Abstract
Studies of the vertebrate hindbrain have revealed parallel mechanisms that establish sharp segments with a distinct and homogeneous regional identity. Recent work has revealed roles of cell identity regulation and its relationships with cell segregation. At early stages, there is overlapping expression at segment borders of the Egr2 and Hoxb1 transcription factors that specify distinct identities, which is resolved by reciprocal repression. Computer simulations show that this dynamic regulation of cell identity synergises with cell segregation to generate sharp borders. Some intermingling between segments occurs at early stages, and ectopic egr2-expressing cells switch identity to match their new neighbours. This switching is mediated by coupling between egr2 expression and the level of retinoic acid signalling, which acts in a community effect to maintain homogeneous segmental identity. These findings reveal an interplay between cell segregation and the dynamic regulation of cell identity in the formation of sharp patterns in the hindbrain and raise the question of whether similar mechanisms occur in other tissues.
Keywords: Boundary formation, hindbrain, segmentation
Introduction
Many tissues are patterned by graded signals which regulate the spatial expression of transcription factors that specify regional identity. The initial expression domains of these transcription factors have fuzzy borders, and the proliferation and movements of cells during tissue morphogenesis can potentially cause intermingling between adjacent regions 1, 2. Nevertheless, a precise pattern subsequently forms in which all cells within each region have the same identity, and there is a straight interface at the border of adjacent domains. This raises the question of how sharp patterns of regional domains are formed and maintained. One general mechanism, which has been extensively studied, is the segregation of cells and restriction of intermingling between adjacent regions, regulated by effectors—such as cadherins or Eph receptors and ephrins—whose expression is coupled to regional identity. This article will focus on recent studies of the vertebrate hindbrain that have revealed a crucial role of the dynamic regulation of cell identity in the establishment of sharp and homogeneous regional domains.
Segments and segregation
The hindbrain is subdivided into segments, termed rhombomeres (r1–r7), which express a distinct set of transcription factors and underlie the patterning of neurons and branchial neural crest cells 3. These include Hox proteins, Egr2 (Krox20), MafB, and Vhnf1, which act in a network to specify the formation and anteroposterior (A-P) specification of segments 4, 5. The spatial expression of these transcription factors is regulated by fibroblast growth factor (Fgf) and retinoic acid (RA) signalling that act in feedback loops to establish a gradient of RA in the hindbrain 6. The borders of segmental gene expression initially are ragged and then sharpen over a period of several hours. Clonal analyses revealed that there is extensive intermingling in the neural epithelium, driven by cell intercalation during cell proliferation and convergent-extension movements 7, 8. However, once segment borders can be seen morphologically, cell intermingling between rhombomeres is restricted 7. Subsequent work revealed key roles of signalling by Eph receptors and ephrins which have complementary segmental expression in the hindbrain in the establishment of sharp borders 9– 13. The mechanisms by which Eph-ephrin signalling drives border sharpening remain unclear but, based on studies in other tissues and in cell culture assays, it likely involves cell segregation driven by contact repulsion or increased cortical tension 14– 17. Following sharpening, there is Eph signalling-dependent formation of actin cables at rhombomere boundaries, which mediate increased actomyosin-dependent tension required to maintain straight borders 18.
Mutually exclusive identity
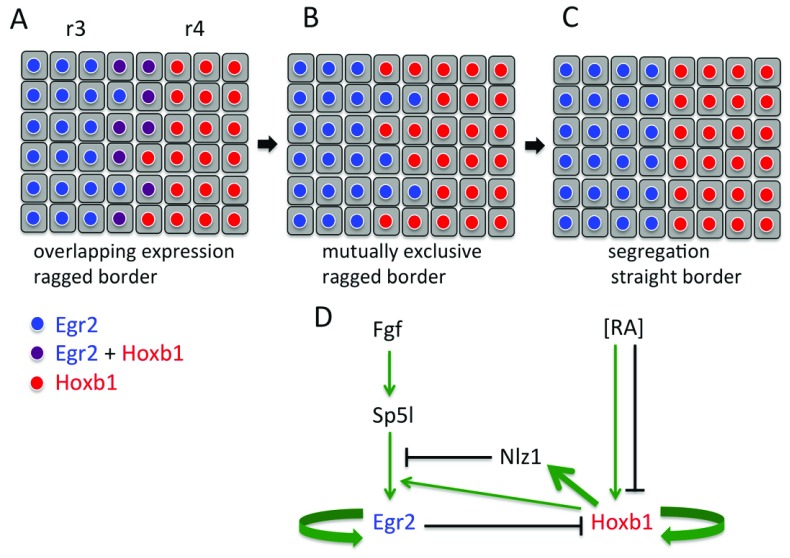
Evidence that cell identity regulation contributes to the formation of sharp borders has come from studies of egr2 and hoxb1. egr2 is expressed in and required for the formation of r3 and r5 19, whereas hoxb1 is expressed in r4 and contributes to A-P specification of this segment 20, 21. At early stages, some cells at the borders of r4 co-express hoxb1 and egr2 ( Figure 1A), and this is likely due to imprecision in the formation and interpretation of graded RA signalling that underlies A-P patterning of the hindbrain 22. The overlap in expression is resolved such that cells express one or the other transcription factor ( Figure 1B). Insights into how this transition occurs have come from studies of the regulation of hoxb1 and egr2 gene expression ( Figure 1D).
