confidence interval for three experiments (n = 9; **P < 0.01, paired Student’s t-test, Bonferroni corrected). (b) The accuracies for classifying two types of phantom movements were evaluated using the currents on the motor cortex contralateral to the phantom hand. Each bar shows the average difference in the accuracy with 95% confidence intervals for each experiment (n = 9, **P < 0.01, paired Student’s t-test, Bonferroni corrected).
We evaluated the classification accuracy using the estimated sensorimotor cortical currents contralateral to the phantom hand for the MEG signals before and after the BMI training. Classification accuracies varied significantly among the training with three decoders (n = 9 each, P = 0.0008, one-way ANOVA) (Fig. 3b). The classification accuracy decreased significantly after training with the real hand decoder compared to the phantom and random decoder (n = 9, P = 0.0013 and 0.009, respectively, paired Student’s t-test, Bonferroni corrected), whereas it increased significantly after training with the phantom decoder compared to the random decoder (n = 9, P = 0.02, paired Student’s t-test).
Discussion
In this study, we have demonstrated that DREZotomy significantly reduced the pain for the patients suffering from shooting pain due to BPRA, although some pain remained even after the surgery in some patients. For patients with phantom limb pain due to BPRA, we tested the effects of MEG–BMI in controlling their pain. Interestingly, we demonstrated that MEG–BMI training significantly altered both the pain and phantom hand representation. MEG–BMI significantly changed the pain in proportion to the classification accuracy of phantom hand movements. In particular, we found that BMI training based on the real hand decoder disrupted the motor information of the original phantom hand representation and significantly decreased pain. These results strongly suggested that the phantom limb pain after BPRA was modulated from the sensorimotor representation of phantom hand movements. By inducing cortical plasticity, MEG–BMI will be a suitable treatment for phantom limb pain.40)
During the training with the real hand decoder, the patients were instructed to associate their phantom hand movements with the movements of the robotic hand, which was actually controlled by a decoder to classify the MEG signals of the intact hand’s movement. This training was expected to associate the phantom hand movements with the cortical representation of the intact hand’s movements, which were different from the original cortical representation of the phantom movements. BMI training with the real hand decoder would accelerate the dissociation of the link between the phantom hand and the original cortical representation by creating a new link to the real hand. The different neural representation might dissociate the robotic hand and the original neural representation of phantom movements even more so than the association of the neural representation and the randomly moved prosthetic hand.
Brain–machine interface training has much in common with mirror therapy. During mirror therapy, the patient tries to move his or her phantom hand while moving his or her intact hand, trying to associate the phantom hand movements with cortical representation of the intact hand’s movements. Therefore, mirror therapy and MEG–BMI training create similar effects if the patient succeeds in thinking that he or she only moved his or her phantom hand without thinking to move the intact hand, although it was very difficult to ignore movement of the intact hands. BMI provides an easier way for the patient to learn how to associate the movements of the phantom hand and the intact hand.
To relieve chronic pain, many treatments targeting peripheral and spinal factors causing pain have been tested. We have demonstrated that DREZotomy was effective for treating the shooting pain after BPRA, while treatments targeting the spinal cord factors could not eliminate chronic pain. Recent studies have demonstrated that chronic pain is partly attributable to a maladaptive neurological state,1,2) and our results demonstrated that treatment targeting cortical factors can relieve chronic pain. By controlling information processing relating to pain, we might be able to control chronic pain. Our results demonstrated that BMI training is a useful and powerful tool that can be used to study and control information processing in the brain. By combining decoding41) and neurofeedback, BMI could be used to treat chronic pain.
In summary, MEG–BMI training provides a novel method to directly change the information content of motor representations and to control phantom limb pain. Our experiments revealed that BMI training deteriorates the phantom hand representation to reduce pain. This strongly suggests that BMI training may be a novel and clinically useful treatment for phantom limb or chronic pain.
Acknowledgments
This research was conducted under the SRPBS by MEXT and AMED. This research was also supported in part by JST PRESTO; Grants-in-Aid for Scientific Research KAKENHI (JP24700419, JP26560467, JP22700435, JP17H06032 and JP15H05710); Brain/MINDS and SICP from AMED; ImPACT; Ministry of Health, Labor, and Welfare (18261201); and the Japan Foundation of Aging and Health and TERUMO foundation for life sciences and arts.
