Summary
Proper regulation of the cell cycle is essential to safeguard the genomic integrity of embryonic stem cells (ESCs) while maintaining the fast proliferation rate. The pluripotency factor OCT4 has been shown to inhibit CDK1 activation, thus preventing mitotic entry and facilitating the maintenance of genomic integrity. Yet, how ESCs enter mitosis in the presence of OCT4 remains unclear. We previously reported that COPS2 promotes the progression through the G2/M phase of mouse ESCs. In this study, through co-immunoprecipitation and mass spectrometric analysis, we found that COPS2 interacts with OCT4 and CDK1. We further demonstrated that COPS2 stimulates the activity of CDK1/CYCLIN B only when OCT4 is present. Consistently, COPS2 promotes the G2/M transition only in the presence of OCT4 in HeLa cells. Mechanistically, COPS2 attenuates the interaction between OCT4 and CDK1 by sequestering OCT4 and forming a COPS2/CDK1 complex, thus blocking the inhibitory effect of OCT4 on CDK1 activation.
Keywords: COPS2, OCT4, CDK1, cell cycle, embryonic stem cells
Highlights
-
•
COPS2 is required for the rapid G2/M transition in mouse embryonic stem cells
-
•
COPS2 counteracts the inhibitory effect of OCT4 on CDK1 activation
-
•
COPS2 accelerates the G2/M transition only in the presence of OCT4
-
•
COPS2 competes with OCT4 in binding to CDK1
Chen and colleagues identified a unique role of COPS2 in maintaining the short G2/M phase of embryonic stem cells. By competing with OCT4 in binding to CDK1, COPS2 alleviates the inhibitory effect of OCT4 on CDK1 activation, thus allowing the rapid G2/M transition in embryonic stem cells.
Introduction
Embryonic stem cells (ESCs) can self-renew indefinitely while maintaining the potential to differentiate into all types of cells in the body. Thus, ESCs hold great promise for regenerative medicine and cell replacement therapy. Compared with differentiated cells, ESCs undergo an accelerated cell cycle with shorter G1 and G2 phase (Becker et al., 2006, Burdon et al., 2002, Fluckiger et al., 2006, Orford and Scadden, 2008, Savatier et al., 2002, White and Dalton, 2005). The fast proliferation rate of ESCs allows quick amplification of cells for subsequent clinical applications. Nevertheless, it raises a safety issue as to how ESCs maintain their genomic integrity during rapid proliferation, even in the absence of checkpoint. Moreover, cell-cycle regulation is tightly associated with controlling the maintenance and dissolution of pluripotency. S- and G2-phase-specific pathways restrict pluripotent state dissolution, while G1 phase is more permissive for ESC differentiation (Gonzales et al., 2015). Human ESCs in early and late G1 phase preferentially differentiate into endoderm and neuroectoderm, respectively (Pauklin and Vallier, 2013). Cell-cycle regulation of developmental regulated transcription factors might account for the differential differentiation propensity of human ESCs at various stages of the cell cycle (Singh et al., 2013). Therefore, understanding the unique cell-cycle regulation in ESCs will facilitate not only the culture of high-quality ESCs that are safe for clinical applications, but also directed differentiation of ESCs.
The molecular mechanism underlying the shortened G1 phase in ESCs has been extensively studied. CYCLIN A/E and CDK2 activity, the primary driving force for the G1 to S progression, is not restricted to the late G1 phase in ESCs. Rather, it remains constantly active throughout the ESC cell cycle (Stead et al., 2002). A high level of deubiquitylase DUB3 in ESCs may stabilize CDC25A, which in turn activates CDK2 and renders CDK2 constitutively active (van der Laan et al., 2013). Interestingly, ESCs lacking all G1 cyclins (D-type and E-type) are able to proliferate at a modest reduced rate and with a prolonged G1 phase (Liu et al., 2017). Whether the extended CYCLIN A-CDK2 activity compensates the loss of G1 cyclins and allows ESC proliferation remains to be tested. In addition, the retinoblastoma protein (RB) is hyperphosphorylated, and thus inactive, throughout the ESC cell cycle (Savatier et al., 1994). Consistently, knockout of all the three RB-related genes, Rb, p107, and p130, does not affect the proliferation of mouse ESCs (Sage et al., 2000). Phosphorylated RB and extended CYCLIN E/A-CDK2 activity mutually promote each other and maintain the shortened G1 phase in ESCs (Malumbres and Barbacid, 2009, White et al., 2005). Moreover, highly expressed EMI1 in ESCs suppresses anaphase-promoting complex/cyclosome (APC/C) mediated degradation of the DNA replication factor CDT1. Elevated level of CDT1 protein licenses DNA for replication and ensures fast entry into the S phase (Ballabeni et al., 2011). Some ESC-specific microRNAs, members of the miR-290 family, also contribute to the shortened G1 phase by suppressing negative regulators of G1/S transition, such as CDKN1A, RBL2, and LATS2 (Wang et al., 2008).
