Summary
Cartilage pellets generated from ectomesenchymal progeny of human pluripotent stem cells (hPSCs) in vitro eventually show signs of commitment of chondrocytes to hypertrophic differentiation. When transplanted subcutaneously, most of the surviving pellets were fully mineralized by 8 weeks. In contrast, treatment with the adenylyl cyclase activator, forskolin, in vitro resulted in slightly enlarged cartilage pellets containing an increased proportion of proliferating immature chondrocytes that expressed very low levels of hypertrophic/terminally matured chondrocyte-specific genes. Forskolin treatment also enhanced hyaline cartilage formation by reducing type I collagen gene expression and increasing sulfated glycosaminoglycan accumulation in the developed cartilage. Chondrogenic mesoderm from hPSCs and dedifferentiated nasal chondrocytes responded similarly to forskolin. Furthermore, forskolin treatment in vitro increased the frequency at which the cartilage pellets maintained unmineralized chondrocytes after subcutaneous transplantation. Thus, the post-transplantational fate of chondrocytes originating from hPSC-derived chondroprogenitors can be controlled during their genesis in vitro.
Keywords: pluripotent stem cell, neural crest, paraxial mesoderm, chondrocyte, hypertrophy, cAMP, forskolin, cartilage, mineralization, endochondral ossification
Graphical Abstract
Highlights
-
•
Forskolin/cAMP suppresses/delays BMP-induced chondrocyte maturation in vitro
-
•
Forskolin supports chondrocyte proliferation and hyaline chondrogenesis in vitro
-
•
Forskolin suppresses osteogenesis and BMP signaling gene expression in cartilage
-
•
In vitro forskolin treatment improves in vivo maintenance of uncalcified cartilage
In this article, Naoki Nakayama and colleagues show that suppression of hypertrophic differentiation and terminal maturation of chondrocytes by forskolin/cAMP treatment during in vitro chondrogenesis from human pluripotent stem cell-derived chondroprogenitors leads to the formation of cartilage consisting of an increased proportion of proliferating, immature chondrocytes with significantly improved ability to be maintained in an unmineralized state in vivo.
Introduction
Healthy cartilage of joints is stably maintained, but damaged cartilage is not spontaneously repaired in large animals and humans, leading to severe wear of the whole joint cartilage and consequent osteoarthritis (Buckwalter et al., 2014). The current cell-based and tissue engineering-based therapies, which use mesenchymal stromal cells (MSCs) and dedifferentiated articular chondrocytes, are far from ideal on a number of fronts (Steinert et al., 2007). One of the major challenges is to prevent the (re)generated cartilage from entering an endochondral ossification program after transplantation (Somoza et al., 2014). Such a program results in hypertrophic differentiation, mineralization/calcification, and the death of chondrocytes, followed by angiogenesis and bone growth, as in the growth plate. Similar changes are also observed during osteoarthritic cartilage degeneration (Pitsillides and Beier, 2011, van der Kraan and van den Berg, 2012).
Chondrogenesis is most active during prenatal and early postnatal stages. Surface injury introduced into fetal joint cartilage in utero is repaired spontaneously and completely, even in a large animal model (Namba et al., 1998), suggesting that embryonic epiphyseal chondrocytes may possess the capacity to regenerate joint articular cartilage without being committed to endochondral ossification. For humans, pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced PSCs (iPSCs), are the only practical source of embryonic cells. Chondroprogenitors have already been developed from mouse (m) and human (h) PSCs and characterized in vitro and in vivo (Nakayama et al., 2016, Nakayama and Umeda, 2011). Interestingly, we and others have recently shown that tissue-engineered cartilage produced from mesodermal progeny of hPSCs under scaffold-free conditions in vitro (i.e., cartilage pellet) tended to stay in an unmineralized state when transplanted at ectopic sites in immunocompromised rodents (Craft et al., 2015, Umeda et al., 2015, Yamashita et al., 2015), as did a piece of articular cartilage (Figure S1A). This tendency is preserved even when cartilage is formed in the presence of bone morphogenetic protein (BMP) (Umeda et al., 2015, Yamashita et al., 2015), which enhances chondrogenesis but also hypertrophic differentiation of chondrocytes (Minina et al., 2002, Tsumaki et al., 2002). However, when such mesodermal progeny were expanded and maintained in culture, the resulting cartilage pellets matured readily and became fully mineralized after transplantation (Figure S1B). So too did the chondrogenic ectomesenchymal cells developed by an expansion culture of neural crest-like progeny of hPSCs (Umeda et al., 2015). Thus, regardless of the developmental origin, expanded chondroprogenitors may have a tendency to give rise to growth-plate-like, endochondral ossification-ready chondrocytes. However, expansion of chondrogenic cells such as MSCs is often needed to gain sufficient cells for treatment.
One way to avoid endochondral ossification in chondrocytes developed from expanded chondroprogenitors is to mimic the parathyroid hormone-related peptide (PTHrP) signaling that keeps growth-plate chondrocytes in the proliferative state and suppresses their hypertrophic differentiation (Amizuka et al., 1994, Karaplis et al., 1994). During embryonic skeletogenesis, early epiphyseal chondrocytes express PTHrP and low levels of its receptor. Later, the Indian hedgehog (IHH)-PTHrP negative feedback loop controls the speed of hypertrophic differentiation and terminal maturation of growth-plate chondrocytes (Lanske et al., 1996, Vortkamp et al., 1996). Increased cAMP is responsible for such effects of PTHrP (Sakamoto et al., 2005). The rise in cAMP leads to activation of Sox9, the master regulator of (immature) chondrocytes, through the action of cAMP-dependent protein kinase (PKA) (Huang et al., 2001, Huang et al., 2000), which also leads to inhibition of Mef2c action and suppression of chondrocyte hypertrophic differentiation (Kozhemyakina et al., 2009). PTHrP causes a decrease in the mRNA level of Runx2, the master regulator for mineralization (bone formation) and hypertrophic differentiation of chondrocytes (Li et al., 2004), and stimulates degradation of Runx2 protein (Zhang et al., 2009), which delays chondrocyte hypertrophic differentiation (Guo et al., 2006). Furthermore, Pthrp mutant mice show mineralization of nasal cartilage (Chen et al., 2008), suggesting that nasal cartilage is maintained permanently in an unmineralized state via PTHrP signaling.
Here, we report that treatment with the small-molecule activator of adenylyl cyclase forskolin, which directly increases intracellular cAMP levels during chondrogenesis from hPSC-derived ectomesenchymal cells, suppresses hypertrophic differentiation and terminal maturation of developed chondrocytes potentially via maintaining their proliferative state. Forskolin also increases their capacity to produce hyaline cartilage matrix. Furthermore, forskolin treatment prevents cartilage pellets to varying degrees from becoming mineralized bony tissues when transplanted at an ectopic site in immunocompromised mice. Thus, the implementation of cAMP signaling seems to be an effective means of generating a long-lasting, endochondral ossification-resistant cartilage construct.
Results
Forskolin Selectively Suppresses Type X Collagen Gene Expression in Hyaline-like Cartilage Pellets Induced with BMP4 from hPSC-Derived Ectomesenchymal Cells
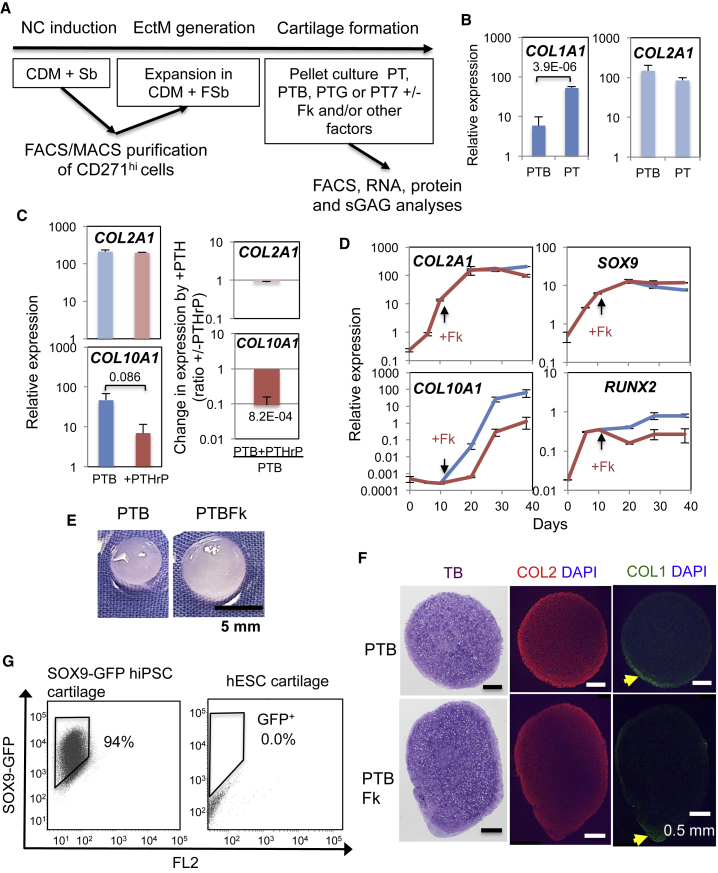
We used ectomesenchymal cells (Figure 1A) as a model hPSC-derived chondroprogenitor that forms large hyaline-like cartilage (i.e., type I collagen [COL1]lo, type II collagen [COL2]+) pellets in the presence of BMP (Figures 1B and 1E) (Umeda et al., 2012) and expresses type X collagen (COL10) mRNA (COL10A1), a marker of hypertrophic chondrocytes (Figures 1C and 1D) (Umeda et al., 2015) to screen for signaling modifiers that counteract the effect of BMP on hypertrophic differentiation of chondrocytes without disturbing its effect on hyaline chondrogenesis in vitro. We aimed to manipulate signaling mechanisms of canonical WNT (stabilizing β-catenin), PTHrP (increasing cAMP), and natriuretic peptide (NP, increasing cGMP). WNT and NP promote, and PTHrP antagonizes, hypertrophic differentiation of chondrocytes in the growth plate (Nakayama et al., 2016).
