Key Teaching Points.
-
•
The inferoseptal process (ISP) is an infrequent site of ventricular ectopy.
-
•
The coronary sinus (CS) has a complex anatomical relationship with the ISP.
-
•
The proximal CS can potentially be a useful site to target the ISP.
Introduction
Radiofrequency catheter ablation is widely used to treat idiopathic ventricular arrhythmias. However, arrhythmic foci arising from the inferoseptal process (ISP) of the left ventricle (LV) that are deep intramural in origin may not be amenable to radiofrequency catheter ablation from the LV endocardium. The ISP of the LV, also referred to as the posterior-superior process of the LV, has been described as an inferoposterior region of the septal area of the LV that connects the muscular portion of the interventricular septum to the posterior portion of the LV free wall.1, 2, 3 Ventricular arrhythmias arising from the ISP of the LV have been previously reported and were mapped and successfully ablated from the adjacent inferomedial right atrium (RA) overlying the ISP.3 The ISP is an ill-defined area, and the coronary sinus (CS) is superiorly adjacent to this area.2 Herein, we describe 2 cases in whom the ISP arrhythmogenic focus could be targeted effectively only from within the CS.
Case reports
Case 1
A 72-year-old man with coronary artery disease with a prior coronary artery bypass graft procedure and implantable cardioverter-defibrillator insertion and ischemic cardiomyopathy presented with evidence of declining LV ejection fraction, from a baseline of 40%–45% to 25%–29%, associated with incessant symptomatic premature ventricular contractions (PVCs). In light of the ejection fraction decrease without other precipitating factors, PVCs were considered a reasonable cause and the patient was scheduled for a complete electrophysiology study and PVC catheter ablation. An apical scar unrelated to the ectopy had been reported on a myocardial perfusion scan.
Electroanatomic mapping (CARTO, Biosense Webster, Diamond Bar, CA) of the RA and CS and of the right ventricle and LV was performed using a 7-F decapolar electrode with 2-8-2-mm electrode spacing (DECANAV catheter, Biosense Webster), guided by an intracardiac echocardiography catheter (SOUNDSTAR, Biosense Webster). PVCs were suppressed by sedation despite stimulation with isoproterenol, and activation mapping became impractical. The site of origin of PVCs was determined by detailed pace mapping. Pace maps were obtained using an 8-F ablation catheter with a 3.5-mm open irrigated tip (SmartTouch Surround Flow, Biosense Webster) and reached 83% PVC match at the LV base. Based on the report by Santangeli and colleagues,3 we mapped the posterior-superior process from the RA. The pacing output required to capture the LV via the RA for pace mapping was 25 mA at 1 ms, and it did not work consistently. The best matching (96%) pace map was obtained inside the proximal CS, beyond the CS ostium (Figure 1). In this case, the CS was relatively high and the proximal CS coursed downward, hugging the LV base and the ISP. We delivered 35 W of radiofrequency ablation for 2 minutes in this location, achieving PVC elimination. After completion of ablation, no further PVCs were observed with and without isoproterenol. In the next 48 hours of continuous electrocardiographic monitoring, no PVCs were observed.
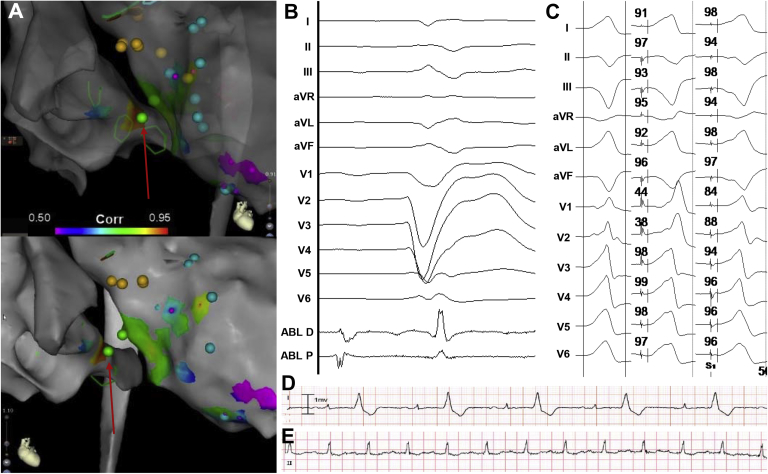
Figure 1.