How ESCs progress rapidly through the G2/M phase remains largely unexplored. It has been shown that the transcription factor B-MYB is required for the rapid G2/M transition in mouse ESCs, likely through regulating the transcription of multiple cell-cycle-related genes (Tarasov et al., 2008, Zhan et al., 2012). In addition, RAD51, a recombinase critical for homologous recombination, contributes to G2/M transition in mouse ESCs (Yoon et al., 2014). However, the mechanism for RAD51 to regulate the G2/M transition is unclear. By contrast, a key pluripotency factor OCT4 interacts with CDK1 to inhibit the activation of CDK1, consequently slowing down the G2/M progression and maintaining genomic integrity (Zhao et al., 2014). As OCT4 is abundantly expressed in ESCs and plays an essential role in pluripotency maintenance (Nichols et al., 1998), ESCs must have an unknown mechanism to antagonize the cell-cycle regulatory function of OCT4 and maintain the shortened G2/M phase.
The COP9 signalosome (CSN) is a highly conserved complex from yeast to human. It is composed of eight subunits, COPS1 to COPS8 (Chamovitz, 2009, Kato and Yoneda-Kato, 2009, Wei et al., 2008). The major function of the CSN is to regulate protein degradation by suppressing the activities of the cullin-RING-E3 ligases through deneddylation of cullins (Lyapina et al., 2001, Yang et al., 2002). Other functions of the CSN, including transcriptional regulation, protein phosphorylation, and subcellular distribution, have been reported (Bech-Otschir et al., 2001, Claret et al., 1996, Seeger et al., 1998, Tomoda et al., 2002, von Arnim and Deng, 1994). We previously showed that knockdown of COPS2, but not any other subunits of the CSN, compromises the self-renewal and pluripotency of mouse ESCs. Notably, downregulation of COPS2 also leads to G2/M arrest of ESCs (Zhang et al., 2016).
In this study, we aimed to elucidate the molecular mechanism for COPS2 to accelerate the G2/M progression of mouse ESCs. Through co-immunoprecipitation (coIP) and mass spectrometric analysis, CDK1 and OCT4 were identified as COPS2-interacting proteins. We further demonstrated that COPS2 counteracts the suppression effect of OCT4 on CDK1, and promotes the G2/M transition only in the presence of OCT4. In summary, we revealed a mechanism for mouse ESCs to maintain a short G2 phase in the presence of OCT4.
Results
Knockdown of COPS2 Increases the Fraction of ESCs in the G2 and M Phases
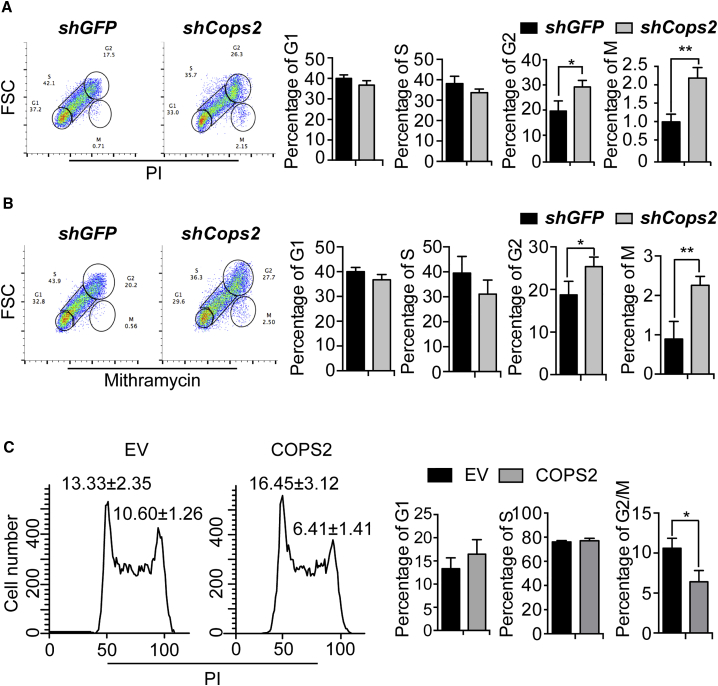
We previously showed that knockdown of COPS2 results in G2/M arrest of ESCs (Zhang et al., 2016). However, it is unclear whether COPS2 knockdown ESCs are arrested at the G2 or M phase. To discriminate cells in the M phase from G1, S, and G2 cells, we performed cell-cycle analysis of nuclei suspensions prepared by nonionic detergent, fixed by formaldehyde, and stained with propidium iodide (PI) or mithramycin. With this special preparation of cells, mitotic nuclei have increased fluorescence of the DNA-fluorochromes and reduced light-scattering signal compared with those of G2 nuclei (Larsen et al., 1986). The results revealed that the fraction of G2 cells is significantly enhanced after COPS2 knockdown, while the number of mitotic cells is also slightly increased (Figures 1A and 1B). Conversely, overexpression of COPS2 reduces the fraction of G2/M ESCs (Figure 1C), indicating accelerated G2/M transition.
