Abstract
Objective
The pathophysiology of chronic pain is complex, with most of our knowledge being derived from preclinical studies. The search for biomarkers mirroring the pathophysiology of chronic pain is ongoing, and there is an increasing interest in saliva as a diagnostic tool. Given what is known about salivary substance P and salivary gland innervation, we hypothesized that salivary substance P and/or beta-endorphin might reflect the basal activity of these neuropeptides in the central nervous system, thereby perhaps mirroring a general propensity to chronic pain. Based on this overall hypothesis, our aim was to compare salivary levels of these neuropeptides in chronic neuropathic pain patients with healthy controls. An additional aim was to relate salivary levels to plasma levels.
Materials and methods
We compared salivary concentrations of beta-endorphin and substance P in 14 chronic neuropathic pain patients with concentrations in 18 healthy controls using a Luminex technology kit. Salivary-to-plasma quotients were also calculated.
Results
We found no significant difference between the groups' salivary concentrations of substance P and beta-endorphin. No correlation was found between salivary and plasma concentrations of each neuropeptide, which we hypothesize might point to local production of beta-endorphin and/or substance P in the salivary glands. Given high substance P salivary-to-plasma quotients, such a local production seems more likely for substance P than for beta-endorphin.
Conclusions
Propensity to neuropathic chronic pain was not substantiated by our analysis of salivary levels of substance P and/or beta-endorphin. However, we report salivary-to-plasma quotients that give potentially important physiological insight about these neuropeptides.
Keywords: Neurology, Neuroscience
1. Introduction
Chronic pain is a widespread problem with a prevalence of 19% among the adult population [1]. Generally speaking, the development of chronic pain can be said to be caused by the combination of (a) events that trigger acute pain and (b) inter-individual differences in the propensity for acute pain to develop into chronic pain [2]. There are several well-known risk factors underlying such a propensity to chronic pain [3]. However, no measureable biological test has hitherto been developed to predict the development of e.g. chronic post-surgical pain.
Interest in saliva as a diagnostic tool has increased over the past decades [4]. Saliva mirrors the physiological state of the body [5, 6], molecules found in blood can also be found in saliva [7], and sample collection and analysis is easy, non-invasive, and relatively inexpensive [5, 8, 9, 10]. Saliva is a plasma ultra-filtrate and hence contains proteins derived from blood, but it also contains proteins produced by the acini epithelial cells in the salivary glands [5]. It is composed of >97% water, electrolytes, immunoglobins, enzymes, and other proteins involved in different aspects of physiological processes [8, 11, 12, 13].
Our group has previously shown that levels of beta-endorphin (BE) in the cerebrospinal fluid (CSF) of patients with chronic neuropathic pain were lower than in healthy controls; plasma levels did not differ [14]. In the same study, we also measured Substance P (SP). For obvious reasons, taking CSF samples is much more complicated than taking saliva samples.
Previous studies found the concentrations of SP to be higher in saliva than in plasma [15, 16]. It has been hypothesized that SP does not passively leak from blood to saliva, but instead actively enters saliva by an unknown mechanism [15]. The salivary glands are innervated [17], SP-neurons have been demonstrated [18], and it has been suggested that a possible route for peptide access to saliva may be through release from nerve terminals in and around the salivary glands [19]. Therefore, rather than merely mirroring plasma levels, levels of salivary SP could conceptually perhaps reflect the overall SP “tone” of the nervous system, putatively altered levels of basal activity in the nervous system hence translating into altered saliva levels. The same could potentially be true of BE whose function in saliva is, if any, currently unknown.
In the context of the on-going search for biomarkers reflecting chronic pain pathophysiology in humans [20], one may therefore hypothesize that a putative general propensity to chronic pain could perhaps be mirrored in altered levels of salivary SP and/or BE in chronic pain patients. The aim of the present study was therefore to compare the concentrations of BE and SP in saliva from chronic neuropathic pain patients with healthy controls. An additional aim was to explore the relationship between plasma and saliva concentrations of these neuropeptides in order to better understand their salivary physiology.
2. Materials and methods
2.1. Patients
All 14 pain patients included in this study were participating in a clinical trial of intrathecal bolus injections of the analgesic ziconotide [21]. Patients with peripheral, postoperative/posttraumatic, neuropathic pain were invited to participate. Inclusion criteria were 1) patient, at least 18 years of age, suffering from chronic (≥6 months) pain, who had failed on conventional pharmacological treatment; only patients with peripheral neuropathic pain due to trauma or surgery were included; 2) Visual Analogue Scale Pain Intensity during week before inclusion ≥40 mm (VASPI); 3) patient capable of judgment, i.e. able to understand information regarding the drug, the mode of administration and evaluation of efficacy and side effects; 4) signed informed consent. Exclusion criteria were 1) limited life expectancy (investigator's judgement); 2) intrathecal chemotherapy; 3) known or suspected intracranial hypertension; 4) known liver or kidney disease, defined as serum transaminases, total bilirubin, alkaline phosphatase or creatinine >1.2 Upper Limit of Normal; 5) advanced cardio-pulmonary disease (investigator's judgment); 6) ongoing infection, whether systemically or locally in the lumbar area; 7) coagulopathy (including medication with warfarin, clopidogrel and heparin); 8) allergy to ziconotide or any of the excipients in the ziconotide vial; 9) history of psychiatric disorders which in the investigator's opinion would put the patient at risk; 10) pregnant or lactating woman (menstruating women had to use contraceptives during the trial period); 11) participation in another clinical trial during the last 30 days.
