Abstract
Background:
Toluene Diisocyanate (TDI) is a known respiratory sensitizer linked to occupational asthma (OA). To better manage worker risks, an appropriate characterization of the TDI-OA dose-risk relationship is needed.
Methods:
The literature was reviewed for data suitable for dose-response modeling. Previous study data were fit to models to derive prospective occupational exposure limits (OELs), using benchmark dose (BMD) and low-dose extrapolation approaches.
Results:
Data on eight TDI-exposed populations were suitable for analysis. There were 118 OA cases in a population contributing 13,590 person-years. The BMD-based OEL was 0.4 ppb. The OEL based on low-dose extrapolation to working lifetime extra risk of 1/1000 was 0.3 ppb.
Conclusions:
This study synthesized epidemiologic data to characterize the TDI-OA dose-risk relationship. This approach yielded prospective OEL estimates below recent recommendations by the American Conference of Governmental Industrial Hygienists, but given significant study limitations, this should be interpreted with caution. Confirmatory research is needed.
Keywords: occupational asthma, isocyanates, epidemiology, dose-response, risk assessment
INTRODUCTION
Asthma is a complex heterogeneous respiratory disease characterized by variable airflow limitation and/or airway hyperresponsiveness.1 The disease is among the most common chronic illnesses in the human experience, affecting about 300 million people worldwide.2 The incidence of new-onset adult asthma appears on the rise in industrialized countries, with estimates ranging from <1 to 11 cases per 1000 person-year among published reports,3–6 but settling on about 2 per 1000 person-years among persons aged 20–50 years in large prospective studies.7 The broad category of work-related asthma comprises adult onset asthma cases that are either caused by workplace exposures (i.e., occupational asthma) or are a preexisting disease that is worsened by work factors (work-exacerbated asthma).8 Estimates of the fraction of adult asthma that is work-related have widely varied, but typically range between from 10% to 25% in industrialized countries.9–11
De novo occupational asthma (OA) is induced via sensitization to a specific substance in the workplace, or by exposure to an inhaled irritant at work.8 There are over 300 causal agents for OA, which are broadly separated into proteinaceous substances (e.g., natural rubber latex, enzymes, and animal-derived antigens), and low-molecular weight (LMW) chemicals (e.g., isocyanates and acid anhydrides).12,13 Among known LMW agents, isocyanates, such as methylene diphenyl diisocyanate (MDI), hexamethylene diisocyanate (HDI), and toluene diisocyanate (TDI), are widely used in the manufacture of flexible and rigid polyurethane (PUR) foams, sealants, elastomers, adhesives, and coatings. In particular, TDI (usually a mixture of toluene-2,4-diisocyanate and toluene-2,6-diisocyanate) is a highly volatile and reactive chemical long recognized as a potent respiratory sensitizer and irritant.1,14 Given its volatility, inhalation is a major exposure pathway.14 Longitudinal studies of TDI-exposed workers have reported annual OA incidence of around 1% related to average airborne TDI concentrations less than 5 ppb.15–19 In 2016, the American Conference of Governmental Industrial Hygienists (ACGIH) lowered its recommended 8-hour Threshold Limit Value Time Weighted Average (TLV-TWA) for TDI from 5.0 ppb to 1.0 ppb.20 The ACGIH recommendation was based primarily on a qualitative synthesis of human data, with supporting evidence from toxicologic studies, to protect against TDI respiratory sensitization. The current study extends this analysis using quantitative risk assessment methods to derive a set of alternative occupational exposure limits (OELs) on TDI inhalation exposure. These prospective OELs add to the body of information informing TDI risk management decisions.
MATERIALS AND METHODS
Database Search Strategy
The aim of the research was to combine existing epidemiologic data on the association between OA and TDI inhalation exposure for dose-response analyses supporting prospective OEL estimates. To construct the database, the English literature was searched for reports on epidemiologic studies of occupational asthma in TDI-exposed working populations that were published from January 1, 1950 to September 11, 2017. The search was conducted using keyword searches of public domain citation databases (e.g. PubMed, Medline, Embase, and Scopus), using terms such as: “toluene diisocyanate”, “TDI”, “occupational asthma”, “work-related asthma”, “asthmagen”, “diisocyanate”, “occupational exposure”, “sensitization”, “sensitizer” and others. Abstracts were reviewed to determine the applicability of articles under consideration. Citations within informative articles and reviews were also considered to uncover studies that may have been missed in database searches. Study authors were not contacted.
Studies judged suitable for dose-response analyses were those reporting data sufficient to estimate three key variables for dose-response modeling: 1) the number of potential OA incidence cases, 2) the average TDI airborne exposure level over the observation period, and 3) the number of person-years at risk. In the absence of reported person-years (e.g., cross-sectional data), estimates were derived from the population size and average employment duration. Data sources were limited to study populations exposed to average TDI concentrations below 20 ppb. Greater average workplace concentrations may be indicative of poorer workplace conditions that may influence employment and increase the potential for selection bias. In addition, exposure to higher TDI levels may result in irritation symptoms that appear similar to those from low-dose sensitization; therefore, the potential for errors in case ascertainment is increased.
Exposure
Data on the appropriate exposure index for dose-response modeling are uncertain.21–23 It remains unclear whether TDI-induced asthma is a consequence of low cumulative exposure, exposure intensity, or some combination that also accounts for time ordering of intermittent exposure. “Cumulative exposure” is the product of intensity and duration, while “intensity” is measured as the time-weighted average TDI concentration or intermittent, peak exposure.24–26 The current study uses intensity as the exposure metric because it is largely believed to be more relevant to the development of OA than cumulative exposure.23 Average exposure is often used to describe exposure intensity in health-effect studies because time-specific data needed to assess peak exposures is generally lacking.24 This was observed in existing TDI studies, which provided sparse information on the magnitude, time-order, and frequency of short-term exposures. For this study, it is assumed that the risk of TDI sensitization is related to average exposure, which may also be a correlate of peak exposures.18,21,27
Where feasible, average TDI concentration values were abstracted precisely from study reports. Three eligible studies did not report average exposure directly, but sufficient data were available to estimate average TDI concentrations using either weighted averages (n=2) or statistical methods assuming exposures were lognormally distributed. Data on exposure modifiers, such as measurement techniques, control technology, and respiratory protection, were inconsistently reported; therefore, were not considered in exposure estimates.
