Abstract
The N-methyl-D-aspartate receptor (NMDAR) is mechanistically involved in the behavioral and neurophysiological effects of alcohol, but the specific role of the GluN2A subunit remains unclear. Here, we exposed mice with constitutive GluN2A gene knockout (KO) to chronic intermittent ethanol vapor (CIE) and tested for EtOH consumption/preference using a two-bottle choice paradigm, as well as NMDAR-mediated transmission at basolateral amygdala synapses via ex vivo slice electrophysiology. Results showed that GluN2A KO mice attained comparable blood EtOH levels in response to CIE exposure, but did not exhibit the significant increase in EtOH drinking that was observed in CIE-exposed wildtypes. GluN2A KO mice also showed no alterations in BLA NMDAR-mediated synaptic transmission after CIE, relative to air-exposed, whereas C57BL/6 J mice showed an attenuated synaptic response to GluN2B antagonism. Taken together, these data add to mounting evidence supporting GluN2A-containing NMDARs as a mechanism underlying relative risk for developing EtOH dependence after repeated EtOH exposure.
Keywords: Alcohol, CIE, NMDA, Glutamate, Addiction, Amygdala
There is compelling evidence that glutamate signaling through the N-methyl-D-aspartate receptor (NMDAR) is a crucial mechanism contributing to the behavioral and neurophysiological effects of acute and chronic alcohol [1–3]. One outstanding issue that remains unresolved, however, is the relative roles of the specific subunits (GluN2A-D, GluN3-B) that constitute, in various combinations with the obligatory GluN1 subunit, the heteromeric NMDAR. However, a convergence of recent data from human and rodent genetic studies [4–8] and rodent pharmacological [9–13] work point to a key role for GluN2A in the effects of alcohol. Here, we sought to extend current understanding of the role of GluN2A in mediating behavioral and neurophysiological sequelae of chronic EtOH. To this end, we exposed mice with constitutive GluN2A gene knockout (KO) to CIE and tested for related increases in EtOH consumption [9,14–16] and NMDAR-mediated synaptic transmission in the BLA, a limbic brain region where glutamatergic neuroplasticity may play a role in the development and maintenance of an addictive phenotype [17].
Subjects used were adult (> 4 months old) female and male mice, housed 2 per cage, in a temperature- (72 ± 5 °F) and humidity- (45 ± 15%) controlled vivarium under a 12 h light/dark cycle (lights on at 0600 h). GluN2A KO mice were generated as previously described [18–21] and repeatedly backcrossed to the C57BL/6 J strain. Mice heterozygous for the GluN2A mutation were bred, in house, to produce KO mice and wildtype (WT) littermate controls. Male C57BL/6 J mice used for slice recordings were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The numbers of mice used in each experiment are given in the figure legends. All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill, and followed the US National Institutes of Health guidelines outlined in ‘Using Animals in Intramural Research.’
Chronic EtOH exposure was achieved using a vapor inhalation procedure, as previously described [16,22]. Mice were removed from the home cages and individually or pair housed in standard mouse cages in Plexiglas vapor chambers (60 ×36 × 60 cm, PlasLabs, Lansing, MI) (up to 6 cages/chamber) and exposed to continuous vaporized ethanol (EtOH). EtOH was volatized by passing air through a vaporization stone submerged in EtOH (95%) and mixed with fresh air to deliver 19–22 mg EtOH/L of air at a rate of ~10 L/min. CIE-induced blood EtOH concentrations (BECs) were confirmed weekly via blood samples taken from dedicated ‘sentinel’ mice exposed to EtOH simultaneously with the test mice. Sentinel mice were pricked with a lancet on the submandibular vein to collect the blood into heparinized capillary tubes. Blood samples were then centrifuged for 30 min at 15,000 rpm at 4 degrees Celsius prior to determination of sentinel BECs using an Analox AM1 alcohol analyzer (Analox Instruments USA, Lunenburg, MA, USA). Sentinels were age- and genotype-specific to the animals being exposed in each of the respective vapor chambers.
