Abstract
Microbiome alterations have been shown to affect stroke outcome. However, to what extent the presence of a gut microbiome per se is affecting post-stroke neuroinflammation has not been tested. By comparing germfree mice with recolonized (Ex-GF) and conventional SPF mice, we were able to demonstrate that bacterial colonization reduces stroke volumes. Bacterial colonization increased cerebral expression of cytokines as well as microglia/macrophage cell counts in contrast to improved stroke outcome. Interestingly, the microbiome-mediated brain protection was absent in lymphocyte-deficient mice. These findings support the concept of lymphocyte-driven protective neuroinflammation after stroke under control of the microbiome.
Keywords: Stroke, microbiota, germfree, T cells, microglia, neuroinflammation
Introduction
Stroke triggers a neuroinflammatory reaction which encompasses activation of brain resident microglia and invasion of leukocytes.1 T cells are potent contributors to post-stroke neuroinflammation. While pro-inflammatory Thelper cell subpopulations (e.g. Th1, Th17) promote neuroinflammation, other populations with anti-inflammatory properties (e.g. Treg cells) can be cerebroprotective. Current findings have highlighted the role of commensal gut microbiota in the regulation of T cell responses to brain ischemia.2,3 Recently, we have shown that stroke changes the bacterial composition in the gut, which was causally linked to a pro-inflammatory T cell polarization and worse stroke outcome.2 Similarly, a study by Benakis et al.3 demonstrated the impact of antibiotic treatment-induced dysbiosis on stroke outcome. Although these first studies demonstrated a previously unrecognized key role of changes in microbiota composition to affect stroke outcome, we are still lacking the proof-of-concept that bacterial colonization per se affects stroke outcome and post-stroke neuroinflammation. In order to fill this gap, we investigated stroke outcome and neuroinflammation in GF animals and colonized littermates (Ex-GF) and specifically analyzed the role of T cells as potential mediators along the gut-brain axis in stroke.
Material and methods
Detailed material and methods can be found in the online Supplementary material. All original raw data of this study as well as detailed protocols are available from the corresponding author upon reasonable request.
All animal experiments were performed under the institutional guidelines for the use of animals for research and were approved by the governmental ethics committee of Upper Bavaria (Regierungspraesidium Oberbayern). GF mice were housed in sterile HAN-gnotocages and received sterile food pellets and water. All surgical procedures including stroke induction, mouse handling and cage changes were performed under sterile conditions in a sterile microbiological laminar flow (Supplementary Figure 1(a)). Germfree status of GF mice was confirmed after surgery and survival period by 16 s PCR of fecal samples. Littermates of GF mice were colonized through co-housing with conventional specific pathogen-free (SPF) C57BL/6 J mice for three weeks and co-housing was maintained also after stroke surgery. Animals were randomized to the treatment groups and all analyses were performed by investigators blinded to group allocation. Unblinding was done after the completion of statistical analysis. All animal experiments were performed and reported according to the ARRIVE guidelines.1
Results