Figure 1. Model of border sharpening by reciprocal repression and cell segregation.
( A) At early stages, some cells at the r3/r4 border co-express egr2 and hoxb1 because of imprecision in the regulation of segmental gene expression by graded retinoic acid (RA). ( B) Reciprocal repression of egr2 and hoxb1 leads to mutually exclusive expression, but the border is still ragged. ( C) Cell segregation leads to sharpening to form a straight border. The synergistic role of gene regulation and cell segregation is supported by computer simulations. ( D) Simplified depiction of the network of gene regulation that upregulates egr2 in r3/r5 and hoxb1 in r4 and underlies mutual repression. r, rhombomere.
hoxb1 is initially expressed in a broad domain up to the r3/r4 boundary, which then becomes restricted to r4. The early expression is initiated by RA signalling acting through a conserved 3′ RA response element 23. Subsequently, expression in r4 is maintained by an enhancer that binds Hoxb1, Hoxa1, and Hoxb2 as well as Pbx and Meis cofactors and thus involves both auto-regulation and cross-regulation from other hox genes 5. Expression of hoxb1 becomes restricted to r4 through repression in r3 and r5 mediated by a 5′ RA response element and by binding of Egr2 5, 24.
Transcriptional regulation of the egr2 gene in r3 and r5 also involves elements that initiate and maintain expression 25– 28. Consistent with studies of factors required for egr2 expression 29– 33, the initiator elements have combinatorial input from Fgf signalling and Hox transcription factors, including positive input from low levels of Hoxb1 expression 25, 28. This drives a pulse of Egr2 protein, which in turn acts on an autoregulatory element that maintains and increases the level of egr2 expression 27. This is counterbalanced by inhibitory loops that limit egr2 expression to the appropriate level 34– 36. Whereas low levels of Hoxb1 promote egr2 expression, the high-level expression of Hoxb1 in r4 represses egr2 and acts via the upregulation of Nlz1 28. Remarkably, recent work has found that an enhancer which initiates egr2 expression in r3 is required for the autoregulatory element to function 37. Furthermore, there are long-range physical interactions within the egr2 regulatory region that likely enable this potentiation of the autoregulatory element by the initiator element 37. The cooperation between regulatory elements may help ensure that inappropriate autoregulation does not occur.
egr2 and hoxb1 thus act in a bistable switch in which they autoregulate and reciprocally repress each other, such that any cells which initially co-express both transcription factors at segment borders come to express one or the other. Computer simulations suggest that noise in the RA gradient leads to the initial rough borders of gene expression and that fluctuations in hoxb1 and egr2 levels enable the transition from co-expression to mutually exclusive expression 22. In recent work, a multi-scale model has been developed to simulate both mechanical forces and plasticity of cell identity. These computer simulations have been used to address whether border formation can be accounted for by the generation of mutually exclusive identity or by cell segregation or both 38. It was found that cell segregation alone is not able to drive the formation of a sharp border if there is a wide ‘transition zone’ in which there is a mixture of cells with distinct identity (that is, when the border is very fuzzy) 38. This is due to the mechanics of cell segregation driven by adhesion or repulsion, which require that cells with the same properties are in contact with each other: isolated cells will segregate to the relevant segment only if they happen to come into contact with it. The regulation of mutually exclusive cell identity facilitated by noise narrows the transition zone but alone is not able to fully sharpen the border 38. In contrast, border sharpening does occur in a model in which plasticity in identity and cell segregation are combined 38. The dynamic regulation of segmental identity narrows the transition zone and this enables cell segregation to efficiently drive border sharpening. These simulations thus suggest that identity regulation and cell segregation have complementary strengths and act synergistically to sharpen borders ( Figure 1A–C).
