Abstract
IN BRIEF Each year, the American Diabetes Association updates its Standards of Medical Care in Diabetes to inform clinicians on components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. In the 2018 Standards, recommendations related to the use of antihyperglycemic therapy in adults with type 2 diabetes were updated in consideration of new evidence published since the last iteration in 2017. This brief review summarizes key recommendations from the 2018 Standards related to the pharmacologic management of people with type 2 diabetes. In so doing, it additionally highlights drug- and patient-specific factors to consider when intensifying antihyperglycemic therapy.
According to estimates from the Centers for Disease Control and Prevention, 30.3 million people in the United States currently live with diabetes (1). For people living with diabetes, optimization of glycemic control is one of several key treatment approaches central to avoiding or delaying the microvascular and macrovascular complications of the disease. Although lifestyle interventions are integral to any diabetes management plan, this brief review will focus on current recommendations from the American Diabetes Association’s (ADA’s) Standards of Medical Care in Diabetes—2018 (Standards) for the use of antihyperglycemic therapies in the management of people with type 2 diabetes.
Glycemic Goal Setting
Table 1 provides a summary of general glycemic recommendations for non-pregnant adults with diabetes (2). It should be noted that, although general recommendations for A1C, preprandial glucose, and postprandial glucose levels are provided, all glycemic goals should be individualized based on person-specific considerations (2). Factors that may inform glycemic goals for an individual may include risk of hypoglycemia and other adverse drug events, type 2 diabetes disease duration, life expectancy, comorbidity burden, presence of vascular complications, attitudes and treatment expectations of the individual, and resources and support available to implement a given treatment plan (2,3). Ultimately, the ADA recommends that treatment decisions should be made and glycemic goal-setting should occur in collaboration with individual patients whenever possible to incorporate their needs, preferences, and values (2).
TABLE 1.
Summary of Glycemic Recommendations for Many Non-Pregnant Adults With Diabetes
More or less stringent goals may be appropriate for individual patients. Adapted from ref. 2.
Initial Antihyperglycemic Therapy
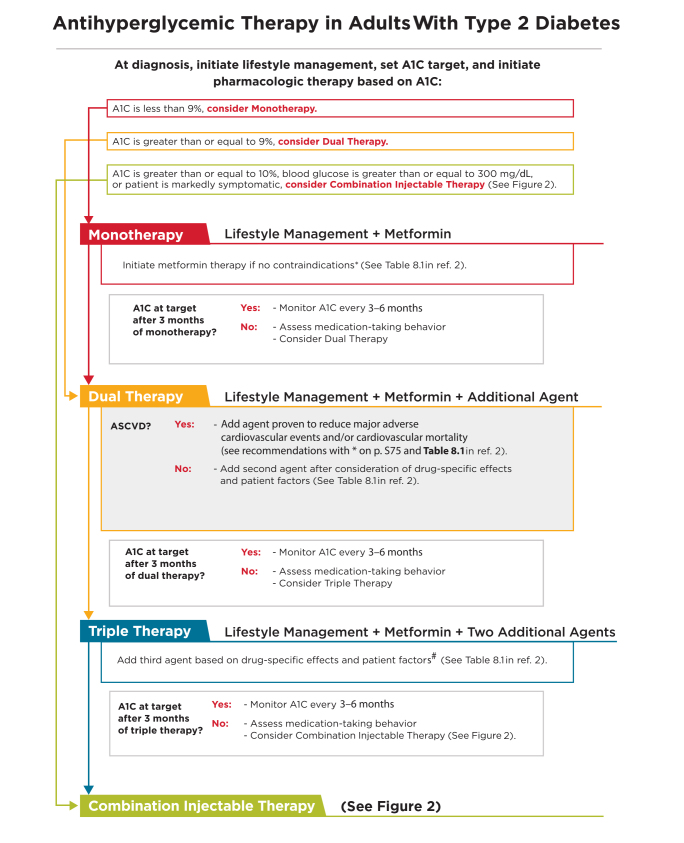
At the time of a diagnosis of type 2 diabetes, the ADA recommends prompt initiation of lifestyle management, determination of appropriate glycemic targets (inclusive of A1C goals), and initiation of pharmacologic therapy (2). Figure 1 provides a summary of general recommendations from the ADA’s Standards of Medical Care in Diabetes—2018 for the use of antihyperglycemic therapy in adults with type 2 diabetes (2). As noted at the top of the figure, the ADA suggests the following initial pharmacologic treatment approaches based on the measured A1C value at the time of diagnosis:
A1C <9.0%: consider antihyperglycemic monotherapy
A1C ≥9.0%: consider antihyperglycemic dual therapy
A1C ≥10.0%, blood glucose ≥300 mg/dL, or patient is markedly symptomatic: consider combination injectable therapy (as outlined in Figure 2)
FIGURE 1.
