Abstract
The objective of the study was to estimate the prevalence of periodontitis at state and local levels across the United States by using a novel, small area estimation (SAE) method. Extended multilevel regression and poststratification analyses were used to estimate the prevalence of periodontitis among adults aged 30 to 79 y at state, county, congressional district, and census tract levels by using periodontal data from the National Health and Nutrition Examination Survey (NHANES) 2009–2012, population counts from the 2010 US census, and smoking status estimates from the Behavioral Risk Factor Surveillance System in 2012. The SAE method used age, race, gender, smoking, and poverty variables to estimate the prevalence of periodontitis as defined by the Centers for Disease Control and Prevention/American Academy of Periodontology case definitions at the census block levels and aggregated to larger administrative and geographic areas of interest. Model-based SAEs were validated against national estimates directly from NHANES 2009–2012. Estimated prevalence of periodontitis ranged from 37.7% in Utah to 52.8% in New Mexico among the states (mean, 45.1%; median, 44.9%) and from 33.7% to 68% among counties (mean, 46.6%; median, 45.9%). Severe periodontitis ranged from 7.27% in New Hampshire to 10.26% in Louisiana among the states (mean, 8.9%; median, 8.8%) and from 5.2% to 17.9% among counties (mean, 9.2%; median, 8.8%). Overall, the predicted prevalence of periodontitis was highest for southeastern and southwestern states and for geographic areas in the Southeast along the Mississippi Delta, as well as along the US and Mexico border. Aggregated model-based SAEs were consistent with national prevalence estimates from NHANES 2009–2012. This study is the first-ever estimation of periodontitis prevalence at state and local levels in the United States, and this modeling approach complements public health surveillance efforts to identify areas with a high burden of periodontitis.
Keywords: epidemiology, dental health surveys, oral health, population surveillance, small area estimation, US population
Introduction
Periodontitis is widespread among adults in the United States, and national surveys report almost half of those aged 30 y or older are affected (Brown et al. 1996; Albandar et al. 1999; Eke, Dye, et al. 2012). A Healthy People 2020 objective focuses on the reduction of moderate and severe periodontitis among the US adult population (US Department of Health and Human Services 2010). Knowing where the prevalence of periodontitis is highest at state and local levels is essential to developing and targeting efficient programs to address this objective. Although much is known about the prevalence of periodontitis nationally, there is a critical gap in the understanding of the prevalence and distribution of periodontitis at state and local levels, where most public health programs are implemented.
In response to the need for state and local assessments of periodontitis, the Centers for Disease Control and Prevention (CDC) began a Periodontal Disease Surveillance Initiative in 2003 with the American Academy of Periodontology (AAP) to seek alternative, valid, reliable, and less resource-demanding approaches for estimating the prevalence of periodontitis at subnational levels. The initiative explored the use of self-report measures that can be integrated into existing state and local surveys (Dietrich et al. 2007; Eke and Genco 2007; Genco et al. 2007; Gilbert and Litaker 2007; Taylor and Borgnakke 2007; Eke and Dye 2009; Eke, Thornton-Evans, et al. 2012). The findings from this initiative and other studies have consistently demonstrated that the modeling of combined sociodemographic, poverty, and risk factor measures (e.g., smoking status) is promising for predicting the prevalence of periodontitis among adult populations (Slade 2007; Eke and Dye 2009; Eke et al. 2013; Zhan 2014). Thus, the combined distribution of these characteristics among communities can be a useful predictor of the overall burden of periodontitis.
Individual and community-level predictors can be combined in a multilevel modeling framework to better predict the prevalence of periodontitis and risk factors for communities and smaller area populations. Currently, there are rich sources of publicly available data containing individual and community characteristics from health surveys and censuses, such as the Behavioral Risk Factor Surveillance System (BRFSS), the American Community Survey (ACS), and the US decennial census. These data sets have been successfully applied collectively in multilevel modeling frameworks to generate valid small area estimates (SAEs) for several chronic diseases and risk factors, such as diabetes (Congdon and Lloyd 2010), obesity (Li et al. 2009; Zhang et al. 2013), chronic obstructive pulmonary disease (COPD) (Zhang et al. 2014), and smoking (Li et al. 2009). The potential for use of modeling in population health at the state and local levels is now recognized and was the focus of a recent Institute of Medicine (IOM) workshop (National Academies of Sciences, Engineering, and Medicine 2015).
