Abstract
A number of industrial chemicals and therapeutic agents cause liver tumors in rats and mice by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPARα). The molecular and cellular events by which PPARα activators induce rodent hepatocarcinogenesis have been extensively studied elucidating a number of consistent mechanistic changes linked to the increased incidence of liver neoplasms. The weight of evidence relevant to the hypothesized mode of action (MOA) for PPARα activator-induced rodent hepatocarcinogenesis is summarized here. Chemical-specific and mechanistic data support concordance of temporal and dose–response relationships for the key events associated with many PPARα activators. The key events (KE) identified in the MOA are PPARα activation (KE1), alteration in cell growth pathways (KE2), perturbation of hepatocyte growth and survival (KE3), and selective clonal expansion of preneoplastic foci cells (KE4), which leads to the apical event–increases in hepatocellular adenomas and carcinomas (KE5). In addition, a number of concurrent molecular and cellular events have been classified as modulating factors, because they potentially alter the ability of PPARα activators to increase rodent liver cancer while not being key events themselves. These modulating factors include increases in oxidative stress and activation of NF-kB. PPARα activators are unlikely to induce liver tumors in humans due to biological differences in the response of KEs downstream of PPARα activation. This conclusion is based on minimal or no effects observed on cell growth pathways and hepatocellular proliferation in human primary hepatocytes and absence of alteration in growth pathways, hepatocyte proliferation, and tumors in the livers of species (hamsters, guinea pigs and cynomolgus monkeys) that are more appropriate human surrogates than mice and rats at overlapping dose levels. Despite this overwhelming body of evidence and almost universal acceptance of the PPARα MOA and lack of human relevance, several reviews have selectively focused on specific studies that, as discussed, contradict the consensus opinion and suggest uncertainty. In the present review, we systematically address these most germane suggested weaknesses of the PPARα MOA.
Keywords: human relevancy framework, key events, liver cancer, mode of action, NF-kB, oxidative stress, peroxisome proliferator-activated receptor α (PPARα)
Background
Published reports in the 1970s linked treatment of rodents with a variety of seemingly structurally diverse chemicals to increased incidence of hepatocellular adenomas and carcinomas. Because all of these compounds increased the number and size of peroxisomes, they were originally termed “peroxisome proliferators” (reviewed in Rao and Reddy, 1996). Found in almost all eukaryotic cells, peroxisomes are subcellular organelles involved in (among many functions) long chain fatty acid catabolism through the β- and/or ω-oxidation cycle (de Duve, 1996). In responsive species, peroxisomes increase in number and/or size following exposure to physiological and metabolic stressors, especially those that perturb fatty acid homeostasis. Chemicals that induce peroxisome proliferation in the rodent liver include several experimental (WY-14,643 (WY; also called pirinixic acid)) and marketed pharmaceutical agents (clofibrate, gemfibrozil, fenofibrate, nafenopin, bezafibrate, and ciprofibrate) as well as environmentally relevant compounds such as phthalate ester plasticizers or their metabolites (di(2-ethylhexyl)phthalate (DEHP)), pesticides (2,4-dichlorophenoxyacetic acid), solvents (perchloroethylene, trichloroethylene) and other industrial chemicals (perfluorooctanoic acid (PFOA)) (additional chemicals that cause peroxisome proliferation and associated responses are found in Klaunig et al., 2003 and Judson et al., 2010). In addition to the increased occurrence of hepatic tumors, chronic exposure of rats and mice to peroxisome proliferators is linked to several hepatic adaptive responses, including hepatocellular hypertrophy and hyperplasia, changes in apoptosis rates, and oxidative stress (Corton et al., 2014).
The seminal identification of a previously uncharacterized “orphan” nuclear receptor, the peroxisome proliferator-activated receptor α (PPARα), led to the discovery that many chemicals, despite their structural diversity, mediate at least some of their transcriptional effects through this receptor (Issemann and Green, 1990). PPARα along with two family members PPARβ/δ and PPARγ possess the typical structure of a nuclear receptor including DNA binding and ligand binding domains. The three subtypes possess different but sometimes overlapping expression patterns, subcellular distributions, ligand specificities, and biological functions. PPARα is expressed in metabolically active tissues, including the liver, kidney, brown fat and heart, which exhibit pleiotropic responses to peroxisome proliferators. An understanding of the biological functions and role in chemical effects of PPARα has been facilitated by the use of a mouse model that lacks a functional PPARα (the Pparα-null mouse) (Lee et al., 1995). Many of the effects of peroxisome proliferators have been shown to be mediated by PPARα as these effects are not observed in similarly treated Pparα-null mice. This includes the regulation of a large battery of genes that in turn regulate lipid catabolism, lipid transport, and peroxisome proliferation (Kersten, 2014), cellular effects that lead to hepatomegaly including alteration in hepatocyte fate (Corton et al., 2014), and many other normal, physiological effects.
The mechanism by which PPARα regulates gene expression is similar to other nuclear receptors. PPARα is functional when heterodimerized with another nuclear receptor family member, retinoid X receptor (RXR), the receptor for 9-cis-retinoic acid. The PPARα-RXR heterodimer binds to peroxisome proliferator response elements (PPREs), usually found in the promoter or enhancer regions of genes regulated by PPARα. The PPRE consensus sequence consists of the sequence 5’-AACT AGGTCA A AGGTCA-3’ (or variant), with PPARα occupying the 5’ position. Binding of ligand bound PPARα-RXR heterodimers to PPREs in chromatin is dynamic, because there are fluctuating endogenous ligands present in most cells that cause binding. Another level of regulation is through co-repressor proteins that dissociate from PPARα upon ligand binding coincident with recruitment of the transcriptional machinery (Escher and Wahli, 2000; Gottlicher et al., 1992). Importantly, the expression level of PPARα in the cell, the presence or absence of endogenous/exogenous ligands, and the availability of chromatin for receptor binding, are all under constant dynamic regulation.
In this review, “PPARα activator” is used in place of the more traditional but outdated term “peroxisome proliferator” to denote the central role PPARα plays in mediating the pleiotropic effects of these compounds. “Activator” is used in place of the more commonly used “agonist” as very few compounds have been shown to activate PPARα through direct binding. PPARα activators are here defined as those chemicals or their proximate metabolites that interact directly or indirectly with PPARα. There is evidence for indirect interactions that require metabolic activation (e.g., DEHP) or activate PPARα secondary to increases in the availability of natural ligands through perturbation of lipid homeostasis (Luebker et al., 2002).
Comprehensive reviews of the underlying mode of action (MOA) for PPARα-mediated rodent liver cancer and the relevance of the rodent MOA to human risk have been published (Klaunig et al., 2003; Corton et al., 2014). The MOA is defined as a biologically plausible sequence of key events (KEs), starting with interaction of an agent with a molecular target, proceeding through cellular and physiological changes ultimately resulting in an observed biological effect, supported by robust experimental observations and mechanistic data. The MOA describes key molecular, biochemical or cytological events that are both measurable and necessary for the observed adverse effect (Sonich-Mullin et al., 2001). A KE is defined as “an empirically observable precursor step that is itself a necessary element of the MOA or is a biologically-based marker for such an element” (US EPA, 2005). The two aforementioned reviews (Klaunig et al., 2003; Corton et al., 2014) on the role of PPARα in liver cancer were the consensus of lengthy literature synthesis and debate among many stakeholders including those from industry, academia, and regulatory agencies. The analysis of the MOA included assessment of the associations between the KEs and liver tumor formation with respect to: (1) strength, consistency and specificity, (2) temporal relationships between the KEs and the liver tumors, (3) the dose–response aspects of the KEs, biological plausibility and coherence of the KEs, and (4) evaluation of possible alternative MOAs (Boobis et al., 2008; Julien et al., 2009; Meek, 2008). The participants in these efforts uniformly agreed that there was enough information to conclude that there is an established MOA for rodent liver tumor induction by PPARα activators (Klaunig et al., 2003; Corton et al., 2014), and that the MOA is either “not relevant” or “not likely to be relevant” to humans (Corton et al., 2014).
Since the publication of the Klaunig et al. (2003) review, two additional reviews were published discussing the MOA and human relevance of liver tumor induction by PPARα activators (Kesheva and Caldwell, 2006; Guyton et al., 2009). Guyton et al. argued that KEs in the rodent MOA are neither necessary nor sufficient and alternative MOAs should be considered. Much of their argument was based on two studies (Ito et al., 2007; Yang et al., 2007), which appeared to contradict the MOA. A number of primary studies and review articles have cited the Guyton et al. analysis of the Ito et al. (2007) study as evidence that the PPARα-dependent carcinogenesis lacks a scientific basis and more specifically, DEHP causes liver cancer through a PPARα-independent mechanism (Benninghoff et al., 2011; Caldwell, 2012; Gentry et al., 2011; Henkler et al., 2010; Pazienza et al., 2012; Polvani et al. 2014; Rigden et al., 2015; Romagnolo et al., 2014; Steenland et al., 2010; Tateno et al., 2015).
In the present review, we describe the KEs in the PPARα-mediated liver cancer MOA and summarize the large body of data which overwhelmingly supports the rodent MOA by PPARα activators for multiple chemicals. To assist in the evaluation of the evidence that a chemical may cause cancer through this MOA, we examine the criticisms of the established MOA as detailed in the two aforementioned review articles (Kesheva and Caldwell, 2006; Guyton et al., 2009).
The MOA for PPARα-mediated liver cancer
A previously published consensus for a hypothesized MOA proposed a series of KEs that must occur for PPARα activators to increase the incidence of hepatocellular adenomas and carcinomas in mice and rats (Klaunig et al., 2003). This MOA was reexamined in a more recent review of studies published since 2003, which included those that mechanistically determined the interdependency of the KEs (Corton et al., 2014). The overlapping KEs identified in these two reviews included activation of PPARα by PPARα activators (KE1), alteration in cell growth pathways (KE2), alteration in hepatocyte growth including effects on proliferation and apoptosis (KE3), and clonal expansion of preneoplastic initiated hepatocytes (KE4) which leads to increases in hepatocellular adenomas and carcinomas (KE5). In the more recent analysis, a number of molecular changes previously termed associative events were described as modulating factors. Associative events are “biological processes that are themselves not causally necessary KEs for the MOA, but are reliable indicators or markers for KEs.” (Corton et al., 2014). Associative events can be used as surrogate markers for a KE in a MOA evaluation or as indictors of exposure to a xenobiotic that has stimulated a KE. In the context of the PPARα MOA, these include regulation of genes involved in lipid metabolism and peroxisome proliferation, which have been used as markers of PPARα activation. Modulating factors are defined as those that “could modulate the dose–response behavior or probability of inducing one or more KEs or the adverse outcome.” The modulating factors considered were increases in oxidative stress and activation of the transcription factor NF-kB. Below, we review the evidence supporting the KEs in the MOA.
Key Event 1-PPARα activation
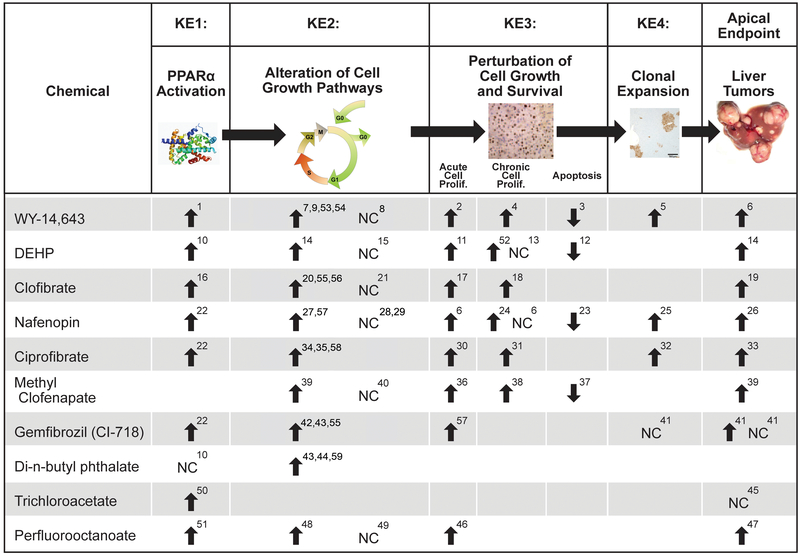
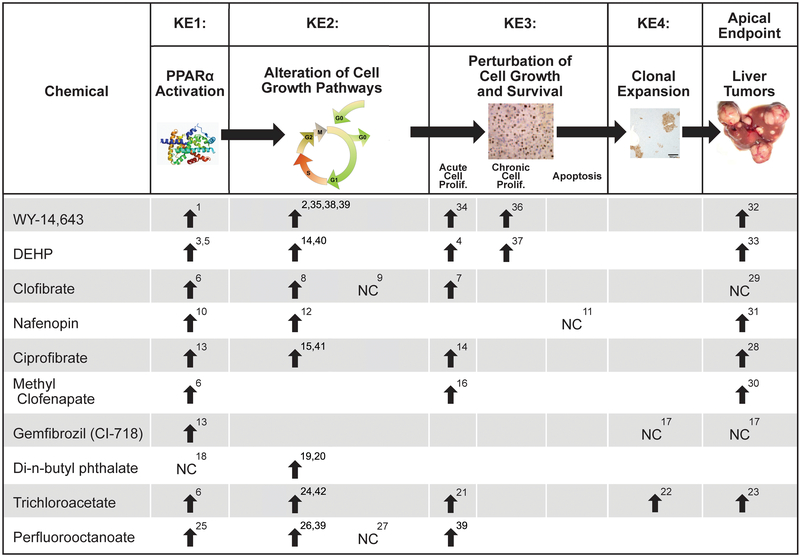
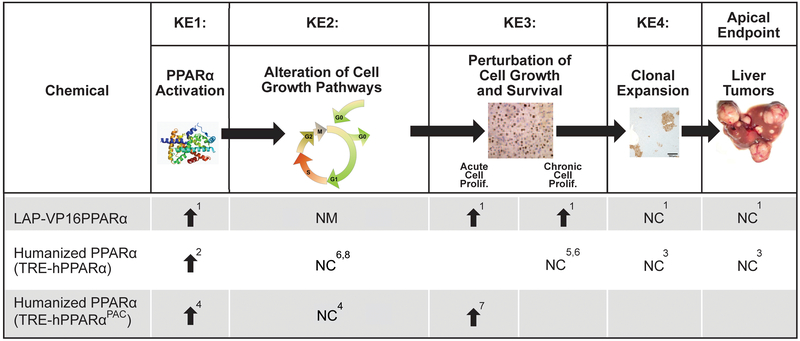
The activation of PPARα leads to the downstream KEs that culminate in liver cancer. Chemical-specific data show excellent concordance among PPARα activation, the KEs in the MOA, and liver cancer (see Figures 1 and 2 for examples of 10 PPARα activators in rats and mice, respectively). There is overwhelming evidence that PPARα activation is the initiating event in the PPARα activator MOA for liver tumor induction. Activation of PPARα can be assessed by trans-activation assays (Corton et al., 2000) or by measuring associative events, which can include increased expression of genes involved in fatty acid β-oxidation or peroxisome proliferation, increased palmitoyl-CoA oxidase activity, or peroxisome proliferation in hepatocytes. The potency of PPARα activation is roughly proportional to the potency of the chemical as an inducer of the liver tumor response (summarized in Klaunig et al., 2003 and discussed below). Importantly, all studies using Pparα-null mice do not show hepatocyte-specific changes associated with the PPARα MOA, indicating the requirement of the activation of this nuclear receptor to mediate these events (Figure 3).
Figure 1. Occurrence of Key Events in the PPARα Mode of Action (MOA) in Rats.
- Marsman et al., 1988; Smith-Oliver and Butterworth, 1987; Isenberg et al., 2000; Hasmall et al., 2000b; Soames et al., 1999; Busser and Lutz, 1987; Hasmall and Roberts, 2000
- Cattley et al., 1987; Marsman et al., 1998
- Marsman et al., 1992; Tanaka et al., 1992; Barrass et al., 1993; Busser and Lutz, 1987; Armacher et al., 1997
- Calfee-Mason et al., 2004; Li et al., 1996
- Barrass et al., 1993; Styles et al., 1988; Hasmall and Roberts, 2000
- Abdellatif et al., 1990; Abdellatif et al., 1991; Biegel et al., 2001
- Corton, 2004
- Armacher et al., 1997
Figure 2. Occurrence of Key Events in the PPARα Mode of Action (MOA) in Mice.
- Styles et al., 1988
- Ward et al., 1988 (evidence for cytotoxicity at the high dose)
Figure 3. Occurrence of Key Events in the PPARα Mode of Action (MOA) in Mice in PPARα Transgenic Mouse Models.
- Cheung et al., 2004; Morimura et al., 2006; Nakajima et al., 2009; Nakagama et al., 2012; Hayashi et al., 2011; Ito et al., 2012
- Cheung et al., 2004 (8 weeks of WY in the diet)
- Morimura et al., 2006 (cell cycle genes only at 38-44 weeks of WY in the diet)
- Yang et al., 2008 (2 weeks of WY in the diet)
Transcript profiling has also been used to comprehensively determine whether PPARα is required to alter gene expression. Alterations of gene expression by WY was almost completely abolished in the livers of Pparα-null mice at multiple time points (Anderson et al., 2004a,b; Corton et al., 2004; Rosen et al., 2008a,b; Woods et al., 2007c). The hypolipidemic drug, fenofibrate required PPARα for 99% of the gene expression changes in the mouse liver (Sanderson et al., 2008). Four perfluorinated compounds (PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate) as well as the phthalate ester, DEHP have been examined by microarrays in wild-type and Pparα-null mice. The results indicate that ~76-94% of the genes were regulated in a PPARα-dependent manner (Ren et al., 2009, 2010; Rosen et al., 2008a,b, 2010; Rosen et al., 2017). The genes that were dependent on PPARα included those involved in lipid homeostasis and the cell cycle. PPARα-independent genes often included those regulated by another nuclear receptor, constitutive activated/androstane receptor (CAR) (discussed in greater detail below).
Mouse studies showed that increased hepatocyte proliferation after PPARα activator exposure was PPARα-dependent. The compounds examined included WY, diisononyl phthalate (DINP), and trichloroethylene (TCE). In each case, wild-type mice showed increases in hepatocyte proliferation, which was abolished in the Pparα-null mice (Laughter et al., 2004; Peters et al., 1998; Valles et al., 2003). PFOA at 10 mg/kg/day produced increases in hepatocyte proliferation in both wild-type and Pparα-null mice (Wolf et al., 2008). We discuss below how PFOA may cause cell proliferation in Pparα-null mice through activation of CAR. Additionally, suppression of apoptosis occurred in primary hepatocytes isolated from wild-type mice but not from Pparα-null mice (Hasmall et al., 2000a).
Chronic treatment with WY or bezafibrate produced hepatocellular neoplasia in 100% of wild-type mice while there were no significant increases in the number of liver neoplasms in Pparα-null mice (Hays et al., 2005; Peters et al., 1997). These two studies provide strong support for the causal role of PPARα activation in rodent carcinogenesis by PPARα activators. A third bioassay with DEHP in wild-type and Pparα-null mice (Ito et al., 2007) showed equivocal results and is discussed below.
Key event 2-Alteration in cell growth pathways
Many studies have been carried out to identify the mechanistic events that lead to alterations in cell growth by PPARα activators. Increases in the expression of cyclins and or c-Myc have been observed in the livers of rats (Ma et al., 1997; Rininger et al., 1996; Amacher et al., 1997; Jolly et al., 2005; Gill et al., 1998; Perrone and Williams, 1998; Urbanek-Olejnik et al., 2016) and mice (Peters et al., 1998; Wolf et al., 2008; Lee and Lim, 2011; Calfee-Mason et al., 2008; Nelson et al., 1990) treated with different PPARα activators (Figures 1 and 2). There is support for two non-exclusive mechanisms linking PPARα activation to hepatocyte proliferation. The first mechanism involves the activation of non-parenchymal cells (NPCs), particularly Kupffer cells. Once activated Kupffer cells produce and secrete cytokines such as tumor necrosis factor α (TNFα), interleukin-1α (IL-1α), and interleukin-1β (IL-1β) that affect hepatocyte fate (Grivennikov and Karin, 2011). The level of TNFα mRNA more than doubled in response to PPARα activators in two studies (Bojes et al., 1997; Rolfe et al.,1997) but did not change in other acute studies (Anderson et al., 2001; Holden et al., 2000). One study showed that PPARα activators increased the level of TNFα protein by bioactivation or by releasing existing TNFα protein from Kupffer cells (Holden et al., 2000). TNFα by itself increased proliferation and decreased apoptosis in cultured rodent hepatocytes (Holden et al., 2000; Rolfe et al., 1997). Hepatocyte proliferation can be prevented in vivo by pretreatment with antibodies to either TNFα (Bojes et al., 1997; Rolfe et al., 1997) or TNFα receptor 1 (West et al., 1999).
Experiments in which hepatocytes are cultured with or without Kupffer cells, provide additional evidence that activated Kupffer cells play a role in the proliferative response of hepatocytes to PPARα activators. In vitro studies have been carried out in which primary hepatocyte cultures exposed to PPARα activators were assessed for cell proliferation by themselves or in the presence of Kupffer cells. Highly purified hepatocyte cell cultures lacking Kupffer cells did not exhibit a proliferative response seen in cultures containing NPCs exposed to the PPARα activators WY and nafenopin. Chemical-induced proliferation was restored by adding back the Kupffer cells to the culture or by adding media from cultured Kupffer cells treated with PPARα activators (Hasmall et al., 2000a; Parzefall et al., 2001). These studies support a model in which soluble factors from the Kupffer cells are crucial for hepatocyte proliferation after PPARα activator exposure. In contrast, there is one study that did not show a requirement for Kupffer cells or growth factors derived from Kupffer cells for the proliferation of hepatocytes after exposure to PPARα activators (Plant et al., 1998). It should be noted that in this study, there was no reported information about the level of purity of the hepatocyte preparation that was used, leaving open the possibility that these cultures may have contained Kupffer cells.
Studies with Pparα-null mice showed that PPARα activation is required for hepatocyte-specific changes associated with exposure to PPARα activators (Christensen et al., 1998; Hasmall et al., 2000b; Lee et al., 1995; Peters et al., 1997, 1998). A conundrum is that while there is evidence that Kupffer cells are required for the cell proliferation response, Kupffer cells do not express detectable levels of PPARα but do express PPARβ/δ and PPARγ (Peters et al., 2000). Kupffer cells from Pparα-null mice restored the proliferative response to PPARα activators of isolated hepatocytes from wild-type mice (Hasmall et al., 2000c). Cell proliferation does not occur in co-cultures of hepatocytes and Kupffer cells from Pparα-null mice demonstrating the absolute requirement of PPARα for induction of cell proliferation (Hasmall et al., 2000a). The in vitro data suggest that the proliferative response of hepatocytes to PPARα activators involves factors secreted by Kupffer cells including TNFα and is PPARα-dependent.
Further evidence that hepatocyte proliferation is dependent on soluble factors in vivo comes from work by Weglarz and Sandgren (2004) who examined chimeric livers composed of wild-type and Pparα-null hepatocytes generated in either wild-type or Pparα-null mice. Exposure to a PPARα activator led to induction of peroxisome proliferation and fatty acid β-oxidation only in wild-type hepatocytes, indicating that these responses require PPARα. Hepatocytes in chimeric livers responded to treatment with increases in proliferation whether or not they contained an intact PPARα as long as there was a population of hepatocytes within the liver that were from wild-type mice. These results indicate that hepatocytes lacking an intact PPARα retain the ability to respond to the proliferative effects of PPARα activators (Weglarz and Sandgren, 2004) and imply that secreted factors from Kupffer cells affect Pparα-null hepatocytes.
The potential role of PPARβ/δ or PPARγ in mediating effects in NPC has not been ruled out. PPARα, PPARβ/δ and PPARγ can all be activated by a large overlapping set of environmentally relevant chemicals, including phthalates (summarized in Lapinskas and Corton, 2005), solvents, and perfluorinated compounds (Maloney and Waxman, 1999). In addition, hypolipidemic drugs and environmentally relevant chemicals activate not only PPARα but also PPARγ and, to a lesser extent for some compounds, PPARβ/δ, as assessed in trans-activation assays (Corton et al., 2000). However, it is critical to point out that trans-activation assays are artificial systems and do not reflect normal physiology. For example, trans-activation assays sometimes use chimeric proteins that do not occur in normal cells, chromatin remodeling is not required for detection of “activity”, and many times trans-activation assays utilize culture medium that lacks serum and reporter constructs that contain multiple copies of the DNA response element required to measure activity. Collectively, these limitations must be considered when determining whether a compound actually activates a nuclear receptor as they could overestimate the ability of a chemical to activate a receptor. PPARγ but not PPARα or PPARβ/δ is expressed in Kupffer cells (Rusyn et al., 2000). Whether PPARγ could be playing a role in Kupffer cell activation upon exposure to compounds that can activate both PPARγ and PPARα cannot be determined from trans-activation assays alone and requires further study.
The mechanism by which PPARα activators activate Kupffer cells may involve activation of NF-kB, which acts as a coordinator of adaptive and innate immune responses and plays a critical role in cancer development and progression (Arsura and Cavin, 2005; Karin, 2006). NF-kB is activated under conditions of inflammation and oxidative stress (Czaja, 2007; Gloire et al., 2006). The evidence that oxidative stress induced by PPARα activators activates NF-kB is discussed in the section “Activation of NF-kB” below.
The second possible nonexclusive mechanism for increases in cell proliferation involves a microRNA (miRNA) cascade that culminates in increased expression of the c-Myc growth regulatory gene central to the hepatoproliferative response (Shah et al., 2007; Qu et al., 2014). Profiling of miRNA expression demonstrated that PPARα regulates expression of the miRNA, let-7c, in the liver. In the absence of exposure to PPARα activators, let-7c was shown to target and down-regulate the expression of the c-Myc gene. Following acute or chronic treatment with WY, let-7c was downregulated, leading to increased expression of the c-Myc gene. These molecular events were abolished in Pparα-null mice. Let-7c overexpression by itself decreased c-Myc expression and suppressed the growth of Hepa-1 cells, an in vitro model of mouse hepatocyte growth. The Shah et al. study (2007) provides evidence for a PPARα-dependent let-7c signaling cascade critical for PPARα activator-induced liver proliferation. Because other PPARα activators were not tested in this or follow-up studies, it is not possible to determine if let-7c is important for PPARα activators other than WY.
