Abstract
Phytoplankton may serve as a key entry for methylmercury (MeHg) into aquatic food webs however very few studies have quantified the bioconcentration of MeHg in marine phytoplankton from seawater, particularly for non-diatoms. Experiments using 203Hg to measure MeHg uptake rates and concentration factors in six marine phytoplankton species belonging to different algal classes were conducted and the influence of light, temperature, and nutrient conditions on MeHg bioaccumulation were determined. All algal species greatly concentrated MeHg out of seawater, with volume concentration factors (VCFs) ranging from 0.2 × 105 to 6.4 × 106. VCFs were directly related to cellular surface area-to-volume ratios. Most of the cellular MeHg was found in the cytoplasm. Temperature, light, and nutrient additions did not directly affect MeHg uptake in most species, with the exception that the dinoflagellate Prorocentrum minimum displayed significantly greater uptake per cell at 18°C than at 4°C, suggesting an active uptake for this species. Passive transport seemed to be the major pathway for most phytoplankton to acquire MeHg and was related to the surface area-to-volume ratio of algal cells. Environmental conditions that promoted cell growth resulted in more total MeHg associated with cells, but with lower concentrations per unit biomass due to biodilution. The very high bioconcentration of MeHg in marine phytoplankton is by far the largest bioconcentration step in marine food chains and variations in algal uptake may account for differences in the amount of MeHg that ultimately builds up in different marine ecosystems.
Introduction
Interest in the biogeochemical cycling of methylmercury (MeHg) in marine ecosystems stems in part from the fact that its neurotoxicity can cause adverse health effects to exposed wildlife and humans (Wiener et al. 2003; Grandjean et al. 2010; Mason et al. 2012). Human exposure to MeHg is primarily from diet, especially seafood consumption (Sunderland 2007). Although MeHg concentrations in seawater are extremely low, typically in the subpico- to femto- molar range (Fitzgerald et al. 2007; Lamborg et al. 2014; Bowman et al. 2015), MeHg is the only mercury species to build up in aquatic food chains and display biomagnification, resulting in high MeHg levels in some common seafood items.
However, our understanding of the factors controlling MeHg accumulation in marine food webs is surprisingly limited. Most research in marine environments has focused on Hg (total Hg or MeHg) levels in commercial fish consumed by humans due to public health concerns (Morel et al. 1998; Selin 2009; Karimi et al. 2012). Very few studies have investigated Hg and MeHg interactions with primary producers at the base of marine food webs (Mason et al. 2012). Phytoplankton are the most critical entry where metals enter marine food webs (Fisher and Reinfelder 1995). What little lab and field data exist on this issue show that MeHg is concentrated from ambient water into phytoplankton by at least a factor of 105 (Watras et al. 1998; Pickhardt and Fisher 2007; Hammerschmidt et al. 2013; Gosnell and Mason 2015), which is by far the largest bioconcentration step in aquatic food webs. Phytoplankton then serve as a highly enriched source of MeHg for herbivores which can pass this compound on to animals higher in the food chain. Subsequent enrichment with increasing trophic levels in aquatic food chains is much smaller, almost always less than a factor of 10.
To date, most studies involving algal uptake of MeHg have considered freshwater phytoplankton (Watras et al. 1998; Miles et al. 2001; Gorski et al. 2006; Pickhardt and Fisher 2007; Luengen et al. 2012), and its trophic transfer in freshwater ecosystems (Pickhardt et al. 2002; Pickhardt et al. 2005). The few studies on marine environments are confined to a coastal diatom and cyanobacterium (Mason et al. 1996; Lawson and Mason 1998; Zhong and Wang 2009; Kim et al. 2014). Published data on MeHg interactions with other marine phytoplankton species are sparse. Generally, bioconcentration factors of metals can vary substantially among different algal species, and for any given metal generally increase with surface area-to-volume ratios (SA/V) of cells (increasing inversely with cell size) (Fisher 1986; Fisher and Reinfelder 1995). Since the composition of phytoplankton assemblages often shifts seasonally, MeHg uptake by the prevailing algal assemblage might also vary, consequently affecting overall MeHg accumulation in organisms at higher trophic levels.
In this study, we examined the uptake of MeHg by six marine phytoplankton species belonging to different algal classes to determine how biological attributes (e.g., size, surface area-to-volume ratio, cell wall composition, and metabolic rate) and environmental conditions (light, temperature, salinity, and nutrient levels) affect MeHg bioaccumulation in diverse marine algae. This information, combined with field data, could ultimately be used by global biogeochemical models describing Hg cycling in the marine environment.