In this study, we extended a multilevel regression and post-stratification (MRP) approach (Zhang et al. 2014; Zhang et al. 2015) by incorporating additional individual-level risk factors (poverty and smoking statuses) confirmed by periodontitis epidemiologic studies and to better estimate the prevalence of adult periodontitis, including severe periodontitis, at the state, county, congressional district, and census tract levels in the United States. Aggregated model-based SAEs of periodontitis prevalence were validated against national estimates from the National Health and Nutrition Examination Survey (NHANES) 2009–2012.
Materials and Methods
Data Sources
Details of the data set used (NHANES, BRFSS, ACS, and US census 2010) and variables are reported in the supplemental Appendix.
Statistical Modeling
Briefly, NHANES 2009–2012 data were first used to construct regression models to estimate associations between periodontitis outcomes and individual-level age, gender, race or ethnicity, poverty, and the dominant risk factor: smoking (Tomar and Asma 2000). Then, to predict state and local estimates for the entire US population from the fitted models on the basis of NHANES, we constructed population counts for these variables at the US census block level, which is the smallest US census unit. Smoking and poverty statuses are not collected by the US census. Thus, we used the BRFSS data and the US census 2010 Summary File 1 population data to estimate population counts by smoking status for the selected demographic characteristics (age, gender, race or ethnicity) at the census block level. Then, we further assigned poverty status to these population counts with smoking status via bootstrapping by using ACS 2008–2012 poverty estimates at the census block level. Finally, using the estimated population counts having demographics and smoking and poverty statuses, we then applied the fitted models from NHANES to generate the prevalence estimates of periodontitis at the census block level and further aggregated upward to other larger geographic levels of interest, such as counties, congressional districts, and states. Details of each of these steps are as follows:
Step 1. Fitting logistic regression models for periodontitis outcomes
NHANES 2009–2010 (N = 3,743) and 2011–2012 (N = 3,323) data were used to construct logistic regression models that quantify the associations between periodontitis and individual-level population demographic characteristics (age, gender, race/ethnicity), poverty, and smoking status. The probability of periodontitis (Pijklm) was assumed to be associated with 5 individual-level factors: age (4 groups), gender (2 groups), race/ethnicity (4 groups), smoking status (3 groups), and poverty status (2 groups) via a logit link:
| (1) |
where yijklm is the periodontitis status (1 = yes or 0 = no) for an individual of age groups (i = 1–4), gender (j = 1, 2), race/ethnicity (k = 1–4), smoking status (l = 1–3), and poverty status (m = 1, 2); ai gj, rk, and sl, and qm are the regression coefficients corresponding to 4 age groups (30–44, 45–54, 55–64, and 65–79 y), 2 genders (male and female), 4 race/ethnicity groups (non-Hispanic white, non-Hispanic black, non-Hispanic other, and Hispanic), 3 smoking statuses (former, current, and never smokers), and 2 poverty statuses (poverty income ratio [PIR] <1.5 or PIR ≥1.5), respectively. The logistic regression models were fitted in SUDAAN (RTI International, Research Triangle Park, NC, USA), and the complex survey design components (stratification, clustering, and weight) were accounted for from NHANES.
After fitting the model (equation 1), for any one individual within given demographics and smoking status, the expected probability (risk) of periodontitis was determined by
| (2) |
In total, there were 192 predicted values for all possible combinations of 32 demographic groups from NHANES: age × gender × race/ethnicity (4 × 2 × 4), 3 smoking statuses (1 [current smoker], 2 [former smoker], 3 [never smoker]), and 2 poverty statuses (1 [PIR <1.5], 2 [PIR ≥1.5]).
Step 2. Estimating census block–level population counts by smoking status
Because the 2010 US census Summary File population data do not have a characteristic for smoking status, the 2012 BRFSS and US census 2010 population count data were used to estimate census block–level population counts by smoking status via MRP. In this study, 2 prevalence models were constructed on the basis of BRFSS 2012.