After informed consent, the following additional data were registered: basic demographic data; pain diagnosis; pain duration; present and past medical history; concomitant medication. A physical examination was performed. Then, within a month, the patients came back for body fluid sampling as described below.
All patients had at least probable neuropathic pain according to the criteria published by Treede et al. [22], meaning that all had pain with a neuroanatomically plausible distribution (criterion 1), all had a history suggestive of a relevant lesion affecting the somatosensory system (criterion 2), and all had congruent neurological signs (criterion 3) and/or a relevant diagnostic test confirming the lesion (criterion 4). Pain diagnoses according to the International Classification of Diseases version 10 (ICD 10) were as follows: 6 patients had ICD 10 code S34.2 (injury of nerve root of lumbar and sacral spine, i.e., failed back surgery syndrome with radiculopathy), and the remaining 8 patients had one of the following diagnoses: S14.2 (injury of nerve root of cervical spine), S34.3 (injury of cauda equina), S54.0 (injury of ulnar nerve), S54.9 (injury of unspecified nerve at forearm level), S74.0 (injury of sciatic nerve at hip and thigh level), S74.1 (injury of femoral nerve at hip), S94.9 (injury of unspecified nerve at ankle and foot level), and G62.9 (polyneuropathy).
2.2. Healthy controls
Eighteen healthy controls were recruited by local advertisement at the Faculty of Health Sciences, Linköping University, Sweden, and by contacting healthy subjects from earlier studies. After informed consent, a structured interview was conducted to ensure the absence of any significant medical condition.
2.3. Sample collection and preparation
For each subject in this study, body fluid samples were collected as follows. First, whole saliva was gathered from subjects using a Salivette® (Sarstedt, Germany). Samples were collected at approximately the same time point for each subject (8.00–11.00 AM). Subjects were not allowed to have eaten within 30 minutes of saliva collection. Subjects rinsed their mouth with water, waited 10 minutes and placed the swab in their mouth for 3 minutes. The swab was placed back into the Salivette and in order to minimize degradation of the proteins the samples were kept on ice. Then, a 10 mL venous blood sample was drawn using an ethylenediaminetetraacetic acid tube. Each sample was immediately cooled on ice and transported to the Painomics® laboratory, Linköping University Hospital. Salivettes were centrifuged for 10 minutes at 4 °C to extract the saliva, which yielded roughly 1 mL saliva per subject. Ocular inspection of saliva was performed to rule out blood contamination. Saliva was then aliquoted and stored at –70 °C until analysis. Blood samples were centrifuged for 10 minutes at 1000 × g at 4 °C within 30 minutes of blood collection; plasma was removed, aliquoted, and stored at –70 °C until analysis.
2.4. Multiplex assay
BE and SP were quantified by using the MILLIPLEX® Map Human Neuropeptide Magnetic Panel, HNPMAG-35 K (EMD Millipore Corporation, Billerica, MA, USA). This is a well-established Luminex technology kit that allows simultaneous quantification of BE and SP in the same assay, and contains all the necessary components (buffers, standards, and microplate) for the full assay procedure. BE and SP were extracted from both saliva and plasma by acetonitrile method precipitation according to the manufacturer's manual. Fifty microliters of the saliva samples and 50 μL of extracted plasma were analyzed in the Luminex 200 instrument (Life Technologies, Invitrogen Stockholm, Sweden). The concentrations were calculated by reference to a 7-point five-parameter logistic standard curve for each substance using MasterPlex QT 2010 (MiraiBio Inc., San Diego, CA, USA).
2.5. Statistical analysis
P ≤ 0.05 was considered statistically significant in all tests. The IBM Statistical Package for the Social Sciences (SPSS, IBM Corporation, Somers, NY, USA) version 24.0 was used. All data are reported as median (25th–75th percentiles) unless stated otherwise. For comparisons between groups the Mann-Whitney U-test or, for categorical data, the Chi-square test or Fisher's exact test were used. Spearman's nonparametric rank correlation coefficient (rs) was used for correlation analysis. When calculating BE quotients (BEQ) and SP quotients (SPQ) between saliva and plasma concentrations, all calculations are based on [saliva value]/[plasma value].
