Synopsis
Uncontrolled growth is a hallmark of cancer; imaging cell proliferation provides an early indicator of cancer therapeutic response. This capability is especially well-matched to the emerging cell-cycle specific chemotherapeutics with the goal of identifying patients that benefit from these treatments early in the course of treatment to guide personalized treatment. This review focuses on currently investigational cell proliferation imaging PET radiotracers to evaluate tumor proliferation in the setting of cell cycle targeted chemotherapy and endocrine therapy for metastatic breast cancer.
Introduction
Breast cancer is one of the most common cancers and the second leading cause of cancer-related death among women in the United States. Up to 6% of breast cancers are advanced or metastatic at the time of diagnosis, requiring chemotherapy1, 2. Accelerated growth is a hallmark of cancer3, including breast cancer. The rapid expansion of treatments targeted to aberrant cell growth – for example, cell cycle targeted chemotherapies for the treatment of metastatic breast cancer – allows for precise targeting of specific alterations in tumor cell proliferation pathway with the goal of reducing tumor cellular proliferation and increasing tumor cell death while minimizing toxicities associated with chemotherapy. The growing application of these targeted therapies motivates cell proliferation imaging techniques that can reflect the treatment response from cell-cycle inhibition before morphologic and anatomic changes.
Evaluation of Ki-67 expression on biopsy samples is currently considered the gold standard for evaluating cell proliferation. A major drawback for clinical use of Ki-67 is the fact that it requires serial biopsies of the primary tumor sites and/or metastatic lesions to assess changes in cell proliferation in response to therapy. Therefore, this technique is invasive and also prone to sampling errors and underestimation of tumor heterogeneity4, 5. An imaging biomarker is an attractive non-invasive alternative that can provide spatial data of both primary and metastatic disease. Imaging cell proliferation can provide an early noninvasive indicator of cancer therapeutic response that can be used to guide personalized treatment with identifying patients that benefit from those that might not need or benefit from the therapy, early in the course of treatment to avoid toxicity and additional costs.
This review focuses on currently investigational cell proliferation imaging PET radiotracers to evaluate tumor proliferation in the setting of cell cycle targeted chemotherapy and endocrine therapy for metastatic breast cancer. We review the underlying biology associated with cancer proliferation and cell cycle-targeted drugs, followed by a review of the mechanistic underpinnings of cell proliferation tracers, and finally, their application to therapy targeted to aberrant breast cancer proliferation.
Cell cycle and proliferation control: Enhancing endocrine therapy with cell cycle targeting agents
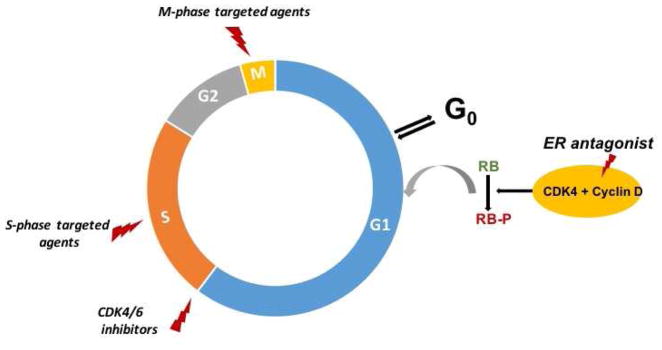
Proliferating cells must progress through 4 phases of cell cycle (G1, S, G2 and M); however, they may exit the cell cycle and enter quiescence (G0) when stressed or deprived of biological stimuli (for example in breast cancer with estrogen deprivation)6, 7. Cyclin-dependent kinases (CDKs) play a key role in controlling cell cycle progression. Among these kinases, CDK4/6 is the key regulator of G1 to S transition by controlling transcription of genes necessary for cell cycle progression. This kinase is activated upon binding to cyclin D, leading to expression of genes required for S-phase entry8. The tightly regulated cyclin D-CDK4/6 complex is frequently disrupted in breast cancer, with subsequent inactivation of the G1-S checkpoint which can lead to aberrant growth and ultimately tumor formation8, 9.
Another important player in breast cancer is estrogen pathway. Estrogen stimulates the proliferation of estrogen receptor (ER)-positive cancer cells via activation of cyclin D10 (Fig. 1). Approximately 70–75% of breast cancers express hormone receptors and most of these cancers depend on estrogen signaling for their growth and survival11. Endocrine therapy has been the mainstay of treatment in the patients with metastatic ER-positive disease and when compared to conventional chemotherapy, it is primarily cytostatic12. Endocrine therapy induced growth inhibition traps cancer cells at the G0/G1 phase of the cell cycle13. Subsequently, the apoptotic pathway will be activated for some of these cells resulting in cell death. However, fractions of the cells might remain in quiescence and evidence suggests that these cells may play an important role in recurrences of hormone-responsive breast cancer, reflecting underlying tumor dormancy14.
Figure 1.
Cell cycle targeted chemotherapeutics and estrogen modulators.
Tamoxifen is a selective ER modulator and is used to treat both early and advanced ER-positive breast cancer. In breast tissue, tamoxifen is a selective estrogen receptor down-regulator resulting in blockade of the estrogen signaling pathway15. Prior studies demonstrated clear survival benefits in patients with ER-positive breast cancer. For example, the EBCTCG study reported approximately 30% reduction in breast cancer mortality for 15 years after diagnosis along with substantial reduction in cancer recurrence in patients reviving Tamoxifen16. Fulvestrant is another ER modulator, approved for the treatment of ER-positive metastatic breast cancer after standard antiestrogen therapy17. This agent inhibits estrogen signaling and tumor proliferation by competing with estradiol binding to the ER, and by down-regulating ER receptor with subsequent reduction in tumor proliferation18.
Additional cancer therapies that target ER pathway deplete the agonist ligand, estradiol. Aromatase inhibitors work by blocking aromatase enzyme activity resulting in reduction of circulating and local estrogen levels. Several meta-analyses underscore clear advantage of third-generation aromatase inhibitors (Anastrozole and Letrozole) compared to Tamoxifen19, 20 for post-menopausal patients, for whom the lack of ovarian synthesis makes aromatase inhibition an effective strategy.
Despite efficacy of various ER antagonists, eventual resistance to such therapies unfortunately can occur 21, sometimes leading to relapse and death. Several mechanisms of resistance to endocrine therapy have been described including the cross-talk between the ER pathway and cell cycle and growth factors such high cyclin D1 expression and Rb phosphorylation22. Changes in these key cell cycle check points result in dysregulated cell cycle progression contributing to loss of endocrine responsiveness23. Different strategies have been used to delay acquired resistance and improve the outcome of patients with ER-positive metastatic breast cancer. One of the emerging treatments is combining endocrine therapy with a cell cycle targeted agent.