Figure 1.
Suppression of Hypertrophic Chondrocyte Gene Expression during Chondrogenesis from hESC-derived Ectomesenchymal Cells
(A) Graphical representation of the experimental procedure. CDM, chemically defined medium; F, FGF2; Sb, SB431542; PT, PDGF + TGF-β; PTB, PT + BMP4; PTG, PT + GDF5; PT7, PT + BMP7.
(B) BMP4 suppression of COL1 gene (COL1A1) expression during chondrogenesis. Mean relative expression levels from n = 5–9 (PTB) and n = 6 (PT) are shown with SEM as thin vertical lines and p value.
(C) PTHrP suppression of COL10A1 expression without affecting COL2A1 expression. Mean value from n = 4 with SEM and p value. Right: Fold changes in expression by PTHrP treatment. Mean value of the “expression in PTHrP-treated pellets divided by that in untreated pellets” with SEM and p value.
(D) Time-dependent changes in gene expression during chondrogenesis under PTB with (brown) or without (blue) forskolin (Fk) treatment (n = 2). Thin vertical line, SD.
(E) Translucent cartilage pellet formation from H9 hESC-derived ectomesenchymal cells under PTB with or without Fk.
(F) Histological and immunofluorescence staining of the cartilage pellet formed as in (E). Representative results of n = 3. TB, toluidine blue. The yellow arrow indicates the COL1+ area.
(G) Composition of SOX9+ chondrocytes within the cartilage pellets formed under PTBFk from SOX9-GFP hiPSC-derived ectomesenchymal cells (left), analyzed by fluorescence-activated cell sorting (FACS). Those derived from H9 hESCs were used for the non-GFP control (right). Representative results of n = 8. Gene expression data are provided in Figure S3C.
We first tested the effect of PTHrP directly (Figure 1C). Addition of PTHrP(1–34) peptide enhanced the growth of cartilage pellets and suppressed COL10A1 expression without affecting COL2 mRNA (COL2A1) levels during chondrogenesis in the standard medium (PTB), which includes platelet-derived growth factor (PDGF), transforming growth factor (TGF) β3, and BMP4 (Umeda et al., 2012, Umeda et al., 2015). During chondrogenesis, COL2A1 expression was stimulated by TGF-β added from day 6 until the levels plateaued around days 20–30, whereas COL10A1 expression became detectable after BMP4 treatment initiated on day 10 (Figure 1D). Accordingly, to restrict its effect on the BMP-promoted chondrocyte hypertrophic differentiation, we added PTHrP on days 11–12, when COL2A1+COL10A1– immature chondrocytes were being made and BMP signal had just begun.
We further investigated small molecules known to directly elevate cAMP (forskolin), to inhibit canonical WNT signaling (iCRT14 and KY02111), to inhibit all WNT signaling (Verapamil and Wnt-C59), or to inhibit cGMP signaling (KT5824) (Figures S2A–S2C). Initial screening demonstrated that forskolin strongly suppressed (Figure S2A), and Wnt-C59 weakly suppressed (Figure S2C), the expression of COL10A1, without significantly affecting COL2A1 expression and growth of the cartilage pellet. Other agents failed to show a significant effect on COL10A1 expression and were somewhat inhibitory to the growth of cartilage pellets.
Therefore, we focused on cAMP signaling activated by forskolin for further analysis. Forskolin added at 20–30 μM (Figure S2D) during chondrogenesis under PTB reproducibly decreased and delayed the expression of COL10A1 and to a lesser degree RUNX2 (one of the critical COL10A1 transcription factors), without affecting the level and kinetics of expression of the chondrocyte genes COL2A1 and SOX9 (the major COL2A1 transcription factor) (Figure 1D). Immunohistological analysis showed that uniform metachromatic staining by toluidine blue (TB; i.e., uniform sulfated glycosaminoglycan [sGAG] accumulation) overlapped with COL2 immunostaining within the cartilage pellet but that COL1 was only detectable in the layer of cells at the periphery of pellets, more so in those formed without forskolin (Figure 1F). In support, the forskolin-treated cartilage pellets consisted mostly of SOX9+ chondrocytes, assessed as the proportion of GFP+ cells in cartilage generated from SOX9-GFP hiPSC-derived ectomesenchymal cells (mean % SOX9-GFP+ cells, 82.9 ± 11.7 [SEM]; Figure 1G). Thus, although forskolin and PTHrP(1–34) can be inhibitory for chondrogenesis from MSCs (Fischer et al., 2014), no sign of inhibition of chondrogenesis was observed in the hPSC-derived ectomesenchymal cells.
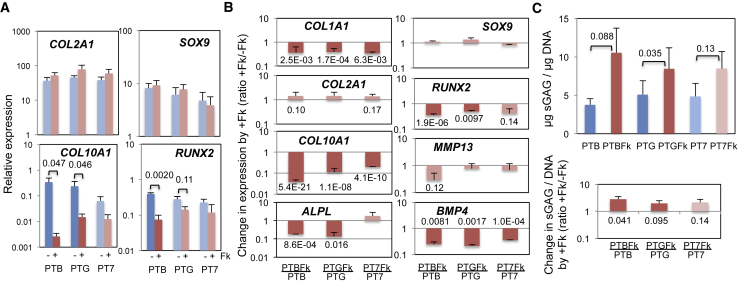
The Suppression of Hypertrophic Chondrocyte Gene Expression and Enhancement of Sulfated Glycosaminoglycan Production by Forskolin Are Not Affected by the Type of BMP Used
GDF5 and BMP7, members of the BMP family, are known to support chondrocyte hypertrophic differentiation only weakly (Caron et al., 2013, Enochson et al., 2014, Hatakeyama et al., 2004). However, under PTG or PT7 conditions, in which BMP4 in PTB is replaced with GDF5 or BMP7, respectively, the expression of COL10A1 and RUNX2 was induced during chondrogenesis to levels not significantly different from those under PTB (blue; Figure 2A). In contrast, forskolin treatment suppressed the expression of both genes (brown): e.g., expression of COL10A1 was suppressed by 98% in PTB (from the relative expression of 0.25 to 0.0045) and 94% in PTG (from 0.26 to 0.015), while that of COL2A1 and SOX9 was not significantly affected. Changes in the expression of individual genes by forskolin treatment (+Fk) are summarized in Figure 2B, which shows the +Fk expression level normalized by the corresponding −Fk level (where −Fk = 1). Among hypertrophy genes, forskolin suppression of COL10A1 expression was statistically significant in all BMP types tested. For the alkaline phosphatase gene (ALPL) and RUNX2, reproducible suppression was observed under PTB and PTG but not under PT7 conditions. Forskolin effects on MMP13 expression were not significant under any of the BMP conditions tested. As expected, changes of the (immature) chondrocyte genes, COL2A1 and SOX9, were weak and statistically insignificant. However, expression of COL1A1 was significantly reduced by forskolin (Figures 2B and S3A), suggesting that it may enhance hyaline chondrogenesis. Forskolin treatment also significantly reduced mRNA levels of BMP4 in all BMP conditions.
Figure 2.