Identity switching
Cell lineage analysis in the chick hindbrain revealed that there is some intermingling between segments at early stages, prior to the formation of morphological boundaries 7. Furthermore, single cells transplanted between hindbrain segments in mouse or zebrafish were found to change their identity to match the new location 39, 40. Such switching did not occur for groups of transplanted cells, suggesting that cell identity is regulated by community signalling, but the nature of the signals was unknown. Based on these findings, it was proposed that identity switching of cells that intermingle acts in parallel with cell segregation to maintain homogeneous segments 41, 42. This idea was challenged by a study which found no intermingling between segments in zebrafish 18, but this work used reporters detected during and after border sharpening, when cell segregation mechanisms are in place. A recent study has shown that intermingling between segments does occur in zebrafish and has elucidated the mechanism of cell identity switching 43. Since Egr2 is a direct regulator of the ephA4 gene 44, it is likely that intermingling of egr2-expressing cells into adjacent segments would occur only during an early time window, when the expression level of EphA4 is not high enough to drive cell segregation. By generating a transgenic reporter line in which expression of a stable fluorescent protein is driven directly from the egr2 locus, investigators found that some cells do intermingle between r3/r5 and adjacent segments 43. These ectopic cells downregulate egr2 expression and, when present in r4, upregulate hoxb1 expression 43.
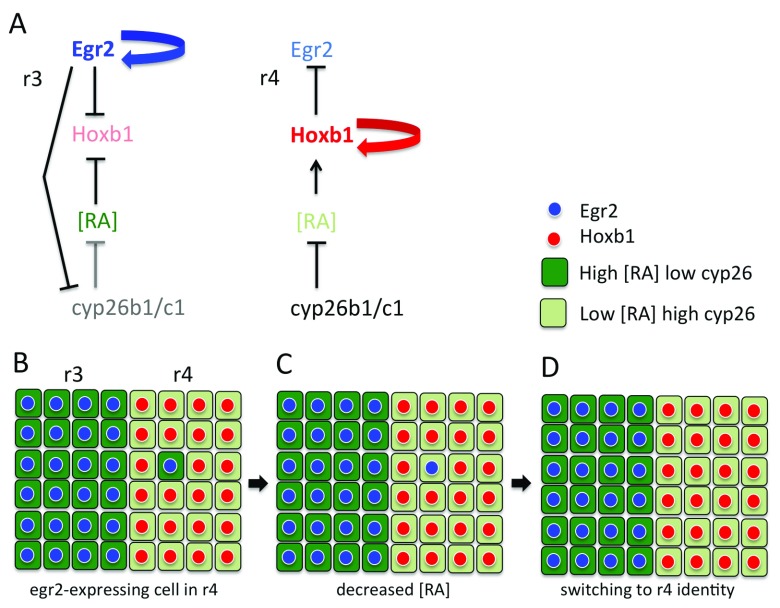
Previous studies have shown that the degradation of RA by Cyp26 family members has a crucial role in establishing differential levels of RA that underlie A-P patterning in the hindbrain. The cyp26a1 gene is regulated by the level of RA and Fgf signalling, which leads to expression in an anterior-to-posterior gradient in the early zebrafish hindbrain 6. In contrast, cyp26b1 and cyp26c1 are not directly regulated by RA signalling and have dynamic segmental expression that progresses from anterior to posterior 45, 46. As strong disruption of A-P patterning occurs only after blocking the function of all three family members 45, they are thought to have parallel roles in regulating the level of RA. cyp26b1 and cyp26c1 are expressed at lower levels in r3 and r5 than in r2, r4, and r6, and it was found that this odd-versus-even difference is regulated downstream of egr2 43. Since Cyp26 enzymes have a strong cell-autonomous and weak non-autonomous effect on RA levels 6, 47, 48, this suggests that r3 or r5 cells that intermingle into adjacent segments move from a high- to a low-RA environment. Furthermore, ectopic expression of egr2 in even-numbered segments was found to be downregulated following blocking of RA signalling, whereas knockdown of cyp26b1 and cyp26c1 leads to a failure to downregulate egr2 in cells that have intermingled from r3 and r5 43. These findings suggest that cyp26b1 and cyp26c1 switch the identity of egr2-expressing cells that have intermingled into even-numbered segments by non-autonomously decreasing RA levels ( Figure 2A–D). In r4, the switching involves upregulation of hoxb1, which in turn represses egr2 expression 43.