CDK4/6 inhibitors have recently become an important tool for breast cancer treatment. CDK4/6 inhibitors work by blocking the phosphorylation of Rb, inducing G1 arrest and halting proliferation and resulting in tumor cell senescence24. As ER-positive tumors usually retain Rb activity and can have cyclin-D1 amplification, they are good targets for CDK4/6 inhibitors. ER signaling upregulates cyclin D1 levels and potentiate multiple signaling pathways largely culminating in upregulation of CDK4/6 activity25, 26. One main outcome of such treatment is the arrest of the cancer cells at G1 so when combined with other agents such as ER selective modulators they will synergistically inhibit cancer growth27. Three CDK4/6 inhibitors have been evaluated in clinical trials in patients with hormone receptor-positive early and metastatic breast cancer: palbociclib, ribociclib, and abemaciclib. Palbociclib recently received FDA approval as the first-line treatment of metastatic ER-positive breast cancer in combination with endocrine therapy, in women with prior endocrine-resistant cancer28. Ribociclib also has been approved by the FDA as a combination therapy with aromatase inhibitor as initial therapy in postmenopausal women with ER-positive HER2-negative breast cancer29.
Response to endocrine therapy has been evaluated using conventional anatomic imaging as well as FDG-PET. Metabolic response assessed by [18F]FDG-PET in patients with metastatic breast cancer undergoing endocrine therapy has been shown to be predictive of progression-free survival30. Recently [18F]F-fluoroestradiol (FES) PET, which is a specific ER-targeted molecular probe for PET for evaluation of ER expression has successfully been used for quantifying in-vivo ER expression in patients with breast cancer. Numerus clinical studies evaluated role of FES-PET as a predictive biomarker for assessing in vivo pharmacodynamic response to endocrine therapy31. Although clinical studies have shown efficacy of different PET imaging biomarkers in predicting the response to endocrine therapy32, increasing application of combination cell cycle targeted therapies raises the need for imaging targeting cell proliferation and the cell cycle to evaluate response to targeted treatments.
Tissue proliferation assays
Numerous techniques have been used as measures of cell proliferation using patients’ blood/tissue samples. These techniques mainly focus on evaluation of cells in S-phase after tissue biopsy. Mitotic index was one of the initial techniques which is still widely used and often integrated into tumor grading systems. Historically, thymidine labelling index was used to measure the S-phase fraction, requiring incubation of fresh tissue section with tritiated thymidine (3HTdR) in-vitro followed by autoradiography of the slides33. On the other hand, flow cytometric analysis of cell cycle by evaluating cell DNA content is another common method for measuring proliferation in the clinical setting. As mentioned under introduction section, expression of a nuclear protein during the cell cycle, Ki-67, is currently the standard technique for assessing cell proliferation. Studies have confirmed that the Ki67 index correlates with the results of mitotic index and flow cytometry34, 35. Inwald et al. studied the associations between Ki-67 and common histopathological parameters in a large cohort of 3,658 patients36. They demonstrated an association between Ki-67 expression and tumor grading. In multivariable analysis, Ki-67 was a prognostic parameter both for disease-free survival as well as overall survival, independent of clinical and histopathological factors. As mentioned earlier, clinical application of the tissue based proliferation assays is largely limited by the heterogeneity of the proliferation process in tumors as well as the invasive nature of these methods.
Imaging cell proliferation: Tracer development
I. Early tracers
PET imaging has been used to evaluate in-vivo tumor proliferation. Much of the work on proliferation imaging has focused on radiotracers that are precursors for DNA synthesis such as 11C and 18fluorine labeled nucleosides. Nucleoside based imaging enables assessment of tumor proliferation by rapid incorporation of radiolabeled labeled nucleosides into newly synthesized DNA. Thymidine has been the main target for such imaging as it is a nucleotide that is exclusively incorporated into DNA and not RNA37. Briefly, thymidine from the blood stream enters the biochemical pathway to DNA synthesis via the “salvage” pathway, where it is transported into the cell, phosphorylated by thymidine kinase, and incorporated into DNA38.
Historically, tritiated thymidine was used to evaluate tumor proliferation;38, 39 quantitative autoradiography of the animal tissues to record emissions from the tritium was correlated to pathologic measures of thymidine incorporation. Although this technique can quantify the fraction of the cells at the S phase, it cannot provide information about the cells in other phases of cell cycle, as well as the pace of cell proliferation. Another important pitfall was the limitation of this technique for clinical application, such as the need for tumor biopsy and radiation exposure due to long-lived tracer.
Early studies paved the road for development of thymidine based radiotracers for PET imaging using short-lived tracers with minimal radiation burden. [11C]thymidine was one of the first PET proliferation radiotracers used in the setting of cancer imaging, in analogy with early work using[14C]thymidine as an in-vitro marker of cellular proliferation38. The most successful version of [11C]thymidine was labeled with 11C at the C2-position in the pyrimidine ring or at the 5-methyl position. Preclinical and clinical studies demonstrated good correlation between this radiotracer, DNA synthesis, and proliferation40. Shields et al. performed one of the early studies to investigate the ability of [11C]thymidine to measure early response to chemotherapy after one week of therapy and compared the findings to metabolic changes in the tumor as measured by FDG-PET37. They reported significant decrease in radiotracer uptake in the responder group; although these differences were present in the FDG-PET, the changes in [11C]thymidine-PET uptake were better correlated with response. Despite the early promising result, this probe was not practical for the clinical setting due to the short half-life (half-life 20 minutes) and rapid metabolism40. Another drawback was the complexity of imaging interpretation needing both mathematical modeling and accurate measurements of the circulating metabolites41.
Subsequent studies investigated the pyrimidine probes labeled with 18F with the goal of longer half-life (half-life 110 minutes) and less catabolism to enable clinical imaging with these tracers. 3′-deoxy-3′[18F]fluorothymidine (FLT) and 2′-fluoro-5-([11C]-methyl)-1-beta-D-arabinofuranosyluracil (FMAU) are the more favorable radiotracers that have been used in both preclinical and clinical settings. Conti et al. reported FMAU as a promising radiotracer for cellular proliferation42. They suggested that this radiotracer shares some important in-vivo features of thymidine such as phosphorylation by kinase, incorporation into DNA, and limited catabolism. Thus, FMAU has potential for providing simplified kinetic models for determination of cellular proliferation using PET imaging. On the other hand, as FMAU also takes part in cytosolic DNA synthesis along with the nuclear DNA leading this might be a limitation as this background incorporation into mitochondrial DNA leads to nonspecific FMAU retention, making this radiotracer less sensitive than FLT for assessment of changes in cell proliferation43.