The first prevalence model, which was based on the full sample of BRFSS, assessed the association between either current or former status (yes vs. no) and individual covariates (age, gender, and race), county-level poverty, and county-level and state-level random effects. The probability of smoking, Ps(ys = 1), was assumed to be associated with 3 factors: individual-level, county-level, and state-level factors via a logit link:
| (3a) |
The second prevalence model, which was based on only BRFSS survey sampled populations who are smokers (both former and current smokers), was used to model the association between current smoking (yes vs. no) compared with former smokers. For the BRFSS respondents who are smokers (ys = 1), the probability of being a current smoker, Pcs (ycs = 1 | ys = 1), was assumed to be associated with 3 factors: individual-level, county-level, and state-level factors via a logit link:
| (3b) |
In both equations 3a and 3b, X is the vector of fixed-effect covariates, including individual covariates from the BRFSS: respondents’ age (30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, and 75–79 y), gender (men and women), and race or ethnicity (non-Hispanic white, non-Hispanic black, American Indian and Alaska Native, Asian, Hawaii Native and other Pacific Islanders, other single races, 2 or more races, and Hispanic). In addition, Z is the vector of state- and county-level random effects associated with the sampled subjects’ residential counties and states, β and α are the vectors of regression coefficients corresponding to fixed and random effects, and ε is the vector of random errors.
After fitting the above 2 prevalence models with the BRFSS, for any one individual given demographics (age group [i], gender [j], race/ethnicity group [k] in a county [c] within a state [s]), the expected probability of being a current or former smoker was given by
| (4a) |
P̂s (ys = 1) is the predicted probability of smoking for a person defined by age, gender, race, and residential county and states; β̂ is the estimated regression coefficients for individual age, gender, race, and county-level poverty; and α̂ is the estimated random-effect coefficients for US counties and states from the multilevel prevalence model of being a smoker (equation 3a).
In the same way, for any individual smoker, the expected probability of being a current smoker was given by
| (4b) |
is the predicted probability of being a current smoker for a person defined by age, gender, race, and residential county and states; is the estimated regression coefficients for individual age, gender, race, and county-level poverty; and is the estimated random-effect coefficients for US counties and states from the multilevel prevalence model of being a current smoker (equation 3b).
From the 2010 US census, we have the population counts (nagr) for each census block by age (a), gender (g), and race/ethnicity (r), corresponding to the demographic groups based on BRFSS survey data: age (30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, and 75–79 y), gender (men and women), and race/ethnicity (non-Hispanic white, non-Hispanic black, American Indian and Alaska Native, Asian, Hawaii Native and other Pacific Islanders, other single races, 2 or more races, and Hispanic).
Thus, we now have the number of smokers within a census block by age (a), gender (g), and race/ethnicity (r) as
| (5a) |
the number of nonsmokers within a census block by age, gender, and race estimated as
| (5b) |
the number of current smokers within a census block by age, gender, and race estimated as
| (5c) |
and the number of former smokers within a census block by age, gender, and race estimated as
| (5d) |
This process provided the census block–level population counts (nagr) by age, gender, race, and smoking status (nonsmoker [ ], former smoker [ ], and current smoker [ ]) for the entire United States.
Step 3. ACS 2008–2012 five-year estimate of census block–level poverty
County-level poverty rates below 150% of the federal poverty level were obtained from the ACS 2007–2012 five-year estimates. The ACS 5-y estimate of census tract–level poverty (percentage of population under 150% federal poverty level) was used to assign individual poverty status for census block–level population counts by age, gender, race/ethnicity, and smoking status via bootstrapping. Thus, the census block–level population counts by age, gender, and smoking status (nonsmoker [ ], former smoker [ ], and current smoker [ ]) were further classified into nonsmoker with poverty status ( ), former smoker with poverty status ( ), and current smoker with poverty status ( ).
Step 4. Applied estimated census block–level population counts
Finally, we applied the estimated census block–level population counts with smoking and poverty statuses from step 3 to the predicted probability of periodontitis by age, gender, race, smoking, and poverty from step 1 to estimate the number of population counts with periodontitis within a census block.
