Abstract
Animal models have shown that social isolation and other forms of social stress lead to depressive- and anxiety-relevant behaviors, as well as neuroendocrine and physiological dysfunction. The goal of this study was to investigate the effects of prior social isolation on neurotransmitter content following acute restraint in prairie voles. Animals were either paired with a same-sex sibling or isolated for four weeks. Plasma adrenal hormones and ex vivo tissue concentrations of monoamine neurotransmitters and their metabolites were measured following an acute restraint stressor in all animals. Isolated prairie voles displayed significantly increased circulating adrenocorticotropic hormone (ACTH) levels, as well as elevated serotonin and dopamine levels in the hypothalamus, and potentially decreased levels of serotonin in the frontal cortex. However, no group differences in monoamine levels were observed in the hippocampus or raphe. The results suggest that social stress may bias monoamine neurotransmission and stress hormone function to subsequent acute stressors, such as restraint. These findings improve our understanding of the neurobiological mechanisms underlying the consequences of social stress.
Keywords: prairie vole, dopamine, serotonin, hypothalamus, social stress
Introduction
Social bonds play an important role in mediating both physical and psychological health. The lack of social bonds negatively affects health and increases susceptibility to later stressors (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Kikusui, Winslow, & Mori, 2006; Uchino, 2006; Uchino, Uno, & Holt-Lunstad, 1999). In addition, individuals who are lonely experience high rates of mood disorders and physical health disturbances (Cacioppo, Hawkley, & Berntson, 2003; Cacioppo et al., 2002; Cacioppo, Hawkley, & Thisted, 2010; Kaplan et al., 1988; Shankar, McMunn, Banks, & Steptoe, 2011; Uchino, 2006). Exemplifying this, decreased social engagement is associated with an increased risk of cardiovascular disease mortality in men (Ramsay et al., 2008). Further, perceived social isolation is associated with elevated blood pressure, endocrine dysregulation, decreases in physical activity, and reduced cognitive performance and life satisfaction (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Cacioppo & Hawkley, 2009; Gow, Pattie, Whiteman, Whalley, & Deary, 2007; Hawkley, Masi, Berry, & Cacioppo, 2006; Hawkley, Thisted, & Cacioppo, 2009; Wen, Hawkley, & Cacioppo, 2006). Together, these studies indicate that the lack or loss of social relationships is associated with increased morbidity and mortality in humans.
In addition to evidence that social stressors have negative consequences on human health, the disruption of social bonds and social isolation are associated with behavioral and physiological disturbances in animals (Grippo et al., 2011; Grippo, Gerena, et al., 2007; Grippo, Lamb, Carter, & Porges, 2007; Manuck, Clarkson, Lusso, Taub, & Miller, 1983; Peuler, Scotti, Phelps, McNeal, & Grippo, 2012; Shively et al., 2005). For example, adult female cynomolgus monkeys living as socially stressed subordinates display low levels of physical activity, autonomic dysregulation, endocrine disturbances, and increased mortality (Shively et al., 2005; Shively, Laber-Laird, & Anton, 1997; Shively, Grant, Ehrenkaufer, Mach, & Nader, 1997; Watson, Shively, Kaplan, & Line, 1998; Shively, 1998; Williams, Shively, & Clarkson, 1994). Rats and mice exposed to social subordination or defeat stress show depression- and anxiety-like behaviors, disturbances in corticosterone and altered stress response feedback to new stressors (Carnevali et al., 2012; Davies et al., 2016; Haller, Halasz, & Makara, 2000; Heinrichs et al. 1994; Lukkes, Mokin, Scholl, & Forster, 2009; Pisu et al., 2016; Ruis et al., 1999; Weiss et 2004). These studies demonstrate that social stress negatively affects the behavior and physiological health of animals, thus enabling them to be used as tools for understanding the mechanisms underlying deleterious effects of environmental stress. An example of an animal model that has been used to investigate the neurobiological mechanisms underlying responses to social stress is the prairie vole.
Prairie voles are rodents that form monogamous social bonds similar to humans (Carter, DeVries, & Getz, 1995; Carter & Getz, 1993; Young & Wang, 2004), display depression- and anxiety-relevant behaviors, and several physiological alterations following long-term social isolation (Grippo, Cushing, & Carter, 2007; Grippo, Gerena, et al., 2007; Grippo et al., 2012). Isolated adult prairie voles show physiological disruptions similar to other species including autonomic dysregulation and elevated stress hormones during periods of low activity and when exposed to additional short-term stressors (Grippo, Gerena, et al., 2007; Grippo, Sgoifo, Mastorci, McNeal, & Trahanas, 2010; McNeal et al., 2014). Further, both young and adult prairie voles display elevations in plasma corticosterone and increased immunostaining of corticotropin-releasing hormone cells and fibers in the paraventricular nucleus of the hypothalamus as a function of social isolation (Grippo, Gerena, et al., 2007; Ruscio, Sweeny, Hazelton, Suppatkul, & Carter, 2007). The accumulated previous evidence that social isolation in prairie voles is associated with behavioral and physiological changes also reported in humans who experience isolation as a chronic stressor indicates that this species is a valuable animal model for investigating the complex interactions of social isolation with brain function. Combined, the previous findings using the prairie vole model provide evidence that social stress contributes to poor health outcomes.
The prairie vole model can be used to better understand how chronic social stress may alter the neurobiological mechanisms regulating the response to acute stressors. Exposure to stress alters central neurotransmitter systems, and these changes are hypothesized to mediate the behavioral and physiological effects observed following stress, including social stress (McKittrick, Blanchard, Hardy, & Blanchard, 2009). Social separation of male rats for 14 days increased serotonin turnover in the hippocampus (dos Santos, de Andrade, & Graeff, 2010), and this has been replicated following chronic variable stress as evidenced by an increase in 5-hydroxyindoleacetic acid (5HIAA)/serotonin ratio (Gamaro, Manoli, Torres, Silveira, & Dalmaz, 2003). Further, subordinate rats living in a complex social system displayed greater serotonin metabolite levels, 5HIAA, in several forebrain regions and in the preoptic area of the hypothalamus compared to dominant rats (Blanchard et al., 1991). Social isolation rearing decreased dopamine and its metabolite, 3, 4-dihydroxyphenylacetic acid (DOPAC), in the frontal cortex in rats (Möller, Du Preez, Viljoen, Berk, & Harvey, 2013), while three episodes of social defeat increased DOPAC in forebrain regions in mice (Puglisi-Allegra & Cabib, 1990). In vivo neurotransmitter monitoring has demonstrated that prior exposure to repeated social stress or isolation potentiates stress-induced increases in dopamine and serotonin release in the prefrontal cortex following an additional stressor (Ago et al., 2013; Miura, Qiao, Kitagami, Ohta, & Ozaki, 2005; Tidey & Miczek, 1996). The increased neurotransmitter response to an acute stressor suggests that prior exposure to chronic social stress affects the neurotransmitter system’s ability to respond to a subsequent stressor, which could then influence hypothalamic-pituitary-adrenal (HPA) activity, subsequent behavior, and overall health.