Materials and methods
Six phytoplankton species Thalassiosira pseudonana (diatom), Dunaliella tertiolecta (chlorophyte), Rhodomonas salina (cryptophyte), Prorocentrum minimum (dinoflagellate), Emiliania huxleyi (coccolithophore), and Synechococcus bacillaris (cyanobacterium), belonging to different algal classes, were used in this study (Table 1). All species were held in clonal, unialgal cultures maintained axenically for generations in an incubator at 18°C under a light:dark cycle (14 h:10 h, 200 μmol quanta m−2 s−1) provided by cool white fluorescent lamps. The f/2 medium (Guillard and Ryther 1962) was prepared with sterile filtered (0.2 μm, Nuclepore polycarbonate membrane) surface seawater (35 psu, DOC = ~2 mg L−1) collected 8 km off Southampton, New York (Lat. 40.77°N, Long. 72.43°W), and was used for maintaining routine cultures. However, to avoid the potential effects on MeHg speciation due to the presence of ethylenediaminetetraacetic acid (EDTA), inocula for experimental algal cultures were grown in separate flasks with amended f medium (nutrients at f/20 level and without EDTA) for 7 to 10 d prior to experiments.
Table 1.
Phytoplankton species used in this study; ranges of measured cell volumes (μm3), calculated surface areas (μm2), and mean surface area-to-volume ratios (μm−1).
| Phytoplankton type | Species | Clone | Volume μm3 | Surface area μm2 | SA/V ratio* μm−1 |
|---|---|---|---|---|---|
| Diatom | Thalassiosira pseudonana | CCMP1335 | 43–62 | 63–89 | 1.44 |
| Chlorophyte | Dunaliella tertiolecta | CCMP1320 | 141–227 | 127–204 | 0.90 |
| Cryptophyte | Rhodomonas salina | CCMP1319 | 160–231 | 133–192 | 0.83 |
| Dinoflagellate | Prorocentrum minimum | CCMP696 | 540–778 | 313–451 | 0.58 |
| Coccolithophore | Emiliania huxleyi | CCMP375 | 40–75 | 51–97 | 1.28 |
| Cyanobacterium | Synechococcus bacillaris | CCMP1333 | 1.1–1.6 | 4.4–6.6 | 4.13 |
Surface area-to-volume ratio.
The gamma-emitting radioisotope, 203Hg (t½ = 46.6 d) was used to trace the transfer of Hg between dissolved and particulate phases in all cultures. Commercially available 203Hg(II) was converted to monomethylmercury (CH3203Hg+ or Me203Hg) from inorganic 203HgCl2 following established methods (Rouleau and Block 1997; Luengen et al. 2012). Briefly, inorganic 203Hg solution purchased from Eckert and Ziegler Isotope Products (Valencia, California) (specific activity: 5 Ci g−1) was mixed with methylcobalamin (C63H91CoN13O14P) and acetate buffer at pH 5, allowing the reaction to proceed in the dark for 18 h to 24 h and then forming Me203Hg. Following extraction by dichloromethane (CH2Cl2) and purification procedures, Me203Hg was then re-dissolved in Milli-Q® water and was ready to use. The conversion yield (fraction of total 203Hg recovered as Me203Hg) was 95 ± 3% (n = 6). A series of experiments was conducted using Me203Hg to track the partitioning of MeHg between water and phytoplankton. Me203Hg activities in experiments ranged from 4.46 kBq L−1 to 9.29 kBq L−1, corresponding to concentrations of 0.29 nM to 0.42 nM. These MeHg concentrations were at the high end of those found in natural waters, however previous studies have shown that bioconcentration factors and uptake rate constants for metals (including Hg) in algal cells are not affected by modestly elevated metal concentrations that are below toxic levels (Fisher et al. 1984). As noted in numerous previous gamma-emitting radiotracer studies, this approach provides a nondestructive, noninvasive and direct measurement of metal bioaccumulation while using low metal concentrations that are in the range of naturally occurring levels (Fisher 2002).
The MeHg uptake experiments generally followed protocols described for metal uptake by marine phytoplankton (Fisher et al. 1984; Stewart and Fisher 2003b). The seawater used in all experiments except those involving nitrate or chloride additions was 0.2 μm filtered surface seawater (35 psu, collected 8 km off Southampton, New York), without addition of any nutrients. Trace metal clean glass-stoppered Erlenmeyer flasks, each containing 150 mL of seawater and microliter quantities of Me203Hg solution (added 1 h prior to inoculation with phytoplankton cells to reach equilibrium) were incubated at 18°C under the light:dark cycle. Inocula of algal cells were concentrated by resuspending cells off 1 μm membranes or by centrifugation at 1000 g for 10 min from late log-phase cultures (without EDTA). Considering the difference in algal biomasses, initial cell densities ranged from 6 × 103 to 6 × 104 cells mL−1, depending on algal species. Water and cell samples were periodically collected into glass tubes and onto 1 μm polycarbonate membranes, respectively for radioassay. This approach (Fisher et al. 1983a) measures the total radioactivity in 1 mL of suspension (water plus cells) and, in a separate sample taken at the same time, the radioactivity in 10-mL of suspension caught on a 1 μm polycarbonate membrane (vacuum pressure < 100 mmHg) that was then washed with 2 × 5 mL of unlabeled, filtered seawater. With this approach, the fraction of total radioactivity in suspension associated with cells and the fraction in the dissolved phase could be determined. We performed both short term (t = 1, 2, 3, 4, 8 h) and long term (t = 2, 6, 12, 24, 48, 72 h) experiments to evaluate the uptake rate constants and bioconcentration factors of MeHg for each species. For each culture, cell density and volume were monitored simultaneously over time using a Multisizer™ Coulter Counter® and cell surface area was calculated using appropriate geometric equations (Table 1).