Figure 1.
Knockdown of COPS2 Increases the Fraction of G2 and M ESCs
(A) Mouse ESCs were transfected with short hairpin RNA plasmids targeting GFP or Cops2. Seventy-two hours after transfection, cells were permeabilized by nonionic detergent, fixed by formaldehyde, and stained with propidium iodide (PI). Quantification of the percentage of G1, S, G2, and M phase from three independent experiments was plotted. Data are shown as mean ± SD of three independent replicates.
(B) Experiments were performed as described in (A), except that cells were stained by mithramycin.
(C) Mouse ESCs were transfected with empty vector or COPS2 overexpression plasmid. Forty-eight hours after transfection, the cells were stained with PI and analyzed by flow cytometry. Quantification of the percentage of G1, S, and G2/M phase from three independent experiments was plotted.
Data are shown as mean ± SD of three independent replicates. ∗∗p < 0.01, ∗p < 0.05.
COPS2 Interacts with OCT4 and CDK1
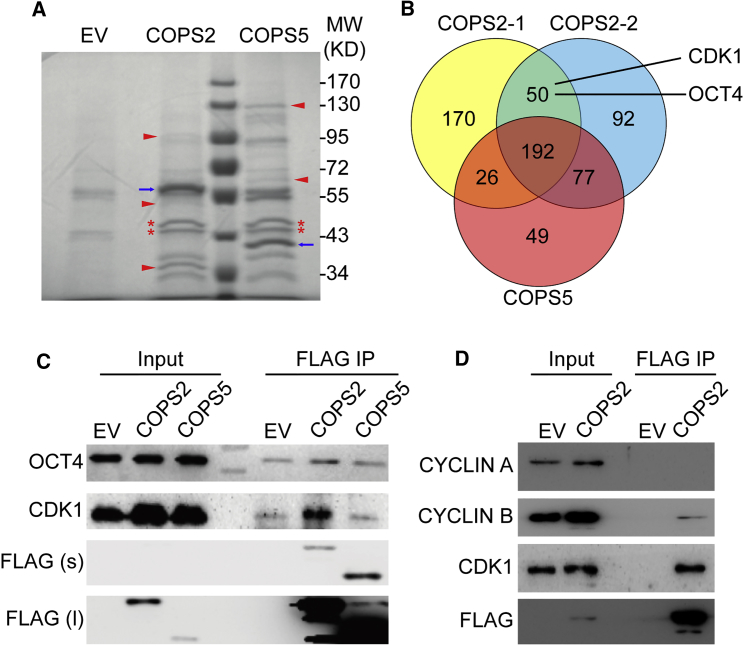
To understand the molecular mechanism for COPS2 to regulate cell-cycle progression, our strategy was to identify COPS2-interacting proteins. Given that knockdown of COPS2, but not COPS5 or COPS8, leads to G2/M arrest of ESCs (Zhang et al., 2016), we believed that the cycle regulatory function of COPS2 is independent of the CSN. Thus, we aimed to identify proteins that specifically interact with COPS2 but not with the CSN. CoIP was performed in ESCs overexpressing FLAG-tagged COPS2 or COPS5. The coIP samples were resolved in an SDS-PAGE gel, demonstrating the distinct pattern of COPS2- or COPS5-interacting proteins, as well as some overlapping bands in these two coIP samples (Figure 2A). Two COPS2 coIP samples and one COPS5 coIP sample were run in SDS-PAGE gel briefly, and the unresolved gel slices were subjected to mass spectrometric analysis to identify COPS2- and COPS5-interacting proteins (Table S1). Fifty proteins were identified in two COPS2 coIP samples, but not in the COPS5 coIP sample (Figure 2B and Table S2). Among these 50 COPS2 specifically interacting proteins, OCT4 and CDK1 came to our immediate attention, because activation of CDK1 is essential for the G2/M transition (Malumbres and Barbacid, 2009) and OCT4 binds to CDK1, blocking the activation of CDK1 by CYCLIN B (Zhao et al., 2014). We then validated the interactions of COPS2 with OCT4 and CDK1 by coIP and western blot. The results demonstrated that COPS2, but not COPS5, interacts with OCT4 and CDK1 (Figure 2C). In addition, COPS2 is associated with CYCLIN B, but not CYCLIN A (Figure 2D), while both CYCLIN A and B are present in OCT4 immunoprecipitations (Zhao et al., 2014). Based on these data, we hypothesized that COPS2 modulates the interaction between OCT4 and CDK1/CYCLIN B to promote the G2/M transition of ESCs.
Figure 2.
COPS2 Interacts with CDK1 and OCT4
(A) Colloidal blue-stained gel of coIP samples. Cell extracts of ESCs overexpressing empty vector (EV), COPS2-FLAG, or COPS5-FLAG were subjected to coIP using anti-FLAG M2 beads. CoIP samples were resolved in an SDS-PAGE gel followed by colloidal blue staining. Blue arrows mark the bands of COPS2-FLAG and COPS5-FLAG. Red triangles and asterisks highlight distinct and identical bands present in COPS2-FLAG and COPS5-FLAG coIP samples, respectively.