Footnotes
Conflicts of Interest Disclosure
Ben Seymour received the research funding from Wellcome Trust and Arthritis Research UK. Hiroshi Yokoi received the research funding from JSPS KAKENHI (25249025), Grant-in-Aid for Scientific Research on Innovative Areas “Understanding brain plasticity on body representations to promote their adaptive functions” (Grant Number 26120008) and Brain Machine Interface Development from AMED. Other authors have no COI to be disclosed.
References
- 1).Kuner R, Flor H: Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 18: 20–30, 2016 [DOI] [PubMed] [Google Scholar]
- 2).Baliki MN, Apkarian AV: Nociception, pain, negative moods, and behavior selection. Neuron 87: 474–491, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Vachon-Presseau E, Tétreault P, Petre B, et al. : Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 139: 1958–1970, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Flor H, Birbaumer N: Phantom limb pain: cortical plasticity and novel therapeutic approaches. Curr Opin Anaesthesiol 13: 561–564, 2000 [DOI] [PubMed] [Google Scholar]
- 5).Ramachandran VS, Rogers-Ramachandran D, Cobb S: Touching the phantom limb. Nature 377: 489–490, 1995 [DOI] [PubMed] [Google Scholar]
- 6).Flor H, Nikolajsen L, Staehelin Jensen T: Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci 7: 873–881, 2006 [DOI] [PubMed] [Google Scholar]
- 7).Wolff A, Vanduynhoven E, van Kleef M, Huygen F, Pope JE, Mekhail N: 21. Phantom pain. Pain Pract 11: 403–413, 2011 [DOI] [PubMed] [Google Scholar]
- 8).Shankar H, Hansen J, Thomas K: Phantom pain in a patient with brachial plexus avulsion injury. Pain Med 16: 777–781, 2015 [DOI] [PubMed] [Google Scholar]
- 9).Flor H, Elbert T, Knecht S, et al. : Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375: 482–484, 1995 [DOI] [PubMed] [Google Scholar]
- 10).Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N: Phantom movements and pain. An fMRI study in upper limb amputees. Brain 124: 2268–2277, 2001 [DOI] [PubMed] [Google Scholar]
- 11).Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H: Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci 21: 3609–3618, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Makin TR, Scholz J, Henderson Slater D, Johansen-Berg H, Tracey I: Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain 138: 2140–2146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Makin TR, Scholz J, Filippini N, Henderson Slater D, Tracey I, Johansen-Berg H: Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun 4: 1570, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Ali M, Saitoh Y, Oshino S, et al. : Differential efficacy of electric motor cortex stimulation and lesioning of the dorsal root entry zone for continuous vs paroxysmal pain after brachial plexus avulsion. Neurosurgery 68: 1252–1257; discussion 1257–1258, 2011 [DOI] [PubMed] [Google Scholar]
- 15).Kishima H, Saitoh Y, Oshino S, et al. : Modulation of neuronal activity after spinal cord stimulation for neuropathic pain; H(2)15O PET study. Neuroimage 49: 2564–2569, 2010 [DOI] [PubMed] [Google Scholar]
- 16).Hosomi K, Seymour B, Saitoh Y: Modulating the pain network—neurostimulation for central poststroke pain. Nat Rev Neurol 11: 290–299, 2015 [DOI] [PubMed] [Google Scholar]
- 17).Saitoh Y, Shibata M, Sanada Y, Mashimo T: Motor cortex stimulation for phantom limb pain. Lancet 353: 212, 1999 [DOI] [PubMed] [Google Scholar]
- 18).Yanagisawa T, Fukuma R, Seymour B, et al. : Induced sensorimotor brain plasticity controls pain in phantom limb patients. Nat Commun 7: 13209, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Orsborn AL, Moorman HG, Overduin SA, Shanechi MM, Dimitrov DF, Carmena JM: Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control. Neuron 82: 1380–1393, 2014 [DOI] [PubMed] [Google Scholar]