II. [18F]FLT-PET
3′-deoxy-3′[18F]fluorothymidine is a metabolized thymidine analog that can serve as a surrogate of proliferation by targeting the activity of thymidine salvage pathway of DNA synthesis44. This radiotracer has better metabolic stability when compared to prior labeled thymidine analogs such as IUdR and [11C]thymidine. FLT enters tissue by Na+ dependent active transporters dominated by human equilibrative nucleoside transporter 1. The level of this transporter increases as proliferating cells enter the cell cycle, accordingly the expression is upregulated in the setting of cancer45 and this might influence radiotracer uptake and signal in the in-vivo setting46. [18F]FLT is phosphorylated in the cells by thymidine kinase-1 resulting in intracellular trapping and accumulation of this tracer, but unlike thymidine, FLT is not incorporated into the DNA structure. Thymidine kinase-1 activity is a rate limiting factor in the salvage pathway of DNA synthesis thus its activity is closely correlated with DNA synthesis38. Concentration of this enzyme increases almost 10-fold during the S-phase of cell cycle and subsequently [18F]FLT-PET uptake increases which can indirectly quantify the S-phase fraction of cycling cancer cells, reflecting cell proliferation47. Semi-routine production of [18F]FLT has been established in many centers, including some commercial suppliers, and it has been widely studied in clinical trials for imaging cell proliferation of various tumor types such as lung, head and neck, and breast cancer48.
[18F]FLT uptake in primary breast cancer as a prognostic factor
[18F]FLT uptake has been shown to correlate with cell proliferation in untreated patients with breast cancer. Smyczek-Gargya et al. used [18F]FLT-PET in the setting of primary breast cancer and compared its performance to [18F]FDG-PET and reported uptake of both radiotracers in cancer tissues. The SUVs of primary tumors and axillary lymph node metastases were lower in FLT-PET when compared to FDG study (SUVFLT: 3.2 vs. SUVFDG: 4.7 in primary tumors and SUVFLT: 2.9 vs SUVFDG: 4.6 in lymph node metastases). However, given very low background FLT uptake in the mediastinum, the tumor-to-mediastinum ratio was significantly higher in [18F]FLT-PET studies49. Of note, however, the relatively high level of [18F]FLT uptake in the liver (due to hepatic clearance) and proliferating bone marrow makes visualization and quantification of uptake in liver and bone metastases challenging.
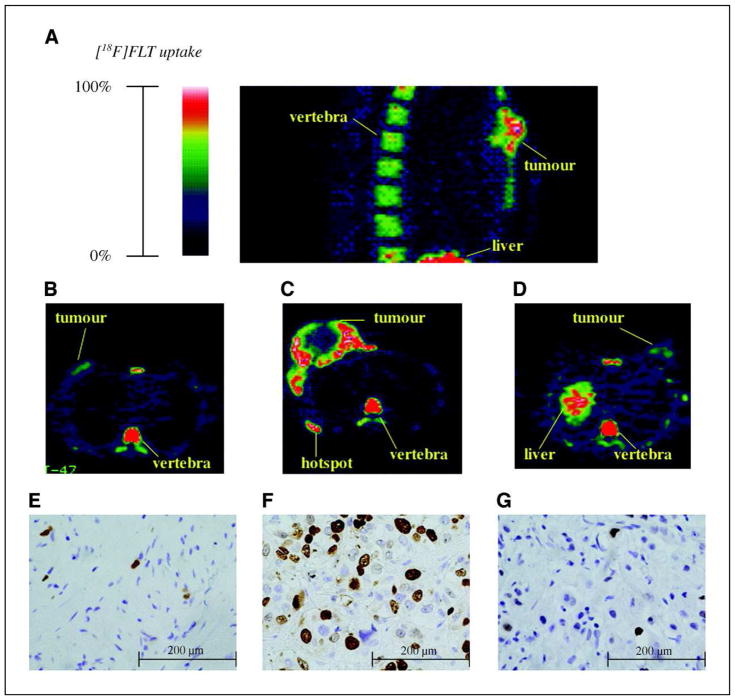
Kenny et al. demonstrated [18F]FLT accumulation in primary tumor, nodal disease, and lung metastases in patients with breast cancer (Fig. 2)50. They also noted heterogeneity of radiotracer uptake, which was related to heterogeneity of cell proliferation within and between disease sites50. The delivery and retention of [18F]FLT was shown to be higher when compared to normal breast tissue (p-value < 0.0001) and that FLT retention correlated with Ki-67 labeling index from tumor biopsies.
Figure 2.
FLT-PET in breast cancer: A, sagittal section of an [18F]FLT-PET image showing high uptake in the vertebrae and liver. B and E, [18F]FLT-PET retention in a lobular carcinoma with corresponding histologic section stained for Ki-67 LI (4.9%). C and F, large primary ductal carcinoma with high uptake of [18F]FLT around the periphery of the tumor, central necrosis, a hotspot on the sixth rib was also confirmed on a radioisotope bone scan; Ki-67 LI (45.2%). D and G, an inflammatory breast carcinoma, and high uptake in the apex of the liver and in a vertebra; Ki-67 LI (8.8%).
Adapted from Kenny LM1, Vigushin DM, Al-Nahhas A, et al. ‘Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods.’ Cancer Res. 2005 Nov 1;65(21):10104–12; with permission.
Tumor expression of Ki-67 has been established as a marker of proliferation as well as a prognostic marker in breast cancer. A meta-analysis explored the correlation between FLT uptake and Ki-67 expression and showed a correlation, independent of cancer type. Additionally, subgroup analysis supported a correlation between FLT uptake and expression in breast cancer51.
[18F]FLT-PET for early treatment response prediction to chemotherapy
[18F]FDG-PET/CT has been widely used for assessing response to chemotherapy, targeted therapy, and endocrine treatment in breast cancer52, 53; however this imaging technique does not capture changes in proliferation in response to therapy. Contractor et al, evaluated the early changes in [18F]FLT-PET uptake after 2 weeks of initiating the first or second cycle of docetaxel in a prospective study in 20 patients with breast cancer54. They demonstrated a significant decrease in SUVmax and SUVmean at 60 minutes which was significantly different in responders when compared to the non-responders (40% vs. 11%). This reduction in tumor SUV was associated with target lesion size changes mid-therapy (after three cycles). Another study of [18F]FLT-PET in the neoadjuvant setting failed to demonstrated any significant association between baseline, post chemotherapy, or change in SUVmax and pathological response, despite sizeable reduction in SUVmax in most of the cases. However, the baseline SUVmax was significantly associated with Ki-67, confirming the role of FLT-PET as a proliferation biomarker55. A prospective cohort pilot study of patients with potentially operable, locally advanced T2–3 breast cancer undergoing anthracycline/taxane-based neoadjuvant chemotherapy followed by surgical resection of cancer evaluated the role of [18F]FLT-PET in predicting treatment response before, after the first cycle, and at the end of neoadjuvant therapy (Fig. 3)56. After the first cycle of chemotherapy, changes in the SUVmax of primary tumor demonstrated a good predictive power for identifying complete and near complete responders with overall accuracy of 93.3 %, sensitivity of 83.3 %, the specificity of 100.0 %, and the positive and negative predictive values of 100.0 and 90.0 %, respectively56.
Figure 3.