Effect of Different BMPs on the Forskolin Suppression of Hypertrophic Chondrocyte Gene Expression and Forskolin Enhancement of Sulfated Glycosaminoglycan Production
(A) Effect of replacement of BMP4 in PTB (PDGF + TGF-β+BMP4) with GDF5 (PTG) or BMP7 (PT7) on gene expression during chondrogenesis with (brown) or without (blue) forskolin (Fk). Mean relative expression levels from n = 3–5 (COL2A1), 3–6 (SOX9 and COL10A1), and 3 (RUNX2) with SEM and p values. Additional data are provided in Figure S3A.
(B) Fold changes in expression by Fk addition. Mean values from n = 3–12 shown with SEM and p values. Table S7 shows all n and p values.
(C) Quantitative comparison of the capacity of chondrocytes generated under PTB, PTG, and PT7 with (brown) or without (blue) Fk to produce sulfated glycosaminoglycan (sGAG). Top: Mean values of μg sGAG/μg DNA (n = 4–5) shown with SEM and p values. Bottom: Mean fold changes in the μg sGAG/μg DNA values by Fk treatment with SEM and p values (n = 4).
A high level of sGAG is a key indicator of healthy articular cartilage. Therefore, we investigated the effect of forskolin on sGAG accumulation in cartilage pellets. Under both PTB and PTG conditions, forskolin treatment resulted in a significant increase in the capacity of chondrocytes to produce sGAG (Figure 2C). The PT7 condition showed the same tendency but with only a weak statistical significance. Thus, forskolin supports hyaline cartilage formation by suppressing COL1A1 expression and stimulating sGAG production from chondrocytes, consistent with a previous suggestion (Malemud et al., 1986), regardless of the type of BMPs used.
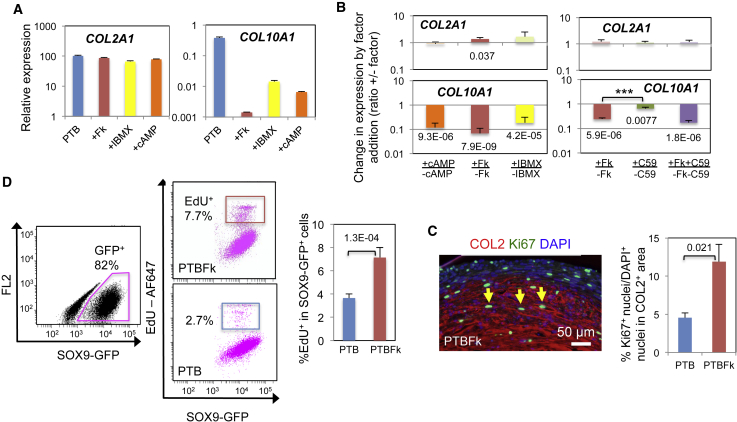
Exogenous cAMP Replaces Forskolin to Suppress COL10A1 Expression during Chondrogenesis
Next, we set out to confirm that the suppressive effect of forskolin on COL10A1 gene expression during chondrogenesis is dependent on the cAMP signaling pathways: i.e., one through PKA and/or another through the exchange protein directly activated by cAMP (EPAC) (Breckler et al., 2011). We tested the effects of the cAMP analogs N6-benzoyl cAMP (Bnz-cAMP) and 8-pCPT-2′-O-Me-cAMP-AM (CPT-cAMP), which preferentially bind and activate PKA and EPAC, respectively (Christensen et al., 2003, Poppe et al., 2008). We also tested the effect of IBMX, an inhibitor of phosphodiesterase, the enzyme that degrades cAMP and cGMP. The PKA-activating Bnz-cAMP significantly inhibited COL10A1 expression during chondrogenesis under PTB (+cAMP; Figures 3A and 3B) in a dose-dependent manner (Figure S4A). IBMX at 0.5 mM also significantly reduced the COL10A1 levels (+IBMX). These treatments had no significant effect on the level of COL2A1 expression (Figures 3A and 3B). In contrast, the EPAC-activating CPT-cAMP reduced the COL10A1 levels, albeit to a much smaller degree (Figure S4A), and the cGMP antagonist Rp-8-Br-PET-cGMPS had no effect on COL10A1 (Figure S4B). These results suggest that suppression by forskolin of COL10A1 expression during chondrogenesis involves the cAMP-PKA pathway.
Figure 3.
Mechanistic Insight into the Effect of Forskolin on the Formation In Vitro of COL2A1+COL10A1lo Cartilage from hESC-Derived Ectomesenchymal Cells
(A) Effects of the cAMP analog, Bnz-cAMP, and PDE-inhibitor, IBMX, on COL2A1 and COL10A1 expression during chondrogenesis. Representative results of n = 3–4. Thin vertical line, SD of three technical repeats. cAMP, 80 μM Bnz-cAMP; IBMX, 0.5 mM. Additional data are provided in Figures S4A and S4B.
(B) Mean fold changes in COL2A1 and COL10A1 expression by addition of a factor during chondrogenesis under PTB (PDGF + TGF-β + BMP4) are shown with SEM and p values. Table S7 shows all n and p values. Factors: cAMP, 100 μM Bnz-cAMP; IBMX, 0.5 mM; C59, 30 nM Wnt-C59. Changes by +Fk (forskolin) versus +C59: ∗∗∗p = 0.0031.
(C and D) Quantitation of proliferating chondrocytes within cartilage pellets formed under PTB with or without Fk.
(C) Left: Immunofluorescence image of Ki67 and COL2 staining of a PTBFk pellet. Right: Mean value of the % Ki67+ nuclei/total (DAPI+) nuclei of the COL2+ area from n = 4 with SEM with p value. The yellow arrow indicates the Ki67+ nucleus. Additional data are provided in Figure S4C.
(D) EdU incorporation. Left: EdU labeled chondrocytes formed from the SOX9-GFP hiPSC-derived ectomesenchymal cells under PTB and PTBFk were FACS analyzed. Representative image of n = 4. The pink dot plots represent cells gated with the pink gate (i.e., SOX9-GFP+ chondrocytes). Right: Mean % EdU+ cells within the SOX9-GFP+ cell population are shown with SEM and p value. Additional results are provided in Figures S4D and S4E.
Effect of Porcupine Inhibitor on the Forskolin-Dependent Decrease in COL10A1 Expression during Chondrogenesis
Even in the presence of forskolin, COL10A1 expression was induced, albeit slowly (only after day 20) and weakly (brown; Figure 1D), resulting in low but variable levels of background expression by day 28 of chondrogenesis (i.e., relative expression of COL10A1 of between 0.0045 and 0.012; Figure 2A). We hypothesized that the hypertrophy-inducing canonical WNT signaling might contribute to such background expression. The weak (<50%) inhibitory effect of Wnt-C59 (Figure S2C) on COL10A1 gene expression observed in the initial screening was reproducible (Figure 3B), consistent with Narcisi et al. (2015). However, addition of Wnt-C59 with forskolin did not significantly enhance the inhibition of COL10A1 expression due to forskolin alone (Figure 3B). Therefore, the residual expression of COL10A1 in forskolin-treated cartilage pellets was probably not supported by endogenous WNT signaling.
Forskolin Promotes Accumulation of Proliferating Chondrocytes within Cartilage Pellets
Forskolin-treated cartilage may maintain COL2A1+COL10A1lo hyaline chondrocytes either by blocking their hypertrophic differentiation or by supporting their proliferation and survival. PTHrP is known to maintain immature chondrocytes in a proliferative state in growth plate (Amizuka et al., 1994, Karaplis et al., 1994). Since forskolin treatment caused a slight enlargement of cartilage pellets (1.31 ± 0.089 [SEM] fold increase in diameter, n = 7, p = 6.2E−04; Figure 1E), we tested whether forskolin mimicked the proliferative effect of PTHrP by immunostaining 30-day-old cartilage pellets with antibodies for Ki67, the protein marker for cell proliferation, and COL2. Forskolin treatment led to a significant increase in the proportion of chondrocytes expressing Ki67 in the cartilage pellets produced under PTB (indicated by the ratio of Ki67+ nuclei/DAPI+ nuclei in the COL2+ area; Figure 3C), although cartilage pellets formed under PTG and PT failed to show statistically significant differences (Figure S4C). Next, we conducted a 5-ethynyl-2′-deoxyuridine (EdU) incorporation study on day 26–28 cartilage pellets. The study demonstrated that addition of forskolin significantly increased the proportion of DNA-replicated chondrocytes (SOX9-GFP+EdU+ cells) within the cartilage pellets formed under PTB from SOX9-GFP hiPSC-derived ectomesenchymal cells (Figures 3D, S4D, and S4E). These results support our hypothesis that forskolin increases the proportion of immature chondrocytes within the formed cartilage by stimulating chondrocyte proliferation.