- 20).Ganguly K, Dimitrov DF, Wallis JD, Carmena JM: Reversible large-scale modification of cortical networks during neuroprosthetic control. Nat Neurosci 14: 662–667, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Wander JD, Blakely T, Miller KJ, et al. : Distributed cortical adaptation during learning of a brain-computer interface task. Proc Natl Acad Sci USA 110: 10818–10823, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Nakanishi Y, Yanagisawa T, Shin D, et al. : Decoding fingertip trajectory from electrocorticographic signals in humans. Neurosci Res 85: 20–27, 2014 [DOI] [PubMed] [Google Scholar]
- 23).Nakanishi Y, Yanagisawa T, Shin D, et al. : Prediction of three-dimensional arm trajectories based on ECoG signals recorded from human sensorimotor cortex. PLoS One 8: e72085, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Yanagisawa T, Hirata M, Saitoh Y, et al. : Electrocorticographic control of a prosthetic arm in paralyzed patients. Ann Neurol 71: 353–361, 2012 [DOI] [PubMed] [Google Scholar]
- 25).Yanagisawa T, Hirata M, Saitoh Y, et al. : Real-time control of a prosthetic hand using human electrocorticography signals. J Neurosurg 114: 1715–1722, 2011 [DOI] [PubMed] [Google Scholar]
- 26).Yanagisawa T, Hirata M, Saitoh Y, et al. : Neural decoding using gyral and intrasulcal electrocorticograms. Neuroimage 45: 1099–1106, 2009 [DOI] [PubMed] [Google Scholar]
- 27).Yanagisawa T, Yamashita O, Hirata M, et al. : Regulation of motor representation by phase-amplitude coupling in the sensorimotor cortex. J Neurosci 32: 15467–15475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Yanagisawa T, Hirata M, Kishima H, et al. : Movement induces suppression of interictal spikes in sensorimotor neocortical epilepsy. Epilepsy Res 87: 12–17, 2009 [DOI] [PubMed] [Google Scholar]
- 29).Nishimura Y, Perlmutter SI, Eaton RW, Fetz EE: Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron 80: 1301–1309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Clancy KB, Koralek AC, Costa RM, Feldman DE, Carmena JM: Volitional modulation of optically recorded calcium signals during neuroprosthetic learning. Nat Neurosci 17: 807–809, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Buch ER, Modir Shanechi A, Fourkas AD, Weber C, Birbaumer N, Cohen LG: Parietofrontal integrity determines neural modulation associated with grasping imagery after stroke. Brain 135: 596–614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Fukuma R, Yanagisawa T, Yorifuji S, et al. : Closed-loop control of a neuroprosthetic hand by magnetoencephalographic signals. PLoS One 10: e0131547, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Toda A, Imamizu H, Kawato M, Sato MA: Reconstruction of two-dimensional movement trajectories from selected magnetoencephalography cortical currents by combined sparse Bayesian methods. Neuroimage 54: 892–905, 2011 [DOI] [PubMed] [Google Scholar]
- 34).Buch E, Weber C, Cohen LG, et al. : Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke 39: 910–917, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Fukuma R, Yanagisawa T, Saitoh Y, et al. : Real-Time control of a neuroprosthetic hand by magnetoencephalographic signals from paralysed patients. Sci Rep 6: 21781, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Maruo T, Nakae A, Maeda L, et al. : Validity, reliability, and assessment sensitivity of the Japanese version of the short-form McGill pain questionnaire 2 in Japanese patients with neuropathic and non-neuropathic pain. Pain Med 15: 1930–1937, 2014 [DOI] [PubMed] [Google Scholar]
- 37).Dale AM., Fischl B, Sereno MI: Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194, 1999 [DOI] [PubMed] [Google Scholar]
- 38).Cohen LG, Bandinelli S, Findley TW, Hallett M: Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain 114: 615–627, 1991 [DOI] [PubMed] [Google Scholar]
- 39).Yoshioka T, Toyama K, Kawato M, et al. : Evaluation of hierarchical Bayesian method through retinotopic brain activities reconstruction from fMRI and MEG signals. Neuroimage 42: 1397–1413, 2008 [DOI] [PubMed] [Google Scholar]
- 40).Shibata K, Watanabe T, Sasaki Y, Kawato M: Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science 334: 1413–1415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E: An fMRI-based neurologic signature of physical pain. N Engl J Med 368: 1388–1397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]