Abstract
In Brief In February 2017, the American Association of Clinical Endocrin-ologists and the American College of Endocrinology published updated “Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease.” The update encompassed recent important clinical trial outcomes and additional research related to the treatment of dyslipidemia. This article summarizes key recommendations from this important guideline.
In February 2017, the American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) published updated “Guidelines for the Management of Dyslipidemia and Prevention of Cardiovascular Disease” (1). The update encompassed recent important clinical trial outcomes and additional research related to the treatment of dyslipidemia as identified by the 16-member AACE/ACE writing committee. The update retains LDL cholesterol treatment goals, which are supported by clinical trials and have been useful to both clinicians and patients. However, for the first time, it extends an LDL cholesterol goal to <55 mg/dL for patients who are at “extreme risk.”
The updated guidelines include 87 graded recommendations and 695 evidence-ranked references. Fifteen clinically related questions are addressed by the recommendations and supported by the evidence base (Table 1). The complete AACE/ACE guidelines, including both an executive summary and a comprehensive evidence base, are available from www.aace.com/files/lipid-guidelines.pdf. The remainder of this article summarizes key points from the guidelines.
TABLE 1.
Specific Clinical Questions Guiding the AACE/ACE Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease (1)
| The following questions form the basis of the 87 recommendations included in the AACE/ACE clinical practice guidelines: 1. What are risk factors for ASCVD? 2. What risk categories does AACE/ACE recommend? 3. How is risk assessed? 4. How does type 1 diabetes affect risk? 5. Who should be screened for ASCVD risk and when? 6. What are secondary causes of dyslipidemia? 7. Which screening tests should be used? 8. What are lipid treatment goals? 9 What treatments are available for dyslipidemia? 10. What special considerations should be given to women? 11. What special considerations should be given to children and adolescents? 12. How does treatment of dyslipidemia affect ASCVD risk? 13. How are different drug categories used to treat dyslipidemia? 14. How should treatment be monitored? 15. Is the treatment of dyslipidemia and prevention of ASCVD cost-effective? |
Assessing Risk
The 10-year risk of a coronary event should be determined by assessment using one or more of the following tools: 1) the Framingham Risk Assessment Tool, 2) the Reynolds Risk Score, 3) the Multi-Ethnic Study of Atherosclerosis (MESA) 10-year ASCVD (atherosclerotic cardiovascular disease) Risk with Coronary Artery Calcification (CAC) Calculator, and 4) the UK Prospective Diabetes Study (UKPDS) Risk Engine for patients with type 2 diabetes (1).
Although each of these tools can be used to predict 10-year risk, the MESA risk score is emerging as the preferred tool using traditional risk factors and CAC to predict 10-year coronary heart disease (CHD) risk. The incorporation of CAC into this risk score significantly improves risk prediction in patients with or without traditional risk factors and in patients with a family history of premature CHD. CAC is also the strongest predictor of cardiovascular disease (CVD) in low-risk patients (1–4).
Using a 10-year risk assessment tool is useful in patients with diabetes when clinical features do not clearly indicate high, very high, or extreme risk status (Table 2).
TABLE 2.
AACE/ACE ASCVD Risk Categories and LDL Cholesterol Treatment Goals
| Risk Category | Risk Factors/10-Year Risk | Treatment Goals | ||
|---|---|---|---|---|
| LDL Cholesterol (mg/dL) | Non-HDL Cholesterol (mg/dL) | Apo B (mg/dL) | ||
| Extreme risk | • Progressive ASCVD, including unstable angina in individuals after achieving an LDL cholesterol <70 mg/dL • Established clinical CVD in individuals with diabetes, stage 3 or 4 CKD, or HeFH • History of premature ASCVD (age <55 years male, <65 years female) |
<55 | <80 | <70 |
| Very high risk | • Established or recent hospitalization for ACS or coronary, carotid, or peripheral vascular disease; 10-year risk >20% • Diabetes or stage 3 or 4 CKD with one or more risk factor • HeFH |
<70 | <100 | <80 |
| High risk | • Two or more risk factors and 10-year risk 10–20% • Diabetes or stage 3 or 4 CKD with no other risk factors |
<100 | <130 | <90 |
| Moderate risk | Two or fewer risk factors and 10-year risk <10% | <100 | <130 | <90 |
| Low risk | Zero risk factors | <130 | <160 | NR |
NR, not recommended.
Extreme Risk
A new “extreme risk” category is introduced for the first time as an ASCVD risk category for patients considered at risk beyond “very high risk” (1). This was based on recent clinical trial outcome data (from Improved Reduction of Outcomes: Vytorin Efficacy International Trial [IMPROVE-IT] [5] and Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk [FOURIER] [6]) and large supporting meta-analyses such as the Cholesterol Treatment Trialists’ Collaboration 2010 (7), which revealed that further lowering of LDL cholesterol beyond prior recommendations produced better outcomes in individuals with CVD. IMPROVE-IT demonstrated lower rates of cardiovascular events, primarily in patients with diabetes and acute coronary syndrome (ACS), when LDL cholesterol levels were lowered to 53 mg/dL when combining ezetimibe with simvastatin compared to simvastatin alone, where an average LDL cholesterol of 69 mg/dL was achieved (5). The FOURIER trial validated this category by demonstrating a no-threshold continuum of CVD event reduction by achieving an average LDL cholesterol of 30 mg/dL in patients treated with evolocumab and high-intensity statin compared to those treated with high-intensity statin alone. At 26 months, myocardial infarction (MI) was reduced by 27%, stroke by 21%, and coronary revascularization by 22% in the evolocumab-treated patients (6). Patients with extreme risk, which includes patients with diabetes and clinical ASCVD, have an LDL cholesterol treatment goal of <55 mg/dL. Patients with diabetes may be at high, very high, or extreme risk (1).
