Summary
Although the functional roles of long noncoding RNAs (lncRNAs) have been increasingly identified, few lncRNAs that control the naïve state of embryonic stem cells (ESCs) are known. Here, we report a naïve-state-associated lncRNA, LincU, which is intrinsically activated by Nanog in mESCs. LincU-deficient mESCs exhibit a primed-like pluripotent state and potentiate the transition from the naïve state to the primed state, whereas ectopic LincU expression maintains mESCs in the naïve state. Mechanistically, we demonstrate that LincU binds and stabilizes the DUSP9 protein, an ERK-specific phosphatase, and then constitutively inhibits the ERK1/2 signaling pathway, which critically contributes to maintenance of the naïve state. Importantly, we reveal the functional role of LincU to be evolutionarily conserved in human. Therefore, our findings unveil LincU as a conserved lncRNA that intrinsically restricts MAPK/ERK activity and maintains the naïve state of ESCs.
Keywords: naïve state, embryonic stem cells, lincRNA, DUSP9, MAPK signaling
Graphical Abstract
Highlights
-
•
LincU is integral and sufficient to maintain the naïve state of mESCs
-
•
LincU binds and stabilizes DUSP9 protein to inhibit the ERK1/2 phosphorylation
-
•
LincU is a direct target of NANOG in naïve-state mESCs
-
•
The functional role of LincU is conserved in human ESCs
Kang and colleagues report a naïve-state-associated long noncoding RNA (LincU), which is directly regulated by Nanog and stabilizes the ERK-specific phosphatase DUSP9 by protecting it from ubiquitin-proteasome mediated degradation to maintain the naïve state of mESCs. Moreover, LincU is functionally conserved in human ESCs.
Introduction
Early embryonic development involves dynamic cellular conversion events that are characterized by dramatic changes in both extrinsic signaling pathways and intrinsic epigenetic and transcriptional programs (Young, 2011). Preimplantation blastocysts have the capacity to differentiate into both somatic cells and germ cells and are thus considered to display a naïve pluripotent state (Bradley et al., 1984, Leitch and Smith, 2013). Upon implantation, the inner cell mass (ICM) develops into the epiblast, which is characterized by the incipient expression of germ layer markers and disintegration of the naïve pluripotency network, considered to be a primed pluripotent state (Nichols and Smith, 2009). In addition, the inaccessibility of these transient cell populations in vivo further restricts the study of this process directly. Fortunately, mouse embryonic stem cells (mESCs), derived from the ICM, provide a versatile model for studying this transient process in vitro (Evans and Kaufman, 1981, Martin, 1981). When cultured in vitro, mESCs exhibit two distinct pluripotent states, the original naïve state and the primed state (epiblast-like cells), which display distinct morphological, transcriptional, and epigenetic profiles (Hackett and Surani, 2014, Kalkan and Smith, 2014). Naïve-state clones show round morphology while primed-state clones are flat, similar to human ESCs (hESCs) (Nichols and Smith, 2009, Tesar et al., 2007). Nanog, Klf2 and Esrrb have been defined as the core transcription factors that determine the naïve state of mESCs (Dunn et al., 2014, Festuccia et al., 2012, Stuart et al., 2014), whereas Brachyury, Fgf5, and Eomes are unique genes expressed in the primed state (Nichols and Smith, 2009). The transition from the naïve state to the primed state is accompanied by global upregulation of H3K27me3 and DNA methylation (Marks et al., 2012). Further understanding the core regulatory network that dominates these two pluripotent states will be pivotal to studying preimplantation embryonic development and applying this knowledge to regenerative medicine.
Signaling pathways are commonly acknowledged as the main extrinsic factors that trigger the transition between naïve and primed pluripotent states (Hackett and Surani, 2014, Kunath et al., 2007). The leukemia inhibitory factor (LIF) and bone morphogenetic protein 4 (BMP4) signaling pathways have been shown to be critical for the self-renewal of naïve-state stem cells but are dispensable for the primed state (Niwa et al., 2009, Yoshida et al., 1994). Additionally, activation of the ERK1/2 signaling pathway triggers the transition from the naïve state to the primed state, and this pathway collaborates with the ACTIVIN/NODAL signaling pathway to maintain the primed state (Brons et al., 2007, Hackett and Surani, 2014, Ogawa et al., 2007). Interestingly, LIF also induces the activation of the ERK1/2 signaling pathway in the naïve pluripotent state regardless of the primarily activated LIF signaling pathway (Nichols et al., 2001). In addition, while ERK1/2 signaling drives lineage priming in mESCs, it is also required for the maintenance of self-renewal in hESCs (Greber et al., 2010). Moreover, the evolutionarily conserved fibroblast growth factor (FGF)/ERK signaling pathway has been reported to control a multitude of early embryonic developmental processes, including proliferation, survival, migration, metabolism, and differentiation (Roskoski, 2012). All of these observations underscore the importance of the ERK1/2 signaling pathway in stem cell fate decisions. Strikingly, maintenance of a homogeneous naïve pluripotent state is enabled by the elimination of differentiation-inducing signals from the ERK1/2 signaling pathway (Nichols et al., 2009, Ying et al., 2008). Therefore, fine-tuning of ERK1/2 activity is critical for determining the pluripotent state of stem cells. Recently, exogenous BMP4 was reported to steadily attenuate ERK1/2 activity by upregulating the expression of the pluripotency-specific protein dual-specificity phosphatase 9 (Dusp9), which binds to ERK1/2 and inhibits its phosphorylation in the naïve pluripotent state (Li et al., 2012). However, the intrinsic modifiers that modulate the activity of ERK1/2 signaling pathway are rarely uncovered.
Long noncoding RNAs (lncRNAs), with lengths more than 200 bp, are more versatile in distinct biological processes, accounting for their higher-ordered structures and flexible expression patterns (Batista and Chang, 2013, Guttman and Rinn, 2012, Rinn and Chang, 2012). Based on their genomic location, lncRNAs can be classified as sense, antisense, bidirectional, intronic, or intergenic (lincRNA) (Fatica and Bozzoni, 2014). By functioning as molecular scaffolds, lncRNAs participate in the regulation of gene expression and the post-translational modification of proteins, including ubiquitination and phosphorylation (Nagano et al., 2008, Pandey et al., 2008, Taniue et al., 2016, Wang et al., 2014). The p53-induced lincRNA-p21 represses the translation of CTNNB1 and JUNB by directly interacting with their mRNAs (Yoon et al., 2012). Furthermore, linc00673 can reinforce the interaction between PTPN11 and PRPF19 and promote the ubiquitination and degradation of PTPN11 (Zheng et al., 2016). A previous study in dendritic cells revealed that the lincRNA lnc-DC could directly interact with STAT3 and sustain its phosphorylation at tyrosine-705 by preventing SHP1-mediated dephosphorylation (Wang et al., 2014). Recently, lncRNAs that collaborate with epigenetic remodeling complexes to directly regulate gene expression for the maintenance of pluripotency and lineage commitment in pluripotent stem cells have been discovered (Dinger et al., 2008, Guttman et al., 2011). Nevertheless, lncRNAs involved in the regulation of core signaling pathway activity in pluripotent stem cells remain largely unexplored.
Here, we report a naïve mESC specifically expressed lincRNA, LincU (previously referred as linc1483 [Guttman et al., 2011]), whose deficiency promotes the transition from naïve state to primed state, and its overexpression inhibits this process. Mechanistically, we found that LincU specifically restricts the activity of the mitogen-activated protein kinase (MAPK)/ERK signaling pathway by binding and stabilizing the ERK-specific phosphatase DUSP9. Our results suggest that LincU acts as an intrinsic inhibitor of MAPK/ERK signaling and critically controls the naïve pluripotent state of mESCs.