To summarize, extensive research has been carried out to identify the underlying mechanisms for cell proliferation after exposure to PPARα activators. Several possible mechanisms for the induction of cell proliferation have been described including a role for cell proliferation dependent on the secretion of soluble growth factors and a role for cellular c-Myc induction. While the precise mechanism for induction in cell growth and suppression of apoptosis by PPARα activators is not known, it can be reasoned that cell fate changes cannot occur without alteration in one or more signaling pathways that impact cell growth. Overall, the data support the conclusion that alteration of growth control pathways is a KE in the PPARα activator MOA.
Key Event 3-Perturbation of cell growth and survival
PPARα activators produce several tumor precursor effects, including increased hepatocyte DNA synthesis and cell proliferation in both normal and preneoplastic hepatocytes. The induction of cell proliferation in liver by PPARα activators is believed to enhance the rate of fixation of DNA damage in genes controlling cell growth leading to silencing and/or mutations of tumor suppressor genes or activation of oncogenes. These changes facilitate clonal expansion of initiated cells, leading to the formation of hepatic focal lesions (Cattley et al., 1991, 1998; Huber et al., 1991) or the selective clonal expansion of already present spontaneous preneoplastic cells (Isenberg et al, 1997; Kolaja et al., 1996a,b). The role of PPARα activators in direct and indirect DNA damage is discussed below. Here, we summarize the data supporting the relationships between PPARα activation and alteration in hepatocyte proliferation and apoptosis.
Increases in cell proliferation.
All PPARα activators that have been examined produce transient increases in replicative DNA synthesis during the first few days or weeks of exposure (Figures 1 and 2) followed by a return to baseline levels. This increase in hepatocyte proliferation along with increases in cell size from proliferation of the smooth endoplasmic reticulum results in liver enlargement. Potent PPARα activators at high doses also exhibit sustained or chronic increases in cell proliferation, although the levels are much lower than those observed after acute exposures. One PPARα activator (DEHP) did not always induce this chronic cell proliferation, even though the acute hepatocyte proliferation is clearly observed. It should be noted that minor increases above variable background levels of cell proliferation are difficult to detect which could preclude observing this sustained proliferation for weak activators.
Effects on apoptosis.
Many non-genotoxic carcinogens including PPARα activators suppress hepatocyte apoptosis. Suppression of apoptosis could inhibit the ability of the liver to remove DNA-damaged pre-neoplastic hepatocytes that arise spontaneously or through direct damage (Bayly et al., 1994; James and Roberts, 1996; Oberhammer and Qin, 1995; Schulte-Hermann et al., 1981). Because of the difficulty in measuring the suppression of already low levels of apoptosis in vivo, most of the evidence for apoptosis suppression comes from in vitro studies. These studies show that the PPARα activators nafenopin, methylclofenapate, and WY suppress spontaneous hepatocyte apoptosis as well as that induced by a negative regulator of liver growth, transforming growth factor β1 (TGF β1) (Bayly et al., 1994; Oberhammer and Qin, 1995) or induced by diverse stimuli such as DNA damage or ligation of Fas, a receptor related to the tumor necrosis factor α (TNFα) family of cell surface receptors (Gill et al., 1998). Four in vivo studies showed suppression of apoptosis after acute dosing with nafenopin, DEHP, or WY within the first few days of initial exposure (Bursch et al., 1984; James et al., 1998a,b; Youssef et al., 2003).
Suppression of apoptosis by PPARα activators occurs under conditions of acute exposure concomitantly with hepatocyte proliferation resulting in increased liver size. However, once a steady state of liver size is reached, levels of apoptosis likely return to background levels or to levels that balance the low level of cell proliferation that occurs for potent PPARα activators. Two studies show that chronic exposure of rats and mice to the PPARα activator WY under conditions that result in chronic low level hepatocyte proliferation leads to increases in apoptosis (Burkhardt et al., 2001; Marsman et al., 1992). The ability of the liver to respond to apoptosis inducers in vivo is altered by PPARα activators. Sensitivity to two apoptosis inducers (conconavalin A and Jo2 antibody) was dramatically increased after exposure to WY for one week in wild-type but not Pparα-null mice (Xiao et al., 2006). The data indicate that a physiological function of PPARα activation is to increase hepatocyte growth through an increase in hepatocyte proliferation or a decrease in apoptosis or a combination of both effects. The end result is an increase in the size and number of hepatocytes followed by maintenance of the system at a new steady state.
To summarize, alterations in the balance between hepatocyte proliferation and apoptosis have been observed after exposure to PPARα activators at different stages of carcinogenesis. Liver tumor growth requires alterations in hepatocyte proliferation and apoptosis. On the basis of these findings, the alteration of hepatocyte fate through induction of cell proliferation and/or inhibition of apoptosis is a KE in the MOA of PPARα activator-induced liver tumors.
Key event 4-Selective clonal expansion of preneoplastic foci cells
Non-genotoxic compounds that induce liver cancer cause selective clonal expansion of the pre-neoplastic liver cell population. PPARα activators promote the growth of chemically- and spontaneously-induced lesions through enhanced cell replication (Cattley and Popp, 1989; Cattley et al., 1991; Isenberg et al., 1997; Marsman et al., 1988). These activators selectively stimulate growth of initiated cells that have molecular characteristics different from cells in either spontaneous tumors or in tumors induced by other non-genotoxic chemicals such as phenobarbital (Rao et al., 1986). Foci induced by PPARα activators are predominantly basophilic and do not express proteins such as glutathione S-transferase–placental form or gamma-glutamyl transpeptidase, which are normally associated with foci and tumors induced by other non-genotoxic carcinogens or DNA-damaging agents (Rao et al., 1988). Once early lesions are formed, continued exposure to PPARα activators causes selective increases in DNA replication in these liver foci (Isenberg et al., 1997) while replication of normal hepatocytes in the surrounding liver is increased only slightly (Grasl-Kraupp et al., 1993a,b,c). Furthermore, the preneoplastic foci respond to the cell replicative effects but not the peroxisome proliferative effects of PPARα activators, suggesting that the growth stimulus, but not the peroxisome proliferative effect, is the important effect for carcinogenic action (Grasl-Kraupp et al., 1993a,b,c). While it has been reported that apoptosis increased in these foci and in adenomas (Isenberg et al., 1997; Grasl-Kraupp et al., 1997), the lesions continue to grow, because the increase in cell replication over ran any increase in cell death. Progression from initiated cell to hepatic carcinomas is dependent on the continued presence of the PPARα activator. Five weeks after withdrawal of nafenopin, there was a 20% reduction in the number of hepatocytes in the non-involved tissue but an 85% reduction of cells in foci, adenomas and carcinomas (Grasl-Kraupp et al., 1997). These data indicate that continual activation of PPARα is necessary for the growth of the altered cells in foci, adenomas, and carcinomas in the livers of mice and rats. Overall, the findings of a large number of studies are consistent with selective clonal expansion of preneoplastic foci cells as a KE in the PPARα activator-induced liver tumor MOA.
Modulating Factors
In the Corton et al. (2014) analysis, a number of other molecular and cellular events were considered as KEs including oxidative stress and activation of NF-kB. However, the workgroup agreed that for oxidative stress and activation of NF-kB there was not enough evidence to designate these effects as key events. Because they have the potential to alter the ability of PPARα activators to increase liver cancer, these events were defined as modulating factors (Corton et al., 2014).
Increases in oxidative stress
Increases in oxidative stress through increases in reactive oxygen species (ROS) has been proposed as a possible KE for PPARα activators (Corton, 2010; Klaunig et al., 2003). There are consistent relationships between increases in ROS and increased incidence of liver cancer by PPARα activators. Overproduction of oxidants are thought to cause DNA damage leading to mutations and cancer (Reddy & Rao, 1989; Yeldandi et al., 2000). Alternatively, increases in ROS lead to increased activation of signaling pathways that alter cell fate (Rusyn et al., 2006). Markers of hepatic oxidative stress determined by measuring lipid peroxidation (thiobarbituric acid reactive substances, conjugated dienes, lipofuscin, malondialdehyde, F2-isoprostanes), oxidized glutathione, or hydrogen peroxide, were consistently increased by PPARα activators in rats and mice (Figures 1 and 2). There were only a few studies that did not detect increases in these markers. These negative studies are difficult to interpret, because other key or associating events were not simultaneously analyzed (e.g., Huber et al., 1991, 1997) and inconsistencies could be attributed to insufficient dose or time of exposure. There were two studies in which one assay for oxidative stress was positive but another negative (Conway et al., 1989; Fischer et al., 2002). Despite some inconsistencies, oxidative stress is induced upon activation of PPARα.
Sources of ROS induced by exposure to PPARα activators include enzyme-induced hydrogen peroxide that oxidizes DNA, lipids and other molecules. Enzymes regulated by PPARα activators produce hydrogen peroxide as a byproduct of metabolism, including the peroxisomal, mitochondrial and microsomal oxidases such as fatty acyl-CoA oxidase (ACO) in hepatocytes (Becuwe & Dauca, 2005). Administration of PPARα activators can also lead to decreased levels of enzymes that degrade ROS, which may contribute to increases in oxidative stress upon exposure (Glauert et al., 1992; O’Brien et al., 2001a,b). The individual contributions of these enzymes involved in the production or metabolism of ROS to increases in oxidative stress and downstream KEs has not been quantitatively addressed.
The other major source of oxidative stress upon PPARα activator exposure is proposed to be NADPH oxidase, which plays an important role in generating superoxide radical in response to Kupffer cell activators (De Minicis et al., 2006). The role of NADPH oxidase was determined directly by measuring oxidative stress and cell proliferation after PPARα activator exposure in mice that lack one of the regulatory subunits of NADPH oxidase (p47Phox-null mice). After a 7d treatment with WY, the p47Phox-null mice lacked increases in oxidative stress and hepatocyte proliferation observed in wild-type mice (Rusyn et al., 2000). In a subsequent 3 week WY exposure study, increases in indicators of oxidative stress, palmitoyl-CoA oxidase activity, and cell proliferation were independent of the status of the p47Phox gene but were dependent on PPARα (Woods et al., 2007b,c). Differences in the results of these two studies might be due to compensatory mechanisms in the longer term exposure which triggers conditions that allow bypass of p47Phox dependence.
The data indicate that PPARα activators consistently increase the levels of ROS and oxidative stress through multiple mechanisms. There is little evidence that increases in oxidative stress leads to direct or indirect DNA damage after PPARα activator exposure (discussed in Corton et al., 2014). The weight of evidence is not sufficient to conclusively link direct or oxidatively-induced DNA damage as part of the MOA. However, it is concluded that the level of oxidative stress could be a modulating event in determining liver tumor induction especially under conditions when background oxidative stress from endogenous PPARα activators could add to chemical-induced oxidative stress.
Activation of NF-kB
PPARα activator exposure leads to activation of NF-kB. Activation of NF-kB can be measured by the ability of a heterodimer composed of p50 and p65 subunits to bind to a NF-kB response element in an electrophoretic mobility shift assay (EMSA). Four activators (WY, ciprofibrate, gemfibrozil, and di-n-butyl phthalate) increased NF-kB activity in rat or mouse liver (Figures 1 and 2). Nafenopin on the other hand did not induce NF-kB; this finding could be due in part to differences in the manner in which this one lab carried out EMSA (Menegazzi et al., 1997; Ohmura et al., 1996). NF-kB was shown to be activated in both Kupffer cells and hepatocytes. Activation occurs at different times in the two cell types; a single gavage dose of WY in rats caused increased NF-kB activity in Kupffer cells as early as 2 h while in hepatocytes the peak occurred 6 h later and was not as pronounced compared to that in Kupffer cells (Rusyn et al., 1998). NF-kB was activated by a PPARα activator in the H4IIEC3 rat hepatoma cell line, responsive to the proliferative effects of PPARα activators (Li et al., 2000a).
Addressing concerns regarding perceived inconsistencies in the rodent MOA.
The PPARα MOA described in Klaunig et al. (2003) has been criticized by Guyton et al. (2009) and Kesheva and Caldwell (2006). Much of the criticism of the MOA was based on two studies that were interpreted to support alternative MOAs for PPARα activators. Below we address the major problems with the arguments raised by these authors.
1. The DEHP bioassay study of Ito et al. (2007) in Pparα-null mice.
The carcinogenic effects of DEHP were examined in wild-type and Pparα-null mice treated for 22 months; a small but statistically significant increase in total number of liver tumors was observed in Pparα-null mice (Ito et al., 2007). No increase in liver tumors were observed in wild-type mice. Guyton et al. state “PPAR-α activation and the subsequent KEs in the hypothesized MOA do not appear to represent the sole cause of DEHP liver tumorigenesis….the mechanisms by which DEHP induces hepatocarcinogenesis remain unknown.”
There are major weaknesses in the Ito et al. study not fully discussed in the Guyton et al. (2009) review. First, Ito et al. combined all liver tumors including hepatoblastomas to achieve statistical significance. Typically, statistical tests in carcinogenesis studies are determined using incidences of hepatocellular adenomas or hepatocellular carcinomas separately, and also on combined hepatocellular adenomas and carcinomas. Hepatoblastomas originate from a different cell population and adding these tumors to hepatocellular adenomas and carcinomas is not an appropriate method to determine statistical significance of liver tumors. Given that the authors did not report the results of the statistical test for the combined adenomas and carcinomas, we can assume that those minor increases were not significant.
The second major weakness of the Ito et al. study was that the two doses of DEHP used in the study (0.01% and 0.05%) did not cause an increase in liver tumors in the wild-type mice, complicating the interpretation of the Pparα-null mouse results. Guyton et al. (2009) attempted to address this issue by comparing the level of tumors in Pparα-null mice (on the SV/129 background) with liver tumor incidence from another study carried out in B6C3F1 mice (David et al., 2000a). This is an inappropriate comparison due not only to strain differences in response but to differences inherent in conducting bioassays in different labs.
Guyton et al. (2009) used flawed logic to extrapolate from effects of DEHP in Pparα-null mice to wild-type mice indicating that the PPARα-dependent MOA is not relevant in wild-type mice even though there were no increases in liver tumors in the wild-type mice in the Ito et al. study. Importantly, they failed to evaluate the weight of evidence of effects of DEHP in wild-type mice and compare the responses to those observed in Pparα-null mice.
There are clear differences in responses observed in the different strains, which indicate that the liver tumor response in wild-type mice is PPARα-dependent. Ito et al. (2007) found that Pparα-null mice exhibit greater levels of background and DEHP-inducible levels of a marker of oxidative stress (8-OHdG) than wild-type mice. There were increases in the expression of p65 and Jun proteins in treated Pparα-null mice but not wild-type mice. Using RT-PCR, there were increases in the gene expression of Hadha in wild-type mice only and Nfkb1 in Pparα-null mice only and decreases in the gene expression of Bax in Pparα-null mice only. In a follow-up study, Takashima et al. (2008) performed a microarray analysis on the liver tumors from the Ito et al. (2007) study and found that there was no overlap in the gene expression patterns between wild-type mice and Pparα-null mice. Furthermore, Takashima et al. validated differences in key genes involved in cell proliferation and apoptosis in the tumors by RT-PCR including increases in Gadd45a and Apaf1 in wild-type but not Pparα-null mice and increases in Ccnb2 and Mcl1 in Pparα-null mice but not wild-type mice. Thus, all data points to the fact that the molecular environments in the treated wild-type and Pparα-null mice were different and that the liver tumors exhibit different molecular profiles.
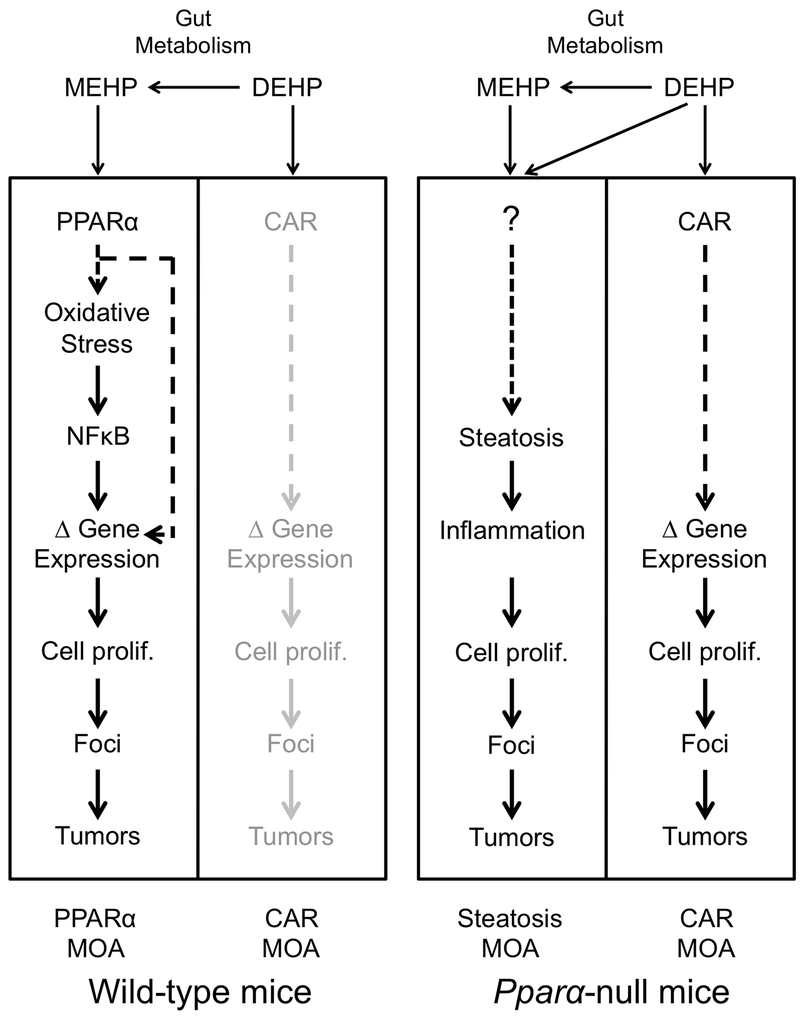
If we assume that the minor increases in the hepatocellular adenomas and carcinomas in the DEHP-treated Pparα-null mice were significant (unlikely for the reason stated above), there are two mechanistic explanations for the increases in the tumors, both of which are related to the biology and physiology of the Pparα-null mice and are PPARα-independent (Figure 4). The first explanation is that Pparα-null mice exhibit increased hepatic lipid accumulation and associated inflammation. Pparα-null mice are known to accumulate hepatic lipids as compared to wild-type mice due to reduced constitutive expression of lipid metabolizing enzymes (Aoyama et al., 1998; Kersten et al., 1999; Leone et al., 1999). Since increased lipid accumulation in the liver is causally associated with liver cancer, it is not surprising that Pparα-null mice allowed to age to 1.5–2 years in the absence of exogenous chemical exposure have significant increases in spontaneous hepatocellular carcinomas and multiple hepatocellular adenomas compared to similarly aged wild-type mice (Howroyd et al., 2004). Given the increased background incidence of liver tumors in Pparα-null mice, the significance of this in DEHP-treated Pparα-null mice could be a chance finding, and not a biologically significant effect of treatment. This is not possible to determine without a larger body of historical control data for liver tumor incidence in the Pparα-null mice. Pparα-null mice are also more susceptible to diethylnitrosamine (DEN)-induced hepatocellular carcinomas compared to wild-type mice possibly because of increased background inflammation (Zhang et al., 2014). Ito et al. (2007) did note increases in inflammatory cell infiltration in DEHP-exposed Pparα-null mice that also had tumors. Chemical-induced augmentation of steatosis and inflammation in the Pparα-null mice has also been observed in other studies. Ammonium perfluorooctanoate (APFO), PFHxS, and PFNA caused or augmented the basal hepatic steatosis in Pparα-null mice (Das et al., 2017; Nakagawa et al., 2012), and APFO caused increases in lobular inflammatory cells in Pparα-null mice but not wild-type mice (Nakagawa et al., 2012). In humanized PPARα mice that express the human PPARα in the absence of the mouse PPARα, there was lipid accumulation and focal necrosis with inflammatory cells after exposure to DEHP, DBP or DEHA (Ito et al., 2012). Combined, these findings indicate that it is more likely that DEHP caused a low incidence of liver tumors (Ito et al., 2007) through a mechanism that involved steatosis and inflammation (Figure 4). Importantly, this explanation does not rule out a PPARα MOA in wild-type mice after exposure to DEHP.
Figure 4. Different mechanisms of liver tumor induction in wild-type and Pparα-null mice by DEHP.
DEHP is metabolized to MEHP in the gut by esterases. (Left panel) MEHP activates PPARα in wild-type mice triggering a cascade of events, including oxidative stress, activation of NFκB, modulation (Δ) of gene expression, leading to liver tumor induction (the PPARα MOA). DEHP weakly activates CAR in wild-type mice, but it is not known if weak activation of CAR leads to downstream key events other than weak activation of some CAR-dependent genes (the CAR MOA; lightened to illustrate the low probability of activation by DEHP). (Right panel) The molecular responses in Pparα-null mice are different than in wild-type mice. DEHP and MEHP may exacerbate the background level of hepatic lipid accumulation and/or inflammation that contributes to liver tumors (the steatosis MOA). Alternatively, DEHP and/or MEHP may be completely ancillary and have nothing to do with the steatosis MOA, as the liver tumors could develop simply from hepatic lipid accumulation and/or inflammation. DEHP also activates CAR to a greater extent in Pparα-null mice than in wild-type mice and may contribute to liver tumors in Pparα-null mice (the CAR MOA). See text for further description.
A second explanation for the increased albeit low incidence of liver tumors observed in DEHP-treated Pparα-null mice in the Ito et al. (2007) study involves activation of another hepatocyte nuclear receptor involved in rodent hepatocarcinogenesis, specifically CAR. DEHP is an inducer of the CAR target gene, Cyp2b10, in wild-type mice (Currie et al., 2005; Eveillard et al., 2009a,b; Ren et al., 2010) but activates Cyp2b10 and by extension CAR to higher levels in Pparα-null mice (Ren et al., 2010). These results suggest that in Pparα-null mice, DEHP could also activate CAR directly (without metabolism to MEHP) resulting in increases in liver tumors through a CAR-dependent mechanism. However, even in the absence of PPARα expression, the level of Cyp2b10 activation was only ~4-fold (Ren et al., 2009) compared to the large inductions (>50-fold) associated with the CAR activator phenobarbital exposures that lead to liver tumors (Geter et al., 2014). It should be noted that no measurements of CAR activation were performed in the original study (Ito et al., 2007) or in the follow-up analysis of the tumors (Takashima et al., 2008), so the CAR hypothesis remains to be established.
In summary, Guyton et al. overemphasized the significance of the Ito et al. (2007) study in the absence of a comprehensive analysis of DEHP effects in wild-type and Pparα-null mice. Further, the review by Guyton et al. neglects to mention viable mechanisms or potential for chance findings, illustrated in the present review that are more likely to contribute to the observed phenotype in DEHP-treated Pparα-null mice. The Ito et al study (2007) has serious flaws including marginal (if any) statistical significance of the liver tumors in the Pparα-null mice and no liver tumors in the corresponding wild-type mice thus precluding a comparison between strains in the same study. The study was not adequately performed or reported to properly evaluate liver tumor induction. Guyton et al. make an inappropriate extrapolation of effects in the Pparα-null mice to that in wild-type mice claiming that because tumors were observed in Pparα-null mice (debatable as discussed above), the liver tumors observed in wild-type mice in other studies (David et al., 2000a) are therefore PPARα-independent. Guyton et al. fail to adequately use a weight of evidence approach to determine the role of PPARα in mediating DEHP effects in the wild-type liver. DEHP exposure leads to consistent effects of the KEs in the MOA. Dose response analysis shows that in mice (described below) and rats (Corton et al., 2014) early KEs are activated at lower doses than those more proximate to the apical event, and global gene expression analysis in the livers of mice treated with DEHP that PPARα is required for over 94% of gene changes in wild-type mice with the remaining 6% consisting of many CAR-regulated genes.
2. Perceived weaknesses of the Ppar α-null mouse model.
The Pparα-null mouse line has been extensively used to determine the molecular and cellular effects of chemical exposures that require PPARα. Kesheva and Caldwell (2006) stated that “….concerns have been raised regarding the adequacy of this model. These are related to both existing study designs (e.g., a less-than-lifetime analysis of tumor induction) and to whether the intrinsic characteristics of these knockout mice mean that they exhibit responses that differ from those of wild-type mice independent of effects related to PPARα agonism.” The mice do exhibit phenotypic differences with wild-type mice that include increases in hepatic steatosis, differences in serum lipid components, and reduced constitutive activity of fatty acid metabolizing enzymes (Aoyama et al., 1998; Kersten et al., 1999; Leone et al., 1999). One could assume that Kesheva and Caldwell are suggesting the Pparα-null mouse line is inappropriate for chemical exposure studies. Interestingly, Pparα-null mice are resistant to apoptosis inducers, Jo2 and Conconavalin A (Xiao et al., 2006). There is evidence that Pparα-null mice are more susceptible to liver toxicity upon chemical exposure. Primary hepatocytes from Pparα-null mice exhibit greater damage after treatment with cadmium or paraquat than hepatocytes from wild-type mice, and Pparα-null mice are more sensitive to damage after carbon tetrachloride and acetaminophen treatment (Anderson et al., 2004b; Chen et al., 2000). Hepatocytes in Pparα-null mice do have the ability to respond to proliferative stimuli. Using hepatocyte transplantation to generate chimeric livers composed of Pparα-null and positive hepatocytes in Pparα-null hosts, Weglarz and Sandgren (2004) showed that hepatocytes in Pparα-null mice respond to WY proliferative signals if adjacent to wild-type hepatocytes (Weglarz and Sandgren, 2004). Pparα-null mouse livers also respond with a proliferative response after a partial hepatectomy, albeit with a slightly delayed onset (Anderson et al., 2002; Wheeler et al., 2003). These results indicate that the livers of Pparα-null mice are not inherently resistant to proliferative stimuli and are thus a relevant model to assess effects of chemicals that cause liver cancer through a PPARα MOA.