Patients with clinical ASCVD and stage 3 or 4 chronic kidney disease (CKD) or heterozygote familial hypercholesterolemia (HeFH) are at extreme risk (1). Growing evidence suggests that individuals with CKD, who comprise a growing population, have increased risk for ASCVD (8). It appears that the increased risk of ASCVD does not occur only in individuals with end-stage renal disease, but also in those with mild to moderate chronic renal dysfunction. These findings led the National Kidney Foundation to consider CKD as an ASCVD equivalent (9).
Familial hypercholesterolemia (FH) is caused by genetic mutations passed on by one parent (HeFH) or both parents (homozygous FH [HoFH]) (10). HoFH prevalence ranges from 1 in 160,000 to 1 in 250,000. Individuals with HoFH have extremely high LDL cholesterol levels (>500 mg/dL) and premature cardiovascular risk. Many with HoFH experience their first coronary event in childhood or adolescence. HeFH prevalence ranges from 1 in 200 to 1 in 250 (11,12). Individuals with HeFH can present with LDL cholesterol levels of 90–500 mg/dL and have premature cardiovascular risk. On average, individuals with HeFH experience their first coronary event at the age of 42 years (∼20 years younger than the general population). Early treatment is recommended for all individuals with FH, with a goal of reducing LDL cholesterol levels by 50% from baseline (13) (Table 2).
ASCVD Risk Categories and LDL Cholesterol Treatment Goals
LDL cholesterol treatment goals, which should be personalized according to levels of risk, and CVD risk categories are as follows (1).
For individuals at low risk (i.e.,with no risk factors), an LDL cholesterol goal <130 mg/dL (non-HDL cholesterol <160 mg/dL) is recommended.
For individuals at moderate risk (i.e., with two or fewer risk factors and a calculated 10-year risk <10%), an LDL cholesterol goal <100 mg/dL (non-HDL cholesterol [total cholesterol minus HDL cholesterol] <130 mg/dL, apolipoprotein [apo] B <90 mg/dL) is recommended.
For individuals at high risk (i.e., those with an ASCVD equivalent including diabetes or stage 3 or 4 CKD with no other risk factors or those with two or more risk factors and a 10-year risk of 10–20%), an LDL cholesterol goal <100 mg/dL (non-HDL cholesterol <130 mg/dL, apo B <90 mg/dL) is recommended.
For individuals at very high risk (i.e., with established or recent hospitalization for ACS; coronary, carotid, or peripheral vascular disease; diabetes or stage 3 or 4 CKD with one or more risk factor(s); a calculated 10-year risk >20%; or HeFH), an LDL cholesterol goal <70 mg/dL (non-HDL cholesterol <100 mg/dL, apo B <80 mg/dL) is recommended.
For individuals at extreme risk (i.e., with progressive ASCVD, including unstable angina that persists after achieving an LDL cholesterol <70 mg/dL or established clinical ASCVD in individuals with diabetes, stage 3 or 4 CKD, and/or HeFH, or in individuals with a history of premature ASCVD [age <55 years for males or <65 years for females]), an LDL cholesterol goal <55 mg/dL (non-HDL cholesterol <80 mg/dL, apo B <70 mg/dL) is recommended.
HDL Cholesterol, non-HDL Cholesterol, Apo B, and Triglycerides Treatment Goals
HDL cholesterol should be >40 mg/dL, but also as high as possible, primarily through the use of lifestyle interventions (e.g., weight loss, physical activity, and tobacco cessation) and, if risk factors are present (e.g., borderline elevated LDL cholesterol levels, a family history of premature ASCVD, or a personal history of ASCVD), also through the use of pharmacotherapy primarily focused on reducing LDL cholesterol (1).
For most individuals, a non-HDL cholesterol goal 30 mg/dL higher than the individual’s specific LDL cholesterol goal is recommended. For individuals at extreme risk, a non-HDL cholesterol goal 25 mg/dL higher than the individual-specific LDL cholesterol goal is recommended (1).
For individuals at increased risk of ASCVD, including those with diabetes, an optimal apo B goal is <90 mg/dL, whereas for individuals with established ASCVD or diabetes plus one or more additional risk factor(s), an optimal apo B goal is <80 mg/dL, and for individuals at extreme risk, an optimal apo B goal is <70 mg/dL (1).
A triglyceride goal <150 mg/dL is recommended. Lowering triglycerides when significantly elevated is important in reducing the risk for pancreatitis. Hypertriglyceridemia usually identifies insulin resistance, and evidence suggests that because hypertriglyceridemia is closely associated with highly atherogenic lipoprotein particles, it is an independent risk factor for ASCVD (1,14).
Screening for ASCVD Risk
Screening guidelines for dyslipidemia vary by age-group; however, the decision to screen should always be based on clinical judgment. Specific indications exist to alert health care providers to conduct screenings.