Forskolin Suppresses COL10A1 Expression without Affecting COL2A1 Expression during Chondrogenesis in Different Cell Systems
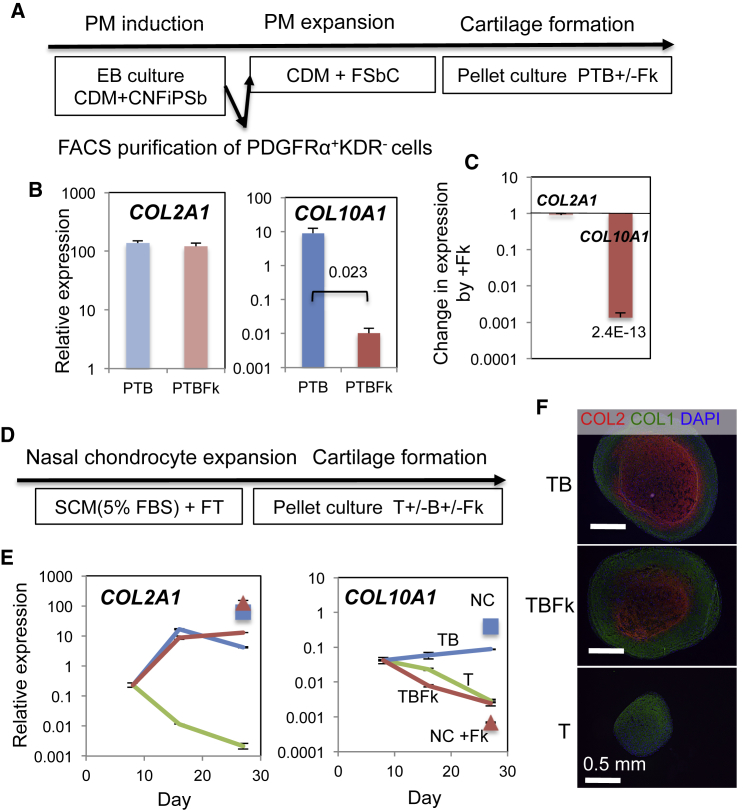
The SOX9-GFP hiPSC-derived ectomesenchymal cells that underwent chondrogenesis under PTB or PTG conditions also showed that the expression of COL10A1 was selectively decreased by forskolin treatment (Figure S3C). In addition, when chondrogenic paraxial mesoderm was derived from hESCs, expanded in a chemically defined medium (CDM) containing fibroblast growth factor (FGF) 2 and SB431542 and CHIR99021 (FSbC condition; Figure 4A) and subjected to pellet chondrogenesis culture under PTB, we observed a similar selective reduction in the COL10A1 expression. The levels of COL2A1 transcript were unchanged (Figures 4B and 4C).
Figure 4.
Effects of Forskolin during Chondrogenesis from hESC-derived Paraxial Mesoderm and from Dedifferentiated Human Adult Nasal Chondrocytes
(A) Graphical representation of the experimental procedure for developing chondrogenic paraxial mesoderm (PM). C, CHIR; N, Noggin; Fi, PD173074; P, PDGF; Sb, SB431542; T, TGFβ3; B, BMP4.
(B and C) Mean relative expression levels of COL2A1 and COL10A1 in cartilage pellets formed under PTB and PTB with forskolin (PTBFk) (B), and mean fold changes in expression of COL2A1 and COL10A1 by Fk treatment (C) are shown with SEM and p value (n = 5).
(D) Graphical representation of the experimental procedure for human nasal chondrocytes. SCM, serum-containing medium; FBS, fetal bovine serum.
(E) COL2A1 and COL10A1 expression during chondrogenesis under T (green) and TB conditions with (TBFk, brown) or without (TB, blue) Fk. Square, cartilage from hESC-ectomesenchymal cells formed under PTB (NC); triangle, cartilage formed under PTBFk (NC + Fk). Thin vertical line, SD of three technical repeats. Results from two other patient samples are shown in Figure S5A.
(F) Immunofluorescence images of the cartilage pellets generated under T, TB, and TBFk conditions. Toluidine blue staining for sGAG is shown in Figure S5B.
Nasal chondrocytes originate from cranial neural crest (i.e., ectomesenchymal cells). Therefore, adult nasal chondrocytes isolated from three patients were propagated (and inevitably dedifferentiated) and subjected to in vitro chondrogenesis as described (Pelttari et al., 2014) (Figures 4D, 4E, and S5A). The low levels of COL2A1 and COL10A1 mRNA detected on days 6–9 of differentiation declined to a near undetectable level (relative expression level of 0.001) by day 28 in the presence of TGFβ3 alone (T, green). However, the addition of BMP4 on day 10 stimulated COL2A1 expression and to a much lesser degree, COL10A1 expression (TB, blue). The addition of forskolin (TBFk, brown) on day 12 selectively knocked down the BMP4-supported COL10A1 expression without affecting the BMP4-enhanced COL2A1 expression. Furthermore, immunohistological analyses supported the finding that in nasal cartilage pellets, the accumulation of COL2 (Figure 4F) and sGAG (Figure S5B) was dependent on BMP4 and independent of forskolin.
Thus, chondrogenesis from human neural-crest-derived ectomesenchymal cells, human paraxial mesoderm-derived chondroprogenitors, and dedifferentiated adult human nasal chondrocytes is sensitive to forskolin treatment, which leads to the suppression of the BMP-stimulated commitment of the developed chondrocytes to hypertrophic differentiation without affecting the enhancement of chondrogenesis by BMP.
Forskolin Treatment Maintains the Expression of Genes Representing “Proliferative” and “Primitive” Stages of Chondrocytes
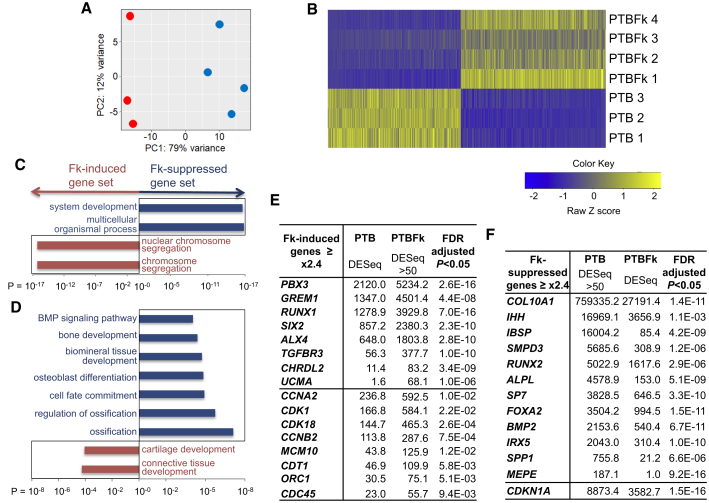
To elucidate the type of cartilage that forskolin forms preferentially and the potential molecular mechanisms involved in the process, we performed genome-wide, comparative transcriptome analyses on the cartilage pellets formed from three to four independent pellet cultures of ectomesenchymal cells under PTB conditions with or without forskolin treatment. RNAs were isolated on day 26–28 and mRNA-sequencing analyses were performed. The principal component analysis and heatmap demonstrated that forskolin-treated cartilage pellets were distinct from forskolin-untreated cartilage pellets (Figures 5A and 5B). Gene ontology (GO) analysis on differentially expressed genes (DEGs, genes showing more than a 2-fold difference in the expression level between forskolin-treated and untreated pellets; Tables S1 and S2) indicated that forskolin treatment during chondrogenesis would likely enhance “(nuclear) chromosome segregation” in the cells, suggesting the enhancement of the cell division cycle of chondrocytes (Figure 5C, Table S1). In support, the forskolin-induced gene set contained various cell-cycle regulator genes, including CCNA2/B2, CDK1/18, and CDC45 (Otto and Sicinski, 2017), and DNA replication genes such as MCM10, ORC1, and CDT1 (Sclafani and Holzen, 2007) (Figure 5E, Table S3). Consistently, the forskolin-suppressed gene set included the p21CIP1 cell-cycle inhibitor gene, CDKN1A (Figure 5F, Table S4).
Figure 5.
Comparative Transcriptome Analysis of Cartilage Pellets Using the RNA-seq Technology
(A) Principal component analysis of the expression pattern of protein-coding genes between PTB (PDGF + TGF-β + BMP4, red) and PTBFk (PTB + forskolin, blue) cartilage pellets.