In another criticism of the PPARα mode of action, Kesheva and Caldwell also suggested that because the Pparα-null mice were exposed to less than lifetime treatments to WY or bezafibrate, this limits the suitability of this model. However, it should be noted that under the conditions of chronic exposure (~10-11 months), the Pparα-null mice did not exhibit phenotypic effects typically associated with PPARα-induced hepatocarcinogenesis (Hays et al., 2005; Peters et al., 1997). These effects included relative liver weight (WY only; Pparα-null mice treated with bezafibrate exhibited a minor increase), replicative DNA synthesis labeling indices, expression of DNA repair genes, and alterations in proteins involved in the regulation of cell cycle and lipid metabolism. Thus, it is extremely unlikely that even if the Pparα-null mice were treated for a longer length of time with a PPARα activator, they would develop liver tumors given the lack of shorter-term effects associated with hepatocarcinogenesis.
3. The Yang et al. VP16PPARα mouse study.
The second major study that has been used to argue against the PPARα MOA is one that examined the effects of a fusion protein, which has constitutive PPARα activity in the absence of an exogenous PPARα activator (Yang et al., 2007). We discussed above that there are strong mechanistic links between PPARα-mediated hepatocyte proliferation and liver tumors. To determine whether hepatocyte-specific PPARα activation could cause hepatocarcinogenesis without involvement of other liver cell types, the effects of a transgenic mouse model that expressed a hepatocyte-specific PPARα fusion protein was examined. This transgenic mouse line expresses a constitutive PPARα in the absence of an exogenous PPARα activator (VP16PPARα transgenic) (Figure 3). It is critical to note that the receptor expressed in the hepatocytes of this transgenic mouse is a fusion protein containing the trans-activation domain from the herpes simplex virus protein VP16 ligated in-frame with the full-length mouse PPARα. Expression of the VP16PPARα fusion protein in the hepatocytes led to increases in typical markers of PPARα activation including expression of genes involved in fatty acid β-oxidation. Replicative DNA synthesis in hepatocytes and relative liver weight were also increased in VP16PPARα as compared to controls. Untreated transgenic VP16PPARα mice allowed to age to ~1 year did not develop liver tumors despite constitutive increases in replicative DNA synthesis in hepatocytes and in relative liver weights (Yang et al., 2007). By contrast, replicative DNA synthesis in hepatocytes and relative liver weights were also increased in wild-type mice treated with WY, and there was an increase in liver tumors in wild-type mice treated with WY as compared to controls. The authors of this study concluded that hepatocyte-specific activity of PPARα was insufficient to cause liver cancer, and that NPCs were required to cause PPARα-dependent liver cancer.
Guyton et al. (2009) interpreted the study by Yang et al. (2007) in a manner different than the authors who performed the study. Guyton et al. (2009) suggested that the study by Yang and colleagues demonstrated that there can be no mechanistic link between cell proliferation and liver tumor induction in the PPARα MOA. More specifically, Guyton et al. wrote: “Thus, the Yang et al. (2007) study provides evidence that, by itself, PPAR-α activation (and its sequelae) is not sufficient to induce hepatocarcinogenesis. These data are therefore inconsistent with the hypothesis that effects mediated through PPAR-α activation constitute a complete MOA for carcinogenesis” There are multiple problems with this interpretation. Guyton et al. did not consider many differences between activation of the VP16PPARα fusion protein and activation of the endogenous PPARα by a PPARα activator such as WY. For example, when ligands bind to wild-type PPARα there are many conformational changes that lead to loss of bound co-repressors, recruitment of co-activators, remodeling of chromatin, binding of a PPARα/RXR/co-factor complex with response elements on chromatin, and increased and decreased expression of many target genes that ultimately leads to biological effects. This dynamic regulation can also be influenced by relative expression and function of co-repressors and co-activators in different cell types, and/or relative expression and function of other proteins involved in the remodeling of chromatin. Importantly, all of these interactions can also be influenced by multiple equilibriums between proteins and/or endogenous/exogenous PPARα ligands. By contrast, the VP16PPARα fusion protein modulates gene expression and subsequent biological functions through different mechanisms. The viral VP16 transactivation domain causes distinctly different protein-protein interactions with general transcription factors TFIIA, TFIIB, the TATA-binding protein, and TAFII40 components of the multisubunit TFIID, as well as direct recruitment of RNA polymerase (Hagmann et al., 1997). The VP16PPARα model is likely similar to other transcription factor-VP16 fusion proteins, that while they retain some ability to transactivate, the fusion proteins cannot induce all typical phenotypes observed when the transcription factor is activated through endogenous pathways (Schwarz et al., 1992). These differences help to explain the molecular basis for why the VP16PPARα fusion protein lacks the ability to induce all of the molecular changes required for hepatocarcinogenesis, and why in contrast PPARα activation by chemical activators is actually sufficient to induce hepatocarcinogenesis by the PPARα MOA.
Guyton et al. (2009) also did not account for the fact that there are molecular differences between the mechanism of hepatocyte proliferation induced by VP16PPARα and that induced by ligand-activated PPARα. Indeed, global transcriptional responses compared between wild-type mice treated with WY and VP16PPARα transgenic mice, revealed a class of genes linked to hepatocyte proliferation and DNA repair induced by WY but not VP16PPARα (Qu et al., 2010). For example, c-Myc, a critical regulator of hepatocyte proliferation was unchanged in the VP16PPARα transgenic mouse liver but is consistently induced by PPARα activators (Cherkaoui-Malki et al., 1990; Miller et al., 1996; Shah et al., 2007; Qu et al., 2014). It has also been shown that c-Myc is required for WY-dependent increases in hepatocyte proliferation (Qu et al., 2014). The difference in transcriptional activation between wild-type mice treated with WY and VP16PPARα transgenic mice cannot be explained by an alternative target of WY as these transcripts are also absent in treated PPARα-null mice (Rosen et al., 2017; Qu et al., 2010). While the VP16PPARα transgenic mouse line is an interesting experimental model, the experiments by Yang and colleagues did not demonstrate uncoupling of cell proliferation and liver cancer by PPARα activators. Rather as noted by the authors, the studies provided evidence that PPARα activity in NPCs appears to be required to cause liver cancer. The model does not provide evidence that there is no link between cell proliferation and liver tumor induction in wild-type mice as part of the PPARα MOA as claimed by Guyton et al. (2009). The observed “uncoupling” of hepatocyte proliferation and liver cancer in the VP16PPARα transgenic mouse line as compared to mice exposed to PPARα activators is due to vastly different molecular events that exist between the two models. Wild-type mice treated with PPARα activators require a c-Myc-dependent pathway for hepatocyte proliferation as well as effects in NPCs for hepatocarcinogenesis, both of which do not occur in the transgenic mice expressing a VP16PPARα fusion protein, due to differences in the function of the VP16PPARα fusion protein, not a target other than PPARα in wild-type mice.
4. Mechanistic links between KEs.
The mechanistic links between the KEs in the PPARα MOA can be assessed by perturbing a KE and determining whether the downstream KEs are altered in a consistent manner. If there is a mechanistic link, there should be effects on those KEs that are downstream from the perturbed KE but not necessarily on the preceding KEs. Guyton et al. (2009) suggested that the KEs in the PPARα MOA were not mechanistically linked but were correlative in nature. Guyton et al. wrote “The limited database of other studies that empirically challenge the necessity or sufficiency of the PPAR-α activation MOA in hepatocarcinogenesis per se also motivates a reexamination of whether this MOA hypothesis should be used as the basis for dismissing the human relevance of effects observed in laboratory animals.” However, Guyton et al. (2009) did not discuss the large number of studies published before their review that demonstrate mechanistic links between the KEs as determined in both genetic and biochemical inhibition studies (Figure 5).
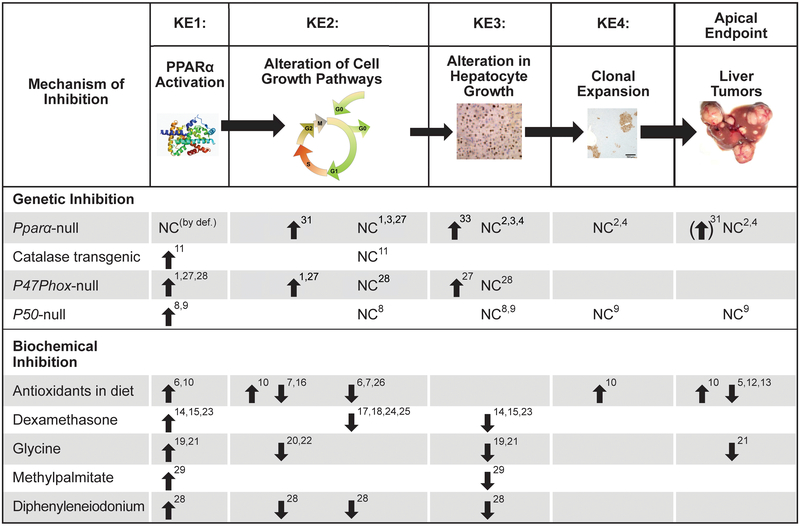
Figure 5. Effects of genetic and biochemical inhibition of key events in the PPARα MOA.
Various transgenic and null mouse models have been used to examine the PPARα MOA, including Pparα-null mice, catalase transgenic mice, p47Phox-null mice and p50-null mice. See details in text. Biochemical inhibitors have also been used as an alternative strategy to determine the relative requirement for specific KEs in the PPARα MOA. An upward pointing arrow indicates that the chemical or genetic inhibition had little, if any effect on that KE/endpoint after exposure to a PPARα activator. A downward pointing arrow indicates suppression of the KE/endpoint. NC (no change) indicates that the inhibition did not change the KE/endpoint.
References: 1Woods et al., 2007a; 2Peters et al., 1997; 3Peters et al., 1998; 4Hays et al., 2005; 5Rao et al., 1984 (ethoxyquin, 2(3)-tertbutyl-14-hydroxyanisole); 6Calfee-Mason et al., 2004 (Vit E); 7Li et al., 2000a (in vitro studies with Vit E treated H4IIE3C cells); 8Tharappel et al., 2003; 9Glauert et al., 2006; 10Glauert et al., 1990 (Vit E increases the number of tumors while depleting glutathione reserves); 11Nilakantan et al., 1998; 12Rao and Subbarao, 1999 (dimethylthiourea); 13Rao and Subbarao, 1997a (deferoxamine – iron chelator); 14Lawrence et al., 2001a; 15Rao and Subbarao, 1997b (dexamethasone); 16Stanko et al., 1995 (Vit E); 17Ray and Prefontaine, 1994; 18Widen et al., 2003; 19Rose et al., 1997a,b; 20Rose et al., 1999a (superoxide production in Kupffer cells); 21Rose et al., 1999b; 22Rusyn et al., 2001 (free radicals in bile); 23Ohmura et al., 1996 (measured peroxisomal bifunctional enzyme as PPARα marker); 24Chang et al., 1997; 25De Bosscher et al., 2006 (review); 26Rusyn et al., 1998 (allopurinol); 27Woods et al., 2007b ; 28Rusyn et al., 2000; 29Rose et al., 1997b; 32Ito et al., 2007; 33Wolf et al., 2008
If the KEs are mechanistically linked, inhibition of the first KE in the PPARα MOA should inhibit the occurrence of the downstream KEs. The effects of PPARα activators in Pparα-null mice are summarized in Figure 3. Two studies assessed the effect of PPARα activators on markers of oxidative stress in wild-type and Pparα-null mice. In the first study, abasic sites (i.e., sites that lack either a purine or a pyrimidine) in genomic DNA were used as a measure of oxidative stress. These sites were increased in wild-type but not Pparα-null mice after exposure to WY for 5 months (Woods et al., 2007b). In the second study, electron spin resonance (ESR) identified an increase in free radicals in the bile of wild-type but not Pparα-null mice after up to 3 week exposures to WY or DEHP. Bezafibrate administered to wild-type and Pparα-null mice at relatively high (100 mg/kg/day) or low (10 mg/kg/day) doses increased hepatic lipid peroxides in a PPARα-dependent manner (Nakajima et al., 2010). Hepatic NF-kB activation was observed in wild-type but not Pparα-null mice after exposure to WY (Woods et al., 2007a,b). Using global gene expression profiling, altered gene expression by WY, PFOA, PFOS, PFHXS, PFNA, DEHP, or ciprofibrate was almost completely abolished (~76-99%) in Pparα-null mice at multiple time points (Anderson et al., 2004a,b; Corton et al., 2004; Woods et al., 2007c; Rosen et al., 2008a,b; Sanderson et al., 2008; Rosen et al., 2017; Ren et al., 2010). The increased expression of the cell cycle control proteins CDK-1, CDK-2, CDK-4 and PCNA proteins and CDK-1, CDK-4 and CYCLIN D1 mRNA was observed in wild-type but not Pparα-null mice fed WY (Peters et al., 1998). Wild-type mice treated with PPARα activators exhibited increased hepatocyte proliferation compared to controls while no increases in hepatocyte proliferation were observed in Pparα-null mice after exposure to WY, diisononyl phthalate, or trichloroethylene (Peters et al., 1997, 1998; Valles et al., 2003; Laughter et al., 2004). In contrast, PFOA exposure led to increased hepatocyte proliferation in both wild-type and Pparα-null mice at 10 mg/kg for 7 days (Wolf et al., 2008) under conditions that activated CAR (Oshida et al., 2015), indicating CAR was responsible for induction of cell proliferation at these high levels of PFOA in Pparα-null mice. The ability of PPARα activators to suppress apoptosis was mitigated in similarly treated hepatocytes isolated from Pparα-null mouse livers (Hasmall et al., 2000a). Chronic treatment with WY or bezafibrate resulted in very high percentages in the incidence of hepatocellular neoplasia in wild-type mice while the Pparα-null mice were essentially unaffected (Peters et al., 1997; Hays et al., 2005; Morimura et al. 2006). A single adenoma was found in one bezafibrate-treated Pparα-null mouse but as noted above for the Ito et al. study, the tumor was most likely due to the presence of lipid accumulation, inflammation and other molecular changes associated with these changes, and not due to the PPARα MOA. Collectively, these studies demonstrate that all of the KEs in the PPARα MOA that are induced by PPARα activators are abolished in the absence of a functional PPARα.
Two transgenic mouse models have been used to determine the relationships between different sources of oxidative stress and downstream events. Catalase converts hydrogen peroxide to water and oxygen. In catalase-transgenic mice that exhibit increased expression and activity of catalase in the liver, there were decreased levels of NF-kB activation and decreased hepatocyte proliferation upon exposure to ciprofibrate (Nilakantan et al., 1998). NADPH oxidase in Kupffer cells plays an important role in generating superoxide radicals in response to Kupffer cell activators (De Minicis et al., 2006). NADPH oxidase is activated by PPARα activators and is important in cell proliferation after short-term PPARα activator exposure. Mice that lack one of the subunits of NADPH oxidase (the p47Phox-null mice) did not exhibit increases in oxidative stress, NF-kB activation, and hepatocyte proliferation after short-term PPARα activator exposure (Rusyn et al., 2000). However, exposure of mice to WY for three weeks led to increases in indicators of oxidative stress (including PCO activity), NF-kB activation, and cell proliferation, independent of the status of the p47Phox gene; these KEs were dependent on PPARα (Woods et al., 2007a,b). Longer-term exposure may allow bypass of p47Phox dependence by increasing oxidative stress through activation of enzymes that produce hydrogen peroxide. Although not performed in a transgenic mouse model, overexpression of ACO (encoding PCO activity) in COS-1 cells, in the presence of a hydrogen peroxide-generating substrate was found to activate an NF-kB-regulated reporter gene in the absence of a PPARα activator (Li et al., 2000b).
NF-kB activation is involved in modulation of hepatocyte fate in response to inducers of oxidative stress (e.g., Maeda et al., 2005) including PPARα activators. Wild-type mice and mice deficient in the p50 subunit of NF-kB (p50-null mice) were fed a diet with or without 0.01% ciprofibrate for 10 days. NF-kB DNA binding activity was increased after ciprofibrate treatment in wild-type mice but not p50-null mice. Ciprofibrate treated p50-null mice exhibited lower levels of hepatocyte proliferation than similarly treated wild-type mice (Tharappel et al., 2003). The p50-null mice were resistant to liver tumor induction after activation of PPARα. Wild-type mice treated with DEN only exhibited a low incidence of liver tumors (25%). Wild-type mice treated with both DEN + WY showed a liver tumor incidence of 63%. In contrast, no increase in liver tumors was found in the DEN only or DEN + WY treated p50-null mice, demonstrating that the p50 subunit of NF-kB was required for the promotion of hepatic tumors by the PPARα activator WY. These studies demonstrate that disruption of NF-kB expression leads to downstream suppression of cell proliferation and liver tumor induction.
Studies using chemical inhibitors of oxidative stress or inflammation also highlight linkages of the KEs in the PPARα MOA. In these studies, animals were pretreated with the inhibitor prior to PPARα activator exposure or co-treated with a PPARα activator and the inhibitor. The free radical scavenger, allopurinol, inhibited the activation of NF-kB in the livers of WY-treated rats compared to controls (Rusyn et al., 1998). In in vitro studies, the anti-oxidants vitamin E or N-acetylcysteine blocked the ability of NF-kB to activate a reporter gene in ciprofibrate-treated HIIE3C cells (Li et al., 2000a). The antioxidant vitamin E inhibited clofibrate-induced increases in lipofuscin-like products, a measure of oxidative stress and ciprofibrate-induced increases in NF-kB activation in the absence of effects on markers of PPARα activation (Stanko et al., 1995; Calfee-Mason et al., 2004). Co-treatment with ciprofibrate and one of two anti-oxidants, 2(3)-tert-butyl-14-hydroxyanisole or ethoxyquin decreased the incidence and size of liver tumors in rats compared to ciprofibrate treatment alone (Rao et al., 1984). In similar studies, the antioxidants dimethylthiourea or deferoxamine decreased the incidence of liver tumors in rats fed ciprofibrate (Rao and Subbarao, 1997a, 1999). Paradoxically, the antioxidant vitamin E depleted levels of the antioxidant glutathione and the animals exhibited increased tumor numbers after ciprofibrate treatment (Glauert et al., 1990). These studies demonstrate that suppression of oxidative stress blocks, or suppresses, the downstream events of NF-kB activation and liver tumor induction.
Inhibition of downstream KEs by compounds that alter NF-kB and Kupffer cell activation has been observed in multiple studies. The glucocorticoid receptor agonist dexamethasone is an anti-inflammatory agent that decreases the ability of NF-kB to be activated under a variety of inflammatory conditions (Ray and Prefontaine, 1994; Chang et al., 1997; De Bosscher et al., 2006). Dexamethasone decreased PPARα activator-induced hepatocyte proliferation after acute exposures (Lawrence et al., 2001a; Rao and Subbarao, 1997b; Ohmura et al., 1996) while having either no effect (Lawrence et al., 2001a; Rao and Subbarao, 1997b) or modest decreases (Ohmura et al., 1996) on markers of PPARα activation. Compounds that inhibit Kupffer cell activation (glycine, methylpalmitate) or inhibit NADPH oxidase (diphenyleneiodonium) attenuated increases in oxidative stress and NF-kB activation after exposure to PPARα activators but had no effects on markers of PPARα activation (Rose et al., 1997a,b; Rose et al., 1999a,b; Rusyn et al., 2001; Rusyn et al., 2000). While pretreatment with diphenyleneiodonium, glycine or methylpalmitate decreased acute cell proliferation (Rose et al., 1997a,b; Rusyn et al., 2000; Rose et al., 1999a), glycine had no effect on chronic cell proliferation (Rose et al., 1999b). However, under these same conditions, glycine did decrease the size and number of tumors (Rose et al., 1999b).
Reddy and coworkers originally proposed that peroxisomal ACO (encoded in mice by Acox1) is the enzyme responsible for oxidative stress-induced DNA damage in liver tumors by PPARα activators (Nemali et al., 1988). ACO was not only found to be dispensable for increases in oxidative stress, but control Acox1-null mice exhibited the phenotype of wild-type mice exposed to PPARα activators including increases in oxidative stress, increased hepatocyte proliferation, and induction of liver tumors that were dependent on PPARα (Fan et al., 1998; Hashimoto et al., 1999). The molecular profile of the spontaneously-induced tumors in the Acox1-null mice was very similar to that for liver tumors induced by the PPARα activator ciprofibrate based on microarray analysis, indicating that the mechanisms leading to the induction of the tumors were similar in the Acox1-null mice and mice treated with a PPARα activator (Meyer et al., 2003). Additional mouse models nullizygous for other genes involved in fatty acid β-oxidation have been created that have phenotypes indicative of constitutive PPARα activation (Jia et al., 2003), but no studies to date have examined aged nullizygous mice to evaluate background tumor incidence. Importantly, a mouse model of hepatitis C virus (HCV)-induction of hepatocellular carcinoma in which the HCV core protein is overexpressed showed that PPARα was required for liver tumor induction in 2-year-old mice (Tanaka et al., 2008a,b). In these studies, changes in a number of the key events or modulating factors involved in the PPARα MOA were similar to that of a typical PPARα activator including induction of oxidative stress and increases in cell proliferation. These results demonstrate that the PPARα MOA is operational in the absence of exogenous chemical exposure. PPARα activators, whether they are endogenous nutritional components or exogenous chemicals, can activate the PPARα MOA resulting in liver tumors. Taken together, these biochemical and genetic inhibition studies demonstrate the large number of interconnecting linkages of the KEs in the PPARα activator MOA that were not discussed in the Guyton et al. review.
5. Consistency of responses across chemicals
The Guyton et al. review (2009) also suggested that, “These considerations also highlight the need for a more robust database for compounds of environmental concern that activate PPAR-α, such as phthalates, perfluorinated acids, chlorinated solvents, and chloroacetic acids, either alone or in combinations relevant to human exposures.” Most of the data to support the evidence of KE modulation by many chemicals are reported in the Klaunig et al. (2003) review. As noted above, the effects of 10 structurally diverse PPARα activators in the livers of mice and rats on the KEs in the PPARα MOA are summarized from the Corton et al. (2014) review (Figures 1 and 2). The chemicals were selected for analysis, because of the large number of studies examining the effects of these compounds on endpoints that measure key events in the PPARα MOA. The information is presented in a way that showed the relationships between PPARα activator exposure and KE modulation. Overall, there was remarkable consistency in effects on the KEs for these compounds including members of the chemical classes mentioned by Guyton et al (2009).
6. Use of WY as the test agent.
Another criticism of the evidence supporting the rodent PPARα MOA is that mechanistic analyses was based largely on one PPARα activator, WY. Guyton et al. (2009) suggested that “The extensive research focus on WY-14,643 is particularly problematic, because a) it is typically administered at necrogenic doses well above those required for maximal responses; b) it is one of the few agonists that produce sustained, as opposed to only transient, enhancement of DNA synthesis in hepatocytes; c) unlike many other agonists, it preferentially activates rodent forms of PPAR-α (with humans exhibiting ~20-fold less sensitivity); and d) humans apparently have never been exposed to it, either in an experimental or clinical setting.” These statements are misleading or not based on the facts. WY is a very specific activator of PPARα. As mentioned above, global transcript profiling experiments showed that PPARα is required for WY to alter the expression of ~98-99% of all genes in the mouse liver (Anderson et al., 2004a,b; Corton et al., 2004; Rosen et al.,2008a,b; Woods et al., 2007c; Rosen et al., 2017). Thus, WY is an excellent established model compound to study PPARα-dependent effects in the absence of “off-target” effects that complicate studies with other (less specific) compounds. It is true that WY has never been administered to humans in the clinic, and that WY has often been used as a model PPARα agonist especially in transgenic or nullizygous studies carried out in a number of laboratories (e.g., Qu et al., 2010; Shah et al., 2007; Morimura et al., 2006; Anderson et al., 2004a,b). However, most of the data that supports the MOA including the mechanistic studies mentioned above were obtained from experiments that used other PPARα activators (see Figures 1-3). The idea that all studies using WY not only lead to PPARα activation but to necrosis/cytotoxicity, is not supported by the literature. For example, many studies have used dietary WY at a concentration of 0.1% or lower to examine effects induced by PPARα. Necrosis is not observed in mouse liver after WY treatment unless a concentration 0.1% is fed to mice (Cunningham, 2007). Similarly, Woods et al. (2007) reported that WY induced liver cytotoxicity but only in rodents treated with exceptionally high doses of WY. To our knowledge, there are no comprehensive studies that have been performed to date demonstrating that, “WY-14,643….is typically administered at necrogenic doses well above those required for maximal responses” (Guyton et al., 2009). This is a misleading statement not supported by the scientific literature. As indicated in Figures 1 and 2, out of the 6 compounds tested, 5 compounds (WY, clofibrate, nafenopin, ciprofibrate, and methylclofenapate) clearly exhibited sustained hepatocyte proliferation in rats. The sixth compound DEHP, caused increases in chronic proliferation in one study but not the other. In mice, out of the two compounds examined, both WY and DEHP caused sustained increases in cell proliferation. Although Guyton et al. (2009) do not explain the basis for saying that human PPARα is ~20-fold less sensitive to WY than the rodent PPARα, Maloney and Waxman (1999) did find ~20-fold difference in the concentration required to maximally activate in transactivation assays. The differences between mouse and human PPARα activation responses for WY are consistent with many other compounds tested: most compounds have either about the same potency or less potency for the human PPARα vs. the mouse PPARα in trans-activation studies. Differences in responsiveness of human vs. rodent PPARα are discussed below. It is critical to note that differences in trans-activation do not necessarily parallel functional effects on gene activation due to many factors that are missing in trans-activation assays (discussed above). In side by side comparisons between activation of mouse or human PPARα in the context of primary hepatocytes, equal concentrations of WY did not lead to greater numbers or fold-changes of gene expression changes in mouse hepatocytes versus human hepatocytes (Rosen et al., 2013). In summary, a thorough analysis of the literature shows that the PPARα MOA is based not only on mechanistic studies using WY but on many structurally-diverse chemicals that exhibit consistent effects on the KEs in the PPARα MOA.