Children and adolescents: In children at risk for FH, screen at 3 years of age, again between the ages of 9 and 11 years, and at 18 years of age. Screen adolescents >16 years of age every 5 years or more frequently if they have ASCVD risk factors (1).
Young adults (men 20–45 years and women 20–55 years of age): Evaluate all adults ≥20 years of age for dyslipidemia every 5 years as part of a global risk assessment (1).
Middle-aged adults (men 45–65 and women 55–65 years of age): In the absence of ASCVD risk factors, screen for dyslipidemia every 1–2 years. More frequent lipid testing is recommended when multiple ASCVD risk factors are present. The frequency of lipid testing should be based on individual clinical circumstances (1).
Adults with diabetes: Annually screen all adults with type 1 or type 2 diabetes for dyslipidemia (1).
Older adults: Annually screen older adults with zero or one ASCVD risk factors for dyslipidemia. Patients with multiple ASCVD risk factors should undergo lipid assessment. The frequency of lipid testing in older adults should be based on individual clinical circumstances (1).
FH: Screen for FH when there is a family history of premature ASCVD (<55 years of age in father or other first-degree male relative or <65 years of age in mother or other first-degree female relative) or in individuals with elevated lipid levels consistent with FH (1).
Screening Tests
A fasting lipid profile ensures the most precise lipid assessment; this should include total cholesterol, LDL cholesterol, triglycerides, and non-HDL cholesterol. Lipids, including triglycerides, can be measured in the non-fasting state if fasting determinations are impractical (1).
LDL cholesterol may be estimated using the Friedewald equation (Eq. 1). However, this method is valid only for values obtained during the fasting state, becomes increasingly inaccurate when triglyceride levels are >200 mg/dL, and becomes invalid when triglyceride levels are >400 mg/dL. LDL cholesterol should be directly measured in certain high-risk individuals such as those with fasting triglyceride levels >250 mg/dL and those with diabetes or known vascular disease. Measurement of HDL cholesterol should be included in screening tests for dyslipidemia.
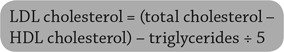 |
EQ. 1 |
Non-HDL cholesterol should be calculated to assist in risk stratification in individuals with moderately elevated triglycerides (200–500 mg/dL), diabetes, and/or established ASCVD. If insulin resistance is suspected, non-HDL cholesterol should be evaluated to gain useful information regarding the individual’s total atherogenic lipoprotein burden.
Triglyceride levels also should be part of routine lipid screening. Moderate elevations (≥150 mg/dL) may identify individuals at risk for insulin resistance syndrome, and levels ≥200 mg/dL may identify individuals at substantially increased ASCVD risk (1).
Apo B and/or an apo B/apo A1 ratio calculation and evaluation may be useful in at-risk individuals (those with triglycerides ≥150 mg/dL, HDL cholesterol <40 mg/dL, prior ASCVD event, type 2 diabetes, and/or insulin resistance syndrome even when at target LDL cholesterol levels) to assess residual risk and guide decision-making. Apo B measurements (reflecting the particle concentration of LDL and all other atherogenic lipoproteins) can be useful to assess and affirm the success of LDL cholesterol–lowering therapy (1,15,16).
Additional Screening Tests
CAC measurement has been shown to be of high predictive value and is useful in refining risk stratification to determine the need for more aggressive treatment strategies. CAC measurements are not necessary routinely in patients with diabetes because, in most instances, diabetes is considered a CVD equivalent and risk stratification is not required. However, in individual circumstances in which risk is unclear in a person with diabetes, assessing CAC can be useful. In the short term in people with diabetes, the absence of calcified plaque appears to have the same predictive value as in individuals without diabetes.
High-sensitivity C-reactive protein(hsCRP) can help stratify ASCVD risk in individuals with a standard risk assessment that is borderline and in those with an intermediate or higher risk with an LDL cholesterol concentration <130 mg/dL (1).
Lipoprotein-associated phospho-lipase A2, which in some studies has demonstrated more specificity than hsCRP, may be measured when necessary to further stratify ASCVD risk, especially when hsCRP is elevated (1).
Lipoprotein (a) assessment is not generally recommended because of wide variability in populations and a lack of standardization, although it may provide useful information to ascribe risk in Caucasians with ASCVD and in those with an unexplained family history of early ASCVD, as well as in those with unknown family history, such as adopted individuals (1).
Because the benefits of routine measurement of homocysteine, plasminogen activator inhibitor-1, or other inflammatory markers have not been demonstrated, these assessments are not recommended (1).
Carotid intima media thickness assessment may be considered in certain individuals to refine risk stratification and determine the need for more aggressive ASCVD preventive strategies (1).
Diabetes as a Risk Factor
Type 2 Diabetes
Based on several epidemiological studies, individuals with type 2 diabetes should be considered to be at high, very high, or extreme risk for ASCVD (1). Approximately 65% of diabetes-related mortality is due to heart disease and cerebrovascular accident (CVA). Compared to individuals who do not have diabetes, individuals with type 2 diabetes have a significantly increased risk of ASCVD. For example, individuals with diabetes plus a previous MI have been shown to have a 2.5-fold greater risk of subsequent ASCVD events than individuals with ASCVD but no diabetes (17,18). Epidemiological data from Finland similarly suggest that individuals with type 2 diabetes and no history of MI have cardiovascular risk (for fatal MI, nonfatal MI, CVA, or overall cardiovascular mortality) equivalent to those without diabetes and a history of MI. This same study found that individuals with type 2 diabetes and previous MI were at the highest risk, with a 7-year incidence of fatal or nonfatal MI of 45% (19). The Emerging Risk Factors Collaboration is a larger, more recent study showing similar evidence of diabetes as a cardiovascular risk equivalent (20). Although diabetes is a dominant CVD risk factor, the dyslipidemia associated with diabetes is characterized by elevated triglycerides, low HDL cholesterol, and normal, high-normal, or mildly elevated LDL cholesterol levels (1).