Patient 1. A: Pace-map correlation maps showing a highest correlation in the proximal coronary sinus (CS; red arrow). B: Signals recorded at the ablation site in sinus. C: Premature ventricular contraction (left panel) and paced QRS from the left ventricle (middle panel) and from the CS (right panel). An average pace-map match of 96% was found while pacing from the CS. D, E: Rhythm strips before and after the procedure, respectively.
Case 2
A 58-year-old man with a history of hypertension, dyslipidemia, and a prior episode of cryptogenic cerebrovascular accident presented with frequent, symptomatic, and monomorphic PVCs refractory to drug therapy. The patient was referred and underwent a complete electrophysiology study and PVC catheter ablation.
Electroanatomic mapping (CARTO, Biosense Webster) of the RA and CS and of the right ventricle and LV was performed using a 7-F decapolar electrode with 2-8-2-mm electrode spacing (DECANAV catheter, Biosense Webster), guided by an intracardiac echocardiography catheter (SOUNDSTAR, Biosense Webster). The patient exhibited monomorphic PVCs identical to the clinical PVCs when stimulated with isoproterenol (2–4 μg/min). The site of origin of PVCs was determined using detailed activation and pace mapping, which were obtained using an 8-F ablation catheter with a 3.5-mm open irrigated tip (SmartTouch Surround Flow, Biosense Webster). The earliest activation site and a PVC pace-map match of 95% were obtained at the LV inferobasal site. We delivered 30 W for 6 minutes in this location, and initially, PVC elimination was achieved; however, once radiofrequency delivery ceased, PVCs returned. Similar to case 1, we mapped the ISP of the LV from the RA (Santangeli and colleagues3). The best matching pace map (98%) was obtained inside the proximal CS, beyond the CS ostium, and we delivered 20 W of radiofrequency ablation in this site (Figure 2). No further PVCs were observed with and without isoproterenol after completion of ablation in this location.
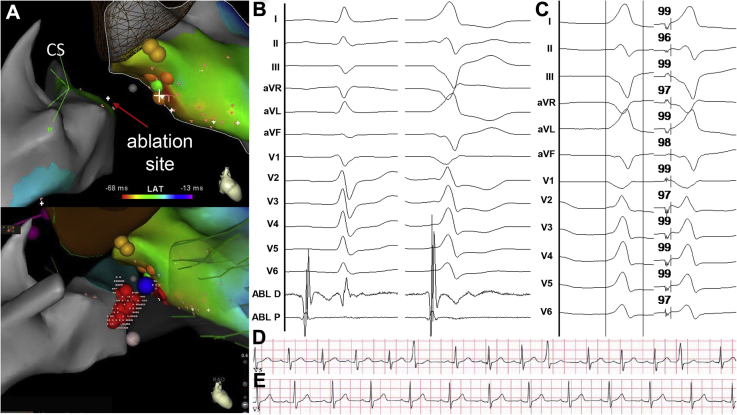
Figure 2.
Patient 2. A: Activation maps showing earliest activation time in the proximal coronary sinus (CS). B: Signals recorded at the ablation site in sinus (left panel) and during premature ventricular contraction (right panel). C: Premature ventricular contraction and paced QRS while pacing from the CS. A pace-map match of 98% was found from the CS. D, E: Rhythm strips before and after the procedure, respectively.
Follow-up
Both patients made an uneventful recovery and have been symptom-free for 10 months since the ablation procedure.