All experiments included control flasks without algal cells which were used to correct for the potential adsorption of Me203Hg onto filter membranes. In addition, sorption of MeHg onto culture flasks was examined by acid washing the flask walls after cell exposures. Sample activities were determined using an LKB Wallac 1282 Compugamma NaI(Tl) gamma detector. 203Hg activity was assessed at 279 keV. All samples were counted with standards and decay-corrected. Propagated counting errors were < 5%.
To examine the effect of low temperature (4°C) on MeHg uptake by phytoplankton, algal cells cultured in medium without EDTA were acclimated at 4°C for 6 h prior to experimental inoculation, after which the experimental procedures described above were followed. To assess MeHg uptake by phytoplankton without light, experimental cultures were held in the dark (18°C). In an experiment evaluating the effects of nitrate on MeHg uptake, different levels of nitrate additions (0, 5, 10, 50 μM) to artificial seawater (prepared by adding salts to Milli-Q® water; 35 psu; DOC = ~1 mg L−1) (Kester et al. 1967) were used as the experimental media; phosphate and silicate concentrations were fixed at 1 μM and 10 μM, respectively. The nutrient concentrations were representative of nutrient levels reported for Long Island Sound, New York (Gobler et al. 2006). Four algal species, including the diatom, the chlorophyte, the dinoflagellate, and the coccolithophore were tested in the nutrient experiment. In a parallel experiment assessing the influence of the culture medium’s ionic strength on MeHg uptake, the euryhaline diatom, T. pseudonana, was exposed to MeHg under four different salinities, reflected by chloride concentrations of 5.90, 27.9, 55.4, and 550 mM, prepared by mixing WCL-1 medium (Guillard 1975) and artificial seawater. The highest chloride concentration corresponds with 35 psu seawater. The diatoms were cultured in these media for more than two generations to make sure they were fully acclimated to low salinity environments.
The cytoplasmic distribution of MeHg in phytoplankton cells was examined in all six species after exposure to Me203Hg for 3 d (two to three cell divisions) to uniformly radiolabel the cells (Fisher et al. 1983b; Reinfelder and Fisher 1991; Stewart and Fisher 2003b).
Results
Algal growth at 18°C under a 14:10 light:dark cycle varied among species, with population doublings during the 72 h exposure period ranging from one for the dinoflagellate P. minimum to four for the diatom T. pseudonana (Fig. 1). All algal species accumulated MeHg (Fig. 1), however two patterns emerged. For those cultures (cryptophyte R. salina, chlorophyte D. tertiolecta, coccolithophore E. huxleyi) where- < 40% of the total MeHg was associated with the cells over the 72 h period, uptake generally followed increases in algal biomass, whereas when > 60% of the MeHg was associated with cells (diatom T. pseudonana, dinoflagellate P. minimum, cyanobacterium S. bacillaris), uptake slowed over time even as growth continued (Fig. 1), probably because there was little bioavailable MeHg remaining in the dissolved phase to support further uptake. For all species, most of the MeHg was found in the cytoplasmic fraction of the cells (Table 2). Among the algal species, no general patterns were observed for MeHg penetration into the cytoplasm among the various culture treatments.
Fig. 1.
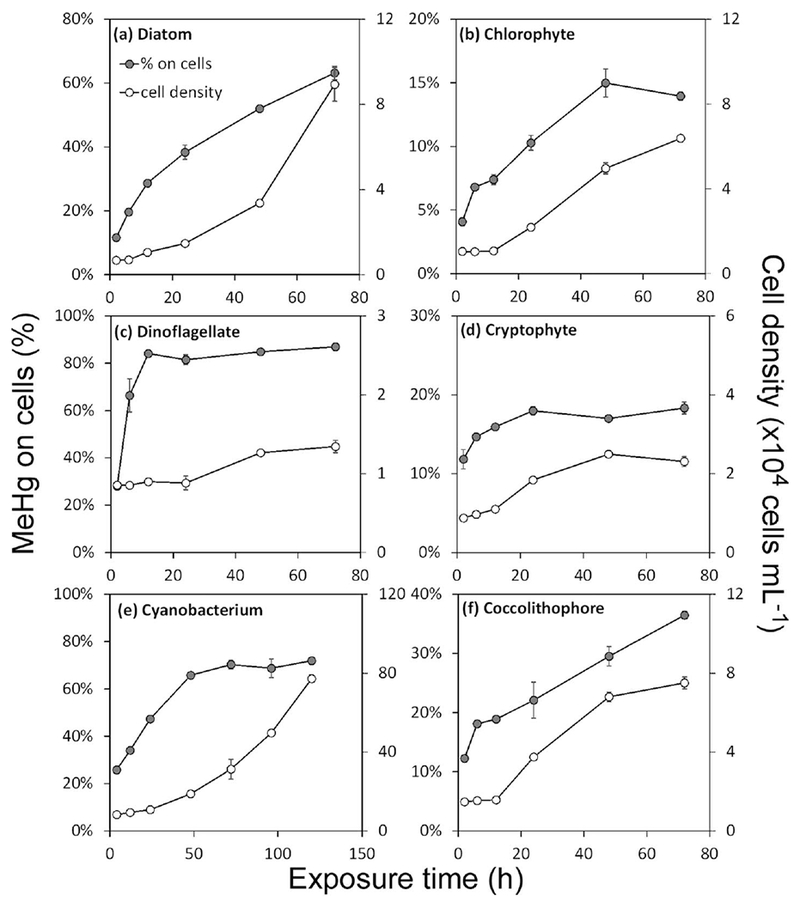
MeHg uptake and growth of algal cells among six phytoplankton species over time. The solid circles (left axis) represent the percentage (%) of total MeHg in the cultures associated with the particulate (>1 μm) phase. The open circles (right axis) represent the cell densities (×104 cells mL−1). Data points are the means from three replicates cultures with error bars of one standard deviation. Note the different scales on each graph.