Type 1 Diabetes
Based on epidemiological and prospective cohort studies, individuals with type 1 diabetes, including late-onset diabetes (at >30 years of age) and duration >15 years or with two or more major CVD risk factors (e.g., albuminuria, stage 3 or 4 CKD, and initiation of intensive control >5 years after diagnosis), poorly controlled A1C, or insulin resistance with metabolic syndrome and an hsCRP >3.0 mg/dL should be considered to have a risk equivalent to that of people with type 2 diabetes (1).
Treatment Recommendations
A comprehensive strategy to control lipid levels and address associated metabolic abnormalities and modifiable risk factors is recommended, primarily using lifestyle changes and patient education, with pharmacotherapy as needed to achieve evidence-based targets (1).
1. Physical Activity
A reasonable and feasible approach to fitness therapy (i.e., exercise programs that include at least 30 minutes of moderate-intensity physical activity [consuming 4–7 kcal/min] four to six times weekly, with an expenditure of at least 200 kcal/day) is recommended. Suggested activities include brisk walking, riding a stationary bike, water aerobics, cleaning/scrubbing, mowing the lawn, and sporting activities (1).
Daily physical activity goals can be met in a single session or in multiple sessions throughout the course of a day (10-minute minimum per session). For some individuals, breaking activity up throughout the day may help improve adherence with physical activity programs. In addition to aerobic activity, muscle-strengthening activity is recommended at least 2 days per week (1).
2. Medical Nutrition Therapy
For adults, a reduced-calorie diet consisting of fruits and vegetables (combined five or more servings/day), grains (primarily whole grains), fish, and lean meats is recommended. For adults, the intake of saturated fats, trans fats, and cholesterol should be limited. LDL cholesterol–lowering macronutrient intake should include plant stanols/sterols (∼2 g/day) and soluble fiber (10–25 g/day). Primary preventive nutrition consisting of healthy lifestyle habits is recommended in all healthy children (1).
3. Smoking Cessation
Smoking cessation should be strongly encouraged and facilitated.
Pharmacologic Therapy
In individuals at risk for ASCVD, aggressive pharmacological lipid-modifying therapy is recommended to achieve appropriate LDL cholesterol goals (Table 3) (1).
TABLE 3.
Primary Lipid-Lowering Drug Classes
| Drug Class | Metabolic Effect* | Main Considerations† |
|---|---|---|
| HMG-CoA reductase inhibitors (statins: lovastatin, pravastatin, fluvastatin, simvastatin, atorvastatin, rosuvastatin, pitavastatin) | • Primarily ↓ LDL-C 21–55% by competitively inhibiting rate-limiting step of cholesterol synthesis in the liver, leading to upregulation of hepatic LDL receptors • Effects on TG and HDL-C are less pronounced (↓ TG 6–30% and ↑ HDL-C 2–10%) |
• Liver function test before therapy and as clinically indicated thereafter • Myalgias and muscle weakness in some individuals • Potential for drug–drug interaction between some statins and cytochrome P450 3A4 inhibitors, cyclosporine, warfarin, and protease inhibitors • Myopathy/rhabdomyolysis in rare cases; increased risk with coadministration of some drugs (see product labeling) • Simvastatin dosage should not exceed 40 mg in most individuals; dosage of 80 mg is no longer recommended except in those who have tolerated 80 mg for ≥12 months without muscle toxicity • Do not exceed 20 mg simvastatin daily with amlodipine or ranolazine • Plasma elevations of rosuvastatin may be higher among Asian people than other ethnic groups • New-onset diabetes is increased in individuals treated with statins; however, it is dose-related, occurs primarily in individuals with metabolic syndrome, appears to be less common with pravastatin and possibly pitavastatin, and occurs overall to a lesser extent than the associated decrease in ASCVD |
| Cholesterol absorption inhibitors (ezetimibe) | • Primarily ↓ LDL-C 10–18% by inhibiting intestinal absorption of cholesterol and decreasing delivery to the liver, leading to upregulation of hepatic LDL receptors • ↓ Apo B 11–16% • In combination with statins, additional ↓ in LDL-C 25%; total ↓ LDL-C 34–61% • In combination with fenofibrate, ↓ LDL-C 20–22% and↓ apo B 25–26% without reducing ↑ HDL-C |
• Myopathy/rhabdomyolysis (rare) • When co-administered with statins or fenofibrate, risks associated with those drugs remain (e.g., myopathy/ rhabdomyolysis and cholelithiasis) |
| PCSK9 inhibitors (alirocumab, evolocumab) | • ↓ LDL-C 48–71%, ↓ non-HDL-C 49–58%, ↓ TC 36–42%, ↓ apo B 42–55% by inhibiting PCSK9 binding with LDLRs, increasing the number of LDLRs available to clear LDL, and lowering LDL-C levels | • Requires subcutaneous self-injection; refrigeration is generally needed • Adverse reactions resulted in discontinuation in 2.2% overall (1.2% more than placebo) for evolocumab and 5.3% overall (0.2% more than placebo) for alirocumab; overall levels of adverse reactions and discontinuation are very low • Adverse reactions with significantly different rates between drug and placebo were: local injection site reactions (1.9% greater for alirocumab vs. placebo, 0.7% greater for evolocumab vs. placebo) and influenza (1.2% greater for alirocumab vs. placebo, 0.2% for evolocumab vs. placebo) • The most common adverse reactions with similar rates for drug versus placebo were for: • Alirocumab (4–12%; most common to least common: nasopharyngitis, influenza, urinary tract infections, diarrhea, bronchitis, and myalgia) • Evolocumab (2–4%; most common to least common: nasopharyngitis, back pain, and upper respiratory tract infection) |