Antihyperglycemic therapy in adults with type 2 diabetes. *If patient does not tolerate or has contraindications to metformin, consider agents from another class in Table 8.1 of ref. 2. #GLP-1 receptor agonists and DPP-4 inhibitors should not be prescribed in combination. If a patient with ASCVD is not yet on an agent with evidence of cardiovascular risk reduction, consider adding. Reprinted with permission from the American Diabetes Association (2).
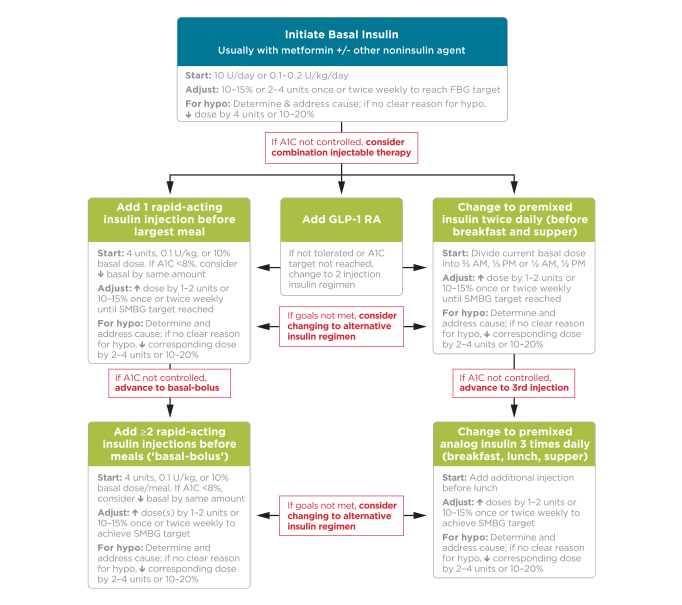
FIGURE 2.
Combination injectable therapy for type 2 diabetes. hypo, hypoglycemia; SMBG, self-monitoring of blood glucose. Reprinted with permission from the American Diabetes Association (2).
It should be noted that, although initial combination injectable therapy (basal-bolus insulin, basal insulin/glucagon-like peptide 1 [GLP-1] receptor agonist, or a premixed insulin formulation) is recommended initially for individuals who have an A1C ≥10.0%, who have a blood glucose level ≥300 mg/dL, or are markedly symptomatic, recently approved fixed-dose basal insulin/GLP-1 receptor agonist combination products are not indicated for use in this situation (4,5). Both currently approved products (insulin degludec/liraglutide and insulin glargine/lixisenatide) are indicated for use in patients who are already receiving one of the agents contained within the fixed-dose combination product (i.e., the insulin degludec/liraglutide product is indicated in people inadequately controlled on basal insulin or liraglutide) (4,5).
Focusing on situations in which monotherapy is indicated, metformin is recommended as the preferred first-line therapy at the time of diagnosis provided there are no contraindications for its use (such as an estimated glomerular filtration rate [eGFR] <30 ml/min/1.73 m2) (2). Metformin is preferred as a first-line agent because of its demonstrated efficacy, safety, low cost, and possible benefits in reducing the risks of cardiovascular events and death (6,7). For patients with a contraindication to or significant intolerance of metformin, initial monotherapy can begin with an agent from another medication class, with the choice of agent determined based on the specific needs and preferences of the individual. Table 2 provides a summary of select drug characteristics that may be considered when selecting an appropriate agent for use in a person with type 2 diabetes. Following are key recommendations provided in the 2018 Standards related to metformin use, along with their evidence grades. (For an explanation of the evidence grades, see the full 2018 Standards [2]).
Metformin, if not contraindicated and if tolerated, is the preferred initial pharmacologic agent for the treatment of type 2 diabetes. A
Long-term use of metformin may be associated with biochemical vitamin B12 deficiency, and periodic measurement of vitamin B12 levels should be considered in metformin-treated patients, especially in those with anemia or peripheral neuropathy. B
Metformin should be continued when used in combination with other agents, including insulin, if not contraindicated and if tolerated. A
In patients with type 2 diabetes with stable congestive heart failure (CHF), metformin may be used if eGFR remains >30 mL/min/1.73 m2 but should be avoided in unstable or hospitalized patients with CHF. B
TABLE 2.