Table 2.
Percentage of total cellular MeHg in the cytoplasmic fraction under three environmental conditions.
| Algal species | Conditions |
||
|---|---|---|---|
| 18°C, L/D* | 18°C Dark | 4°C, L/D | |
| T. pseudonana | 26 | 53 | 61 |
| D. tertiolecta | 75 | 73 | 53 |
| R. salina | 77 | 74 | 79 |
| P. minimum | 27 | 39 | 57 |
| E. huxleyi | 78 | 88 | 82 |
| S. bacillaris | 38 | 91 | 73 |
L/D: 14:10 light-dark cycle.
In each flask, sorption of Me203Hg to the glass walls accounted for < 5% of the total Me203Hg, and at least 90% of the total Me203Hg could be accounted for by summing dissolved, particulate, and flask wall activities of the added Me203Hg (Fig. 2). The exception was for the coccolithophore (E. huxleyi) cultures, where a substantial loss of Me203Hg (~30%) was consistently observed, implying strong volatilization from the cultures. The cryptophyte R. salina and the cyanobacterium S. bacillaris cells were prone to attach to flask walls and may not have been completely removed by acid rinsing of the walls. This may explain that 203Hg recoveries for these cultures were such that mass balances were not able to achieve ~100%.
Fig. 2.
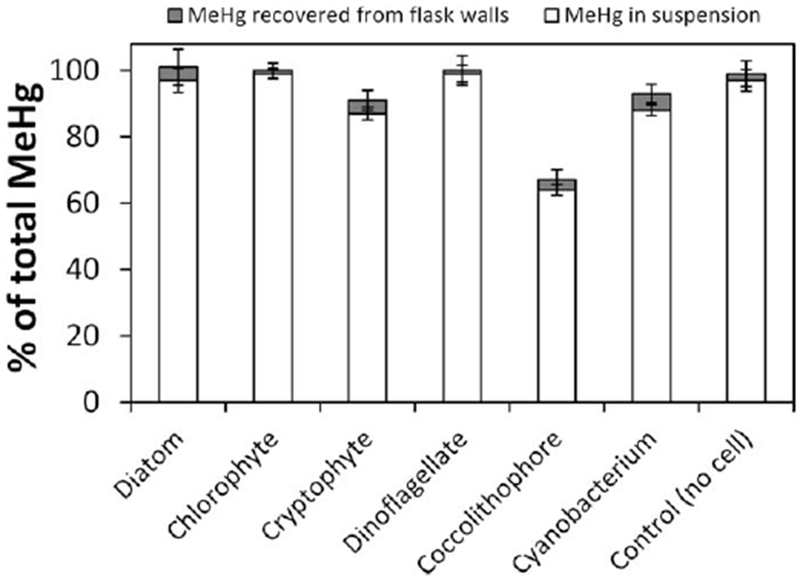
MeHg mass balance in cultures at 18°C and 14:10 light:dark cycle. The white bar represents the percentage of total MeHg remaining in suspension (dissolved plus particulate fractions after 72 h exposure). The gray bar represents the MeHg recovered from the flask wall by acid rinsing at 72 h. Recovery of less than 100% of total Me203Hg from each culture is presumed attributable to evasion into the air.
Volume concentration factors (VCFs) were calculated ([Bq Me203Hg μm−3]cell/[Bq Me203Hg μm−3]solution), showing the relative degree of enrichment in the algal cells relative to the ambient water. Figure 3 shows VCFs of six species over time under different environmental conditions. At 18°C, VCFs ranged from 0.2 × 105 for the chlorophyte D. tertiolecta to 6.4 ×106 for the cyanobacterium S. bacillaris. Within the first 12 h, there were no differences in MeHg VCFs among environmental conditions for all species but the dinoflagellate. After 12 h of exposure, VCFs in the dark and low temperature treatments leveled off. However, VCFs at 18°C and light/dark conditions decreased slightly and then leveled off. This decreasing trend was particularly obvious in the cultures of the diatom T. pseudonana and the cyanobacterium S. bacillaris for which growth was most pronounced. To compare MeHg sorption to abiotic and living particles, the MeHg accumulation by suspended glass beads (diameter ~5 μm), added at a density of 105 beads mL−1, was determined. Only about 1% of the MeHg adsorbed onto the surface of the glass beads, resulting in a VCF of about 103 (data not shown).