(B) Heatmap of the differentially expressed genes (DEG).
(C and D) Gene ontology (GO) analysis. GO categories for forskolin (Fk)-induced genes (brown bars) and Fk-suppressive genes (blue bars). (C) Top GO categories (p < 1.0E−17) from Table S1. (D) Skeletogenesis-related GO terms (p < 1.0E−04) from Table S2.
(E and F) Selected lists of Fk-induced genes (E) from Table S3 and Fk-suppressive genes (F) from Table S4.
Furthermore, GO analysis suggested that forskolin treatment during chondrogenesis would promote “cartilage development” and “connective tissue development,” but suppress “ossification,” “osteoblast differentiation,” “biomineral tissue development,” “bone development,” and the “BMP signaling pathway” (Figure 5D, Table S2). In support, the forskolin-induced gene set contained genes associated with chondrogenesis such as RUNX1 (Yoshida and Komori, 2005), PBX3 (Capellini et al., 2006), and TGFBR3, genes involved in nasal cartilage specification such as ALX4 (Beverdam et al., 2001) and SIX2 (He et al., 2010), the mineralization inhibitor gene, UCMA (Surmann-Schmitt et al., 2008), and BMP inhibitor genes such as GREM1 (Leijten et al., 2012) and CHRDL2 (Nakayama et al., 2004) (Figure 5E, Table S3). In contrast, the forskolin-suppressed gene set included inducers of chondrocyte hypertrophy such as RUNX2 (de Crombrugghe and Akiyama, 2009), Osterix/SP7 (Nakashima et al., 2002), and FOXA2 (Ionescu et al., 2012) as well as IHH (Scotti et al., 2010), indicator genes of chondrocyte hypertrophy such as COL10A1 and ALPL, genes associated with (chondrocyte) mineralization and bone formation such as IBSP, SPP1, MEPE (Bonewald et al., 2009), and IRX5 (Askary et al., 2015), and those associated with BMP and its signaling targets such as BMP2 and SMPD3 (Kakoi et al., 2014) (Figure 5F, Table S4). Real-time RT-PCR of some of these genes has confirmed the differential expression (Figure S3B).
These results are strikingly consistent with our previous observations that forskolin treatment keeps the chondrocytes in an immature state by suppressing their commitment to hypertrophic differentiation, probably through enhancement of chondrocyte proliferation (Figures 3C and 3D).
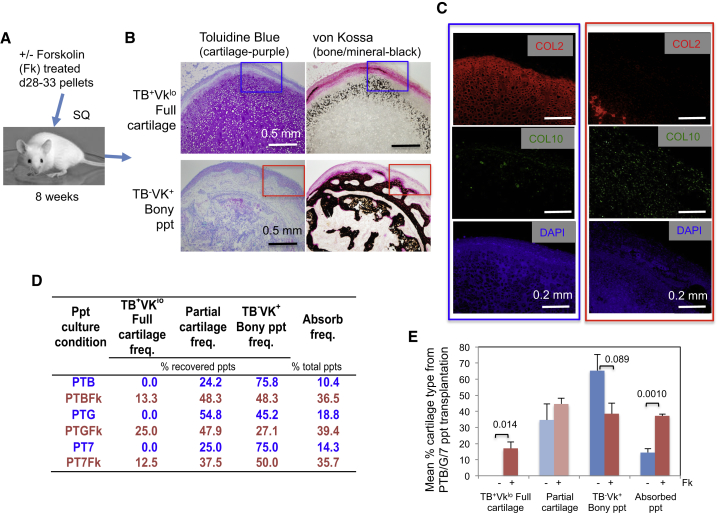
Forskolin-Treated Cartilage Pellets Are Better Maintained in an Unmineralized State In Vivo than Untreated Pellets
Based on a prediction from the report of Scotti et al. (2010), we examined whether the suppression by forskolin of the commitment of chondrocytes to hypertrophic differentiation in cartilage developed in vitro would lead to suppression of post-transplantational mineralization of the cartilage. We subjected ectomesenchymal cells to pellet culture for 28–33 days under PTB, PTG, and PT7 conditions with or without forskolin treatment. The resulting pellets were transplanted subcutaneously into NSG mice, harvested 8 weeks later (Figure 6A), and analyzed immunohistochemically. Metachromatic staining of TB, which indicates the presence of sGAG, would confirm the presence of active chondrocytes, whereas black von Kossa staining (VK), a sign of calcium accumulation would indicate chondrocyte mineralization and bone formation. Figures S6A–S6C classify the types of cartilage pellets recovered. Analyses were focused on the TB+VKlo full-cartilage pellet that maintained cartilaginous matrix (sGAG [TB+] and COL2) with minimal signs of mineralization (VKlo) and COL10 expression, and the TB−VK+ bony pellets that lost sGAG (TB−) and were fully mineralized (VK+) but still contained COL10+ hypertrophic chondrocytes (Figures 6B and 6C).
Figure 6.
In Vivo Stability of Cartilage Pellets Formed from hESC-Derived Ectomesenchymal Cells in the Presence of Forskolin
(A and B) Graphical representation of the experimental procedure and examples of recovered cartilage: near-intact toluidine blue (TB)+, von Kossa (VK)lo full cartilage, and completely mineralized TB−VK+ bony pellet (ppt). The criteria for the different types of cartilage pellets are summarized in Figures S6A–S6C.
(C) Immunofluorescence study of the TB+VKlo full cartilage (blue) and the TB−VK+ bony ppt (red). See Figure S6D.
(D) Mean frequencies (freq.) of full cartilage, partial cartilage, and bony ppt recovery from transplantation experiments using cartilage pellets (ppts) generated under PTB (PDGF + TGF-β + BMP4), PTB + forskolin (PTBFk), PTG (PDGF + TGF-β + GDF5), PTGFk, PT7 (PDGF + TGF-β + BMP7), or PT7Fk conditions. Mean frequencies of absorption are also shown. Figures S7A and S7B shows graphical representations with statistics.
(E) Mean frequencies of full cartilage, partial cartilage, and bony ppt recovery from all three BMP conditions tested (n = 3) with or without Fk treatment are presented with SEM and p values. Cumulative data and analyses are presented in Figures S7C–S7E.
As shown in Figure 6D, the addition of forskolin to PTB-based pellet culture increased the recovery of TB+VKlo full-cartilage pellets from 0% to 13.3% of total recovered pellets (n [independent transplantation] = 9–10, p = 0.18; not statistically significant; Figure S7A), and led to a concomitant decrease in the frequency of TB−VK+ bony pellets from 75.8% to 48.3% (p = 0.050; Figure S7A). Similarly, forskolin addition to PTG culture significantly improved the maintenance in vivo of TB+VKlo cartilage from 0% to 25.0% (n = 7–8, p = 0.023; Figure S7A). Forskolin addition to PT7 culture also improved TB+VKlo cartilage maintenance from 0% to 12.5% (n = 5–6, p = 0.24, not statistically significant; Figure S7A). Interestingly, however, the recoveries of TB+VKlo full cartilage (increased by forskolin, p = 0.014) and TB−VK+ bony pellet (decreased by forskolin, p = 0.089) were surprisingly consistent, regardless of the type of BMP used to promote cartilage pellet formation (Figure 6E). The analysis based on cumulative data led to similar results (Figures S7C–S7E).
In conclusion, while forskolin treatment did not completely inhibit the BMP-promoted commitment of chondrocytes to hypertrophic differentiation during in vitro chondrogenesis from ectomesenchymal cells, it significantly and specifically counteracted such effects of BMP (but not BMP-facilitated hyaline chondrogenesis), and improved the maintenance of cartilage at an unmineralized or less mineralized state in vivo without loss of the capacity to produce cartilage matrix.