| Fibric acid derivatives (gemfibrozil, fenofibrate, fenofibric acid) | • Primarily ↓ TG 20–35%, ↑ HDL-C 6–18% by stimulating lipoprotein lipase activity • Fenofibrate may ↓ TC and LDL-C 20–25% • Lower VLDL-C and LDL-C; reciprocal rise in LDL-C transforms the profile into a less atherogenic form by shifting fewer LDL particles to larger size • Fenofibrate ↓ fibrinogen level |
• Gemfibrozil may ↑ LDL-C 10–15% • Gastrointestinal symptoms, possible cholelithiasis • May potentiate effects of orally administered anticoagulants • Gemfibrozil may ↑ fibrinogen level‡ • Gemfibrozil and fenofibrate can ↑ homocysteine independent of vitamin concentrations§ • Myopathy/rhabdomyolysis when used with statin (uncommon with gemfibrozil, but increased risk with all statins except fluvastatin); interaction less likely with fenofibrate or fenofibric acid (no apparent difference by statin) • Fibrates are associated with increased serum creatinine levels, which may not reflect renal dysfunction; fenofibrate dose should be cut by two-thirds and gemfibrozil by one-half when eGFR is 15–60 mL/min per 1.73 m2, and fibrates should be avoided when eGFR is <15 mL/min per 1.73 m2 • May cause muscle disorders • Can improve diabetic retinopathy |
| Niacin (nicotinic acid) | • ↓ LDL-C 10–25%, ↓ TG 20–30%, ↑ HDL-C 10–35% by decreasing hepatic synthesis of LDL-C and VLDL-C • ↓ Lipoprotein (a) • Transforms LDL-C to less atherogenic form by increasing average particle size and also decreases LDL particle concentration |
• Potential for frequent skin flushing, pruritus, abdominal discomfort, hepatoxicity (rare but may be severe), nausea, peptic ulcer, atrial fibrillation • Deleterious effect on serum glucose at higher dosages • Increases uric acid levels; may lead to gout |
| Bile acid sequestrants (cholestyramine, colestipol, colesevelam hydrochloride) | • Primarily ↓ LDL-C 15–25% by binding bile acids and preventing their reabsorption in the ileum (causing hepatic cholesterol depletion and LDL receptor upregulation) • Colesevelam ↓ glucose and A1C (∼0.5%); FDA-approved to treat type 2 diabetes |
• May ↑ serum TG • Frequent constipation and/or bloating, which can reduce adherence • Many potential drug interactions (decreased drug absorption); less so with colesevelam (see product labeling) May reduce absorption of folic acid and fat-soluble vitamins such as vitamins A, D, and K |
| MTP inhibitor (lomitapide) | • ↓ LDL-C up to 40%, TC 36%, apo B 39%, TG 45%, and non-HDL-C 40% (depending on dose) in individuals with HoFH by binding and inhibiting MTP, which inhibits synthesis of chylomicrons and VLDL | • Can cause increases in transaminases (ALT, AST); monitoring of ALT, AST, alkaline phosphatase, and total bilirubin before initiation and of ALT and AST during treatment is required per FDA REMS • Causes increases in hepatic fat (steatosis) with or without concomitant elevated transaminases, which may be a risk for progressive liver diseases • Also causes steatosis of the small intestine with resulting abdominal pain and steatorrhea unless a very-low-fat diet is followed; may also cause fat-soluble vitamin deficiency unless vitamin supplements are taken • Caution should be exercised when used with other drugs with potential hepatoxicity; because of hepatoxicity risk, only available through REMS program |
| Antisense apo B oligonucleotide (mipomersen subcutaneous injection) | • ↓ LDL-C 21%, TC 19%, apo B 24%, and non-HDL-C 22% in individuals with HoFH by degrading mRNA for apo B-100, the principal apolipoprotein needed for hepatic synthesis of VLDL (and subsequent intra-plasma production of IDL and LDL) | • Can cause increases in transaminases (ALT, AST); monitoring of ALT, AST, alkaline phosphatase, and total bilirubin before initiation and of ALT and AST during treatment is recommended • Causes increases in hepatic fat (steatosis) with or without concomitant elevated transaminases, which may be a risk for progressive liver diseases • Caution should be exercised when used with other drugs with potential hepatoxicity; because of hepatoxicity risk, only available through REMS program |
| Omega-3 fish oil (icosapent ethyl, omega-3-acid ethyl esters) | • ↓ TG 27–45%, TC 7–10%, VLDL-C 20–42%, apo B 4%, and non-HDL-C 8–14% in individuals with severe hypertriglyceridemia most likely by reducing hepatic VLDL-TG synthesis and/or secretion and enhancing TG clearance from circulating VLDL particles; other potential mechanisms of action include increased ß-oxidation, inhibition of acyl-CoA, 1,2-diacylglyceral acyltransferase, decreased hepatic lipogenesis, and increased plasma lipoprotein activity • Icosapent ethyl ↓ LDL-C 5%, whereas,omega-3-acid ethyl esters ↑ LDL-C 45% |