Statistical approach
Mathematical models were developed to estimate prospective OELs. These OELs follow the two forms of health- and risk-based, as posited by the Scientific Committee on Occupational Exposure Limits.28 A ‘health-based’ OEL is defined as an OEL that is applied to an agent that has an exposure level below which the excess risk of the adverse effect is considered negligible (i.e., a threshold). Some agents may not have an exposure-response threshold; therefore, there is an associated risk for any exposure. In this case, a ‘risk-based’ OEL can be derived under a condition of ‘target risk’, which is a level of residual risk chosen to initiate risk management. For this study, the target risk was defined as an extra risk of one case per 1000 workers who are continuously exposed to TDI over a 45-year working lifetime. The health-based OEL used a standard benchmark dose approach and applied the EPA Benchmark Dose Software (BMDS), Version 2.6.0.1 (Build 88, 6/15/2015).29 Modeling for low-dose risk extrapolation was conducted by Poisson regression using SAS software, Version 9.4 (2002–2012).30
For the health-based OEL, the analysis assumed that a practical population-averaged threshold resides below the observable range in epidemiologic data, and applying uncertainty factors to an estimate of some point of departure (PoD) in the observable range is a reasonable approach for estimating a safe level of exposure.31 A benchmark dose approach was used to identify a PoD based on a benchmark response (BMR) of 1% extra risk, as determined by parametric dose-response modeling. The BMR value was selected a priori based on evidence from existing studies, the range of data available for analysis, and to achieve reasonable statistical power in the low dose range (i.e., TDI concentrations <5 ppb). A clear advantage of one model over several biologically-plausible models was not apparent a priori; therefore, data were fitted to a suite of non-threshold binomial regression models, comprising: quantal-linear, logprobit, gamma, log-logistic, Weibull, probit, linear-quadratic (LQ), and logistic forms. Additionally, a no-intercept LQ model was fit to examine the dose-response curve under an assumption that the data contained no background asthma cases. The adequacy of model fit was judged by likelihood goodness-of-fit using a critical value of 0.1. Reasonable agreement in estimates among the set of models suggested little model dependence; therefore, the ‘best’ model was selected based on the minimum Akaike Information Criterion (AIC).32 To account for sample variance, the 95% lower confidence limit on the average TDI concentration at the BMD (i.e., BMDL) was selected as the PoD. The OEL was estimated by dividing the BMDL by an uncertainty factor to account for variation in human sensitivity (UFH). The default value UFH =10 was used given disease severity and in lieu of specific information on human toxicokinetics or toxicodynamics.33,34
For low-dose extrapolation, general relative rate models were fit using Poisson regression. Two rate functions were assumed. First, a linear no-threshold (i.e., LNT) response was assumed given its frequent use in risk assessment. The model follows the form: Y|τ, d ~ poisson(λ0[1+ βd]τ), where OA incidence counts, Y, are conditional on person-time, τ, and the average TDI concent (dose, d) where the slope parameter, β, is the excess relative rate (ERR) at unit dose. The variable λ0 represents the baseline rate. The failure probability of OA conditional on 𝜏 and d is given by: P(d,τ) = 1-e-λ0τ[1+ βd]. Extra risk was calculated by: Similarly, models were fit assuming that the incidence rate was quadratic in dose, based on its superior fit among binomial models specified for the health-based OEL.
Heterogeneity in data among studies could substantively influence estimates. To account for heterogeneity, scaled residuals and plots of dose-response curves were examined for outlying data. Studies with scaled residuals >2.0 were considered outlying and effects on model estimates were examined by series removal of suspect studies. Final model estimates were obtained using study data excluding studies judged as outlying. In addition to removing outliers, leave-one-out analyses were conducted on the reduced set of studies to examine the influence of any one remaining study. Random effects models were also fit to examine heterogeneity and Poisson assumption validity.
Analyses were conducted using data restricted to: 1) longitudinal studies to examine the effects of combining cross-sectional and longitudinal data, and 2) naïve populations to examine the potential for healthy worker selection effects. Naïve populations comprise workers who are first exposed at or after the beginning of observation in longitudinal studies, or time of survey for cross-sectional studies. To examine the effects from limiting exposure levels, models were fit that included the next available study with average exposure of 20 ppb or more.
RESULTS
Literature review
There were 29 reports providing information on the association between TDI exposure and OA. Of these, 13 lacked sufficient data for dose-response modeling and five were excluded for reporting average exposures ≥20 ppb (Supplemental Materials, Table SI). Among studies ineligible for dose-response analyses, four were longitudinal designs. Thus, 11 (41%) reports contributed to the current study by providing information on eight TDI-exposed populations that was suitable for quantitative analysis (Table I). Most eligible workers were employed in TDI production or PUR foam manufacturing. There were 118 OA cases in the combined cohort contributing 13,590 person-years, resulting in a crude aggregate incidence rate of about 0.9% per year. The observation period ranged over three decades from 1971 to 1999. There were three cross-sectional studies with sufficient temporal information to estimate OA incidence, the remaining studies were longitudinal designs. Average TDI exposures ranged from 0.23 ppb to 4.2 ppb, providing a person-time weighted average of 2.2 ppb. All but one study reported data from 8-hour TWA measurements. Information on individual studies is provided below and in Table I.
Table I.
Study data.
| ID | Description | Study Design | Exposure period | Recruitment at inception1 | Ascertainment method2 | Asthma Cases | Person-years | Average TDI Level (ppb) | Study Reference No. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PUR foam workers employed at 1 US production facility | Cross-sectional | 1986–1999 | survivors | 1 | 20 | 1482 | 0.23 | 42 |
| 2 | Japanese male workers employed at 4 TDI production plants (n=87) and 2 research facilities (n=19) | Cross-sectional | 1980–1982 | survivors | 3 | 6 | 954 | 0.7 | 41 |
| 3 | Swedish PUR foam workers | Cross-sectional | 1972–1979 | naïve | 2 | 3 | 288 | 1.8 | 40 |
| 4 | PUR foam workers employed at 12 UK facilities | Longitudinal | 1981–1986 | survivors | 2 | 36 | 3874 | 2.1 | 15, 35–37 |
| 5 | PUR foam workers at 2 US facilities | Longitudinal | 1982–1987 | survivors | 2&3 | 12 | 1930 | 2 | 16 |
| 6 | US TDI production workers at 1 US facility | Longitudinal | 1973–1978 | naïve | 2&3 | 12 | 1200 | 3.5 | 17, 38 |
| 7 | TDI production workers at 1 US facility | longitudinal | 1967–1992 | naïve | 2&3 | 19 | 1779 | 4.2 | 18 |
| 8 | TDI production workers at 1 US facility | Longitudinal | 1971–1997 | naïve | 2 | 10 | 2083 | 2.3 | 19 |
“Inception” refers to the beginning of observation in longitudinal studies and time of survey for cross-sectional studies. “Survivors” are those workers who were actively employed and exposed prior to inception while “Naïve” refers to those workers first exposed at or after inception.
Case decision based on: 1) self-reported symptoms, 2) decision rendered by occupational medicine clinician based on symptoms; 3) OA diagnoses supported by one or more clinical tests (e.g., pulmonary function, immunologic, and TDI challenge tests). An assignment of “2&3” indicates that not all cases were confirmed by clinical tests.
Limited to exposed workers.