Figure 2. Model of how homogeneous segments are maintained by identity switching.
( A) Relationship between egr2, hoxb1, and retinoic acid (RA) levels regulated by cyp26b1 and cyp26c1. In r3 and r5, high-level egr2 expression downregulates cyp26b1 and cyp26c1 expression. This creates a high-RA environment that suppresses hoxb1 expression. In r4, there is high cyp26b1 and cyp26c1 expression. This creates a low-RA environment that promotes hoxb1 expression. ( B) Owing to coupling between egr2 expression and cyp26b1/c1 expression levels, an egr2-expressing cell that intermingles into r4 initially has a high-RA level. ( C) There is non-autonomous depletion of RA by high cyp26b1/c1 expression in adjacent cells. ( D) This leads to upregulation of hoxb1 and thus downregulation of egr2 expression. r, rhombomere.
Relationships between cell segregation and identity
Taken together, these studies have found that cell identity regulation not only acts to refine borders in combination with cell segregation but also maintains the homogeneity of segments despite intermingling that occurs before segregation mechanisms have been upregulated. The latter mechanism involves the coupling of the level of cyp26b1 and cyp26c1 expression and RA signalling to segmental identity. Single egr2-expressing cells that intermingle switch identity, as they are surrounded by neighbours with a higher level of cyp26 expression. In contrast, positive feedback between segmental identity and cyp26 expression potentially maintains RA levels in groups of cells. The coupling thus underlies a community effect that may explain the results of transplantation experiments with single cells or groups of cells 39, 40.
Consistent with this model, non-autonomous effects of egr2 overexpression were found to depend upon cell organisation. In early work, it was shown that forced mosaic expression of egr2 in the chick hindbrain induced the upregulation of egr2 in adjacent cells in even-numbered segments 49. The recent study in zebrafish found that this non-autonomous induction occurs when cells overexpressing egr2 are intermingled with r2/r4/r6 cells but not following Eph-mediated segregation of the egr2-expressing cells 43. This suggests that cell identity regulation depends upon how many neighbours are of the same or different type. The regulation of both ephA4 and the level of cyp26b1 and cyp26c1 expression by egr2 creates segregated communities with different levels of RA signalling. Cells at a sharp border have a sufficient number of neighbours with the same cyp26 expression and thus maintain their identity. In contrast, cells with distinct identity that enter the adjacent community are surrounded and switch to match their new neighbours ( Figure 2B–D). It will be interesting to explore whether the sharpening of fuzzy borders ( Figure 1) involves RA signalling dependent upon how many neighbours have the same or different cyp26 expression level.
These findings raise a number of new questions. It will be important to understand cell identity switching by quantitative modelling of the gene regulatory networks in r3/r5 and r4. How does a change in the level of RA switch the network from one state to the other? Since cells do not switch identity if transplanted between segments at late stages 40, autoregulation or other mechanisms (or both) may increasingly lock the network in each segment into one state. A key issue is to measure the segmental level of RA signalling, but, owing to limitations in the sensitivity and noise of the available techniques, this is currently not possible at the spatial resolution of hindbrain segments 50– 52. A further important question is how the segmental levels of cyp26b1 and cyp26c1 expression are regulated. Since Egr2 usually acts as an activator, the repression of cyp26b1 and cyp26c1 expression in r3 and r5 may involve intermediary factors, but Egr2 can in some contexts act as a repressor 53, 54. hox genes have been found to regulate the expression of components of the RA signalling pathway 4, 55– 57, and it will be interesting to test whether this underlies feedback that maintains segmental identity. Finally, a broader issue raised by studies of the hindbrain is whether a similar interplay between dynamic cell identity regulation and cell segregation refines patterning elsewhere, such as other boundaries in the developing brain 58, in particular at early stages when there are extensive morphogenetic movements and cell fate is plastic.
Abbreviations
A-P, anteroposterior; fgf, fibroblast growth factor; r, rhombomere; RA, retinoic acid.