In order to induce intoxication and produce stabilized BECs, the EtOH group received intraperitoneal (i.p.) injections of 71.6 mg/kg of the alcohol dehydrogenase inhibitor pyrazole (Sigma, St. Louis, MO) combined with 1.5 g/kg 20% (v/v) EtOH, in a volume of 10 mL/kg body weight, prior to being placed into the vapor chambers. Vapor exposure lasted 16 h per day (in at 1700 h, 1 h before start of the 12-hr circadian dark phase, out at 0900 h), followed by an 8-h withdrawal period. For 1 cycle of exposure, there were 4 consecutive days of intermittent exposure (Monday-Friday) and then a longer, 80-h, withdrawal (Friday-Monday). This cycle was repeated 4 times. Air controls also received an injection of pyrazole, 68.1 mg/kg, to control for this treatment, before being placed into dedicated air vapor chambers (located adjacent to the EtOH chambers) which received vaporized air at ~10 L/min for the same periods described for the CIE-exposed group.
EtOH consumption was measured using a two-bottle choice paradigm, as previously described [16,23]. Mice were individually housed in ‘Space Saver’ cages (Model 1145 T, Tecniplast, Buguggiate, Italy) with lids fitted for 2 fluid bottles [Model 1145 T482SUDB Polysulfone cage-top to accommodate 2 water bottles (top flow design; Tecniplast)]: contained 15% (v/v) EtOH in water and the other contained tap water. Every 2 days, bottle position was switched, body weight was recorded and EtOH and water consumption determined by measuring the change in weight, correcting for evaporation and spillage (i.e., the average loss of fluid measured from bottles in two empty ‘dummy’ cages was subtracted from the amount drank). Food was available ad libitum throughout.
Physiological and behavioral data were analyzed via Student’s ttests or two-way analysis of variance (ANOVA) followed by Sidak’s multiple comparison post hoc test, using GraphPad Prism 7.01 (GraphPad Software Inc, La Jolla, CA, USA). The threshold for statistical significance was set at P < .05.
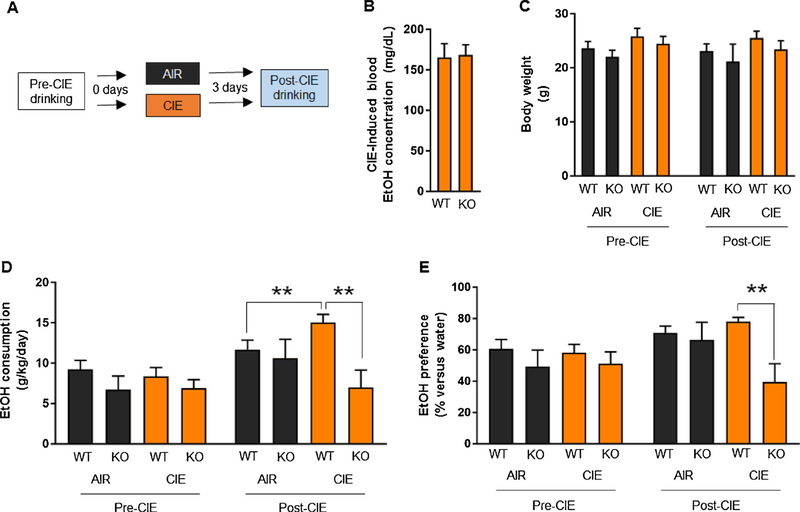
Pre-CIE consumption and preference (=amount drank from the EtOH-containing bottle / amount drank from both bottles * 100) was measured for 14 days to provide a reference for post-CIE drinking. Mice were assigned to CIE exposure groups by matching the groups for average pre-CIE drinking. EtOH was not available for drinking (water bottle only) during CIE exposure. Beginning on the third day (72 h) after CIE exposure (as in prior studies [9,15,16] to avoid confounding effects of acute withdrawal on behavior, such as transient aversion to EtOH), the mice were again presented with the water and EtOH-containing bottles for an additional 7 days to measure post-CIE consumption and preference. For a schematic of the experimental design and timeline, see Fig. 1A. We confirmed that the CIE exposure parameters used produced similar BECs in GluN2A KO and WT sentinel mice (Fig. 1B) and that body weight was also similar between genotypes, both during the pre-CIE and post-CIE periods (Fig. 1C).