Abbreviations: PUR, polyurethane; TDI, toluene diisocyanate
There were several reports on a longitudinal study of 1462 TDI workers from 12 plants within the flexible foam manufacturing industry in the United Kingdom.15,35–37 Initial followup was from 1981 through 1986 and data from this period was used in the current study. TDI respiratory sensitization was defined by self-report of recurrent work-related symptoms. Personal air sampling provided estimates of TDI 8-hour TWA concentrations, although the mean of these values was not reported. For the current study, the average exposure concentration (2.1 ppb) was calculated from the daily dose distribution data (in ppb-hours) among exposed workers, as reported by Bugler et al. (1991), assuming exposure data were lognormally distributed.15 About 19% of 8-hour TWA exposures contained short-term peak concentrations in excess of 20 ppb, with about 9% of peaks exceeding the upper limit of detection of 40 ppb. The authors stated that respiratory protection was worn frequently, but not always, when these short-term concentrations occurred. Data analyses were restricted to process workers known to be exposed (n=946). The exposed group included 694 workers who could not be verified unexposed prior to enrollment.36 About 70% of workers were employed at study facilities prior to observation. During the observation period, workers accrued 3874 person-years and 43 cases of respiratory sensitization were observed. There were seven cases reporting symptoms prior to observation that were excluded from the current study. Among 36 incidence cases, 9 (25%) presented only with minimal upper respiratory symptoms.
There were multiple reports from a longitudinal study following 277 U.S. TDI production workers from April 1973 through October 1978.17,38,39 Information was mostly abstracted from the comprehensive report by Weill et al. (1981).17 There were nine data collection visits made over the course of the study, during which 71 workers were permanently lost to followup. Data on the reasons for leaving employment were not available. The cohort contributed 1200 person-years and included process workers with continuous exposures, maintenance and support personnel with intermittent exposures, and persons assigned to unexposed jobs; however, data separated by exposure groups were not available. TDI TWA exposures were assessed by personal monitoring of 143 participants from 1975 to study end. The overall average 8-hour TWA from 1949 samples was 3.5 ppb. The average percentage of time spent above 5 ppb and 20 ppb was 14.6% and 3%, respectively. There were 12 cases defined from self-report of respiratory signs and symptoms. Cases included workers who presented with minimal upper respiratory symptoms. Half of the cases had been involved in TDI spills or equipment malfunction resulting in significant short-term peak TDI exposure. A fourth of the cases were diagnosed atopic. All but one case worked in process areas. Of six cases given a TDI challenge, two reacted to levels below 20 ppb.
Jones et al. (1992) examined 386 PUR foam workers employed in one of two U.S. plants and who agreed to one or more of four interviews between 1982 and 1987 (88.7% of eligible workers).16 Details on losses to followup were not available; however, the authors acknowledged more lost from exam refusal than leaving work. Study participants included workers with potential exposure prior to observation. Time at risk (n=1930 person-years) was set equal to the product of the cohort size and study period. There were 12 sensitization cases identified by clinical assessment of self-reported symptoms; however, the authors stated that a loss of medical services during the study period might have led to missed cases. There was no distinction made between new onset and prevalent cases. Case definitions were unclear; however, half were confirmed by TDI challenge test. Exposures were assessed as 8-hour TWA concentrations using personal monitoring data. The weighted average TWA from 4845 samples was 2 ppb. Short-term (12-minute) peak concentrations exceeded 5 ppb and 20 ppb in about 9% and 1% of samples, respectively.
Bodner et al. (2001) examined U.S. TDI production workers ever employed for at least 3 consecutive months in TDI-related departments from January 1, 1971 (beginning of production), through September 18, 1997.19 During this period, the average followup was 7.8 years. Of 305 eligible workers, 267 (87.5%) completed at least one medical examination within the observation period and contributed 2083 person-years. There were 17 asthma cases diagnosed; however, seven cases identified at baseline were excluded. The case definition was unclear; but the decision on case status appeared rendered by a clinician based on a review or medical charts that included self-reported symptoms and spirometry. TDI exposures were determined by 8-hour TWAs using 449 samples collected from personal monitoring. At last observation, the average and maximum TDI concentrations were 2.3 and 5.2 ppb respectively. The average dose was reported as 96.9 ppb-months and the average duration of exposure was 46 months. According to plant occupational physicians, there were no instances of transferring workers out of exposed jobs because of sensitization. Bodner et al. (2001) examined the potential for selection bias by conducted analyses restricted to 3 years from initial TDI exposure and found no potential for strong bias.
Ott et al. (2000) conducted a longitudinal study of workers employed in a U.S. TDI production facility for at least 3 months from 1967 (plant startup) to 1992.18 Followup was through 1996 and accrued 1779 person-years (average 5.7 years). The cohort comprised process workers, supervision and maintenance personnel, but excluded clerical and administrative staff. OA case ascertainment (n=19) was accomplished by review of site physicians’ assessments recorded in company medical charts available for 297 eligible workers (94.9%). These assessments were primarily based on symptomology (n=13); however, a single occurrence of symptoms was generally insufficient to diagnose OA. It was also noted that assessments might have considered available information from immunologic and pulmonary tests. Cases were not confirmed by specific inhalation challenge test, and bronchial hyperresponsiveness was not evaluated. The reported average 8-hour TWA concentration from personal monitoring was 4.2 ppb. Co-exposures to phosgene was possible. The average 8-hour phosgene concentration between 1977 and 1988 was 7 ppb; however, incident levels in excess of 10 ppm-minutes were reported. Among OA cases, 5 (26%) were linked to previous incidents of acute phosgene exposure.
A 1979 cross-sectional study, Belin et al (1983) also reported longitudinal data on workers who were employed in TDI PUR foam operations that began in 1972.40 Work practices required exclusion of workers with a medical history of “strong atopy” or respiratory dysfunction from TDI work. During this 6-year period, three workers were transferred to other jobs because of asthma-like symptoms, presumably on a physician’s recommendation. These data were preliminary to a cross-sectional survey of the same workforce; however, it is not clear whether the previously identified cases were also among subjects surveyed, who were absent of clinically identified OA cases. Coexposures to the amines N-methylmorpholine and triethylenediamine (1,4-diazabicyclo-(2,2,2) octane) were likely. Work area airborne concentrations were determined by sparse ambient air monitoring in three work areas. Using all available sample data, weighted average concentrations for total TDI, triethylenediamine, and N-methylmorpholine were about 1.85 ppb (n=12), 79 ppb (n=7), and 7 ppm (n=7), respectively.
Omae et al. (1984) conducted a cross-sectional study of 106 male workers employed in four TDI-producing plants (n=87) and two research laboratories (n=19) in Japan.41 The average employment duration among these workers was 9 years. The time-at-risk was estimated as the product of study size and employment duration (954 person-years). Information on losses to followup was not available. Case definitions were unclear; however, six workers were found to be in “latent or subclinical states” of OA, which was ascertained at time of survey based on symptoms and pulmonary function tests. The authors also stated that none of these workers suffered from asthmatic reactions under observed working conditions between the initial survey in 1980 and a followup survey in 1982. There was some effort to exclude cases (n=2) thought not to be related to TDI exposure. The reported average among 8-hour TWA concentrations from 165 air samples collected in 1980 was 0.7 ppb, with values twice exceeding 20 ppb. Short-term peak concentrations exceeded 20 ppb in 15 samples (0.9%). Potential coexposures varied by facility and included irritants such as phosgene, amines, chlorine, nitric acid, sulfuric acid, and other isocyanates.