As stated above, the patients were part of a clinical trial of intrathecal ziconotide, and the saliva samples were hence collected based on “convenience”. Therefore, no pre-study sample size calculation pertaining to salivary levels of the studied neuropeptides was done. However, we effectuated a two-tailed, post hoc power calculation using the G*Power software (version 3.1.9.2) on salivary SP levels (we chose SP because it had no missing values, as opposed to salivary BE); see Results section.
2.6. Ethics
Ethical approval was granted by the Regional Ethics Committee in Linköping (RECL), Sweden (Dnr M136-06 and Dnr 2012/94-32). The clinical trial, from which patient data were derived, was conjointly approved by the Swedish Medical Products Agency (EudraCT 2010-018920-21) and by the RECL (Dnr 2011/48-31). The clinical trial was monitored by the Linköping Academic Research Centre and was conducted according to the standards of Good Clinical Practice.
3. Results
3.1. Saliva
Patients were significantly older than healthy controls, 58 (51–70) years vs 27 (22–40) years, P < 0.001. We found no correlation between age and salivary BE, neither in patients (rs = −0.029, P = 0.923), in healthy controls (rs = 0.077, P = 0.793), or in all individuals taken together (rs = −0.052, P = 0.794). We found no correlation between age and salivary SP, neither in patients (rs = −0.130, P = 0.658), in healthy controls (rs = 0.278, P = 0.265), or in all individuals taken together (rs = −0.087, P = 0.635). For other characteristics of patients and healthy controls and comparisons between the groups, see Table 1. BE could be detected in saliva in all patients, however it could only be detected in 14 out of 18 healthy controls due to technical errors when analyzing these samples. SP could be detected in saliva of all studied subjects. Therefore, BE saliva results are based on 14 patients and 14 controls, whereas SP saliva results are based on 14 patients and 18 controls.
Table 1.
Characteristics of patients and healthy controls.
| Patients |
Healthy Controls |
Statistics |
|
|---|---|---|---|
| (N = 14) | (N = 18) | P value | |
| Age in years | 58 (51–70) | 27 (22–44) | <0.001* |
| Sex (% female) | 36 | 39 | 1.000 |
| Pain intensity at inclusiona | 70 (59–79) | 0 | <0.001* |
| Pain duration in months | 69 (38–120) | 0 | <0.001* |
| Opioid doseb | 0 (0–75) | 0 | 0.007* |
| On opioids (%) | 36 | 0 | 0.010* |
| On tricyclics or duloxetine (%) | 29 | 0 | 0.028* |
| On gabapentinoids (%) | 29 | 0 | 0.028* |
| On paracetamolc (%) | 50 | 0 | 0.001* |
| On NSAIDc,d (%) | 7 | 0 | 0.438 |