7. Pleiotropy of PPARα activator effects.
It is widely recognized that environmentally relevant chemicals can mediate toxicity by interactions with one or more molecular targets. Even drugs designed to specifically modulate one target have “off-target” effects. Numerous examples of this pleiotropy are found in the ToxCast screening program in which ~1000 compounds have been examined for their ability to cause effects in ~330 high throughput assays (Sipes et al., 2013). Guyton et al. (2009) have expressed concern that despite the overwhelming evidence for the rodent PPARα MOA that there could be other effects important in mediating hepatocarcinogenesis. Guyton et al. (2009) state, “Indeed, the compounds that activate PPAR-α are pleiotropic and have been reported to exhibit a diversity of responses in addition to the hallmark effect of peroxisome proliferation, including genotoxicity (reviewed by Melnick 2001), epigenetic alterations (e.g., hypomethylation) (Pogribny et al. 2007), oxidative stress (reviewed in O’Brien et al. 2005), and effects on other receptors (e.g., Guo et al. 2007) and other organelles (e.g., mitochondria) within parenchymal cells (Lundgren et al. 1987; Scatena et al. 2003; Youssef and Badr 1998; Zhou and Wallace 1999).” (See Guyton et al. for references.) It is important to note that Guyton et al. (2009) do not provide scientific evidence that any of these effects support a different PPARα MOA from that presented above. Thus, the statement above misleads readers not familiar with the primary literature into thinking that there must be other mechanisms that explains how these chemicals cause liver cancer. Thorough examination of the literature indicates that the consensus among experts is that PPARα activators are not directly genotoxic (reviewed in Klaunig et al., 2003). As discussed above there is evidence that PPARα activators may damage DNA indirectly through increases in oxidative stress. PPARα activators may cause effects by interacting with other receptors including CAR (discussed above in the context of the Ito et al. 2007 study), PPARγ (discussed above regarding results of trans-activation assays), and estrogen receptor (Rosen et al., 2017). Epigenetic effects can be found after exposure to most if not all compounds (reviewed in Pogribny, 2009; Romagnolo et al., 2014). Global DNA methylation, methylation of histone H4K20 and histone H3K9 were PPARα-dependent as they occurred in wild-type but not Pparα-null mice after treatment of WY at 1, 5, and 21 weeks (Pogribny et al., 2007). Some PPARα activators have effects on mitochondrial bioenergetics and biogenesis, but these changes have not been linked to liver cancer (Zhou and Wallace, 1999; Walters et al., 2009). Keshiva and Caldwell (2006) frequently mentioned effects of PPARα activators in other tissues even though there are no known linkages between those effects and the PPARα MOA for liver cancer. The collective data indicate that in spite of this diversity of responses, the fact that the KEs in the PPARα MOA are altered in predictable ways by structurally diverse compounds provides strength to the weight of evidence supporting the PPARα MOA.
8. Comparison of potencies and dose-response relationships of PPARα activators.
If the rodent PPARα MOA is correct, the potency for PPARα activation or downstream KEs should be quantitatively related to the relative carcinogenic potency between PPARα activators. If so, differences in carcinogenesis sensitivity could be approximated using quantitative information derived from dose-response assessments of the KEs. Guyton et al. (2009) compared disparate dose-response data from studies examining the effects of PPARα activators on PPARα activation in in vitro trans-activation studies and on liver cancer. Cancer data came from the Carcinogenic Potency Database (CPDB), which summarizes multiple carcinogenesis studies for a compound and derives a TD50 (mg/kg/day) defined as the daily dose inducing tumors in half of the mice that would otherwise have remained tumor-free (Gold et al. (2005). Guyton et al. (2009) stated that “WY-14,643 and MEHP activate PPAR-α at comparable concentrations when directly compared in the transactivation assay” even though WY was “>65-fold” more potent than DEHP in inducing liver cancer. Guyton et al. (2009) conclude that “Together, these findings underscore the significant chemical-specific quantitative differences in these markers that limit their utility for predicting carcinogenic dose–response relationships.” A closer inspection of all relevant data indicates that their conclusion was not correct.
Guyton et al. (2009) used transactivation data from the Maloney and Waxman (1999) study who examined the effects of a number of PPARα activators, using WY as a reference compound, for activation of the mouse PPARα. Guyton et al. (2009) somehow calculated EC50s and EC2-folds from the Maloney and Waxman data even though Maloney and Waxman did not calculate or report these values. Guyton et al. (2009) claimed that the EC50s for WY and MEHP were 0.63 and ~0.7 uM, respectively and for EC2-fold were ~0.4 and ~0.7 uM, respectively. It is entirely unclear how Guyton et al. (2009) derived these values in the absence of the raw data, and these values are not consistent with what is found in the Maloney and Waxman study. Even by “eyeballing” the graphs, an EC50 for WY can be approximated as ~0.02 μM as the response was maximal at 1 μM (from Figure 1 of Maloney and Waxman, 1999) and an EC50 for MEHP can be approximated at ~0.5 μM as the maximal response was at 20 μM (from Figure 3C of Maloney and Waxman, 1999). The analysis results by Guyton et al. (2009) are not only inconsistent with the data in the Maloney and Waxman study but inconsistent with the results in three independent studies that examined activation of PPARα by MEHP using WY as a positive control. Issemann and Green (1990) carried out a dose response for six structurally-diverse compounds and showed that WY and MEHP have ED50s of 1.5 μM and 50 μM, respectively, a ~33-fold difference in potency. Bility et al. (2004) showed that MEHP exhibits markedly lower activity (~3-fold) compared to WY at approximately equal concentrations. Lapinskas et al. (2005) showed that a reporter gene was activated to a much higher level by WY compared to MEHP at any but the highest dose tested (200 μM). Additionally, MEHP binding to human PPARα was ~10-fold weaker than WY (Ki = 83 μM vs. 9 μM for MEHP and WY, respectively) (Lapinskas et al., 2005). It is important to note that while there are differences in the types of trans-activation assays performed by these different research groups, the results are consistent. Thus, the weight of evidence indicates that WY activates PPARα at lower concentrations than MEHP and is more efficacious resulting in greater levels of activation than MEHP (Isseman and Green, 1990; Lapinskas et al., 2005).
The “> 65-fold” greater potency of WY than DEHP in liver tumor induction as suggested in the Guyton et al. (2009) analyses is consistent with WY being a more potent activator of PPARα than MEHP in vitro. Guyton et al. (2009) did not discuss the fact that we cannot assume that there will always be a strict quantitative relationship between potencies of PPARα activators in vitro and potencies of PPARα activators in vivo in the absence of assessment of factors that allow in vitro to in vivo extrapolations. In this regard Guyton et al. (2009) did not thoroughly discuss the contribution of differences between DEHP and WY in absorption, distribution, metabolism, and excretion that determine tissue dose. This is especially important in the present case as DEHP but not WY requires metabolism in the gut to MEHP. Questions remain as towhether the metabolism in gut and liver is saturable (Rowland, 1974; Kessler et al., 2004). If the data were available, a more accurate comparison of potencies would be to compare the levels of MEHP and WY in the blood or liver under conditions that lead to liver cancer and compare those levels to the activation of KEs in the PPARα MOA.
Dose-response relationships between activation of KEs can provide another test of the predicted sequence of KEs in a MOA. If the MOA is relevant, doses that activate one KE should also be sufficient to activate preceding KEs but not necessarily those KEs that are more proximate to cancer. Very few examples are found in the literature in which comparisons can be made of the dose-dependent relationships between most of the KEs in the PPARα MOA. For three PPARα activators, the findings are consistent with the linkage of the KEs. For DEHP, the KEs closer to the apical event (liver tumor induction) require greater DEHP levels in the diet to be induced (Isenberg et al., 2000; David et al., 1999; David et al., 2000a; David et al., 2000b). At 500 ppm, markers of PPARα were induced, while at 2500 ppm there were alterations of cell growth pathways (in this case, inhibition of gap junction intercellular communication), cell proliferation, and increases in hepatocellular adenomas.
Downstream KEs are induced by gemfibrozil at doses higher than those that activate PPARα (analysis described in Corton et al., 2014 using data from Cunningham et al., 2010). The effective concentration for a 50% increase in the response (EC50) was approximately the same for fatty acid β-oxidation (used here as a surrogate for PPARα activation) and relative liver weight (EC50 = 3862 and 3297 ppm gemfibrozil in the diet, respectively), whereas hepatic cell proliferation was induced at higher concentrations in the diet (EC50 = 17,309 ppm).
For trichloroethylene (TCE), palmitoyl-CoA oxidase activity was increased consistently at 100 mg/kg/day, hepatocyte proliferation was increased at 300 mg/kg/day, and liver cancer was increased at 850 mg/kg/day (analysis described in Corton, 2008). These three examples highlight the consistency in the dose-response relationships between surrogates of PPARα activation, downstream KEs, and liver cancer.
Species differences in the PPARα MOA
Studies conducted in numerous test species demonstrate that while mice and rats are responsive to PPARα activator-induced liver cancer and associated responses, other species (e.g. Syrian hamsters, guinea pigs, New and Old World primates and humans) are less sensitive or insensitive (Ashby et al., 1994; Bentley et al., 1993; Cattley et al., 1998; Doull et al., 1999). Figure 6 summarizes PPARα MOA KEs in Syrian hamsters, guinea pigs, cynomolgus monkeys, and humans. Because of the paucity of data for KEs in species other than rats and mice, other endpoints more commonly measured in these studies and associated with exposure to PPARα activators are discussed (i.e., relative liver weight and hypolipidemic effects). It is worth noting that there are inherent difficulties in extrapolating data from animal models to humans, due in large part to the lack of comparable data that are not available due to ethical reasons.
Figure 6. Species Differences in the Responses to PPARα Agonists.
The effects of various PPARα activators have been examined in different species including Syrian hamsters, guinea pigs, cynomologus monkeys, and humans. Note: PPARα activation is a summary of trans-activation data as well as response of markers such as ACO (or PCO) and CYP4A, which are biomarkers of PPARα activation and are dependent on level of PPARα expression. Hypolipidemic effects are measured by decreases in triglycerides or VLDL-triglycerides. The table does not include PCO data from monkey species other than cynomolgus monkeys as other monkey data (which is almost universally negative) is summarized in Klaunig et al., 2003. An upward pointing arrow indicates that the chemical was found to lead to the KE/endpoint. A downward pointing arrow indicates suppression of the KE/endpoint. Upward arrows in parentheses indicated weak increases. NC (no change) indicates that the chemical did not change the KE/endpoint. Compounds used to treat humans or primary human hepatocytes are indicated in the footnotes.
- Styles et al., 1988
- Lake et al., 2000; Styles et al., 1988
- Hanefeld et al., 1983 (clofibrate)
- Hanefeld et al., 1980 (clofibrate); De La Iglesia et al., 1982 (gemfibrozil); Blumcke et al., 1983 (fenofibrate); Gariot et al., 1983 (fenofibrate) (review); Bentley et al., 1993 (review); Shaw et al., 2002 (monoisononylphthalate)
- Perrone et al., 1998 (ciprofibrate; clofibric acid); Goll et al., 1999 (ciprofibrate; bezafibrate; nafenopin; clofibrate; DEHP); Hasmall et al., 1999 (monoethylhexylphthalate; diisononylphthalate); Hasmall et al., 2000b (DEHP); Shaw et al., 2002 (monoisononylphthalate)
- Hasmall et al., 1998 (nafenopin); Goll et al., 1999 (ciprofibrate; bezafibrate; nafenopin; clofibrate; DEHP); Hasmall et al., 1999 (monoethylhexylphthalate; diisononylphthalate); Shaw et al., 2002 (monoisononylphthalate)
- Cherkaoui Malki et al., 1999 (no change in myc)
- Hoivik et al., 2004 (cyclins)
- Thomas et al., 2015 (myc and cyclins)
A partial PPARα activator response was observed in Syrian hamsters and guinea pigs even though they are often considered “non-responsive” species compared to rats and mice. PPARα activators WY and methylclofenapate decreased triglycerides or VLDL-triglycerides in Syrian hamsters and guinea pigs. No changes in Myc were observed in guinea pigs treated with ciprofibrate. Five of the six PPARα activators examined increased relative liver weights in Syrian hamsters. Only one chemical out of seven examined in guinea pigs increased relative liver weight and for that chemical (perfluorodecanoic acid), there was conflicting evidence of increases in the two studies. Studies measuring changes in hepatocyte proliferation in Syrian hamsters showed either a weak response, no response, or inconsistent results. Guinea pigs consistently did not exhibit increases in cell proliferation after exposure to four different chemicals. Syrian hamsters exhibited suppression of apoptosis after exposure to nafenopin. Guinea pigs exhibited suppression of apoptosis with nafenopin but not with methylclofenapate. WY did not activate NF-kB in the livers of hamsters, indicating that this response is species-specific. Cancer bioassays performed in Syrian hamsters with nafenopin, WY, and DEHP were all negative (Lake et al., 1993; Schmezer et al., 1988). In summary, Syrian hamsters and to a lesser extent guinea pigs exhibited changes in endpoints associated with PPARα activation (hypolipidemic effects and changes in fatty acid metabolizing enzymes). However, these species do not exhibit consistent changes in KEs in the liver cancer PPARα MOA.
In vitro and in vivo data from cynomolgus monkeys (Figure 6) and from other species of monkeys (marmoset, Rhesus) indicate that the KEs following PPARα activation do not occur. Palmitoyl-CoA oxidase activity was evaluated in monkeys after in vivo exposure to a variety of PPARα activators (e.g., bezafibrate, clofibrate, DEHP, MEHP, fenofibrate, nafenopin and LY171883); the changes were minimal or did not change relative to controls (summarized in Klaunig et al., 2003). No changes in cyclins were observed after exposure to ciprofibrate or fenofibrate. Moreover, cynomolgus monkeys failed to exhibit an increase in hepatocyte proliferation following exposure to DEHP, di-isononyl phthalate (DINP), or clofibrate (Doull et al., 1999; Pugh et al., 2000). After a two-week treatment with clinically relevant doses of fenofibrate or ciprofibrate, cynomolgus monkeys exhibited increases in the number of hepatic peroxisomes but not peroxisome area (Hoivik et al., 2004). In this study, ciprofibrate but not fenofibrate, significantly increased relative liver weights; hepatocyte proliferation was not observed after either exposure. Transcript profiling was used to characterize the genes altered by ciprofibrate exposure in the livers of treated monkeys from the Hoivik et al. (2004) study. Many genes involved in fatty acid metabolism and mitochondrial oxidative phosphorylation exhibited increased expression reflecting the known effects of exposure on lipid metabolism, but the magnitude of induction in the β-oxidation pathway was substantially less in monkeys compared to mice and rats (Cariello et al., 2005). Consistent with the lack of hepatocyte proliferation, exposure led to decreased expression of a number of key hepatocyte proliferation regulatory genes including members of the JUN, MYC and NF-kB families; in contrast, rats exposed to the peroxisome proliferator BR-931 exhibited increased expression of JUN and MYC gene expression (Hsieh et al., 1991). Additionally, there were no transcriptional changes typical of DNA damage or oxidative stress observed in the monkey livers (Cariello et al., 2005). Lastly, marmosets exposed for 6.5 years to clofibrate at clinically relevant doses (94 mg/kg day or higher) did not develop liver tumors or increases in other indicators of KEs in the MOA over the duration of this study (Tucker and Orton, 1995), but it should be noted that the duration of this study did not represent a lifetime exposure for marmosets. Taken together, these studies in monkeys and marmosets indicate that there is no evidence that the KEs downstream of PPARα activation are activated in primates treated with PPARα activators at doses similar to which mice and rats have been exposed.
There is overwhelming evidence that humans are not responsive to the carcinogenic effects of PPARα activators. One study measured changes in liver size in patients treated with fenofibrate and no changes were noted (Gariot et al., 1987). Biopsies from the livers of humans treated with hypolipidemic drugs or primary human hepatocytes treated with PPARα activators were almost uniformly negative for peroxisome proliferation (reviewed in Bentley et al., 1993). In one out of five studies there was a statistically significant increase in peroxisome number (~50%) but in the absence of a corresponding increase in volume of peroxisomes (Blumcke et al., 1983; De La Iglesia et al., 1982; Gariot et al., 1983; Hanefeld et al., 1980, 1983).
While there are no data on human hepatocyte proliferation in vivo, non-human primate data from in vivo studies collectively show that hepatocyte proliferation was not induced by PPARα activators (Figure 6 and reviewed in Doull et al., 1999). No increases in c-Myc and cyclins were seen in human primary hepatocytes treated with WY. The consistent lack of proliferation response in human primary hepatocytes in multiple studies is described below.
The effects of fenofibrate were investigated using a hepatocyte-humanized chimeric mouse model in which mouse hepatocytes were replaced with >70% human hepatocytes. Fenofibrate induced hepatocellular hypertrophy, cell proliferation, and peroxisome proliferation in livers of mice containing all mouse hepatocytes, as expected, but not in the human hepatocytes in the chimeric mouse livers (Tateno et al., 2014).
Molecular basis for species differences
There are many differences in the structural and functional properties of PPARα that exist between species. These include the cellular expression patterns of PPARα and many other co-effector proteins that interact with PPARα, cellular expression patterns of chromatin remodeling proteins, the relative availability of chromatin for PPARα binding sites, and differences in the stoichiometry and relative binding affinities between all of these variables. These differences likely determine, at least in part, the underlying basis for human-rodent differences in PPARα activator biological effects. The full-length human PPARα is fairly comparable in overall structure from that in rodents (Mukherjee et al., 1994; Sher et al., 1993; Tugwood et al., 1996), and thus differences in responses must be based on other characteristics of the human receptor. PPARα expression is the most often cited factor for determining species-specific differences in PPARα activator responsiveness. In a side-by-side comparison, mice exhibited ~3-fold more PPARα mRNA expression than partially responsive Syrian hamsters and ~10-fold more PPARα mRNA than nonresponsive guinea pigs (Choudhury et al., 2004). Studies of human liver indicate that PPARα is expressed at lower levels compared to responsive species. Palmer et al. (1998) used electrophoretic mobility shift assays (EMSA) to determine the level of PPARα protein in liver samples capable of binding the human CYP4A6 PPRE. In lysates from seven individual human livers in which PPARα could be detected by the assay, the levels of PPARα protein were ~10-fold lower than those detected in the livers of CD-1 or BALB/cByJ mice. For the remaining 13 individual human livers, the levels were below detection (>20-fold less than mouse liver). A ~3-fold variation in the expression of the full-length PPARα mRNA between human samples was noted. In another study using mouse and human hepatocyte cultures, the authors found that PPARα mRNA in humans was only slightly lower compared to mice (Rakhshandehroo et al., 2009). It should be noted that this study did not evaluate protein expression or expression of the truncated form of PPARα (discussed below). Overall, the data suggest that PPARα mRNA and protein may be expressed at lower levels in human liver than in rodent liver.
A common PPARα protein variant has been identified in a number of labs and is called hPPARα-8/14 (Tugwood et al., 1996), hPPARSV (Palmer et al., 1998), PPARαtr (Gervois et al., 1999), PPARα2 (Hanselman et al., 2001), and PPARα-tr (Thomas et al., 2015). Due to alternative splicing this truncated form lacks exon 6, resulting in premature termination of the protein. The resulting protein lacks the hinge region between the DNA binding domain and the ligand domain as well as the ligand binding domain itself. This form acts as a dominant negative of the full length PPARα, inhibiting the ability of the wild-type receptor to activate transcription (Thomas et al., 2015), possibly by titrating out limiting amounts of co-activators (Gervois et al., 1999). The mRNA level of the truncated form ranges from 10% to 50% of full-length hPPARα mRNA (Gervois et al., 1999; Hanselman et al., 2001; Palmer et al., 1998; Roberts et al., 2000) similar to that found in Cynomolgus monkeys (Hanselman et al., 2001). By comparison, mice and rats express the truncated protein at less than 10% of the full length receptor (Hanselman et al., 2001). In a recent study with a large cohort of samples (n=150), mean absolute transcript levels of PPARα-tr were ~5-fold lower compared to the full length receptor, whereas the truncated protein was expressed at ~3-fold lower than the wild type protein (Thomas et al., 2015). Selective gene silencing of either form in primary human hepatocytes showed that while the full-length PPARα regulates metabolic genes including those involved in metabolism of lipids and lipoproteins, the truncated PPARα functions as an endogenous inhibitor of proliferative and pro-inflammatory genes (Thomas et al., 2015). Thomas et al. suggest that the truncated PPARα splice variant functions as an endogenous inhibitor of proliferative and pro-inflammatory genes in human hepatocytes the absence of which in the mouse may explain species-specific differences in PPARα activator-induced hepatocarcinogenesis.
Differences in the sequence of the LBD between rodent and human PPARα could lead to differences in the efficacy (maximum level of activation) and potency usually measured at the effective concentration that leads to a half maximal response (EC50). In side-by-side assays human PPARα is generally less sensitive than rodent PPARα to activation by PPARα activators. Most PPARα activators activate mouse or rat PPARα better than human PPARα or exhibit no differences between species. Hypolipidemic agents and environmentally relevant PPARα activators were able to activate rat or mouse PPARα at lower concentrations or to higher absolute levels than human PPARα in side-by-side trans-activation studies. The PPARα activators included WY (Keller et al., 1997; Maloney and Waxman, 1999; Takacs and Abbott, 2007), perfluorooctanesulfonate (PFOS) (Shipley et al., 2004; Takacs and Abbott, 2007), and a number of phthalate ester metabolites (Bility et al., 2004; Lapinskas et al., 2005). Some PPARα activators showed no differences in activation between mouse and human PPARα, including trichloroacetate (TCA), dichloroacetate, 2-ethylhexanoic acid (Maloney and Waxman, 1999), a number of phthalates (Bility et al., 2004), and clofibrate (Keller et al., 1993). PFOA was found to be less potent in activating human PPARα compared to the mouse PPARα (Maloney and Waxman, 1999). In another study, PFOA significantly activated the human and mouse PPARα at 50 uM and above while the rat PPARα was activated at 100 uM and above (Vanden Heuvel, 2006). Perfluorooctanesulfonamide (Shipley et al., 2004) was shown to modestly activate the human but not the rodent PPARα at one lower dose (25 μM versus 34 μM in human versus mouse assays, respectively). There is only one example in the literature of a compound (an experimental hypolipidemic drug [compound 1 (3-chloro-4-((3-((3-phenyl-7-propyl-1-benzofuran-6-yl)oxy)propyl)thio)phenyl)acetic acid] (Merck Research Laboratories, Rahway, NJ)) that activated the human PPARα at much lower doses than the mouse PPARα (EC50=16 nM versus >10 μM for human PPARα versus mouse PPARα, respectively) (Lawrence et al., 2001a, b). Despite this one example, the data collectively indicate that human PPARα is generally less sensitive than the mouse or rat PPARα to activation by environmentally relevant PPARα activators.
Allelic variants of human PPARα have been identified which have properties somewhat different from the original cloned human PPARα. The L162V variant containing an amino acid change in the DNA-binding domain is found at an allelic frequency of 0.025–0.073 in an ethnically diverse set of populations (Flavell et al., 2000; Lacquemant et al., 2000; Tai et al., 2002). In subjects from Northern India by contrast, this allele is found at high frequencies (0.745) (Sapone et al., 2000). This variant lacks a response to low doses of WY but exhibits greater ligand-induced activity at higher doses compared to the wild-type receptor (up to ~4-fold difference between activation by the variant and the wild-type PPARα) (Flavell et al., 2000; Sapone et al., 2000). Humans carrying this variant exhibited a greater decrease in total serum cholesterol when administered the hypolipidemic, bezafibrate (Flavell et al., 2000). Three Asian populations studied carry a PPARα variant (V227A) within the hinge region at frequencies of 0.003–0.051 (Chan et al., 2006; Yamakawa-Kobayashi et al., 2002). This allele has been associated with decreased serum cholesterol and triglycerides in a Japanese population (Yamakawa-Kobayashi et al., 2002) and in Chinese women (Chan et al., 2006). Due to increased interactions with the nuclear receptor co-repressor (NCoR), the V227A variant of PPARα exhibited decreased responsiveness to PPARα activators (Liu et al., 2008). The human PPARα-6/29 variant containing four amino acid substitutions acts as a dominant negative that can bind to a PPRE but cannot be activated by PPARα activators (James et al., 1998a). This variant is likely very rare, as it was not detected in any of 173 human subjects from two studies (Roberts, 1999; Sapone et al., 2000). Overall, some PPARα allelic heterogeneity exists in human populations. However, no variants have been identified that exhibit differential sensitivity to low, environmentally relevant doses of PPARα activators compared to the “wild-type” human receptor.
In summary, species differences in response to PPARα activators may be due to a number of factors including relative expression of the full-length receptor to expression of a dominant negative truncated form of the receptor. Because the human receptor does not regulate genes involved in hepatocyte growth, species differences in the structure of the promotors/enhancers of these genes may also be the molecular basis for species differences in growth response to PPARα activators.
Addressing concerns regarding perceived weaknesses of data used to assess human relevance of the rodent MOA.
1. Lack of response of human primary hepatocytes to increases in proliferation.
A number of studies examined proliferative responses in human primary hepatocytes. In contrast to the studies in rat and mouse primary hepatocytes that consistently demonstrated increases in proliferation and suppression of apoptosis (discussed above), PPARα activators uniformly do not induce cell proliferation or suppress apoptosis in human hepatocytes cultured in vitro (Goll et al., 1999; Hasmall et al., 1999, 2000b; Perrone et al., 1998; Williams and Perrone, 1995). The lack of response was consistent across seven different PPARα activators tested in multiple labs. Rat primary hepatocytes treated with the same PPARα activators were used as positive controls.
A criticism of these studies from the Guyton et al. review suggested that: “The culture conditions, including lack of co-cultured nonparenchymal cells (e.g., Kupffer cells), may limit the in vitro hepatocyte proliferative response, as observed for other species (e.g., Parzefall et al. 2001).” The Parzefall et al. study demonstrated that in the absence of NPCs, the rat primary hepatocytes lose the ability to proliferate after PPARα activator exposure. The method used by Parzefall et al. to purify hepatocytes from the NPCs (Kreamer et al., 1986) included three low speed sedimentations followed by a centrifugation over Percoll to remove the NPCs. These additional steps were not used in any of the studies examining the effects of PPARα activators on proliferation of human hepatocytes, and thus it can be confidently assumed that the preparations used in these studies contained NPCs. The presence of NPCs was validated by the fact that the rat primary hepatocytes isolated using similar procedures consistently responded to PPARα activators with a proliferative response. Despite the presence of the NPCs, the human hepatocyte preparations lacked the ability to proliferate, consistent with the conclusion that human liver is refractory to the hepatoproliferative effects of PPARα activators.