Fig. 1. Absence of CIE-potentiation of EtOH drinking in GluN2A knockout mice.
(A) Schematic of the timeline for pre-CIE EtOH drinking, CIE (or air) exposure and post-CIE drinking in GluN2A knockout (KO) mice and their wild-type (WT) litter mate controls. (B) CIE-induced blood EtOH concentration (BECs) did not differ between genotypes. (C) Body weight did not differ between genotypes, irrespective of CIE-exposure. (D) Pre-CIE EtOH consumption did not differ between genotypes. Post-CIE EtOH consumption was significantly higher in CIE-exposed WT mice, as compared to either air-exposed WT mice or CIE-exposed KO mice. (E) Pre-CIE EtOH preference did not differ between genotypes. Post-CIE EtOH preference was significantly higher in CIE-exposed WT mice relative to CIE-exposed KO mice. Data are mean ± SEM. n =6–12 per genotype per treatment group. *P < .05 WT AIR versus WT CIE or WT CIE versus KO CIE.
We then examined EtOH drinking and preference both prior to and following CIE. EtOH consumption did not differ between genotypes during the pre-CIE period, but differed in a genotype and treatment dependent manner, post-CIE (genotype x treatment interaction: F1,34 = 5.01, P < .05). Post hoc analysis showed that the amount of EtOH consumed was significantly higher in WT mice exposed to CIE than in WT mice exposed to air (P < .05), whereas GluN2A KO mice showed no such difference (Fig. 1D). EtOH consumption levels were also significantly higher in CIE-exposed WT mice than CIE-exposed KO mice (post hoc test: P < .05) and higher in WT mice post-CIE, as compared to pre-CIE (post hoc test: P < .05). Pre-CIE, EtOH preference was similar between genotypes, but differed as a function of genotype post-CIE (genotype x treatment interaction: F1,34 = 5.84, P < .05). EtOH preference was significantly higher in CIE-exposed WT mice than similarly-exposed GluN2A KO mice (post hoc test: P < .05) and preference was higher in WT mice post-CIE as compared to pre-CIE F2,44 = 18.52, P < .01) (Fig. 1E). Baseline and post-CIE water consumption did not differ irrespective of genotype or treatment group (Table 1). As previously reported [16,35,36], air controls exhibited an increase in EtOH consumption compared to pre-exposure baseline (Fig. 1D). One plausible explanation for this increase is that pre-vapor drinking experience, followed by a period of forced abstinence (i.e., during the air exposure), produces a modest deprivation-like increase in EtOH consumption [14].
Table 1.
Water consumption during two-bottle choice experiment. Neither genotype or CIE exposure significantly altered water consumption (n = 6–12 per genotype pe treatment group). Data are mean ± SEM.
| Baseline water consumption (g/day) | Post-CIE water consumption (g/day) | ||||||
|---|---|---|---|---|---|---|---|
| WT | NR2A KO | WT | NR2A KO | ||||
| Air | CIE | Air | CIE | Air | CIE | Air | CIE |
| 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.3 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.2 | 1.6 ± 0.3 |
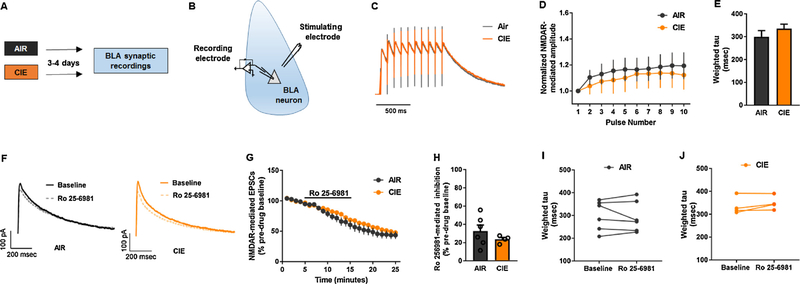
To explore potential neural correlates of the GluN2A KO EtOH drinking phenotype, we next examined synaptic transmission in BLA neurons of these mice after CIE, using ex vivo slice physiology, as previously described [24,25]. Naïve cohorts of GluN2A KO and C57BL/6 J mice were exposed to CIE, as described above, and 72–96 h later anaesthetized with isoflurane and decapitated (for a schematic of the experimental design and timeline, see Fig. 2A and B). The brain was quickly removed and transferred to ice-cold, oxygenated sucrose artificial cerebrospinal fluid (ACSF) [(in mM): 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10 glucose, and 26 NaHCO3 saturated with 95% O2/5% CO2]. Three-hundred μm thick coronal slices containing the BLA were prepared using a tissue slicer Leica vibratome 1200S (Buffalo Grove, IL, USA). Slices were incubated in a heated holding chamber maintained at approximately 32 °C containing normal ACSF [(in mM): 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3 saturated with 95% O2/5% CO2]. Slices were then transferred to a submerged recording chamber (Warner Instruments, Hamden, CT, USA) and perfused with heated (28–30 °C), oxygenated ACSF maintained at ~2 mL/min and incubated for 30 min.