Note: Data are presented as median (25th–75th percentiles) except for percentages. Furthest to the right is the result of the statistical comparisons between patients and healthy controls. * denotes statistically significant group differences.
Patients were asked to grade their average pain intensity for the last week on a visual analogue scale 0–100 mm (VASPI), whereas pain status in healthy controls was investigated by means of an extensive structured interview. All controls were free of pain.
In oral morphine equivalents, mg/day.
Excluding treatment “as needed.”
NSAID = non-steroidal anti-inflammatory drug.
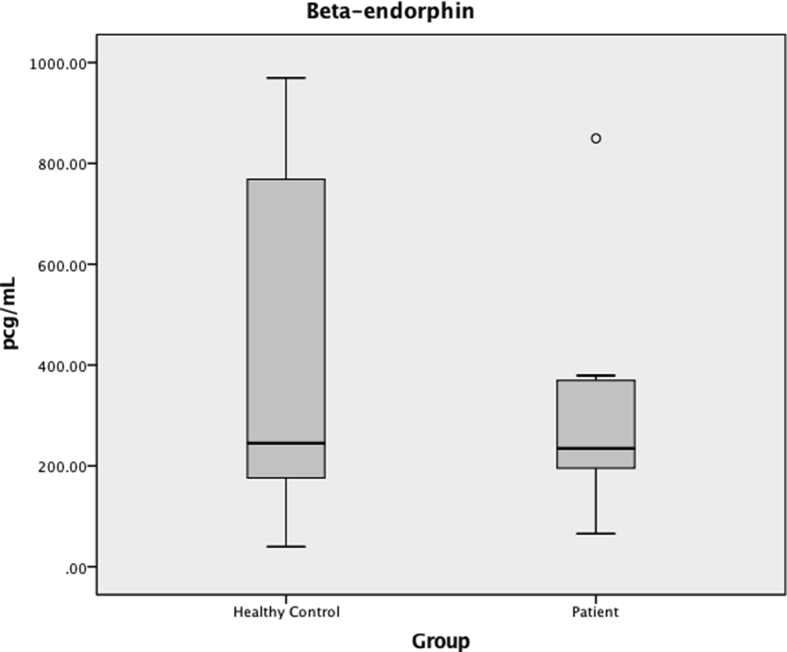
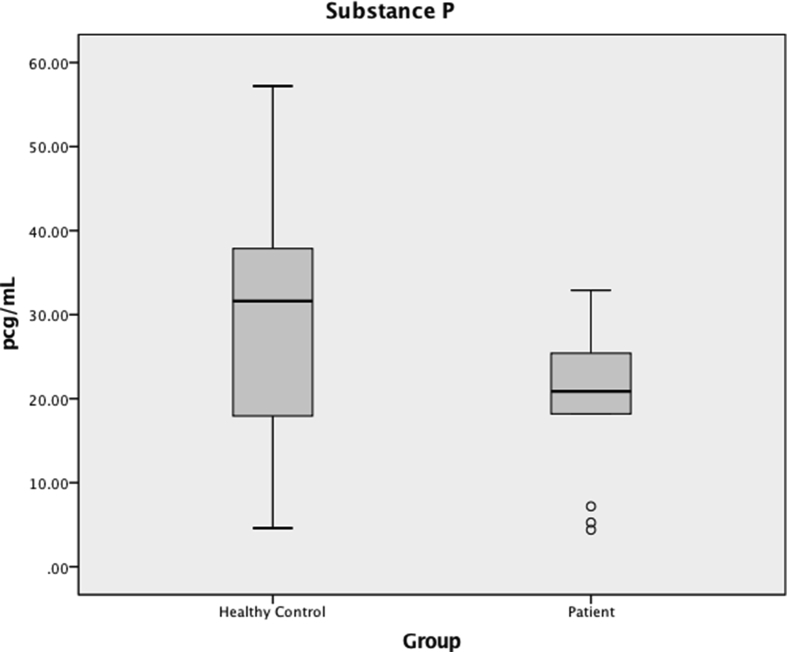
BE levels in saliva did not differ between groups (235 (183–371) pg/mL in patients vs 245 (162–779) pg/mL in healthy controls, P = 0.435) (Fig. 1). Likewise, SP levels in saliva did not differ between groups (21 (15–27) pg/mL in patients vs 32 (18–39) pg/mL in healthy controls, P = 0.111) (Fig. 2). When excluding patients on opioids, no differences were found between BE and SP levels in patients and healthy controls (BE 221 (170–374) pg/mL vs 245 (162–779) pg/mL, respectively, P = 0.529; SP 25 (12–32) pg/mL vs 32 (18–39) pg/mL, respectively, P = 0.237). Likewise, when excluding patients on tricyclics or duloxetine, no differences were found between BE and SP levels in patients and healthy controls (BE 241 (183–371) pg/mL vs 245 (162–779) pg/mL, respectively, P = 0.682; SP 21 (18–31) pg/mL vs 32 (18–39) pg/mL, respectively, P = 0.250).
Fig. 1.
Beta-endorphin (BE) in saliva, in pg/mL. Median values are represented by horizontal lines, and the interquartile ranges by boxes. The ends of the whiskers represent minimum and maximum values and dots represent outliers. There was no difference between salivary concentrations of BE in patients compared to healthy controls (P = 0.435).
Fig. 2.
Substance P (SP) in saliva, in pg/mL. Median values are represented by horizontal lines, and the interquartile ranges by boxes. The ends of the whiskers represent minimum and maximum values and dots represent outliers. There was no difference between salivary concentrations of SP in patients compared to healthy controls (P = 0.111).
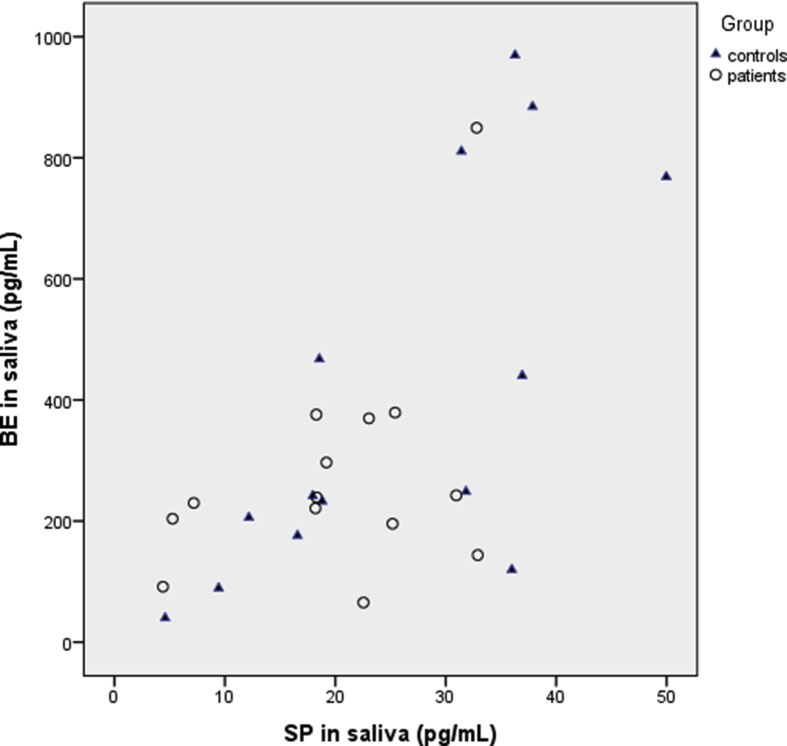
For all subjects taken together, we found a significant correlation between the levels of BE and SP in saliva (rs = 0.602, P = 0.001). A higher significant correlation was found between salivary BE and SP in healthy controls (rs = 0.741, P = 0.002) than in patients (rs = 0.305, P = 0.288) (Fig. 3). In patients, there was no correlation between pain VASPI and BE or SP, respectively, and there was no correlation between pain duration and BE or SP, respectively (data not shown).