Although the effects of social stress on brain function have been investigated in rats and mice, there has been little study of neurotransmitter changes in the prairie vole following social isolation. Therefore, the current experiment measured neurotransmitter and metabolite levels in key neuronal structures that play a role in regulating responses to stress, including the hippocampus, hypothalamus, frontal cortex, and raphe (Ago et al., 2013; Cryan, Valentino, & Lucki, 2005; Dent & Neill, 2012; Dronjak & Gavrilovic, 2006; Krystal & Neumeister, 2009; Möller et al., 2013). Specifically, this study assessed dopamine, serotonin, and their metabolites, as well as circulating hormones levels, following an acute restraint stressor in prairie voles that experienced prior long-term social isolation or control (paired) housing. We hypothesized that social isolation would negatively influence neurotransmitter levels and neuroendocrine function following acute restraint stress, compared to paired control housing.
Methods
Animals
Fifteen male prairie voles (60–90 days old) were used from the colony at Northern Illinois University. All prairie voles were removed from breeding pairs at 21 days of age, and housed in same-sex sibling pairs until the commencement of experimentation. Animals were allowed ad libitum access to food and water, maintained at a room temperature of 20–21°C and a relative humidity of 40–50%, under a standard 14:10 light/dark cycle (lights on at 06:30). All experimental protocols were approved by the Northern Illinois University Institutional Animal Care and Use Committee and followed National Institute of Health guidelines as stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
General Procedure
Prairie voles were randomly divided into two groups of either paired (control; n = 8 isolated (n = 7) conditions for four weeks. After this period, all animals were exposed to a 60 minute restraint session (Smith, Lieberwirth, & Wang, 2013). Immediately following the restraint, plasma and brain tissue samples were collected. Serotonin, dopamine, and their metabolites were assayed via high-performance liquid chromatography (HPLC) from tissue dissections of the frontal cortex, hippocampus, hypothalamus, and raphe. Finally, circulating adrenocorticotropic hormone (ACTH) and corticosterone were measured via enzyme-linked immunosorbent assay (ELISA) from plasma samples. All handling, cage changing, and collection of tissue and plasma (described below) were matched between the two groups.
Housing Conditions
Isolated animals were separated from their siblings and housed individually for four weeks, without visual, olfactory or auditory cues from the sibling. Paired control animals were continually housed with their same-sex siblings. For each group, only one animal from each pair was used in this investigation. The four-week manipulation period was selected based on previous results demonstrating that this time frame is sufficient to induce disruptions of affective behaviors and associated health consequences (Grippo et al., 2010; Grippo, Cushing, et al., 2007; Grippo, Gerena, et al., 2007).
Restraint
All prairie voles were exposed to 60 minutes of restraint during the light period in a custom-designed apparatus (Pournajafi-Nazarloo et al., 2009). Briefly, each animal was placed in a wire mesh screen that wrapped around the animal and was closed with binder clips (Dayas, Buller, Crane, Xu, & Day, 2001).
Sample Collection
Immediately following the 60-minute restraint session, animals were anesthetized for sample collection using procedures described previously (Grippo, Gerena, et al., 2007; McNeal et al., 2014). Briefly, voles were deeply anesthetized with a mixture of ketamine (67 mg/kg, subcutaneous (sc); NLS Animal Health, Owings Mills, MD) and xylazine (13.33mg/kg, sc; NLS Animal Health). Blood was sampled within two minutes of the anesthetic injection, from the periorbital sinus via a heparinized capillary tube, and was collected during a period not exceeding 1.5 minutes. The blood was placed immediately on ice, and then centrifuged at 4°C, at 3500rpm, for 15 minutes to obtain plasma. Plasma aliquots were stored at −80°C until assayed for circulating ACTH and corticosterone via ELISA. Immediately after blood was collected, animals were sacrificed and brains were carefully removed. The hypothalamus, raphe, frontal cortex, and hippocampus were dissected, placed in Eppendorf tubes, frozen on dry ice, and then stored at −80°C until assayed for neurotransmitter and metabolite levels via high performance liquid chromatography (HPLC).
Enzyme-Linked Immunosorbent Assay
Stress hormone levels were determined using commercially available ELISA kits (ACTH, EK-001–21, Phoenix Pharmaceuticals, Burlingame, CA; corticosterone, ADI-900–097, Enzo Life Sciences, Farmingdale, NY). Plasma samples were analyzed according to the kit instructions, and diluted to give results reliably within the linear portion of the standard curve (ACTH, 1:7; corticosterone, 1:500). The sensitivity of the kit for ACTH is 0.08 ng/ml (range 0–25 ng/ml), and for corticosterone is 27.0 pg/ml (range 32–20,000 pg/ml).
High Performance Liquid Chromatography
For the neurotransmitter measures, dissected brain tissue samples were thawed, sonicated in 0.1 M perchloric acid and then centrifuged at 11,000 × g for 6 minutes. The supernatant was assayed for dopamine and dopamine metabolites homovanillic acid (HVA) and DOPAC, as well as, serotonin and the serotonin metabolite 5HIAA, using HPLC procedures described previously (Matuszewich & Yamamoto, 2004). Protein content was determined for each sample using the Quick Start Bradford protein assay (Bio-Rad, Hercules, CA, USA) and read on a Multiscan Plus microplate reader at 620 nm (Thermo Electron Corporation, Waltham, MA, USA).