Results
LincU Is Both Essential and Sufficient for Maintaining the Naïve Pluripotency of mESCs
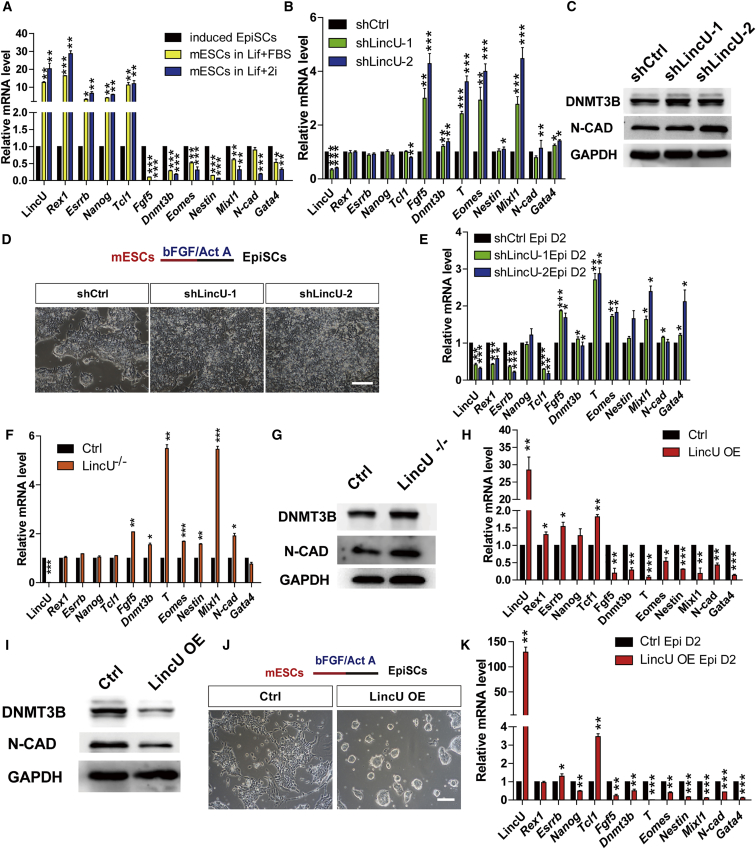
Naïve pluripotency holds great promise for the clinical application of pluripotent stem cells (Batista and Chang, 2013). Although thousands of lncRNAs have been identified and integrated into the pluripotency regulation network (Guttman et al., 2011), functional lncRNA that plays a pivotal role in the maintenance of naïve pluripotency has rarely been found. As Nanog is considered a core element in naïve-state maintenance (Chambers et al., 2003, Chambers et al., 2007, Mitsui et al., 2003) we found that Nanog can bind the promoter region of LincU, which is located on chromosome 16 (9,133,405–9,138,147) (mm9) (Murakami et al., 2016). Similar to that of Nanog, the expression pattern of LincU is dramatically and rapidly downregulated after LIF withdrawal (Figure S1A). Thus, to identify the functional role of LincU in the maintenance of naïve pluripotency, we assessed the expression levels of LincU in the mESCs at different pluripotent states, including those at the primed pluripotent state (sustained with basic FGF [bFGF] and activin A, bFGF + activin A), naïve pluripotent state (maintained with LIF and 2i, LIF + 2i), and heterogeneous pluripotent state (cultured in medium supplemented with LIF and fetal bovine serum [FBS], LIF + FBS). As expected, the naïve pluripotency-specific genes Rex1, Esrrb, Nanog, and Tcl1 are highly expressed in the heterogeneous pluripotent state and even more highly expressed in the naïve pluripotent state, while the primed-state genes Fgf5, Dnmt3b, T, and Eomes together with the developmental genes Nestin, Mixl l, N-cad, and Gata4 are highly expressed in the primed pluripotent state (Figure 1A). Compared with ESCs cultured in bFGF + activin A and LIF + FBS, ESCs cultured in LIF + 2i showed higher expression level of LincU (Figure 1A), which is in accordance with the expression patterns of naïve pluripotency-specific genes, indicating a potentially pivotal role of LincU in the maintenance of the naïve pluripotent state.
Figure 1.
LincU Is Both Essential and Sufficient for Maintaining the Naïve Pluripotency of mESCs
(A) LincU is highly expressed in the heterogeneous pluripotent state and even more highly expressed in the naïve pluripotent state. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test.
(B and C) shLincU mESCs exhibit a primed-like state when cultured in LIF + FBS medium, as shown by qPCR (B) and western blot (C) analysis of primed-state-related and developmental genes. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test.
(D and E) LincU knockdown accelerates the epiblast induction process as shown by microscopy (D) and qPCR (E) analysis of epiblast cells induced for 2 days. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test. Scale bar, 100 μm.
(F and G) LincU−/− mESCs express higher levels of primed-state-specific genes and developmental genes when cultured in LIF + FBS medium, as shown by qPCR (F) and western blot (G) analysis. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test.
(H and I) LincU-overexpressing mESCs express lower levels of primed-state-related genes and developmental genes when cultured in LIF + FBS medium, as shown by qPCR (H) and western blot (I) analysis. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test.
(J and K) LincU-overexpressing mESCs fail to differentiate into the primed pluripotent state, as shown by microscopy (J) and qPCR (K) analysis of day-2 induced epiblast cells. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test. Scale bar, 100 μm.
Employing a nucleocytoplasmic separation assay, we observed that the LincU transcripts were abundant in the cytoplasm of naïve-state mESCs (Figure S1B). Thus, we designed two efficient short hairpin RNAs (shRNAs) that target LincU transcripts to knock down its expression (shLincU-1 and shLincU-2) in mESCs via a lentivirus delivery system; a scrambled shRNA (shCtrl) served as the negative control. When cultured in LIF + FBS medium, mESCs fluctuate between the naïve and primed pluripotent states, which is termed the heterogeneous pluripotent state (Festuccia et al., 2012), and provides a more accessible platform to study the transition between the naïve and primed states. After maintaining shLincU and shCtrl mESCs in this serum-containing medium, shLincU mESCs exhibited a primed-like pluripotent state, which was characterized by higher expression levels of primed-state-specific genes, including Fgf5, Dnmt3b, T, Eomes, Mixl1, and N-cadherin, although LincU knockdown had no apparent influence on the expression of naïve-state-related genes (Figure 1B). Western blot also confirmed the elevated expression levels of primed-state-specific proteins (Figures 1C and S1C). Increased expression level of DNMT3B, a DNA methyltransferase, will enhance DNA methylation, which is well known to be a dominant feature of the primed pluripotent state (Borgel et al., 2010).
To validate the primed-like pluripotent state of LincU knockdown mESCs, we directly induced these mESCs to enter the primed pluripotent state by applying N2B27 + bFGF + activin A medium (Hayashi et al., 2011). As expected, LincU knockdown mESCs exhibited accelerated epiblast induction compared with shCtrl mESCs, as determined by more notable epiblast-like morphology, decreased expression of naïve-state-related genes, and increased expression of primed-state-related genes after 2 days of induction (Figures 1D and 1E). Furthermore, when we replated the induced primed pluripotent cells (cultured in N2B27 + activin A + FGF2) into LIF + FBS medium, LincU knockdown cells failed to form alkaline phosphatase (AP)-positive colonies (Figures S1D and S1E) (Karwacki-Neisius et al., 2013). These data demonstrated that LincU knockdown mESCs exhibit a primed-like pluripotent state.