Discussion
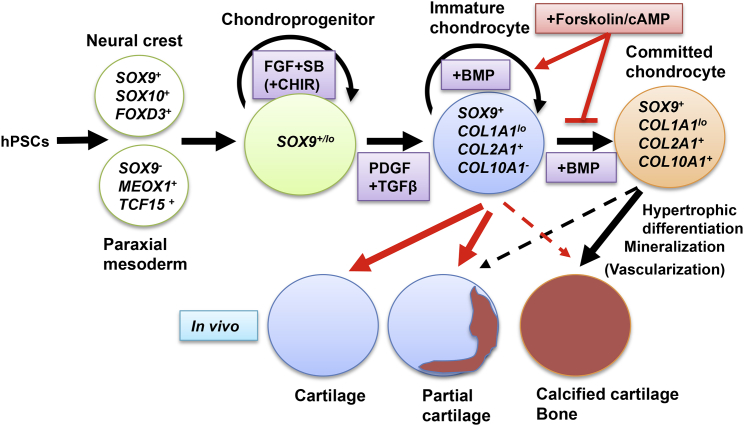
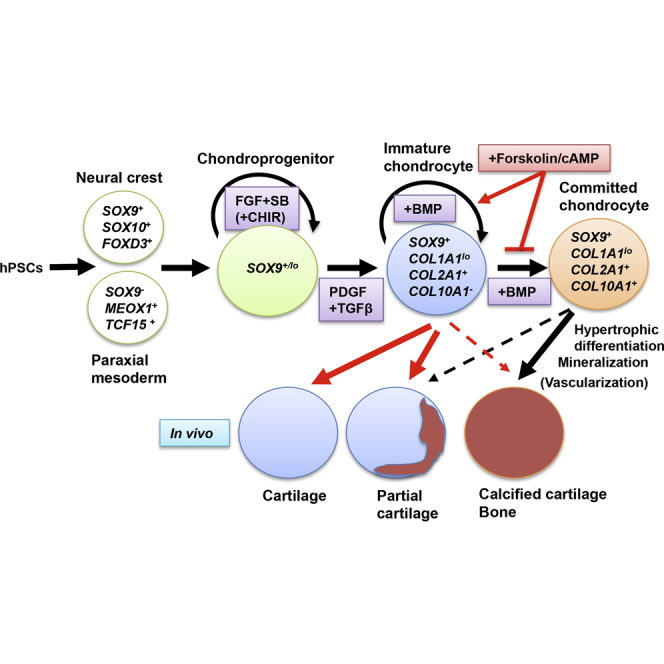
We have demonstrated that the commitment of chondrocytes in cartilage pellets developed in vitro from the hPSC-derived chondroprogenitors to hypertrophic differentiation can be inhibited by forskolin treatment, and such in vitro treatment can have a lasting effect on the fate of the resulting chondrocytes; namely inhibition of further maturation, mineralization, and bone formation, even after transplantation (Figure 7). Furthermore, forskolin achieved this effect, at least in part, by maintaining chondrocytes in a primitive, proliferative state.
Figure 7.
Schematic Representation of the Role of In Vitro Forskolin Treatment on the Pre- and Post-transplantational Fate of Cartilage Developed from hPSCs
We used forskolin as a PTHrP mimic. Attempts to use PTHrP or PTHrP(1–34) peptide to suppress hypertrophic differentiation of chondrocytes developed in vitro from MSCs have thus far yielded contradictory results. In earlier studies, PTHrP enhanced COL2 expression and weakly suppresses COL10 expression (Kim et al., 2008). PTHrP in chondrocyte conditioned medium also suppressed COL10 expression (Fischer et al., 2010). However, in later studies, PTHrP was inhibitory for chondrogenesis and suppressed the expression of both COL2A1 and COL10A1 (Weiss et al., 2010), although intermittent administration of PTHrP(1–34) was found to alleviate the situation to some extent (Fischer et al., 2014). Chondrogenesis from adult MSCs in the presence of TGF-β induced the expression of COL2A1 and COL10A1 simultaneously (Fischer et al., 2014, Weiss et al., 2010). In contrast, chondrogenesis from the hPSC-derived chondroprogenitors showed sequential induction of COL2A1 then COL10A1, so resembling the maturation process of chondrocytes in the growth plate. It is therefore tempting to speculate that our success in the use of forskolin to reproducibly suppress signs of chondrocyte hypertrophic differentiation in vitro without affecting hyaline chondrogenesis may be attributed to the stage-specific administration of forskolin (i.e., after COL2A1 but before COL10A1 induction) used in our hPSC-derived cell system.
BMPs are known to facilitate the hypertrophic differentiation of chondrocytes in vitro at different efficiencies dependent on the BMP used; e.g., GDF5 and BMP7 are not very effective or sometimes inhibitory (Caron et al., 2013, Enochson et al., 2014, Hatakeyama et al., 2004). In fact, BMP7 weakly induced COL10A1 and ALPL expression, and GDF5 induced it to levels between those achieved with BMP7 and BMP4 (Figures 2 and S3A). These differences, however, did not reflect the in vivo stability of developed cartilage pellets, so that no cartilage remained as TB+VKlo full cartilage after 8 weeks regardless of BMP (Figures 6D and S7). In addition, conditions without exogenous BMPs (PT condition) have thus far showed no significant improvements (Figure S7), contrary to the expectation from the previous studies using mesodermal progenitors (Craft et al., 2015). Therefore, the use of no BMP, or a particular type of BMP, could not substitute effectively for forskolin in the promotion of long-lasting cartilage formation from ectomesenchymal cells.
We performed comparative bioinformatics analyses with Wu et al. (2013) human embryonic chondrocyte databases (GEO: GSE51812), but found no significant indications of forskolin-promotion of articular chondrocyte formation in our cultures (Tables S5 and S6), similar to the effects of GREM1, FRZB, and DKK1 on the MSC chondrogenesis (Leijten et al., 2012). However, the GO analysis implicated forskolin suppression of the “BMP signaling pathway” among the most probable biological mechanisms of forskolin (Figure 5D). In fact, we observed that forskolin induced BMP inhibitor gene expression and suppressed BMP signaling gene expression (Figures 2B, 5E, 5F, and S3B, Tables S3 and S4). Since exogenous forskolin and BMPs were not present in vivo, suppression of the intrinsic capacity of cartilage pellets to activate BMP signaling may contribute to the improved maintenance of transplanted cartilage pellets at an unmineralized or less mineralized state for 8 weeks.
Significant fractions of the forskolin-treated cartilage pellets recovered from ectopic transplantation were mineralized (Figures 6, S6, and S7), possibly due in part to preferential absorption of unmineralized cartilage pellets generated in vitro with forskolin. We have noted that forskolin treatment resulted in increased pellet loss, although only the PTB condition gave statistically significant differences: from 10.4% (PTB) to 36.5% (PTBFk) (p = 0.084; Figure S7B). However, the mean increased rate of loss of forskolin-treated pellets was consistent, regardless of the type of BMP used for cartilage pellet formation (p = 0.0010; Figure 6E). Furthermore, the results may be due to the background expression of hypertrophy-inducer genes such as RUNX2, SP7, BMP2, and BMP4, which was not completely suppressed by forskolin. These gene products may be upregulated and become functional after transplantation to promote mineralized cartilage formation. Additional blocking of WNT signaling by a porcupine inhibitor did not significantly improve the in vitro effect (Figure 3B) and in vivo consequence (data not shown) of forskolin treatment. Therefore, manipulation of other mechanisms may be needed.
Thus, one of the advantages of the hPSC-derived chondroprogenitor cell system for generating tissue-engineered cartilage is that the post-transplantational fate of developed chondrocytes can be more robustly controlled at a pre-transplantational stage (i.e., in vitro), compared with the adult MSC systems reported (Fischer et al., 2010, Narcisi et al., 2015, Weiss et al., 2010). Mechanistic studies to understand how these processes work are necessary, not only to improve the efficacy of hPSC-based cartilage regenerative therapy but also to apply the mechanism to the more clinically relevant adult stem cells for better therapeutic outcomes.
Experimental Procedures
Human Pluripotent Stem Cell Culture
H9 (WA09) hESCs from WiCell were maintained on mouse embryonic fibroblast feeder cells. CY2-SOX9-2A-ZsGreen-2A-Puro (SOX9-GFP) hiPSCs from NIH were maintained in E8 medium as described (Umeda et al., 2015). Human PSC experiments were under the regulation of SCRO for the University of Texas Health Science Center at Houston (UTHealth).
Generation and Expansion of Ectomesenchymal Cells from hPSCs through Neural Crest Specification
Human ESCs and iPSCs were differentiated, and the neural crest-like progeny were purified by fluorescence-activated cell sorting (FACS) and expanded as described (Umeda et al., 2015).
Generation and Expansion of Paraxial Mesoderm from hPSCs
Human ESCs were differentiated using the improved embryoid-body-forming culture method (Umeda et al., 2015). Paraxial mesoderm cells were isolated by FACS as described (Umeda et al., 2012), and were then expanded in CDM (Umeda et al., 2015) supplemented with FGF2, PDGF, SB431542, and CHIR99021 (FPSbC medium). At passage 2, PDGF was removed (FSbC).
Expansion of Human Adult Nasal Chondrocytes
Human adult nasal chondrocytes from one female (11F) and two male patients (12M, 13M) were independently isolated and expanded to passage 3 as described (Centola et al., 2013).