Tissue samples were analyzed for dopamine, serotonin, and metabolites with HPLC electrochemical detection. A Rheodyne injector (Cotati, CA, USA) with a 20 μl loop delivered the sample onto a reverse phase Synergi 4 C18 column 150 mm × 2 mm (Phenomenex, Torrance, CA). A Shimadzu 10 ADVP pump continuously pumped mobile phase (32 mM citric acid, 54.3 mM sodium acetate, 0.074 mM ethylenediaminetetraacetic acid, 0.32 mM octyl sodium sulfate and 3% methanol) at a flow rate of 0.20 ml/minute. Compounds were detected with an LC-4B amperometric detector (Bioanalytical Systems, West Lafayette, IN, USA), with a 3 mm glassy carbon working electrode maintained at a potential of +0.5V relative to an Ag/AgCl reference electrode. Data were collected using ChromPerfect Spirit Software (Justice Innovations, Inc., Denville, NJ, USA).
Statistical Analyses
Statistical analyses of intracellular neurotransmitter content in the hippocampus, raphe, hypothalamus, and frontal cortex, and circulating hormones, were compared between prairie voles that were previously isolated or pair housed using independent-samples t-tests for each dependent variable. Effect size was calculated using Cohen’s d by taking the mean difference and dividing by the pooled standard deviation. For interpretation, a small effect size was classified as d=.2, a medium effect size was classified as d=.5 and a large effect size was classified as d=.8 and greater (Cohen, 1988). The data presented in the figures represent means ± standard error of the mean (SEM). All data were analyzed using SPSS 22 (IBM Corporation Somers, NY, USA). A probability value of p<.05 was considered statistically significant. Using HPLC, we were unable to assess metabolites in some brain regions because of low metabolite levels, however, the attrition of sample size was similar between both conditions. The range of samples sizes for metabolites varies by brain region (hippocampus n= 3–5, raphe n= 4–6, frontal cortex n= 4–7, hippocampus n= 5–8).
Results
Serotonin
Serotonin levels in the hypothalamus were significantly greater in prairie voles previously isolated compared to pair house animals following the restraint stressor (t(13)=2.985, p=.011; d =1.49; see Figure 1). Frontal cortex serotonin was marginally decreased in animals that were previously isolated compared to animals that were pair housed (t(9)= −2.058, p=.070; d =1.43). There were no group differences in serotonin levels in the hippocampus (t(13)= .648) or in the raphe (t(10) = −1.475) following restraint stress.
Figure 1.
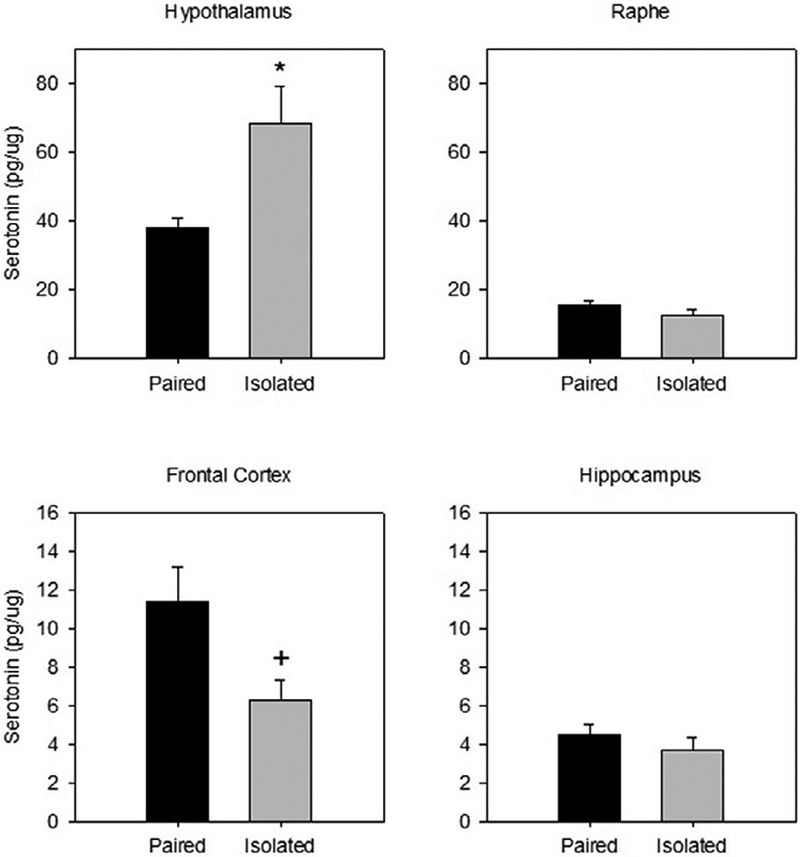
Mean (± SEM) serotonin concentration observed in the hypothalamus (top left), raphe (top right), frontal cortex (bottom left), and hippocampus (bottom right) following 60 minutes of restraint in prairie voles exposed to prior social isolation or pairing. Social isolation was associated with altered serotonin levels in the hypothalamus and frontal cortex but not in the hippocampus or raphe. * indicates p<.05 vs. paired group, + indicates p<.10 vs. paired group. Note the scale differences among the 4 panels.
Despite differences in serotonin levels in the hypothalamus and frontal cortex, there were no differences between paired and isolated animals in the serotonin metabolite, 5HIAA in any of the brain regions (hypothalamus: t(4)= 1.888; frontal cortex: t(9) = .456; hippocampus: t(13)= − 1.319; raphe: t(8) = 1.306; see Table 1). There were also no group differences in the turnover ratio of 5HIAA to serotonin (hypothalamus: t(4)= −.673; frontal cortex: t(9) = 1.351; hippocampus: t(13)= 738; raphe: t(10) = 1.560; see Table 1).
Table 1.