Daftarian et al. (2000) reported findings from a 1999 cross-sectional study of workers employed in the manufacture of PUR foam cushions for automobile seats.42 The study was commissioned on behalf of labor representatives based on worker safety concerns. Participation was voluntary and only 39% of workers available at time of survey completed the questionnaire. OA cases (n=20) were identified using a self-administered questionnaire to distinguish respiratory symptoms that improved on non-workdays. Of these cases, two were observed in non-production workers. The incidence rate was estimated by dividing the number of prevalent cases by product of average employment duration (n=13 years) and study population (n=114). TDI exposures were assessed as 8-hour TWA concentrations by personal monitoring of 104 study participants. The reported average and maximum total TDI concentrations were 0.23 ppb and 1.15 ppb, respectively. The authors reported that a delay in processing samples might have led to instability in some samples, which suggested a systematic underestimation in the range of 12–14%. Primary coexposures for this cohort included formaldehyde, solvents, and Bis [2-dimethylaminoethyl] ether (DMAEE).
Statistical Modeling
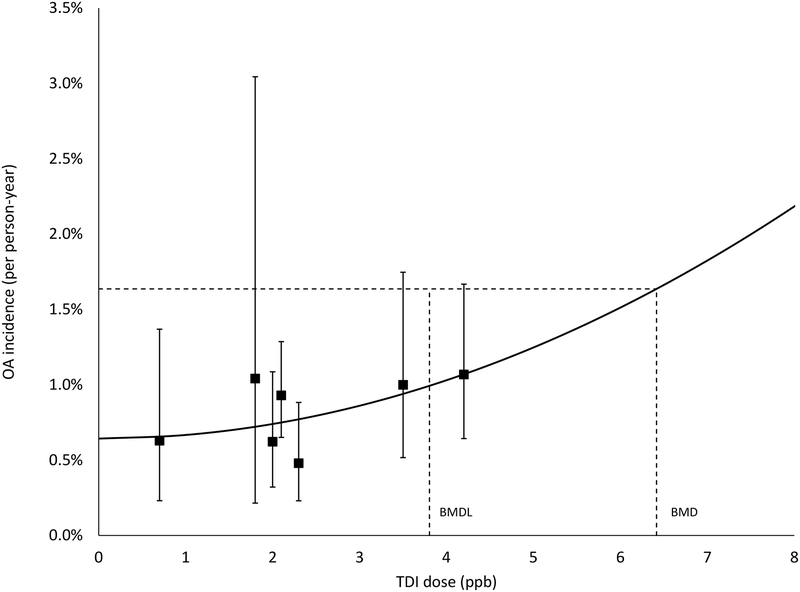
In fitting binomial models for BMD, none of the models met the goodness-of-fit criteria when using all study data (e.g., p-values <0.1 for all models). Inspection of the scaled residuals and dose-response plots revealed that the study by Daftarian et al. (2000) was outlying, with a scaled residual under the best fitting model of 2.23. Removal of this study significantly improved model fit for all models, with goodness-of-fit p-values ranging from 0.30 to 0.44 (Table II). Within the suite of models fitted to the reduced dataset, the AIC was lowest for the LQ model with an intercept (Figure 1), which had resolved to a simple quadratic form with a positive but not statistically significant dose parameter (β2 =2.44 ×10−4; 95% CI: −8.86 × 10−5, 5.77 × 10−4) and a BMDL value of 4.3 ppb. There was negligible fit differences observed between the quadratic, logistic, probit, and quantal-linear forms, all reporting similar BMDL results with a positive, but not statistically significant dose-response (Table III). The no-intercept LQ model fit had the greatest AIC value and the largest scaled residual at the lowest observed dose. The quadratic term (β2) in this model was statistically significant and negative (β2 = −6.8 ×10−4; 95% CI: −1.3 ×10−4, −6.2 ×10−5).
Table II.
Benchmark dose modeling results (BMR=0.01) from final study selection (n=7)
| Model | TDI (ppb) | Goodness of fit (p-value) | AIC | |
|---|---|---|---|---|
| BMD | BMDL1 | |||
| quadratic | 6.42 | 4.32 | 0.44 | 1140.97 |
| logistic | 6.66 | 4.11 | 0.44 | 1141.02 |
| probit | 6.78 | 4.09 | 0.44 | 1141.03 |
| quantal-linear | 7.99 | 3.75 | 0.43 | 1141.11 |
| gamma | 6.03 | 3.81 | 0.30 | 1142.96 |
| log-logistic | 5.99 | 2.75 | 0.30 | 1142.96 |
| log-probit | 6.15 | 2.75 | 0.30 | 1142.96 |
| Weibull | 5.98 | 3.81 | 0.30 | 1142.96 |
| no intercept, LQ | 3.74 | 2.30 | 0.17 | 1143.68 |
95% lower confidence level on dose for a 1% change in response level (i.e., BMD).
Abbreviations: BMD, benchmark dose (in this case annual average TDI concentration); BMDL, lower 95% confidence interval on the benchmark dose; LQ, linear-quadratic.
Figure 1.
Occupational asthma (OA) incidence (per person-year) per ppb TDI. Quadratic model with benchmark dose (BMD) and its 95% lower confidence limit (BMDL) shown for a benchmark response (BMR) of 1% extra risk. The squares represent individual study responses. The whiskers represent the 95% confidence interval on the response.
Table III.
Dose-response modeling parameter estimates from select binomial models used in BMD modeling.
| Model | Parameter estimates (95% CI) | |
|---|---|---|
| Intercept | Dose | |
| Quadratic | 6.4 × 10−23 (3.8 × 10−3, 9.0 ×10−3) | 2.4 × 10−4 (−8.9 × 10−5, 5.8 × 10−4) |
| Logistic | −5.2 (−5.8, −4.6) | 0.16 (−0.046, 0.36) |
| Probit | −2.5 (−2.8, −2.3) | 0.057 (−0.017, 0.13) |
| Quantal-Linear | 5.0 × 10−3 (8.2 ×10−4, 9.2 × 10−3) | 1.3 × 10−3 (−4.4 × 10−4, 3.0 × 10−3) |
| NILQ | NA | β1 = 5.3 × 10−3 (3.2 × 10−3, 7.2 × 10−3) β2 =−6.8 × 10−4 (−1.3 × 10−3, − 6.2 × 10−5) |
Abbreviation: NILQ, no-intercept linear-quadratic (2nd order polynomial)
Except for the no-intercept LQ model, excluding cross-sectional studies (n=3) did not change the order of model fit (Supplemental Materials, Table SII). The fit of the no-intercept LQ was markedly improved without cross-sectional data, resulting in the fifth lowest AIC value. The quadratic term remained negative but was no longer statistically significant. All models met goodness-of-fit, with p-values ranging from 0.11 to 0.22. The BMD and BMDL values from the quadratic model without cross-sectional data were 6.3 and 4.2 ppb, respectively. The quadratic model slope factor increased by about 3%. Similar results were observed when restricting analyses to naïve populations (n=4), although BMD and BMDL estimates were lower compared to estimates in main analyses (Supplemental Materials, Table SIII). Again, a simple quadratic model fit best (p-value =0.32), with BMD and BMDL values of 5.0 and 3.6 ppb, respectively. The relative change in slope factor was about +66%. However, after applying UFH and rounding, prospective health-based OELs from models restricted to either longitudinal or naïve studies did not differ from main analyses.
