Summary
Hydrothermal fluids (341°C and 19°C) were collected < 1 m apart from a black smoker chimney and a tubeworm mound on the Boardwalk edifice at the Endeavour Segment in the northeastern Pacific Ocean to study anaerobic microbial growth in hydrothermal mineral deposits. Geochemical modelling of mixed vent fluid and seawater suggests the mixture was anoxic above 55°C and that low H2 concentrations (79 μmol kg−1 in end-member hydrothermal fluid) limit anaerobic hydrogenotrophic growth above this temperature. A thermophilic, hydrogenotrophic sulfur reducer, Desulfurobacterium strain HR11, was isolated from the 19°C fluid raising questions about its H2-dependent growth kinetics. Strain HR11 grew at 40–77°C (Topt 72–75°C), pH 5–8.5 (pHopt 6–7) and 1–5% (wt vol−1) NaCl (NaClopt 3–4%). The highest growth rates occurred when S2O32− and S° were reduced to H2S. Modest growth occurred by NO3− reduction. Monod constants for its growth were Ks of 30 μM for H2 and Ks of 20 μM for S2O32− with a μmax of 2.0 h−1. The minimum H2 and S2O32− concentrations for growth were 3 μM and 5 μM respectively. Possible sources of S2O32− and S° are from abiotic dissolved sulfide and pyrite oxidation by O2.
Introduction
Deep-sea hydrothermal vents are seafloor expressions of biogeochemical processes that occur deeper within the subseafloor (Deming and Baross, 1993; Orcutt et al., 2011). Based on thermodynamic predictions of the energy available for redox reactions in mixtures of hydrothermal fluid and seawater, chemolithoautotrophy is generally dominated by aerobic H2S oxidation at mesophilic growth temperatures (e.g. below 50°C) and by anaerobic H2 oxidation at higher temperatures at most hydrothermal vents (McCollom and Shock, 1997; Amend et al., 2011). The amount of H2 available for growth in hydrothermal fluids varies significantly based on host rock composition and frequency of volcanic activity (for summaries see Von Damm, 1995; Amend et al., 2011; Holden et al., 2012). The Aquificales and the Methanococcales are among the more common H2-oxidizing autotrophs found in hydrothermal vents (Huber and Holden, 2008). The Aquificales are strictly autotrophic and largely thermophilic H2 oxidizers that use various sulfur compounds, NO3− and sometimes O2 as electron acceptors (Huber and Eder, 2006). The Methanococcales are mesophilic-to-hyperthermophilic methanogens that are generally obligate hydrogenotrophs, although a few can also use formate (Whitman and Jeanthon, 2006).
In some terrestrial anoxic environments such as freshwater sediments and sewage treatment plants, CH4 formation is inhibited when SO42− concentrations are high (Lovley and Goodwin, 1988). Mesophilic sulfate-reducing bacteria (e.g. Desulfovibrio) have lower H2 half-saturation constants (Ks) for H2 uptake and growth and higher maximum H2 utilization and growth rates than mesophilic methanogens (e.g. Methanobacterium, Methanobrevibacter, Methanospirillum and Methanosarcina) (Kristjansson et al., 1982; Lovley et al., 1982; Robinson and Tiedje, 1984; Karadagli and Rittmann, 2005). This enables sulfate reducers to inhibit methanogen growth by lowering the partial pressure of H2 to concentrations below levels that methanogens can use for growth. This is in keeping with the traditional hierarchy of anaerobic metabolisms, in which methanogenesis occurs only when all other electron acceptors are absent (Lovley and Goodwin, 1988). Unlike hydrothermal systems, the H2 in these terrestrial environments is derived from the microbial breakdown of organic matter, and the minimum thresholds for syntrophic microbial H2 uptake are at nanomolar concentrations (Lovley and Goodwin, 1988). However, methanogens can coexist with sulfate-reducing bacteria in the presence of SO42− where the outcome of competition is a function of the rate of H2 supply, relative population sizes and SO42− availability (Lovley et al., 1982).