Mean (± SEM) metabolite and turnover ratio within the hypothalamus, raphe, frontal cortex, and hippocampus following 60 minutes of restraint in prairie voles exposed to prior social isolation or pairing. There were no statistically significant differences in metabolite or turnover ratios between the two groups. + indicates p<.10 vs. paired group; - indicates that the metabolite was not detectable. 5HT=serotonin, DA=dopamine.
| Metabolites | Turnover Ratio | |||||
|---|---|---|---|---|---|---|
| 5HIAA | DOPAC | HVA | 5HIAA/5HT | DOPAC/DA | HVA/DA | |
| Hypothalamus | ||||||
| Paired | 41.55 ± 0.41 | 0.56 ± 0.10 | 1.85 ± 0.66 | 1.32 ± 0.05 | 0.07 ± 0.01 | 0.29 ± 0.10 |
| Isolated | 74.51 ± 17.45 | 1.04 ± 0.38 | 3.09 ± 0.25 # | 1.21 ± 0.15 | 0.08 ± 0.03 | 0.34 ± 0.09 |
| Raphe | ||||||
| Paired | 2.24 ± 0.22 | 4.79 ± 0.70 | - | 0.31 ± 0.02 | 0.04 ± 0.01 | - |
| Isolated | 3.47 ± 1.14 | 4.45 ± 0.33 | - | 0.38 ± 0.04 | 0.05 ± 0.01 | - |
| Frontal Cortex | ||||||
| Paired | 1.22 ± 0.44 | 0.68 ± 0.15 | 0.66 ± 0.36 | 0.11 ± 0.03 | 2.32 ± 0.68 | 1.53 ± 0.61 |
| Isolated | 1.59 ± 0.76 | 0.54 ± 0.08 | - | 0.24 ± 0.12 | 1.31 ± 0.50 | - |
| Hippocampus | ||||||
| Paired | 19.80 ± 2.39 | 0.31 ± 0.15 | 0.94 ± 0.22 | 5.28 ± 1.47 | 1.96 ± 1.00 | 4.27 ± 1.10 |
| Isolated | 15.33 ± 2.38 | 0.07 ± 0.01 | 0.85 ± 0.15 | 4.10 ± 0.23 | 0.43 ± 0.05 | 5.00 ± 0.84 |
Dopamine
Prairie voles that were previously isolated had significantly greater dopamine in the hypothalamus following restraint compared to those that were previously paired (t(13)= 2.372 p=.034; d =1.19; see Figure 2). However, there were no group differences in dopamine levels in the frontal cortex (t(9) = 1.476), hippocampus (t(13)= −1.088), or raphe (t(10) = −1.636).
Figure 2.
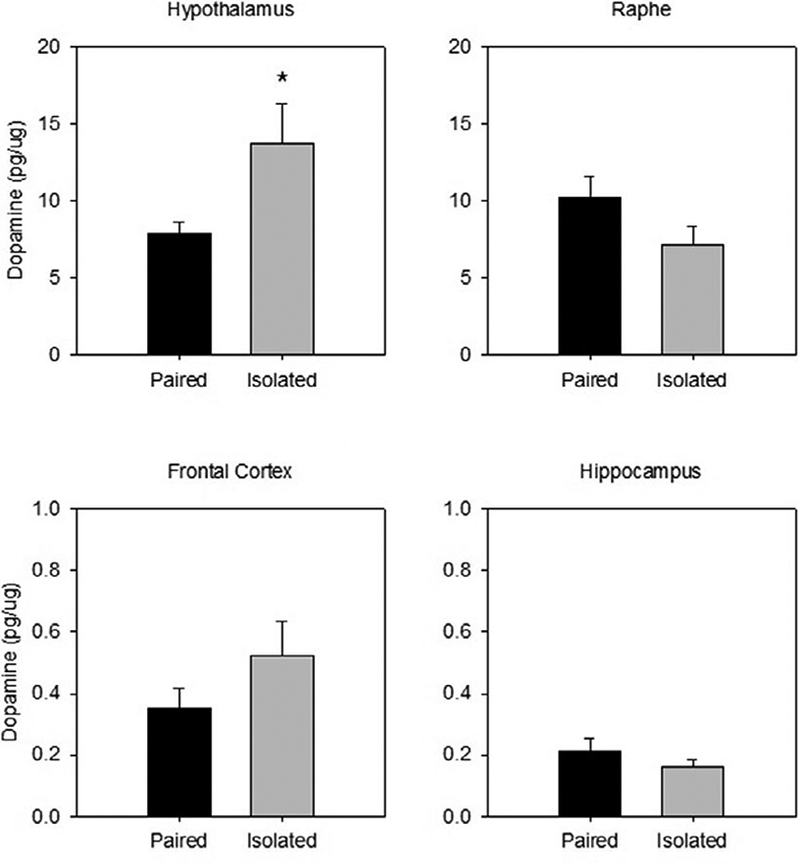
Mean (± SEM) dopamine concentration observed in the hypothalamus (top left), raphe (top right), frontal cortex (bottom left), and hippocampus (bottom right) following 60 minutes of restraint in prairie voles exposed to prior social isolation or pairing. Social isolation was associated with altered dopamine levels in the hypothalamus but not in the other brain regions. * indicates p<.05 vs. paired group. Note the scale differences among the 4 panels.
Similar to serotonin metabolite, there were no group differences in the dopamine metabolite, DOPAC, in the brain regions of interest (hypothalamus: t(6)= 1.546; frontal cortex: t(9) = −.647; hippocampus: t(13)= −1.441; raphe: t(10) = −.454). However, homovanillic acid (HVA) in the hypothalamus was slightly higher in isolated compared to paired animals (t(6)= 2.114, p=.079; d =1.38), while there was no group difference in the hippocampus (t(9)= −.340). There were also no group differences in the ratio of DOPAC to dopamine (hypothalamus: t(6)= .672; frontal cortex: t(9) = −1.028; hippocampus: t(13)= −1.425; raphe: t(10) = .836) or the ratio of HVA to dopamine (hypothalamus: t(6)= .371; hippocampus: t(9)= .512; see Table 1).
ACTH and Corticosterone
Prairie voles that were previously isolated displayed higher plasma levels of ACTH following the restraint compared to animals that were pair housed (t(13) = 2.476, p=.028; d=1.23; Figure 3). Corticosterone levels did not differ following restraint stress in animals that were previously isolated or pair housed (t(13)= .326; Figure 3).
Figure 3.