Key Characteristics of Preferred Antihyperglycemic Drugs
| Medication Class | Compound(s) | Efficacy | Hypoglycemia | Weight | Cost | Oral/SQ | Additional Considerations |
|---|---|---|---|---|---|---|---|
| Biguanides | • Metformin | High | No | Neutral | Low | Oral | • Gastrointestinal side effects common |
| • Potential for vitamin B12 deficiency | |||||||
| SGLT2 inhibitors | • Canagliflozin | Intermediate | No | Loss | High | Oral | • Benefit in patients with ASCVD and CHF (canagliflozin and empagliflozin) |
| • Dapagliflozin | • Benefit in progression of DKD (canagliflozin and empagliflozin) | ||||||
| • Empagliflozin | • Amputation and bone fracture risk (canagliflozin) | ||||||
| • Ertugliflozin | • Risk of genitourinary infections, volume depletion, and DKA | ||||||
| GLP-1 receptor agonists | • Dulaglutide | High | No | Loss | High | SQ | • Gastrointestinal side effects (nausea, vomiting, diarrhea) |
| • Exenatide | • Benefit in patients with ASCVD (liraglutide) | ||||||
| • Exenatide ER | • Benefit in progression of DKD (liraglutide) | ||||||
| • Liraglutide | • Potential risk of thyroid C-cell tumors and acute pancreatitis | ||||||
| • Lixisenatide | |||||||
| DPP-4 inhibitors | • Alogliptin | Intermediate | No | Neutral | High | Oral | • Potential risk in CHF (alogliptin and saxagliptin) |
| • Linagliptin | • Potential risk of acute pancreatitis | ||||||
| • Saxagliptin | |||||||
| • Sitagliptin | |||||||
| Thiazolidinediones | • Pioglitazone | High | No | Gain | Low | Oral | • Potential benefit in ASCVD (pioglitazone) • Increased risk in CHF • Risk of bone fracture |
| • Rosiglitazone | |||||||
| Sulfonylureas (second generation) | • Glimepiride | High | Yes | Gain | Low | Oral | |
| • Glipizide | |||||||
| • Glyburide | |||||||
| Insulin | Human insulin | Highest | Yes | Gain | Low | SQ | • Injection site reactions relatively common |
| • Short-acting: regular human insulin | |||||||
| • Intermediate-acting: NPH | • Higher risk of hypoglycemia with human insulin versus insulin analogs | ||||||
| Insulin analogs | High | ||||||
| • Rapid-acting: | |||||||
| Insulin aspart | |||||||
| Insulin glulisine | |||||||
| Insulin lispro | |||||||
| Inhaled human insulin | |||||||
| • Long-acting: | |||||||
| Insulin detemir | |||||||
| Insulin glargine (U-100) | |||||||
| • Ultra-long-acting: | |||||||
| Insulin degludec | |||||||
| Insulin glargine (U-300) |
Adapted from ref. 2 and ref. 3. DKA, diabetic ketoacidosis; DKD, diabetic kidney disease; ER, extended release; SQ, subcutaneous.
As shown in Figure 1, A1C should be measured 3 months after initiation of monotherapy (2). If the individualized A1C goal is not achieved after 3 months of consistent monotherapy, combination therapy should be considered.
Combination Therapy
For individuals requiring dual combination therapy to meet individualized glycemic goals, the agent(s) recommended for add-on to metformin are stratified based on the presence or absence of atherosclerotic cardiovascular disease (ASCVD) (2). For people without ASCVD, the ADA recommends considering a combination of metformin plus another agent from one of the following preferred medication classes: sulfonylurea, thiazolidinedione, dipeptidyl peptidase 4 (DPP-4) inhibitor, sodium–glucose cotransporter 2 (SGLT2) inhibitor, GLP-1 receptor agonist, or basal insulin (2). The choice of which agent to use in combination with metformin should be based on drug- and person-specific considerations. For those with ASCVD, however, the ADA recommends a combination of metformin with an agent proven to reduce major adverse cardiovascular events (MACE) and/or cardiovascular mortality (2). Based on current evidence, the ADA recommends the consideration of liraglutide, empagliflozin, or canagliflozin in such individuals (2). The 2018 Standards include the following specific recommendations for the selection of an agent for add-on to metformin in those requiring additional glucose lowering (2,8–10).
In patients without ASCVD, if monotherapy or dual therapy does not achieve or maintain the A1C goal over 3 months, add an additional antihyperglycemic agent based on drug-specific and patient factors. A
In patients with type 2 diabetes and established ASCVD, antihyperglycemic therapy should begin with lifestyle management and metformin and subsequently incorporate an agent proven to reduce MACE and cardiovascular mortality (currently empagliflozin and liraglutide), after considering drug-specific and patient factors. A
In patients with type 2 diabetes and established ASCVD, after lifestyle management and metformin, the antihyperglycemic agent canagliflozin may be considered to reduce MACE, based on drug-specific and patient factors. C
All three of the recommendations highlighted above make note of the importance of drug-specific and patient factors when selecting an appropriate therapy as add-on to metformin. Factors outlined in Table 2 can be considered when selecting an agent in a given individual (2). Beyond factors such as efficacy, hypoglycemia risk, weight effects, cost, and route of administration, recently published data have highlighted the additional benefits of GLP-1 receptor agonists and SGLT2 inhibitors on cardiovascular and renal outcomes (8–10). For example, in dedicated cardiovascular outcome trials with empagliflozin, liraglutide, and canagliflozin, all were shown to slow the progression of nephropathy, with empagliflozin and canagliflozin showing benefit in preventing hospitalization for heart failure (8–10). Such considerations may drive the use of these agents in a given individual based on the presence of key comorbidities.