To further confirm the functional role of LincU in the maintenance of naïve pluripotency, we directly generated LincU−/− mESCs via CRISPR/Cas9-mediated genome deletion (Li et al., 2017). Knockout efficiency was validated by genomic DNA PCR (Figure S1F) and qPCR (Figure 1F). Similarly, LincU−/− mESCs expressed higher levels of primed-state-specific genes when maintained in a heterogeneous pluripotent state (Figures 1F, 1G, and S1G). Moreover, we evaluated whether LincU−/− cells could contribute to chimeras and found that mESCs lacking LincU failed to form chimeras, thereby demonstrating that LincU-deficient mESCs have lost naïve pluripotency (Figures S2A and S2B). Additionally, we performed the teratoma formation assay and our results showed that LincU−/− mESCs formed larger teratomas with higher expression levels of primed-state and lineage-specific genes (Figures S2C and S2D). These results demonstrated that LincU is required for maintaining the naïve pluripotency of mESCs.
Next, we built the LincU-overexpressing mESCs by integrating CAG promoter-driven LincU expression into the Rosa26 locus using CRISPR/Cas9-mediated homologous recombination (Li et al., 2017). After confirming the constitutive overexpression of LincU transcripts, we found that LincU-overexpressing mESCs expressed lower expression levels of primed-state-related and developmental genes than control mESCs when cultured in LIF + FBS medium (Figure 1H). Additionally, a slight increase in the expression of naïve-state-related genes was observed (Figure 1H). Western blot assay also verified the decreased DNMT3B and N-CADHERIN protein levels (Figures 1I and S2E). These observations indicated that LincU-overexpressing mESCs exhibit a naïve-like pluripotent state. To further confirm this hypothesis, we induced control and LincU-overexpressing mESCs to differentiate to the primed pluripotent state. Notably, the LincU-overexpressing mESCs failed to transit to the primed state, as shown by a naïve-state morphology and a much lower expression level of primed-state-related genes than that in control mESCs (Figures 1J and 1K). Moreover, the induced EpiSCs derived from LincU-overexpressing mESCs were still able to form AP-positive colonies after being replated into LIF + FBS medium (Figures S2F and S2G), showing that overexpression of LincU severely disrupted the transition from the naïve pluripotent state to the primed pluripotent state. Similarly, we performed the teratoma formation assay with control and LincU-overexpressing mESCs, whereby LincU overexpression resulted in a smaller teratoma size and lower expression level of lineage-specific genes than those in control mESCs (Figures S2H and S2I). These in vivo results further confirmed that LincU-overexpressing mESCs maintain a naïve-like pluripotent state that is resistant to the initiation of differentiation. Collectively, these results demonstrated that LincU is both essential and sufficient for maintaining the naïve pluripotency of mESCs.
LincU Specifically Inhibits the MAPK-ERK Signaling Pathway
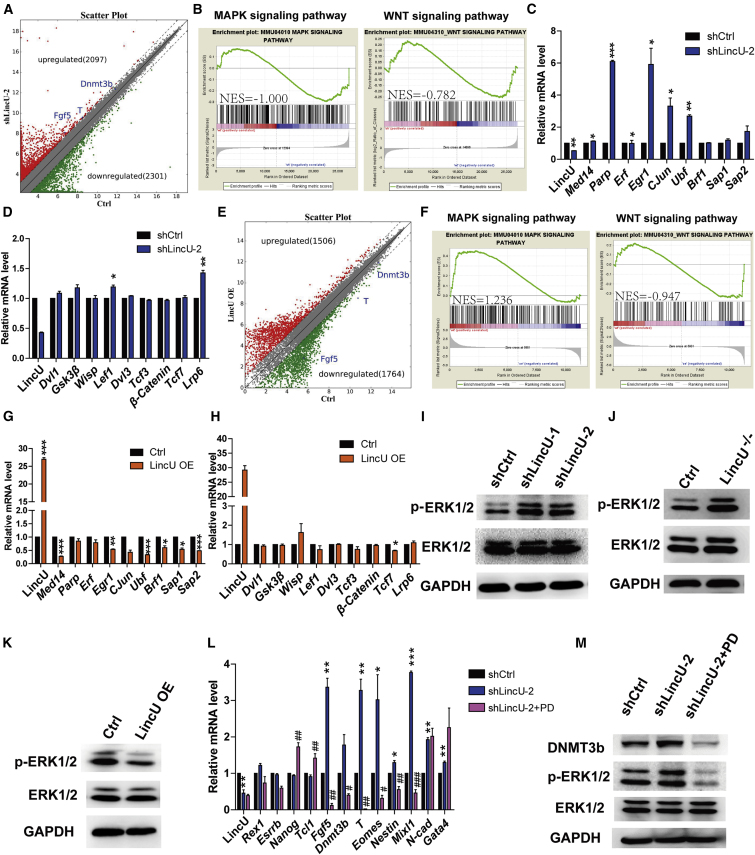
To determine the mechanism underlying LincU-induced key phenotypes in mESCs, we performed global transcriptome analysis of both LincU knockdown and LincU-overexpressing mESCs, which were maintained in the heterogeneous pluripotent state. Consistently, primed-state-related genes were upregulated upon LincU knockdown and downregulated upon LincU overexpression (Figures 2A and 2E). In addition, gene ontology analysis of these differentially expressed genes showed that LincU deficiency led to significant upregulation of functional terms related to in utero embryonic development, whereas LincU overexpression notably downregulated these functional terms (Figures S3A and S3B). Importantly, KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis of the downregulated genes in LincU-overexpressing mESCs showed that these genes are significantly enriched in the MAPK signaling pathway (Figure S3C). Furthermore, we performed gene set enrichment analysis (GSEA) and analyzed the normalized enrichment scores (NES) for two key signaling pathways that are involved in naïve-state reprogramming and maintenance: the MAPK/ERK and WNT signaling pathways. Our results indicated that MAPK signaling, but not WNT signaling, was significantly affected in both LincU knockdown and LincU-overexpressing mESCs (Figures 2B and 2F). We further examined the expression level of downstream genes of the WNT and MAPK/ERK signaling pathways, and our data indicated that LincU was negatively correlated with the expression level of MAPK/ERK downstream genes, but not the downstream genes of the WNT signaling pathway (Figures 2C, 2D, 2G, and 2H), which suggested that the activity of MAPK/ERK signaling pathway was affected by LincU expression manipulation.
Figure 2.
LincU Specifically Inhibits the MAPK-ERK Signaling Pathway
(A) Scatterplots of global gene expression according to microarray data for shLincU-2 mESCs versus shCtrl mESCs. The gray dashed lines delineate the boundaries of a 2-fold change in gene expression. Upregulated genes are highlighted in red and downregulated genes are shown in green.
(B) GSEA of the MAPK and WNT signaling pathways in shLincU-2 mESCs versus shCtrl mESCs. NES represents the normalized enrichment scores in each category.
(C and D) LincU knockdown increases the expression of downstream genes of the MAPK signaling pathway (C) but barely affects the expression level of WNT target genes (D). Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test.
(E) Scatterplots of global gene expression according to microarray data for LincU-overexpressing mESCs versus Ctrl mESCs. The gray dashed lines delineate the boundaries of a 2-fold change in gene expression. Upregulated genes are highlighted in red and downregulated genes are shown in green.
(F) GSEA of the MAPK and WNT signaling pathways in LincU-overexpressing mESCs versus Ctrl mESCs. NES represents the normalized enrichment scores in each category.
(G and H) LincU overexpression decreases the expression of the downstream genes of the MAPK signaling pathway (G) but barely affects the expression level of WNT target genes (H). Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test.
(I and J) Western blot (n = 3) showing higher levels of ERK1/2 phosphorylation in shLincU mESCs (I) and LincU−/− mESCs (J) cultured in LIF + FBS medium.