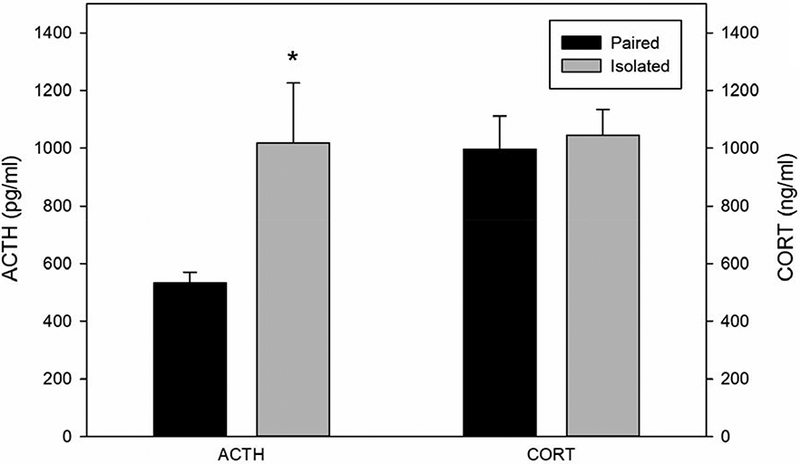
Mean (± SEM) circulating levels of adrenocorticotropic hormone (ACTH) and corticosterone following 60 minutes of restraint in prairie voles exposed to prior social isolation or pairing. Social isolation was associated with increased circulating ACTH but not corticosterone. * indicates p<.05 vs. paired group.
Discussion
The current study investigated the influence that prior social isolation has on neurotransmitter content following acute restraint in prairie voles. Although several studies have focused on the effects of acute or chronic stress on neurotransmission (Ago et al., 2013; Cryan, Valentino, & Lucki, 2005; Dent & Neill, 2012; Dronjak & Gavrilovic, 2006; Krystal & Neumeister, 2009; Möller et al., 2013), it is unknown whether the same neurotransmitter alterations occur as a function of exposure to a combination of chronic and acute stress in prairie voles. Therefore, the current study used the prairie vole model to assess the influence of chronic social isolation or paired housing on tissue neurotransmitter and metabolite concentrations in brain regions that are relevant to stress and circulating hormone concentrations, following subsequent acute restraint. Isolated prairie voles displayed significantly increased serotonin and dopamine levels in the hypothalamus and marginally decreased serotonin levels in the frontal cortex, as well as increased circulating ACTH following restraint, versus pair housed controls. There were no observed group differences in circulating corticosterone or in neurotransmitter, metabolite or ratio of neurotransmitters to metabolite levels in the hippocampus or raphe. Combined, these alterations in neurotransmission offer unique insight into possible mechanisms by which chronic social stress can adversely influence subsequent responses to acute stress.
The present study demonstrated that social isolation influenced central serotonin function in key brain regions associated with stress, emotion, and physiological functioning. Specifically, socially isolated prairie voles showed significantly increased serotonin in the hypothalamus and marginally significantly decreased frontal cortex serotonin following acute restraint. Alterations in central serotonin are associated with both behavioral and physiological dysregulation (Jacobs & Fornal, 1991). In the hypothalamus, serotonin may coordinate behavioral and endocrine responses to stressors, as well as downstream cardiovascular function (Van de Kar & Blair, 1999). For example, serotonin plays a significant role in behaviors that are disrupted during stress (e.g., mood, sleep), and depression is associated with changes in central serotonin signaling (e.g., impaired serotonin synthesis or release, malfunctions at postsynaptic serotonin receptors, alterations in serotonin transporter density) (Cryan, Valentino, & Lucki, 2005; Grippo et al., 2005; Lucki, 1998; Meltzer et al., 2004; Ressler & Nemeroff, 2000; Way & Taylor, 2010). Additionally, altered central serotonin levels have been found in rodents with myocardial ischemia, and a specific serotonin transporter gene polymorphism has been associated with a higher risk of myocardial infarction in men who survived an initial heart attack (Fumeron et al 2002; Sole, Versteeg, de Kloet, Hussain, & Lixfeld, 1983). The elevated hypothalamic serotonin levels displayed by socially isolated animals following restraint may therefore be one mechanism through which social and environmental stressors lead to dysregulation of nuclei that control biological systems and associated behavioral consequences (Nalivaiko,2006).
In addition to serotonin, dopamine neurotransmission is also associated with stress responses and emotion dysregulation (Gambardella, Greco, Sticchi, Bellotti, & Renzo, 1994; Kehoe, Shoemaker, Arons, Triano, & Suresh, 1998; Tidey & Miczek, 1996). The present study demonstrated that prairie voles housed in isolation exhibited significantly increased dopamine in the hypothalamus following restraint compared to prairie voles that were pair housed. These findings are similar to those of Dronjak & Gavrilovic (2006), who observed greater dopamine stores in the hypothalamus following 120 minutes of restraint in rats previously exposed to 21 days of social isolation, compared to group housed rats. The increased dopamine in the hypothalamus in isolated prairie voles observed in the present study may be associated with alterations in hedonic behavior. For instance, prairie voles exposed to long-term social isolation showed a decrease in sucrose intake and preference, suggestive of anhedonia (Grippo, Cushing, & Carter, 2007; Grippo, Gerena, et al., 2007).
The observations of metabolite and metabolite/neurotransmitter ratios in the current study may offer a more complex interpretation of central nervous system changes following a combination of social isolation and acute restraint. Differences in the pathways of dopamine metabolites, HVA and DOPAC, may contribute to the disparity in findings between metabolites (Elsworth & Roth, 1997), and between dopamine and metabolites (for review see Meiser, Weindl, & Hiller, 2013). In the current study, HVA in the hypothalamus showed an upward trend in isolated prairie voles, following a similar pattern of dopamine content in the hypothalamus. Conversely, the other dopamine metabolite DOPAC did now show this trend. Dopamine is metabolized into DOPAC through monoamine oxidase both intra- and extracellularly, whereas HVA is metabolized further via catechol-O-methyl transferase which is exclusively localized extracellularly (for review see Meiser, Weindl, & Hiller, 2013). Thus, DOPAC levels can be influenced by many different synaptic processes such as reuptake and recycling of dopamine, availability of monoamine oxidase, and then the rate of further metabolism to HVA (for review see Meiser, Weindl, & Hiller, 2013). The increase in monoamines in the hypothalamus a without significant changes in metabolite levels may indicate that neurotransmitter synthesis is higher in isolated prairie voles following restraint stress.
In the current experiment with all groups exposed to restraint stress, the increased neurotransmitter content may reflect an increase in synthesis due to chronic isolation rather than the interaction between acute stress and chronic isolation. Similarly, the current study found an increase in hypothalamic serotonin tissue content and a trending decrease in the frontal cortex in prairie voles that were previously isolated, with no differences in 5HIAA. These findings may be the result of increased synthesis of serotonin, while also having a decrease in metabolism resulting in similar 5HIAA levels. Conversely, there may be different levels of reuptake of serotonin from the synapse, resulting in similar levels of degradation and therefore similar levels of 5HIAA. To better understand the impact of chronic isolation with and without an acute stressor, future research could use an in vivo model of measuring both basal and stimulated monoamine and metabolite levels.