By multiplying equation 2 and for the corresponding demographic groups, we could obtain the estimated 2010 US census block counts for periodontitis for current smokers as
| (6a) |
By multiplying equation 2 and for the corresponding demographic groups, we could obtain the estimated 2010 US census block counts for periodontitis for former smokers as
| (6b) |
By multiplying equation 2 and for the corresponding demographic groups, we could obtain the estimated 2010 US census block counts for periodontitis for nonsmokers as
| (6c) |
Thus, the total expected population counts with periodontitis is the sum of equations 6a, 6b, and 6c, and the estimated prevalence of periodontitis ( ) for a census block (b) was calculated as follows:
Popb is the total population count for census block (b). And could be conveniently aggregated to any upper-level geographic units of interest, such as census tract, county, and congressional district or state.
where Popb is the total population count of census block (b), and K is the number of census blocks for the higher geographic units of interest (g), such as census tracts, counties, congressional districts, and states in this study. Monte Carlo simulation was used to repeat steps 2 to 4 one thousand times to generate all the final small area estimates and their related statistics, such as mean, median, standard errors, and percentiles. We used 2.5% and 97.5% percentiles to approximate 5% significant level confidence intervals.
Internal validity tests were performed by comparing summary prevalence estimates (mean, median, minimum, and maximum values) from states, counties, congressional districts, and census tracts with the national prevalence estimate derived from the 2009–2012 NHANES data. Esri Arc GIS was used to generate map estimates for state, county, census tract, and congressional district levels. Boundary data were obtained from US Census Bureau TIGER/Line 2010, joined with estimates by FIPS code.
Results
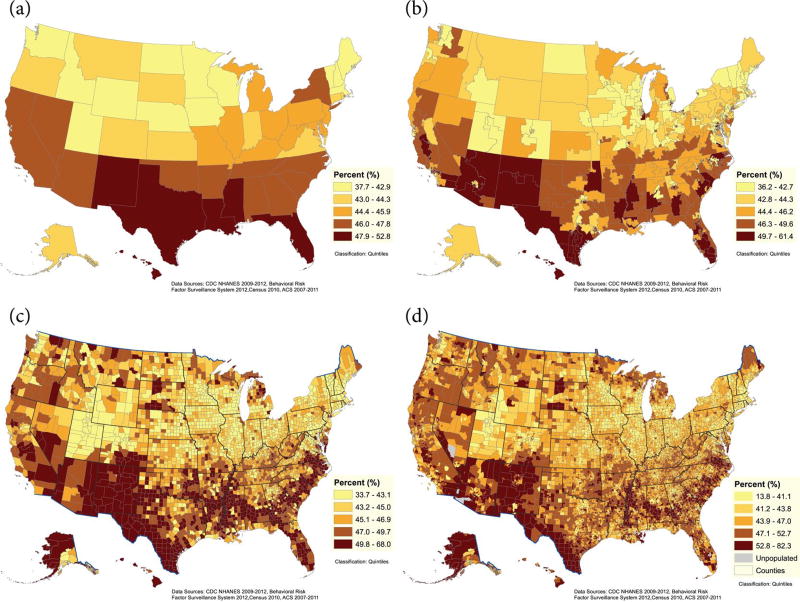
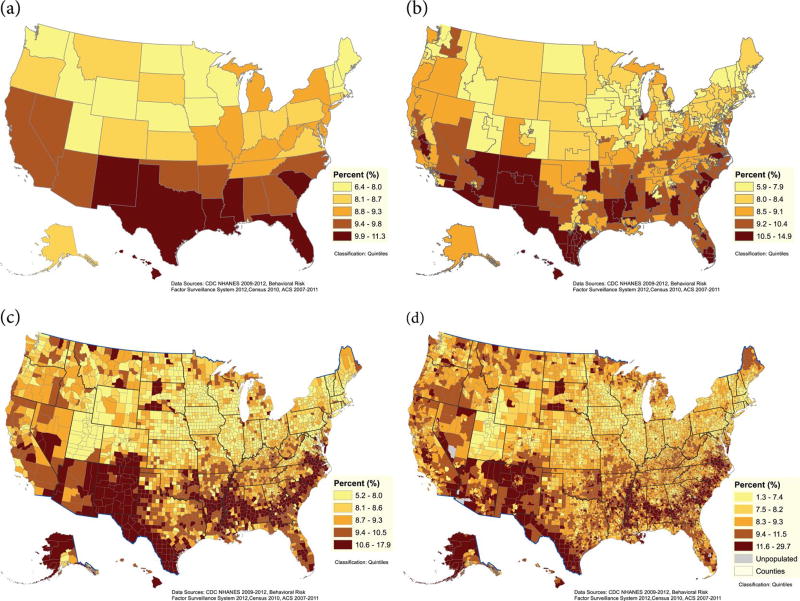
The population distributions in each of the 3 data sets (i.e., NHANES, BRFSS, and US census) used were comparable for gender, age group, race or ethnicity, poverty, and smoking status (Appendix Table 1). Our model-based estimates were statistically similar to estimates generated from NHANES (2009–2012) at the national level and within gender, age, race/ethnicity, poverty, and smoking subgroups (Table 1). The model-estimated prevalence of periodontitis among the states ranged from 37.7% in Utah to 52.7% in New Mexico (mean, 45.1%; median, 44.9%), representing an estimated 15% disparity in prevalence among states. County estimates ranged from 33.7% to 68% (mean, 46.6%; median, 45.9%), representing a much larger disparity of 34% in prevalence among counties. Severe periodontitis ranged from 6.4% in New Hampshire to 11.3% in Louisiana among the states (mean, 8.9%; median, 8.8%) and from 5.2% to 17.9% among counties (mean, 9.2%; median, 8.8%) (Table 2). Detailed estimates for each state are listed in Appendix Table 2.