FLT-PET for prediction of early treatment response: a. Coronal fused PET/CT slice show abnormal areas of FLT uptake corresponding to a primary breast tumor (T) and a dominant axillary metastatic node (N). b, c. Transaxial CT, PET and fused PET/CT images in a NCT responding patient show FLT uptake changes in the primary breast tumor (b) and in the dominant axillary metastatic node (c). Postsurgery histology revealed a complete pathological response both in the mammary gland and in the removed axillary nodes (0/10 nodes). d. Axial fused PET/CT slices in a nonresponding patient. In the interim PET image, the abnormal area of FLT uptake corresponding to the primary cancer (arrow) is larger than in the baseline PET image, in spite of a partial reduction in tracer accumulation (ΔSUVmax=−26%). In the final PET image, there is a further increase in the area of FLT uptake with appearance of a photopenic central area, attributable to tumor necrosis
Adapted from Crippa F, Agresti R, Sandri M, et al.18F-FLT PET/CT as an imaging tool for early prediction of pathological response in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy: a pilot study. Eur J Nucl Med Mol Imaging. 2015 May;42(6):818–30. doi: 10.1007/s00259-015-2995-8. Epub 2015 Feb 12; with permission.
Data from these single-center studies has been promising with good sensitivity of [18F]FLT-PET for assessing changes in tumor proliferation after chemotherapy to predict subsequent treatment response; however, they all came from single center and included small patient populations, suggesting the need for further validation. The American College of Radiology Imaging Network (ACRIN) study 6688 performed a nonrandomized, multicenter phase II study with the aim of correlating changes of [18F]FLT-PET approximately one week after chemotherapy with pathologic response at the end of treatment in patients with primary breast cancer who were undergoing neoadjuvant therapy57. Fifty-one patients underwent FLT-PET/CT at baseline, after 1 cycle and after completion of neoadjuvant therapy. Complete pathologic response was reported in 9 patients (18%) with significant but modest difference when comparing changes in SUVmax between those with complete pathologic response from other patients (p-value = 0.05). They also assessed the correlation between baseline and post-treatment SUVmax with Ki-67 and reported strong correlation only between SUVmax and Ki-67 after completion of neoadjuvant therapy. Although this was a multicenter study, results need further validation since there were a small number of patients. In addition, the chemotherapy regimen was variable among centers and treatment variation was not considered in the analysis. This study supports the potential of FLT-PET, but thus far, has not provided sufficient evidence to support FDA approval of FLT-PET as an early biomarker of treatment response in breast cancer.
A recent meta-analysis of 4 articles including 46 patients and 54 tumors evaluated the diagnostic performance of [18F]FLT-PET for assessing response to chemotherapy in patients with breast cancer and reported a pooled sensitivity of 77.3% and pooled specificity of 68% predicting response for this modality and concluded that this imaging technique is useful to predict response with reasonable diagnostic profile58. The result of this review is limited due to the relatively pooled small studies and patient number as well as utilizing different chemotherapy regimens, imaging techniques, and reference standard across different studies.
[18F]FLT-PET for predicting treatment response to endocrine therapy
In order to commit a patient with breast cancer to endocrine therapy alone, it would be ideal to determine which tumors will respond to such therapy early in the course of treatment. Serial biopsy and Ki-67 analysis demonstrated potential for assessing endocrine therapy response early in the course of treatment, predicting long-term outcome after neoadjuvant endocrine therapy in several clinical trials59, 60. ACOSOG Z1031 trial also tested Ki-67-based algorithms to identify patients who are poorly responsive to neoadjuvant endocrine therapy61. Previous imaging studies supported the ability of FDG-PET, as an indirect measure of cell proliferation, to evaluate early treatment response to endocrine therapy52, in line with Ki-67 based studies. The results from tissue assay studies, however, motivate the evaluation of more direct imaging measures of proliferation, such as FLT-PET. Linden et al. evaluated the associations between parametric analysis of dynamic [18F]FLT-PET and Ki-67 at baseline and following two weeks of endocrine monotherapy in patients with early stage ER-positive breast cancer, prior to definitive surgery. SUVmax declined in all 26 patients except for one, while Ki-67 declined in all lesions. An average decline of 27% in SUVmax after two weeks of treatment was reported correlating to an average of 68% decrease in Ki-6762. This study, still at an early stage of evaluation, motivates investigations of the possible role of [18F]FLT-PET as an early non-invasive biomarker of response in the setting of endocrine therapy.
Further support for FLT as a marker of endocrine therapy response can be found in published pre-clinical literature. Shah et al. performed a preclinical investigation of 3 molecular imaging metrics, correlating breast cancer tumor regression with molecular imaging of apoptosis, glucose metabolism, and cellular proliferation. Analysis of [18F]FLT-PET imaging predicted trastuzumab response in BT474 model of breast cancer but not in MMTV/HER2. This was further investigated in xenograft model of breast cancer with comparing the changes in tumor-to-muscle FLT uptake ratio both in trastuzumab-sensitive and resistant before and after treatment with trastuzumab. They concluded [18F]FLT-PET is a sensitive modality for predicting early molecular changes in trastuzumab-sensitive breast cancer xenografts. They suggested that [18F]FLT-PET may be able to distinguish non-responders from responders earlier during the course of treatment63.
[18F]FLT-PET has been also used in the setting of treatment with investigational drug, steroid sulfatase inhibitor. Steroid sulfatase targets aromatase pathway for estrogen synthesis by converting estrone sulfate to estrone. Irosustat is a first generation, irreversible steroid sulfatase inhibitor that has been recently evaluated in clinical trials64. A pre-surgical window of opportunity study utilized [18F]FLT-PET in postmenopausal women with ER-positive breast cancer at baseline and after 2 weeks of therapy with irosustat to demonstrate proof of concept data that suggest that steroid sulfatase inhibition is effective in reducing tumor proliferation as measured by FLT-PET, in-vivo. They reported significant reduction in FLT uptake and Ki-67 after 2 weeks of treatment65.
Limitation of [18F]FLT-PET for cancer proliferation imaging
Overall, lower uptake of FLT is an inherent limitation of this imaging technique. This could result in low sensitivity for assessment of changes in the small metastatic lesions as well as regional lymph node metastases with more false-negative findings compared with [18F]FDG-PET. Another drawback is related to high background [18F]FLT uptake in liver and bone marrow which has been attributed to glucuronidation of [18F]FLT in liver and high level of cellular proliferation and kinetics of FLT in bone marrow50, 55, 66. This physiologic uptake limits evaluation of extent of disease in these organs that are in fact common sites of metastatic breast cancer.