Bacterial colonization is cerebroprotective after stroke
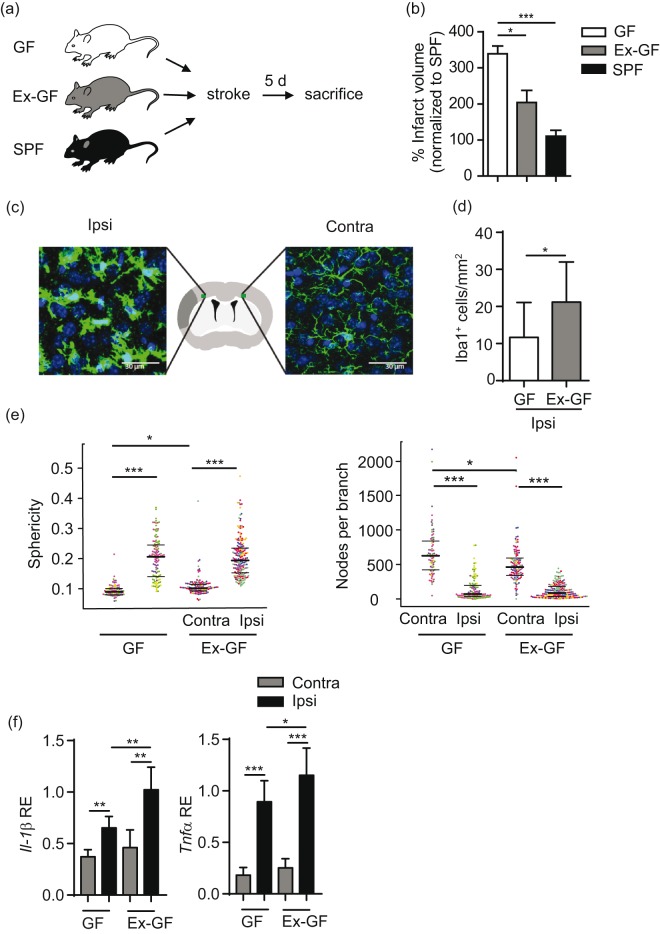
Stroke was induced in GF, Ex-GF and SPF mice in order to test the impact of bacterial colonization on stroke outcome (Figure 1(a)). All surgical procedures were performed in a sterile microbiological safety cabinet (Supplementary Figure 1(a)). Reduced caecum-body weight index increased total fecal DNA and eubacteria amount verified bacterial colonization in Ex-GF mice as well as maintaining the germfree status after surgery in GF mice (Supplementary Figure 1(b) to (d)). Sequencing of bacterial 16s rRNA showed comparable alpha diversity and abundance of bacterial phyla between Ex-GF and SPF mice as a key markers of successful colonization in the Ex-GF group (Supplementary Figure1(e) and (f)). Quantification of infarct volume five days after stroke induction revealed an improved stroke outcome in Ex-GF and SPF mice compared to GF littermates (Figure 1(b)). This substantial effect of colonization on lesion volume has been consistently observed in three independent experiments performed by three different surgeons. After confirmation of comparable outcome and colonization in SPF and Ex-GF animals, we used Ex-GF generated by randomized colonization of GF littermates as the favourable “colonized” control group.
Figure 1.
Bacterial colonization is cerebroprotective after stroke. (a) Schematic illustration of experimental paradigm. (b) Quantification of infarct volume in germfree (GF), colonized (Ex-GF) and SPF mice (n= 12 per group). Data are shown as mean ± SD normalized to the mean of the SPF group as 100%. (c) Representative images of Iba-1 stained microglia/macrophages with schematic illustration of imaging locations in the peri-infarct area 900 µm distant from the border of the infarct core in the deep cortical layer 4/5 and homotypical contralateral area. (d) Analysis of microglia cell counts (n = 5 per group) in the ipsilateral cortex. (e) Analysis of microglial morphology showing increased sphericity (left) and reduced ramification (right) in ipsilateral hemispheres as markers of microglial activation. (f) Gene expression analysis by RT-PCR for the indicated genes of mainly microglial/macrophage origin in ipsilateral and contralateral hemispheres after stroke (n = 10–12 per group, mean ± SD). *p < 0.05, **p < 0.01, ***p < 0.001).
Gut microbiota increase the neuroinflammatory response after stroke
In order to test the impact of bacterial colonization on the local neuroinflammatory milieu, we investigated cell counts and morphology of microglia/macrophages as well as key transcriptional markers of innate immune responses after stroke. In contrast to reduced lesion volumes, we found an increased number of microglia/macrophages in the ischemic hemisphere of Ex-GF mice compared to the GF group (Figure 1(c) and (d)). Automated analysis of microglial morphology4 revealed massive activation of microglia in the peri-infarct area characterized by enhanced sphericity index and reduced ramifications compared to the contralateral side (Figure 1(e)). While bacterial recolonization did not further affect already activated peri-lesional microglia/macrophages, a significant effect was observed on cell sphericity and ramifications in the contralateral hemispheres in Ex-GF mice (Figure 1(e)). Accordingly, we also observed a significant increase in the transcriptional regulation of pro-inflammatory cytokine expression associated with microglial activation such as Il-1β and Tnf-α (Figure 1(f)).