The purpose of this study was to assess the effects of H2 and S2O32− concentration on the growth of a thermophilic, autotrophic sulfur reducer from a marine environment, then compare its growth limitations with those of marine thermophilic methanogens. It might be assumed that sulfur-reducing bacteria would outcompete methanogens for H2 in marine thermal systems, given the evidence from terrestrial systems. However, few measurements of H2 growth kinetics have been made for autotrophic thermophiles. The minimum and Monod half-saturation H2 values for the growth of deep-sea methanogens (Methanocaldococcus) at 70°C and 82°C were 17–23 μM and 67 μM respectively (Ver Eecke et al., 2012). In this study, an obligately hydrogenotrophic, thermophilic bacterium, Desulfurobacterium strain HR11, a member of the Aquificales that reduces S2O32−, S° and NO3−, was isolated from 19°C fluid flowing from the top of the Boardwalk hydrothermal edifice along the Endeavour Segment in the northeastern Pacific Ocean. Its physiological characteristics and minimum Ks values for growth on H2 and S2O32− were measured and compared with those of high-temperature marine methanogens. The geochemistry of pure 341°C hydrothermal fluid collected within a metre of the 19°C fluid used to isolate strain HR11 (Fig. S1) was determined to provide an environmental context for the growth of microbes in that system.
Results and discussion
Fluid chemistry and microbial redox reaction energies
Most of the calculated end-member chemical concentrations for the 341°C hydrothermal fluid emanating from the Boardwalk hydrothermal chimney (Table 1) fall within the range of previously measured values for Endeavour Segment hydrothermal fluids (Lilley et al., 1993; 2003; Butterfield et al., 1994). Hydrogen concentrations were low to normal relative to historical values for Endeavour (Lilley et al., 1993; 2003; Butterfield et al., 1994; Ver Eecke et al., 2012). Hydrogen concentrations in most of the pure (zero-Mg2+) hydrothermal fluids from the Endeavour Segment since 2008 have been below 100 μmol kg−1 (Ver Eecke et al., 2012), which peaked in some vents at >1 mmol kg−1 in 1999 following seismic activity (Lilley et al., 2003). For the Boardwalk edifice in 2011, diluting the 341°C end-member hydrothermal fluid with seawater to 40–75°C results in H2 concentrations of 9–17 μM in the mixed fluid. Using geochemical mixing models, mixed fluids were predicted to be anoxic above 55°C, and their pH were calculated to be above pH 5 below 70°C (Fig. S2).
Table 1.
Chemical composition of end-member hydrothermal vent fluid from the Boardwalk edifice extrapolated to zero-Mg2+ from this study and seawater for modelling purposes.
| Hydrothermal fluid | Seawatera | |
|---|---|---|
| Temperature, max. | 341°C | 2°C |
| pH at 25°C | 4.1 | 7.8 |
| H2 (μmol kg−1) | 79 | 0 |
| CH4 (μmol kg−1) | 2680 | 0 |
| O2 (μmol kg−1) | 0 | 70 |
| Na+ (mmol kg−1) | 506.9 | 441 |
| K+ (mmol kg−1) | 36.2 | 9.8 |
| NH4+ (μmol kg−1) | 833 | – |
| Mg2+ (mmol kg−1) | 0.01 | 54.5 |
| Ca2+ (mmol kg−1) | 48.2 | 10.7 |
| Fe2+ (μmol kg−1) | 1300.4 | 0 |
| Cl− (mmol kg−1) | 621.9 | 550 |
| SO42− (mmol kg−1) | 1.7 | 27.9 |
| HCO3− (mmol kg−1) | 29.4 | 2.2 |
| HS− (mmol kg−1) | 3.4 | 0 |
| SiO2 (mmol kg−1) | 18.1 | 0.13 |
Seawater composition from Amend and colleagues (2011), except the O2 concentration, which is from Richard Thomson (Institute of Ocean Sciences, Fisheries and Oceans Canada, pers. comm.).
At 25–45°C, aerobic oxidation of S2− and CH4 was predicted to provide the largest amount of redox energy for autotrophic catabolism (up to 13.7 J kg−1 and 15.9 J kg−1 of mixed vent fluid respectively) (Fig. S2). They were both limited by the availability of O2 in seawater. The energies for hydrogenotrophic sulfate reduction and methanogenesis increased with temperature due to the increased availability of H2 (up to 0.8 J kg−1 and 0.4 J kg−1 mixed vent fluid, respectively). They were substantially lower than the reaction energy available for mesophilic aerobic S2− and CH4 oxidation (Fig. S2), as reported previously (Amend et al., 2011).