(K) Western blot (n = 3) showing that LincU overexpression decreases the level of ERK1/2 phosphorylation when cultured in LIF + FBS medium.
(L and M) LincU knockdown-induced increases in the expression of primed-state-related genes and developmental genes are significantly compromised by MAPK/ERK inhibitor (PD0325901) treatment, as shown by qPCR (L) and western blot (M) analysis. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001, t test.
To validate the sequential changes of MAPK/ERK signaling pathway activity after modifying the expression of LincU, we examined the level of ERK1/2 phosphorylation in LincU knockdown, LincU knockout, and LincU-overexpressing mESCs. Consistently, the level of ERK1/2 phosphorylation was markedly increased by LincU knockdown or knockout and dramatically decreased by LincU overexpression (Figures 2I–2K and S3D–S3F). Meanwhile, we also observed a negative correlation between the LincU expression level and the level of ERK1/2 phosphorylation in the teratomas derived from these three groups of mESCs (Figures S3G–S3J). Moreover, treatment with a potent MAPK inhibitor, PD0325901, significantly compromised the LincU knockdown-induced upregulation of primed-state-related genes and increased the level of ERK1/2 phosphorylation and DNMT3B protein (Figures 2L, 2M, and S3K). In conclusion, these results proved that LincU specifically inhibits MAPK/ERK signaling activity in mESCs, thereby elucidating the role of LincU in maintaining the naïve state.
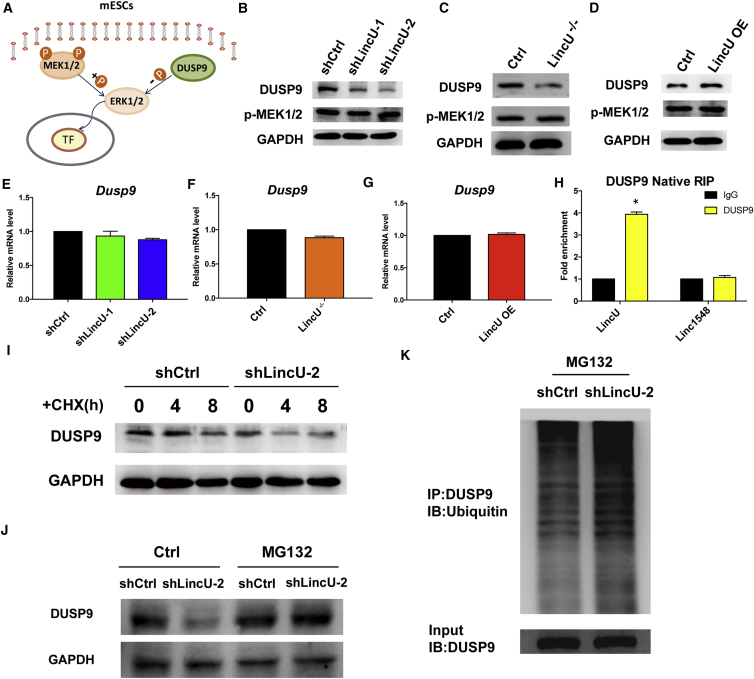
LincU Stabilizes the ERK-Specific Phosphatase DUSP9 in mESCs
Direct competition between phosphorylation and dephosphorylation has been proposed as a major mechanism underlying extracellular stimulation and has been documented in most biological processes (Bononi et al., 2011). The negative correlation between LincU expression and the level of ERK1/2 phosphorylation indicates that LincU modifies ERK1/2 phosphorylation in mESCs. ERK1/2 are serine/threonine kinases that are mainly involved in signal transduction of the Ras-Raf-MEK-ERK cascade. MEK1/2 are well-known kinases that catalyze the phosphorylation of ERK1/2, whereas DUSP9 specifically hydrolyzes the phosphate group on ERK1/2 in mESCs (Figure 3A). We thus examined the protein levels of phosphorylated MEK1/2 and DUSP9 in LincU-deficient and LincU-overexpressing mESCs. Notably, no significant change in the phosphorylated MEK1/2 protein level was observed after either LincU deficiency or overexpression, whereas the protein level of DUSP9 was obviously decreased after LincU deficiency and increased with LincU overexpression (Figures 3B–3D and S4A–S4C). In vivo results also showed that LincU knockout decreased DUSP9 protein levels while LincU overexpression increased DUSP9 protein levels in teratomas (Figures S4D–S4G). Interestingly, the mRNA level of Dusp9 remained unchanged after either LincU deficiency or overexpression (Figures 3E–3G), indicating that LincU might increase Dusp9 protein stability at the post-translational level.
Figure 3.
LincU Stabilizes the ERK-Specific Phosphatase DUSP9 in mESCs
(A) Model illustrating the phosphorylation and dephosphorylation roles of MEK1/2 and DUSP9, respectively, in the regulation of ERK1/2 phosphorylation.
(B–D) Western blot (n = 3) showing the expression level of LincU is positively correlated with the protein level of DUSP9 but not the level of MEK1/2 phosphorylation in shLincU mESCs (B), LincU−/− mESCs (C), and LincU-overexpressing mESCs (D).
(E–G) LincU does not change the mRNA level of Dusp9, as shown by qPCR analysis of shLincU mESCs (E), LincU−/− mESCs (F), and LincU-overexpressing mESCs (G).
(H) Native RIP assays showing the physical interaction between DUSP9 and LincU. Linc1548 acts as a negative control. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, t test.
(I) DUSP9 protein degradation is accelerated in shLincU mESCs compared with shCtrl mESCs after CHX (20 μg/mL) treatment.
(J) DUSP9 protein degradation in shLincU mESCs is rescued by proteasome inhibitor (MG132) treatment.
(K) LincU knockdown induces robust DUSP9 ubiquitination.
Considering that LincU is primarily resident in the cytoplasm, we suspected that LincU could bind to DUSP9 to increase its protein stability. To this end, a native RNA immunoprecipitation (RIP) assay was performed using a specific antibody against DUSP9 in mESCs. After pulling down DUSP9, substantial amounts of LincU were observed in the immunocomplexes (Figure 3H). Moreover, we performed the crosslinked RIP assay with a specific DUSP9 antibody, and our results further showed that LincU indeed interacted with DUSP9 (Figure S4H). To further confirm that LincU is required for the stabilization of DUSP9 protein, we treated shCtrl and shLincU mESCs with cycloheximide to disrupt the translation processes and found that the DUSP9 protein level decreased much more dramatically in LincU-deficient mESCs than in control mESCs (Figures 3I and S4I). Moreover, pretreatment with a potent proteasome inhibitor, MG132, stabilized DUSP9 protein expression in LincU-deficient mESCs, indicating that the ubiquitination-proteasome pathway is involved in LincU deficiency-induced DUSP9 degradation (Figures 3J and S4J). Immunoprecipitation using a specific DUSP9 antibody was performed after pretreatment with MG132 and blotted with an antibody against ubiquitin. Our results clearly showed polyubiquitinated DUSP9 bands, suggesting that LincU knockdown causes DUSP9 ubiquitination (Figures 3K and S4K). Collectively, these results demonstrated that LincU protects DUSP9 protein from ubiquitination-proteasome-mediated degradation in mESCs.