Fig. 2. Absence of CIE effects on NMDAR-mediated synaptic transmission in the BLA of GluN2A KO mice.
(A) Schematic of the timeline for CIE (or air) exposure and post-CIE recordings of synaptic transmission in the BLA of GluN2A knockout (KO) mice. (B) Cartoon of procedure for synaptic recordings in BLA principal neurons. Example normalized traces (C) and pooled normalized amplitude data (D) showing similar temporal summation (from 10 stimulation pulses at 10 Hz) of NMDAR-mediated currents in CIE- and air-exposed GluN2A KO mice (n = 11–15 cells per treatment group from 3 to 4 mice). (E) Pooled data showing similar decay kinetics of NMDAR-mediated excitatory post-synaptic currents (EPSCs) in CIE- and air-exposed GluN2A KO mice (n =4–6 cells per treatment group). (F) Example traces showing similar NMDAR-mediated EPSCs after application of the GluN2B antagonist, Ro 25–6981 (2 μM), in CIE and air-exposed GluN2A KO mice (n =4–6 cells per treatment group from 3 to 4 mice per genotype). (G) Similar time course of Ro 25–6981-induced reduction in NMDAR-mediated EPSC amplitude in CIE and air-exposed GluN2A KO mice. (H) Averaged data showing the reduction in NMDAR-mediated EPSC amplitude during minutes 10–15 after Ro 25–6981 application. Paired data showing the absence of effects of Ro 25–6981 on decay kinetics of NMDAR-mediated excitatory post-synaptic currents (EPSCs) in air-exposed (I) and CIE-exposed (J) GluN2A KO mice (n = 4–6 cells per treatment group from 3 to 4 mice per genotype). Data are mean ± SEM.
Principal neurons in the BLA were identified using infrared differential interference contrast enhanced microscopy (Olympus, Waltham, MA, USA). Borosilicate electrodes with a pipette resistance between 4–6 MΩ were pulled with a Flaming-Brown micropipette puller (Sutter Instruments, Novator, CA, USA). Whole-cell patch clamp recordings were performed in voltage clamp mode with a cesium gluconate based internal solution [(in mM): 117 Cs-gluconate, 20 HEPES, 0.4 EGTA, 5 TEA, 2 MgCl2.6H2O, 4 disodium ATP, and 0.4 disodium GTP, pH =7.3, 290 mOsm]. QX-314.HCl was added at 1 mg/ml to block voltage gated sodium channels.
To isolate NMDAR current, neurons were held at + 40 mV in the presence of picrotoxin (25 μM, Abcam, Cambridge, MA, USA) and NBQX disodium salt (10 μM, Abcam) to block GABA-A receptors and AMPA/kainate receptors, respectively. To record evoked NMDAR-mediated excitatory postsynaptic current (NMDAR-EPSC), a bipolar nichrome electrode was placed in the BLA dorsal to the recorded neurons. NMDAR-EPSCs were evoked at 0.167 Hz for 100–150 micro-seconds using current pulses. After establishing a stable baseline over 5 min, 2 μM Ro 25–6981 maleate (Tocris, Minneapolis, MN, USA) was bath applied for 10 min. The peak amplitude of the evoked response was normalized to the baseline period.