In leave-one-out sensitivity analyses, excluding Studies 2, 3, or 4 did not change the rank order of models. Excluding any of the remaining studies changed the rank order; however, the quadratic model always fit best compared to the linear model. Model convergence and fit acceptance was achieved for the no-intercept LQ model only on removal of Studies 5 and 8. The BMDLs among best-fitting models (n=7) ranged from 2.7 (excluding Study 7) to 4.3 ppb (excluding Study 6). The BMDLs from all alternative models that met fit criteria (n=58) ranged from 1.8 to 4.3 ppb, which was only slightly different from the range from the suite of models fit to all seven studies (2.3–4.1 ppb).
The first study excluded based on high average exposure was a cross-sectional examination of TDI production workers whose average exposure was 20.3 ppb.43 Including these data resulted in a best-fit linear model with a statistically significant positive slope, (1.33 × 10−3; 95% CI: 2.67 ×10−5, 2.62 ×10−3), although no change observed in the prospective OEL (BMD =7.6 ppb, BMDL =4.0 ppb).
Annualized results from the Poisson excess relative rate models were essentially identical to those from corresponding binomial models used in BMD software (results not shown). There was no evidence of study heterogeneity in models including random effects. As before, the quadratic model provided a slightly better fit to the data. For low-dose extrapolation of lifetime risk, the LNT model yielded a risk-based OEL estimate of 0.018 ppb, corresponding to 0.1% excess risk. The 95% lower bound on this estimate was 0.008 ppb. Assuming the response is quadratic in dose (i.e., the best fitting dose-response model), the risk-based OEL increased to 0.3 ppb, with a lower bound of 0.2 ppb. The number of estimated OA cases at various lifetime exposure levels are shown for each rate function in Table IV.
Table IV.
Excess risk of sensitization from continuous TDI exposure over a 45-year working lifetime.
| Average TDI exposure (ppb) | Extra Risk (Cases per 1000 persons) | |
|---|---|---|
| Linear rate function | Quadratic rate function | |
| 5 | 245 | 238 |
| 1 | 55 | 10 |
| 0.1 | 6 | <1 |
| 0.01 | <1 | <1 |
DISCUSSION
Using a BMD approach based on a synthesis of existing epidemiologic data, the estimated health-based OEL was equivalent to an 8-hour TWA concentration of about 0.4 ppb, which is less than half that currently recommended by the ACGIH. This method assumed that keeping average workplace concentrations below this level is sufficient to avert sensitization. There is some evidence of a safe level corresponding to an average workplace TDI level that is ≤1 ppb.37,44 For example, there were no OA cases reported in the 12-year extended followup of 251 UK foam workers.37 The average airborne TDI concentration during the observation period was less than 1 ppb, with only 1.3% of all 8-hour TWAs (n=1004) in excess of 5 ppb. Similarly, no OA cases were diagnosed in a 1-year prospective study of 49 new hires at a recently constructed PUR foam factory in Eastern Europe.44 The average TDI concentration was well below 0.1 ppb; however, short-term (18-minute) concentrations reached 10 ppb. No 8-hour TWA concentration exceeded 5 ppb. The results of this study are tempered by its small size and short followup period.
Data against a safe level ≤1 ppb was provided by Daftarian et al. (2000), who reported 20 OA cases among 114 workers exposed to an average TDI concentration of about 0.2 ppb. Interestingly, this study was deemed outlying in the current study, reporting an unusually high incidence rate (IR) per unit dose (IR per ppb =59 per 1000 person-years). There are several noteworthy limitations supporting its exclusion. First, participation in the study was voluntary and was exceptionally low (39% of eligible workers), prompting the authors’ acknowledgement that volunteers may be more likely to report asthma-related symptoms compared to those not participating. Second, ascertainment was by self-report of symptoms and was not confirmed by clinical diagnosis, this may have led to ascertainment bias. Third, the study was cross-sectional and prevalence is likely to be a poor proxy for OA incidence given sparse information on the observation period and evidence that nearly half (46%) of participants reported TDI exposure elsewhere. Fourth, OA cases were nearly twice as likely to present with dermal symptoms compared to non-cases, stemming from significant cutaneous exposure in production workers.45 Given existing evidence of respiratory sensitization via skin contact,14,46,47 dermal exposures in this study may have significantly contributed to OA risk and may explain the outlying response observed. Lastly, there was some evidence of a potential issue with air sampling methods that may have led to an underestimation of airborne TDI concentrations.
Despite large uncertainty in the mechanisms of chemical respiratory sensitization, available mechanistic data suggest that a sensitization threshold plausibly exists for low molecular weight chemicals, including TDI.48,49 Nevertheless, data on a practical threshold are equivocal. An alternative assumption is that a risk-free level of exposure does not exist. Assuming a target extra risk of 10−3 over a working lifetime of 45 years, the corresponding OELs are 0.02 ppb using LNT extrapolation and 0.3 ppb assuming the response is quadratic in dose. Of course, the target risk was arbitrarily set and is arguably overly conservative for managing TDI-induced OA. At 1% lifetime risk, the OEL increased to 0.2 ppb and 1.0 ppb for linear and quadratic rate functions, respectively.
The current study found that a quadratic model had a slightly improved fit compared to LNT, which is supportive of nonlinear or threshold effects at low doses. If the true association between TDI inhalation and OA results from the frequency of overexposure, then curvature in the dose-response would be expected in lower dose range regardless of a threshold. This is consistent with longitudinal study by Diem et al. (1982), who reported average times spent above 20 ppb per 8-hr shift of 1.3, 8.6, and 28.2 minutes corresponding to 8-hour TWA concentrations of 1.6, 3.2, and 6.8 ppb, respectively.38 Assuming that the true dose-response is quadratic, the health-based OEL (0.4 ppb) lies between estimates based on 0.1% (0.3 ppb) and 1% (1.0 ppb) excess lifetime risk.
Other risk assessments
Meredith et al. (2000) conducted a case-control study of workers employed in TDI manufacture and examined the dose-response using conditional logistic regression while treating exposure as a continuous variable.50 Among cases (n=27), the mean 8-hour TWA exposure to TDI was 1.5 ppb. Controls (n=51) were selected from the same population, matched on work area, start and duration of employment, sex, and age. The authors reported an adjusted odds ratio (OR) of 1.07 (95% CI: 0.99, 1.16) for a 0.1 ppb increase in the 8-hour TWA. This estimate was greater than the current study’s estimate of the relative risk at 0.1 ppb using the linear model (1.02; 95%CI: 0.96, 1.09). Nevertheless, it is difficult to reconcile differences between estimates given many dissimilarities in study design.
Arnold et al. (2012) conducted a risk assessment of environmental exposure to TDI from flexible PUR foam.51 Based on their synthesis of the human observational data, the authors derived a no observable adverse effect level (NOAEL) for TDI respiratory sensitization in workers of 5.0 ppb. In their risk analysis, Arnold et al. (2012) assumed UFH =2; therefore, the adjusted benchmark toxicity value (for workers) was 2.5 ppb compared to 0.4 ppb in the current study. However, the NOAEL was comparable to the BMDL (4.3 ppb) in the current study, thus the disparity in OELs is attributable mainly to the differing UFH values used between the two studies.