(B) Venn diagram of COPS2 and COPS5 interacting proteins identified by coIP and mass spectrometric analysis. COPS2-1 and COPS2-2 are two lists of COPS2-interacting proteins identified in two independent experiments. Detailed information about these proteins is provided in Tables S1 and S2.
(C) CoIP experiments to validate the binding of COPS2 to CDK1 and OCT4. CoIP experiments were performed as described in (A). The resulting coIP samples were subjected to western blot.
(D) CoIP experiments to detect the binding of COPS2 to Cyclins. CoIP experiments were performed as described in (A). The resulting coIP samples were subjected to western blot.
COPS2 Counteracts the Inhibitory Effect of OCT4 on CDK1 Activation
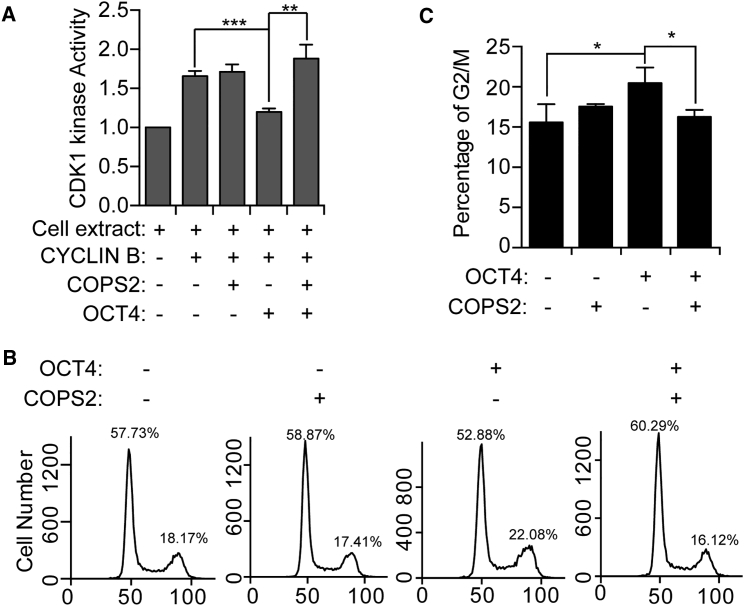
Next, we examined whether and how COPS2 modulates CDK1 kinase activity. CDK1 kinase activity in HeLa cell extract was measured. Consistent with previous reports (Zhao et al., 2014), CYCLIN B activates CDK1, which is blocked by pre-incubation of OCT4 with HeLa cell extract (Figure 3A). In contrast, COPS2 alone does not affect CDK1 activity. However, when OCT4 recombinant protein was present in the kinase reaction, COPS2 increased CDK1 activity (Figure 3A).
Figure 3.
COPS2 Antagonizes with OCT4 to Promote the G2/M Transition
(A) In vitro CDK1 kinase assays using HeLa cell extract, with or without 300 nM CYCLIN B, 1,800 nM OCT4, and/or 1,800 nM COPS2. Data are shown as mean ± SD of three independent replicates.
(B) HeLa cells were transfected with plasmids overexpressing OCT4 or/and COPS2. Forty-eight hours after transfection, the cells were stained with PI and analyzed by flow cytometry.
(C) Quantification of the percentage of G2/M cells from three independent experiments. Data are shown as mean ± SD of three independent replicates.
∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
In vitro kinase assays demonstrated that COPS2 modulates CDK1 activity only when OCT4 protein is present. We then addressed whether COPS2 regulates cell-cycle progression only in the presence of OCT4 protein. Overexpression of COPS2 alone in HeLa cells does not change the cell-cycle distribution, while OCT4 overexpression increases the percentage of G2/M cells. Notably, simultaneous expression of COPS2 and OCT4 counteracts the effect of OCT4, and the fraction of G2/M cells is reduced to the same level of control HeLa cells (Figures 3B and 3C). These results, corroborating the results of in vitro CDK1 kinase assays, suggested that COPS2 antagonizes the suppression effect of OCT4 on CDK1 activity to promote G2/M transition.