2. Perceived weaknesses of the PPARα-humanized mouse models.
Two mouse strains have been created that express human PPARα in Pparα-null mice (PPARα humanized mice) (Figure 3) allowing for analysis of functions of the human PPARα in the context of the mouse liver. In the TRE-hPPARα mouse, PPARα is under the control of a liver-specific promoter and is preferentially expressed in hepatocytes (Cheung et al., 2004). The other humanized mouse (hPPARα PAC mouse) contains a 211-kilobase region encoding the regulatory and structural regions of the human PPARα gene. In this model, human PPARα is expressed in the same tissues as those of the mouse PPARα (Yang et al., 2008). Both strains express human PPARα at levels comparable to or greater than mouse PPARα in wild-type mice. After WY exposure, the humanized mice exhibit many of the typical responses of treated wild-type mice including activation of lipid metabolism and peroxisome genes, increases in peroxisome proliferation, and decreases in serum total triglycerides (Cheung et al., 2004; Morimura et al., 2006). TRE-hPPARα mice exhibited lower responsiveness of lipid metabolism genes than similarly treated wild-type mice. These attenuated responses were observed with 0.1 or 0.3 mg/kg of ammonium perfluorooctanate (APFO) for 2 weeks by gavage (Nakamura et al., 2009), 1 and 5 mg/kg of APFO for 2 weeks by gavage (Nakagama et al., 2012), three doses of DEHP (0.01%, 0.05%, and 0.1%) for ~7 weeks (Hayashi et al., 2011), and two doses of the plasticizers di-n-butyl phthalate (DBP), DEHP, or di(2-ethylhexyl) adipate (DEHA) for 2 weeks (Ito et al., 2012). The TRE-hPPARα mice did not exhibit increases in cell proliferation or expression of cell cycle proteins after WY treatment (Cheung et al., 2004; Morimura et al., 2006). In the hPPARα PAC mouse there was a slight but significant increase in cell proliferation after exposure to WY but no changes in the expression of cell cycle genes cyclin D1 and CDK4 (Yang et al., 2008). In a 38-to 44-week exposure study with WY, the TRE-hPPARα mice were also refractory to liver cancer. Wild-type mice but not the humanized mice exhibited a significant increase in liver tumors despite the fact that the humanized mice were exposed 6 weeks longer than the wild-type mice to the compound (Morimura et al., 2006). These studies showed that human PPARα is pharmacologically active but does not regulate the full spectrum of responses necessary for hepatocarcinogenesis when expressed in the mouse liver.
Concerns were raised by Guyton et al. (2009) about the use of the humanized mice to make conclusions about differences between human and rodent PPARα and liver cancer induction: “….the accuracy of estimates of the extent of this difference is limited by the short exposure duration, the substantial mortality and morbidity in wild-type mice, the small number of animals studied, and potential differences in the interaction of the human receptor with mouse-specific co-activators and response elements”. It is true that the exposures in the carcinogenicity study were less than lifetime. However, similar to the argument made above about the bioassays with WY and bezafibrate in wild-type and Pparα-null mice, less than lifetime exposures would be more of a concern if there were molecular or cellular indicators of carcinogenesis altered in the TRE-hPPARα mice at the time of euthanasia (44 weeks of exposure). Other than mild fatty change, glycogen deposition, minor increases in relative liver weight, and increases in proteins involved in fatty acid homeostasis (ACOX, CYP4A, MCAD, ME), there were no changes in markers of cell proliferation or DNA damage including c-MYC, CD1, CDK1, CDK4, p21, BAX and BCL2 (Morimura et al., 2006). (A slight induction of p53 gene expression was observed, the origin and significance of which is unknown.) The morbidity and mortality of the treated wild-type mice was likely due to the concentration of WY in the diet (0.1%) used to maximize responses given that the human PPARα is less responsive to WY than mouse PPARα based on trans-activation assays. The “small” numbers of animals would be a concern for this study if the results were more equivocal. There were clear differences in hepatocellular adenomas and carcinomas between the wild-type and humanized PPARα mice. Potential differences in the interactions between the human or mouse PPARα and mouse co-regulators could lead to differences in responses. However, the human PPARα was able to efficiently activate genes involved in fatty acid metabolism but not cell cycle genes including c-MYC (Morimura et al., 2006), similar to the functions of the human receptor in humanized livers (Tateno et al., 2014). We stress that the results generated in these models should not be over-interpreted. The models were never developed to derive a quantitative estimation of species differences in sensitivity to carcinogenesis.
3. Perceived weaknesses of the epidemiology studies.
Several large retrospective epidemiological studies examined the relationships between chronic treatment with the hypolipidemic agents and PPARα activators, gemfibrozil and clofibrate, and liver cancer (reviewed in Klaunig et al., 2003; Peters et al., 2005). There was no elevated risk of mortality from liver cancer reported in any of the published reports on the health outcomes associated with over a decade of chronic use of these pharmaceuticals to treat large human cohorts (Frick et al., 1987; Huttunen et al., 1994). A possible exception is one cohort, in which excess mortality due to a higher incidence of the malignant neoplasms of the “liver, gallbladder and intestines” was reported in clofibrate treated subjects (Report from the Committee of Principal Investigators, 1978). However, death rates among the clofibrate-treated group for cancer were similar to the official mortality statistics for individuals from the same area, the number of observed cases of gastrointestinal cancers was very small, and there was no difference among groups in a follow up analysis of the mortality trends in this cohort.
A number of concerns were raised by Guyton et al. (2009) regarding the epidemiology studies to discount risk of liver cancer caused by the fibrate drugs, which are PPARα activators. Guyton et al. suggested that, “the available studies have low power to detect statistical differences in the risk of liver cancer; an estimated five or fewer liver cancer deaths would have been expected in these studies using data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results database (Ries et al. 2008).” Since the Guyton et al. (2009) review, a meta-analysis of 17 randomized controlled trials (RCTs) was carried out by Bonavas et al. (2012). RCTs involving 44,929 participants with an average follow-up of 5.2 years, was included in the analysis. The authors determined two common parameters from the data: relative risk (RR) and confidence intervals (CI). RR is the ratio of the probability of an event occurring in the drug-exposed group compared to the probability of the event occurring in the non-exposed group. CIs are a range of values where there is a specified probability that the value of a parameter lies within it. The authors indicated that, “The quantitative synthesis of data retrieved from the RCTs was not indicative of a fibrate effect on cancer incidence (780 [fibrate] vs 814 [control]; RR = 1.02, 95% CI 0.92–1.12) or cancer death (385 [fibrate] vs 377 [control]; RR = 1.06, 95% CI: 0.92–1.22). When the analysis was restricted to major RCTs, the results did not substantially change. Similarly, we found no evidence of differential effects by length of follow-up or type of fibrate.” (Bonavas et al., 2012). The authors concluded that fibrates have a neutral effect on cancer outcomes, which include those in the liver. In summary, fibrate drugs have been on the market since 1977 without an apparent increase in liver cancer in people taking them chronically.
Summary
There is remarkable consistency in the data supporting the PPARα MOA as originally described by Klaunig et al. (2003) and modified with more recent data (Corton et al., 2014). The consistency occurs across many structurally-diverse PPARα activators. These include not only PPARα activators in consumer use products, but hypolipidemic drugs that patients have been and are exposed to at levels many orders of magnitude higher than environmentally relevant chemicals. All of the 10 PPARα activators examined in the analysis activated most, if not all of the KEs in the MOA in the two responsive species (rats and mice). Mechanistic studies using gene nullizygous models or chemical inhibitors of oxidative stress or inflammation demonstrated that the KEs are mechanistically linked. In these studies, inhibition of the KE leads to effects on the KEs downstream but generally not upstream of the targeted KE. The linkage of the KEs is also supported by dose-response analysis of individual chemicals. KEs that are more proximate to liver cancer require the same or greater doses of chemical for activation. There are striking differences in species responses of the KEs in the PPARα MOA. Syrian hamsters, guinea pigs and non-human primates are better human surrogates than mice and rats because of differences in PPARα expression and activity. While these species exhibit PPARα activation and associated increases in genes and proteins involved in lipid homeostasis which underlie the universal hypolipidemic effects, these species lack the activation of KEs downstream of PPARα including alteration of cell growth pathways, hepatocyte proliferation, and liver cancer. Human hepatocytes in culture or in the context of humanized mouse livers do not respond to exposure with a proliferative response. Epidemiological studies of large numbers of patients that have been prescribed hypolipidemic drugs for up to a decade do not show increases in adverse liver effects or cancer. Taken together, the weight of evidence strongly supports the rodent MOA for PPARα-induced liver tumors and the conclusion that this PPARα MOA is either “not relevant” or “unlikely to be relevant” in humans (Corton et al., 2014).
Criticisms of the rodent MOA (Klaunig et al., 2003) have been articulated in two reviews (Kesheva and Caldwell, 2006; Guyton et al., 2009). Here, we have systematically addressed the most germane perceived weaknesses of the PPARα MOA made by these groups. The Guyton et al. (2009) analysis focused in part on two studies that appeared to provide evidence that the KEs in the MOA are not mechanistically linked to liver cancer. The Ito et al. (2007) DEHP bioassay performed in wild-type and Pparα-null mice has been suggested by Guyton et al (2009) to show that DEHP does not require PPARα to cause liver tumors in wild-type mice. We detail the weaknesses of the study, which include questionable, if any statistical relevance of the induced tumors in the Pparα-null mice. The Guyton et al. (2009) review argued that because DEHP caused liver tumors in Pparα-null mice, the liver tumors that occur in wild-type mice (from other studies) are PPARα-independent, even though no liver tumors occurred in the wild-type mice from the Ito et al. study. We provide evidence that the liver tumors in the Pparα-null mice occur either through augmenting background hepatic steatosis and inflammation or through activation of CAR, both of which are not relevant to DEHP treated wild-type mice. There is abundant evidence that the liver tumors produced in wild-type mice by DEHP exposures occur through the PPARα MOA. There is consistent activation of all of the KEs in the MOA by DEHP, there is consistent dose and temporal responses regarding the KEs, and DEHP regulates gene expression in the mouse liver almost exclusively through PPARα.
The second study (Yang et al., 2007) describes effects of a constitutively active PPARα (VP16PPARα) that was most notable, because hepatocyte proliferation was observed in the absence of liver tumor induction. These results were used by Guyton et al. (2009) to suggest that the KEs in the MOA are not mechanistically linked. However, the mechanism by which constitutive activation of PPARα in the VP16PPARα transgenic mouse leads to hepatocyte proliferation is not the same as that activated by PPARα activator exposure in wild-type mice. Wild-type mice require activation of a pathway involving the proto-oncogene c-Myc and proliferation of NPCs both of which do not occur in mice that express the VP16PPARα fusion protein. Thus, it is not surprising that cell proliferation caused by the VP16PPARα fusion protein does not lead to liver cancer.
We also addressed additional concerns of Guyton et al. (2009) including the 1) perceived use of one compound for mechanistic studies, 2) perceived weaknesses of the Pparα-null mouse model to provide mechanistic support to the MOA, 3) lack of linkage of KEs, 4) lack of data on environmentally relevant compounds, 5) pleiotropy of PPARα activator effects, and 6) potency differences between chemicals.
Concerns about the perceived weaknesses of species extrapolation of the MOA to human risk have also been argued by Guyton et al. (2009). The present review has addressed three of the main points including artifacts in the isolation of human primary hepatocytes that could lead to a lack of a proliferative response, weaknesses of the humanized PPARα mouse studies, and weaknesses of the epidemiology studies. Guyton et al. (2009) suggested that experiments with human primary hepatocytes were compromised because of the lack of NPCs in the cultures. However, a careful examination of the procedures used to isolate the human hepatocytes demonstrated that the lack of proliferative responses in the primary hepatocytes is not due to lack of NPCs. Procedures to purify the hepatocytes from the NPCs were not carried out in the human primary hepatocyte experiments. Furthermore, in the same studies, rat primary hepatocytes isolated using similar procedures consistently responded with a proliferative response to the same chemicals demonstrating the striking species differences. Criticisms of the humanized PPARα studies included uncertainty regarding the use of the models to quantitate differences in responses between mice and humans. While the humanized mice were refractory to the proliferative and hepatocarcinogenic effects of PPARα activator exposure, the mice were never intended to derive values that can be used to quantitate species differences for risk assessment. These mice lack proliferative responses in the livers consistent with lack of responses in human primary hepatocytes. Thus, under a diverse array of exposure scenarios, humans do not respond the same way as responsive species. While the weaknesses of using individual epidemiology studies to assess risk of liver cancer made by the Guyton et al. (2009) are acknowledged, a meta-analysis study of 17 epidemiology studies was published after the Guyton et al. (2009) review. The meta-analysis study included over ~45,000 patients. The conclusion of the study was that there was no increased risk of any kind of cancer after exposures for up to a decade or more.
In a MOA analysis every molecular detail is not needed to build a MOA and use that information for human risk assessment (Cohen et al, 2003; 2004). While not every molecular event has been defined for PPARα activation, the events which occur between activation of PPARα and liver tumor induction are well established and have been consistently reproduced. Epidemiologist Sir Austin Bradford Hill said: “All scientific work is incomplete – whether it be observational or experimental. All scientific work is liable to be upset or modified by advancing knowledge. That does not confer upon us a freedom to ignore the knowledge we already have….” Over the last 40 years, a large body of data has been generated involving many academic, government and industry labs on a diverse array of chemicals that strongly supports the MOA for PPARα liver tumorigenesis in the rodent and provides equally strong evidence for the lack of relevance to the human.
Acknowledgements
The information in this document has subjected to review by the National Health and Environmental Effects Research Laboratory of the US EPA and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The authors thank Drs. Jennifer Foreman and Barbara Abbott for review of the manuscript, Mr. Chuck Gaul for figure production, and Ms. Emily Beukema for editorial assistance.
The information in this document has been funded by the U.S. Environmental Protection Agency. It has been subjected to review by the National Health and Environmental Research Laboratory and approved for publication. Approval does not signify that the contents necessarily reflect the views and the policies of the Agency, nor does mention of trade names or commercial products not constitute endorsement or recommendation for use.
Abbreviations:
- CAR
constitutive activated receptor
- DEHP
di-(2-ethylhexyl)phthalate
- DINP
diisononyl phthalate
- KE
key event
- MOA
mode of action
- PPARα
peroxisome proliferator-activated receptor α
- PPARβ
peroxisome proliferator-activated receptor β
- PPARγ
peroxisome proliferator-activated receptor γ
- PFHxS
perfluorohexanesulfonic acid
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctane sulfonate
- PPREs
peroxisome proliferator response elements
- TNF
tumor necrosis factor
- WY
WY-14,643
- IL
interleukin
- miRNA
microRNA
- TGF
tumor growth factor
- ROS
reactive oxygen species
- ACO
acyl CoA oxidase
- EMSA
electrophoretic mobility shift assays
- TCA
trichloroacetate
- TCE
trichloroethylene
- DEN
diethylnitrosamine
- APFO
ammonium perfluorooctanoate
- DEHA
bis(2-ethylhexyl) adipate
- CPDB
carcinogenic potency database
- ED50
effective dose, 50
- RCT
randomized controlled trials
- RR
relative risk
- CI
confidence interval
- MEHP
mono-(2-ethylhexyl) phthalate
References
- Abdellatif AG, Preat V, Vamecq J, Nilsson R, Roberfroid M. (1990). Peroxisome proliferation and modulation of rat liver carcinogenesis by 2,4-dichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid, perfluorooctanoic acid and nafenopindichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid, perfluorooctanoic acid and nafenopin. Carcinogenesis 11(11): 1899–1902. [DOI] [PubMed] [Google Scholar]
- Alsarra IA, Brockmann WG, Cunningham ML, Badr MZ. (2006). Hepatocellular proliferation in response to agonists of peroxisome proliferator-activated receptor alpha: a role for Kupffer cells? J Carcinog 5: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amacher DE, Beck R, Schomaker SJ, Kenny CV. Hepatic microsomal enzyme induction, beta-oxidation, and cell proliferation following administration of clofibrate, gemfibrozil, or bezafibrate in the CD rat. Toxicol Appl Pharmacol. 1997. January;142(1):143–50. [DOI] [PubMed] [Google Scholar]
- Anderson SP, Dunn C, Laughter A, Yoon L, Swanson C, Stulnig TM, Steffensen KR, Chandraratna RA, Gustafsson JA, Corton JC. (2004a). Overlapping transcriptional programs regulated by the nuclear receptors peroxisome proliferator-activated receptor alpha, retinoid X receptor, and liver X receptor in mouse liver. Mol Pharmacol 66(6):1440–52. [DOI] [PubMed] [Google Scholar]
- Anderson SP, Dunn CS, Cattley RC, Corton JC. (2001). Hepatocellular proliferation in response to a peroxisome proliferator does not require TNFαlpha signaling. Carcinogenesis 22: 1843–1851. [DOI] [PubMed] [Google Scholar]
- Anderson SP, Howroyd P, Liu J, Qian X, Bahnemann R, Swanson C, Kwak MK, Kensler TW, Corton JC. (2004b). The transcriptional response to a peroxisome proliferator-activated receptor alpha agonist includes increased expression of proteome maintenance genes. J Biol Chem 279(50):52390–8. [DOI] [PubMed] [Google Scholar]
- Anderson SP, Yoon L, Richard EB, Dunn CS, Cattley RC, Corton JC. (2002). Delayed liver regeneration in peroxisome proliferator-activated receptor-alpha-null mice. Hepatology 36(3):544–54. [DOI] [PubMed] [Google Scholar]
- Aoyama T, . Peters J, Iritani N, Nakajima T, Furihata K, Hashimotot T, Gonzalez FJ. et al. (1998). Altered Constitutive Expression of Fatty Acid-metabolizing Enzymes in Mice Lacking the Peroxisome Proliferator-activated Receptor α (PPARα). J Biol Chem 273 (10) 5678–5684. [DOI] [PubMed] [Google Scholar]
- Arsura M, Cavin LG. (2005). Nuclear factor-kappaB and liver carcinogenesis. Cancer Lett 229(2):157–69. [DOI] [PubMed] [Google Scholar]
- Ashby J, Brady A, Elcombe CR, Elliott BM, Ishmael J, Odum J, Tugwood JD, Kettle S, Purchase IF. (1994). Mechanistically-based human hazard assessment of peroxisome proliferator-induced hepatocarcinogenesis. Hum Exp Toxicol 13 Suppl 2: S1–117. [DOI] [PubMed] [Google Scholar]
- Austin EW, Okita JR, Okita RT, Larson JL, Bull RJ. (1995). Modification of lipoperoxidative effects of dichloroacetate and trichloroacetate is associated with peroxisome proliferation. Toxicology 97(1-3): 59–69. [DOI] [PubMed] [Google Scholar]
- Barrass NC, Price RJ, Lake BG, Orton TC. (1993). Comparison of the acute and chronic mitogenic effects of the peroxisome proliferators methylclofenapate and clofibric acid in rat liver. Carcinogenesis 14(7): 1451–6. [DOI] [PubMed] [Google Scholar]
- Bayly AC, Roberts RA, Dive C. (1994). Suppression of liver cell apoptosis in vitro by the non-genotoxic hepatocarcinogen and peroxisome proliferator nafenopin. J Cell Biol 125(1): 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becuwe P, Dauça M (2005). Comparison of cytotoxicity induced by hypolipidemic drugs via reactive oxygen species in human and rodent liver cells. Int J Mol Med 16(3):483–92. [PubMed] [Google Scholar]
- Bell AR, Savory R, Horley NJ, Choudhury AI, Dickins M, Gray TJ, Salter SM, Bell DR. (1998). Molecular basis of non-responsiveness to peroxisome proliferators: the guinea-pig PPARα is functional and mediates peroxisome proliferator-induced hypolipidaemia. Biochem J 332 (Pt 3): 689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DR, Plant NJ, Rider CG, Na L, Brown S, Ateitalla I, Acharya SK, Davies MH, Elias E, Jenkins NA, et al. (1993). Species-specific induction of cytochrome P-450 4A RNAs: PCR cloning of partial guinea-pig, human and mouse CYP4A cDNAs. Biochem J 294 ( Pt 1):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol Sci. 2011. March;120(1):42–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Calder I, Elcombe C, Grasso P, Stringer D, Wiegand HJ. (1993). Hepatic peroxisome proliferation in rodents and its significance for humans. Food Chem Toxicol 31:857–907. [DOI] [PubMed] [Google Scholar]
- Biegel LB, Hurtt ME, Frame SR, O’Connor JC, Cook JC. (2001). Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci 60(1): 44–55. [DOI] [PubMed] [Google Scholar]
- Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, Peters JM. (2004). Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci 82(1): 170–182. [DOI] [PubMed] [Google Scholar]
- Blumcke S, Schwartzkopff W, Lobeck H, Edmondson NA, Prentice DE, Blane GF (1983). Influence of fenofibrate on cellular and subcellular liver structure in hyperlipidemic patients. Atherosclerosis 46:105–116. [DOI] [PubMed] [Google Scholar]
- Bojes HK, Germolec DR, Simeonova P, Bruccolrei A, Schoonhoven R, Luster MI, Thurman RG. (1997). Antibodies to tumor necrosis factor alpha prevent increases in cell replication in liver due to the potent peroxisome proliferator WY14,643. Carcinogenesis 18:669–674. [DOI] [PubMed] [Google Scholar]
- Bonovas S, Sitaras NM. Editorial: prophylactic treatment with antiviral agents to prevent infection and disease. Curr Med Chem. 2012;19(35):5923. [PubMed] [Google Scholar]
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. (2008). IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol 38(2):87–96. [DOI] [PubMed] [Google Scholar]
- Bull RJ, Orner GA, Cheng RS, Stillwell L, Stauber AJ, Sasser LB, Lingohr MK, Thrall BD. (2002). Contribution of dichloroacetate and trichloroacetate to liver tumor induction in mice by trichloroethylene. Toxicol Appl Pharmacol 182(1): 55–65. [DOI] [PubMed] [Google Scholar]
- Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ. (1990). Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology 63(3): 341–59. [DOI] [PubMed] [Google Scholar]
- Burkhardt S, Mellert W, Reinacher M, Bahnenmann R. (2001). Zonal evaluation of proliferation and apoptosis in mice reveals new mechanistic data for PB, WY 14,643 and CH. Toxicologist 60:286. [Google Scholar]
- Bursch W, Lauer B, Timmermann-Trosiener I, Barthel G, Schuppler J, Schulte-Hermann R. (1984). Controlled death (apoptosis) of normal and putative preneoplastic cells in rat liver following withdrawal of tumor promoters. Carcinogenesis 5(4):453–8. [DOI] [PubMed] [Google Scholar]
- Busser MT, Lutz WK. (1987). Stimulation of DNA synthesis in rat and mouse liver by various tumor promoters. Carcinogenesis 8(10): 1433–7. [DOI] [PubMed] [Google Scholar]