Scaffold-free Cartilage Formation: Pellet Culture
Chondrogenesis was induced by pellet culture as described (Umeda et al., 2015). Chondrogenesis from human nasal chondrocytes was performed as described (Pelttari et al., 2014).
Isolation of Chondrocytes from Cartilage Pellets
A cartilage pellet was treated with 4 mg/mL collagenase in the pellet culture medium (Umeda et al., 2015) at 37°C for 3 hr and dissociated to single cells by repetitive pipetting.
Isolation and Quantification of DNA, RNA, and sGAG from Cartilage
DNAs and sGAGs from cartilage pellets were isolated, quantified, and analyzed as described (Umeda et al., 2015).
EdU Incorporation Assay
Cartilage pellets were labeled with EdU for 21–25 hr. Then chondrocytes were isolated and processed using the Click-iT plus EdU Alexa Fluor 647 Kit (Invitrogen) and analyzed by FACS.
Gene Expression Profiling
Real-time RT-PCR experiments were performed as described (Umeda et al., 2015). The results are presented as mean relative expression levels (against EEF1A1) with SEM shown by thin error lines. The change in expression was a relative expression of a gene in treated (+) pellets normalized against that in the corresponding untreated (−) pellets. RNA sequencing (RNA-seq) was performed on an Illumina Nextseq500. Sequenced reads (GEO: GSE116173) were mapped against the human reference genome (hg19). Expression levels were calculated as normalized gene counts from DESeq2 (Figure 5, Tables S1–S4).
Subcutaneous Transplantation of Cartilage Particles
The subcutaneous transplantation was performed as described (Umeda et al., 2015), under the regulation of IACUC for UTHealth.
Immunohistological Staining
The cartilage pellets made in vitro were fixed with Zn-formalin, paraffin embedded, sectioned, and subjected to immunofluorescence detection of COL1, COL2, and Ki67.
Statistical Analysis
Statistical differences between groups were determined by Student's t test (2 categories) or one-way ANOVA(>2 categories) followed by the Student-Newman-Keuls multiple comparisons. n is the number of independent experiments. p < 0.2 values are shown as numbers in the bar graphs. Mean values that give p ≥ 0.1 from the +/− treatment comparisons are shown in light-colored bars.
Note Added in Proof
During revision of this manuscript, Wu et al. (2017) published a report demonstrating that inhibition of κ-opioid receptor/cAMP signaling accelerated the degeneration of injured articular cartilage.
Author Contributions
J.Y.L. performed the initial hPSC-derived ectomesenchymal cell experiments and RT-PCR analyses, analyzed data, and wrote the first draft of the manuscript; N.M. performed immunofluorescence staining and transplantation experiments; A.P. performed cartilage pellet formation and RT-PCR; B.K.A. performed additional ectomesenchymal cell experiments; M.L. made the RNA-seq libraries and performed sequencing; and J.L. and D.S. performed bioinformatics analyses. Y.H. oversaw the RNA-seq and bioinformatics analyses; S.P. and I.M. isolated and expanded human adult nasal chondrocytes; J.H. provided knowledge through regular discussion of experiments and revised the manuscript; and N.N. designed and directed the research, performed additional experiments, analyzed data, and completed the manuscript. N.M. and A.P. contributed equally.
Acknowledgments
We would like to acknowledge A. Hazen and A. Blancas for cell sorting, A. Blancas, Z. Mao, S. Amra, and M. Starbuck for histological analyses, and Z. Mao for immunofluorescence analyses. This work was supported by the Brown Foundation Institute of Molecular Medicine start-up fund (N.N.), the Annie and Bob Graham Distinguished Chair in Stem Cell Biology (N.N.), Cancer Prevention and Research Institute of Texas (RR140053, Y.H.), the John S. Dunn Foundation Collaborative Research Award (Y.H.), the NIH (R01HL134780, Y.H.), and an allocation from the Texas A&M University start-up funds (Y.H. and D.S.).
Published: July 26, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and seven tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.06.021.
Supplemental Information
References
- Amizuka N., Warshawsky H., Henderson J.E., Goltzman D., Karaplis A.C. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J. Cell Biol. 1994;126:1611–1623. doi: 10.1083/jcb.126.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askary A., Mork L., Paul S., He X., Izuhara A.K., Gopalakrishnan S., Ichida J.K., McMahon A.P., Dabizljevic S., Dale R. Iroquois proteins promote skeletal joint formation by maintaining chondrocytes in an immature state. Dev. Cell. 2015;35:358–365. doi: 10.1016/j.devcel.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverdam A., Brouwer A., Reijnen M., Korving J., Meijlink F. Severe nasal clefting and abnormal embryonic apoptosis in Alx3/Alx4 double mutant mice. Development. 2001;128:3975–3986. doi: 10.1242/dev.128.20.3975. [DOI] [PubMed] [Google Scholar]
- Bonewald L.F., Dallas S.L., Gorski J.P. Bone mineralization. In: Pourquié O., editor. The Skeletal System. Cold Spring Harbor Laboratory Press; 2009. pp. 277–295. [Google Scholar]
- Breckler M., Berthouze M., Laurent A.C., Crozatier B., Morel E., Lezoualc'h F. Rap-linked cAMP signaling Epac proteins: compartmentation, functioning and disease implications. Cell Signal. 2011;23:1257–1266. doi: 10.1016/j.cellsig.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Buckwalter J.A., Marsh J.L., Brown T., Amendola A., Martin J.A. Articular cartilage injury. In: Lanza R., Langer R., Vacanti J.P., editors. Principles of Tissue Engineering. Academic Press; 2014. pp. 1253–1266. [Google Scholar]
- Capellini T.D., Di Giacomo G., Salsi V., Brendolan A., Ferretti E., Srivastava D., Zappavigna V., Selleri L. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development. 2006;133:2263–2273. doi: 10.1242/dev.02395. [DOI] [PubMed] [Google Scholar]
- Caron M.M., Emans P.J., Cremers A., Surtel D.A., Coolsen M.M., van Rhijn L.W., Welting T.J. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthritis Cartilage. 2013;21:604–613. doi: 10.1016/j.joca.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Centola M., Abbruzzese F., Scotti C., Barbero A., Vadala G., Denaro V., Martin I., Trombetta M., Rainer A., Marsano A. Scaffold-based delivery of a clinically relevant anti-angiogenic drug promotes the formation of in vivo stable cartilage. Tissue Eng. Part A. 2013;19:1960–1971. doi: 10.1089/ten.tea.2012.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Macica C.M., Nasiri A., Broadus A.E. Regulation of articular chondrocyte proliferation and differentiation by Indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58:3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A.E., Selheim F., de Rooij J., Dremier S., Schwede F., Dao K.K., Martinez A., Maenhaut C., Bos J.L., Genieser H.G. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J. Biol. Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- Craft A.M., Rockel J.S., Nartiss Y., Kandel R.A., Alman B.A., Keller G.M. Generation of articular chondrocytes from human pluripotent stem cells. Nat. Biotechnol. 2015;33:638–645. doi: 10.1038/nbt.3210. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Akiyama H. Transcriptional control of chondrocyte differentiation. In: Pourquié O., editor. The Skeletal System. Cold Spring Harbor Laboratory Press; 2009. pp. 147–170. [Google Scholar]
- Enochson L., Stenberg J., Brittberg M., Lindahl A. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthritis Cartilage. 2014;22:566–577. doi: 10.1016/j.joca.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Fischer J., Dickhut A., Rickert M., Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- Fischer J., Aulmann A., Dexheimer V., Grossner T., Richter W. Intermittent PTHrP(1-34) exposure augments chondrogenesis and reduces hypertrophy of mesenchymal stromal cells. Stem Cells Dev. 2014;23:2513–2523. doi: 10.1089/scd.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Chung U.I., Yang D., Karsenty G., Bringhurst F.R., Kronenberg H.M. PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev. Biol. 2006;292:116–128. doi: 10.1016/j.ydbio.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Hatakeyama Y., Tuan R.S., Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J. Cell. Biochem. 2004;91:1204–1217. doi: 10.1002/jcb.20019. [DOI] [PubMed] [Google Scholar]
- He G., Tavella S., Hanley K.P., Self M., Oliver G., Grifone R., Hanley N., Ward C., Bobola N. Inactivation of Six2 in mouse identifies a novel genetic mechanism controlling development and growth of the cranial base. Dev. Biol. 2010;344:720–730. doi: 10.1016/j.ydbio.2010.05.509. [DOI] [PubMed] [Google Scholar]