Pauluhn (2014) examined elicitation by TDI inhalation in skin-sensitized Brown Norway rats.52 The aim of the study was to estimate an elicitation threshold dose in rats that could be extrapolated to humans. His data suggested an 8-hour time-adjusted asthma-related human-equivalent threshold for elicitation of about 3 ppb. In this case, the estimate is remarkably similar to the unadjusted BMDL in the current study; however, an elicitation dose is thought to be much lower in magnitude compared to that required for sensitization. The similarities in estimates may be caused by errors in ascertainment in the epidemiologic studies used to calculate the BMD given uncertainty in separating cases of elicitation from sensitization. There is evidence of elicited asthmatic reactions at exposures as low as 1 ppb in subjects confirmed with OA caused by isocyanates.53
Limitations
There are a number of noteworthy limitations. First, there was a general lack of high quality data on person-time, outcome, and exposure. For example, case ascertainment methods varied widely among contributing studies with differing objectives, with only three studies relying on clinical diagnoses. Challenges of accurately ascertaining OA with or without clinical diagnoses are well known.1,8,54–56 There is no simple diagnostic test for OA. Moreover, asthma and many respiratory symptoms without asthma are common conditions; therefore, onset coincidental to or exacerbated by work cannot be ruled out. This may partly explain the poor fit of the zero intercept LQ model compared to other model forms that allowed for a background OA incidence. Similarly, exposure measurement error is likely from sparse information on TDI concentrations from measurement techniques that have differed greatly among studies and over time. Contributing to this error is the uncertainty in the appropriate exposure metric for dose-response analysis. Although, cumulative exposure appears appropriate for most chronic illnesses, peak exposures might contribute disproportionately to disease when effects are nonlinear.24,25 Moreover, Esmen proposed that the time-ordering of fractionated exposure may have an important role in the dose-response for TDI sensitization.26 Data on peak exposures were sparse among eligible studies; therefore, analyses were limited to exposure intensity measured by average exposure. Finally, analyses lack statistical power, caused in part by limiting eligibility to studies with average exposures <20ppb. Marked improvement in estimate precision was achieved by including data from one study having average exposure of about 20 ppb. Interestingly, including these data did not appreciably change the dose-response slope; therefore, there is no evidence of strong bias from low power in the current study. Additional longitudinal studies with sufficient power, a well-defined OA definition, and adequate personal exposure monitoring at low doses are needed to confirm results.
Another study weakness is the potential for selection bias from asthma or asthma symptoms that influence recruitment (selection in) and retention (selection out) of study participants. For example, cross-sectional studies examine a population at a point in time; therefore, persons enrolled have survived to the time of survey and may represent a healthier group compared to all workers ever exposed. Longitudinal studies recruiting persons employed in TDI-jobs prior to cohort inception (i.e., prevalent hires) may also be subject to this bias.57,58 The potential for these effects is reduced in study populations that are unexposed and disease free at the beginning of observation (i.e., naïve participants), as in studies with cohort inception at plant startup. In studies enrolling survivors, the potential for selection effects can be examined by comparing risks between survivors and naïve participants. For example, albeit slightly increased, there was no statistical difference in the risk among new hires compared to prevalent hires in the UK Foam Workers Study.15 This finding supports the notion that effects from selection into this cohort have a limited potential to bias risk estimates. Sensitivity analyses restricted to longitudinal data or naïve populations provided some evidence of healthy worker effects. Most notably, the dose-response slope increased by nearly 70% in the quadratic model restricted to naïve populations.
Selection bias in longitudinal studies can occur from study participants who become sick and leave before they are ascertained as a case. Again, a healthier group is retained resulting in a downward bias in risk estimates. The potential for this bias is reduced by improved followup (e.g., frequent examinations during observation), and investigating causes for losses to followup. For example, detailed surveys of participants in the UK Foam Workers Study reported that only 2.3% of persons leaving employment did so because of respiratory illness.37 Survivor effects are likely to increase with increasing exposure, which would attenuate the observed dose-response in the high dose region. Interestingly, the no-intercept LQ model resulted in a negative quadratic term, suggesting this attenuation. However, this finding is tempered by the model’s poor fit compared to other models.
Effects on study findings from unmeasured lifestyle factors (e.g., alcohol use, smoking, and obesity), co-exposure to other respiratory irritants and sensitizers (e.g., amines), co-morbidities (e.g., atopy and bronchial hyperresponsiveness), and other known or suspect risk factors for adult-onset asthma cannot be ruled out.6,59 Many of these factors have not been considered in the set of studies examined, or in data used in dose-response models. For example, the lack of accounting for risks associated with unmeasured dermal exposure may have distorted dose-response estimates for inhaled TDI. Additional research is needed to better understand the potential effects on the TDI-OA dose-response association that result from other risk factors, including effects from dermal exposure to TDI.
Lastly, eligible studies were limited mostly to working populations from TDI production and PUR foam industries operating prior to 2000. The characteristics of contemporary exposures, such as fraction of dermal exposure and frequency of TDI incidents, may greatly differ today, especially in other industry settings (e.g., automotive body, paint and interior repair and maintenance, building insulation, and furniture manufacture). New efforts are needed to characterize current exposures to TDI workers.
CONCLUSIONS
TDI is a chemical in wide industrial use that is also a known respiratory sensitizer and irritant that is causally linked to OA. An appropriate characterization of the dose-risk relationship between TDI and OA is needed to manage worker risks. Previous examinations have reported on dose-response trends between TDI and OA; however, these analyses have been largely qualitative.20,27,60 The current study used a quantitative approach to synthesize existing epidemiologic data and characterize the TDI-OA dose-response. Positive, but not statistically significant, dose-responses were observed in several models that allowed for estimates of candidate health and risk-based OELs. These prospective OELs were consistently below current recommendations. For example, the ACGIH TLV-TWA for TDI is over twice that of the health-based OEL reported in the current study. This discordance is likely explained, at least in part, by differences in the qualitative approach used by ACGIH and the quantitative synthesis reported herein. Nevertheless, the available data were limited and risk estimates supporting prospective OELs were largely uncertain. This uncertainty should factor into decisions regarding the applicability of the OELs in risk management practices, which is beyond the scope of this report.
Supplementary Material
Acknowledgements:
The author expresses his thanks to Robert Park and Randall Smith for their insightful discussions throughout the course of this work.
Funding: The author reports that there was no funding source for the work that resulted in the article or the preparation of the article.
Footnotes
Institution at which work was performed: National Institute for Occupational Safety and Health (NIOSH), Cincinnati, Ohio, USA.
Institution and Ethics approval and informed consent: This work was conducted at the National Institute for Occupational Safety and Health, which is part of the Centers for Disease Control and Prevention (CDC) under the United States Department of Health and Human Services. All data was abstracted from the published literature; therefore, human subjects’ institutional review was not required.