Acknowledgements
I thank Qiling Xu for comments on this article.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Dalit Sela-Donenfeld, Koret School of Veterinary Medicine, The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot, Israel
Kyo Yamasu, Division of Life Science, Graduate School of Science and Engineering, Saitama University, Sakura-ku, Saitama, Japan
Robb Krumlauf, Stowers Institute for Medical Research, Kansas City, MO, USA
Funding Statement
Our work is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001217), the UK Medical Research Council (FC001217), and the Wellcome Trust (FC001217).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Dahmann C, Oates AC, Brand M: Boundary formation and maintenance in tissue development. Nat Rev Genet. 2011;12(1):43–55. 10.1038/nrg2902 [DOI] [PubMed] [Google Scholar]
- 2. Batlle E, Wilkinson DG: Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb Perspect Biol. 2012;4(1):a008227. 10.1101/cshperspect.a008227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lumsden A, Krumlauf R: Patterning the vertebrate neuraxis. Science. 1996;274(5290):1109–15. 10.1126/science.274.5290.1109 [DOI] [PubMed] [Google Scholar]
- 4. Parker HJ, Krumlauf R: Segmental arithmetic: Summing up the Hox gene regulatory network for hindbrain development in chordates. Wiley Interdiscip Rev Dev Biol. 2017;6(6):e286. 10.1002/wdev.286 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Tümpel S, Wiedemann LM, Krumlauf R: Hox genes and segmentation of the vertebrate hindbrain. Curr Top Dev Biol. 2009;88:103–37. 10.1016/S0070-2153(09)88004-6 [DOI] [PubMed] [Google Scholar]
- 6. White RJ, Nie Q, Lander AD, et al. : Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 2007;5(11):e304. 10.1371/journal.pbio.0050304 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Fraser S, Keynes R, Lumsden A: Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344(6265):431–5. 10.1038/344431a0 [DOI] [PubMed] [Google Scholar]
- 8. Kimmel CB, Warga RM, Kane DA: Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development. 1994;120(2):265–76. [DOI] [PubMed] [Google Scholar]
- 9. Xu Q, Alldus G, Holder N, et al. : Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development. 1995;121(12):4005–16. [DOI] [PubMed] [Google Scholar]
- 10. Xu Q, Mellitzer G, Robinson V, et al. : In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399(6733):267–71. 10.1038/20452 [DOI] [PubMed] [Google Scholar]
- 11. Cooke JE, Kemp HA, Moens CB: EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr Biol. 2005;15(6):536–42. 10.1016/j.cub.2005.02.019 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Kemp HA, Cooke JE, Moens CB: EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev Biol. 2009;327(2):313–26. 10.1016/j.ydbio.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sela-Donenfeld D, Kayam G, Wilkinson DG: Boundary cells regulate a switch in the expression of FGF3 in hindbrain rhombomeres. BMC Dev Biol. 2009;9:16. 10.1186/1471-213X-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rohani N, Canty L, Luu O, et al. : EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 2011;9(3):e1000597. 10.1371/journal.pbio.1000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fagotto F, Rohani N, Touret AS, et al. : A molecular base for cell sorting at embryonic boundaries: Contact inhibition of cadherin adhesion by ephrin/ Eph-dependent contractility. Dev Cell. 2013;27(1):72–87. 10.1016/j.devcel.2013.09.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Taylor HB, Khuong A, Wu Z, et al. : Cell segregation and border sharpening by Eph receptor-ephrin-mediated heterotypic repulsion. J R Soc Interface. 2017;14(132): pii: 20170338. 10.1098/rsif.2017.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canty L, Zarour E, Kashkooli L, et al. : Sorting at embryonic boundaries requires high heterotypic interfacial tension. Nat Commun. 2017;8(1):157. 10.1038/s41467-017-00146-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Calzolari S, Terriente J, Pujades C: Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries. EMBO J. 2014;33(7):686–701. 10.1002/embj.201386003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Schneider-Maunoury S, Topilko P, Seitandou T, et al. : Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993;75(6):1199–214. 10.1016/0092-8674(93)90329-O [DOI] [PubMed] [Google Scholar]