Fig. 3.
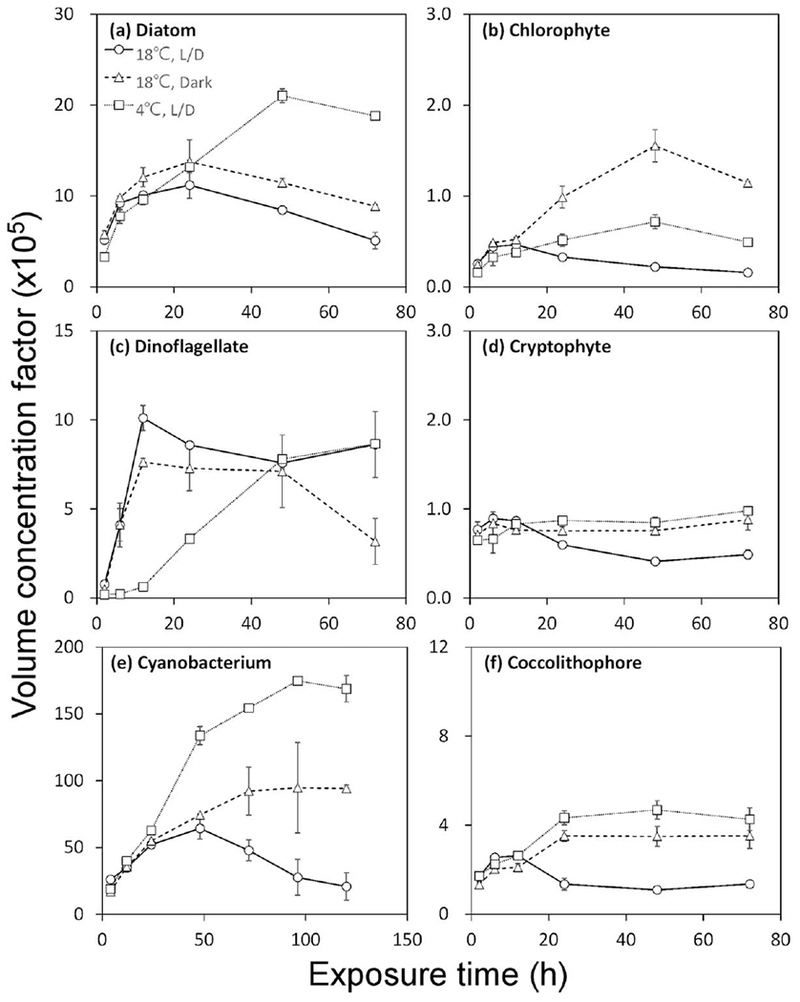
MeHg volume concentration factors over time under different environmental conditions. L/D represents 14:10 light:dark cycle. Data points are the means from two replicate cultures shown with 1 SD error bars. Note the different scales on each graph.
In the short term exposure experiments, it was clear that light had no significant effect on MeHg uptake rates for any species (Fig. 4) but temperature did have a pronounced effect on MeHg uptake rates in the dinoflagellate P. minimum and to a lesser extent in the cyanobacterium S. bacillaris. The short term uptake rate constant, which was normalized to the initial dissolved MeHg concentrations in the media, was calculated on per cell, per μm2 cell surface, and per μm3 cell volume bases for the first 4 h of exposure during which time MeHg uptake was linear (Fig. 5; Table 3). At 18°C, the uptake rate constants on cell surface area or cell volume bases were comparable for the diatom, the cyanobacterium, and the dinoflagellate, all of which were significantly higher than the other species, sometimes by more than an order of magnitude.
Fig. 4.