In addition to the central measures in this study, corticosterone and ACTH were measured following restraint, to better understand the influence that chronic isolation had on stress-induced alterations of the HPA axis. Relative to paired prairie voles, isolated animals in the current study displayed increased ACTH levels following restraint; however, corticosterone did not differ between paired and isolated groups. This finding is not entirely consistent with previous studies that have investigated the combination of isolation and an acute stressor using the prairie vole model. For example, social isolation combined with a 5 minute resident-intruder stressor elevated neither ACTH nor corticosterone in male prairie voles (however these hormones were elevated in female prairie voles; Grippo, Gerena, et al., 2007). By contrast, social isolation combined with a 5 minute forced swimming stressor increased both ACTH and corticosterone in male and female prairie voles (McNeal et al., 2014). The inconsistencies in HPA axis reactivity to a combination of social isolation and additional acute stressors may be a function of the specific type of acute stressor employed. HPA axis reactivity may also differ as a function of the timing or predictability of the acute stressor. For example, female prairie voles exposed to restraint at predictable times displayed a habituation of corticosterone levels; however, those that received restraint sessions at unpredictable times did not habituate (Smith, Lieberwirth, & Wang, 2013). Further research is therefore necessary to determine the specific conditions under which social isolation produces exaggerated HPA axis reactivity to additional acute stressors.
Taken together, the present results demonstrate that social isolation may bias neurotransmission toward a subsequent acute stressor in global brain regions associated with stress, emotion, behavior, and endocrine regulation. The lack of group differences in dopamine and serotonin in the hippocampus, frontal cortex, and raphe in the present study may be a function of the analysis of entire brain regions, therefore preventing conclusions regarding possible subregion changes. For example, the ventral hippocampus has been related to stress whereby the dorsal hippocampus is related to both stress and cognitive functions (Fanselow & Dong, 2010). Social isolation may have produced changes in one subregion of the hippocampus such as the ventral, but not dorsal, region. Future studies will extend the current findings by incorporating discrete subregion analyses and experimental approaches that would allow for better localizations of neurotransmitter alterations.
The changes in neurotransmitter concentrations and hormone expression observed in the present study provide evidence that long-term social isolation influences the neurobiological responses to an acute stressor. Mechanistically, stress may bias an organism to negative affective behaviors by decreasing serotonergic neurotransmission from the raphe to the prefrontal cortex (via GABAergic inhibition of transmission in the raphe; Cryan et al., 2005). Further, chronic stress has been shown to decrease dendritic spine density, branch, and length within the prefrontal cortex (Holmes & Wellman, 2009; Radley et al., 2006), which may suggest emotional dysregulation and cognitive inflexibility. Stress also influences the hypothalamus which is involved in both the HPA axis and the hypothalamic-pituitary-gonadal axis. Because of the involvement in both axes, alterations within the hypothalamus can affect a variety of hormones, thereby influencing behaviors ranging from reproduction to stress responses (for review see Toufexis, Rivarola, Lara, & Viau, 2014). Together these neurobiological changes may be associated with decreased intracellular serotonin within the frontal cortex as a function of long-term social isolation. Given the significant reliance on the social environment in prairie voles, studies that focus on central neurotransmission may reveal a unique pattern of responses (versus other rodent species whose behavior and physiology are comparatively less dependent on the surrounding social context). The current findings provide a foundation for future studies using the prairie vole model to improve our understanding of social and behavioral influences on central nervous system functioning.
Acknowledgments
We would like to thank the following individuals for their assistance: Stephanie Allen, Danielle Chandler, Susan Bates, and Melissa-Ann Scotti, PhD.
This work was supported by the NIH under Grant HL 112350 (AJG). The first two authors, NM and EA, contributed equally to the work reported in this paper.
References
- Adam EK, Hawkley LC, Kudielka BM, & Cacioppo JT (2006). Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences, 103(45), 17058–17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y, Araki R, Tanaka T, Sasaga A, Nishiyama S, Takuma K, & Matsuda T (2013). Role of social encounter-induced activation of prefrontal serotonergic systems in the abnormal behaviors of isolation-reared mice. Neuropsychopharmacology, 38(8), 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Cholvanich P, Blanchard RJ, Clow DW, Hammer RP Jr., Rowlett JK, & Bardo MT (1991). Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res, 568(1–2), 61–66. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, & Hawkley LC (2009). Perceived social isolation and cognition. Trends in Cognitive Sciences, 13(10), 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, & Berntson GG (2003). The anatomy of loneliness. Current Directions in Psychological Science, 12(3), 71–74. [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, . . . Berntson GG (2002). Loneliness and health: Potential mechanisms. Psychosom Med, 64(3), 407–417. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, & Thisted RA (2010). Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol Aging, 25(2), 453–463. doi: 10.1037/a0017216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L, Mastorci F, Graiani G, Razzoli M, Trombini M, Pico-Alfonso MA, ... Sgoifo A (2012). Social defeat and isolation induce clear signs of a depression-like state, but modest cardiac alterations in wild-type rats. Phsyiology & Behavior, 106, 142–150. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, & Getz LL (1995). Physiological substrates of mammalian monogamy: The prairie vole model. Neuroscience and Biobehavioral Reviews, 19, 303–314. [DOI] [PubMed] [Google Scholar]
- Carter CS, & Getz LL (1993). Monogamy and the prairie vole. Scientific American, 268, 100–106. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cryan JF, Valentino RJ, & Lucki I (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev, 29(4–5), 547–569. doi: 10.1016/j.neubiorev.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Davies DR, Olson D, Meyer DL, Scholl JL, Watt MJ, Manzerra P, . . . Forster GL (2016). Mild traumatic brain injury with social defeat stress alters anxiety, contextual fear extinction, and limbic monoamines in adult rats. Front Behav Neurosci, 10, 71. doi: 10.3389/fnbeh.2016.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, & Day TA (2001). Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci, 14(7), 1143–1152. [DOI] [PubMed] [Google Scholar]