Temporal summation of NMDAR was examined by measuring the responses to bursts of stimulation (10 pulses) at 10 Hz. The amplitude of the subsequent pulses was normalized to the 1 st pulse. Decay kinetics for NMDAR-EPSC was calculated by fitting the average normalized baseline and post-drug responses using 2 exponential simplex function in Clampfit 10.6 and the weighted Tau (Tw) was calculated using the equation: Tw = (T1*A1) + (T2*A2); where T1 and T2 are the 2 time constants and A1 and A2 are their relative amplitudes. Input resistance, holding current and series resistance were monitored throughout the experiments and cells in which changes in series resistance were greater than 20% were excluded from the analysis. All signals were acquired using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA), digitized at 10 kHz and low-pass filtered at 3 kHz and analyzed in pClamp 10.6.
Results showed there was no difference between CIE- and air-exposed GluN2A KO mice in the pooled normalized amplitude of NMDARmediated currents (i.e., temporal summation) at BLA synapses (Fig. 2C and D). The decay kinetics of NMDAR-mediated EPSCs was also similar between the groups (Fig. 2E). Our next step was to assess whether the function of the GluN2B-containing NMDARs was affected by CIE in the GluN2A KO mice. The time course and overall inhibition of NMDAR-mediated EPSCs following application of the GluN2B antagonist, Ro 256981, was not affected by CIE-exposure, as compared to air-exposure, in these mice (Fig. 2F–H). Finally, the decay kinetics of Ro 25–6981 -induced inhibition were also no different between CIE- and air-exposed GluN2A KO mice (Fig. 2I and J).
For comparison, BLA recordings were also conducted in CIE- and air-exposed C57BL/6 J mice. There were no differences observed in the neurophysiological measurements made, with the exception that the decrease in decay kinetics of NMDAR-mediated EPSCs produced Ro 25–6981 was attenuated in CIE-exposed counterparts (Supplementary Fig. S1). Given this effect of the GluN2B antagonist was absent in the GluN2A KO mice, these data offer initial evidence that GluN2A deletion may perturb CIE-induced adaptations in other NMDAR subtypes within the BLA. The contribution of GluN2B to NMDAR-mediated transmission observed in wildtype mice was greater than that from GluN2A KO counterparts. In contrast to our findings, others have reported a greater contribution of GluN2B to NMDAR-mediated transmission in GluN2A knockout mice [35–37]. Of note, however, these earlier studies examined brain regions other than the amygdala (i.e., the dorsal striatum and bed nucleus of the stria terminalis). As such, in addition to methodological and species differences, there could be as yet unidentified regional differences accounting for these apparent discrepancies. The conclusions regarding the contribution of NMDAR subunits remains tentative in lieu of replication and additional investigation.
To reiterate, the major findings of these various behavioral and physiological results were that GluN2A KO mice did not exhibit increases in EtOH drinking or alterations in Ro 25–6981 mediated alteration of decay kinetics of NMDAR-mediated synaptic transmission following CIE exposure. These data add to growing support for GluN2Acontaining NMDARs as an important mechanism underlying the neural and molecular adaptations that drive EtOH dependence. For instance, the current data align with rodent work showing that selection of high two-bottle choice EtOH preference in (heterogeneous stock-collaborative cross) mice and (high alcohol drinking) rats associates with the Grin2a gene locus [7,8] and differential nucleus accumbens expression of Grin2a [26]. Speaking to the translational relevance of these observations in rodents, variation in the human GRIN2A gene has been associated with alcohol dependence [4–6], but not alcohol cue-induced prefrontal cortical (PFC) BOLD activation [30].
We have previously reported that GluN2A KO mice show normal two-bottle choice EtOH drinking and behavioral (stimulant, ataxic, hypothermic, sedative/hypnotic) responses to acute EtOH challenge, but loss of EtOH conditioned place preference and attenuated sedative/hypnotic responses to co-administration of the NMDAR antagonist, MK801, and EtOH [27–29]. In terms of repeated EtOH exposure, there are reports of upregulation of GluN2A-mediated synaptic transmission and protein levels in the medial PFC, orbitofrontal cortex, BLA, and bed nucleus of the stria terminalis [9–12], while other studies find no change or downregulation in cortex, nucleus accumbens or hippocampus [11,31–34]. While significant variation, not only in brain region examined, but also the type and duration of exposure and the timing of the analysis relative to withdrawal complicates direct comparison of these findings, taken together they indicate dynamic alterations in GluN2A in response to chronic EtOH.