Thiosulfate and sulfur are the preferred terminal electron acceptors for the growth of Desulfurobacterium strain HR11 (see below), but their concentrations in hydrothermal fluids are unknown. Thiosulfate is a key intermediate in the oxidation of HS− to SO42−, especially where O2 concentrations are below saturation (Cline and Richards, 1969; Jørgensen, 1990). O2 concentrations at 2200 m depth in the northeast Pacific Ocean near North America are low (~70 μmol kg−1) due to an oxygen minimum zone in the region (Hartnett et al., 1998). Thiosulfate also forms from pyrite from within hydrothermal chimney walls. Pyrite is abiotically oxidized by Fe3+, which adsorbs to the pyrite and forms Fe2+ and S2O32−, although the S2O32− is rapidly oxidized to SO42− if additional Fe3+ is present (Luther, 1987; Moses et al., 1987). Pyrite is also oxidized by O2. The reaction rate is 10-fold slower than with Fe3+ as an oxidant, but S2O32− is present in higher concentrations due to its slow oxidation rate with O2 (Luther, 1987; Moses et al., 1987).
Characteristics of strains HR11
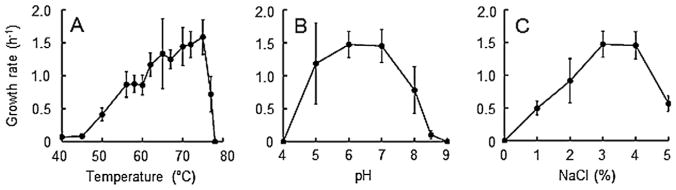
Strain HR11 was isolated at 55°C from the 19°C hydrothermal fluid emitted from the Boardwalk edifice and produced H2S in modified DSM 282 medium. Based on its 16S rRNA gene sequence, it is phylogenetically most closely related (>99% identity) to Desulfurobacterium thermolithotrophum (L’Haridon et al., 1998) (Fig. S3). Electron microscopy revealed short oblong rods, 0.5 μm by 1–2 μm, with a typical Gram-negative bacterial cell envelope and lophotrichous flagellation with three flagella (Fig. S4). Growth was observed between 40°C and 77°C with an optimum of 72–75°C (Fig. 1A), between pH 5.0 and pH 8.5 with an optimum of pH 6.0–7.0 (Fig. 1B), and between 1% and 5% NaCl with an optimum of 3–4% (Fig. 1C). Metabolite measurements showed that the organism produced up to 6 mM H2S. Strain HR11 is an obligate hydrogenotrophic autotroph that did not utilize yeast extract, maltose, tryptone, acetate or formate as an alternative source of carbon or electrons. In bottles, it grew at the same rate with elemental sulfur as the sole electron acceptor (1.56 ± 0.17 h−1) as it did with Na2S2O3 (1.59 ± 0.26 h−1) and showed modest growth (0.24 ± 0.21 h−1) when KNO3 was the terminal electron acceptor. Strain HR11 did not grow when Na2SO3, Na2SO4, Fe(III)-citrate, Fe(III) (oxy)hydroxide or O2 were supplied as the terminal electron acceptor. It did not grow on modified DSM 282 medium without the addition of an electron acceptor.
Fig. 1.
Growth rates for strain HR11 grown over its ranges of temperature (A), pH (B) and NaCl concentration (C). Strain HR11 was grown in 10 ml of modified DSM 282 medium in sealed Balch tubes, with 1 g l−1 S2O32− as the electron acceptor and 2 atm 80:20 H2:CO2 headspace, and growth determined via cell counts on a Petroff–Hausser chamber. Error bars represent 95% confidence intervals.
Monod kinetics for Desulfurobacterium strain HR11
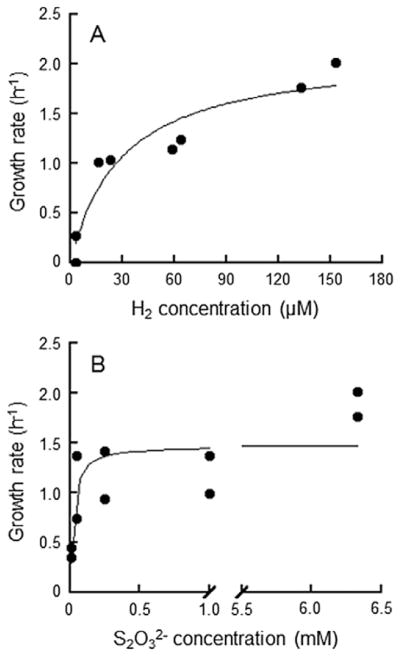
Desulfurobacterium strain HR11 was grown in a gas flow-controlled bioreactor at 72°C to determine the effect of H2 and S2O32− concentration on growth. It had longer doubling times and lower maximum cell concentrations with decreasing H2 and S2O32− concentrations. The minimum H2 concentration for growth was 3 μM and the Ks for growth on H2 was 30 μM (Fig. 2A). When grown on excess H2 (>100 μM), strain HR11 grew on as little as 5 μM S2O32− and its Ks for growth was 20 μM (Fig. 2B). The maximum growth rate (μmax) in the reactor was 2.0 h−1. Ver Eecke and colleagues (2012) previously measured the minimum and Ks values of H2 for the growth of three methanogens (Methanocaldococcus spp.) grown at 70°C and 82°C in the same reactor. All three organisms had minimum H2 requirements of 17–23 μM, a Ks for H2 of 67 μM and a μmax of 0.8–1.2 h−1. In this study, Desulfurobacterium strain HR11 had a lower minimum H2 requirement, a lower H2 Ks and a higher μmax than those reported for Methanocaldococcus. The μmax/Ks ratios for H2 indicate that Desulfurobacterium strain HR11 has a growth advantage over Methanocaldococcus species (0.067 h−1 μM−1 versus 0.015 h−1 μM−1).