Similar to the decision-making process discussed above for transitioning from monotherapy to dual therapy in those not achieving or maintaining glycemic goals, triple combination therapy is recommended for those not achieving or maintaining treatment goals with dual therapy alone (2). If triple therapy is proven insufficient to meet glycemic goals, consideration of combination injectable therapy is recommended.
Combination Injectable Therapy
Figure 2 provides recommendations for the implementation of combination injectable therapy in people with type 2 diabetes (2). The figure, starting at the top, provides some guidance on the initiation and titration of basal insulin, inclusive of a recommended starting dose (10 units/day or 0.1–0.2 units/kg/day) and guidance on titration of the basal insulin to achieve an individualized fasting blood glucose (FBG) target (2). If goal A1C is not met following basal insulin optimization and achieving the target FBG, the addition of injectable agents targeting postprandial glucose excursions is recommended. For this scenario, Figure 2 outlines three recommended combination injectable approaches after basal insulin optimization: 1) the addition of one rapid-acting insulin injection with the largest meal, 2) the addition of a GLP-1 receptor agonist, or 3) transition to a premixed insulin product administered twice daily (2). Although basal insulin in combination with a GLP-1 receptor agonist is associated with less hypoglycemia and the potential for weight loss instead of weight gain when compared to a basal-bolus insulin approach (11,12), these potential benefits must be weighed against the potential for gastrointestinal intolerance and the cost of treatment. It should be noted that if one of these approaches proves to be ineffective or is otherwise not tolerated, changing to an alternative approach might be appropriate.
Moving downward in Figure 2, if initial combination injectable therapy does not result in adequate glycemic control, treatment can be further intensified to include basal insulin in combination with two or more rapid-acting insulin injections per day or the use of a premixed insulin analog three times daily with meals (2). Of note, it is recommended that metformin therapy be continued in patients receiving combination injectable therapy, if not contraindicated and if tolerated, for further glycemic benefits (2).
Conclusion and Key Takeaway Points
A key message of the 2018 Standards is that the care of people with diabetes should be individualized. The recommendations are quite clear that a “one-size-fits-all” approach is not practical or effective when managing people with type 2 diabetes. Accordingly, the recommendations briefly reviewed here provide a framework for clinicians to intensify antihyperglycemic therapy based on individualized needs and treatment goals.
The following are key takeaway messages from the 2018 Standards regarding the pharmacologic approach to glycemic treatment:
Glycemic goals and treatment approaches should be individualized based on the individual’s clinical needs and preferences.
Metformin is recommended as the preferred first-line pharmacologic agent in people with type 2 diabetes unless they have a contraindication or significant intolerance to treatment.
The choice of agent to use in combination therapy for the treatment of type 2 diabetes should be driven by patient- and drug-specific characteristics and considerations and patient preferences.
For people with ASCVD who require combination therapy to meet glycemic goals, the addition of an agent proven to reduce MACE and/or cardiovascular mortality should be considered.
Combination injectable therapy is recommended for patients requiring multiple pharmacological agents to meet glycemic treatment goals.
For additional details and discussion regarding pharmacologic approaches to glycemic management, please refer to the full 2018 Standards (2), available online at professional.diabetes.org/standards.
Duality of Interest
J.J.N. and G.E.U. served on the ADA Professional Practice Committee in 2017 and participated in the writing of the Standards of Medical Care in Diabetes—2018. J.J.N. is also editor-in-chief of Diabetes Spectrum.
Author Contributions
J.J.N. and G.E.U. researched data and wrote the manuscript. J.J.N. is the guarantor of this work and, as such, had full access to all of the references cited and takes responsibility for the accuracy of the content.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017. Estimates of Diabetes and Its Burden in the United States. Available from www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 1 April 2018 [Google Scholar]
- 2.American Diabetes Association Pharmacologic approaches to glycemic treatment. Sec. 8 in Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S73–S85 [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 4.Soliqua [insulin glargine and lixisenatide injection] Prescribing information. Bridgewater, N.J., Sanofi-aventis, October 2017 [Google Scholar]
- 5.Xultophy 100/3.6 [insulin degludec and liraglutide injection] Prescribing information. Plainsboro, N.J., Novo Nordisk, November 2016 [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 7.Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–751 [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 10.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamant M, Nauck MA, Shaginian R, et al.; 4B Study Group . Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care 2014;37:2763–2773 [DOI] [PubMed] [Google Scholar]
- 12.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 2014;384:2228–2234 [DOI] [PubMed] [Google Scholar]