In this context, in a clear parallel with the current data, we recently found that GluN2A KO mice did not develop tolerance to the EtOH’s sedative/hypnotic challenge after CIE exposure [13]. Adding to these data, we show here an absence of the marked alterations in synaptictransmission that have been repeatedly documented in the BLA and proposed as a critical substrate for EtOH-induced changes in, for example, anxiety-like behavior [17]. Tolerance is one of the hallmarks of EtOH-dependence and could explain why we failed to observe a post-CIE induced increase in EtOH consumption and preference, and alterations in NMDAR-mediated synaptic transmission in NR2A KO mice. Whether this physiological phenotype also contributes to the loss of CIE-induced EtOH-dependence we found in the current study remains to be fully investigated in future studies.
Supplementary Material
Funding sources and acknowledgements
Research supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program and NIH awards U01AA020911, R01AA019454 and P60AA01165 to TLK.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.bbr.2018.06.029.
References
- [1].Morisot N, Ron D, Alcohol-dependent molecular adaptations of the NMDA receptor system, Genes Brain Behav. 16 (1) (2017) 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Holmes A, Spanagel R, Krystal JH, Glutamatergic targets for new alcohol medications, Psychopharmacology 229 (3) (2013) 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hopf FW, Do specific NMDA receptor subunits act as gateways for addictive behaviors? Genes Brain Behav. 16 (1) (2017) 118–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reimers MA, Riley BP, Kalsi G, Kertes DA, Kendler KS, Pathway based analysis of genotypes in relation to alcohol dependence, Pharmacogenom. J 12 (4) (2012) 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke T-K, Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior, Arch. Gen. Psychiatry 65 (7) (2008) 826–838. [DOI] [PubMed] [Google Scholar]
- [6].Domart MC, Benyamina A, Lemoine A, Bourgain C, Blecha L, Debuire B, Reynaud M, Saffroy R, Association between a polymorphism in the promoter of a glutamate receptor subunit gene (GRIN2A) and alcoholism, Addict. Biol 17 (4) (2012) 783–785. [DOI] [PubMed] [Google Scholar]
- [7].Lo CL, Lossie AC, Liang T, Liu Y, Xuei X, Lumeng L, Zhou FC, Muir WM, High resolution genomic scans reveal genetic architecture controlling alcohol preference in bidirectionally selected rat model, PLoS Genet. 12 (8) (2016) e1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carr LG, Habegger K, Spence J, Ritchotte A, Liu L, Lumeng L, Li TK, Foroud T, Analyses of quantitative trait loci contributing to alcohol preference in HAD1/LAD1 and HAD2/LAD2 rats, Alcohol. Clin. Exp. Res 27 (11) (2003) 1710–1717. [DOI] [PubMed] [Google Scholar]
- [9].Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A, Chronic EtOH effects on putative measures of compulsive behavior in mice, Addiction biology, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kalluri HS, Mehta AK, Ticku MK, Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment, brain research, Mol. Brain Res 58 (1–2) (1998) 221–224. [DOI] [PubMed] [Google Scholar]
- [11].Nona CN, Li R, Nobrega JN, Altered NMDA receptor subunit gene expression in brains of mice showing high vs. low sensitization to ethanol, Behav. Brain Res 260 (2014) 58–66. [DOI] [PubMed] [Google Scholar]
- [12].den Hartog CR, Gilstrap M, Eaton B, Lench DH, Mulholland PJ, Homanics GE, Woodward JJ , Effects of repeated ethanol exposures on NMDA receptor expression and locomotor sensitization in mice expressing ethanol resistant NMDA receptors, Front. Neurosci 11 (2017) 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Daut RA, Busch EF, Ihne J, Fisher D, Mishina M, Grant SG, Camp M, Holmes A, Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95, Addict. Biol 20 (2) (2015) 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Becker HC, Lopez MF, Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice, Alcohol. Clin. Exp. Res 28 (12) (2004) 1829–1838. [DOI] [PubMed] [Google Scholar]