- Huang W., Zhou X., Lefebvre V., de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol. Cell. Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Chung U.I., Kronenberg H.M., de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc. Natl. Acad. Sci. USA. 2001;98:160–165. doi: 10.1073/pnas.011393998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu A., Kozhemyakina E., Nicolae C., Kaestner K.H., Olsen B.R., Lassar A.B. FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev. Cell. 2012;22:927–939. doi: 10.1016/j.devcel.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoi H., Maeda S., Shinohara N., Matsuyama K., Imamura K., Kawamura I., Nagano S., Setoguchi T., Yokouchi M., Ishidou Y. Bone morphogenic protein (BMP) signaling up-regulates neutral sphingomyelinase 2 to suppress chondrocyte maturation via the Akt protein signaling pathway as a negative feedback mechanism. J. Biol. Chem. 2014;289:8135–8150. doi: 10.1074/jbc.M113.509331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaplis A.C., Luz A., Glowacki J., Bronson R.T., Tybulewicz V.L., Kronenberg H.M., Mulligan R.C. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Kim H.J., Im G.I. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem. Biophys. Res. Commun. 2008;373:104–108. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- Kozhemyakina E., Cohen T., Yao T.P., Lassar A.B. Parathyroid hormone-related peptide represses chondrocyte hypertrophy through a protein phosphatase 2A/histone deacetylase 4/MEF2 pathway. Mol. Cell. Biol. 2009;29:5751–5762. doi: 10.1128/MCB.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanske B., Karaplis A.C., Lee K., Luz A., Vortkamp A., Pirro A., Karperien M., Defize L.H., Ho C., Mulligan R.C. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Leijten J.C., Emons J., Sticht C., van Gool S., Decker E., Uitterlinden A., Rappold G., Hofman A., Rivadeneira F., Scherjon S. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64:3302–3312. doi: 10.1002/art.34535. [DOI] [PubMed] [Google Scholar]
- Li T.F., Dong Y., Ionescu A.M., Rosier R.N., Zuscik M.J., Schwarz E.M., O'Keefe R.J., Drissi H. Parathyroid hormone-related peptide (PTHrP) inhibits Runx2 expression through the PKA signaling pathway. Exp. Cell Res. 2004;299:128–136. doi: 10.1016/j.yexcr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Malemud C.J., Mills T.M., Shuckett R., Papay R.S. Stimulation of sulfated-proteoglycan synthesis by forskolin in monolayer cultures of rabbit articular chondrocytes. J. Cell. Physiol. 1986;129:51–59. doi: 10.1002/jcp.1041290108. [DOI] [PubMed] [Google Scholar]
- Minina E., Kreschel C., Naski M.C., Ornitz D.M., Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Umeda K. From pluripotent stem cells to lineage-specific chondrocytes: essential signalling and cellular intermediates. In: Atwood C., editor. Embryonic Stem Cells: The Hormonal Regulation of Pluripotency and Embryogenesis. INTECH; 2011. pp. 621–648. [Google Scholar]
- Nakayama N., Han C.Y., Cam L., Lee J.I., Pretorius J., Fisher S., Rosenfeld R., Scully S., Nishinakamura R., Duryea D. A novel chordin-like BMP inhibitor, CHL2, expressed preferentially in chondrocytes of developing cartilage and osteoarthritic joint cartilage. Development. 2004;131:229–240. doi: 10.1242/dev.00901. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Lee J.Y., Matthias N., Umeda K., Yan Q., Huard J. Cartilage regeneration using pluripotent stem cell-derived chondroprogenitors: promise and challenges. In: Tomizawa M., editor. Pluripotent Stem Cells. INTECH; 2016. pp. 385–425. [Google Scholar]
- Namba R.S., Meuli M., Sullivan K.M., Le A.X., Adzick N.S. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J. Bone Joint Surg. Am. 1998;80:4–10. doi: 10.2106/00004623-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Narcisi R., Cleary M.A., Brama P.A., Hoogduijn M.J., Tuysuz N., ten Berge D., van Osch G.J. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Reports. 2015;4:459–472. doi: 10.1016/j.stemcr.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T., Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelttari K., Pippenger B., Mumme M., Feliciano S., Scotti C., Mainil-Varlet P., Procino A., von Rechenberg B., Schwamborn T., Jakob M. Adult human neural crest-derived cells for articular cartilage repair. Sci. Transl. Med. 2014;6:251ra119. doi: 10.1126/scitranslmed.3009688. [DOI] [PubMed] [Google Scholar]
- Pitsillides A.A., Beier F. Cartilage biology in osteoarthritis–lessons from developmental biology. Nat. Rev. Rheumatol. 2011;7:654–663. doi: 10.1038/nrrheum.2011.129. [DOI] [PubMed] [Google Scholar]
- Poppe H., Rybalkin S.D., Rehmann H., Hinds T.R., Tang X.B., Christensen A.E., Schwede F., Genieser H.G., Bos J.L., Doskeland S.O. Cyclic nucleotide analogs as probes of signaling pathways. Nat. Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- Sakamoto A., Chen M., Kobayashi T., Kronenberg H.M., Weinstein L.S. Chondrocyte-specific knockout of the G protein G(s)alpha leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. J. Bone Miner. Res. 2005;20:663–671. doi: 10.1359/JBMR.041210. [DOI] [PubMed] [Google Scholar]
- Sclafani R.A., Holzen T.M. Cell cycle regulation of DNA replication. Annu. Rev. Genet. 2007;41:237–280. doi: 10.1146/annurev.genet.41.110306.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti C., Tonnarelli B., Papadimitropoulos A., Scherberich A., Schaeren S., Schauerte A., Lopez-Rios J., Zeller R., Barbero A., Martin I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somoza R.A., Welter J.F., Correa D., Caplan A.I. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014;20:596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert A.F., Ghivizzani S.C., Rethwilm A., Tuan R.S., Evans C.H., Noth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmann-Schmitt C., Dietz U., Kireva T., Adam N., Park J., Tagariello A., Onnerfjord P., Heinegard D., Schlotzer-Schrehardt U., Deutzmann R. Ucma, a novel secreted cartilage-specific protein with implications in osteogenesis. J. Biol. Chem. 2008;283:7082–7093. doi: 10.1074/jbc.M702792200. [DOI] [PubMed] [Google Scholar]
- Tsumaki N., Nakase T., Miyaji T., Kakiuchi M., Kimura T., Ochi T., Yoshikawa H. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J. Bone Miner. Res. 2002;17:898–906. doi: 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- Umeda K., Zhao J., Simmons P., Stanley E., Elefanty A., Nakayama N. Human chondrogenic paraxial mesoderm, directed specification and prospective isolation from pluripotent stem cells. Sci. Rep. 2012;2:455. doi: 10.1038/srep00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K., Oda H., Yan Q., Matthias N., Zhao J., Davis B.R., Nakayama N. Long-term expandable SOX9(+) chondrogenic ectomesenchymal cells from human pluripotent stem cells. Stem Cell Reports. 2015;4:712–726. doi: 10.1016/j.stemcr.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kraan P.M., van den Berg W.B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Vortkamp A., Lee K., Lanske B., Segre G.V., Kronenberg H.M., Tabin C.J. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Weiss S., Hennig T., Bock R., Steck E., Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J. Cell. Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- Wu L., Bluguermann C., Kyupelyan L., Latour B., Gonzalez S., Shah S., Galic Z., Ge S., Zhu Y., Petrigliano F.A. Human developmental chondrogenesis as a basis for engineering chondrocytes from pluripotent stem cells. Stem Cell Reports. 2013;1:575–589. doi: 10.1016/j.stemcr.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Zhang S., Shkhyan R., Lee S., Gullo F., Eliasberg C.D., Petrigliano F.A., Ba K., Wang J., Lin Y. Kappa opioid receptor signaling protects cartilage tissue against posttraumatic degeneration. JCI Insight. 2017;2:e88553. doi: 10.1172/jci.insight.88553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A., Morioka M., Yahara Y., Okada M., Kobayashi T., Kuriyama S., Matsuda S., Tsumaki N. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports. 2015;4:404–418. doi: 10.1016/j.stemcr.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida C.A., Komori T. Role of Runx proteins in chondrogenesis. Crit. Rev. Eukaryot. Gene Expr. 2005;15:243–254. doi: 10.1615/critreveukargeneexpr.v15.i3.60. [DOI] [PubMed] [Google Scholar]
- Zhang M., Xie R., Hou W., Wang B., Shen R., Wang X., Wang Q., Zhu T., Jonason J.H., Chen D. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J. Cell Sci. 2009;122:1382–1389. doi: 10.1242/jcs.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.