Dusp9 Lies Downstream of LincU to Preserve the Naïve Pluripotency of mESCs
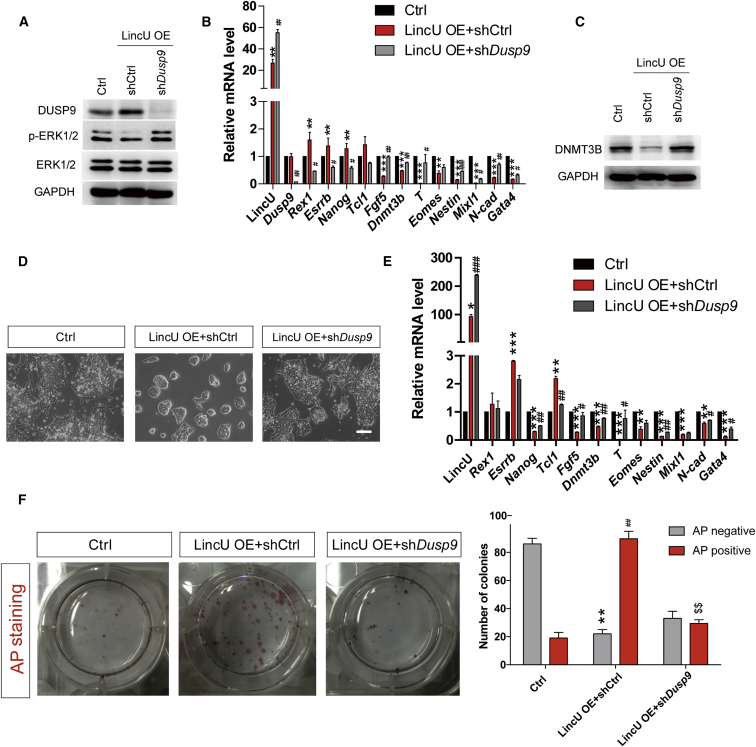
To certify that Dusp9 lies downstream of LincU to maintain the naïve state of mESCs, we designed a potent shRNA targeting the CDS (coding sequence) region of Dusp9 mRNA to knock down Dusp9 in LincU-overexpressing mESCs. The knockdown efficiency was verified by qPCR and western blot (Figures 4A, 4B, and S5A). In addition, we found that both the decreased levels of ERK1/2 phosphorylation and the decreased expression levels of primed-state-related genes triggered by LincU overexpression were significantly compromised by Dusp9 knockdown (Figures 4A, 4B, and S5B). Moreover, the LincU-inhibited expression of DNMT3B was rescued by Dusp9 knockdown (Figures 4C and S5C). Additionally, LincU-overexpressing mESCs recuperated their ability to transition to the primed state when Dusp9 was downregulated (Figures 4D–4F). Taken together, our data demonstrated that Dusp9 lies downstream of LincU to preserve the naïve pluripotency of mESCs.
Figure 4.
Dusp9 Lies Downstream of LincU to Preserve the Naïve Pluripotency of mESCs
(A) Western blot (n = 3) showing Dusp9 knockdown rescues the LincU overexpression-induced decrease in ERK1/2 phosphorylation.
(B and C) LincU overexpression-induced inhibition of primed-state-related and developmental gene expression is significantly compromised by Dusp9 knockdown, as shown by qPCR (B) and western blot (C) analysis in mESCs. Data are shown as the mean ± SEM (n = 3). ∗∗p < 0.01, ∗∗∗p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001, t test.
(D and E) Dusp9 knockdown restores the capacity of LincU-overexpressing mESCs to undergo epiblast induction, as shown by microscopy (D) and qPCR (E) analysis of day-2 induced epiblast cells. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, #p < 0.05, ##p < 0.01, ###p < 0.001, t test. Scale bar, 100 μm.
(F) Colony-formation assay of single cells from Ctrl group and LincU-overexpression group along with shCtrl and shDusp9, respectively, 48 hr post epiblast induction. Colonies were stained for AP activity on day 6 of culture in LIF + FBS medium and deemed as either AP-positive or AP-negative. Data are shown as the mean ± SEM (n = 3). ∗∗p < 0.01, ##p < 0.01, $$p < 0.01, t test.
LincU Is the Direct Target of NANOG in Naïve-State mESCs
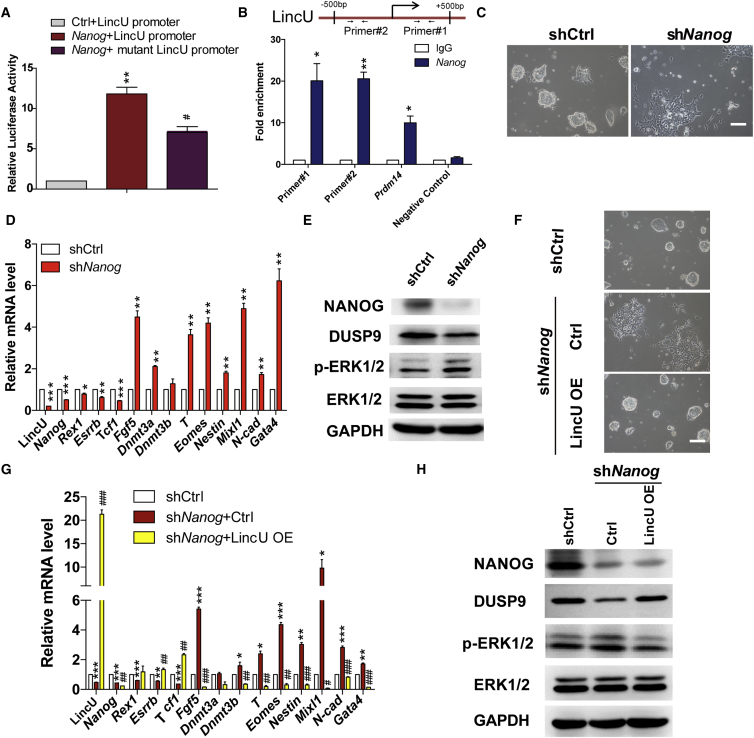
Chromatin immunoprecipitation sequencing (ChIP-seq) data for ESCs from CODEX (http://codex.stemcells.cam.ac.uk/) indicated that the core transcription factors NANOG, SOX2, ESRRB, KLF4, and SMAD1 do bind to the promoter of LincU (Sanchez-Castillo et al., 2015). Thus, we tested the LincU-promoter luciferase reporter with Sox2, Esrrb, Klf4, and Smad1 as well as Nanog. Our results showed that NANOG induced a stronger activation of LincU promoter than KLF4 and SMAD1, while SOX2 and ESRRB showed no significant activation of LincU promoter (Figure S5D). Next, we deleted two possible Nanog-binding motifs in LincU promoter (mutant LincU promoter) and found that reporter activity was reduced (Figure 5A). These results indicated that Nanog is one important upstream regulator of LincU, and these other transcription factors might regulate LincU together with NANOG. Furthermore, ChIP-PCR assay using a specific antibody against NANOG confirmed that NANOG directly binds to the promoter of LincU in naïve-state mESCs (Figure 5B).
Figure 5.
LincU Is the Direct Target of NANOG in Naïve-State mESCs
(A and B) NANOG directly binds to the promoter region of LincU, as shown by the luciferase reporter assay (A) and ChIP-qPCR with anti-NANOG antibody (B). Prdm14 acts as a positive control. The negative control amplifies an intergenic region. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, #p < 0.05, t test.
(C and D) Nanog knockdown induces obvious differentiation of mESCs, as shown by microscopy (C) and qPCR (D) analysis 48 hr post shNanog lentivirus infection. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test. Scale bar, 100 μm.
(E) Western blot (n = 3) showing Nanog knockdown clearly decreases the protein level of DUSP9 and elevates the level of ERK1/2 phosphorylation.
(F and G) Overexpressing LincU markedly blocks the differentiation of mESCs triggered by Nanog knockdown, as shown by microscopy (F) and qPCR analysis (G). Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, #p < 0.5, ##p < 0.01, ###p < 0.001, t test. Scale bar, 100 μm.
(H) Western blot (n = 3) showing the decreased protein level of DUSP9 and elevated level of ERK1/2 phosphorylation in shNanog mESCs are significantly compromised by LincU overexpression.