- 20. Studer M, Lumsden A, Ariza-McNaughton L, et al. : Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384(6610):630–4. 10.1038/384630a0 [DOI] [PubMed] [Google Scholar]
- 21. Goddard JM, Rossel M, Manley NR, et al. : Mice with targeted disruption of Hoxb-1 fail to form the motor nucleus of the VIIth nerve. Development. 1996;122(10):3217–28. [DOI] [PubMed] [Google Scholar]
- 22. Zhang L, Radtke K, Zheng L, et al. : Noise drives sharpening of gene expression boundaries in the zebrafish hindbrain. Mol Syst Biol. 2012;8:613. 10.1038/msb.2012.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marshall H, Studer M, Pöpperl H, et al. : A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994;370(6490):567–71. 10.1038/370567a0 [DOI] [PubMed] [Google Scholar]
- 24. Studer M, Pöpperl H, Marshall H, et al. : Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994;265(5179):1728–32. 10.1126/science.7916164 [DOI] [PubMed] [Google Scholar]
- 25. Wassef MA, Chomette D, Pouilhe M, et al. : Rostral hindbrain patterning involves the direct activation of a Krox20 transcriptional enhancer by Hox/Pbx and Meis factors. Development. 2008;135(20):3369–78. 10.1242/dev.023614 [DOI] [PubMed] [Google Scholar]
- 26. Chomette D, Frain M, Cereghini S, et al. : Krox20 hindbrain cis-regulatory landscape: Interplay between multiple long-range initiation and autoregulatory elements. Development. 2006;133(7):1253–62. 10.1242/dev.02289 [DOI] [PubMed] [Google Scholar]
- 27. Bouchoucha YX, Reingruber J, Labalette C, et al. : Dissection of a Krox20 positive feedback loop driving cell fate choices in hindbrain patterning. Mol Syst Biol. 2013;9:690. 10.1038/msb.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Labalette C, Wassef MA, Desmarquet-Trin Dinh C, et al. : Molecular dissection of segment formation in the developing hindbrain. Development. 2015;142(1):185–95. 10.1242/dev.109652 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Maves L, Jackman W, Kimmel CB: FGF3 and FGF8 mediate a rhombomere 4 signaling activity in the zebrafish hindbrain. Development. 2002;129(16):3825–37. [DOI] [PubMed] [Google Scholar]
- 30. Walshe J, Maroon H, McGonnell IM, et al. : Establishment of hindbrain segmental identity requires signaling by FGF3 and FGF8. Curr Biol. 2002;12(13):1117–23. 10.1016/S0960-9822(02)00899-0 [DOI] [PubMed] [Google Scholar]
- 31. Marín F, Charnay P: Hindbrain patterning: FGFs regulate Krox20 and mafB/kr expression in the otic/preotic region. Development. 2000;127(22):4925–35. [DOI] [PubMed] [Google Scholar]
- 32. Weisinger K, Wilkinson DG, Sela-Donenfeld D: Inhibition of BMPs by follistatin is required for FGF3 expression and segmental patterning of the hindbrain. Dev Biol. 2008;324(2):213–25. 10.1016/j.ydbio.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 33. Weisinger K, Kayam G, Missulawin-Drillman T, et al. : Analysis of expression and function of FGF-MAPK signaling components in the hindbrain reveals a central role for FGF3 in the regulation of Krox20, mediated by Pea3. Dev Biol. 2010;344(2):881–95. 10.1016/j.ydbio.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 34. Mechta-Grigoriou F, Garel S, Charnay P: Nab proteins mediate a negative feedback loop controlling Krox-20 activity in the developing hindbrain. Development. 2000;127(1):119–28. [DOI] [PubMed] [Google Scholar]
- 35. García-Gutiérrez P, Juárez-Vicente F, Gallardo-Chamizo F, et al. : The transcription factor Krox20 is an E3 ligase that sumoylates its Nab coregulators. EMBO Rep. 2011;12(10):1018–23. 10.1038/embor.2011.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kayam G, Kohl A, Magen Z, et al. : A novel role for Pax6 in the segmental organization of the hindbrain. Development. 2013;140(10):2190–202. 10.1242/dev.089136 [DOI] [PubMed] [Google Scholar]
- 37. Thierion E, Le Men J, Collombet S, et al. : Krox20 hindbrain regulation incorporates multiple modes of cooperation between cis-acting elements. PLoS Genet. 2017;13(7):e1006903. 10.1371/journal.pgen.1006903 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Wang Q, Holmes WR, Sosnik J, et al. : Cell Sorting and Noise-Induced Cell Plasticity Coordinate to Sharpen Boundaries between Gene Expression Domains. PLoS Comput Biol. 2017;13(1):e1005307. 10.1371/journal.pcbi.1005307 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Trainor P, Krumlauf R: Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat Cell Biol. 2000;2(2):96–102. 10.1038/35000051 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Schilling TF, Prince V, Ingham PW: Plasticity in zebrafish hox expression in the hindbrain and cranial neural crest. Dev Biol. 2001;231(1):201–16. 10.1006/dbio.2000.9997 [DOI] [PubMed] [Google Scholar]