Fig. 2.
Growth rates for strain HR11 grown over its ranges of H2 concentration (A) and initial Na2S2O3 concentration (B). Strain HR11 was grown in 1.5 l of modified DSM 282 medium in a 2 l bioreactor, gassed with H2, CO2 and N2 as required to achieve experimental H2 concentrations. Growth was determined as for Balch tube experiments. The line is a Michaelis–Menten fit to the data.
For terrestrial mesophilic microbes, the Monod H2 Ks is 2–4 μM for Desulfovibrio strain G11 and 6–7 μM for Methanospirillum hungatei JF-1 (Robinson and Tiedje, 1984). Similarly, the H2 uptake Ks is 1–2 μM for five Desulfovibrio spp.; 3–7 μM for Methanobrevibacter, Methanobacterium and Methanospirillum species; and 13 μM for Methanosarcina barkeri strain MS (Kristjansson et al., 1982; Robinson and Tiedje, 1984). These differences in substrate affinities confer a competitive advantage for sulfate-reducing bacteria over methanogens when SO42− is not limiting. However, both groups of organisms can coexist in anoxic environments when both H2 and SO42− are plentiful (Lovley et al., 1982). A global survey of low-temperature hydrothermal fluids with co-localized phylogenetic and chemical analyses shows that Desulfurobacterium and the Methanococcales are both present in vent environments with H2 concentrations predicted to be above 17 μM at 72°C, and both are generally absent below this threshold (Table S1). This includes the 19°C hydrothermal fluid in this study where a thermophilic methanogen (Methanothermococcus strain BW11) was also isolated (Fig. S2). These data suggest that S2O32− and S° are not at limiting concentrations in these hydrothermal systems and that generally there is sufficient H2 flux in many vent systems to support both groups of organisms.
The diversity of thermophilic anaerobes in hydrothermal vents is relatively low, making pure cultures of these organisms useful for modelling growth and competition in these systems. Thermophilic, autotrophic sulfur reducers such as Desulfurobacterium spp. and thermophilic methanogens such as Methanothermococcus and Methanocaldococcus spp. are common in vent systems; grow over the same temperatures, pHs and salinities; and compete for H2, making them ideal candidates for environmental modelling. Although Desulfurobacterium appears to have a kinetic growth advantage over the Methanococcales as long as S2O32− or S° is present, the two functional groups appear to coexist where the flux of H2 is sufficient. Important future research questions are how these organisms respond physiologically to H2 limitation, whether spatial heterogeneity separates them in situ, and if they have physiological mechanisms to compete for resources.
Supplementary Material
Fig. S1. Boardwalk hydrothermal vent sampling site showing the black smoker (bottom) that was the source of the 341°C hydrothermal fluid and the tubeworm mound (left side) that was the source of the 19°C fluid. The image is a video frame grab from ROV Jason dive J2-576.
Fig. S2. Predicted catabolic energies (per kilogram of mixed fluid) available for hydrogenotrophic sulfate reduction (●), hydrogenotrophic methanogenesis (○), aerobic sulfide oxidation (▲) and aerobic methane oxidation (△) at varying temperatures in mixed abiotic hydrothermal-seawater solutions flowing from the Boardwalk edifice. The calculated pH for the mixed fluid is also shown (×).