Fig. 3.
Scatter plot of the relationship between substance P (SP) and beta-endorphin (BE) in saliva of chronic neuropathic pain patients (circles) and healthy controls (triangles). All values are in pg/mL.
3.2. Relationship between saliva and plasma levels
For plasma, BE and SP could be detected in all controls. However, BE and SP in plasma could only be detected in 12 out of 14 patients due to technical errors when analyzing these samples. BEQ was therefore calculated in 12 patients and 14 controls, and SPQ was calculated in 12 patients and 18 controls. Plasma levels (but not their relationship to salivary levels) have previously been published and are therefore not repeated here [14].
There was no correlation between saliva and plasma levels of BE (rs = 0.197, P = 0.334); this was also the case when looking at each group separately (data not shown). Likewise, there was no correlation between saliva and plasma levels of SP (rs = –0.077, P = 0.687); this was also the case when looking at each group separately (data not shown).
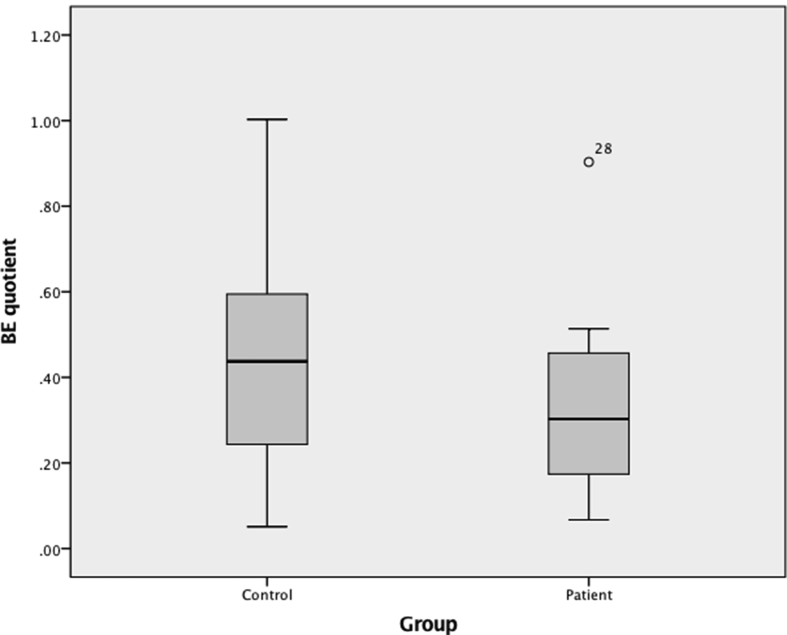
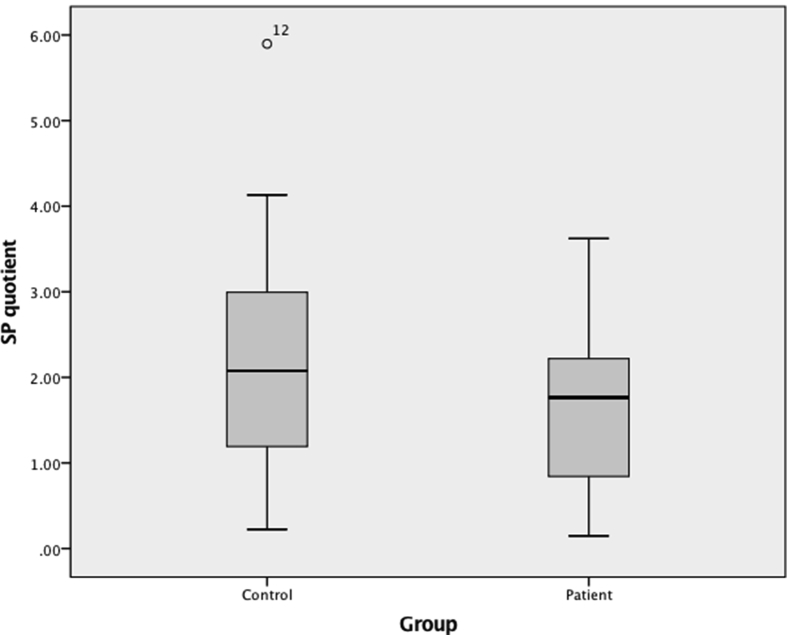
BEQ did not differ between patients and healthy controls (0.28 (0.17–0.47) vs 0.44 (0.22–0.64), respectively, P = 0.280), and neither did SPQ (1.87 (0.84–2.80) vs 2.08 (1.19–3.03), respectively, P = 0.611) (Table 2, and Figs. 4 and 5). For all subjects taken together (i.e., patients and healthy controls together) BEQ and SPQ were 0.35 (0.18–0.54) and 2.00 (1.09–2.95), respectively.