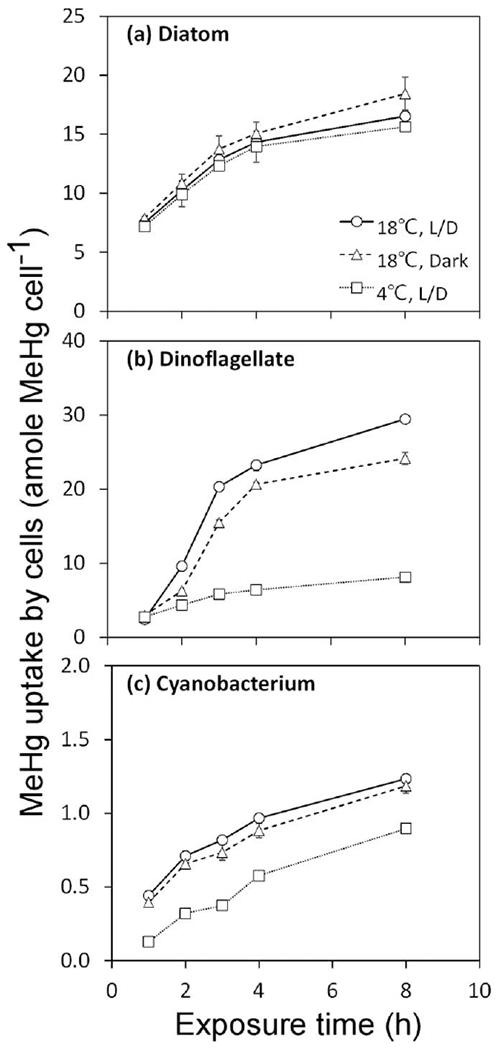
Uptake of MeHg in cells over short-term exposures for T. pseudonana (diatom), P. minimum (dinoflagellate), and S. bacillaris (cyanobacterium) under different environmental conditions. L/D represents 14:10 light:dark cycle. Data points are the means from two replicate cultures shown with 1 SD error bars.
Fig. 5.
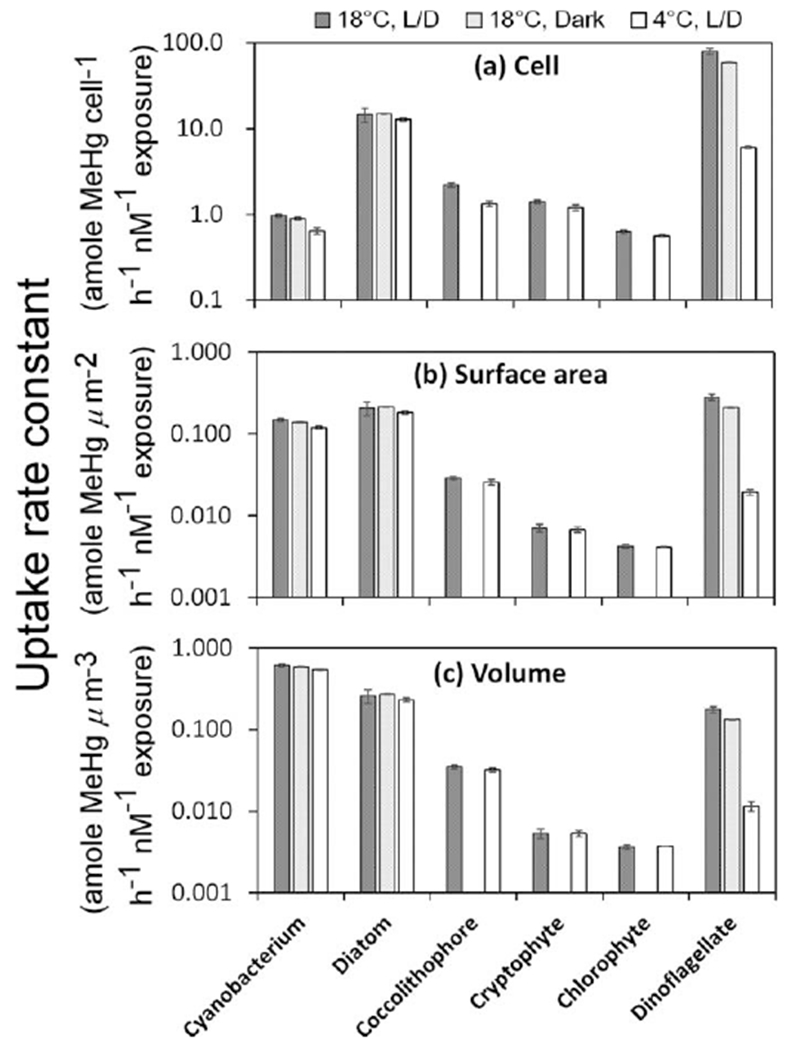
Uptake rate constants of MeHg in six different algal species over short-term exposures. Values are normalized to the initial dissolved MeHg concentrations in the media and are expressed on per cell, per μm2 cell surface area, and per μm3 cell volume bases for the first 4 h of exposure. Algal species on the x axis are arranged in increasing size order, with the cyanobacterium being the smallest cell and the dinoflagellate the largest cell.
Table 3.