Cerebroprotection is mediated by microbiome-induced T cell priming
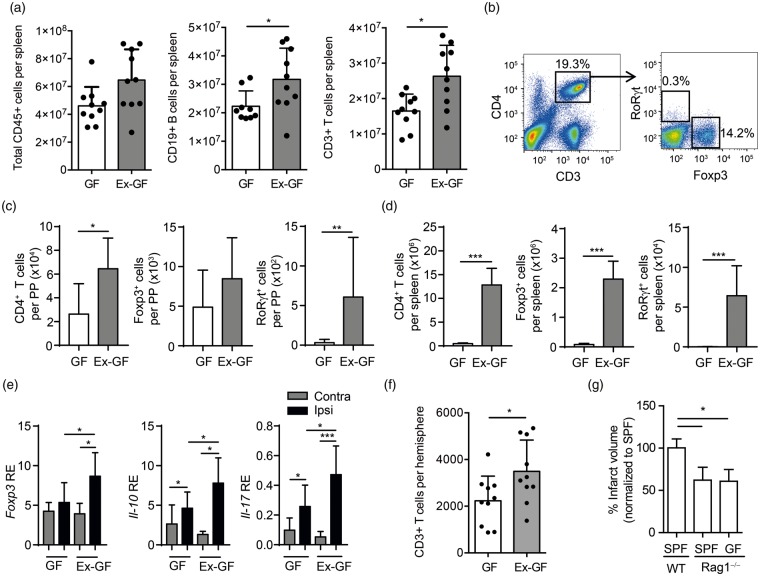
Previous studies using models of altered microbiota composition have proposed modification in lymphocyte priming and particularly in T cell polarization as key mediators of the microbiome effect on stroke outcome.2,3,5 Therefore, we first sought to determine the impact of the gut microbiome on T cell priming in secondary lymphatic organs by flow cytometry. While the overall leukocyte count was unaltered between GF and Ex-GF animals, we detected increased T and B cells counts in spleens of Ex-GF compared to GF animals (Figure 2(a)). More specifically, we observed an increase in overall Thelper cell (CD4+) counts as well as polarized regulatory T cells (Foxp3+) and Th17 cells (RoRγt+) in the intestinal immune compartments of the Peyer’s patches (Figure 2(b) and (c)) and even more pronounced in the spleens of Ex-GF compared to GF mice at five days after stroke (Figure 2(d)). Interestingly, we observed a similar pattern in the ischemic brain with increased mRNA levels for specific Thelper cell subpopulations including Foxp3 and Il-10 (Treg) and Il-17 (Th17) in Ex-GF animals compared to GF littermates (Figure 2(e)). Additionally, we observed by flow cytometry also an increase in total T cell counts in the ischemic hemispheres of Ex-GF compared to GF animals (Figure 2(f)). These results implicate that a physiological gut microbiome is required for generating an adequate lymphocyte-driven immune reaction in response to brain injury and execute tissue protection. Because of this pronounced effect of bacterial colonization on post-stroke T cell responses, we examined the role of lymphocytes in mediating the cerebroprotective effects of the microbiome in lymphocyte-deficient Rag1−/− mice. Lesions were smaller in Rag1−/− mice compared to wild-type C57BL/6 J mice. However, we found no difference in lesion volumes between GF Rag1−/− mice and conventional Rag1−/− mice, supporting the finding that lymphocytes play an important role in the microbiome-mediated effects on stroke (Figure 2(g)). Additionally, we did not detect a significant impact of gut bacterial colonization on blood–brain barrier integrity or cortical capillary density (Supplementary Figure 2).
Figure 2.
T cell priming by the gut microbiome is cerebroprotective. (a) Flow cytometric analysis of absolute cell counts per spleen in GF and Ex-GF animals for total leukocytes (CD45+), B cells (CD19+) and T cells (CD3+). While total leukocytes counts were unaffected, bacterial colonization significantly increase T and B cells ounts in Ex-GF mice. (b) Representative gating strategy for the flow cytometric analysis of Thelper cell counts and subsets of Foxp3+ (Treg) and RoRγt+ (Th17) cells in (c) the Peyer’s patches and in (d) the spleen. (e) (c) RT-PCR for expression Thelper cell markers Foxp3, Il-10 and Il-17 in ipsilateral and contralateral brain hemispheres five days after stroke (n = 10–12 per group). (f) Flow cytometric analysis of ipsilateral brain hemispheres five days after stroke in GF and Ex-GF revealing significantly increased T cell counts in the ischemic hemispheres of recolonized Ex-GF animals. (g) Brain infarct volumes in SPF WT, SPF Rag1−/− and GF Rag1−/− mice (n = 7–9 per group, mean ± SD normalized to SPF group as 100%). *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
This study provides a proof-of-concept that the gut microbiome per se is cerebroprotective in experimental stroke. These are novel and relevant insights for the gut-brain axis field in stroke as well as other models of acute brain injury because previous studies have so far exclusively investigated the impact of alterations in bacterial composition on disease outcome but did not test the relevance of the microbiome as a bona fide modulator of secondary neuroinflammation.
These experiments were so far lacking due to the technical difficulties of GF mouse handling and surgical stroke induction. We have overcome these technical limitations by on one side ensuring germfree status of animals despite surgical manipulation and on the other side establishing natural recolonization as comparable to conventional SPF mice on stroke outcome, which allows the study of littermates (GF and Ex-GF) for improved comparability. Ex-GF mice were chosen as a superior control group for colonized animals compared to SPF mice which have substantial disadvantages including potential differences due to genetic drift, epigenetic modifications and housing conditions.