Table 2.
Quotients between saliva and plasma concentrations of beta-endorphin (BEQ) and substance P (SPQ).
| Variables | Patients | Healthy controls | Statistics |
|---|---|---|---|
| P value | |||
| BEQa | 0.28 (0.17–0.47) | 0.44 (0.22–0.64) | 0.280 |
| SPQb | 1.87 (0.84–2.80) | 2.08 (1.19–3.03) | 0.611 |
All quotients are based on [saliva value]/[plasma value] and are expressed as median (25th–75th percentiles).
These deviations from the original n:s are due to missing values for either BE or SP in either saliva or plasma, which in turn were caused by technical errors during analysis.
For BEQ, calculations are based on patients, n = 12 and healthy controls, n = 14.
For the SPQ, calculations are based on patients, n = 12 and healthy controls, n = 18.
Fig. 4.
Boxplots of beta-endorphin quotients (BEQ). Quotients between the groups did not differ (P = 0.280).
Fig. 5.
Boxplots of substance P quotients (SPQ). Quotients between the groups did not differ (P = 0.611). Note: one outlier (patient #22 with SPQ = 22.27) has been excluded from the SPQ boxplot in order to make interpretation easier.
For all subjects taken together, we found a significant correlation between BEQ and SPQ (rs = 0.391, P = 0.048); looking at each group separately, we found a better correlation in healthy controls than in patients (rs = 0.626, P = 0.017, and rs = –0.007, P = 0.983, respectively).
3.3. Post hoc power calculation
Mean salivary SP was 29 pg/mL in healthy controls. A post hoc power calculation showed that our sample size had a power of 88% to detect a difference between groups of at least 14.5 pg/mL (i.e., an increase or decrease of 50% in mean SP of patients compared with controls) at a pooled standard deviation of 12.5 pg/mL and alpha = 0.05.
4. Discussion
Although several previous studies have investigated neuropeptide levels in CSF and plasma in various chronic pain conditions [14, 23, 24, 25], fewer have investigated neuropeptide levels in saliva [16, 26]. To the best of our knowledge, no previous study has investigated both salivary BE and SP levels in saliva and in plasma from chronic neuropathic pain patients.
4.1. Salivary levels of BE and SP did not differ between groups
The primary finding in our study was that there was no difference between the groups' salivary peptide levels. Considering that SP is pro-nociceptive, it seems to make sense to hypothesize that chronic pain patients would have a general tendency towards higher SP levels. However, our current study shows that salivary SP was not elevated in patients.
Previous studies on the levels of salivary SP in chronic pain patients report conflicting results. One study found decreased levels of salivary SP in patients with chronic back pain syndromes [26], while others have shown higher levels of salivary SP in patients with chronic migraine [16]. Also, when looking at other body fluids such as CSF, the results are conflicting. One study reported lower levels of SP in CSF of chronic neuropathic pain patients [23], another showed a non-significant trend towards lower SP levels in patients [14], yet others have reported elevated levels of SP in CSF of patients with fibromyalgia [24, 25]. Similarly, when looking at plasma, findings are inconsistent. One study reported elevated levels of SP in plasma of patients with chronic migraine [16], and another reported no difference in SP levels between controls and patients with chronic tension-type headache [27]. It is possible that different chronic pain syndromes may be characterized by different combinations of increased or decreased protein concentrations in different body fluids, reflecting possible pathophysiological differences across different chronic pain conditions.
Salivary BE did not differ between groups in the present study. To the best of our knowledge, there is no other comparable study measuring salivary BE in chronic pain patients.
4.2. Salivary and plasma levels do not correlate, but we report interesting quotients
Because we found no correlation between plasma and saliva levels, we hypothesize that a substantial part of BE and SP found in saliva may be a result of production in the salivary glands, rather than the peptides being merely an ultra-filtrate from systemic circulation. However, our results are not in line with the findings of Jang et al [16] who found a significant correlation between plasma and saliva levels of SP. Of course, both mechanisms (i.e. ultrafiltration from plasma and direct local production in the salivary glands) may be at work simultaneously.