MeHg uptake rate constants (normalized to the initial dissolved MeHg concentrations in the media) on per cell, per μm2 cell surface area, and per μm3 cell volume bases for the first 4 h of exposure.
| Algal species |
|||||||
|---|---|---|---|---|---|---|---|
| Uptake rate constant | Conditions | T. pseudonana | D. tertiolecta | R. salina | P. minimum | E. huxleyi | S. bacillaris |
| Cell basis* | 18°C, L/D | 15±3 | 0.63±0.03 | 1.4±0.1 | 80±6 | 2.2±0.1 | 0.97±0.03 |
| 18°C, Dark | 15±0.1 | 59±0.2 | 0.89±0.03 | ||||
| 4°C, L/D | 13±1 | 0.56±0.02 | 1.2±0.1 | 6.1±0.1 | 1.3±0.1 | 0.64±0.06 | |
| Surface area basis† | 18°C, L/D | 0.21±0.04 | 0.0042±0.0002 | 0.0071±0.0007 | 0.28±0.02 | 0.029±0.001 | 0.15±0.01 |
| 18°C, Dark | 0.21±0.00 | 0.21±0.00 | 0.14±0.00 | ||||
| 4°C, L/D | 0.18±0.01 | 0.0041±0.0000 | 0.0067±0.0005 | 0.02±0.00 | 0.026±0.002 | 0.12±0.01 | |
| Volume basis‡ | 18°C, L/D | 0.26±0.05 | 0.0036±0.0002 | 0.0053±0.0007 | 0.18±0.01 | 0.035±0.002 | 0.62±0.03 |
| 18°C, Dark | 0.27±0.00 | 0.13±0.01 | 0.59±0.01 | ||||
| 4°C, L/D | 0.23±0.01 | 0.0037±0.0000 | 0.0054±0.0004 | 0.01±0.00 | 0.032±0.002 | 0.55±0.01 | |
unit:
amole MeHg cell−1 h−1 nM−1 exposure
amole MeHg μm−2 h−1 nM−1 exposure
amole MeHg μm−3 h−1 nM−1 exposure
Experiments to assess the effects of nitrate level on MeHg uptake showed no significant effect in four algal species. MeHg was accumulated over time but no differences were found between high and low nutrient treatments (Fig. 6). However, higher nutrient levels led to significant cell growth after 48 h to 72 h (Fig. 7a–d). Such increases in algal biomass would result in a “dilution” of MeHg in algal cells, and lower VCFs in high nutrient treatments were indeed observed at the end of the experiment (Fig. 7e–h). For the ionic strength (salinity) experiment involving the diatom T. pseudonana, VCF values increased with chloride concentrations (Fig. 8a). Uptake of MeHg was essentially linear over the first 4 h exposure, and at this time the MeHg VCFs in the diatoms was linearly related to the log chloride concentration (Fig. 8b).
Fig. 6.
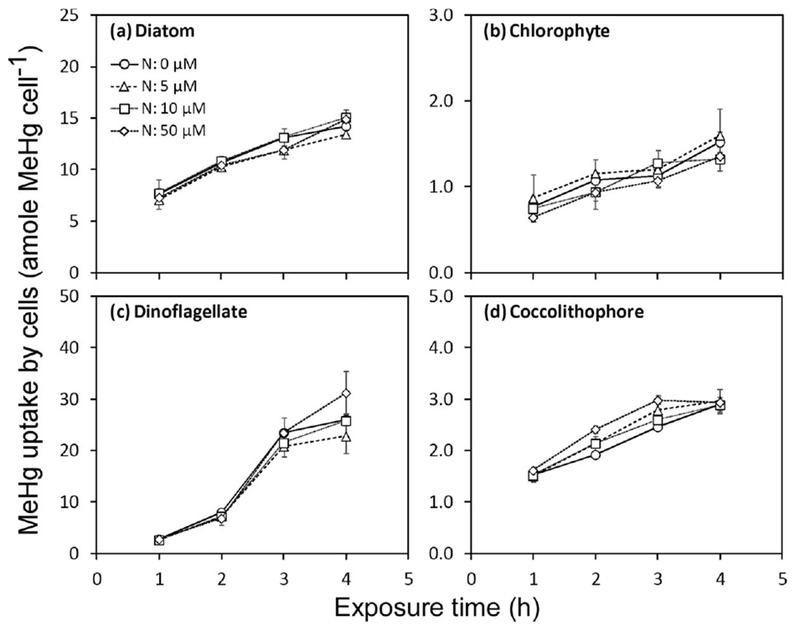
Uptake of MeHg by cells exposed under four nitrate treatments for T. pseudonana (diatom), D. tertiolecta (chlorophyte), P. minimum (dinoflagellate), and E. huxleyi (coccolithophore). Data points are the means from two replicate cultures shown with 1 SD error bars.
Fig. 7.