To further confirm that LincU is downstream of Nanog, we performed knockdown of Nanog and observed a dramatic decrease of LincU transcription after Nanog knockdown (Figures 5C and 5D). Consistent with the results obtained from LincU knockdown, Nanog knockdown upregulated primed-state-related genes (Figure 5D), decreased DUSP9 protein expression and increased ERK1/2 phosphorylation (Figures 5E and S5E). Notably, Nanog knockdown-accelerated primed-state transition, which was characterized by a flat morphology and high expression levels of primed-state-related genes, was significantly compromised by ectopic LincU expression (Figures 5F and 5G). Meanwhile, the decreased DUSP9 protein expression and increased level of ERK1/2 phosphorylation triggered by Nanog knockdown were also rescued by LincU overexpression (Figures 5H and S5F). Together, these data verified that LincU is the direct target of Nanog in naïve mESCs.
The Functional Role of LincU Is Conserved in hESCs
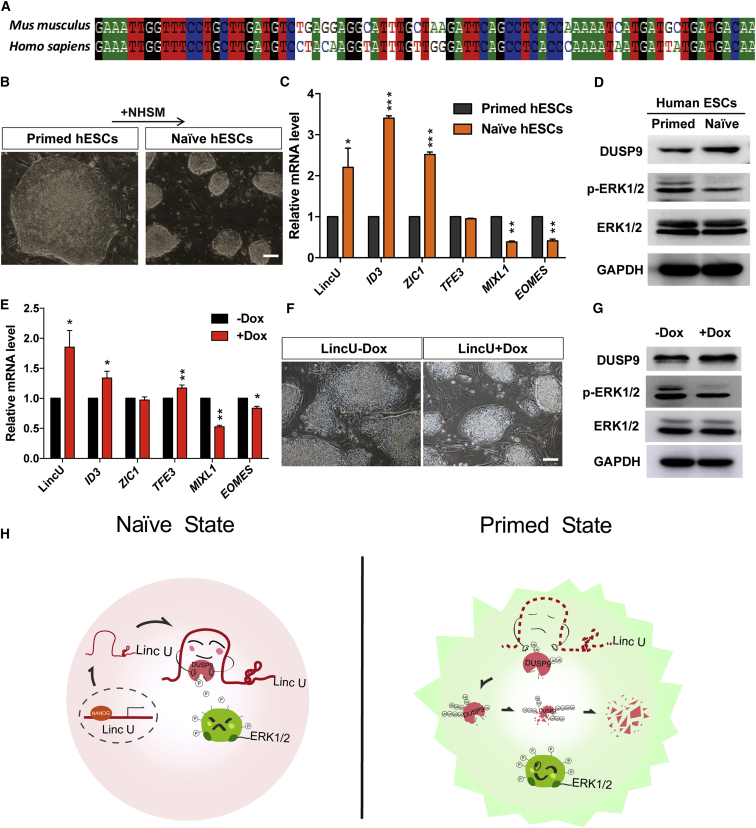
Naïve-state hESCs show great promise for clinical cellular supplementation therapy (Batista and Chang, 2013). In addition, lincRNAs commonly exhibit high mutation frequencies and are rarely conserved from rodents to humans. Thus, conserved and functional lincRNAs involved in the transition between naïve and primed hESCs are rarely reported. Fortunately, mouse LincU shares a homology region with human genome (Figure 6A), indicating that LincU is an evolutionarily conserved lincRNA. We thus designed a specific pair of primers for the qPCR amplification of human LincU and examined the relative expression levels between naïve and primed hESCs (Figure 6B). The results showed that the expression of LincU was higher in naïve-state hESCs than that in primed-state hESCs, which is similar to the expression patterns of reported human naïve-state-specific genes (Gafni et al., 2013) (Figure 6C). Importantly, DUSP9 protein expression levels were higher and ERK1/2 phosphorylation levels were lower in naïve hESCs than in primed-state hESCs (Figures 6D and S6A), indicating the conserved role of LincU in stabilizing the DUSP9 protein.
Figure 6.
Functional Role of LincU Is Conserved in hESCs
(A) The matched sequence of mouse LincU in the human genome indicates that LincU is conserved in humans.
(B) Morphology of primed hESCs and naïve hESCs. Scale bar, 100 μm.
(C) LincU is highly expressed in naïve hESCs compared with primed hESCs. Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, t test.
(D) Western blot (n = 3) showing higher protein levels of DUSP9 and lower levels of ERK1/2 phosphorylation are detected in naïve hESCs than in primed hESCs.
(E and F) LincU overexpression leads to naïve-state-like hESCs when cultured in NHSM without PD0325901, as shown by qPCR (E) and microscopy (F). Data are shown as the mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, t test. Scale bar, 100 μm.
(G) Western blot (n = 3) showing that overexpressing LincU increases the protein level of DUSP9 and decreases the level of ERK1/2 phosphorylation.
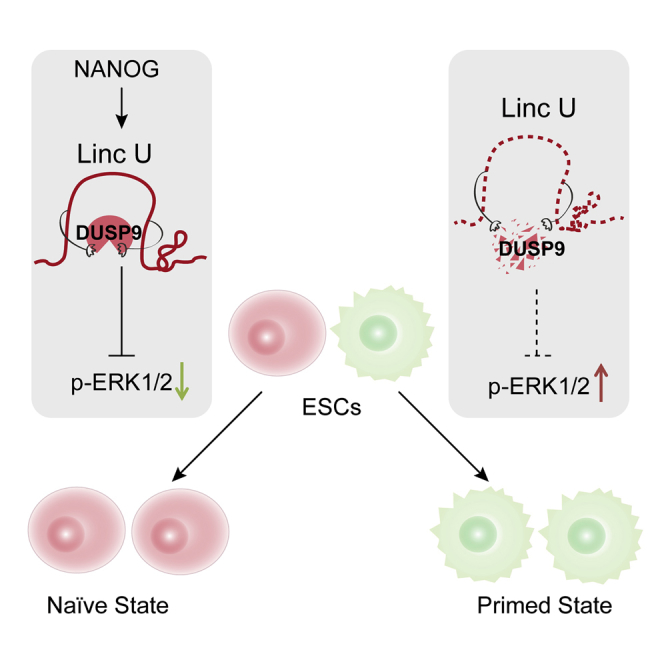
(H) Model showing that Nanog-activated LincU directly binds and stabilizes the ERK-specific phosphatase DUSP9 and then inhibits MAPK/ERK signaling activity to maintain the naïve pluripotent state of ESCs.
Moreover, ectopic expression of human LincU in hESCs successfully induced hESCs to a naïve-like state under NHSM (naïve human stem cell medium) without an MAPK signaling pathway inhibitor, as characterized by a more domed morphology, high expression levels of naïve-state-related genes, and lower expression levels of primed-state-related genes (Figures 6E and 6F). Notably, human LincU overexpression in hESCs also protected DUSP9 from degradation to a greater extent and inhibited the phosphorylation of ERK1/2 (Figures 6G and S6B). Taken together, our findings uncovered an evolutionarily conserved naïve-state-associated lincRNA named LincU that plays a pivotal role in maintaining the naïve state of both mouse and human ESCs. Nanog-induced LincU directly binds and stabilizes DUSP9 protein, which then constitutively inhibits the ERK1/2 signaling pathway, critically contributing to the maintenance of the naïve state (Figure 6H).