Table 1.
Comparison Estimates of Total and Severe Periodontitis Prevalence among Adults Aged 30–79 y, by Selected Characteristics, Using the National Health Examination and Nutrition Examination Survey (NHANES) 2009–2012 and Multilevel Model-Based Estimation.
| Total Periodontitis, % (95% CI) | Severe Periodontitis, % (95% CI) | |||
|---|---|---|---|---|
|
|
|
|||
| Outcomes | NHANES | Model Based | NHANES | Model Based |
| United States | 44.8 (41.7, 48.0) | 45.96 (45.94, 45.97) | 8.9 (7.7, 10.1) | 9.08 (9.07, 9.09) |
| Gender | ||||
| Men | 54.2 (50.8, 57.5) | 55.59 (55.57, 55.62) | 13.3 (11.6, 15.2) | 13.54 (13.53, 13.56) |
| Women | 35.7 (32.3, 39.3) | 36.95 (36.93, 36.98) | 4.6 (3.6, 5.7) | 4.91 (4.90, 4.91) |
| Age group, y | ||||
| 30–44 | 30.7 (27.6, 34.1) | 29.21 (29.18, 29.23) | 4.8 (4.1, 5.7) | 4.37 (4.37, 4.38) |
| 45–54 | 46.8 (42.4, 51.2) | 46.97 (46.94, 47.00) | 10.6 (8.5, 13.1) | 10.38 (10.37, 10.39) |
| 55–64 | 54.9 (49.5, 60.2) | 55.96 (55.93, 55.99) | 12.5 (10.2, 15.2) | 12.88 (12.87, 12.90) |
| 65–79 | 65.9 (60.8, 70.6) | 66.78 (66.74, 66.82) | 11.4 (8.7, 14.9) | 12.11 (12.09, 12.13) |
| Race or ethnicity | ||||
| Non-Hispanic white | 39.1 (35.2, 43.2) | 41.50 (41.48, 41.52) | 6.7 (5.4, 8.3) | 7.42 (7.41, 7.43) |
| Non-Hispanic black | 58.7 (54.0, 63.3) | 58.45 (58.41, 58.50) | 15.5 (12.8, 18.6) | 14.77 (14.75, 14.79) |
| Hispanic | 58.7 (55.7, 61.6) | 56.37 (56.32, 56.42) | 12.8 (10.5, 15.5) | 12.20 (12.18, 12.22) |
| Non-Hispanic other | 51.0 (44.0, 57.9) | 50.74 (50.71, 50.79) | 11.8 (8.3, 16.4) | 10.63 (10.61, 10.65) |
| Below 150% poverty level | ||||
| Yes | 60.6 (57.4, 63.6) | 59.17 (59.09, 59.25) | 14.4 (12.3, 16.8) | 13.77 (13.73, 13.80) |
| No | 39.6 (36.2, 43.1) | 41.96 (41.93, 41.99) | 7.0 (5.8, 8.3) | 7.66 (7.65, 7.67) |
| Smoking status | ||||
| Never smoked | 36.8 (33.9, 39.8) | 37.35 (37.34, 37.37) | 5.3 (4.3, 6.6) | 5.58 (5.57, 5.58) |
| Former smoker | 47.2 (42.2, 52.2) | 49.96 (49.94, 49.96) | 9.6 (7.6, 12.0) | 10.26 (10.25, 10.27) |
| Current smoker | 66.5 (62.7, 70.0) | 66.35 (66.33, 66.37) | 18.8 (15.6, 22.5) | 18.06 (18.05, 18.08) |
There are 2 decimals for model-based estimates because of their very narrow confidence intervals (CIs).