- Cai Y, Appelkvist EL, DePierre JW. (1995). Hepatic oxidative stress and related defenses during treatment of mice with acetylsalicylic acid and other peroxisome proliferators. J Biochem Toxicol 10(2): 87–94. [DOI] [PubMed] [Google Scholar]
- Caira F, Clémencet MC, Cherkaoui-Malki M, Dieuaide-Noubhani M, Pacot C, Van Veldhoven PP, Latruffe N. (1998). Differential regulation by a peroxisome proliferator of the different multifunctional proteins in guinea pig: cDNA cloning of the guinea pig D-specific multifunctional protein 2. Biochem J 330 (Pt 3):1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JC. DEHP: genotoxicity and potential carcinogenic mechanisms-a review. Mutat Res. 2012. Oct-Dec;751(2):82–157. [DOI] [PubMed] [Google Scholar]
- Calfee-Mason KG, Lee EY, Spear BT, Glauert HP. Role of the p50 subunit of NF-kappaB in vitamin E-induced changes in mice treated with the peroxisome proliferator, ciprofibrate. Food Chem Toxicol. 2008. June;46(6):2062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee-Mason KG, Spear BT, Glauert HP. (2004). Effects of vitamin E on the NF-kappaB pathway in rats treated with the peroxisome proliferator, ciprofibrate. Toxicol Appl Pharmacol 199(1): 1–9. [DOI] [PubMed] [Google Scholar]
- Cariello NF, Romach EH, Colton HM, Ni H, Yoon L, Falls JG, Casey W, Creech D, Anderson SP, Benavides GR, Hoivik DJ, Brown R, Miller RT. (2005). Gene expression profiling of the PPAR-alpha agonist ciprofibrate in the cynomolgus monkey liver. Toxicol Sci 88(1):250–64. [DOI] [PubMed] [Google Scholar]
- Cattley RC, Conway JG, Popp JA. (1987). Association of persistent peroxisome proliferation and oxidative injury with hepatocarcinogenicity in female F-344 rats fed di(2-ethylhexyl)phthalate for 2 years. Cancer Lett 38(1-2): 15–22. [DOI] [PubMed] [Google Scholar]
- Cattley RC, DeLuca J, Elcombe C, Fenner-Crisp P, Lake BG, Marsman DS, Pastoor TA, Popp JA, Robinson DE, Schwetz B, Tugwood J, Wahli W. (1998). Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans? Regul Toxicol Pharmacol 27: 47–60. [DOI] [PubMed] [Google Scholar]
- Cattley RC, Marsman DS, Popp JA. (1991). Age-related susceptibility to the carcinogenic effect of the peroxisome proliferatorWY14,643 in rat liver. Carcinogenesis 12:469–473. [DOI] [PubMed] [Google Scholar]
- Cattley RC, Popp JA. (1989). Differences between the promoting activities of the peroxisome proliferator WY-14,643 and phenobarbital in rat liver. Cancer Res 49(12): 3246–51. [PubMed] [Google Scholar]
- Chan E, Tan CS, Deurenberg-Yap M, Chia KS, Chew SK, Tai ES. (2006). The V227A polymorphism at the PPARΑ locus is associated with serum lipid concentrations and modulates the association between dietary polyunsaturated fatty acid intake and serum high density lipoprotein concentrations in Chinese women. Atherosclerosis 187(2):309–15. [DOI] [PubMed] [Google Scholar]
- Chang CK, Llanes S, Schumer W. Effect of dexamethasone on NF-kB activation, tumor necrosis factor formation, and glucose dyshomeostasis in septic rats. J Surg Res. 1997. October;72(2):141–5. [DOI] [PubMed] [Google Scholar]
- Chen C, Hennig GE, Whiteley HE, Corton JC, Manautou JE. Peroxisome proliferator-activated receptor alpha-null mice lack resistance to acetaminophen hepatotoxicity following clofibrate exposure. Toxicol Sci. 2000. October;57(2):338–44. [DOI] [PubMed] [Google Scholar]
- Chen H, Huang CY, Wilson MW, Lay LT, Robertson LW, Chow CK, Glauert HP. (1994). Effect of the peroxisome proliferators ciprofibrate and perfluorodecanoic acid on hepatic cell proliferation and toxicity in Sprague Dawley rats. Carcinogenesis 15(12): 2847–50. [DOI] [PubMed] [Google Scholar]
- Cheung C, Akiyama TE, Ward JM, Nicol CJ, Feigenbaum L, Vinson C, Gonzalez FJ. (2004). Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor alpha. Cancer Res 64(11):3849–54. [DOI] [PubMed] [Google Scholar]
- Chinje E, Kentish P, Jarnot B, George M, Gibson G. (1994). Induction of the CYP4A subfamily by perfluorodecanoic acid: the rat and the guinea pig as susceptible and non-susceptible species. Toxicol Lett 71(1):69–75. [DOI] [PubMed] [Google Scholar]
- Choudhury AI, Chahal S, Bell AR, Tomlinson SR, Roberts RA, Salter AM, Bell DR. (2000). Species differences in peroxisome proliferation; mechanisms and relevance. Mutat Res 448(2):201–12. [DOI] [PubMed] [Google Scholar]
- Choudhury AI, Sims HM, Horley NJ, Roberts RA, Tomlinson SR, Salter AM, Bruce M, Shaw PN, Kendall D, Barrett DA, Bell DR. (2004). Molecular analysis of peroxisome proliferation in the hamster. Toxicol Appl Pharmacol 197(1):9–18. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Gonzales AJ, Cattley RC, Goldsworthy TL. (1998). Regulation of apoptosis in mouse hepatocytes and alteration of apoptosis by nongenotoxic carcinogens. Cell Growth Differ 9(9): 815–25. [PubMed] [Google Scholar]
- Cohen SM, Klaunig J, Meek ME, Hill RN, Pastoor T, Lehman-McKeeman L, Bucher J, Longfellow DG, Seed J, Dellarco V, Fenner-Crisp P, Patton D. (2004). Evaluating the human relevance of chemically induced animal tumors. Toxicol Sci 78(2):181–6. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Meek ME, Klaunig JE, Patton DE, Fenner-Crisp PA. (2003). The Human Relevance of Information on Carcinogenic Modes of Action: Overview. Crit Rev Toxicol 33(6):581–589. [DOI] [PubMed] [Google Scholar]
- Committee of Principal Investigators (1978). A cooperative trial in the primary prevention of ischemic heart disease using clofibrate. Br Heart J 40: 1069–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JG, Tomaszewski KE, Olson MJ, Cattley RC, Marsman DS, Popp JA. (1989). Relationship of oxidative damage to the hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)phthalate and WY-14,643. Carcinogenesis 10(3): 513–9. [DOI] [PubMed] [Google Scholar]
- Cornu-Chagnon MC, Dupont H, Edgar A. (1995). Fenofibrate: Metabolism and species differences for peroxisome proliferation in cultured hepatocytes. Fundam Appl Toxicol 26:63–74. [DOI] [PubMed] [Google Scholar]
- Corton JC, Anderson SP, Stauber A. (2000). Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annu Rev Pharmacol Toxicol 40: 491–518. [DOI] [PubMed] [Google Scholar]
- Corton JC, Apte U, Anderson SP, Limaye P, Yoon L, Latendresse J, Dunn C, Everitt JI, Voss KA, Swanson C, Kimbrough C, Wong JS, Gill SS, Chandraratna RA, Kwak MK, Kensler TW, Stulnig TM, Steffensen KR, Gustafsson JA, Mehendale HM. (2004). Mimetics of caloric restriction include agonists of lipid-activated nuclear receptors. J Biol Chem 279(44):46204–12. [DOI] [PubMed] [Google Scholar]
- Corton JC, Lapinskas PJ. (2005). Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol Sci 83(1): 4–17. [DOI] [PubMed] [Google Scholar]
- Corton JC. (2008). Evaluation of the role of peroxisome proliferator-activated receptor alpha (PPARα in mouse liver tumor induction by trichloroethylene and metabolites. Crit Rev Toxicol 38(10):857–75. [DOI] [PubMed] [Google Scholar]
- Corton JC. (2010). Mode of Action Analysis and Human Relevance of Liver Tumors Induced by PPARα Activation In Cancer Risk Assessment: Chemical Carcinogenesis from Biology to Standards Quantification, Hsu Ching-Hung and Stedeford Todd (editors), John Wiley and Sons, Inc., Hoboken, New Jersey. [Google Scholar]
- Cunningham ML, Collins BJ, Hejtmancik MR, Herbert RA, Travlos GS, Vallant MK, Stout MD. (2010). Effects of the PPARα agonist and widely used antihyperlipidemic drug gemfibrozil on hepatic toxicity and lipid metabolism. PPAR Research pii: 681963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ML. Toxicity studies of WY-14,643 (CAS No. 50892-23-4) administered in feed to male Sprague-Dawley rats, B6C3F1 mice, and Syrian hamsters. Toxic Rep Ser. 2007. October;(62):1–136. [PubMed] [Google Scholar]
- Currie RA, Bombail V, Oliver JD, Moore DJ, Lim FL, Gwilliam V, Kimber I, Chipman K, Moggs JG, Orphanides G. (2005). Gene ontology mapping as an unbiased method for identifying molecular pathways and processes affected by toxicant exposure: application to acute effects caused by the rodent non-genotoxic carcinogen diethylhexylphthalate. Toxicol Sci 86(2):453–69. [DOI] [PubMed] [Google Scholar]
- Czaja MJ. (2007). Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis 27(4):378–89. [DOI] [PubMed] [Google Scholar]
- Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, Corton JC, Abbott BD. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology. 2017. March 1;378:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David RM, Moore MR, Cifone MA, Finney DC, Guest D. (1999). Chronic peroxisome proliferation and hepatomegaly associated with the hepatocellular tumorigenesis of di(2-ethylhexyl)phthalate and the effects of recovery. Toxicol Sci 50(2):195–205. [DOI] [PubMed] [Google Scholar]
- David RM, Moore MR, Finney DC, Guest D. (2000a). Chronic toxicity of di(2-ethylhexyl)phthalate in mice. Toxicol Sci 58(2):377–85. [DOI] [PubMed] [Google Scholar]
- David RM, Moore MR, Finney DC, Guest D. (2000b). Chronic toxicity of di(2-ethylhexyl)phthalate in rats. Toxicol Sci 55(2):433–43. [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene. 2006. October 30;25(51):6868–86. [DOI] [PubMed] [Google Scholar]
- de Duve C. (1996). The peroxisome in retrospect. Ann N Y Acad Sci 804:1–10. [DOI] [PubMed] [Google Scholar]
- de la Iglesia FA, Lewis JE, Buchanan RA, Marcus EL, McMahon G. (1982). Light and electron microscopy of liver in hyperlipoproteinemic patients under long-term gemfibrozil treatment. Atherosclerosis 43:19–37. [DOI] [PubMed] [Google Scholar]
- De Minicis S, Bataller R, Brenner DA. (2006). NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology 131(1):272–5. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, Daniel FB, McMillan L, Wernsing P, Savage RE Jr. (1989). Species and strain sensitivity to the induction of peroxisome proliferation by chloroacetic acids. Toxicol Appl Pharmacol 101(2): 285–98. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, Daniel FB, Most BM, Olson GR. (1997). Failure of monochloroacetic acid and trichloroacetic acid administered in the drinking water to produce liver cancer in male F344/N rats. J Toxicol Environ Health 52(5): 425–45. [DOI] [PubMed] [Google Scholar]
- Dees C, Travis C. (1994). Trichloroacetate stimulation of liver DNA synthesis in male and female mice. Toxicol Lett 70(3): 343–55. [DOI] [PubMed] [Google Scholar]
- Dostalek M, Hardy KD, Milne GL, Morrow JD, Chen C, Gonzalez FJ, Gu J, Ding X, Johnson DA, Johnson JA, Martin MV, Guengerich FP. (2008). Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems. J Biol Chem 283(25):17147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M. (1999). A cancer risk assessment of di(2-ethylhexyl)phthalate: Application of the new U.S. EPA Risk Assessment Guidelines. Regul Toxicol Pharmacol 29:327–357. [DOI] [PubMed] [Google Scholar]
- Dwivedi RS, Alvares K, Nemali MR, Subbarao V, Reddy MK, Usman MI, Rademaker AW, Reddy JK, Rao MS. (1989). Comparison of the peroxisome proliferator-induced pleiotropic response in the liver of nine strains of mice. Toxicol Pathol 17(1 Pt 1): 16–26. [DOI] [PubMed] [Google Scholar]
- Elcock FJ, Chipman JK, Roberts RA (1998). The rodent nongenotoxic hepatocarcinogen and peroxisome proliferator nafenopin inhibits intercellular communication in rat but not guinea-pig hepatocytes, perturbing S-phase but not apoptosis Archives of Toxicology 72(7): 439–444.F [DOI] [PubMed] [Google Scholar]
- Elliott BM, Elcombe CR. (1987). Lack of DNA damage or lipid peroxidation measured in vivo in the rat liver following treatment with peroxisomal proliferators. Carcinogenesis 8(9): 1213–8. [DOI] [PubMed] [Google Scholar]
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000. March 17;448(2):121–38. [DOI] [PubMed] [Google Scholar]
- Eveillard A, Lasserre F, de Tayrac M, Polizzi A, Claus S, Canlet C, Mselli-Lakhal L, Gotardi G, Paris A, Guillou H, Martin PG, Pineau T. (2009b). Identification of potential mechanisms of toxicity after di-(2-ethylhexyl)-phthalate (DEHP) adult exposure in the liver using a systems biology approach. Toxicol Appl Pharmacol. 236(3):282–92. [DOI] [PubMed] [Google Scholar]
- Eveillard A, Mselli-Lakhal L, Mogha A, Lasserre F, Polizzi A, Pascussi JM, Guillou H, Martin PG, Pineau T. (2009a). Di-(2-ethylhexyl)-phthalate (DEHP) activates the constitutive androstane receptor (CAR): a novel signalling pathway sensitive to phthalates. Biochem Pharmacol. 77(11):1735–46. [DOI] [PubMed] [Google Scholar]
- Fan CY, Pan J, Usuda N, Yeldandi AV, Rao MS, Reddy JK. (1998). Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J Biol Chem 273:15639–15645. [DOI] [PubMed] [Google Scholar]
- Fischer JG, Glauert HP, Yin T, Sweeney-Reeves ML, Larmonier N, Black MC. (2002). Moderate iron overload enhances lipid peroxidation in livers of rats, but does not affect NF-kappaB activation induced by the peroxisome proliferator, WY-14,643. J Nutr 132(9): 2525–31. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JE, Sanyer JL, Schardein JL, Lake RS, McGuire EJ, de la Iglesia FA. (1981). Carcinogen bioassay and mutagenicity studies with the hypolipidemic agent gemfibrozil. J Natl Cancer Inst 67(5): 1105–16. [PubMed] [Google Scholar]
- Flavell DM, Pineda Torra I, Jamshidi Y, Evans D, Diamond JR, Elkeles RS, Bujac SR, Miller G, Talmud PJ, Staels B, Humphries SE. (2000). Variation in the PPARα gene is associated with altered function in vitro and plasma lipid concentrations in Type II diabetic subjects. Diabetologia 43(5):673–80. [DOI] [PubMed] [Google Scholar]
- Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, et al. (1987). Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317(20): 1237–45. [DOI] [PubMed] [Google Scholar]
- Gariot P, Barrat E, Drouin P, Genton P, Pointel JP, Foliguet B, Kolopp M, Debry G. (1987). Morphometric study of human hepatic cell modifications induced by fenofibrate. Metabolism 36:203–210. [DOI] [PubMed] [Google Scholar]
- Gariot P, Barrat E, Mejean L, Pointel JP, Drouin P, Debry G. (1983). Fenofibrate and human liver. Lack of proliferation of peroxisomes. Arch Toxicol 53(2):151–63. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Clewell HJ 3rd, Clewell R, Campbell J, Van Landingham C, Shipp AM. Challenges in the application of quantitative approaches in risk assessment: a case study with di-(2-ethylhexyl)phthalate. Crit Rev Toxicol. 2011. August;41 Suppl 2:1–72. [DOI] [PubMed] [Google Scholar]
- Gervois P, Torra IP, Chinetti G, Grotzinger T, Dubois G, Fruchart JC, Fruchart-Najib J, Leitersdorf E, Staels B. (1999). A truncated human peroxisome proliferator-activated receptor alpha splice variant with dominant negative activity. Mol Endocrinol 13:1535–1549. [DOI] [PubMed] [Google Scholar]
- Gill JH, Brickell P, Dive C, Roberts RA. The rodent non-genotoxic hepatocarcinogen nafenopin suppresses apoptosis preferentially in non-cycling hepatocytes but also elevates CDK4, a cell cycle progression factor. Carcinogenesis. 1998. October;19(10):1743–7. [DOI] [PubMed] [Google Scholar]
- Gill JH, Roberts RA, Dive C. (1998). The nongenotoxic hepatocarcinogen nafenopin suppresses rodent hepatocyte apoptosis induced by TGFb1, DNA damage and FAS. Carcinogenesis 19:299–304. [DOI] [PubMed] [Google Scholar]
- Glauert HP, Beaty MM, Clark TD, Greenwell WS, Tatum V, Chen LC, Borges T, Clark TL, Srinivasan SR, Chow CK. (1990). Effect of dietary vitamin E on the development of altered hepatic foci and hepatic tumors induced by the peroxisome proliferator ciprofibrate. J Cancer Res Clin Oncol 116(4): 351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert HP, Eyigor A, Tharappel JC, Cooper S, Lee EY, Spear BT. (2006). Inhibition of hepatocarcinogenesis by the deletion of the p50 subunit of NF-kappaB in mice administered the peroxisome proliferator WY-14,643. Toxicol Sci 90(2): 331–6. [DOI] [PubMed] [Google Scholar]
- Glauert HP, Srinivasan S, Tatum VL, Chen LC, Saxon DM, Lay LT, Borges T, Baker M, Chen LH, Robertson LW, et al. (1992). Effects of the peroxisome proliferators ciprofibrate and perfluorodecanoic acid on hepatic cellular antioxidants and lipid peroxidation in rats. Biochem Pharmacol 43(6): 1353–9. [DOI] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. (2006). NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72(11):1493–505. [DOI] [PubMed] [Google Scholar]
- Goel SK, Lalwani ND, Reddy JK. (1986). Peroxisome proliferation and lipid peroxidation in rat liver. Cancer Res 46(3): 1324–30. [PubMed] [Google Scholar]
- Gold LS, Manley NB, Slone TH, Rohrbach L, Garfinkel GB. Supplement to the Carcinogenic Potency Database (CPDB): results of animal bioassays published in the general literature through 1997 and by the National Toxicology Program in 1997-1998. Toxicol Sci. 2005. June;85(2):747–808. [DOI] [PubMed] [Google Scholar]
- Goll V, Alexandre E, Viollon-Abadie C, Nicod L, Jaeck D, Richert L. (1999). Comparison of the effects of various peroxisome proliferators on peroxisomal enzyme activities, DNA synthesis, and apoptosis in rat and human hepatocyte cultures. Toxicol Appl Pharmacol 160:21–32. [DOI] [PubMed] [Google Scholar]
- Gottlicher M, Widmark E, Li Q, Gustafsson JA. (1992). Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A 89(10): 4653–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Huber W, Just W, Gibson G, Schulte-Hermann R. (1993c). Enhancement of peroxisomal enzymes, cytochrome P-452 and DNA synthesis in putative preneoplastic foci of rat liver treated with the peroxisome proliferator nafenopin. Carcinogenesis 14(5): 1007–12. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Huber W, Timmermann-Trosiener I, Schulte-Hermann R. (1993a). Peroxisomal enzyme induction uncoupled from enhanced DNA synthesis in putative preneoplastic liver foci of rats treated with a single dose of the peroxisome proliferator nafenopin. Carcinogenesis 14:2435–2437. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Ruttkay-Nedecky B, Mullauer L, Taper H, Huber W, Bursch W, Schulte-Hermann R. (1997). Inherent increase of apoptosis in liver tumors: Implications for carcinogenesis and tumor regression. Hepatology 25:906–912. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Waldhor T, Huber W, Schulte-Hermann R. (1993b). Glutathione S-transferase isoenzyme patterns in different subtypes of enzyme-altered rat liver foci treated with the peroxisome proliferator nafenopin or with phenobarbital. Carcinogenesis 14(11): 2407–12. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011. March;70 Suppl 1:i104–8. [DOI] [PubMed] [Google Scholar]
- Guo D, Sarkar J, Suino-Powell K, Xu Y, Matsumoto K, Jia Y, Yu S, Khare S, Haldar K, Rao MS, Foreman JE, Monga SP, Peters JM, Xu HE, Reddy JK. (2007). Induction of nuclear translocation of constitutive androstane receptor by peroxisome proliferator-activated receptor alpha synthetic ligands in mouse liver. J Biol Chem 282: 36766–76. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Chiu WA, Bateson TF, Jinot J, Scott CS, Brown RC, Caldwell JC. (2009). A reexamination of the PPAR-α activation mode of action as a basis for assessing human cancer risks of environmental contaminants. Environmental Health Perspectives 117(11):1664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann M, Georgiev O and Schaffner. (1997) The VP16 Paradox: Herpes Simplex Virus VP16 Contains a Long-Range Activation Domain but within the Natural Multiprotein Complex Activates Only from Promoter-Proximal Positions. Journal of Virology 71 (8) 5952–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler JA, Seed CB, Bradford BU, Thurman RG. (1992). Induction of peroxisomes by treatment with perfluorooctanoate does not increase rates of H2O2 production in intact liver. Toxicol Lett 60(1): 61–8. [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Kemmer C, Kadner E. (1983). Relationship between morphological changes and lipid lowering action of p-chlorphenoxyisobutyric acid (CPIB) on hepatic mitochondria and peroxisomes in man. Atherosclerosis 46:239–246. [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Kemmer C, Leonhardt W, Kunze KD, Jaross W, Haller H. (1980). Effects of p-chlorophenoxyisobutyric acid (CPIB) on the human liver. Atherosclerosis 36(2):159–72. [DOI] [PubMed] [Google Scholar]
- Hanselman JC, Vartanian MA, Koester BP, Gray SA, Essenburg AD, Rea TJ, Bisgaier CL, Pape ME. (2001). Expression of the mRNA encoding truncated PPAR alpha does not correlate with hepatic insensitivity to peroxisome proliferators. Mol Cell Biochem 217(1-2):91–7. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Fujita T, Usuda N, Cook W, Qi C, Peters JM, Gonzalez FJ, Yeldandi AV, Rao MS, Reddy JK. (1999). Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor alpha and peroxisomal fatty acyl-CoA oxidase. Genotype correlation with fatty liver phenotype. J Biol Chem 274(27):19228–36. [DOI] [PubMed] [Google Scholar]
- Hasmall SC, James NH, Macdonald N, Gonzalez FJ, Peters JM, Roberts RA. (2000a). Suppression of mouse hepatocyte apoptosis by peroxisome proliferators: role of PPARα and TNFαlpha. Mutat Res 448(2): 193–200. [DOI] [PubMed] [Google Scholar]
- Hasmall SC, James NH, Macdonald N, Soames AR, Roberts RA. (2000b). Species differences in response to diethylhexylphthalate: suppression of apoptosis, induction of DNA synthesis and peroxisome proliferator activated receptor alpha-mediated gene expression. Arch Toxicol 74(2): 85–91. [DOI] [PubMed] [Google Scholar]
- Hasmall SC, James NH, Macdonald N, West D, Chevalier S, Cosulich SC, Roberts RA. (1999). Suppression of apoptosis and induction of DNA synthesis in vitro by the phthalate plasticizers monoethylhexylphthalate (MEHP) and diisononylphthalate (DINP): a comparison of rat and human hepatocytes in vitro. Arch Toxicol 73(8-9): 451–6. [DOI] [PubMed] [Google Scholar]
- Hasmall SC, James NH, Soames AR, Roberts RA. (1998). The peroxisome proliferator nafenopin does not suppress hepatocyte apoptosis in guinea-pig liver in vivo nor in human hepatocytes in vitro. Arch Toxicol 72(12):777–83. [DOI] [PubMed] [Google Scholar]
- Hasmall SC, West DA, Olsen K, Roberts RA. (2000c). Role of hepatic non-parenchymal cells in the response of rat hepatocytes to the peroxisome proliferator nafenopin in vitro. Carcinogenesis 21(12): 2159–65. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ito Y, Yamagishi N, Yanagiba Y, Tamada H, Wang D, Ramdhan DH, Naito H, Harada Y, Kamijima M, Gonzales FJ, Nakajima T. Hepatic peroxisome proliferator-activated receptor α may have an important role in the toxic effects of di(2-ethylhexyl)phthalate on offspring of mice. Toxicology. 2011. October 28;289(1):1–10. [DOI] [PubMed] [Google Scholar]
- Hays T, Rusyn I, Burns AM, Kennett MJ, Ward JM, Gonzalez FJ, Peters JM. (2005). Role of peroxisome proliferator-activated receptor-alpha (PPARα) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis 26(1): 219–27. [DOI] [PubMed] [Google Scholar]
- Henkler F, Brinkmann J, Luch A. The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers (Basel). 2010. April 8;2(2):376–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herren-Freund SL, Pereira MA, Khoury MD, Olson G. (1987). The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicol Appl Pharmacol 90(2): 183–9. [DOI] [PubMed] [Google Scholar]
- Hinton RH, Mitchell FE, Mann A, Chescoe D, Price SC, Nunn A, Grasso P, Bridges JW. (1986). Effects of phthalic acid esters on the liver and thyroid. Environ Health Perspect 70: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoivik DJ, Qualls CW Jr, Mirabile RC, Cariello NF, Kimbrough CL, Colton HM, Anderson SP, Santostefano MJ, Morgan RJ, Dahl RR, Brown AR, Zhao Z, Mudd PN Jr, Oliver WB Jr, Brown HR, Miller RT. (2004). Fibrates induce hepatic peroxisome and mitochondrial proliferation without overt evidence of cellular proliferation and oxidative stress in cynomolgus monkeys. Carcinogenesis 25(9):1757–69. [DOI] [PubMed] [Google Scholar]
- Holden P, Hasmall S, James N, West D, Brindle R, Gonzalez F, Peters J, Roberts R. (2000). Tumour necrosis factor a (TNFα): Role in suppression of apoptosis by peroxisome proliferators. Cell Mol Biol 46:29–39. [PubMed] [Google Scholar]
- Howroyd P, Swanson C, Dunn C, Cattley RC, Corton JC. (2004). Decreased longevity and enhancement of age-dependent lesions in mice lacking the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα). Toxicol Pathol 32(5): 591–9. [DOI] [PubMed] [Google Scholar]
- Hsieh LL, Shinozuka H, Weinstein IB. (1991). Changes in expression of cellular oncogenes and endogenous retrovirus-like sequences during hepatocarcinogenesis induced by a peroxisome proliferator. Br J Cancer 64(5):815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W, Kraupp-Grasl B, Esterbauer H, Schulte-Hermann R. (1991). Role of oxidative stress in age dependent hepatocarcinogenesis by the peroxisome proliferator nafenopin in the rat. Cancer Res 51(7): 1789–92. [PubMed] [Google Scholar]