Tumors are heterogeneous growing asynchronously with tumor cells in different phases of cell cycle. Thus, measuring only S-fraction as obtained by FLT-PET will underestimate the pool of proliferating cells as it cannot differentiate between proliferating cells in G1, G2, and M phases vs. quiescent cells at G0. This may have important implications for determining appropriate treatment regimen. For example, cell cycle targeted chemotherapies are effective in tumors with a high index of cell proliferation, while other agents should be used in the setting of low proliferating tumors67. McKinley et al. addressed the mixed results about accuracy of [18F]FLT in quantification of changes in proliferation by assessing the quantitative relationships between [18F]FLT-PET and cellular metrics of proliferation in human breast cancer model. They demonstrated that the de novo pathway of thymidine synthesis is one of the causes of decoupling of [18F]FLT uptake from Ki-67 expression in the tumors that mainly depend on this de novo thymidine synthesis pathway68.
III. Alternative approach to imaging proliferation: [18F]ISO-1-PET
Proliferative status is one of the principal measures of cell proliferation and is defined as the ratio of proliferating cells (in G1, S, G2 or M phases) to quiescent cells (P:Q ratio). As discussed earlier, estimation of S-fraction by [18F]FLT is insufficient to provide a comprehensive estimate of proliferative status as it measures DNA synthesis which happens primarily in the S-phase. An alternative approach targets the σ-receptors, which has been investigated as a biomarker of proliferative status69. This marker originally was classified as opiate receptors with two subtypes, σ1 and σ2. The σ2-receptor subtype been shown to be unregulated during transition from quiescent state to G1 and is it has been reported to be overexpressed in some tumors, including breast cancer70. Accordingly, the expression is higher in proliferating cells making it a particularly appealing receptor-based biomarker of cell proliferation in breast cancer. Mach et al. compared the density of σ2-receptors between proliferating and quiescent mouse mammary adenocarcinoma cell line 66 and showed that it is approximately 10 times higher in the proliferating breast cancer cells69.
11C labeled σ2 receptor ligands were developed initially. Mach et al. reported four 11C-labeled benzamide analogs with high affinity and selectivity for σ2 versus σ1-receptors71. They suggested one of these compounds ((2-methoxy-11C)-N-(4-(3,4-dihydro-6,7-dimethoxy-isoquinolin-2(1H)-yl)butyl)-5- methylbenzamide) has potential for imaging the breast tumors proliferation given the high tumor uptake and suitable tumor to background ratio72. They also established comparison of this compound with [18F]FLT-PET which is currently performed as a measure of cell proliferation and demonstrated similar tumor/lung and tumor/fat ratios for both imaging markers while their radiotracer for imaging σ2 receptor had a higher tumor/blood and tumor/muscle ratio.
18F-radiolabeled σ2- receptor were also validated in a variety of cancer animal models, such as pancreatic cancer and breast cancer73. Correlative analysis of the tissue using micro-PET imaging revealed preferential expression of σ2 in tumor as opposed to normal tissues. A18F-radiolabeled σ2-receptors were synthesized and analysis of the chemistry and biodistribution data showed two fluorine-containing benzamide analogs that can be used for proliferation imaging74.
Preclinical data: [18F]ISO-1 for imaging the proliferative status of tumors
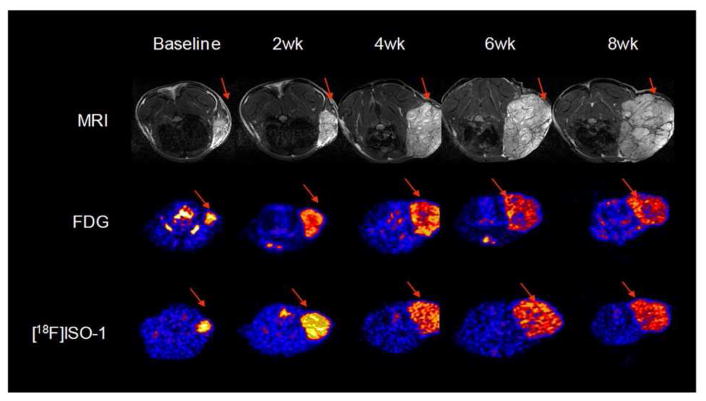
One of the early preclinical studies evaluated the treatment response to bexarotene in mouse mammary tumor 66 with σ2 receptor ligand 2-(2-[18F]fluoroethoxy)-N-(4-(3,4-dihydro-6,7-dimethoxyisoquinolin-2(1H)-yl)butyl)-5-methylbenzamide, ([18F]ISO-1) and findings were compared to [18F]FDG micro-PET and MRI75. This imaging finding was also correlated with P:Q ratio as determined by flow cytometric cell cycle analysis. [18F]ISO-1 revealed good contrast with high tumor to background uptake ratio when compared to [18F]FDG-PET. Accordingly, [18F]ISO-1 imaging demonstrated a strong linear correlation between the radiotracer uptake ratio and P:Q ratio (R= 0.87); while this correlation was poor for [18F]FDG-PET (R= 0.37). Using another model of breast cancer (MNU-induced tumors), they assessed tumor growth rate in relation to [18F]ISO-1 uptake. The changes of [18F]ISO-1 uptake significantly correlated with relative changes in tumor volume as defined by changes in tumor volume based on MRI (Fig. 4) 75. One important clinical implications of this study was to provide evidence for the potential role of [18F]ISO-1 as a predictive measure of tumor growth rate.
Figure 4.
Three modalities for treatment response assessment: Representative time course imaging of MNU-induce tumors at baseline, 2 weeks, 4 weeks, 6 weeks, and 8 weeks with MRI, FDG and [18F]ISO-1.
Adapted from Shoghi KI, Xu J, Su Yi, et al. Quantitative Receptor-Based Imaging of Tumor Proliferation with the Sigma-2 Ligand [18F]ISO-1 PLoS One. 2013; 8(9): e74188. doi: 10.1371/journal.pone.0074188; with permission.
Early clinical data: [18F]ISO-1 safety and feasibility studies
The first study evaluating [18F]ISO-1 in humans was performed in 30 adult patients with different cancers, including 13 patients with newly diagnosed breast cancer76. The primary aim of the study was to evaluate the safety and dosimetry of [18F]ISO-1. [18F]ISO-1 uptake was assessed semi-quantitatively by obtaining SUVmax, tumor to normal tissue and tumor to muscle, and relative distribution volume ratios. Significant correlation between tumor SUVmax as well as tumor-to-muscle ratio with Ki-67 was reported (p-value= 0.04 and p-value=0.003, respectively). They also suggested that [18F]ISO-1 uptake can be helpful in stratification of the tumor sites to high and low proliferative tumors and this information can be potentially used in treatment planning to provide personalized treatment.
Preliminary results of an ongoing pilot phase I trial study utilizing [18F]ISO-1, as a σ2 selective PET radiotracer demonstrated increased [18F]ISO-1 uptake both in primary breast cancer and nodal metastatic sites, suggesting this is a feasible technique for assessing σ2-receptor expression in-vivo77. Preliminary analysis demonstrated that [18F]ISO-1 uptake in ER positive tumors significantly correlates with expression levels of Ki-6777. Apart from the early clinical data on imaging σ2-receptors, these receptors have been also evaluated as therapeutic targets for different disease processes. The suggested oncologic implication mainly focuses on the role of σ2 ligands in inducing apoptosis in tumor cells78. The potentials of this biomarker hold promise for a targeted diagnostic and therapeutic radiotracer in breast cancer.