Disclosure (Authors): The authors declares no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the author and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- 1.Bernstein IL, Chan-Yeung M, Malo J-L, Bernstein DI. Asthma in the Workplace. Boca Raton FL: CRC Press, Taylor & Francis Group; 2006. [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469–478. [DOI] [PubMed] [Google Scholar]
- 3.Lundback B, Ronmark E, Jonsson E, Larsson K, Sandstrom T. Incidence of physician-diagnosed asthma in adults--a real incidence or a result of increased awareness? Report from The Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2001;95(8):685–692. [DOI] [PubMed] [Google Scholar]
- 4.Ekerljung L, Ronmark E, Larsson K, et al. No further increase of incidence of asthma: incidence, remission and relapse of adult asthma in Sweden. Respir Med. 2008;102(12):1730–1736. [DOI] [PubMed] [Google Scholar]
- 5.Toren K, Gislason T, Omenaas E, et al. A prospective study of asthma incidence and its predictors: the RHINE study. Eur Respir J. 2004;24(6):942–946. [DOI] [PubMed] [Google Scholar]
- 6.Anto JM, Sunyer J, Basagana X, et al. Risk factors of new-onset asthma in adults: a population-based international cohort study. Allergy. 2010;65(8):1021–1030. [DOI] [PubMed] [Google Scholar]
- 7.Toren K, Ekerljung L, Kim JL, et al. Adult-onset asthma in west Sweden--incidence, sex differences and impact of occupational exposures. Respir Med. 2011;105(11):1622–1628. [DOI] [PubMed] [Google Scholar]
- 8.Tarlo SM, Balmes J, Balkissoon R, et al. Diagnosis and management of work-related asthma: American College of Chest Physicians consensus statement. Chest. 2008;134(3 SUPPL.):1S–41S. [DOI] [PubMed] [Google Scholar]
- 9.Balmes J, Becklake M, Blanc P, et al. American Thoracic Society Statement: Occupational contribution to the burden of airway disease. Am J Respir Crit Care Med. 2003;167(5):787–797. [DOI] [PubMed] [Google Scholar]
- 10.Arif AA, Whitehead LW, Delclos GL, Tortolero SR, Lee ES. Prevalence and risk factors of work related asthma by industry among United States workers: Data from the third national health and nutrition examination survey (1988–94). Occup Environ Med. 2002;59(8):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kogevinas M, Zock JP, Jarvis D, et al. Exposure to substances in the workplace and new-onset asthma: an international prospective population-based study (ECRHS-II). Lancet. 2007;370(9584):336–341. [DOI] [PubMed] [Google Scholar]
- 12.Van Kampen V, Merget R, Baur X. Occupational airway sensitizers: An overview on the respective literature. Am J Ind Med. 2000;38(2):164–218. [DOI] [PubMed] [Google Scholar]
- 13.Baur X, Bakehe P. Allergens causing occupational asthma: An evidence-based evaluation of the literature. Int Arch Occup Environ Health. 2014;87(4):339–363. [DOI] [PubMed] [Google Scholar]
- 14.Bello D, Herrick CA, Smith TJ, et al. Skin exposure to isocyanates: Reasons for concern. Environ Health Perspect. 2007;115(3):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugler J, Clark RL, Hill H, Mcdermont M. The acute and long-term respiratory effects of aromatic diisocyanates A five year longitudinal study of polyurethene foam workers. Report No. 10848 Manchester, UK: International Isocyanates Institute; 1991. [Google Scholar]
- 16.Jones RN, Rando RJ, Glindmeyer HW, et al. Abnormal lung function in polyurethane foam producers: Weak relationship to toluene diisocyanate exposures. Am Rev Respir Dis. 1992;146(4):871–877. [DOI] [PubMed] [Google Scholar]
- 17.Weill H, Butcher B, Dharmarajan V, et al. Respiratory and immunologic evalatuion of isocyanate expsoure in a new manufacturing plant. Morgantown, WV: National Institute for Occupational Safety and Health; 1981. [Google Scholar]
- 18.Ott MG, Klees JE, Poche SL. Respiratory health surveillance in a toluene di-isocyanate production unit, 1967–97: Clinical observations and lung function analyses. Occup Environ Med. 2000;57(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodner KM, Burns CJ, Randolph NM, Salazar EJ. A longitudinal study of respiratory health of toluene diisocyanate production workers. J Occup Environ Med. 2001;43(10):890–897. [DOI] [PubMed] [Google Scholar]
- 20.American Conference of Governmental Industrial Hygienists (ACGIH). Toluene-2,4 or 2,6-Diisocyanate (Or As A Mixture): TLV(R) Chemical Substances 7th Edition Documentation. Product ID: 7DOC-600, Cincinnati, OH: ACGIH; 2016. [Google Scholar]
- 21.Ott MG, Diller WF, Jolly AT. Respiratory effects of toluene diisocyanate in the workplace: a discussion of exposure-response relationships. Crit Rev Toxicol. 2003;33(1):1–59. [DOI] [PubMed] [Google Scholar]
- 22.Högberg J, Larsson K, Albin M, et al. Letter to the editor: Comments on “respiratory effects of toluene diisocyanate in the workplace: A discussion of exposure-response relationships” (multiple letters). Crit Rev Toxicol. 2005;35(5):459–462. [DOI] [PubMed] [Google Scholar]
- 23.Sastre J, Vandenplas O, Park HS. Pathogenesis of occupational asthma. Eur Respir J. 2003;22(2):364–373. [DOI] [PubMed] [Google Scholar]
- 24.Checkoway H, Rice CH. Time weighted averages, peaks, and other indices of exposure in occupational epidemiology. Am J Ind Med. 1992;21(1):25–33. [DOI] [PubMed] [Google Scholar]
- 25.Rappaport SM. Assessment of long-term exposures to toxic substances in air. Ann Occup Hyg. 1991;35(1):61–122. [DOI] [PubMed] [Google Scholar]
- 26.Esmen N On estimation of occupational health risks In: Esmen NA, Hatch TF, and Mehlman MA, ed. Occupational and Industrial Hygiene: Concepts and Methods. Princeton NJ: Princeton Scientific Publishers; 1984:45–84. [Google Scholar]
- 27.Ott MG. Occupational asthma, lung function decrement, and toluene diisocyanate (TDI) exposure: a critical review of exposure-response relationships. Appl Occup Environ Hyg. 2002;17(12):891–901. [DOI] [PubMed] [Google Scholar]
- 28.Scientifc Committee on Occupational Exposure Limits (SCOEL). 013. Methodology for the Derivation of Occupational Exposure Limits: Key Documentation (Version 7). Brussels, Belgium: European Commssion, Employment, Social Affairs &Inclusion; 2013. [Google Scholar]
- 29.United States Environmental Protection Agency (U.S. EPA). Benchmark Dose Software (BMDS) Version 2.6.0.1 (Build 88, 6/25/2015). National Center for Environmental Assessment; Available from http://bmds.epa.gov (Accessed 09/10/2017). [Google Scholar]