- [15].DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, Lovinger D, Holmes A, Chronic alcohol alters rewarded behaviors and striatal plasticity, Addict. Biol 20 (2) (2015) 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jury NJ, DiBerto JF, Kash TL, Holmes A, Sex differences in the behavioral sequelae of chronic ethanol exposure, Alcohol 58 (2017) 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McCool BA, Christian DT, Diaz MR, Lack AK, Glutamate plasticity in the drunken amygdala: the making of an anxious synapse, Int. Rev. Neurobiol 91 (2010) 205–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kiselycznyk C, Jury NJ, Halladay LR, Nakazawa K, Mishina M, Sprengel R, Grant SG, Svenningsson P, Holmes A, NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism, Behav. Brain Res 287 (2015) 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. , Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit, Nature 373 (6510) (1995) 151–155. [DOI] [PubMed] [Google Scholar]
- [20].Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A, Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit, Learn. Mem 15 (2) (2008) 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A, Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability, J. Neurosci 30 (15) (2010) 5357–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lopez MF, Becker HC, Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice, Psychopharmacology 181 (4) (2005) 688–696. [DOI] [PubMed] [Google Scholar]
- [23].Radke AK, Jury NJ, Delpire E, Nakazawa K, Holmes A, Reduced ethanol drinking following selective cortical interneuron deletion of the GluN2B NMDA receptors subunit, Alcohol 58 (2017) 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Masneuf S, Lowery-Gionta E, Colacicco G, Pleil KE, Li C, Crowley N, Flynn S, Holmes A, Kash T, Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior, Neuropharmacology 85 (2014) 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL, Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala, Neuropharmacology 99 (2015) 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Colville AM, Iancu OD, Oberbeck DL, Darakjian P, Zheng CL, Walter NA, Harrington CA, Searles RP, McWeeney S, Hitzemann RJ, Effects of selection for ethanol preference on gene expression in the nucleus accumbens of HS-CC mice, Genes Brain Behav. 16 (4) (2017) 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palachick B, Chen YC, Enoch AJ, Karlsson RM, Mishina M, Holmes A, Role of major NMDA or AMPA receptor subunits in MK-801 potentiation of ethanol intoxication, Alcohol. Clin. Exp. Res 32 (8) (2008) 1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boyce-Rustay JM, Holmes A, Functional roles of NMDA receptor NR2A and NR2B subunits in the acute intoxicating effects of ethanol in mice, Synapse 56 (4) (2005) 222–225. [DOI] [PubMed] [Google Scholar]
- [29].Boyce-Rustay JM, Holmes A, Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit, Psychopharmacology 187 (4) (2006) 455–466. [DOI] [PubMed] [Google Scholar]
- [30].Bach P, Kirsch M, Hoffmann S, Jorde A, Mann K, Frank J, Charlet K, Beck A, Heinz A, Walter H, Rietschel M, Kiefer F, Vollstadt-Klein S, The effects of single nucleotide polymorphisms in glutamatergic neurotransmission genes on neural response to alcohol cues and craving, Addict. Biol 20 (6) (2015) 1022–1032. [DOI] [PubMed] [Google Scholar]
- [31].Carpenter-Hyland EP, Woodward JJ, Chandler LJ, Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors, J. Neurosci 24 (36) (2004) 7859–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Qiang M, Denny AD, Ticku MK, Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons, Mol. Pharmacol 72 (1) (2007) 95–102. [DOI] [PubMed] [Google Scholar]
- [33].Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, Carter E, Alexander NP, McCool BA, Souza-Formigoni ML, Weiner JL, Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration, J. Neurosci 33 (11) (2013) 4834–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cozzoli DK, Kaufman MN, Nipper MA, Hashimoto JG, Wiren KM, Finn DA, Functional regulation of PI3K-associated signaling in the accumbens by binge alcohol drinking in male but not female mice, Neuropharmacology 105 (2016) 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang J, Lanfranco MF, Gibb SL, Ron D, Ethanol-mediated long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum, Channels 5 (2011) 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D, Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse, J. Neurosci 30 (2010) 10187–10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kash TL, Baucum AJ 2nd, Conrad KL, Colbran RJ, Winder DG, Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis, Neuropsychopharmacology 34 (2009) 2420–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.