Table 2.
Summary Statistics of Model-Based Small Area Estimations (SAEs) of Total and Severe Periodontitis Prevalence, 2009–2012.
| Outcome | Geography | n | Minimum | Q1 | Median | Q3 | Maximum | Mean |
|---|---|---|---|---|---|---|---|---|
| Total periodontitis | State | 51 | 37.7 | 42.9 | 44.9 | 47.2 | 52.8 | 45.1 |
| County | 3,143 | 33.7 | 43.6 | 45.9 | 48.7 | 68.0 | 46.6 | |
| Census tract | 72,468 | 13.8 | 41.8 | 45.2 | 50.8 | 82.3 | 46.8 | |
| Congressional district | 436 | 36.2 | 43.1 | 44.9 | 48.1 | 61.4 | 46.1 | |
| Severe periodontitis | State | 51 | 6.4 | 8 | 8.8 | 9.5 | 11.3 | 8.9 |
| County | 3,143 | 5.2 | 8.1 | 8.8 | 9.8 | 17.9 | 9.2 | |
| Census tract | 72,468 | 1.3 | 7.6 | 8.6 | 11 | 29.7 | 9.4 | |
| Congressional district | 436 | 5.9 | 8.1 | 8.7 | 9.9 | 14.9 | 9.1 |
When aggregated to the national level, summary measures for these modeled estimates of periodontitis had a mean and median state prevalence of 45.1% and 44.9%, respectively, and 46.6% and 45.9%, respectively, for counties (Table 2). These summary measures compare with the estimated national prevalence of periodontitis among US adults of 44.8% (95% CI: 41.7, 48.0) from NHANES 2009–2012. Similarly, summary measures for severe periodontitis at the state and county levels compared with the estimated national prevalence of severe periodontitis.
The geographic distribution of estimated periodontitis at the state, county, congressional district, and census track levels is presented in Figure 1a–d. Overall, the highest estimated prevalence of periodontitis was observed among southeastern and southwestern states, concentrated in pockets stretching along the Southeast, in the Mississippi Delta, along the US-Mexican border, and among Native American reservations. Other areas with an estimated high prevalence of periodontitis were southern Florida, Hawaii, and remote areas of western Alaska. Overall, similar geographic distribution patterns were determined for severe periodontitis (Fig. 2a–d). Estimated prevalence for each state is provided in Appendix Table 2.
Figure 1.
Percent estimates of periodontitis among adults (aged 30–79 y) by states (a), congressional districts (b), counties (c), and census tracts (d), 2009 to 2012.
Figure 2.
Percent estimates of severe periodontitis among adults (aged 30–79 y) by states (a), congressional districts (b), counties (c), and census tracts (d), 2009 to 2012.
Discussion
This study shows that multilevel regression and poststratification modeling of individual and community-level data can be a practical and statistically valid approach to generate predicted estimates of the prevalence of adult periodontitis at the state and local levels in the United States. The extended MRP approach also demonstrates the flexibility to incorporate additional individual-level risk factors for predicting population health outcomes, such as individual poverty and smoking statuses used in this study. Predicted estimates were generated solely from modeling measures collected from publicly available national health surveillance data sets and the US census. Our predicted estimates were comparable with national estimates from NHANES and within subgroups by age, gender, race or ethnicity, and poverty. In addition, aggregated summary estimates from state and local levels were comparable to national estimates of periodontitis from NHANES. For the first time, our modeling approach offers insights into the geographic distribution of periodontitis at state and local levels in the United States.