Discussion
The ISP has only recently been described as a site of origin of ventricular arrhythmias. Santangeli and colleagues3 described 5 cases in whom the ISP was successfully targeted from the anatomical RA, in its annular septal region, where it is adjacent to the ISP. Our cases illustrate that the proximal CS can also be a successful ablation site. The CS is anatomically an atrial structure, yet far-field ventricular signals are frequently recorded from it, given its annular location (Figure 3). In our cases, the ventricular sites of origin could be reached from the CS. Undoubtedly, ISP ectopy is ventricular in origin, but deep intramural (septal) origin makes it difficult to reach from endocardial ventricular sites. The proximal CS may allow targeting those sites effectively in selected cases. The CS (epicardial) approach entails ablation in the pyramidal space, which is traversed by the atrioventricular node artery. Therefore, atrioventricular node function could be affected. In addition, the areas ablated are adjacent to the slow pathway region ablated for the treatment of atrioventricular nodal reentry. Junctional rhythm—typical of such ablation—has been reported during ablation of ISP ventricular ectopy.4 We did not elicit junctional rhythm in the cases described.
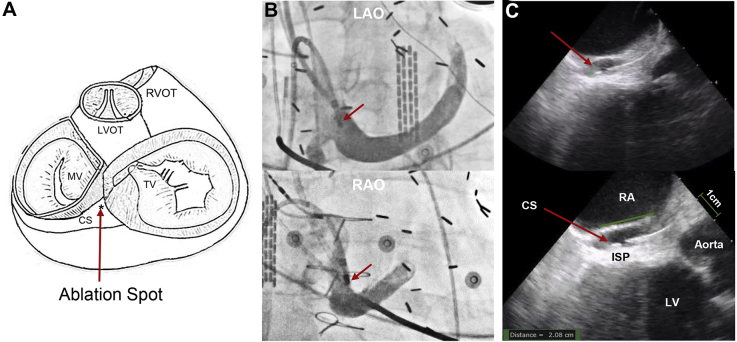
Figure 3.
Anatomical relationship between the coronary sinus (CS) and the inferoseptal process (ISP) of the left ventricle (LV). A: Schematic drawing of the heart showing the anatomical relationship between the CS and its adjacent structures. B: Left anterior oblique (LAO) and right anterior oblique (RAO) fluoroscopic views showing the position of the ablation catheter (red arrows) in the proximal CS. C: Intracardiac echocardiography showing the catheter position inside the CS. The ISP of the LV is immediately adjacent to the CS. LVOT = left ventricular outflow tract; MV = mitral valve; RA = right atrium; RVOT = right ventricular outflow tract; TV = tricuspid valve.
Conclusion
These cases illustrate that certain anatomical variations can make the CS approach a suitable approach to target the ISP of the LV. This approach should be considered when ablating arrhythmias arising from the ISP.
Acknowledgments
We thank Zachary Paulson, MEng, for his technical assistance with the CARTO images.
Footnotes
This work was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute (grant no. R01 HL 115003) and the Charles Burnett III endowment (to Dr Valderrábano).
References
- 1.McAlpine W.A. Springer; New York, NY: 1975. Heart and Coronary Arteries. [Google Scholar]
- 2.Menasche P. Anatomic bases of the surgical division of Kent bundles in the posterior septal area of the heart. Surg Radiol Anat. 1986;8:109–114. doi: 10.1007/BF02421377. [DOI] [PubMed] [Google Scholar]
- 3.Santangeli P., Hutchinson M.D., Supple G.E., Callans D.J., Marchlinski F.E., Garcia F.C. Right atrial approach for ablation of ventricular arrhythmias arising from the left posterior-superior process of the left ventricle. Circ Arrhythm Electrophysiol. 2016:9. doi: 10.1161/CIRCEP.116.004048. [DOI] [PubMed] [Google Scholar]
- 4.Li A, Zuberi Z, Bradfield JS, Zarif JK, Ward DE, Anderson RH, Shivkumar K, Saba MM. Endocardial ablation of ventricular ectopic beats arising from the basal inferoseptal process of the left ventricle [published online ahead of print April 28, 2018]. Heart Rhythm. 10.1016/j.hrthm.2018.04.029. [DOI] [PubMed]