Discussion
Until recently the functional roles of lncRNAs, which were once underestimated and regarded as “junk” in the genome, have been revealed in multiple biological processes, including early embryonic development and adult aging (Balakirev and Ayala, 2003, Grote et al., 2013, Lee and Bartolomei, 2013, Ulitsky et al., 2011, Zhang et al., 2012). Although an increasing number of lncRNAs that participate in controlling the pluripotency of mESCs have been discovered (Guttman et al., 2011), identification of functional lncRNAs involved in maintaining the naïve state of mESCs, which are closer to early-stage embryos and have higher pluripotency, is still missing. Here, we found that LincU is specifically and highly expressed in naïve mESCs and that its expression decreases dramatically as mESCs exit the naïve state. Moreover, we verified that LincU is both indispensable and sufficient to maintain the naïve state of mESCs with both in vivo and in vitro assays, similar to those well-defined naïve-state-associated transcription factors, such as Klf2, Klf4, Esrrb, and Nanog. Therefore, our research identified a vital naïve-state-associated lincRNA that is involved in maintaining the naïve state.
Early embryonic development is a complicated and elaborate process that is precisely controlled by extrinsic signaling transduction and intrinsic epigenetic and transcriptional regulation. In addition, extrinsic signaling pathways are consistently acknowledged as the main force underlying early lineage commitment (Kalkan and Smith, 2014). Among those pathways, MAPK/ERK signaling has been identified as a pivotal signaling pathway involved in early embryonic development both in vivo and in vitro, playing roles in gastrulation, transition from the naïve to the primed state, stabilization of primed-state pluripotent stem cells, and early neural lineage commitment (Kunath et al., 2007, Nichols et al., 2009, Nichols and Smith, 2009). However, the intrinsic factor that tactically modulates the activity of MAPK/ERK signaling remains unelucidated. In the present study, we identified LincU as an intrinsic inhibitor of the MAPK/ERK signaling pathway in naïve-state mESCs. Notably, we revealed that LincU stabilizes the DUSP9 protein, a BMP4/SMADs-induced and mESC-specific ERK1/2 phosphatase, by interacting with DUSP9 and preventing its ubiquitination/degradation cascade (Li et al., 2012). Although the details regarding the protective effects of LincU on DUSP9 must be further explored, the present study clearly demonstrates that intrinsic inhibition of the MAPK/ERK signaling pathway by the LincU/DUSP9 axis is indispensable for the maintenance of the naïve state.
When cultured in medium supplemented with LIF and serum, mESCs are a hodgepodge of both naïve and primed mESCs, which are well defined by the expression level of Nanog (Festuccia et al., 2012). Nanog has been reported to be the gateway to naïve pluripotency, and forced Nanog expression is sufficient to drive the cytokine-independent self-renewal of naïve-state mESCs (Chambers et al., 2007). Interestingly, inhibition of the MAPK/ERK signaling pathway leads to effects similar to those of Nanog overexpression. However, the relationship between Nanog and MAPK/ERK signaling in naïve-state mESCs remains to be elucidated. In the present study, we verified a negative correlation between Nanog expression and the activity of MAPK/ERK signaling. In addition, we demonstrated that Nanog directly activates the expression of LincU, which further stabilizes DUSP9 protein expression and results in inhibition of the MAPK/ERK signaling pathway in naïve-state mESCs. Importantly, we found that forced expression of LincU can inhibit the MAPK/ERK signaling pathway and drive the self-renewal of Nanog-deficient mESCs. Collectively, our study clearly revealed that the intrinsic transcription factor Nanog inhibits MAPK/ERK signaling pathway activity by directly inducing the transcription of LincU in naïve-state mESCs.
Species differences in early embryonic development, especially the stage around implantation, cause the results acquired from animal models to be suboptimal for human applications. Most famously, signaling pathways implicated in the maintenance of mESCs involve activation of the LIF/STAT3 and BMP signaling pathways or inhibition of the FGF/ERK and GSK3β signaling pathways, whereas the self-renewal of hESCs requires activation of the bFGF/ERK and activin/nodal signaling pathways (James et al., 2005, Li et al., 2007). Recently, numerous studies have identified a combination of five kinase inhibitors, including an ERK1/2 inhibitor together with LIF and activin A (5i/L/A), that enables the conversion of pre-existing hESCs to the naïve state, indicating the conserved role of MAPK/ERK signaling in the maintenance of naïve-state hESCs (Gafni et al., 2013). Here, we demonstrated that LincU is conserved and more highly expressed in naïve-state hESCs, along with higher protein level of DUSP9 and lower MAPK/ERK signaling activity in naïve-state hESCs compared with those in primed-state hESCs. Notably, we recapitulated the functional interplay between LincU and DUSP9 in hESCs, and the results coincided exactly with those in mice.
In summary, we report an evolutionarily conserved naïve-state-associated lincRNA, LincU, which plays a pivotal role in maintaining the naïve state of both mouse and human ESCs. Nanog-induced LincU directly binds and stabilizes the DUSP9 protein and then constitutively inhibits the ERK1/2 signaling pathway, which critically contributes to maintenance of the naïve state. Collectively, our data shed light on a crucial role of the intrinsic Nanog/LincU/DUSP9/ERK signaling pathway in naïve-state maintenance, which is potentially beneficial for capturing the highest value of naïve-state ESCs in future research and therapeutic applications.
Experimental Procedures
Animal Studies
All experiments involving animals were approved by the Institutional Animal Care and Use Committee of Tongji University under the Guide for the Care and Use of Laboratory Animals (NIH Guide).
Induction of Epiblast-like Cells
A 6-well plate was coated with 0.1% (w/v) gelatin (Gibco) overnight, and mESCs were trypsinized and plated (3 × 105) into each well in N2B27-based medium containing 1% knockout serum replacement medium (Gibco), 12 ng/mL bFGF (Sino Biological), and 20 ng/mL activin A (R&D Systems).
Colony-Formation Assay
Colony-formation assay was performed as previously described (Karwacki-Neisius et al., 2013). In total, 600 cells were plated into each well of a 6-well plate and cultured in mESC medium for 6 days. The colonies were then fixed with 4% paraformaldehyde in PBS for 1 min at room temperature, and AP staining was performed using an Alkaline Phosphatase Kit (Sigma) for 20 min at 37°C. The AP-positive and -negative colony numbers were calculated.
Statistical Analysis
All statistical data are presented as the mean ± SEM of at least three independent experiments. Statistical significance was determined using unpaired two-tailed Student's t tests (in figures ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, #p < 0.05, ##p < 0.01, ##p < 0.001, $p < 0.05, $$p < 0.01, $$$p < 0.001).
Author Contributions
Z.J. and G.L. contributed equally to this work, including study conception and design, performing the experiments, data analysis, and manuscript writing. D.Y., M.B., J.L., D.S., Y.Y., and F.Z. were involved in manuscript preparation. Y.D. contributed to the microarray data analysis. W.J., W.C., X.G., G.W., and X.W. contributed to the study design and supervised the project. J.K. designed and supervised the project and provided financial support and manuscript writing.
Acknowledgments
This work was supported by grants obtained from the Ministry of Science and Technology (grant number 2016YFA0101300), the National Natural Science Foundation of China (grant numbers 81530042, 31721003, 31571529, 31571519, 31471250, 31701110, 31571390, 31771506, 81600675, and 31671533), Ministry of Education grant IRT_15R51, Science and Technology Commission of Shanghai Municipality (grant number 15JC1403201), the Fundamental Research Funds for the Central Universities (1500219106, 20002310002, 1515219039, and 1515219040), and Shanghai Municipal Medical and Health Discipline Construction projects (grant number 2017ZZ02015).
Published: July 12, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and one table and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.06.010.
Accession Numbers
The NCBI GEO accession number for the microarray data reported in this paper is GEO: GSE107418.