- 30.SAS Institute Inc., SAS 9.4 Help and Documentation, Cary, NC: SAS Institute Inc., 2002–2012. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. [Google Scholar]
- 31.Crump KS. Use of threshold and mode of action in risk assessment. Crit Rev Toxicol. 2011;41(8):637–650. [DOI] [PubMed] [Google Scholar]
- 32.Akaike H Information theory and an extension of the maximum likelihood principle In: Parzen E, Tanabe K, Kitagawa G, eds. Selected Papers of Hirotugu Akaike. New York, NY: Springer New York; 1998:199–213 [Google Scholar]
- 33.Dankovic DA, Naumann BD, Maier A, Dourson ML, Levy LS. The Scientific Basis of Uncertainty Factors Used in Setting Occupational Exposure Limits. J Occup Environ Hyg. 2015;12:S55–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dourson ML, Felter SP, Robinson D. Evolution of science-based uncertainty factors in noncancer risk assessment. Regul Toxicol Pharmacol. 1996;24(2 II):108–120. [DOI] [PubMed] [Google Scholar]
- 35.Clark RL, Bugler J, McDermott M, Hill ID, Allport DC, Chamberlain JD. An epidemiology study of lung function changes of toluene diisocyanate foam workers in the United Kingdom. Int Arch Occup Environ Health. 1998;71(3):169–179. [DOI] [PubMed] [Google Scholar]
- 36.Allport DC. Development Of Respiratory Sensitization in a UK TDI Foam Worker Population Further Evaluation Of III Report 10848: “The Acute And Long-Term Respiratory Effects Of Aromatic Diisocyanates: A Five Year Longitudinal Study Of Polyurethane Foam Workers.” Report Report No. 11115. Manchester, UK: International Isocyanates Institute; 1994. [Google Scholar]
- 37.Clark RL, Bugler J, Paddle GM, Chamberlain JD, Allport DC. A 17-year epidemiological study on changes in lung function in toluene diisocyanate foam workers. Int Arch Occup Environ Health. 2003;76(4):295–301. [DOI] [PubMed] [Google Scholar]
- 38.Diem JE, Jones RN, Hendrick DJ, et al. Five-year longitudinal study of workers employed in a new toluene diisocyanate manufacturing plant. Am Rev Respir Dis. 1982;126(3):420–428. [DOI] [PubMed] [Google Scholar]
- 39.Butcher BT, Jones RN, O’Neil CE, et al. Longitudinal study of workers employed in the manufacture of toluene-diisocyanate. Am Rev Respir Dis. 1977;116(3):411–421. [DOI] [PubMed] [Google Scholar]
- 40.Belin L, Wass U, Audunsson G, Mathiasson L. Amines: Possible causative agents in the development of bronchial hyperreactivity in workers manufacturing polyurethanes from isocyanates. Br J Ind Med. 1983;40(3):251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omae K Two-year observation of pulmonary function in workers exposed to low concentrations of toluene diisocyanate. Int Arch Occup Environ Health. 1984;55(1):1–12. [DOI] [PubMed] [Google Scholar]
- 42.Daftarian HS, Roegner KC, Reh CM. Hazard evaluation and technical assistance report: HETA-98–0011–2801, Woodbridge Corporation, Brodhead, Wisconsin. Cincinnati, OH: National Institute for Occupational Safety and Health; 2000. [Google Scholar]
- 43.Soderlund N, Rees D, Wasserfall C, Roodt L. A survey of a small group of workers exposed to toluene di-isocyanate. South African Medical Journal. 1993;83(2):100–103. [PubMed] [Google Scholar]
- 44.Gui W, Wisnewski AV, Neamtiu I, et al. Inception cohort study of workers exposed to toluene diisocyanate at a polyurethane foam factory: initial one-year follow-up. Am J Ind Med. 2014;57(11):1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daftarian HS, Lushniak BD, Reh CM, Lewis DM. Evaluation of self-reported skin problems among workers exposed to toluene diisocyanate (TDI) at a foam manufacturing facility. J Occup Environ Med. 2002;44(12):1197–1202. [DOI] [PubMed] [Google Scholar]
- 46.Redlich CA. Skin exposure and asthma: is there a connection? Proc Am Thorac Soc. 2010;7(2):134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karol MH, Hauth BA, Riley EJ, Magreni CM. Dermal contact with toluene diisocyanate (TDI) produces respiratory tract hypersensitivity in guinea pigs. Toxicol Appl Pharmacol. 1981;58(2):221–230. [DOI] [PubMed] [Google Scholar]
- 48.Cochrane SA, Arts JHE, Ehnes C, et al. Thresholds in chemical respiratory sensitisation. Toxicology. 2015;333:179–194. [DOI] [PubMed] [Google Scholar]
- 49.Arts JHE, Mommers C, De Heer C. Dose-response relationships and threshold levels in skin and respiratory allergy. Crit Rev Toxicol. 2006;36(3):219–251. [DOI] [PubMed] [Google Scholar]
- 50.Meredith SK, Bugler J, Clark RL. Isocyanate exposure and occupational asthma: a case-referent study. Occup Environ Med. 2000;57(12):830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold SM, Collins MA, Graham C, et al. Risk assessment for consumer exposure to toluene diisocyanate (TDI) derived from polyurethane flexible foam. Regul Toxicol Pharmacol. 2012;64(3):504–515. [DOI] [PubMed] [Google Scholar]
- 52.Pauluhn J Development of a respiratory sensitization/elicitation protocol of toluene diisocyanate (TDI) in Brown Norway rats to derive an elicitation-based occupational exposure level. Toxicology. 2014;319:10–22. [DOI] [PubMed] [Google Scholar]
- 53.Lemière C, Romeo P, Chaboillez S, Tremblay C, Malo JL. Airway inflammation and functional changes after exposure to different concentrations of isocyanates. J Allergy Clin Immunol. 2002;110(4):641–646. [DOI] [PubMed] [Google Scholar]
- 54.Tarlo SM. Canadian Thoracic Society. Guidelines for occupational asthma. Can Respir J. 1998;5(4):289–300. [DOI] [PubMed] [Google Scholar]
- 55.Vandenplas O, Suojalehto H, Cullinan P. Diagnosing occupational asthma. Clin Exp Allergy 2017;47(1):6–18. [DOI] [PubMed] [Google Scholar]
- 56.Mapp CE, Boschetto P, Maestrelli P, Fabbri LM. Occupational asthma. Am J Respir Crit Care Med. 2005;172(3):280–305. [DOI] [PubMed] [Google Scholar]
- 57.Applebaum KM, Malloy EJ, Eisen EA. Left truncation, susceptibility, and bias in occupational cohort studies. Epidemiology. 2011;22(4):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Applebaum KM, Malloy EJ, Eisen EA. Reducing healthy worker survivor bias by restricting date of hire in a cohort study of Vermont granite workers. Occup Environ Med. 2007;64(10):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, Risk Factors, and Mechanisms of Adult-Onset Asthma. Mediators Inflamm. 2015;2015(514868):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diller WF. Frequency and trends of occupational asthma due to toluene diisocyanate: a critical review. Appl Occup Environ Hyg. 2002;17(12):872–877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.