COPS2 Competes with OCT4 in Binding to CDK1
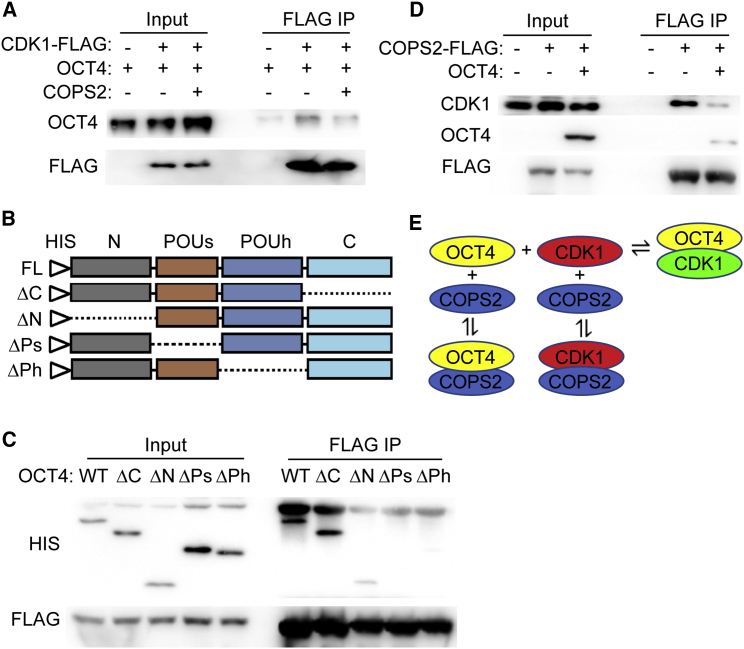
We detected the binding of COPS2 to OCT4 and CDK1, and demonstrated that COPS2 facilitates the activation of CDK1 only in the presence of OCT4. How does COPS2 regulate the interaction between OCT4 and CDK1 to modulate the activity of CDK1? We first asked whether COPS2 enhances or suppresses the interaction between OCT4 and CDK1. CoIP experiments showed that overexpression of COPS2 attenuates the binding between OCT4 and CDK1 (Figures 4A and S1A). Conversely, knockdown of COPS2 in G2/M enriched mouse ESCs enhances the interaction between OCT4 and CDK1 (Figure S1C). We then dissected the region of OCT4 mediating the interaction with COPS2. CoIP assays, using a series of OCT4 truncation mutants, revealed that both POUs (POU-specific) and POUh (POU-homeo) domains are required for COPS2 binding (Figures 4B and 4C). The POUh domain is also essential for OCT4 binding to CDK1 (Zhao et al., 2014). These data imply that COPS2 may occupy the POUh domain of OCT4 and prevent CDK1 from binding to OCT4.
Figure 4.
COPS2 Attenuates the Interaction between OCT4 and CDK1
(A) CoIP experiment to detect the interaction between OCT4 and CDK1 in the presence and absence of COPS2. HEK293T cells were transfected with plasmids expressing OCT4 and CDK1-FLAG, with or without COPS2-expressing plasmid. Forty-eight hours after transfection, cells were harvested for coIP experiments. See also Figure S1.
(B) Schematic illustration of OCT4 truncation mutants.
(C) CoIP experiment to map the domain of OCT4 required for the binding of COPS2. HeLa cells were transfected with plasmid expressing COPS2-FLAG, as well as plasmids expressing His- OCT4 and its truncation mutant. Forty-eight hours after transfection, cells were harvested for coIP experiments.
(D) CoIP experiment to detect the interaction between COPS2 and CDK1 in the presence and absence of OCT4. HEK293T cells were transfected with plasmid expressing COPS2-FLAG, with or without OCT4-expressing plasmid. Forty-eight hours after transfection, cells were harvested for coIP experiments.
(E) Schematic diagram of the interactions among CDK1, COPS2, and OCT4. Red color marks CDK1 activatable by CYCLIN B, and green color indicates a resistant status for CDK1 activation by CYCLIN B.
Notably, COPS2 does not affect CDK1 activity in the in vitro kinase assay, when OCT4 is absent (Figure 3A). It is possible that the binding of COPS2 to CDK1 is dependent on OCT4. Thus, without OCT4, COPS2 does not bind to CDK1. Alternatively, OCT4 is dispensable for the interaction of COPS2 and CDK1, but COPS2 binding has no effect on CDK1 activity. To distinguish these two possibilities, we performed coIP experiments in HEK293T cells in which OCT4 protein is undetectable. The result showed that COPS2 binds to CDK1 in the absence of OCT4 (Figure 4D), supporting the latter possibility. Moreover, OCT4 overexpression attenuates the interaction between COPS2 and CDK1 (Figure 4D), further demonstrating the competition between OCT4 and COPS2 in binding to CDK1.
Discussion
Short G1 and G2 phases allow fast proliferation of ESCs, but compromise cell-cycle checkpoints. How do ESCs maintain genomic integrity while they proliferate rapidly? It has been shown that the pluripotency factor OCT4 blocks CDK1 activation, and hence prevents premature mitotic entry (Zhao et al., 2014). Although it helps the maintenance of genomic integrity, the cell-cycle regulatory function of OCT4 is apparently unfavorable for rapid cell-cycle progression, specifically the G2/M transition. This raises another question: how do ESCs overcome the inhibitory effect of OCT4 to enter mitosis? In this study, we found that COPS2, which promotes the G2/M transition of ESCs, binds to both OCT4 and CDK1 and prevents the formation of OCT4/CDK1 complex (Figure 4E). Therefore, COPS2 may serve as a switch to turn off the inhibitory effect of OCT4 and allow mitotic entry of ESCs. Importantly, the binding between COPS2 and CDK1 does not interfere with CDK1 activity (Figure 3A). Thus, in the absence of OCT4, COPS2 does not accelerate G2/M transition.