- Dent MF, & Neill DB (2012). Dose-dependent effects of prefrontal dopamine on behavioral state in rats. Behav Neurosci, 126(5), 620–639. doi: 10.1037/a0029640 [DOI] [PubMed] [Google Scholar]
- dos Santos L, de Andrade TG, & Graeff FG (2010). Social separation and diazepam withdrawal increase anxiety in the elevated plus-maze and serotonin turnover in the median raphe and hippocampus. J Psychopharmacol, 24, 725–731. doi: 10.1177/0269881109106954 [DOI] [PubMed] [Google Scholar]
- Dronjak S, & Gavrilovic L (2006). Effects of stress on catecholamine stores in central and peripheral tissues of long-term socially isolated rats. Brazilian Journal of Medical and Biological Research, 39(6), 785–790. [DOI] [PubMed] [Google Scholar]
- Elsworth JD & Roth RH (1997). Dopamine synthesis, uptake, metabolism, and receptors: Relevance to gene therapy of Parkinson’s disease. Exp Neurol, 144, 4–9. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, & Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumeron F, Betoulle D, Nicaud V, Evans A, Kee F, Ruidavets JB, . . . Cambien F (2002). Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Temoins de l’Infarctus du Myocarde (ECTIM). Circulation, 105(25), 2943–2945. [DOI] [PubMed] [Google Scholar]
- Gamaro G, Manoli L, Torres I, Silveira R, & Dalmaz C (2003). Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochemistry International, 42(2), 107–114. [DOI] [PubMed] [Google Scholar]
- Gambardella P, Greco AM, Sticchi R, Bellotti R, & Renzo GD (1994). Individual housing modulates daily rhythms of hypothalamic catecholaminergic system and circulating hormones in adult male rats. Chronobiology International, 11(4), 213–221. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Pattie A, Whiteman MC, Whalley LJ, & Deary IJ (2007). Social support and successful aging: Investigating the relationships between lifetime cognitive change and life satisfaction. Journal of Individual Differences, 28(3), 103–115. [Google Scholar]
- Grippo A, Sgoifo A, Mastorci F, McNeal N, & Trahanas D (2010). Cardiac dysfunction and hypothalamic activation during a social crowding stressor in prairie voles. Autonomic Neuroscience: Basic and Clinical, 156, 44–50 doi:10.1016/j.autneu.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Carter CS, McNeal N, Chandler DL, Larocca MA, Bates SL, & Porges SW (2011). 24-hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: implications for the study of depression and cardiovascular disease. Psychosom Med, 73(1), 59–66. doi: 10.1097/PSY.0b013e31820019e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, & Carter CS (2007). Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med, 69(2), 149–157. doi: 10.1097/PSY.0b013e31802f054b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, & Carter CS (2007). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology, 32(8–10), 966–980. doi: 10.1016/j.psyneuen.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, & Porges SW (2007). Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry, 62(10), 1162–1170. doi: 10.1016/j.biopsych.2007.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Sgoifo A, Jepson AJ, Bates SL, Chandler DL, . . . Preihs K (2012). The integration of depressive behaviors and cardiac dysfunction during an operational measure of depression: Investigating the role of negative social experiences in an animal model. Psychosom Med, 74(6), 612–619. doi: 10.1097/PSY.0b013e31825ca8e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, ... Van de Kar LD (2005). Chronic mild stress induces behavioral and phsyiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology, 179, 769–780. [DOI] [PubMed] [Google Scholar]
- Haller J, Halasz J, & Makara GB (2000). Housing conditions and the anxiolytic efficacy of buspirone: the relationship between main and side effects. Behav Pharmacol, 11, 403–412. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, & Cacioppo JT (2006). Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging, 21(1), 152. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Thisted RA, & Cacioppo JT (2009). Loneliness predicts reduced physical activity: Cross-sectional & longitudinal analyses. Health Psychology, 28(3), 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, & Ehlert U (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocal stress. Biological Psychiatry, 54, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT, & Koob GF (1994). Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology, 11, 179–186. [DOI] [PubMed] [Google Scholar]
- Holmes A, & Wellman CL (2009). Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev, 33, 773–783. doi: 10.1016/j.neubiorev.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, & Fornal CA (1991). Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev, 43(4), 563–578. [PubMed] [Google Scholar]
- Kaplan GA, Salonen JT, Cohen RD, Brand RJ, Syme SL, & Puska P (1988). Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. American Journal of Epidemiology, 128(2), 370–380. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker W, Arons C, Triano L, & Suresh G (1998). Repeated isolation stress in the neonatal rat: Relation to brain dopamine systems in the 10-day-old rat. Behav Neurosci, 112(6), 1466. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y (2006). Social buffering: Relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci, 361(1476), 2215–2228. doi: 10.1098/rstb.2006.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, & Neumeister A (2009). Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res, 1293, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I (1998). The spectrum of behaviors influenced by serotonin. Biol Psychiatry, 44(3), 151–162. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, & Forster GL (2009). Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear beahvior, and altered hormonal stress responses. Horm Behav, 55, 248–256. doi: 10.1016/j.yhbeh.2008.10.014 [DOI] [PubMed] [Google Scholar]
- Manuck SB, Clarkson TB, Lusso FM, Taub DM, & Miller EW (1983). Social stress and atherosclerosis in normocholesterolemic monkeys. Science, 220(4598), 733–735. [DOI] [PubMed] [Google Scholar]
- Matuszewich L & Yamamoto BK (2004). Effects of chronic stress on methamphetamine-induced dopamine depletions in the striatum. Ann N Y Acad Sci, 1032, 312–314. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Hardy MP, & Blanchard RJ (2009). Social stress effects on hormones, brain, and behavior. Hormones, Brain and Behavior Online. 333–367). doi: 10.1016/B978-008088783-8.00009-7 [DOI] [Google Scholar]