The geographic distribution of periodontitis in the United States was consistent with the distribution of known population risk factors for periodontitis (Albandar et al. 1999). The highest estimates of periodontitis were correctly predicted among communities characterized by disproportionate populations of racial or ethnic minorities, older adults of lower socioeconomic status, large immigrant populations, high densities of tobacco users, and remote areas with less access to dental care. The geographic distributions of the predicted highest areas of periodontitis coincide with areas disproportionately affected by higher prevalence of chronic conditions known to be associated with periodontitis, such as cardiovascular diseases and diabetes (i.e., among areas often referred to as the Stroke Belt, Diabetes Belt, and Infant Mortality Belt) (Borgnakke and Genco 2013).
The distribution of periodontitis by geography and by known population risk factors and comorbid conditions that occur simultaneously with periodontitis provides information for potential areas of collaboration in public health programs and implementation. In addition, these findings support the view of the World Health Organization (WHO) that periodontal diseases should be considered within the groups of chronic diseases (Petersen and Ogawa 2005, 2012).
The strength, efficiency, and practicality of our modeling approach lie first with the quality and validity of publicly available data. The applied, weighted, predictive coefficients for periodontitis used were generated from the 2009–2012 NHANES data cycle, which is based on optimal clinical surveillance measures of 6 sites per tooth that optimized the true classification of periodontitis cases. These measures generate the most valid population parameters for predicting the prevalence of periodontitis among US adults. Thus, the periodontitis case definition is based on the standard CDC/AAP case definition for surveillance of periodontitis and the 2012 BRFSS, which collected state-level public health information by using landlines and cell phones.
In addition, this approach represents a new step from previous models: a health behavior (i.e., smoking), which has been shown to be a significant predictor of periodontitis, is used in the prediction model, rather than just demographics. Finally, census block–level population counts from the US census were used to estimate the adult population aged 30 to 79 y for each census block for the entire United States, which could then be aggregated to any upper-level geographic units of interest. And because information from several data sources is used, our aggregated state and national estimates have high precision and small confidence intervals.
As a limitation, it is not possible at this time to externally validate estimates from our model because of the absence of any state- or local-level data about periodontitis in the United States. Currently, only NHANES national-level estimates for periodontitis are available for the adult US population. Hence, the only validation option for this study was to compare aggregated national summary estimates for different levels (i.e., states, counties, congressional districts, and census blocks) with NHANES national estimates. We do acknowledge the inherent limitations in directly comparing summary measures with national prevalence estimates. Further studies will be required to externally validate our estimates among smaller communities, where clinical periodontal surveillance is feasible or available.
In conclusion, the modeling approach complements public health surveillance by generating predicted estimates for periodontitis among subnational populations and geographic regions. This information can be used to inform oral health policy decision and for developing intervention strategies at the state and local levels. In addition, our findings suggest potential areas of collaboration between adult oral health and other chronic disease prevention programs at geographical levels. The extended multilevel regression and poststratification approach demonstrates the flexibility of combing information from multiple data resources to better predict population health outcomes at local levels, such as NHANES, BRFSS, the 2010 US census, and ACS, as is modeled in this study.
Supplementary Material
Acknowledgments
The authors acknowledge the pioneering work by the CDC Periodontal Disease Surveillance Workgroup (Genco and Eke 2007). The findings and conclusion in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
Author Contributions
P.I. Eke, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; X. Zhang, contributed to data acquisition and analysis, drafted the manuscript; H. Lu, contributed to data acquisition and interpretation, drafted the manuscript; L. Wei, contributed to data analysis, critically revised the manuscript; G. Thornton-Evans, contributed to data acquisition and analysis, drafted and critically revised the manuscript; K.J. Greenlund, contributed to data acquisition and interpretation, critically revised the manuscript; J.B. Holt, contributed to data analysis and interpretation, critically revised the manuscript; J.B. Croft, contributed to data acquisition, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70(1):13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- Borgnakke WS, Genco RJ. Risk factors for periodontal disease. Periodontol 2000. 2013;62(1):59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- Brown LJ, Brunelle JA, Kingman A. Periodontal status in the United States, 1988–1991: prevalence, extent, and demographic variation. J Dent Res. 1996;75:672–683. doi: 10.1177/002203459607502S07. [DOI] [PubMed] [Google Scholar]