It has been demonstrated that the CSN complex is involved in cell-cycle regulation. Knockdown of individual subunits of the CSN complex in Drosophila cells increases the G1 fraction (Kondo and Perrimon, 2011). The cell-cycle regulatory function of the CSN might be achieved through regulating the degradation of cell-cycle regulators, such as Rb, p27, and APC/C (Kob et al., 2009, Tomoda et al., 2002, Ullah et al., 2007, Yang et al., 2002). However, the G2/M-promoting function of COPS2 in ESCs seems to be independent of the whole CSN complex. First, knockdown of other subunits of the CSN, COPS5 and COPS8, do not lead to G2/M arrest in ESCs (Zhang et al., 2016). Second, overexpression of COPS2 counteracts the G2/M arrest effect of OCT4 in HeLa cells (Figure 2B). Under this condition, overexpression of COPS2 alone unlikely elevates the expression level of the CSN complex. Most importantly, in the in vitro CDK1 kinase assay, purified recombinant COPS2 protein antagonizes the inhibitory effect of OCT4 on CDK1 activity (Figure 2A), demonstrating the CSN-independent function of COPS2.
Whether the binding of COPS2 to OCT4 is constitutive throughout the whole cell cycle or is transiently activated at late G2 phase remains unclear. OCT4, as a transcription factor, is restricted to the nucleus and remains constant throughout the cell cycle to maintain the undifferentiated status of ESCs. In contrast, the majority of COPS2 is distributed in the cytoplasm of ESCs, while only a small fraction of COPS2 is present in the nucleus of ESCs. In the nucleus, a small amount of COPS2 is likely insufficient to counteract the inhibitory effect by relatively abundant OCT4 protein on CDK1. In addition, to allow OCT4 to exert its cell-cycle regulatory function, COPS2 should not bind to OCT4 during interphase and early G2 phase. CYCLIN B, but not CYCLIN A, is associated with the COPS2/CDK1 complex, implying that COPS2 mainly facilitates the activation of CDK1/CYCLIN B at the late G2 phase. Thus, it is more likely that the interaction between COPS2 and OCT4 is triggered at late G2 rather than being constitutive throughout the whole cell cycle. One possible mechanism to regulate the binding of COPS2 and OCT4 is oscillatory expression of COPS2, like cyclins. Alternatively, controlling the cytoplasmic and nuclear shuffling of COPS2 protein may contribute to cell-cycle stage-specific interaction. How the interaction between COPS2 and OCT4 is regulated at various cell-cycle stages of ESCs needs further investigation.
Experimental Procedures
Cell-Cycle Analysis
To distinguish the G2 and M phase, we performed cell-cycle analysis as described elsewhere (Larsen et al., 1986). In brief, 2 × 106 ESCs were harvested and resuspended in 2 mL of pre-cooled detergent buffer (NP-40 0.1%, 0.5 mM EDTA [pH 7.2], dissolved in PBS). After incubation on an ice bath for 5 min, 0.7 mL of pre-cooled 4% paraformaldehyde was added and mixed well, then kept slowly rotating on a rotating mixer for 15 min. The suspension was diluted with 10 mL of detergent buffer and centrifuged. The cell pellet was resuspended in detergent buffer supplemented with 5 μg/mL PI or 20 μg/mL mithramycin, as well as 1 mg/mL RNase A. Cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences).
Samples for conventional cell-cycle analysis were prepared as follows. Cells were harvested by trypsinization and washed once in PBS. Cells were fixed in ice-cold 70% ethanol at 4°C overnight. Following RNase A treatment, total DNA was stained with PI (Sigma). Cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences).
Cell Extract Preparation
HeLa cell extract was prepared as described previously (Deibler and Kirschner, 2010). In brief, cells were resuspended in buffer A (20 mM HEPES [pH 7.7], 5 mM KCl, 1.5 mM MgCl2, and 2 mM DTT) and incubated on ice for 30 min. The cells were then disrupted by 7 strokes of a loose-fitting Dounce homogenizer. Next, nuclei were disrupted in buffer B (50 mM HEPES [pH 7.7], 10 mM MgCl2, 2 mM DTT, 25% sucrose, and 50% glycerol). While stirring on ice, (NH4)2SO4 was added to a final concentration (w/v) of 10%. After centrifugation, the supernatant was dialyzed against extract dialysis buffer (50 mM HEPES [pH 7.7], 75 mM K-glutamate, 4 mM MgCl2, 0.2 mM EDTA, and 2 mM DTT). The extract (5 mg/mL) was frozen in liquid nitrogen and stored at −80°C until use.
Statistical Analysis
All data were analyzed by Student's t test. Statistically significant p values are indicated in figures as ∗∗∗p < 0.001, ∗∗p < 0.01, and ∗p < 0.05.