• TG levels should be carefully assessed before initiating therapy and periodically during therapy • Omega-3-acid ethyl esters can increase LDL-C levels; monitoring LDL-C levels during treatment is recommended • May prolong bleeding time; periodic monitoring of coagulation status should be undertaken in patients receiving treatment with omega-3 fatty acids and other drugs affecting coagulation • Periodic monitoring of ALT and AST levels during treatment is recommended for patients with hepatic impairment; some patients may experience increases in ALT levels only • Caution should be exercised when treating patients with a known hypersensitivity to fish and/or shellfish • In patients with severe hypertriglyceridemia, the effect of omega-3 fatty acids on cardiovascular morbidity and mortality and the risk of pancreatitis has not been determined in patients with severe hypertriglyceridemia • In patients with paroxysmal or persistent AF, therapy with omega-3-acid ethyl esters may be associated with increased frequency of symptomatic AF or flutter, especially within the first 2–3 months after initiation • The most common adverse events in patients receiving omega-3 fatty acids included arthralgia (2.3%), eructation (4%), dyspepsia (3%), and taste perversion (4%); patients may also experience constipation, gastrointestinal disorders, vomiting, rash, or pruritus • Omega-3 fatty acids should be used with caution in nursing mothers and should only be used in pregnant women if the benefits of treatment outweigh the potential risk of fetal harm |
Percentage of change varies depending on baseline lipid variables and dosages. Statin potency and dosages vary.
Most frequent or serious. See prescribing information for complete contraindications, warnings, precautions, and side effects.
Results vary. Gemfibrozil has been shown to decrease, have no effect on, or increase fibrinogen depending on the study.
Results vary. Gemfibrozil has been shown to have no effect on or increase homocysteine. AF, atrial fibrillation; ALT, alanine aminotransferase; AST, aspartate amino transferase; eGFR, estimated glomerular filtration rate; HDL-C, HDL cholesterol; IDL, intermediate-density lipoprotein; LDL-C, LDL cholesterol; LDLR, low-density lipoprotein receptor; MTP, microsomal transfer triglyceride protein; REMS, risk evaluation and mitigation strategies; TC, total cholesterol; TG, triglycerides; VLDL-C, very-low-density lipoprotein cholesterol.
1. Statins
Therapy with statins (also called HMG-CoA [hydroxymethylglutaryl-coenzyme A] reductase inhibitors) is recommended as the primary pharmacological agent to achieve target LDL cholesterol goals on the basis of morbidity and mortality outcome trials (1,21–27). For clinical decision-making, mild elevations in blood glucose levels and/or an increased risk of new-onset type 2 diabetes associated with intensive statin therapy do not outweigh the benefits of statin therapy for ASCVD risk reduction.
In individuals with high or very high risk, further lowering of LDL cholesterol beyond established targets with statins results in additional ASCVD event reduction and may be considered. Very-high-risk individuals with established coronary, carotid, and peripheral vascular disease or diabetes who also have one or more additional risk factors should be treated with statins to target a reduced LDL cholesterol treatment goal of <70 mg/dL. Individuals at extreme risk should be treated with statins to target an even lower LDL cholesterol treatment goal of <55 mg/dL.
The statin dose in all patients, including those with diabetes, is determined by the dose necessary to achieve the appropriate LDL goal alone or in combination with other LDL cholesterol–lowering therapy. Statin intolerance, primarily consisting of myalgias, is a clinical problem of uncertain frequency requiring lowering the statin dose, switching statins, or in some instances avoiding statins entirely (1).
2. Fibrates
Fibrates should be used to treat severe hypertriglyceridemia (triglycerides >500 mg/dL) (1). Based on subclass analyses in several fibrate trials (Helsinki Heart, VA HIT [Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial], Bezafibrate Infarction Prevention Study, FIELD [Fenofibrate Intervention and Event Lowering in Diabetes], and ACCORD [Action to Control Cardiovascular Risk in Diabetes]-Lipid [28–32]), fibrates may improve ASCVD outcomes in primary and secondary prevention when triglyceride concentrations are ≥200 mg/dL and HDL cholesterol concentrations are <40 mg/dL (1).
3. Omega-3 Fish Oil
Prescription omega-3 fish oil 2–4 g daily should be used to treat severe hypertriglyceridemia (triglycerides >500 mg/dL). Dietary supplements are not approved by the U.S. Food and Drug Administration (FDA) for treatment of hypertriglyceridemia and generally are not recommended for this purpose (1).
4. Niacin
Niacin therapy is recommended principally as an adjunct for reducing triglycerides. Niacin should not be used in individuals aggressively treated with a statin because of the absence of additional benefit in patients with well-controlled LDL cholesterol (1).