Summary of current status and future directions
The ongoing development of new chemotherapeutic regimens with the goal of combining agents with different mechanism of actions in order to enhance response and at the same time reduce the toxicity profile challenges our current imaging modalities for assessing treatment response. One emerging application is the increasing use of cell cycle targeted chemotherapeutics such as CDK4/6 inhibitors in combination with previously established regimens, such as endocrine therapy, which motivates the need for clinically applicable non-invasive techniques for assessing early changes to therapy.
The promising results of ACRIN 6688 and the prospective studies pave the road for future multicenter trials exploring the role of the above-mentioned imaging techniques as a prognostic biomarker and an early indicator of cancer therapeutic response that can be used to guide personalized treatment in patients with breast cancer. An ongoing clinical trial in our institution is utilizing [18F]FLT-PET as a non-invasive tool to assess the impact of the CDK4/6 inhibitor and combined CDK4/6 inhibitor and chemotherapy on breast cancer proliferation in patients with recurrent or metastatic breast cancer following a phase II activity and safety study in 37 patients79. The need to synchronize the cell-cycle agents with the timing of cytotoxic chemotherapy in this approach suggests a potential role for FLT-PET to track the pharmacodynamics effect of CDK4/6 inhibitors and its impact on cell cycle arrest. The capability of [18F]ISO-1 to predict proliferative status is especially well-matched to cell-cycle specific chemotherapeutics, such as CDK4/6 inhibitors and may also be applicable to their approved use as a component of first-line treatment for metastatic ER-positive breast cancer in combination with endocrine therapy. Ongoing and future preclinical studies are needed to validate this biomarker before clinical application.
Key points.
Imaging cell proliferation can provide an early measure of treatment response that can be used to guide personalized treatment.
Imaging biomarker of proliferation are especially beneficial in the setting of regimens exploiting cell cycle targeted chemotherapies in combination with endocrine therapy
FLT uptake can quantify the S-phase fraction of cycling cancer cells.
ISO-1 uptake can measure overall proliferative status of the tumor.
Acknowledgments
This work was supported in part by the Susan G. Komen Foundation (Dr. McDonald CCR16376362 and Dr. Mankoff SAC130060, Department of Energy Grant DE-SC0012476, and NIH Grant 5T32EB004311-13 for radiology research track residency. Dr. McDonald is also the 2016–2018 American Roentgen Ray Society Scholar. Grants support related research and support, in part, the effort of the co-authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu J, Steeg PS, Price JE, et al. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009 Jun 15;69(12):4951–4953. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 2.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008 Aug 20;100(16):1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 04;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Sorbye SW, Kilvaer TK, Valkov A, et al. Prognostic impact of CD57, CD68, M-CSF, CSF-1R, Ki67 and TGF-beta in soft tissue sarcomas. BMC Clin Pathol. 2012 May 03;12:7. doi: 10.1186/1472-6890-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishihara M, Mukai H, Nagai S, et al. Retrospective analysis of risk factors for central nervous system metastases in operable breast cancer: effects of biologic subtype and Ki67 overexpression on survival. Oncology. 2013;84(3):135–140. doi: 10.1159/000345321. [DOI] [PubMed] [Google Scholar]
- 6.Mellor HR, Ferguson DJ, Callaghan R. A model of quiescent tumour microregions for evaluating multicellular resistance to chemotherapeutic drugs. Br J Cancer. 2005 Aug 08;93(3):302–309. doi: 10.1038/sj.bjc.6602710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shackney SE, Shankey TV. Cell cycle models for molecular biology and molecular oncology: exploring new dimensions. Cytometry. 1999 Feb 01;35(2):97–116. doi: 10.1002/(sici)1097-0320(19990201)35:2<97::aid-cyto1>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Choi YJ, Anders L. Signaling through cyclin D-dependent kinases. Oncogene. 2014 Apr 10;33(15):1890–1903. doi: 10.1038/onc.2013.137. [DOI] [PubMed] [Google Scholar]
- 9.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011 Jul 07;11(8):558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 10.Quelle DE, Ashmun RA, Shurtleff SA, et al. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993 Aug;7(8):1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 11.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005 Jan;123(1):21–27. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- 12.Schneider PG, Jackisch C, Brandt B. Endocrine management of breast cancer. Int J Fertil Menopausal Stud. 1994;39(Suppl 2):115–127. [PubMed] [Google Scholar]
- 13.Kilker RL, Planas-Silva MD. Cyclin D1 is necessary for tamoxifen-induced cell cycle progression in human breast cancer cells. Cancer Res. 2006 Dec 01;66(23):11478–11484. doi: 10.1158/0008-5472.CAN-06-1755. [DOI] [PubMed] [Google Scholar]
- 14.Telli ML, Sledge GW. The future of breast cancer systemic therapy: the next 10 years. J Mol Med (Berl) 2015 Feb;93(2):119–125. doi: 10.1007/s00109-014-1238-y. [DOI] [PubMed] [Google Scholar]
- 15.Osborne CK, Zhao H, Fuqua SA. Selective estrogen receptor modulators: structure, function, and clinical use. J Clin Oncol. 2000 Sep;18(17):3172–3186. doi: 10.1200/JCO.2000.18.17.3172. [DOI] [PubMed] [Google Scholar]
- 16.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011 Aug 27;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumachi F, Luisetto G, Basso SM, Basso U, Brunello A, Camozzi V. Endocrine therapy of breast cancer. Curr Med Chem. 2011;18(4):513–522. doi: 10.2174/092986711794480177. [DOI] [PubMed] [Google Scholar]
- 18.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010 Oct 20;28(30):4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 19.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006 Sep 20;98(18):1285–1291. doi: 10.1093/jnci/djj357. [DOI] [PubMed] [Google Scholar]
- 20.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010 Jan 20;28(3):509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 21.Yeo B, Turner NC, Jones A. An update on the medical management of breast cancer. BMJ. 2014 Jun 09;348:g3608. doi: 10.1136/bmj.g3608. [DOI] [PubMed] [Google Scholar]