Additional experimental procedures are provided in Supplemental Information.
Author Contributions
P.L., N.D., and W.Z. performed experiments. P.L. and L.C. analyzed the data. L.C. conceived the study and wrote the manuscript.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (grant no. 2018YFA0107002), the National Natural Science Foundation of China (grant nos. 31622038 and 31671497), the 111 Project Grant (B08011), and the Fundamental Research Funds for the Central Universities. We thank Dr. Zhao Rui for providing the plasmids expressing OCT4 truncation mutants.
Published: July 19, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, one figure, and two tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.06.013.
Supplemental Information
References
- Ballabeni A., Park I.H., Zhao R., Wang W., Lerou P.H., Daley G.Q., Kirschner M.W. Cell cycle adaptations of embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2011;108:19252–19257. doi: 10.1073/pnas.1116794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Otschir D., Kraft R., Huang X., Henklein P., Kapelari B., Pollmann C., Dubiel W. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 2001;20:1630–1639. doi: 10.1093/emboj/20.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K.A., Ghule P.N., Therrien J.A., Lian J.B., Stein J.L., van Wijnen A.J., Stein G.S. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J. Cell. Physiol. 2006;209:883–893. doi: 10.1002/jcp.20776. [DOI] [PubMed] [Google Scholar]
- Burdon T., Smith A., Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- Chamovitz D.A. Revisiting the COP9 signalosome as a transcriptional regulator. EMBO Rep. 2009;10:352–358. doi: 10.1038/embor.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret F.X., Hibi M., Dhut S., Toda T., Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453–457. doi: 10.1038/383453a0. [DOI] [PubMed] [Google Scholar]
- Deibler R.W., Kirschner M.W. Quantitative reconstitution of mitotic CDK1 activation in somatic cell extracts. Mol. Cell. 2010;37:753–767. doi: 10.1016/j.molcel.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckiger A.C., Marcy G., Marchand M., Negre D., Cosset F.L., Mitalipov S., Wolf D., Savatier P., Dehay C. Cell cycle features of primate embryonic stem cells. Stem Cells. 2006;24:547–556. doi: 10.1634/stemcells.2005-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales K.A., Liang H., Lim Y.S., Chan Y.S., Yeo J.C., Tan C.P., Gao B., Le B., Tan Z.Y., Low K.Y. Deterministic restriction on pluripotent state dissolution by cell-cycle pathways. Cell. 2015;162:564–579. doi: 10.1016/j.cell.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Kato J.Y., Yoneda-Kato N. Mammalian COP9 signalosome. Genes Cells. 2009;14:1209–1225. doi: 10.1111/j.1365-2443.2009.01349.x. [DOI] [PubMed] [Google Scholar]
- Kob R., Kelm J., Posorski N., Baniahmad A., von Eggeling F., Melle C. Regulation of the anaphase-promoting complex by the COP9 signalosome. Cell Cycle. 2009;8:2041–2049. doi: 10.4161/cc.8.13.8850. [DOI] [PubMed] [Google Scholar]
- Kondo S., Perrimon N. A genome-wide RNAi screen identifies core components of the G(2)-M DNA damage checkpoint. Sci. Signal. 2011;4:rs1. doi: 10.1126/scisignal.2001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J.K., Munch-Petersen B., Christiansen J., Jorgensen K. Flow cytometric discrimination of mitotic cells: resolution of M, as well as G1, S, and G2 phase nuclei with mithramycin, propidium iodide, and ethidium bromide after fixation with formaldehyde. Cytometry. 1986;7:54–63. doi: 10.1002/cyto.990070108. [DOI] [PubMed] [Google Scholar]
- Liu L., Michowski W., Inuzuka H., Shimizu K., Nihira N.T., Chick J.M., Li N., Geng Y., Meng A.Y., Ordureau A. G1 cyclins link proliferation, pluripotency and differentiation of embryonic stem cells. Nat. Cell Biol. 2017;19:177–188. doi: 10.1038/ncb3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyapina S., Cope G., Shevchenko A., Serino G., Tsuge T., Zhou C., Wolf D.A., Wei N., Deshaies R.J. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science. 2001;292:1382–1385. doi: 10.1126/science.1059780. [DOI] [PubMed] [Google Scholar]
- Malumbres M., Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Scholer H., Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Orford K.W., Scadden D.T. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Pauklin S., Vallier L. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013;155:135–147. doi: 10.1016/j.cell.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage J., Mulligan G.J., Attardi L.D., Miller A., Chen S., Williams B., Theodorou E., Jacks T. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 2000;14:3037–3050. doi: 10.1101/gad.843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatier P., Huang S., Szekely L., Wiman K.G., Samarut J. Contrasting patterns of retinoblastoma protein expression in mouse embryonic stem cells and embryonic fibroblasts. Oncogene. 1994;9:809–818. [PubMed] [Google Scholar]