In general, saliva contains low concentrations of analytes [13], i.e. roughly a quarter of the protein concentration found in blood [4, 6]. From that perspective, our SPQ findings point to a significant production of SP in the salivary glands. This hypothesis is strengthened by the fact that the salivary glands are innervated [17], which may further point to a local salivary gland production of SP. The specific salivary roles of SP and BE, if any, remain elusive and more research is needed.
4.3. Correlations between salivary SP and salivary BE
We found a strong significant correlation between salivary BE and SP, and an even stronger correlation between salivary BE and SP in healthy controls than in chronic pain patients, the latter correlation being not significant. This is of interest from a physiological perspective as it may indicate that these peptides, in their physiological state, exist in a structured relationship to each other, an order that appears to become deranged when a person suffers from chronic neuropathic pain. This line of reasoning is strengthened by the fact that we could show a significant correlation between BEQ and SPQ in healthy controls but not in patients.
4.4. Limitations
A major limitation of this study is that patients were significantly older than healthy controls. Even though we could not find any correlation between age and the studied neuropeptides, this limitation has to be acknowledged. In future studies, it will be important to age-match participants in order to eliminate age as a possible confounder.
This study has some other limitations as well. Our study consisted of a small sample population which may have impacted the statistical significance in our calculations, perhaps indicating a type II error, i.e. a lack of “power”, not least for detecting subtle differences between groups (our post hoc power analysis notwithstanding). Moreover, the findings in this pilot study are based on a single measurement time point; the baseline variation within individuals needs to be investigated by multiple saliva samplings in future studies. Further, concomitant medicines are possible confounders which must be accounted for. However, we did not find any difference between groups when excluding patients prescribed opioids (n = 5) or tricyclics/duloxetine (n = 4), indicating that concomitant treatment with these drugs does not seem to affect the concentrations of salivary BE or SP.
5. Conclusion
In conclusion, we found no differences between the groups' salivary concentrations of BE and SP. However, the present study gives new, potentially important physiological information about how the salivary and plasma concentrations of SP and BE relate to each other. Salivary BE and SP do not hence seem to be indicators of neuropathic chronic pain propensity, but future more sophisticated studies could perhaps relate salivary levels of these neuropeptides to, e.g., conditioned pain modulation [28] or quantitative sensory testing data in order to perhaps predict the development of chronic pain after surgery [29].
Declarations
Author contribution statement
Thomas Kallman: Analyzed and interpreted the data; Wrote the paper.
Bijar Ghafouri: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Emmanuel Bäckryd: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by a grant from NEURO Sweden, and by a grant from Sinnescentrum, Region Östergötland. The authors would also like to thank the Region Östergötland Research and Development department for funding this study by providing a summer research grant (grant no. LIO-703411) to Thomas F Kallman.
Competing interest statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at The European Union Clinical Trials Register under the registration number 2010-018920-21.
References
- 1.Breivik H., Collett B., Ventafridda V., Cohen R., Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. S1090-3801(05)00086-8 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Croft P., van der Windt D., Boardman H., Blyth F.M. The potential for prevention: overview. In: Croft P., Blyth F.M., van der Windt D., editors. Chronic Pain Epidemiology: from Aetiology to Public Health. Oxford University Press; Oxford: 2010. pp. 345–358. [Google Scholar]
- 3.van Hecke O., Torrance N., Smith B.H. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013;111(1):13–18. doi: 10.1093/bja/aet123. [DOI] [PubMed] [Google Scholar]
- 4.Pfaffe T., Cooper-White J., Beyerlein P., Kostner K., Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin. Chem. 2011;57(5):675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- 5.Schulz B.L., Cooper-White J., Punyadeera C.K. Saliva proteome research: current status and future outlook. Crit. Rev. Biotechnol. 2013;33(3):246–259. doi: 10.3109/07388551.2012.687361. [DOI] [PubMed] [Google Scholar]