- 22.Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011 Jun;18(3):333–345. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy CG, Dickler MN. Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr Relat Cancer. 2016 Aug;23(8):R337–352. doi: 10.1530/ERC-16-0121. [DOI] [PubMed] [Google Scholar]
- 24.Choi YJ, Li X, Hydbring P, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012 Oct 16;22(4):438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster JS, Henley DC, Bukovsky A, Seth P, Wimalasena J. Multifaceted regulation of cell cycle progression by estrogen: regulation of Cdk inhibitors and Cdc25A independent of cyclin D1-Cdk4 function. Mol Cell Biol. 2001 Feb;21(3):794–810. doi: 10.1128/MCB.21.3.794-810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts CK, Sweeney KJ, Warlters A, Musgrove EA, Sutherland RL. Antiestrogen regulation of cell cycle progression and cyclin D1 gene expression in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1994;31(1):95–105. doi: 10.1007/BF00689680. [DOI] [PubMed] [Google Scholar]
- 27.Dean JL, McClendon AK, Knudsen ES. Modification of the DNA damage response by therapeutic CDK4/6 inhibition. J Biol Chem. 2012 Aug 17;287(34):29075–29087. doi: 10.1074/jbc.M112.365494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015 Jan;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 29.Administration FaD, editor. Ribociclib (Kisqali) 2017. [Google Scholar]
- 30.Mortazavi-Jehanno N, Giraudet AL, Champion L, et al. Assessment of response to endocrine therapy using FDG PET/CT in metastatic breast cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2012 Mar;39(3):450–460. doi: 10.1007/s00259-011-1981-z. [DOI] [PubMed] [Google Scholar]
- 31.Liao GJ, Clark AS, Schubert EK, Mankoff DA. 18F-Fluoroestradiol PET: Current Status and Potential Future Clinical Applications. J Nucl Med. 2016 Aug;57(8):1269–1275. doi: 10.2967/jnumed.116.175596. [DOI] [PubMed] [Google Scholar]
- 32.Kurland BF, Peterson LM, Lee JH, et al. Estrogen Receptor Binding (18F-FES PET) and Glycolytic Activity (18F-FDG PET) Predict Progression-Free Survival on Endocrine Therapy in Patients with ER+ Breast Cancer. Clin Cancer Res. 2017 Jan 15;23(2):407–415. doi: 10.1158/1078-0432.CCR-16-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livingston RB, Johnson P, Goormastic M, Williams GW. Effects on labeling index as a predictor of response to chemotherapy in the 13762 adenocarcinoma. Cancer Chemother Pharmacol. 1982 Dec;10(1):47–50. doi: 10.1007/BF00257238. [DOI] [PubMed] [Google Scholar]
- 34.Barnard NJ, Hall PA, Lemoine NR, Kadar N. Proliferative index in breast carcinoma determined in situ by Ki67 immunostaining and its relationship to clinical and pathological variables. J Pathol. 1987 Aug;152(4):287–295. doi: 10.1002/path.1711520407. [DOI] [PubMed] [Google Scholar]
- 35.Parrado C, Falkmer UG, Hoog A, et al. A technique for automatic/interactive assessment of the proliferating fraction of neoplastic cells in solid tumors. A methodological study on the Ki-67 immunoreactive cells in human mammary carcinomas, including a comparison with the results of conventional S-phase fraction assessments by means of DNA cytometry. Gen Diagn Pathol. 1996 Mar;141(3–4):215–227. [PubMed] [Google Scholar]
- 36.Inwald EC, Klinkhammer-Schalke M, Hofstadter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013 Jun;139(2):539–552. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shields AF, Mankoff DA, Link JM, et al. Carbon-11-thymidine and FDG to measure therapy response. J Nucl Med. 1998 Oct;39(10):1757–1762. [PubMed] [Google Scholar]
- 38.Cleaver JC. Thymidine Metabolism and Cell Kinetics. Amsterdam: North-Holland Publishing Company; 1967. [Google Scholar]
- 39.Livingston RB, Ambus U, George SL, Freireich EJ, Hart JS. In vitro determination of thymidine-3H labeling index in human solid tumors. Cancer Res. 1974 Jun;34(6):1376–1380. [PubMed] [Google Scholar]
- 40.Shields AF, Lim K, Grierson J, Link J, Krohn KA. Utilization of labeled thymidine in DNA synthesis: studies for PET. J Nucl Med. 1990 Mar;31(3):337–342. [PubMed] [Google Scholar]
- 41.Mankoff DA, Shields AF, Graham MM, Link JM, Eary JF, Krohn KA. Kinetic analysis of 2-[carbon-11]thymidine PET imaging studies: compartmental model and mathematical analysis. J Nucl Med. 1998 Jun;39(6):1043–1055. [PubMed] [Google Scholar]
- 42.Conti PS, Bading JR, Mouton PP, et al. In vivo measurement of cell proliferation in canine brain tumor using C-11-labeled FMAU and PET. Nucl Med Biol. 2008 Jan;35(1):131–141. doi: 10.1016/j.nucmedbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Chou TC, Kong XB, Fanucchi MP, et al. Synthesis and biological effects of 2′-fluoro-5-ethyl-1-beta-D-arabinofuranosyluracil. Antimicrob Agents Chemother. 1987 Sep;31(9):1355–1358. doi: 10.1128/aac.31.9.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grierson JR, Schwartz JL, Muzi M, Jordan R, Krohn KA. Metabolism of 3′-deoxy-3′-[F-18]fluorothymidine in proliferating A549 cells: validations for positron emission tomography. Nucl Med Biol. 2004 Oct;31(7):829–837. doi: 10.1016/j.nucmedbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Belt JA, Marina NM, Phelps DA, Crawford CR. Nucleoside transport in normal and neoplastic cells. Adv Enzyme Regul. 1993;33:235–252. doi: 10.1016/0065-2571(93)90021-5. [DOI] [PubMed] [Google Scholar]
- 46.Plotnik DA, Emerick LE, Krohn KA, Unadkat JD, Schwartz JL. Different modes of transport for 3H-thymidine, 3H-FLT, and 3H-FMAU in proliferating and nonproliferating human tumor cells. J Nucl Med. 2010 Sep;51(9):1464–1471. doi: 10.2967/jnumed.110.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002 Sep;43(9):1210–1217. [PubMed] [Google Scholar]
- 48.Bollineni VR, Kramer GM, Jansma EP, Liu Y, Oyen WJ. A systematic review on [(18)F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur J Cancer. 2016 Mar;55:81–97. doi: 10.1016/j.ejca.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Smyczek-Gargya B, Fersis N, Dittmann H, et al. PET with [18F]fluorothymidine for imaging of primary breast cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2004 May;31(5):720–724. doi: 10.1007/s00259-004-1462-8. [DOI] [PubMed] [Google Scholar]
- 50.Kenny LM, Vigushin DM, Al-Nahhas A, et al. Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res. 2005 Nov 01;65(21):10104–10112. doi: 10.1158/0008-5472.CAN-04-4297. [DOI] [PubMed] [Google Scholar]