Supplemental Information
References
- Balakirev E.S., Ayala F.J. Pseudogenes: are they “junk” or functional DNA? Annu. Rev. Genet. 2003;37:123–151. doi: 10.1146/annurev.genet.37.040103.103949. [DOI] [PubMed] [Google Scholar]
- Batista P.J., Chang H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bononi A., Agnoletto C., De Marchi E., Marchi S., Patergnani S., Bonora M., Giorgi C., Missiroli S., Poletti F., Rimessi A. Protein kinases and phosphatases in the control of cell fate. Enzyme Res. 2011;2011:329098. doi: 10.4061/2011/329098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgel J., Guibert S., Li Y., Chiba H., Schubeler D., Sasaki H., Forne T., Weber M. Targets and dynamics of promoter DNA methylation during early mouse development. Nat. Genet. 2010;42:1093–1100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M.H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brons I.G., Smithers L.E., Trotter M.W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- Dinger M.E., Amaral P.P., Mercer T.R., Pang K.C., Bruce S.J., Gardiner B.B., Askarian-Amiri M.E., Ru K., Solda G., Simons C. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008;18:1433–1445. doi: 10.1101/gr.078378.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S.J., Martello G., Yordanov B., Emmott S., Smith A.G. Defining an essential transcription factor program for naive pluripotency. Science. 2014;344:1156–1160. doi: 10.1126/science.1248882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fatica A., Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- Festuccia N., Osorno R., Halbritter F., Karwacki-Neisius V., Navarro P., Colby D., Wong F., Yates A., Tomlinson S.R., Chambers I. Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell. 2012;11:477–490. doi: 10.1016/j.stem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- Greber B., Wu G., Bernemann C., Joo J.Y., Han D.W., Ko K., Tapia N., Sabour D., Sterneckert J., Tesar P. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010;6:215–226. doi: 10.1016/j.stem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Grote P., Wittler L., Hendrix D., Koch F., Wahrisch S., Beisaw A., Macura K., Blass G., Kellis M., Werber M. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Donaghey J., Carey B.W., Garber M., Grenier J.K., Munson G., Young G., Lucas A.B., Ach R., Bruhn L. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J.A., Surani M.A. Regulatory principles of pluripotency: from the ground state up. Cell Stem Cell. 2014;15:416–430. doi: 10.1016/j.stem.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Ohta H., Kurimoto K., Aramaki S., Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–532. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- James D., Levine A.J., Besser D., Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- Kalkan T., Smith A. Mapping the route from naive pluripotency to lineage specification. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwacki-Neisius V., Goke J., Osorno R., Halbritter F., Ng J.H., Weisse A.Y., Wong F.C., Gagliardi A., Mullin N.P., Festuccia N. Reduced Oct4 expression directs a robust pluripotent state with distinct signaling activity and increased enhancer occupancy by Oct4 and Nanog. Cell Stem Cell. 2013;12:531–545. doi: 10.1016/j.stem.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath T., Saba-El-Leil M.K., Almousailleakh M., Wray J., Meloche S., Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- Lee J.T., Bartolomei M.S. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Leitch H.G., Smith A. The mammalian germline as a pluripotency cycle. Development. 2013;140:2495–2501. doi: 10.1242/dev.091603. [DOI] [PubMed] [Google Scholar]
- Li G., Jiapaer Z., Weng R., Hui Y., Jia W., Xi J., Wang G., Zhu S., Zhang X., Feng D. Dysregulation of the SIRT1/OCT6 Axis contributes to environmental stress-induced neural induction defects. Stem Cell Reports. 2017;8:1270–1286. doi: 10.1016/j.stemcr.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang G., Wang C., Zhao Y., Zhang H., Tan Z., Song Z., Ding M., Deng H. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- Li Z., Fei T., Zhang J., Zhu G., Wang L., Lu D., Chi X., Teng Y., Hou N., Yang X. BMP4 signaling acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell. 2012;10:171–182. doi: 10.1016/j.stem.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Marks H., Kalkan T., Menafra R., Denissov S., Jones K., Hofemeister H., Nichols J., Kranz A., Stewart A.F., Smith A. The transcriptional and epigenomic foundations of ground state pluripotency. Cell. 2012;149:590–604. doi: 10.1016/j.cell.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M., Maeda M., Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Murakami K., Gunesdogan U., Zylicz J.J., Tang W.W.C., Sengupta R., Kobayashi T., Kim S., Butler R., Dietmann S., Surani M.A. NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature. 2016;529:403–407. doi: 10.1038/nature16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T., Mitchell J.A., Sanz L.A., Pauler F.M., Ferguson-Smith A.C., Feil R., Fraser P. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–1720. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- Nichols J., Chambers I., Taga T., Smith A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development. 2001;128:2333–2339. doi: 10.1242/dev.128.12.2333. [DOI] [PubMed] [Google Scholar]
- Nichols J., Silva J., Roode M., Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Niwa H., Ogawa K., Shimosato D., Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Saito A., Matsui H., Suzuki H., Ohtsuka S., Shimosato D., Morishita Y., Watabe T., Niwa H., Miyazono K. Activin-Nodal signaling is involved in propagation of mouse embryonic stem cells. J. Cell Sci. 2007;120:55–65. doi: 10.1242/jcs.03296. [DOI] [PubMed] [Google Scholar]
- Pandey R.R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., Nagano T., Mancini-Dinardo D., Kanduri C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol. Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Sanchez-Castillo M., Ruau D., Wilkinson A.C., Ng F.S., Hannah R., Diamanti E., Lombard P., Wilson N.K., Gottgens B. CODEX: a next-generation sequencing experiment database for the haematopoietic and embryonic stem cell communities. Nucleic Acids Res. 2015;43:D1117–D1123. doi: 10.1093/nar/gku895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart H.T., van Oosten A.L., Radzisheuskaya A., Martello G., Miller A., Dietmann S., Nichols J., Silva J.C. NANOG amplifies STAT3 activation and they synergistically induce the naive pluripotent program. Curr. Biol. 2014;24:340–346. doi: 10.1016/j.cub.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniue K., Kurimoto A., Sugimasa H., Nasu E., Takeda Y., Iwasaki K., Nagashima T., Okada-Hatakeyama M., Oyama M., Kozuka-Hata H. Long noncoding RNA UPAT promotes colon tumorigenesis by inhibiting degradation of UHRF1. Proc. Natl. Acad. Sci. USA. 2016;113:1273–1278. doi: 10.1073/pnas.1500992113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Ulitsky I., Shkumatava A., Jan C.H., Sive H., Bartel D.P. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell. 2011;147:1537–1550. doi: 10.1016/j.cell.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Xue Y., Han Y., Lin L., Wu C., Xu S., Jiang Z., Xu J., Liu Q., Cao X. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.H., Abdelmohsen K., Srikantan S., Yang X., Martindale J.L., De S., Huarte M., Zhan M., Becker K.G., Gorospe M. LincRNA-p21 suppresses target mRNA translation. Mol. Cell. 2012;47:648–655. doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Chambers I., Nichols J., Smith A., Saito M., Yasukawa K., Shoyab M., Taga T., Kishimoto T. Maintenance of the pluripotential phenotype of embryonic stem cells through direct activation of gp130 signalling pathways. Mech. Dev. 1994;45:163–171. doi: 10.1016/0925-4773(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Young R.A. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Arun G., Mao Y.S., Lazar Z., Hung G., Bhattacharjee G., Xiao X., Booth C.J., Wu J., Zhang C. The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2:111–123. doi: 10.1016/j.celrep.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Huang X., Tan W., Yu D., Du Z., Chang J., Wei L., Han Y., Wang C., Che X. Pancreatic cancer risk variant in LINC00673 creates a miR-1231 binding site and interferes with PTPN11 degradation. Nat. Genet. 2016;48:747–757. doi: 10.1038/ng.3568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.