- Huber WW, Grasl-Kraupp B, Stekel H, Gschwentner C, Lang H, Schulte-Hermann R. (1997). Inhibition instead of enhancement of lipid peroxidation by pretreatment with the carcinogenic peroxisome proliferator nafenopin in rat liver exposed to a high single dose of corn oil. Arch Toxicol 71(9): 575–81. [DOI] [PubMed] [Google Scholar]
- Huttunen JK, Heinonen OP, Manninen V, Koskinen P, Hakulinen T, Teppo L, Manttari M, Frick MH. (1994). The Helsinki Heart Study: an 8.5-year safety and mortality follow-up. J Intern Med 235(1): 31–9. [DOI] [PubMed] [Google Scholar]
- IARC. (1996). Some pharmaceutical drugs. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Lyon, France: IARC Press, 391–426. [Google Scholar]
- Isenberg JS, Kamendulis LM, Ackley DC, Smith JH, Pugh G Jr, Lington AW, McKee RH, Klaunig JE. (2001). Reversibility and persistence of di-2-ethylhexyl phthalate (DEHP)- and phenobarbital-induced hepatocellular changes in rodents. Toxicol Sci 64(2): 192–9. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Kamendulis LM, Smith JH, Ackley DC, Pugh G Jr, Lington AW, Klaunig JE. (2000). Effects of Di-2-ethylhexyl phthalate (DEHP) on gap-junctional intercellular communication (GJIC), DNA synthesis, and peroxisomal beta oxidation (PBOX) in rat, mouse, and hamster liver. Toxicol Sci 56(1): 73–85. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Kolaja KL, Ayoubi SA, Watkins JB 3rd, Klaunig JE. (1997). Inhibition of WY 14,643-induced hepatic lesion growth in mice by rotenone. Carcinogenesis 18:1511–1519. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S. (1990). Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347(6294): 645–50. [DOI] [PubMed] [Google Scholar]
- Ito Y, Nakamura T, Yanagiba Y, Ramdhan DH, Yamagishi N, Naito H, Kamijima M, Gonzalez FJ, Nakajima T. Plasticizers May Activate Human Hepatic Peroxisome Proliferator-Activated Receptor α Less Than That of a Mouse but May Activate Constitutive Androstane Receptor in Liver. PPAR Res. 2012;2012:201284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee CH, Aoyama T, Ichihara G, Furuhashi K, Kamijima M, Gonzalez FJ, Nakajima T. (2007). Di(2-ethylhexyl)phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor alpha-independent pathway. J Occup Health 49(3):172–82. [DOI] [PubMed] [Google Scholar]
- James NH, Gill JH, Brindle R, Woodyatt NJ, Macdonald N, Rolfe M, Hasmall SC, Tugwood JD, Holden PR, Roberts RA. (1998b). Peroxisome proliferator-activated receptor (PPAR) alpha-regulated growth responses and their importance to hepatocarcinogenesis. Toxicol Lett 102-103:91–6. [DOI] [PubMed] [Google Scholar]
- James NH, Roberts RA. (1996). Species differences in response to peroxisome proliferators correlate in vitro with induction of DNA synthesis rather than suppression of apoptosis. Carcinogenesis 17(8): 1623–32. [DOI] [PubMed] [Google Scholar]
- James NH, Roberts RA. Species differences in response to peroxisome proliferators correlate in vitro with induction of DNA synthesis rather than suppression of apoptosis. Carcinogenesis. 1996. August;17(8):1623–32. [DOI] [PubMed] [Google Scholar]
- James NH, Soames AR, Roberts RA. (1998a). Suppression of hepatocyte apoptosis and induction of DNA synthesis by the rat and mouse hepatocarcinogen diethylhexylphthalate (DEHP) and the mouse hepatocarcinogen 1,4-dichlorobenzene (DCB). Arch Toxicol 72:784–790. [DOI] [PubMed] [Google Scholar]
- Jia Y, Qi C, Zhang Z, Hashimoto T, Rao MS, Huyghe S, Suzuki Y, Van Veldhoven PP, Baes M, Reddy JK. (2003). Overexpression of peroxisome proliferator-activated receptor-alpha (PPARα)-regulated genes in liver in the absence of peroxisome proliferation in mice deficient in both L- and D-forms of enoyl-CoA hydratase/dehydrogenase enzymes of peroxisomal beta-oxidation system. J Biol Chem 278(47):47232–9. [DOI] [PubMed] [Google Scholar]
- Jolly RA, Goldstein KM, Wei T, Gao H, Chen P, Huang S, Colet JM, Ryan TP, Thomas CE, Estrem ST. Pooling samples within microarray studies: a comparative analysis of rat liver transcription response to prototypical toxicants. Physiol Genomics. 2005. August 11;22(3):346–55. [DOI] [PubMed] [Google Scholar]
- Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, Reif DM, Rotroff DM, Shah I, Richard AM, Dix DJ. In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ Health Perspect. 2010. April;118(4):485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien E, Boobis AR, Olin SS. (2009). The key events dose-response framework: A cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Crit Rev Food Sci Nutr 49 (8):682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. (2006). Nuclear factor-kappaB in cancer development and progression. Nature 441(7092):431–6. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Suzuki S, Kozuka H, Sato M, Suzuki Y. (1994). Effects of prolonged administration of perfluorooctanoic acid on hepatic activities of enzymes which detoxify peroxide and xenobiotic in the rat. Toxicology 93(2-3): 85–97. [DOI] [PubMed] [Google Scholar]
- Keller H, Devchand PR, Perroud M, Wahli W. (1997). PPAR alpha structure-function relationships derived from species-specific differences in responsiveness to hypolipidemic agents. Biol Chem 378:651–655. [DOI] [PubMed] [Google Scholar]
- Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. (1993). Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci 90(6):2160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S. et al. (1999) Peroxisome proliferator–activated receptor α mediates the adaptive response to fasting J Clin Inv 103 (11) 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S. Integrated physiology and systems biology of PPARα. Mol Metab. 2014. March 6;3(4):354–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshava N, Caldwell JC. Key issues in the role of peroxisome proliferator-activated receptor agonism and cell signaling in trichloroethylene toxicity. Environ Health Perspect. 2006. September;114(9):1464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler W, Numtip W, Grote K, Csanády GA, Chahoud I, Filser JG. Blood burden of di(2-ethylhexyl) phthalate and its primary metabolite mono(2-ethylhexyl) phthalate in pregnant and nonpregnant rats and marmosets. Toxicol Appl Pharmacol. 2004. March 1;195(2):142–53. [DOI] [PubMed] [Google Scholar]
- Kim MY, Song KS, Park GH, Chang SH, Kim HW, Park JH, Jin H, Eu KJ, Cho HS, Kang G, Kim YC, Cho MH. (2004). B6C3F1 mice exposed to ozone with 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone and/or dibutyl phthalate showed toxicities through alterations of NF-kappaB, AP-1, Nrf2, and osteopontin. J Vet Sci 5(2): 131–7. [PubMed] [Google Scholar]
- Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JM, Roberts RA, Fenner-Crisp PA. (2003). PPARα agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol 33(6):655–780. [DOI] [PubMed] [Google Scholar]
- Kluwe WM, Huff JE, Matthews HB, Irwin R, Haseman JK. (1985). Comparative Chronic Toxicities and Carcinogenic Potentials of 2-Ethylhexyl- Containing Compounds in Rats and Mice, Carcinogenesis 6(11):1577–1583. [DOI] [PubMed] [Google Scholar]
- Kluwe WM, McConnell EE, Huff JE, Haseman JK, Douglas JF, Hartwell WV. (1982). Carcinogenicity Testing Of Phthalate Esters And Related Compounds Environ. Health Perspect. 45:129–133, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaja KL, Stevenson DE, Walborg EF Jr., Klaunig JE (1996a). Dose Dependence of Phenobarbital Promotion of Preneoplastic Hepatic Lesions in F344 Rats and B6C3F1 Mice: Effects on DNA Synthesis and Apoptosis. Carcinogenesis, 17(5), 947–954. [DOI] [PubMed] [Google Scholar]
- Kolaja KL, Stevenson DE, Walborg EF Jr., Klaunig JE (1996b). Reversibility of Promoter Induced Hepatic Focal Lesion Growth in Mice. Carcinogenesis, 17(7), 1403–1409. [DOI] [PubMed] [Google Scholar]
- Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986. April;22(4):201–11. [DOI] [PubMed] [Google Scholar]
- Lacquemant C, Lepretre F, Pineda Torra I, Manraj M, Charpentier G, Ruiz J, Staels B, Froguel P. (2000). Mutation screening of the PPARα gene in type 2 diabetes associated with coronary heart disease. Diabetes Metab 26(5):393–401. [PubMed] [Google Scholar]
- Lake BG, Evans JG, Cunninghame ME, Price RJ. (1993). Comparison of the hepatic effects of nafenopin and WY-14,643 on peroxisome proliferation and cell replication in the rat and Syrian hamster. Environ Health Perspect 101 Suppl 5: 241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake BG, Evans JG, Gray TJ, Körösi SA, North CJ. (1989b). Comparative studies on nafenopin-induced hepatic peroxisome proliferation in the rat, Syrian hamster, guinea pig, and marmoset. Toxicol Appl Pharmacol. 99(1):148–60. [DOI] [PubMed] [Google Scholar]
- Lake BG, Gray TJ, Körösi SA, Walters DG. (1989a). Nafenopin, a peroxisome proliferator, depletes hepatic vitamin E content and elevates plasma oxidised glutathione levels in rats. Toxicol Lett. 45(2-3):221–9. [DOI] [PubMed] [Google Scholar]
- Lake BG, Kozlen SL, Evans JG, Gray TJ, Young PJ, Gangolli SD. (1987). Effect of prolonged administration of clofibric acid and di-(2-ethylhexyl)phthalate on hepatic enzyme activities and lipid peroxidation in the rat. Toxicology 44(2): 213–28. [DOI] [PubMed] [Google Scholar]
- Lake BG, Rumsby PC, Price RJ, Cunninghame ME. (2000). Species differences in hepatic peroxisome proliferation, cell replication and transforming growth factor-beta1 gene expression in the rat, Syrian hamster and guinea pig. Mutat Res. 448(2):213–25. [DOI] [PubMed] [Google Scholar]
- Lapinskas PJ, Brown S, Leesnitzer LM, Blanchard S, Swanson C, Cattley RC, Corton JC. (2005). Role of PPARαlpha in mediating the effects of phthalates and metabolites in the liver. Toxicology. 207(1):149–63. [DOI] [PubMed] [Google Scholar]
- Laughter AR, Dunn CS, Swanson CL, Howroyd P, Cattley RC, Corton JC. (2004). Role of the peroxisome proliferator-activated receptor alpha (PPARα) in responses to trichloroethylene and metabolites, trichloroacetate and dichloroacetate in mouse liver. Toxicology 203(1-3):83–98. [DOI] [PubMed] [Google Scholar]
- Lawrence JW, Li Y, Chen S, DeLuca JG, Berger JP, Umbenhauer DR, Moller DE, Zhou G. (2001c). Differential gene regulation in human versus rodent hepatocytes by peroxisome proliferator-activated receptor (PPAR) alpha. PPAR alpha fails to induce peroxisome proliferation-associated genes in human cells independently of the level of receptor expression. J Biol Chem 276(34): 31521–7. [DOI] [PubMed] [Google Scholar]
- Lawrence JW, Wollenberg GK, DeLuca JG. (2001b). Tumor necrosis factor alpha is not required for WY14,643-induced cell proliferation. Carcinogenesis 22(3): 381–6. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim KT. Plant-originated glycoprotein (24 kDa) has an inhibitory effect on proliferation of BNL CL.2 cells in response to di(2-ethylhexyl)phthalate. Cell Biochem Funct. 2011. August;29(6):496–505. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. (1995). Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15: 3012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999. June 22;96(13):7473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Glauert HP, Spear BT. (2000a). Activation of nuclear factor-kappaB by the peroxisome proliferator ciprofibrate in H4IIEC3 rat hepatoma cells and its inhibition by the antioxidants N-acetylcysteine and vitamin E. Biochem Pharmacol 59(4): 427–34. [DOI] [PubMed] [Google Scholar]
- Li Y, Tharappel JC, Cooper S, Glenn M, Glauert HP, Spear BT. (2000b). Expression of the hydrogen peroxide-generating enzyme fatty acyl CoA oxidase activates NF-kappaB. DNA Cell Biol 19(2): 113–20. [DOI] [PubMed] [Google Scholar]
- Liu MH, Li J, Shen P, Husna B, Tai ES, Yong EL. (2008). A natural polymorphism in peroxisome proliferator-activated receptor-alpha hinge region attenuates transcription due to defective release of nuclear receptor corepressor from chromatin. Mol Endocrinol 22(5):1078–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebker DJ, Hansen KJ, Bass NM, Butenhoff JL, Seacat AM. (2002). Interactions of fluorochemicals with rat liver fatty acid-binding protein. Toxicology 176(3):175–85. [DOI] [PubMed] [Google Scholar]
- Lundgren B, Meijer J, DePierre JW. Induction of cytosolic and microsomal epoxide hydrolases and proliferation of peroxisomes and mitochondria in mouse liver after dietary exposure to p-chlorophenoxyacetic acid, 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid. Biochem Pharmacol. 1987. March 15;36(6):815–21. [DOI] [PubMed] [Google Scholar]
- Ma X, Stoffregen DA, Wheelock GD, Rininger JA, Babish JG. Discordant hepatic expression of the cell division control enzyme p34cdc2 kinase, proliferating cell nuclear antigen, p53 tumor suppressor protein, and p21Waf1 cyclin-dependent kinase inhibitory protein after WY14,643 ([4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid) dosing to rats. Mol Pharmacol. 1997. January;51(1):69–78. [DOI] [PubMed] [Google Scholar]
- Macdonald N, Holden PR, Roberts RA. (1999). Addition of peroxisome proliferator-activated receptor alpha to guinea pig hepatocytes confers increased responsiveness to peroxisome proliferators. Cancer Res 59(19):4776–80. [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. (2005). IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121(7):977–90. [DOI] [PubMed] [Google Scholar]
- Makowska JM, Gibson GG, Bonner FW. (1992). Species differences in ciprofibrate induction of hepatic cytochrome P450 4A1 and peroxisome proliferation. J Biochem Toxicol 7(3):183–91. [DOI] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ. (1999). Trans-activation of PPARα and PPARγamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol 161(2): 209–18. [DOI] [PubMed] [Google Scholar]
- Marsman D. (1995). NTP Technical Report on the Toxicity Studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice. Toxic Rep Ser 30: 1–G5. [PubMed] [Google Scholar]
- Marsman DS, Cattley RC, Conway JG, Popp JA. (1988). Relationship of hepatic peroxisome proliferation and replicative DNA synthesis to the hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)phthalate and [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid (WY-14,643) in rats. Cancer Res 48(23): 6739–44. [PubMed] [Google Scholar]
- Marsman DS, Goldsworthy TL, Popp JA. (1992). Contrasting hepatocytic peroxisome proliferation, lipofuscin accumulation and cell turnover for the hepatocarcinogens WY-14,643 and clofibric acid. Carcinogenesis 13(6): 1011–7. [DOI] [PubMed] [Google Scholar]
- Marsman DS, Popp JA. (1994). Biological potential of basophilic hepatocellular foci and hepatic adenoma induced by the peroxisome proliferator, WY-14,643. Carcinogenesis 15(1): 111–7. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Amanuma T, Tsutsumi M, Tsujiuchi T, Horiguchi K, Denda A, Konishi Y. (1994). Inhibition by catechol and di(2-ethylhexyl)phthalate of pancreatic carcinogenesis after initiation with N-nitrosobis(2-hydroxypropyl)amine in Syrian hamsters. Carcinogenesis. 15(6):1193–6. [DOI] [PubMed] [Google Scholar]
- Meek ME. (2008). Recent developments in frameworks to consider human relevance of hypothesized modes of action for tumours in animals. Environ Mol Mutagen 49(2):110–6. [DOI] [PubMed] [Google Scholar]
- Menegazzi M, Carcereri-De Prati A, Suzuki H, Shinozuka H, Pibiri M, Piga R, Columbano A, Ledda-Columbano GM. (1997). Liver cell proliferation induced by nafenopin and cyproterone acetate is not associated with increases in activation of transcription factors NF-kappaB and AP-1 or with expression of tumor necrosis factor alpha. Hepatology 25(3): 585–92. [DOI] [PubMed] [Google Scholar]
- Meyer K, Lee JS, Dyck PA, Cao WQ, Rao MS, Thorgeirsson SS, Reddy JK. (2003). Molecular profiling of hepatocellular carcinomas developing spontaneously in acyl-CoA oxidase deficient mice: comparison with liver tumors induced in wild-type mice by a peroxisome proliferator and a genotoxic carcinogen. Carcinogenesis 24(5):975–84. Carcinogenesis 24(5):975-84. [DOI] [PubMed] [Google Scholar]
- Miller RT, Shah RS, Cattley RC, Popp JA. The peroxisome proliferations WY-14,643 and methylclofenapate induce hepatocyte ploidy alterations and ploidy-specific DNA synthesis in F344 rats. Toxicol Appl Pharmacol. 1996. June;138(2):317–23. [DOI] [PubMed] [Google Scholar]
- Morimura K, Cheung C, Ward JM, Reddy JK, Gonzalez FJ. (2006). Differential susceptibility of mice humanized for peroxisome proliferator-activated receptor alpha to WY-14,643-induced liver tumorigenesis. Carcinogenesis 27(5):1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R, Jow L, Noonan D, McDonnell DP. (1994). Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. J Steroid Biochem Molec Biol 51: 157–166. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ramdhan DH, Tanaka N, Naito H, Tamada H, Ito Y, Li Y, Hayashi Y, Yamagishi N, Yanagiba Y, Aoyama T, Gonzalez FJ, Nakajima T. Modulation of ammonium perfluorooctanoate-induced hepatic damage by genetically different PPARα in mice. Arch Toxicol. 2012. January;86(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Tanaka N, Li G, Hu R, Kamijo Y, Hara A, Aoyama T. Effect of bezafibrate on hepatic oxidative stress: comparison between conventional experimental doses and clinically-relevant doses in mice. Redox Rep. 2010;15(3):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Ito Y, Yanagiba Y, Ramdhan DH, Kono Y, Naito H, Hayashi Y, Li Y, Aoyama T, Gonzalez FJ, Nakajima T. Microgram-order ammonium perfluorooctanoate may activate mouse peroxisome proliferator-activated receptor alpha, but not human PPARalpha. Toxicology. 2009. November 9;265(1-2):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MA, Sanchez IM, Bull RJ, Sylvester SR. Increased expression of c-myc and c-Ha-ras in dichloroacetate and trichloroacetate-induced liver tumors in B6C3F1 mice. Toxicology. 1990. October;64(1):47–57. [DOI] [PubMed] [Google Scholar]
- Nemali MR, Usuda N, Reddy MK, Oyasu K, Hashimoto T, Osumi T, Rao MS, Reddy JK. (1988). Comparison of constitutive and inducible levels of expression of peroxisomal beta-oxidation and catalase genes in liver and extrahepatic tissues of rat. Cancer Res 48:5316–5324. [PubMed] [Google Scholar]
- Nesfield SR, Clarke CJ, Hoivik DJ, Miller RT, Allen JS, Selinger K, Santostefano MJ. (2005b). Evaluation of the carcinogenic potential of clofibrate in the rasH2 mouse. Int J Toxicol. 24(5):301–11. [DOI] [PubMed] [Google Scholar]
- Nesfield SR, Williams TC, Hoivik DJ, Miller RT, Allen JS, Selinger K, Rickert D, Santostefano MJ. (2005a). Evaluation of the carcinogenic potential of clofibrate in the neonatal mouse. Int J Toxicol. 24(5):341–8. [DOI] [PubMed] [Google Scholar]
- Nicholls-Grzemski FA, Belling GB, Priestly BG, Calder IC, Burcham PC. (2000). Clofibrate pretreatment in mice confers resistance against hepatic lipid peroxidation. J Biochem Mol Toxicol 14(6): 335–45. [DOI] [PubMed] [Google Scholar]
- Nilakantan V, Spear BT, Glauert HP. (1998). Liver-specific catalase expression in transgenic mice inhibits NF-kappaB activation and DNA synthesis induced by the peroxisome proliferator ciprofibrate. Carcinogenesis 19(4): 631–7. [DOI] [PubMed] [Google Scholar]
- O’Brien ML, Cunningham ML, Spear BT, Glauert HP. (2001a). Effects of peroxisome proliferators on glutathione and glutathione-related enzymes in rats and hamsters. Toxicol Appl Pharmacol 171(1): 27–37. [DOI] [PubMed] [Google Scholar]
- O’Brien ML, Twaroski TP, Cunningham ML, Glauert HP, Spear BT. (2001b). Effects of peroxisome proliferators on antioxidant enzymes and antioxidant vitamins in rats and hamsters. Toxicol Sci 60(2): 271–8. [DOI] [PubMed] [Google Scholar]
- Oberhammer FA, Qin HM. (1995). Effect of three tumour promoters on the stability of hepatocytes cultures and apoptosis after transforming growth factor-beta1. Carcinogenesis 16:1363–1371. [DOI] [PubMed] [Google Scholar]
- O’Brien ML, Spear BT, Glauert HP. Role of oxidative stress in peroxisome proliferator-mediated carcinogenesis. Crit Rev Toxicol. 2005. January;35(1):61–88. [DOI] [PubMed] [Google Scholar]
- Ohmura T, Ledda-Columbano GM, Piga R, Columbano A, Glemba J, Katyal SL, Locker J, Shinozuka H. (1996). Hepatocyte proliferation induced by a single dose of a peroxisome proliferator. Am J Pathol 148(3): 815–24. [PMC free article] [PubMed] [Google Scholar]
- Oreskes N, Conway EM. Defeating the merchants of doubt. (2010). Nature 465(7299):686–7. [DOI] [PubMed] [Google Scholar]
- Oshida K, Vasani N, Jones C, Moore T, Hester S, Nesnow S, Auerbach S, Geter DR, Aleksunes LM, Thomas RS, Applegate D, Klaassen CD, Corton JC. Identification of chemical modulators of the constitutive activated receptor (CAR) in a gene expression compendium. Nucl Recept Signal. 2015. April 27;13:e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacot C, Petit M, Rollin M, Behechti N, Moisant M, Deslex P, Althoff J, Lhuguenot JC, Latruffe N. (1996). Difference between guinea pig and rat in the liver peroxisomal response to equivalent plasmatic level of ciprofibrate. Arch Biochem Biophys 327(1):181–8. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF. (1998). Peroxisome proliferator activated receptor-alpha expression in human liver. Mol Pharmacol 53(1): 14–22. [PubMed] [Google Scholar]
- Parzefall W, Berger W, Kainzbauer E, Teufelhofer O, Schulte-Hermann R, Thurman RG. (2001). Peroxisome proliferators do not increase DNA synthesis in purified rat hepatocytes. Carcinogenesis 22(3): 519–23. [DOI] [PubMed] [Google Scholar]
- Pazienza V, Vinciguerra M, Mazzoccoli G. PPARs Signaling and Cancer in the Gastrointestinal System. PPAR Res. 2012;2012:560846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permadi H, Lundgren B, Andersson K, Sundberg C, DePierre JW. (1993). Effects of perfluoro fatty acids on peroxisome proliferation and mitochondrial size in mouse liver: dose and time factors and effect of chain length. Xenobiotica 23(7): 761–70. [DOI] [PubMed] [Google Scholar]
- Perrone CE, Shao L, Williams GM. (1998). Effect of rodent hepatocarcinogenic peroxisome proliferators on fatty acyl-CoA oxidase, DNA synthesis, and apoptosis in cultured human and rat hepatocytes. Toxicol Appl Pharmacol 150: 277–86. [DOI] [PubMed] [Google Scholar]
- Perrone CE, Williams GM. Rodent hepatocarcinogenic peroxisome proliferators induce proliferation of rat hepatocytes in primary mixed cultures with rat liver epithelial cells. Cancer Lett. 1998. January 16;123(1):27–33. [DOI] [PubMed] [Google Scholar]
- Peters JM, Aoyama T, Cattley RC, Nobumitsu U, Hashimoto T, Gonzalez FJ. (1998). Role of peroxisome proliferator-activated receptor alpha in altered cell cycle regulation in mouse liver. Carcinogenesis 19(11): 1989–94. [DOI] [PubMed] [Google Scholar]
- Peters JM, Cattley RC, Gonzalez FJ. (1997). Role of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator WY-14,643. Carcinogenesis 18 (11): 2029–33. [DOI] [PubMed] [Google Scholar]
- Peters JM, Cheung C, Gonzalez FJ. (2005). Peroxisome proliferator-activated receptor-α and liver cancer: where do we stand? J Mol Med 83: 774–785. [DOI] [PubMed] [Google Scholar]
- Peters JM, Rusyn I, Rose ML, Gonzalez FJ, Thurman EG. (2000). Peroxisome proliferator-activated receptor alpha is restricted to hepatic parenchymal cells, not Kupffer cells: implications for the mechanism of action of peroxisome proliferators in hepatocarcinogenesis. Carcinogenesis 21(4): 823–6. [DOI] [PubMed] [Google Scholar]
- Plant NJ, Horley NJ, Dickins M, Hasmall S, Elcombe CR, Bell DR. (1998). The coordinate regulation of DNA synthesis and suppression of apoptosis is differentially regulated by the liver growth agents, phenobarbital and methylclofenapate. Carcinogenesis 19(9): 1521–7. [DOI] [PubMed] [Google Scholar]
- Pogribny IP, Tryndyak VP, Woods CG, Witt SE, Rusyn I. Epigenetic effects of the continuous exposure to peroxisome proliferator WY-14,643 in mouse liver are dependent upon peroxisome proliferator activated receptor alpha. Mutat Res. 2007. December 1;625(1-2):62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogribny IP. MicroRNA dysregulation during chemical carcinogenesis. Epigenomics. 2009. December;1(2):281–90. [DOI] [PubMed] [Google Scholar]
- Polvani S, Tarocchi M, Tempesti S, Galli A. Nuclear receptors and pathogenesis of pancreatic cancer. World J Gastroenterol. 2014. September 14;20(34):12062–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RJ, Evans JG, Lake BG. (1992). Comparison of the effects of nafenopin on hepatic peroxisome proliferation and replicative DNA synthesis in the rat and Syrian hamster. Food Chem Toxicol 30(11):937–44. [DOI] [PubMed] [Google Scholar]
- Pugh G Jr, Isenberg JS, Kamendulis LM, Ackley DC, Clare LJ, Brown R, Lignton AW, Smith JH, Klaunig JE. (2000). Effects of diisononyl phthalate, di-2-ethylhexyl phthalate, and clofibrate in cynomolgus monkeys. Toxicol Sci 56:181–188. [DOI] [PubMed] [Google Scholar]