Fig. S3. Neighbour-joining trees showing the positions of (A) strain HR11 within the genus Desulfurobacterium (870 nt) and (B) strain BW11 within the genus Methanothermococcus (880 nt) based on sequences of the 16S rRNA gene. GenBank/EMBL/DDBJ accession numbers are included in parentheses. The topology of the tree was estimated by bootstraps based on 500 replications. Numbers at the branch point are the percentage support by bootstraps. Bar, 2% sequence divergence.
Fig. S4. Negative staining (A) and thin-section transmission electron micrographs of strain HR11. Bars, 500 nm.
Table S1. Characteristics of various global deep-sea hydrothermal vent sites and the presence or absence of Desulfurobacterium and Methanococcales species.
Table S2. Inorganic redox reactions (from Amend et al., 2011).
Acknowledgments
We wish to thank Nancy Akerman and Julie Huber for their assistance in collecting field samples; the crew and pilots of the RV Thomas G. Thompson and the ROV Jason II for their expertise and assistance; Kevin Roe, Hoang-My Christensen, Ben Larson and Eric Olson for vent fluid analyses; and Dale Callaham for his assistance with the electron microscopy. We also thank Richard Thomson of the Institute of Ocean Sciences, Fisheries and Oceans Canada for providing the dissolved O2 data used to calculate redox energy. This work was supported by grants to J.F.H. and D.A.B. from the Gordon and Betty Moore Foundation (GBMF 3297); to L.C.S and J.F.H. from the NASA Earth and Space Science Fellowship program (NNX11AP78H); to L.C.S. from a Fulbright New Zealand-Ministry of Research, Science and Technology Graduate Award; to D.A.B. from the Joint Institute for the Study of the Atmosphere and Ocean (JISAO) under NOAA Cooperative Agreement No. NA10OAR4320148, Contribution No. 4356; and to M.D.L. from NSF (0819004 and 1037874). The authors have no conflict of interest to declare.
References
- Amend JP, McCollom TM, Hentscher M, Bach W. Catabolic and anabolic energy for chemolithoautotrophs in deep-sea hydrothermal systems hosted in different rock types. Geochim Cosmochim Acta. 2011;75:5736–5748. [Google Scholar]
- Butterfield DA, McDuff RE, Mottl MJ, Lilley MD, Lupton JE, Massoth GJ. Gradients in the composition of hydrothermal fluids from the Endeavour segment vent field: phase separation and brine loss. J Geophys Res. 1994;99:9561–9583. [Google Scholar]
- Cline JD, Richards FA. Oxygenation of hydrogen sulfide in seawater at constant salinity, temperature, and pH. Env Sci Tech. 1969;3:838–843. [Google Scholar]
- Deming JW, Baross JA. Deep-sea smokers: windows to a subsurface biosphere? Geochim Cosmochim Acta. 1993;57:3219–3230. doi: 10.1016/0016-7037(93)90535-5. [DOI] [PubMed] [Google Scholar]
- Hartnett HE, Keil RG, Hedges JI, Devol AH. Influence of oxygen exposure time on organic carbon preservation in continental margin sediments. Nature. 1998;391:572–574. [Google Scholar]
- Holden JF, Breier JA, Rogers KL, Schulte MD, Toner BM. Biogeochemical processes at hydrothermal vents: microbes and minerals, bioenergetics, and carbon fluxes. Oceanography. 2012;25:196–208. [Google Scholar]
- Huber JA, Holden JF. Modeling the impact of diffuse vent microorganisms along mid-ocean ridges and flanks. In: Lowell RP, Seewald JS, Metaxas A, Perfit MR, editors. Magma to Microbe: Modeling Hydrothermal Processes at Ocean Spreading Centers. Washington, DC, USA: American Geophysical Union; 2008. pp. 215–231. [Google Scholar]
- Huber R, Eder W. Aquificales. Prokaryotes. 2006;7:925–938. [Google Scholar]
- Jørgensen BB. A thiosulfate shunt in the sulfur cycle of marine sediments. Science. 1990;249:152–154. doi: 10.1126/science.249.4965.152. [DOI] [PubMed] [Google Scholar]
- Karadagli F, Rittmann BE. Kinetic characterization of Methanobacterium bryantii M.o.H. Environ Sci Technol. 2005;39:4900–4905. doi: 10.1021/es047993b. [DOI] [PubMed] [Google Scholar]
- Kristjansson JK, Schönheit P, Thauer RK. Different Ks values for hydrogen of methanogenic bacteria and sulfate reducing bacteria: an explanation for the apparent inhibition of methanogenesis by sulfate. Arch Microbiol. 1982;131:278–282. [Google Scholar]