- Savatier P., Lapillonne H., Jirmanova L., Vitelli L., Samarut J. Analysis of the cell cycle in mouse embryonic stem cells. Methods Mol. Biol. 2002;185:27–33. doi: 10.1385/1-59259-241-4:27. [DOI] [PubMed] [Google Scholar]
- Seeger M., Kraft R., Ferrell K., Bech-Otschir D., Dumdey R., Schade R., Gordon C., Naumann M., Dubiel W. A novel protein complex involved in signal transduction possessing similarities to 26S proteasome subunits. FASEB J. 1998;12:469–478. [PubMed] [Google Scholar]
- Singh A.M., Chappell J., Trost R., Lin L., Wang T., Tang J., Matlock B.K., Weller K.P., Wu H., Zhao S. Cell-cycle control of developmentally regulated transcription factors accounts for heterogeneity in human pluripotent cells. Stem Cell Reports. 2013;1:532–544. doi: 10.1016/j.stemcr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead E., White J., Faast R., Conn S., Goldstone S., Rathjen J., Dhingra U., Rathjen P., Walker D., Dalton S. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 2002;21:8320–8333. doi: 10.1038/sj.onc.1206015. [DOI] [PubMed] [Google Scholar]
- Tarasov K.V., Tarasova Y.S., Tam W.L., Riordon D.R., Elliott S.T., Kania G., Li J., Yamanaka S., Crider D.G., Testa G. B-MYB is essential for normal cell cycle progression and chromosomal stability of embryonic stem cells. PLoS One. 2008;3:e2478. doi: 10.1371/journal.pone.0002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda K., Kubota Y., Arata Y., Mori S., Maeda M., Tanaka T., Yoshida M., Yoneda-Kato N., Kato J.Y. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J. Biol. Chem. 2002;277:2302–2310. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- Ullah Z., Buckley M.S., Arnosti D.N., Henry R.W. Retinoblastoma protein regulation by the COP9 signalosome. Mol. Biol. Cell. 2007;18:1179–1186. doi: 10.1091/mbc.E06-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan S., Tsanov N., Crozet C., Maiorano D. High Dub3 expression in mouse ESCs couples the G1/S checkpoint to pluripotency. Mol. Cell. 2013;52:366–379. doi: 10.1016/j.molcel.2013.10.003. [DOI] [PubMed] [Google Scholar]
- von Arnim A.G., Deng X.W. Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell. 1994;79:1035–1045. doi: 10.1016/0092-8674(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Wang Y., Baskerville S., Shenoy A., Babiarz J.E., Baehner L., Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat. Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N., Serino G., Deng X.W. The COP9 signalosome: more than a protease. Trends Biochem. Sci. 2008;33:592–600. doi: 10.1016/j.tibs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- White J., Dalton S. Cell cycle control of embryonic stem cells. Stem Cell Rev. 2005;1:131–138. doi: 10.1385/SCR:1:2:131. [DOI] [PubMed] [Google Scholar]
- White J., Stead E., Faast R., Conn S., Cartwright P., Dalton S. Developmental activation of the Rb-E2F pathway and establishment of cell cycle-regulated cyclin-dependent kinase activity during embryonic stem cell differentiation. Mol. Biol. Cell. 2005;16:2018–2027. doi: 10.1091/mbc.E04-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Menon S., Lykke-Andersen K., Tsuge T., Di X., Wang X., Rodriguez-Suarez R.J., Zhang H., Wei N. The COP9 signalosome inhibits p27(kip1) degradation and impedes G1-S phase progression via deneddylation of SCF Cul1. Curr. Biol. 2002;12:667–672. doi: 10.1016/s0960-9822(02)00791-1. [DOI] [PubMed] [Google Scholar]
- Yoon S.W., Kim D.K., Kim K.P., Park K.S. Rad51 regulates cell cycle progression by preserving G2/M transition in mouse embryonic stem cells. Stem Cells Dev. 2014;23:2700–2711. doi: 10.1089/scd.2014.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan M., Riordon D.R., Yan B., Tarasova Y.S., Bruweleit S., Tarasov K.V., Li R.A., Wersto R.P., Boheler K.R. The B-MYB transcriptional network guides cell cycle progression and fate decisions to sustain self-renewal and the identity of pluripotent stem cells. PLoS One. 2012;7:e42350. doi: 10.1371/journal.pone.0042350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Ni P., Mou C., Zhang Y., Guo H., Zhao T., Loh Y.H., Chen L. Cops2 promotes pluripotency maintenance by stabilizing nanog protein and repressing transcription. Sci. Rep. 2016;6:26804. doi: 10.1038/srep26804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Deibler R.W., Lerou P.H., Ballabeni A., Heffner G.C., Cahan P., Unternaehrer J.J., Kirschner M.W., Daley G.Q. A nontranscriptional role for Oct4 in the regulation of mitotic entry. Proc. Natl. Acad. Sci. USA. 2014;111:15768–15773. doi: 10.1073/pnas.1417518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.