5. Bile Acid Sequestrants
Bile acid sequestrants may be considered for reducing LDL cholesterol and apo B and modestly increasing HDL cholesterol but may increase triglycerides (1).
6. Cholesterol Absorption Inhibitors
Ezetimibe may be considered as monotherapy in reducing LDL cholesterol and apo B, especially in statin-intolerant individuals. Ezetimibe can be used in combination with statins to further reduce both LDL cholesterol and ASCVD risk (1).
7. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors should be considered for use in combination with statin therapy for LDL cholesterol–lowering in individuals with FH. PCSK9 inhibitors should be considered in individuals with clinical CVD who are unable to reach LDL cholesterol/non-HDL cholesterol goals with maximally tolerated statin therapy. They should not be used as monotherapy except in statin-intolerant individuals (1).
8. Combination Therapy
Combination therapy with lipid-lowering agents should be considered when the LDL cholesterol/non-HDL cholesterol level is markedly increased and monotherapy (usually with a statin) does not achieve the therapeutic goal (1).
Special Considerations: Women
Women should be evaluated for ASCVD risk and treated with pharmacotherapy if lifestyle intervention is insufficient. Hormone replacement therapy for the treatment of dyslipidemia in postmenopausal women is not recommended (1).
Special Considerations: Children and Adolescents
Pharmacotherapy is recommended for children and adolescents >10 years of age who do not respond sufficiently to lifestyle modification, and particularly for those satisfying any of the following criteria (1):
LDL cholesterol ≥190 mg/dL
LDL cholesterol ≥160 mg/dL and the presence of two or more cardiovascular risk factors, even after vigorous intervention
Family history of premature ASCVD (before 55 years of age)
Overweight, obesity, or other elements of the insulin resistance syndrome
An acceptable LDL cholesterol level in children and adolescents is <100 mg/dL, although some pediatric guidelines consider <110 mg/dL acceptable. A level 100–129 mg/dL is considered borderline, and a level >130 mg/dL is considered high (1).
Follow-Up and Monitoring (1,14,33)
Reassess individuals’ lipid status 6 weeks after therapy initiation and again at 6-week intervals until the treatment goal is achieved.
While on stable lipid therapy, individuals should be tested at 6- to 12-month intervals.
While on stable lipid therapy, the specific interval of testing should depend on individual adherence to therapy and lipid profile consistency; if adherence is a concern or the lipid profile is unstable, the individual will probably benefit from more frequent assessment.
More frequent lipid status evaluation is recommended in situations such as deterioration of diabetes control, use of a new drug known to affect lipid levels, progression of atherothrombotic disease, considerable weight gain, unexpected adverse change in any lipid parameter, development of a new ASCVD risk factor, or convincing new clinical trial evidence or guidelines that suggest stricter lipid goals.
Liver transaminase levels should be measured before and 3 months after initiating niacin or fibric acid treatment because most liver abnormalities occur within 3 months of treatment initiation. Liver transaminase levels should be measured periodically (e.g., semiannually or annually) thereafter.
Creatine kinase levels should be assessed and the statin discontinued, at least temporarily, when individuals report clinically significant myalgias or muscle weakness while on statin therapy.
Cost-Effectiveness
Non-pharmacological interventions such as dietary management and smoking cessation are the most cost-effective options available for ASCVD prevention. When non-pharmacological interventions fail, pharmacological intervention is a cost-effective option for primary and secondary intervention among individuals at moderate to high risk. Among otherwise healthy individuals at low risk, the cost-effectiveness of primary pharmacological intervention varies on the basis of age and sex (1).
Statins have proven to be cost-effective in both primary and secondary prevention of ASCVD events in individuals at moderate to high risk or in individuals with an LDL cholesterol ≥190 mg/dL (14,34,35). Fibrates are cost-effective as monotherapy and in combination therapy for lowering triglycerides and raising HDL cholesterol and reducing CVD events in individuals with triglycerides >200 mg/dL and HDL cholesterol <40 mg/dL (36,37). The cost-effectiveness for ezetimibe is unclear (38,39), but with recent clinical trial outcome data and price reductions, ezetimibe may emerge as a cost-effective strategy to achieve LDL cholesterol goals and reduce CVD events. Assessment of the cost-effectiveness of PCSK9 inhibitors awaits further clinical trial data on already-demonstrated CVD event reduction and a longer analysis to determine a CVD death benefit.
Acknowledgments
The author thanks Yehuda Handelsman, MD, for text commentary and Caitlin Rothermel for editorial assistance.
Duality of Interest
P.S.J. is a speakers bureau member for Amgen, AstraZeneca, Janssen, Merck, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
P.S.J. is the sole author of this article and is the guarantor of this work and takes responsibility for the integrity of the data presented and the accuracy of the data analysis.