- L’Haridon S, Cilia V, Messner P, Rag G, Gamba A, Jeanthon C. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Bacteriol. 1998;48:701–711. doi: 10.1099/00207713-48-3-701. [DOI] [PubMed] [Google Scholar]
- Lilley MD, Butterfield DA, Olson EJ, Lupton JE, Macko SA, McDuff RE. Anomalous CH4 and NH4+ concentrations at an unsedimented mid-ocean-ridge hydrothermal system. Nature. 1993;364:45–47. [Google Scholar]
- Lilley MD, Butterfield DA, Lupton JE, Olson EJ. Magmatic events can produce rapid changes in hydrothermal vent chemistry. Nature. 2003;422:878–881. doi: 10.1038/nature01569. [DOI] [PubMed] [Google Scholar]
- Lovley DR, Goodwin S. Hydrogen concentrations as an indicator of the predominant terminal electronaccepting reactions in aquatic sediments. Geochim Cosmochim Acta. 1988;52:2993–3003. [Google Scholar]
- Lovley DR, Dwyer DF, Klug MJ. Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol. 1982;43:1373–1379. doi: 10.1128/aem.43.6.1373-1379.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther GW., III Pyrite oxidation and reduction: molecular orbital theory considerations. Geochim Cosmochim Acta. 1987;51:3193–3199. [Google Scholar]
- McCollom TM, Shock EL. Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim Cosmochim Acta. 1997;61:4375–4391. doi: 10.1016/s0016-7037(97)00241-x. [DOI] [PubMed] [Google Scholar]
- Moses CO, Nordstrom DK, Herman JS, Mills AL. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim Cosmochim Acta. 1987;51:1561–1571. [Google Scholar]
- Orcutt BN, Sylvan JB, Knab NJ, Edwards KJ. Microbial ecology of the dark ocean above, at, and below the seafloor. Microbiol Mol Biol Rev. 2011;75:361–422. doi: 10.1128/MMBR.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Tiedje J. Competition between sulfate-reducing and methanogenic bacteria for H2 under resting and growing conditions. Arch Microbiol. 1984;137:26–32. [Google Scholar]
- Ver Eecke HC, Butterfield DA, Huber JA, Lilley MD, Olson EJ, Roe KK, et al. Hydrogen-limited growth of hyperthermophilic methanogens at deep-sea hydrothermal vents. Proc Natl Acad Sci USA. 2012;109:13674–13679. doi: 10.1073/pnas.1206632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Damm KL. Controls on the chemistry and temporal variability of seafloor hydrothermal fluids. In: Humphris SE, Zierenberg RA, Mullineaux LS, Thomson RE, editors. Seafloor Hydrothermal Systems: Physical, Chemical, Biological, and Geological Interactions. Washington, DC, USA: American Geophysical Union; 1995. pp. 222–247. [Google Scholar]
- Whitman WB, Jeanthon C. Methanococcales. Prokaryotes. 2006;7:257–273. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Boardwalk hydrothermal vent sampling site showing the black smoker (bottom) that was the source of the 341°C hydrothermal fluid and the tubeworm mound (left side) that was the source of the 19°C fluid. The image is a video frame grab from ROV Jason dive J2-576.
Fig. S2. Predicted catabolic energies (per kilogram of mixed fluid) available for hydrogenotrophic sulfate reduction (●), hydrogenotrophic methanogenesis (○), aerobic sulfide oxidation (▲) and aerobic methane oxidation (△) at varying temperatures in mixed abiotic hydrothermal-seawater solutions flowing from the Boardwalk edifice. The calculated pH for the mixed fluid is also shown (×).
Fig. S3. Neighbour-joining trees showing the positions of (A) strain HR11 within the genus Desulfurobacterium (870 nt) and (B) strain BW11 within the genus Methanothermococcus (880 nt) based on sequences of the 16S rRNA gene. GenBank/EMBL/DDBJ accession numbers are included in parentheses. The topology of the tree was estimated by bootstraps based on 500 replications. Numbers at the branch point are the percentage support by bootstraps. Bar, 2% sequence divergence.
Fig. S4. Negative staining (A) and thin-section transmission electron micrographs of strain HR11. Bars, 500 nm.
Table S1. Characteristics of various global deep-sea hydrothermal vent sites and the presence or absence of Desulfurobacterium and Methanococcales species.
Table S2. Inorganic redox reactions (from Amend et al., 2011).