- 6.Jasim H., Olausson P., Hedenberg-Magnusson B., Ernberg M., Ghafouri B. The proteomic profile of whole and glandular saliva in healthy pain-free subjects. Sci. Rep. 2016;6:39073. doi: 10.1038/srep39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan W., Apweiler R., Balgley B.M., Boontheung P., Bundy J.L., Cargile B.J. Systematic comparison of the human saliva and plasma proteomes. Proteom. Clin. Appl. 2009;3(1):116–134. doi: 10.1002/prca.200800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiappin S., Antonelli G., Gatti R., De Palo E.F. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta. 2007;383(1–2):30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa J.M., Schafer C.A., Schafer J.J., Farrell J.J., Paster B.J., Wong D.T. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013;26(4):781–791. doi: 10.1128/CMR.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y.H., Wong D.T. Saliva: an emerging biofluid for early detection of diseases. Am. J. Dent. 2009;22(4):241–248. [PMC free article] [PubMed] [Google Scholar]
- 11.Lima D.P., Diniz D.G., Moimaz S.A., Sumida D.H., Okamoto A.C. Saliva: reflection of the body. Int. J. Infect. Dis. 2010;14(3):e184–e188. doi: 10.1016/j.ijid.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Ghafouri B., Tagesson C., Lindahl M. Mapping of proteins in human saliva using two-dimensional gel electrophoresis and peptide mass fingerprinting. Proteomics. 2003;3(6):1003–1015. doi: 10.1002/pmic.200300426. [DOI] [PubMed] [Google Scholar]
- 13.Choo R.E., Huestis M.A. Oral fluid as a diagnostic tool. Clin. Chem. Lab. Med. 2004;42(11):1273–1287. doi: 10.1515/CCLM.2004.248. [DOI] [PubMed] [Google Scholar]
- 14.Bäckryd E., Ghafouri B., Larsson B., Gerdle B. Do low levels of Beta-endorphin in the cerebrospinal fluid indicate defective top-down inhibition in patients with chronic neuropathic pain? A cross-sectional, comparative study. Pain Med. 2014;15(1):111–119. doi: 10.1111/pme.12248. [DOI] [PubMed] [Google Scholar]
- 15.Fischer H.P., Eich W., Russell I.J. A possible role for saliva as a diagnostic fluid in patients with chronic pain. Semin. Arthritis Rheum. 1998;27(6):348–359. doi: 10.1016/s0049-0172(98)80014-0. [DOI] [PubMed] [Google Scholar]
- 16.Jang M.U., Park J.W., Kho H.S., Chung S.C., Chung J.W. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis. 2011;17(2):187–193. doi: 10.1111/j.1601-0825.2010.01717.x. [DOI] [PubMed] [Google Scholar]
- 17.Patel S.A., Barros J.A. Introduction. In: Streckfus C.F., editor. Advances in Salivary Diagnostics. Berlin Springer; 2015. pp. 1–16. [Google Scholar]
- 18.Hauser-Kronberger C., Albegger K., Saria A., Hacker G.W. Neuropeptides in human salivary (submandibular and parotid) glands. Acta Otolaryngol. 1992;112(2):343–348. doi: 10.1080/00016489.1992.11665430. [DOI] [PubMed] [Google Scholar]
- 19.Konttinen Y.T., Hukkanen M., Kemppinen P., Segerberg M., Sorsa T., Malmstrom M. Peptide-containing nerves in labial salivary glands in Sjogren's syndrome. Arthritis Rheum. 1992;35(7):815–820. doi: 10.1002/art.1780350717. [DOI] [PubMed] [Google Scholar]
- 20.Bäckryd E. Pain in the blood? Envisioning mechanism-based diagnoses and biomarkers in clinical pain medicine. Diagnostics. 2015;5:84–95. doi: 10.3390/diagnostics5010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bäckryd E., Sorensen J., Gerdle B. Ziconotide trialing by intrathecal bolus injections: an open-label non-randomized clinical trial in postoperative/posttraumatic neuropathic pain patients refractory to conventional treatment. Neuromodulation. 2015;18:404–413. doi: 10.1111/ner.12293. [DOI] [PubMed] [Google Scholar]
- 22.Treede R.D., Jensen T.S., Campbell J.N., Cruccu G., Dostrovsky J.O., Griffin J.W. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 23.Almay B.G., Johansson F., Von Knorring L., Le Greves P., Terenius L. Substance P in CSF of patients with chronic pain syndromes. Pain. 1988;33(1):3–9. doi: 10.1016/0304-3959(88)90197-2. [DOI] [PubMed] [Google Scholar]
- 24.Vaeroy H., Helle R., Forre O., Kass E., Terenius L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain. 1988;32(1):21–26. doi: 10.1016/0304-3959(88)90019-X. [DOI] [PubMed] [Google Scholar]
- 25.Russell I.J., Orr M.D., Littman B., Vipraio G.A., Alboukrek D., Michalek J.E. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37(11):1593–1601. doi: 10.1002/art.1780371106. [DOI] [PubMed] [Google Scholar]
- 26.Parris W.C., Kambam J.R., Naukam R.J., Rama Sastry B.V. Immunoreactive substance P is decreased in saliva of patients with chronic back pain syndromes. Anesth. Analg. 1990;70(1):63–67. doi: 10.1213/00000539-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Ashina M., Bendtsen L., Jensen R., Ekman R., Olesen J. Plasma levels of substance P, neuropeptide Y and vasoactive intestinal polypeptide in patients with chronic tension-type headache. Pain. 1999;83(3):541–547. doi: 10.1016/S0304-3959(99)00159-1. [DOI] [PubMed] [Google Scholar]
- 28.Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain. 2015;156(Suppl. 1):S24–S31. doi: 10.1097/01.j.pain.0000460343.46847.58. [DOI] [PubMed] [Google Scholar]
- 29.Sangesland A., Storen C., Vaegter H.B. Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review. Scand. J. Pain. 2017;15:44–52. doi: 10.1016/j.sjpain.2016.12.002. [DOI] [PubMed] [Google Scholar]