We have previously reported that large stroke lesions induce changes in the gut microbiome, leading to a pro-inflammatory over-activation of peripheral immune responses and worse stroke outcome.2 In contrast, we show here that also the complete lack of a gut flora leads to enlarged brain lesions compared to colonized littermates or conventionally housed SPF mice. This notion contradicts the widely accepted concept that the secondary inflammatory response to tissue injury is per se “detrimental” and—in the case of acute brain injury— “neurotoxic.” 1 However, recent reports revised this paradigm of secondary neuroinflammation as “too much of a bad thing” by considering secondary neuroinflammation to be a physiologically relevant protective mechanism in which both excessive immune activation and immunosuppression can be harmful.6
By investigating germfree, lymphocyte-deficient Rag1−/− mice, we provide evidence supporting a key role for lymphocytes along the gut-brain axis. Our results demonstrate that lymphocytes are required to generate a neuroinflammatory milieu that is associated with a reduction of secondary lesion expansion. We observed that a lack of bacterial colonization was associated significantly with an increased expression of pro-inflammatory cytokines in the ischemic hemisphere. On the other side, germfree animals displayed reduced overall microglia/macrophage cell counts and a reduction in pro-inflammatory cytokine expression. These findings could indicate a dysfunctional, non-physiological microglial response to the ischemic injury which is associated with secondary neurological deterioration. Moreover, lymphocyte-dependent neuroprotection under the control of the gut microbiome is likely to be mediated also via non-immunological mechanisms that contribute to lesion growth and recovery such as growth factor secretion by lymphocytes, modulation of neurogenesis or impaired vascular function7,8 although our results exclude direct major effects on vascular function. Additionally, while the role of B cells is less investigated in stroke-immunology as well as the gut-brain research field compared to T cells, the expansion of splenic B cell counts and the lack of mature B cells in Rag1−/− mice warrant additional experiments investigating the contribution of B cells to the observed phenomenon.
Taken together, future experiments which were beyond the scope of this proof-of-concept study are urgently needed for the in-depth analysis of the potential pathways of lymphocyte–brain interaction that lead to neuroprotection under control of the microbiome. Our findings open up several new questions such as the mode of microbiome interaction with intestinal lymphocytes, the involved bacterial mediators and/or antigens. Finally, the druggability of microbiome-lymphocyte interaction to prime a beneficial, pro-regenerative immune response is of great future interest. More detailed understanding of the gut-immune-brain axis in stroke could open up a new field of microbiome-targeted therapies for stroke patients.
Supplemental Material
Supplemental Material for The gut microbiome primes a cerebroprotective immune response after stroke by Vikramjeet Singh, Rebecca Sadler, Steffanie Heindl, Gemma Llovera, Stefan Roth, Corinne Benakis and Arthur Liesz in Journal of Cerebral Blood Flow & Metabolism
Acknowledgments
DNA sequences have been deposited in MG-RAST (http.//metagenomics.anl.gov/) under accession number #9778. We thank Kathleen McCoy (University of Bern, Switzerland) for providing GF mice.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Excellence cluster of the German research foundation “Munich Cluster for Systems Neurology (SyNergy)” to AL.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
VS, RS, SH, GL, SR, CB performed experiments; VS, RS, CB and AL analyzed data, VS and AL wrote the manuscript; AL conceived the study and supervised the project.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh V, Roth S, Llovera G, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016; 36: 7428–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 2016; 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heindl S, Gesierich B, Benakis C, et al. Automated morphological analysis of microglia after stroke. Front Cell Neurosci. Epub ahead of print 19 April 2018. DOI: 10.3389/fncel.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadler R, Singh V, Benakis C, et al. Microbiota differences between commercial breeders impacts the post-stroke immune response. Brain Behav Immun 2017; 66: 23–30. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz M, Kipnis J. Protective autoimmunity and neuroprotection in inflammatory and noninflammatory neurodegenerative diseases. J Neurol Sci 2005; 233: 163–166. [DOI] [PubMed] [Google Scholar]
- 7.Ziv Y, Ron N, Butovsky O, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 2006; 9: 268–275. [DOI] [PubMed] [Google Scholar]
- 8.De Meyer SF, Denorme F, Langhauser F, et al. Thromboinflammation in Stroke Brain Damage. Stroke 2016; 47: 1165–1172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The gut microbiome primes a cerebroprotective immune response after stroke by Vikramjeet Singh, Rebecca Sadler, Steffanie Heindl, Gemma Llovera, Stefan Roth, Corinne Benakis and Arthur Liesz in Journal of Cerebral Blood Flow & Metabolism