References
- 1.Jellinger PS, Handelsman Y, Rosenblit PD, et al. . American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract 2017;23(Suppl. 2):1–87 [DOI] [PubMed] [Google Scholar]
- 2.Bild DE, Bluemke DA, Burke GL, et al. . Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 3.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. . Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClelland RL, Jorgensen NW, Budoff M, et al. . 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon CP, Blazing MA, Giugliano RP, et al. . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397 [DOI] [PubMed] [Google Scholar]
- 6.Sabatine MS, Giugliano RP, Keech AC, et al. . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722 [DOI] [PubMed] [Google Scholar]
- 7.Baigent C, Keech A, Kearney PM, et al. . Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2010;376:1670–1681 [DOI] [PubMed] [Google Scholar]
- 8.Weiner DE, Tighiouart H, Amin MG, et al. . Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004;15:1307–1315 [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(Suppl. 1):S1–S266 [PubMed] [Google Scholar]
- 10.Zimmerman MP. How do PCSK9 inhibitors stack up to statins for low-density lipoprotein cholesterol control? Am Health Drug Benefits 2015;8:436–442 [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein J, Hobbs H, Brown M. Familial hypercholesterolemia. In The Metabolic and Molecular Bases of Inherited Disease. 7th ed. Scriver C, Beaudet A, Sly W, Valle D, Eds. New York, N.Y, McGraw-Hill, 1995, 1981–2030 [Google Scholar]
- 12.Bouhairie VE, Goldberg AC. Familial hypercholesterolemia. Cardiol Clin 2015;33:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turgeon RD, Barry AR, Pearson GJ. Familial hypercholesterolemia: review of diagnosis, screening, and treatment. Can Fam Physician 2016;62:32–37 [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health; National Heart Lung, and Blood Institute ; 2002. National Cholesterol Education Program. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report (NIH Publication No. 02–5215). Bethesda, Md., National Institutes of Health, 2002 [Google Scholar]
- 15.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A–I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet 2001;358:2026–2033 [DOI] [PubMed] [Google Scholar]
- 16.Shai I, Rimm EB, Hankinson SE, et al. . Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation 2004;110:2824–2830 [DOI] [PubMed] [Google Scholar]
- 17.National Diabetes Data Group; National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases . Diabetes in America. 2nd ed. (NIH Publication No. 95–1468). Bethesda, Md., National Institutes of Health, 1995
- 18.Haffner SM. Management of dyslipidemia in adults with diabetes. Diabetes Care 1998;21:160–178 [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 20.Emerging Risk Factors Collaboration, Di Angelantonio E, Kaptoge S, Wormser D, et al. . Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation 1998;97:1440–1445 [DOI] [PubMed] [Google Scholar]
- 22.Downs JR, Clearfield M, Weis S, et al. . Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615–1622 [DOI] [PubMed] [Google Scholar]
- 23.Sever PS, Dahlof B, Poulter NR, et al. . Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs 2004;64(Suppl. 2):43–60 [DOI] [PubMed] [Google Scholar]
- 24.Colhoun HM, Betteridge DJ, Durrington PN, et al. . Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–696 [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, MacFadyen JG, Fonseca FA, et al. . Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER). Circ Cardiovasc Qual Outcomes 2009;2:616–623 [DOI] [PubMed] [Google Scholar]
- 26.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–1389 [PubMed] [Google Scholar]
- 27.Pfeffer MA, Sacks FM, Moye LA, et al. . Influence of baseline lipids on effectiveness of pravastatin in the CARE Trial (Cholesterol And Recurrent Events). J Am Coll Cardiol 1999;33:125–130 [DOI] [PubMed] [Google Scholar]
- 28.Frick MH, Elo O, Haapa K, et al. . Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia: safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987;317:1237–1245 [DOI] [PubMed] [Google Scholar]
- 29.Robins SJ, Collins D, Wittes JT, et al. . Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA 2001;285:1585–1591 [DOI] [PubMed] [Google Scholar]
- 30.Benzafibrate Infarction Prevention (BIP) Study Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation 2000;102:21–27 [DOI] [PubMed] [Google Scholar]
- 31.Keech A, Simes RJ, Barter P, et al. . Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849–1861 (errata Lancet 2006;368:1415 and Lancet 2006;368:1420) [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg HN, Elam MB, Lovato LC, et al. . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niaspan (niacin extended-release) [prescribing information]. Cranbury NJ, Kos Pharmaceuticals, 2015 [Google Scholar]
- 34.Ohsfeldt RL, Gandhi SK, Fox KM, McKenney JM. Statin cost-effectiveness comparisons using real-world effectiveness data: formulary implications. Value Health 2008;11:1061–1069 [DOI] [PubMed] [Google Scholar]
- 35.Odden MC, Pletcher MJ, Coxson PG, et al. . Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hay JW, Sterling KL. Cost effectiveness of treating low HDL-cholesterol in the primary prevention of coronary heart disease. Pharmacoeconomics 2005;23:133–141 [DOI] [PubMed] [Google Scholar]
- 37.Nyman JA, Martinson MS, Nelson D, et al. . Cost-effectiveness of gemfibrozil for coronary heart disease patients with low levels of high-density lipoprotein cholesterol: the Department of Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial. Arch Intern Med 2002;162:177–182 [DOI] [PubMed] [Google Scholar]
- 38.Kohli M, Attard C, Lam A, et al. . Cost effectiveness of adding ezetimibe to atorvastatin therapy in patients not at cholesterol treatment goal in Canada. Pharmacoeconomics 2006;24:815–830 [DOI] [PubMed] [Google Scholar]
- 39.Mihaylova B, Schlackow I, Herrington W, et al. . Cost-effectiveness of simvastatin plus wzetimibe for cardiovascular prevention in CKD: results of the Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 2016;67:576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]