- 41. Cooke JE, Moens CB: Boundary formation in the hindbrain: Eph only it were simple... Trends Neurosci. 2002;25(5):260–7. 10.1016/S0166-2236(02)02134-3 [DOI] [PubMed] [Google Scholar]
- 42. Pasini A, Wilkinson DG: Stabilizing the regionalisation of the developing vertebrate central nervous system. Bioessays. 2002;24(5):427–38. 10.1002/bies.10085 [DOI] [PubMed] [Google Scholar]
- 43. Addison M, Xu Q, Cayuso J, et al. : Cell Identity Switching Regulated by Retinoic Acid Signaling Maintains Homogeneous Segments in the Hindbrain. Dev Cell. 2018;45(5):606–620.e3. 10.1016/j.devcel.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Theil T, Frain M, Gilardi-Hebenstreit P, et al. : Segmental expression of the EphA4 (Sek-1) receptor tyrosine kinase in the hindbrain is under direct transcriptional control of Krox-20. Development. 1998;125(3):443–52. [DOI] [PubMed] [Google Scholar]
- 45. Hernandez RE, Putzke AP, Myers JP, et al. : Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134(1):177–87. 10.1242/dev.02706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sirbu IO, Gresh L, Barra J, et al. : Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132(11):2611–22. 10.1242/dev.01845 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Rydeen A, Voisin N, D'Aniello E, et al. : Excessive feedback of Cyp26a1 promotes cell non-autonomous loss of retinoic acid signaling. Dev Biol. 2015;405(1):47–55. 10.1016/j.ydbio.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Rydeen AB, Waxman JS: Cyp26 enzymes are required to balance the cardiac and vascular lineages within the anterior lateral plate mesoderm. Development. 2014;141(8):1638–48. 10.1242/dev.105874 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Giudicelli F, Taillebourg E, Charnay P, et al. : Krox-20 patterns the hindbrain through both cell-autonomous and non cell-autonomous mechanisms. Genes Dev. 2001;15(5):567–80. 10.1101/gad.189801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sosnik J, Zheng L, Rackauckas CV, et al. : Noise modulation in retinoic acid signaling sharpens segmental boundaries of gene expression in the embryonic zebrafish hindbrain. eLife. 2016;5:e14034. 10.7554/eLife.14034 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Shimozono S, Iimura T, Kitaguchi T, et al. : Visualization of an endogenous retinoic acid gradient across embryonic development. Nature. 2013;496(7445):363–6. 10.1038/nature12037 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Schilling TF, Sosnik J, Nie Q: Visualizing retinoic acid morphogen gradients. Methods Cell Biol. 2016;133:139–63. 10.1016/bs.mcb.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Desmazières A, Charnay P, Gilardi-Hebenstreit P: Krox20 controls the transcription of its various targets in the developing hindbrain according to multiple modes. J Biol Chem. 2009;284(16):10831–40. 10.1074/jbc.M808683200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mager GM, Ward RM, Srinivasan R, et al. : Active gene repression by the Egr2.NAB complex during peripheral nerve myelination. J Biol Chem. 2008;283(26):18187–97. 10.1074/jbc.M803330200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choe SK, Zhang X, Hirsch N, et al. : A screen for hoxb1-regulated genes identifies ppp1r14al as a regulator of the rhombomere 4 Fgf-signaling center. Dev Biol. 2011;358(2):356–67. 10.1016/j.ydbio.2011.05.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rohrschneider MR, Elsen GE, Prince VE: Zebrafish Hoxb1a regulates multiple downstream genes including prickle1b. Dev Biol. 2007;309(2):358–72. 10.1016/j.ydbio.2007.06.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Makki N, Capecchi MR: Identification of novel Hoxa1 downstream targets regulating hindbrain, neural crest and inner ear development. Dev Biol. 2011;357(2):295–304. 10.1016/j.ydbio.2011.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kiecker C, Lumsden A: Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6(7):553–64. 10.1038/nrn1702 [DOI] [PubMed] [Google Scholar]