- 51.Chalkidou A, Landau DB, Odell EW, Cornelius VR, O’Doherty MJ, Marsden PK. Correlation between Ki-67 immunohistochemistry and 18F-fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. Eur J Cancer. 2012 Dec;48(18):3499–3513. doi: 10.1016/j.ejca.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Dehdashti F, Flanagan FL, Mortimer JE, Katzenellenbogen JA, Welch MJ, Siegel BA. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999 Jan;26(1):51–56. doi: 10.1007/s002590050359. [DOI] [PubMed] [Google Scholar]
- 53.Duch J, Fuster D, Munoz M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009 Oct;36(10):1551–1557. doi: 10.1007/s00259-009-1116-y. [DOI] [PubMed] [Google Scholar]
- 54.Contractor KB, Kenny LM, Stebbing J, et al. [18F]-3′Deoxy-3′-fluorothymidine positron emission tomography and breast cancer response to docetaxel. Clin Cancer Res. 2011 Dec 15;17(24):7664–7672. doi: 10.1158/1078-0432.CCR-11-0783. [DOI] [PubMed] [Google Scholar]
- 55.Woolf DK, Beresford M, Li SP, et al. Evaluation of FLT-PET-CT as an imaging biomarker of proliferation in primary breast cancer. Br J Cancer. 2014 Jun 10;110(12):2847–2854. doi: 10.1038/bjc.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crippa F, Agresti R, Sandri M, et al. (1)(8)F-FLT PET/CT as an imaging tool for early prediction of pathological response in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy: a pilot study. Eur J Nucl Med Mol Imaging. 2015 May;42(6):818–830. doi: 10.1007/s00259-015-2995-8. [DOI] [PubMed] [Google Scholar]
- 57.Kostakoglu L, Duan F, Idowu MO, et al. A Phase II Study of 3′-Deoxy-3′-18F-Fluorothymidine PET in the Assessment of Early Response of Breast Cancer to Neoadjuvant Chemotherapy: Results from ACRIN 6688. J Nucl Med. 2015 Nov;56(11):1681–1689. doi: 10.2967/jnumed.115.160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng SM, Zhang W, Zhang B, Wu YW. Assessment of tumor response to chemotherapy in patients with breast cancer using (18)F-FLT: a meta-analysis. Chin J Cancer Res. 2014 Oct;26(5):517–524. doi: 10.3978/j.issn.1000-9604.2014.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007 Jan 17;99(2):167–170. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 60.Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol. 2001 Nov;12(11):1527–1532. doi: 10.1023/a:1013128213451. [DOI] [PubMed] [Google Scholar]
- 61.Ellis MJ, Suman VJ, Hoog J, et al. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance) J Clin Oncol. 2017 Apr 01;35(10):1061–1069. doi: 10.1200/JCO.2016.69.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts TK, Peterson L, Kurland B, et al. Use of serial 18F-Fluorothymidine (FLT) PET and Ki-67 to predict response to aromatase inhibitors (AI) in women with ER+ breast cancer. Journal of Clinical Oncology. 2016;34(15 suppl) [Google Scholar]
- 63.Whisenant JG, McIntyre JO, Peterson TE, et al. Utility of [18 F]FLT-PET to assess treatment response in trastuzumab-resistant and trastuzumab-sensitive HER2-overexpressing human breast cancer xenografts. Mol Imaging Biol. 2015 Feb;17(1):119–128. doi: 10.1007/s11307-014-0770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmieri C, Stein RC, Liu X, et al. IRIS study: a phase II study of the steroid sulfatase inhibitor Irosustat when added to an aromatase inhibitor in ER-positive breast cancer patients. Breast Cancer Res Treat. 2017 Sep;165(2):343–353. doi: 10.1007/s10549-017-4328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmieri C, Szydlo R, Miller M, et al. IPET study: an FLT-PET window study to assess the activity of the steroid sulfatase inhibitor irosustat in early breast cancer. Breast Cancer Res Treat. 2017 Aug 09;166:1–13. doi: 10.1007/s10549-017-4427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muzi M, Vesselle H, Grierson JR, et al. Kinetic analysis of 3′-deoxy-3′-fluorothymidine PET studies: validation studies in patients with lung cancer. J Nucl Med. 2005 Feb;46(2):274–282. [PubMed] [Google Scholar]
- 67.Loddo M, Kingsbury SR, Rashid M, et al. Cell-cycle-phase progression analysis identifies unique phenotypes of major prognostic and predictive significance in breast cancer. Br J Cancer. 2009 Mar 24;100(6):959–970. doi: 10.1038/sj.bjc.6604924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKinley ET, Ayers GD, Smith RA, et al. Limits of [18F]-FLT PET as a biomarker of proliferation in oncology. PLoS One. 2013;8(3):e58938. doi: 10.1371/journal.pone.0058938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mach RH, Smith CR, al-Nabulsi I, Whirrett BR, Childers SR, Wheeler KT. Sigma 2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res. 1997 Jan 01;57(1):156–161. [PubMed] [Google Scholar]
- 70.Bem WT, Thomas GE, Mamone JY, et al. Overexpression of sigma receptors in nonneural human tumors. Cancer Res. 1991 Dec 15;51(24):6558–6562. [PubMed] [Google Scholar]
- 71.Mach RH, Huang Y, Freeman RA, Wu L, Vangveravong S, Luedtke RR. Conformationally-flexible benzamide analogues as dopamine D3 and sigma 2 receptor ligands. Bioorg Med Chem Lett. 2004 Jan 05;14(1):195–202. doi: 10.1016/j.bmcl.2003.09.083. [DOI] [PubMed] [Google Scholar]
- 72.Tu Z, Dence CS, Ponde DE, et al. Carbon-11 labeled sigma2 receptor ligands for imaging breast cancer. Nucl Med Biol. 2005 Jul;32(5):423–430. doi: 10.1016/j.nucmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Kashiwagi H, McDunn JE, Simon PO, Jr, et al. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy. Mol Cancer. 2007 Jul 15;6:48. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tu Z, Xu J, Jones LA, et al. Fluorine-18-labeled benzamide analogues for imaging the sigma2 receptor status of solid tumors with positron emission tomography. J Med Chem. 2007 Jul 12;50(14):3194–3204. doi: 10.1021/jm0614883. [DOI] [PubMed] [Google Scholar]
- 75.Shoghi KI, Xu J, Su Y, et al. Quantitative receptor-based imaging of tumor proliferation with the sigma-2 ligand [(18)F]ISO-1. PLoS One. 2013;8(9):e74188. doi: 10.1371/journal.pone.0074188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dehdashti F, Laforest R, Gao F, et al. Assessment of cellular proliferation in tumors by PET using 18F-ISO-1. J Nucl Med. 2013 Mar;54(3):350–357. doi: 10.2967/jnumed.112.111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDonald ES, Tchou J, Doot R, et al. Imaging Proliferative Status in Primary Breast Cancer Using the sigma-2 selective ligand, [18]F-ISO-1. J Nucl Med. 2016;57(232 suppl) [Google Scholar]
- 78.McDonald ES, Mankoff J, Makvandi M, et al. Sigma-2 ligands and PARP inhibitors synergistically trigger cell death in breast cancer cells. Biochem Biophys Res Commun. 2017 May 06;486(3):788–795. doi: 10.1016/j.bbrc.2017.03.122. [DOI] [PubMed] [Google Scholar]
- 79.DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015 Mar 01;21(5):995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]