- McNeal N, Scotti MA, Wardwell J, Chandler DL, Bates SL, Larocca M, . . . Grippo AJ (2014). Disruption of social bonds induces behavioral and physiologic dysregulation in male and female prairie voles. Auton Neurosci, 180, 9–16. doi: 10.1016/j.autneu.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, Weindl D, & Hiller K (2013). Complexity of dopamine metabolism. Cell Communication and Signaling, 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, . . . Reynolds CF (2004). Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology, 29(12), 2258–2265. doi: 10.1038/sj.npp.1300556 [DOI] [PubMed] [Google Scholar]
- Miura H, Qiao H, Kitagami T, Ohta T, & Ozaki N (2005). Effects of fluvoxamine on levels of dopamine, serotonin, and their metabolites in the hippocampus elicited by isolation housing and novelty stress in adult rats. Int J Neurosci, 115(3), 367–378. doi: 10.1080/00207450590520984 [DOI] [PubMed] [Google Scholar]
- Möller M, Du Preez JL, Viljoen FP, Berk M, & Harvey BH (2013). N-acetyl cysteine reverses social isolation rearing induced changes in cortico-striatal monoamines in rats. Metab Brain Dis, 28(4), 687–696. doi: 10.1007/s11011-013-9433-z [DOI] [PubMed] [Google Scholar]
- Nalivaiko E (2006). 5-HT(1A) receptors in stress-induced cardiac changes: a possible link between mental and cardiac disorders. Clin Exp Pharmacol Physiol, 33(12), 1259–1264. doi: 10.1111/j.1440-1681.2006.04521.x [DOI] [PubMed] [Google Scholar]
- National Research Council (2011). Guide for the care and use of laboratory animals (8th ed.). Washington, D. C.: The National Academies Press. [Google Scholar]
- Peuler JD, Scotti MA, Phelps LE, McNeal N, & Grippo AJ (2012). Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiol Behav, 106(4), 476–484. doi: 10.1016/j.physbeh.2012.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu MG, Garau A, Boero G, Biggio F, Pibiri V, Dore R, . . . Serra M (2016). Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience, 320, 172–182. doi: 10.1016/j.neuroscience.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L, Sanzenbacher L, Paredes J, Hashimoto K, Azizi F, & Carter SC (2009). Stress differentially modulates mRNA expression for corticotrophin-releasing hormone receptors in hypothalamus, hippocampus and pituitary of prairie voles. Neuropeptides, 43(2), 113–123. doi: 10.1016/j.npep.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, & Cabib S (1990). Effects of defeat experiences on dopamine metabolism in different brain areas of the mouse. Aggressive Behavior, 16(3–4), 271–284. doi: [DOI] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, . . . Morrison JH (2006). Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex, 16, 313–320. doi: 10.1093/cercor/bhi104 [DOI] [PubMed] [Google Scholar]
- Ramsay S, Ebrahim S, Whincup P, Papacosta O, Morris R, Lennon L, & Wannamethee SG (2008). Social engagement and the risk of cardiovascular disease mortality: results of a prospective population-based study of older men. Ann Epidemiol, 18(6), 476–483. doi: 10.1016/j.annepidem.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Ressler KJ, & Nemeroff CB (2000). Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety, 12 Suppl 1, 2–19. doi: [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, . . . Koolhaas JM (1999). Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology, 24, 285–300. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, & Carter CS (2007). Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav, 51(1), 54–61. doi: 10.1016/j.yhbeh.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Shankar A, McMunn A, Banks J, & Steptoe A (2011). Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol, 30(4), 377–385. doi: 10.1037/a0022826 [DOI] [PubMed] [Google Scholar]
- Shively CA (1998). Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry, 44, 882–891. [DOI] [PubMed] [Google Scholar]
- Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, & Nader MA (1997). Social stress, depression, and brain dopamine in female cynomolgus monkeys. Ann N Y Acad Sci, 807, 574–577. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, & Anton RF (1997). Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry, 41, 871–882. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, & Lanier T (2005). Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biol Psychol, 69(1), 67–84. doi: 10.1016/j.biopsycho.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Smith AS, Lieberwirth C, & Wang Z (2013). Behavioral and physiological responses of female prairie voles (Microtus ochrogaster) to various stressful conditions. Stress, 16(5),531–539. doi: 10.3109/10253890.2013.794449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole MJ, Versteeg DH, de Kloet ER, Hussain N, & Lixfeld W (1983). The identification of specific serotonergic nuclei inhibited by cardiac vagal afferents during acute myocardial ischemia in the rat. Brain Res, 265(1), 55–61. [DOI] [PubMed] [Google Scholar]
- Tidey JW, & Miczek KA (1996). Social defeat stress selectively alters mesocorticolimbic dopamine release: An in vivo microdialysis study. Brain Res, 721(1–2), 140–149. [DOI] [PubMed] [Google Scholar]
- Toufexis D, Rivarola MA, Lara H, & Viau V (2014). Stress and the reproductive axis Journal of Neuroendocrinology, 26, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN (2006). Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med, 29(4), 377–387. doi: 10.1007/s10865-006-9056-5 [DOI] [PubMed] [Google Scholar]
- Uchino BN, Uno D, & Holt-Lunstad J (1999). Social support, physiological processes, and health. Current Directions in Psychological Science, 8(5), 145–148. [Google Scholar]
- Van de Kar LD, & Blair ML (1999). Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol, 20(1), 1–48. doi: 10.1006/frne.1998.0172 [DOI] [PubMed] [Google Scholar]
- Watson SL, Shively CA, Kaplan JR, & Line SW (1998). Effects of chronic social separation on cardiovascular disease risk factors in female cynomolgus monkeys. Atherosclerosis, 137, 259–266. [DOI] [PubMed] [Google Scholar]
- Way BM, & Taylor SE (2010). Social influences on health: Is serotonin a critical mediator? Psychosom Med, 72(2), 107–112. doi: 10.1097/PSY.0b013e3181ce6a7d [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, & Feldon J (2004). Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res, 152, 279–295. doi: 10.1016/j.bbr.2003.10.015 [DOI] [PubMed] [Google Scholar]
- Wen M, Hawkley LC, & Cacioppo JT (2006). Objective and perceived neighborhood environment, individual SES and psychosocial factors, and self-rated health: An analysis of older adults in Cook County, Illinois. Social Science & Medicine, 63(10), 2575–2590. [DOI] [PubMed] [Google Scholar]
- Williams JK, Shively CA, & Clarkson TB (1994). Determinants of coronary artery reactivity in premenopausal female cynomolgus monkeys with diet-induced atherosclerosis. Circulation, 90, 983–987. [DOI] [PubMed] [Google Scholar]
- Young LJ, & Wang Z (2004). The neurobiology of pair bonding. Nature Neuroscience, 7, 1048–1054. [DOI] [PubMed] [Google Scholar]