- Qu A, Jiang C, Cai Y, Kim JH, Tanaka N, Ward JM, Shah YM, Gonzalez FJ. Role of Myc in hepatocellular proliferation and hepatocarcinogenesis. J Hepatol. 2014. February;60(2):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu A, Shah YM, Matsubara T, Yang Q, Gonzalez FJ. (2010). PPARαlpha-dependent activation of cell cycle control and DNA repair genes in hepatic nonparenchymal cells. Toxicol Sci 118(2):404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu B, Halliwell B, Ong CN, Lee BL, Li QT. (2000). Caloric restriction prevents oxidative damage induced by the carcinogen clofibrate in mouse liver. FEBS Lett 473(1): 85–8. [DOI] [PubMed] [Google Scholar]
- Rakhshandehroo M, Hooiveld G, Müller M, Kersten S. (2009). Comparative analysis of gene regulation by the transcription factor PPARα between mouse and human. PLoS One 4(8):e6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Dwivedi RS, Subbarao V, Reddy JK. (1988) Induction of peroxisome proliferation and hepatic tumours in C57BL/6N mice by ciprofibrate, a hypolipidaemic compound. Br J Cancer.;58(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Lalwani ND, Scarpelli DG, Reddy JK. (1982). The absence of gamma-glutamyl transpeptidase activity in putative preneoplastic lesions and in hepatocellular carcinomas induced in rats by the hypolipidemic peroxisome proliferator WY-14,643. Carcinogenesis 3(10): 1231–3. [DOI] [PubMed] [Google Scholar]
- Rao MS, Lalwani ND, Watanabe TK, Reddy JK. (1984). Inhibitory effect of antioxidants ethoxyquin and 2(3)-tert-butyl-4-hydroxyanisole on hepatic tumorigenesis in rats fed ciprofibrate, a peroxisome proliferator. Cancer Res 44(3): 1072–6. [PubMed] [Google Scholar]
- Rao MS, Reddy JK. (1996). Hepatocarcinogenesis of peroxisome proliferators. Ann N Y Acad Sci 804:573–87. [DOI] [PubMed] [Google Scholar]
- Rao MS, Subbarao V. (1997a). The effect of deferoxamine on ciprofibrate-induced hepatocarcinogenesis in the rat. In Vivo 11(6): 495–8. [PubMed] [Google Scholar]
- Rao MS, Subbarao V. (1999). Inhibition of ciprofibrate-induced hepatocarcinogenesis in the rat by dimethylthiourea, a scavenger of hydroxyl radical. Oncol Rep 6(6): 1285–8. [DOI] [PubMed] [Google Scholar]
- Rao MS, Subbarao V. Effect of dexamethasone on ciprofibrate-induced cell proliferation and peroxisome proliferation. Fundam Appl Toxicol. 1997b. January;35(1):78–83. [DOI] [PubMed] [Google Scholar]
- Rao MS, Tatematsu M, Subbarao V, Ito N, Reddy JK. (1986). Analysis of peroxisome proliferator-induced preneoplastic and neoplastic lesions of rat liver for placental form of glutathione S-transferase and gamma-glutamyltranspeptidase. Cancer Res 46(10): 5287–90. [PubMed] [Google Scholar]
- Rao MS, Thangada S, Subbarao V. (1991). Peroxisome proliferation in neoplastic nodules and hepatocellular carcinomas induced by ciprofibrate in the rat. Exp Pathol 41(1):44–9. [DOI] [PubMed] [Google Scholar]
- Rao MS, Usuda N, Subbarao V, Reddy JK. (1987). Absence of gamma-glutamyl transpeptidase activity in neoplastic lesions induced in the liver of male F-344 rats by di-(2-ethylhexyl)phthalate, a peroxisome proliferator. Carcinogenesis 8(9): 1347–50. [DOI] [PubMed] [Google Scholar]
- Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1994. January 18;91(2):752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JK, Lalwani ND, Reddy MK, Qureshi SA. (1982). Excessive accumulation of autofluorescent lipofuscin in the liver during hepatocarcinogenesis by methyl clofenapate and other hypolipidemic peroxisome proliferators. Cancer Res 42(1): 259–66. [PubMed] [Google Scholar]
- Reddy JK, Qureshi SA. (1979). Tumorigenicity of the hypolipidaemic peroxisome proliferator ethyl-alpha-p-chlorophenoxyisobutyrate (clofibrate) in rats. Br J Cancer 40(3): 476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JK, Rao MS, Azarnoff DL, Sell S. (1979b). Mitogenic and carcinogenic effects of a hypolipidemic peroxisome proliferator, [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid (WY-14, 643), in rat and mouse liver. Cancer Res.39(1):152–61. [PubMed] [Google Scholar]
- Reddy JK, Rao MS. (1977). Malignant tumors in rats fed nafenopin, a hepatic peroxisome proliferator. J Natl Cancer Inst 59(6): 1645–50. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Rao MS. (1989). Oxidative DNA damage caused by persistent peroxisome proliferation: Its role in hepatocarcinogenesis. Mutat Res 214:63–68. [DOI] [PubMed] [Google Scholar]
- Ren H, Aleksunes LM, Wood C, Vallanat B, George MH, Klaassen CD, Corton JC. (2010). Characterization of peroxisome proliferator-activated receptor alpha--independent effects of PPARαlpha activators in the rodent liver: di-(2-ethylhexyl) phthalate also activates the constitutive-activated receptor. Toxicol Sci. 113(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Vallanat B, Nelson DM, Yeung LW, Guruge KS, Lam PK, Lehman-McKeeman LD, Corton JC. (2009). Evidence for the involvement of xenobiotic-responsive nuclear receptors in transcriptional effects upon perfluoroalkyl acid exposure in diverse species. Reprod Toxicol. 27(3-4):266–77. [DOI] [PubMed] [Google Scholar]
- Rigden M, Pelletier G, Poon R, Zhu J, Auray-Blais C, Gagnon R, Kubwabo C, Kosarac I, Lalonde K, Cakmak S, Xiao B, Leingartner K, Ku KL, Bose R, Jiao J. Assessment of urinary metabolite excretion after rat acute exposure to perfluorooctanoic acid and other peroxisomal proliferators. Arch Environ Contam Toxicol. 2015. January;68(1):148–58. [DOI] [PubMed] [Google Scholar]
- Rininger JA, Wheelock GD, Ma X, Babish JG. Discordant expression of the cyclin-dependent kinases and cyclins in rat liver following acute administration of the hepatocarcinogen [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio] acetic acid (WY14,643). Biochem Pharmacol. 1996. December 13;52(11):1749–55. [DOI] [PubMed] [Google Scholar]
- Roberts RA, James NH, Hasmall SC, Holden PR, Lambe K, Macdonald N, West D, Woodyatt NJ, Whitcome D. (2000). Apoptosis and proliferation in nongenotoxic carcinogenesis: species differences and role of PPARα. Toxicol Lett 112-113:49–57. [DOI] [PubMed] [Google Scholar]
- Roberts RA. (1999). Peroxisome proliferators: mechanisms of adverse effects in rodents and molecular basis for species differences. Arch Toxicol 73(8-9):413–8. [DOI] [PubMed] [Google Scholar]
- Rolfe M, James NH, Roberts RA. (1997). Tumour necrosis factor alpha (TNF-alpha) suppresses apoptosis and induces S-phase in rodent hepatocytes: A mediator of the hepatocarcinogenicity of peroxisome proliferators? Carcinogenesis 18:2277–2280. [DOI] [PubMed] [Google Scholar]
- Romagnolo DF, Zempleni J, Selmin OI. Nuclear receptors and epigenetic regulation: opportunities for nutritional targeting and disease prevention. Adv Nutr. 2014. July 14;5(4):373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose ML, Cattley RC, Dunn C, Wong V, Li X, Thurman RG. (1999b). Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis 20(11): 2075–81. [DOI] [PubMed] [Google Scholar]
- Rose ML, Germolec D, Arteel GE, Schoonhoven R, Thurman RG. (1997a). Dietary glycine prevents increases in hepatocyte proliferation caused by the peroxisome proliferator WY-14,643. Chem Res Toxicol 10(10):1198–204. [DOI] [PubMed] [Google Scholar]
- Rose ML, Germolec DR, Schoonhoven R, Thurman RG. (1997b). Kupffer cells are causally responsible for the mitogenic effect of peroxisome proliferators. Carcinogenesis 18(8):1453–6. [DOI] [PubMed] [Google Scholar]
- Rose ML, Rivera CA, Bradford BU, Graves LM, Cattley RC, Schoonhoven R, Swenberg JA, Thurman RG. (1999a). Kupffer cell oxidant production is central to the mechanism of peroxisome proliferators. Carcinogenesis 20(1): 27–33. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Abbott BD, Wolf DC, Corton JC, Wood CR, Schmid JE, Das KP, Zehr RD, Blair ET, Lau C. (2008a). Gene profiling in the livers of wild-type and PPARα-null mice exposed to perfluorooctanoic acid. Toxicol Pathol 36(4):592–607. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Das KP, Wood CR, Wolf CJ, Abbott BD, Lau C. Evaluation of perfluoroalkyl acid activity using primary mouse and human hepatocytes. Toxicology. 2013. June 7;308:129–37. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, WaalKEs MP, Abbott BD, Lau C, Corton JC. (2008b). Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol Sci 103(1):46–56. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Schmid JR, Corton JC, Zehr RD, Das KP, Abbott BD, Lau C. (2010). Gene expression profiling in wild-type and PPARα-null mice exposed to perfluorooctane sulfonate reveals PPARα-independent effects. PPAR Research pii: 794739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Das Kaberi P., Rooney John, Abbott Barbara, Lau Christopher and Corton J. Christopher. PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Submitted to Toxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland IR. Metabolism of di-(2-ethylhexyl) phthalate by the contents of the alimentary tract of the rat. Food Cosmet Toxicol. 1974. June;12(3):293–303. [DOI] [PubMed] [Google Scholar]
- Rusyn I, Kadiiska MB, Dikalova A, Kono H, Yin M, Tsuchiya K, Mason RP, Peters JM, Gonzalez FJ, Segal BH, Holland SM, Thurman RG. (2001). Phthalates rapidly increase production of reactive oxygen species in vivo: role of Kupffer cells. Mol Pharmacol 59(4): 744–50. [DOI] [PubMed] [Google Scholar]
- Rusyn I, Peters JM, Cunningham ML. (2006). Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol 36(5):459–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, Tsukamoto H, Thurman RG. (1998). WY-14 643 rapidly activates nuclear factor kappaB in Kupffer cells before hepatocytes. Carcinogenesis 19(7): 1217–22. [DOI] [PubMed] [Google Scholar]
- Rusyn I, Yamashina S, Segal BH, Schoonhoven R, Holland SM, Cattley RC, Swenberg JA, Thurman RG. (2000). Oxidants from nicotinamide adenine dinucleotide phosphate oxidase are involved in triggering cell proliferation in the liver due to peroxisome proliferators. Cancer Res 60(17): 4798–803. [PubMed] [Google Scholar]
- Sanderson LM, de Groot PJ, Hooiveld GJ, Koppen A, Kalkhoven E, Müller M, Kersten S. (2008). Effect of synthetic dietary triglycerides: a novel research paradigm for nutrigenomics. PLoS One 3(2):e1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapone A, Peters JM, Sakai S, Tomita S, Papiha SS, Dai R, Friedman FK, Gonzalez FJ. (2000). The human peroxisome proliferator-activated receptor alpha gene: identification and functional characterization of two natural allelic variants. Pharmacogenetics 10(4):321–33. [DOI] [PubMed] [Google Scholar]
- Schmezer P, Pool BL, Klein RG, Komitowski D, Schmähl D. (1988). Various short-term assays and two long-term studies with the plasticizer di(2-ethylhexyl)phthalate in the Syrian golden hamster. Carcinogenesis 9(1):37–43. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R, Ohde G, Schuppler J, Timmermann-Trosiener I. (1981). Enhanced proliferation of putative preneoplastic cells in rat liver following treatment with the tumor promoters phenobarbital, hexachlorocyclohexane, steroid compounds, and nafenopin. Cancer Res 41(6): 2556–62. [PubMed] [Google Scholar]
- Schwarz JJ, Chakraborty T, Martin J, Zhou J and Olson EN (1992) The Basic Region of Myogenin Cooperates with Two Transcription Activation Domains To Induce Muscle-Specific Transcription. Mol Cell Biol 12 (1) 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo KW, Kim KB, Kim YJ, Choi JY, Lee KT, Choi KS. (2004). Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem Toxicol 42(1): 107–14. [DOI] [PubMed] [Google Scholar]
- Shah YM, Morimura K, Yang Q, Tanabe T, Takagi M, Gonzalez FJ. (2007). Peroxisome proliferator-activated receptor alpha regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol Cell Biol 27(12):4238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D, Lee R, Roberts RA. (2002). Species differences in response to the phthalate plasticizer monoisononylphthalate (MINP) in vitro: a comparison of rat and human hepatocytes. Arch Toxicol 76(5-6):344–50. [DOI] [PubMed] [Google Scholar]
- Sher T, Yi HF, McBride OW, Gonzalez FJ. (1993). cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry 32(21): 5598–604. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Hurst CH, Tanaka SS, DeRoos FL, Butenhoff JL, Seacat AM, Waxman DJ. (2004). trans-activation of PPARα and induction of PPARα target genes by perfluorooctane-based chemicals. Toxicol Sci 80(1):151–60. [DOI] [PubMed] [Google Scholar]
- Sipes NS, Martin MT, Kothiya P, Reif DM, Judson RS, Richard AM, Houck KA, Dix DJ, Kavlock RJ, Knudsen TB. Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays Chem Res Toxicol. 2013. June 17;26(6):878–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Oliver T, Butterworth BE. (1987). Correlation of the carcinogenic potential of di(2-ethylhexyl)phthalate (DEHP) with induced hyperplasia rather than with genotoxic activity. Mutat Res 188(1): 21–8. [DOI] [PubMed] [Google Scholar]
- Soames AR, Cliffe S, Pate I, Foster JR. (1999). Quantitative analysis of the lobular distribution of S-phase in rat liver following dietary administration of di(2-ethylhexyl)phthalate. Toxicol Pathol 27(4): 436–40. [DOI] [PubMed] [Google Scholar]
- Soliman MS, Cunningham ML, Morrow JD, Roberts LJ 2nd, Badr MZ. (1997). Evidence against peroxisome proliferation-induced hepatic oxidative damage. Biochem Pharmacol 53(9): 1369–74. [DOI] [PubMed] [Google Scholar]
- Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, Mangelsdorf I, Meek ME, Rice JM, Younes M. (2001). IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol 34 (2):146–152. [DOI] [PubMed] [Google Scholar]
- Stanko RT, Sekas G, Isaacson IA, Clarke MR, Billiar TR, Paul HS. (1995). Pyruvate inhibits clofibrate-induced hepatic peroxisomal proliferation and free radical production in rats. Metabolism 44(2): 166–71. [DOI] [PubMed] [Google Scholar]
- Stauber AJ, Bull RJ. (1997). Differences in phenotype and cell replicative behavior of hepatic tumors induced by dichloroacetate (DCA) and trichloroacetate (TCA). Toxicol Appl Pharmacol 144(2): 235–46. [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect. 2010. August;118(8):1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styles JA, Kelly MD, Pritchard NR, Elcombe CR. (1990). Acute hyperplasia and peroxisome proliferation induced by methylclofenapate: a species comparison and implications for liver carcinogenesis. Prog Clin Biol Res. 331:385–93. [PubMed] [Google Scholar]
- Svoboda DJ, Azarnoff DL. (1979). Tumors in male rats fed ethyl chlorophenoxyisobutyrate, a hypolipidemic drug. Cancer Res 39(9): 3419–28. [PubMed] [Google Scholar]
- Tai ES, Demissie S, Cupples LA, Corella D, Wilson PW, Schaefer EJ, Ordovas JM. (2002). Association between the PPARΑ L162V polymorphism and plasma lipid levels: The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 22(5):805–10. [DOI] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD. (2007). Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci 95(1): 108–17. [DOI] [PubMed] [Google Scholar]
- Takashima K, Ito Y, Gonzalez FJ, Nakajima T. (2008). Different mechanisms of DEHP-induced hepatocellular adenoma tumorigenesis in wild-type and PPAR alpha-null mice. J Occup Health. 50(2):169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Smith PF, Stromberg PC, Eydelloth RS, Herold EG, Grossman SJ, Frank JD, Hertzog PR, Soper KA, Keenan KP. (1992). Studies of early hepatocellular proliferation and peroxisomal proliferation in Sprague Dawley rats treated with tumorigenic doses of clofibrate. Toxicol Appl Pharmacol 116(1): 71–7. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Moriya K, Kiyosawa K, Koike K, Aoyama T. (2008b). Hepatitis C virus core protein induces spontaneous and persistent activation of peroxisome proliferator-activated receptor alpha in transgenic mice: implications for HCV-associated hepatocarcinogenesis. Int J Cancer 122(1):124–31. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T (2008a). PPARα activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest. 118(2):683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno C, Yamamoto T, Utoh R, Yamasaki C, Ishida Y, Myoken Y, Oofusa K, Okada M, Tsutsui N, Yoshizato K. Chimeric mice with hepatocyte-humanized liver as an appropriate model to study human peroxisome proliferator-activated receptor-α. Toxicol Pathol. 2015. February;43(2):233–48. [DOI] [PubMed] [Google Scholar]
- Tateno C, Yamamoto T, Utoh R, Yamasaki C, Ishida Y, Myoken Y, Oofusa K, Okada M, Tsutsui N, Yoshizato K. Chimeric mice with hepatocyte-humanized liver as an appropriate model to study human peroxisome proliferator-activated receptor-α. Toxicol Pathol. 2015. February;43(2):233–48. [DOI] [PubMed] [Google Scholar]
- Tharappel JC, Cunningham ML, Spear BT, Glauert HP (2001). Differential activation of hepatic NF-kappaB in rats and hamsters by the peroxisome proliferators WY-14,643, gemfibrozil, and dibutyl phthalate. Toxicol Sci 62(1): 20–7. [DOI] [PubMed] [Google Scholar]
- Tharappel JC, Nalca A, Owens AB, Ghabrial L, Konz EC, Glauert HP, Spear BT. (2003). Cell proliferation and apoptosis are altered in mice deficient in the NF-kappaB p50 subunit after treatment with the peroxisome proliferator ciprofibrate. Toxicol Sci 75(2): 300–8. [DOI] [PubMed] [Google Scholar]
- Thomas M, Bayha C, Klein K, Müller S, Weiss TS, Schwab M, Zanger UM. The truncated splice variant of peroxisome proliferator-activated receptor alpha, PPARα-tr, autonomously regulates proliferative and pro-inflammatory genes. BMC Cancer. 2015. June 30;15:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thottassery J, Winberg L, Youssef J, Cunningham ML, Badr M. (1992). Regulation of perfluorooctanoic acid--induced peroxisomal enzyme activities and hepatocellular growth by adrenal hormones. Hepatology 15(2): 316–22. [DOI] [PubMed] [Google Scholar]
- Tomaszewski KE, Heindel SW, Jenkins WL, Melnick RL. (1990). Induction of peroxisomal acyl CoA oxidase activity and lipid peroxidation in primary rat hepatocyte cultures. Toxicology 65(1-2): 49–60. [DOI] [PubMed] [Google Scholar]
- Tucker MJ, Orton TC. (1995). Comparative Toxicology of Hypolipidaemic Fibrates. Taylor and Francis, Bristol, PA. [Google Scholar]
- Tugwood JD, Aldridge TC, Lambe KG, Macdonald N, and Woodyatt NJ (1996). Peroxisome proliferator-activated receptors: Structures and function. Ann. NY Acad. Sci 804:252–265. [DOI] [PubMed] [Google Scholar]
- Tugwood JD, Aldridge TC, Lambe KG, Macdonald N, Woodyatt NJ. (1996). Peroxisome proliferator-activated receptors: structures and function. Ann N Y Acad Sci. 804:252–65. [DOI] [PubMed] [Google Scholar]
- Tugwood JD, Holden PR, James NH, Prince RA, Roberts RA. (1998). A PPAR alpha cDNA cloned from guinea pig liver encodes a protein with similar properties to the mouse PPAR alpha: Implications for species differences in response to peroxisome proliferators. Arch. Toxicol 72:169–177. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. (2005) Guidelines for carcinogen risk assessment and supplemental guidance for assessing susceptibility from early-life exposure to carcinogens. Fed. Reg 70:66 , pp. 17765–17817. [Google Scholar]
- Urbanek-Olejnik K, Liszewska M, Winczura A, Kostka G. Changes of c-Myc and DNMT1 mRNA and protein levels in the rat livers induced by dibutyl phthalate treatment. Toxicol Ind Health. 2016. May;32(5):801–8. [DOI] [PubMed] [Google Scholar]; Abdellatif AG, Preat V, Taper HS, Roberfroid M. (1991). The modulation of rat liver carcinogenesis by perfluorooctanoic acid, a peroxisome proliferator. Toxicol Appl Pharmacol 111(3): 530–7. [DOI] [PubMed] [Google Scholar]
- Valles EG, Laughter AR, Dunn CS, Cannelle S, Swanson CL, Cattley RC, Corton JC. (2003). Role of the peroxisome proliferator-activated receptor alpha in responses to diisononyl phthalate. Toxicology 191(2-3):211–25. [DOI] [PubMed] [Google Scholar]
- Van Rafelghem MJ, Mattie DR, Bruner RH, Andersen ME. (1987). Pathological and hepatic ultrastructural effects of a single dose of perfluoro-n-decanoic acid in the rat, hamster, mouse, and guinea pig. Fundam Appl Toxicol 9(3): 522–40. [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. (2006). Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 92(2): 476–89. [DOI] [PubMed] [Google Scholar]
- Wada N, Marsman DS, Popp JA. (1992). Dose-related effects of the hepatocarcinogen, WY-14,643, on peroxisomes and cell replication. Fundam Appl Toxicol 18(1): 149–54. [DOI] [PubMed] [Google Scholar]
- Walters MW, Bjork JA, Wallace KB. Perfluorooctanoic acid stimulated mitochondrial biogenesis and gene transcription in rats. Toxicology. 2009. October 1;264(1-2):10–5. [DOI] [PubMed] [Google Scholar]
- Ward JM, Hagiwara A, Anderson LM, Lindsey K, Diwan BA. (1988). The chronic hepatic or renal toxicity of di(2-ethylhexyl) phthalate, acetaminophen, sodium barbital, and phenobarbital in male B6C3F1 mice: autoradiographic, immunohistochemical, and biochemical evidence for levels of DNA synthesis not associated with carcinogenesis or tumor promotion. Toxicol Appl Pharmacol 96(3):494–506. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Horie S, Yamada J, Isaji M, Nishigaki T, Naito J, Suga T. (1989). Species differences in the effects of bezafibrate, a hypolipidemic agent, on hepatic peroxisome-associated enzymes. Biochem. Pharmacol 38:367–371. [DOI] [PubMed] [Google Scholar]
- Weglarz TC, Sandgren EP. (2004). Cell cross-talk mediates PPARα null hepatocyte proliferation after peroxisome proliferator exposure. Carcinogenesis 25(1): 107–12. [DOI] [PubMed] [Google Scholar]
- West D, James N, Holden P, Brindle R, Rolfe M, Roberts R. (1999). Role for tumour necrosis factor a (TNFα) receptor 1 (TNFR1) and interleukin 1 receptor (IL1R) in the suppression of apoptosis by peroxisome proliferators. Hepatology 30:1417–1424. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Smutney OM, Check JF, Rusyn I, Schulte-Hermann R, Thurman RG. (2003). Impaired Ras membrane association and activation in PPARα knockout mice after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 284(2):G302–12. [DOI] [PubMed] [Google Scholar]
- Williams GM, Perrone C. (1995). Mechanism based risk assessment of peroxisome proliferating rodent hepatocarcinogens. In: Peroxisomes: Biology and Role in Toxicology and Disease, eds. Reddy JK, Suga T, and Mannaerts GP, Ann.NYAcad. Sci 804:554–572. [DOI] [PubMed] [Google Scholar]
- Wolf DC, Moore T, Abbott BD, Rosen MB, Das KP, Zehr RD, Lindstrom AB, Strynar MJ, Lau C. (2008). Comparative hepatic effects of perfluorooctanoic acid and WY 14,643 in PPAR-alpha knockout and wild-type mice. Toxicol. Pathol 36(4):632–9. [DOI] [PubMed] [Google Scholar]
- Woods CG, Burns AM, Bradford BU, Ross PK, Kosyk O, Swenberg JA, Cunningham ML, Rusyn I. (2007c). WY-14,643 induced cell proliferation and oxidative stress in mouse liver are independent of NADPH oxidase. Toxicol Sci. 98(2):366–74. [DOI] [PubMed] [Google Scholar]
- Woods CG, Burns AM, Maki A, Bradford BU, Cunningham ML, Connor HD, Kadiiska MB, Mason RP, Peters JM, Rusyn I. (2007b). Sustained formation of alpha-(4-pyridyl-1-oxide)-N-tert-butylnitrone radical adducts in mouse liver by peroxisome proliferators is dependent upon peroxisome proliferator-activated receptor-alpha, but not NADPH oxidase. Free Radic Biol Med 42(3): 335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods CG, Kosyk O, Bradford BU, Ross PK, Burns AM, Cunningham ML, Qu P, Ibrahim JG, Rusyn I. (2007a). Time course investigation of PPARα- and Kupffer cell-dependent effects of WY-14,643 in mouse liver using microarray gene expression. Toxicol Appl Pharmacol. 225(3):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Anderson SP, Swanson C, Bahnemann R, Voss KA, Stauber AJ, Corton JC. (2006). Activation of peroxisome proliferator-activated receptor alpha enhances apoptosis in the mouse liver. Toxicol Sci. 92(2):368–77. [DOI] [PubMed] [Google Scholar]
- Yamakawa-Kobayashi K, Ishiguro H, Arinami T, Miyazaki R, Hamaguchi H. (2002). A Val227Ala polymorphism in the peroxisome proliferator activated receptor alpha (PPARα) gene is associated with variations in serum lipid levels. J Med Genet. 39(3):189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Ito S, Gonzalez FJ. (2007). Hepatocyte-restricted constitutive activation of PPAR alpha induces hepatoproliferation but not hepatocarcinogenesis. Carcinogenesis. 28(6):1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Nagano T, Shah Y, Cheung C, Ito S, Gonzalez FJ. (2008). The PPAR alpha-humanized mouse: a model to investigate species differences in liver toxicity mediated by PPAR alpha. Toxicol Sci. 101(1):132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeldandi AV, Milano M, Subbarao V, Reddy JK, Rao MS. (1989). Evaluation of liver cell proliferation during ciprofibrate-induced hepatocarcinogenesis. Cancer Lett 47(1-2): 21–7. [DOI] [PubMed] [Google Scholar]
- Yeldandi AV, Rao MS, Reddy JK. (2000). Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat Res. 448(2):159–77. [DOI] [PubMed] [Google Scholar]
- Youssef JA, Bouziane M, Badr MZ. (2003). Age-dependent effects of nongenotoxic hepatocarcinogens on liver apoptosis in vivo. Mech Ageing Dev 124(3): 333–40. [DOI] [PubMed] [Google Scholar]