- Congdon P, Lloyd P. Estimating small area diabetes prevalence in the US using BRFSS. J Data Sci. 2010;8(2010):235–252. [Google Scholar]
- Dietrich T, Stosch U, Dietrich D, Kaiser W, Bernimoulin JP, Joshipura K. Prediction of periodontal disease from multiple self-reported items in a German practice-based sample. J Periodontol. 2007;78(7 Suppl):1421S–1428S. doi: 10.1902/jop.2007.060212. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye B. Assessment of self-report measures for predicting population prevalence of periodontitis. J Periodontol. 2009;80(9):1371–1379. doi: 10.1902/jop.2009.080607. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans G, Genco R. Self-reported measures for periodontitis in U.S. adults. J Dent Res. 2013;92(11):1041–1047. doi: 10.1177/0022034513505621. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ CDC Periodontal Disease Surveillance Workgroup. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Eke PI, Genco RJ. CDC periodontal disease surveillance project: background, objectives, and progress report. J Periodontol. 2007;78(7 Suppl):1366S–1371S. doi: 10.1902/jop.2007.070134. [DOI] [PubMed] [Google Scholar]
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans G, Dye B, Genco R. Advances in surveillance of periodontitis: the Centers for Disease Control and Prevention Periodontal Disease Surveillance Project. J Periodontol. 2012;83(11):1337–1342. doi: 10.1902/jop.2012.110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco RJ, Falkner KL, Grossi S, Dunford R, Trevisan M. Validity of self-reported measures for surveillance of periodontal disease in two western New York population-based studies. J Periodontol. 2007;78(7 Suppl):1439S–1454S. doi: 10.1902/jop.2007.060435. [DOI] [PubMed] [Google Scholar]
- Gilbert GH, Litaker MS. Validity of self-reported periodontal status in the Florida dental care study. J Periodontol. 2007;78(7 Suppl):1429S–1438S. doi: 10.1902/jop.2007.060199. [DOI] [PubMed] [Google Scholar]
- Li W, Land T, Zhang Z, Keithley L, Kelsey JL. Small area estimation and prioritizing communities for tobacco control efforts in Massachusetts. Am J Public Health. 2009;99(3):470–479. doi: 10.2105/AJPH.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. How modeling can inform strategies to improve population health: Workshop summary. Washington (DC): The National Academies Press; 2015. [PubMed] [Google Scholar]
- Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 Suppl):1387S–1399S. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol. 2005;76(12):2187–2193. doi: 10.1902/jop.2005.76.12.2187. [DOI] [PubMed] [Google Scholar]
- Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60(1):15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- Slade GD. Interim analysis of validity of periodontitis screening questions in the Australian population. J Periodontol. 2007;78(7 Suppl):1463S–1470S. doi: 10.1902/jop.2007.060344. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Borgnakke WS. Self-reported periodontal disease: validation in an epidemiological survey. J Periodontol. 2007;78(7 Suppl):1407S–1420S. doi: 10.1902/jop.2007.060481. [DOI] [PubMed] [Google Scholar]
- Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71(5):743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. [accessed 2015 Dec 22];Healthy People 2020. 2010 2010 http://www.healthypeople.gov/ [Google Scholar]
- Zhan Y, Holtfreter B, Meisel P, Hoffmann T, Micheelis W, Dietrich T, Kocher T. Prediction of periodontal disease: modelling and validation in different general German populations. J Clin Periodontol. 2014;41(3):224–231. doi: 10.1111/jcpe.12208. [DOI] [PubMed] [Google Scholar]
- Zhang X, Holt JB, Lu H, Wheaton AG, Ford ES, Greenlund K, Croft JB. Multilevel regression and post stratification for small-area estimation of population health outcomes: a case study of chronic obstructive pulmonary disease prevalence using the Behavioral Risk Factor Surveillance System. Am J Epidemiol. 2014;179(8):1025–1033. doi: 10.1093/aje/kwu018. [DOI] [PubMed] [Google Scholar]
- Zhang X, Holt JB, Yun S, Lu H, Greenlund K, Croft JB. Validation of multilevel regression and post stratification methodology for small area estimation of health outcomes. Am J Epidemiol. 2015;181(12):970–980. doi: 10.1093/aje/kwv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Onufrak S, Holt JB, Croft JB. A multilevel approach to estimating small area childhood obesity prevalence at the census block-group level. Prev Chronic Dis. 2013;10:120252. doi: 10.5888/pcd10.120252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.