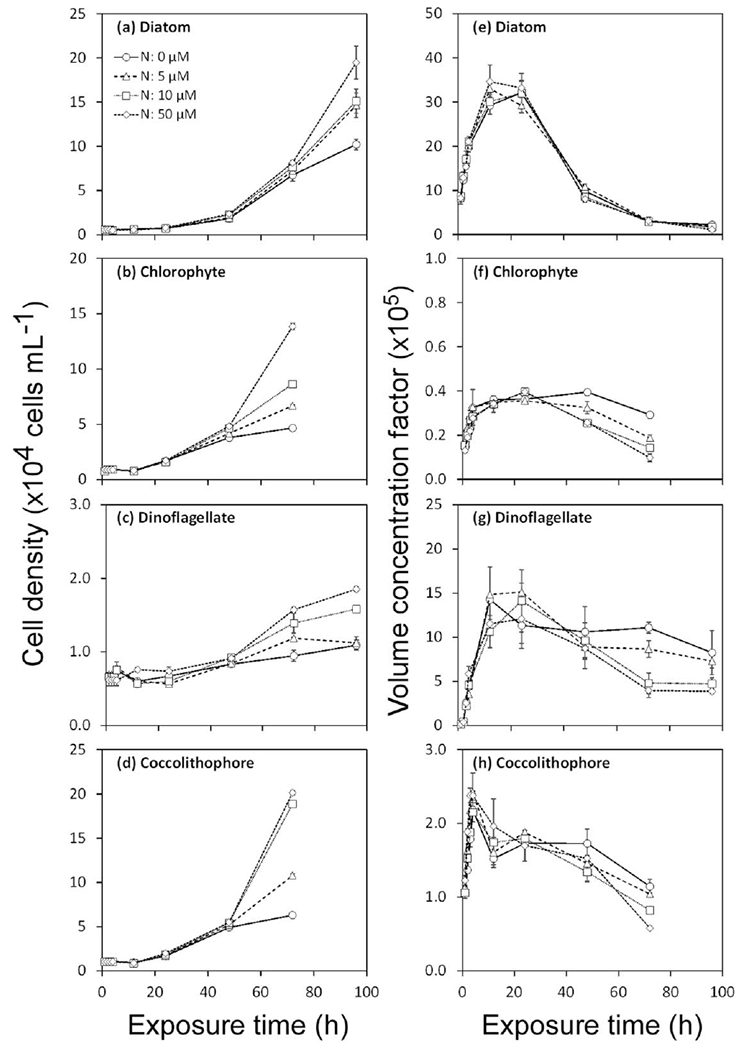
Cell growth (a–d) and MeHg volume concentration factors (×105, e–h) over time in cultures exposed to four different nitrate treatments. Data points are the means from two replicate cultures shown with 1 SD error bars.
Fig. 8.
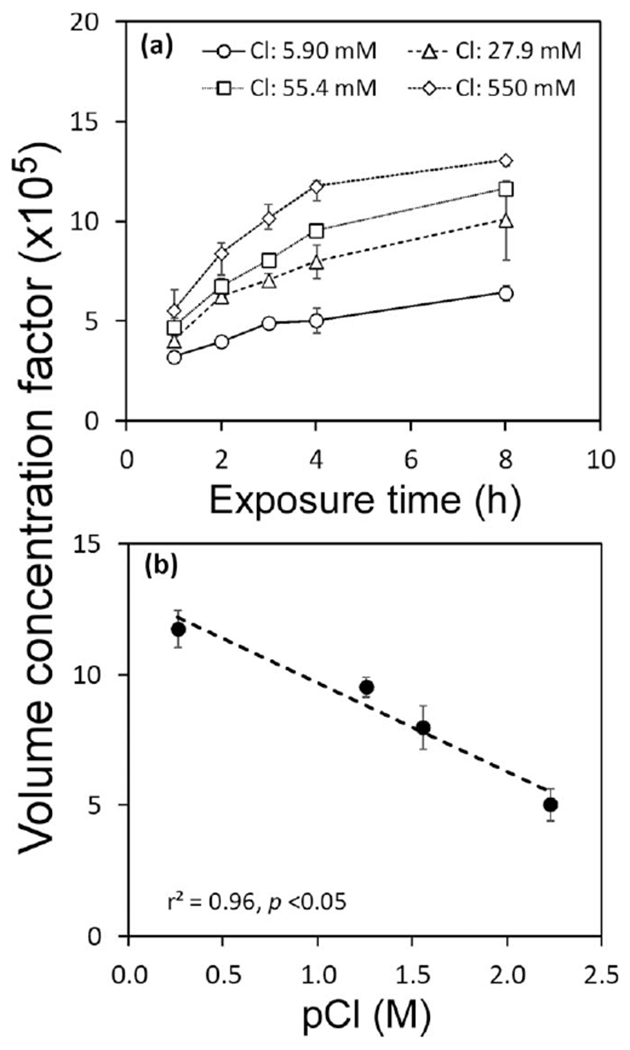
(a) Volume concentration factors (×105) for the diatom, T. pseudonana, over time at four different chloride concentrations. (b) The VCFs (t = 4 h) vs. pCl (−log Cl concentration). The dotted line represents the linear regression relating VCF to pCl (r2 = 0.96, p < 0.05). Data points are the means from three replicate cultures shown with 1 SD error bars.
Discussion
This is the first report describing MeHg uptake by diverse marine phytoplankton cells, and how environmental conditions affect the uptake process. While all species greatly concentrated MeHg out of ambient seawater, significant differences among the species can be explained. VCFs among the different algal species significantly increased with cell surface area-to-volume ratios (Fig. 9) except for the dinoflagellate which may have a different uptake pathway. In general, the greater MeHg enrichment in phytoplankton for smaller cells with greater surface area-to-volume ratios is consistent with findings for many other metals and algal taxa (Fisher and Reinfelder 1995).
Fig. 9.
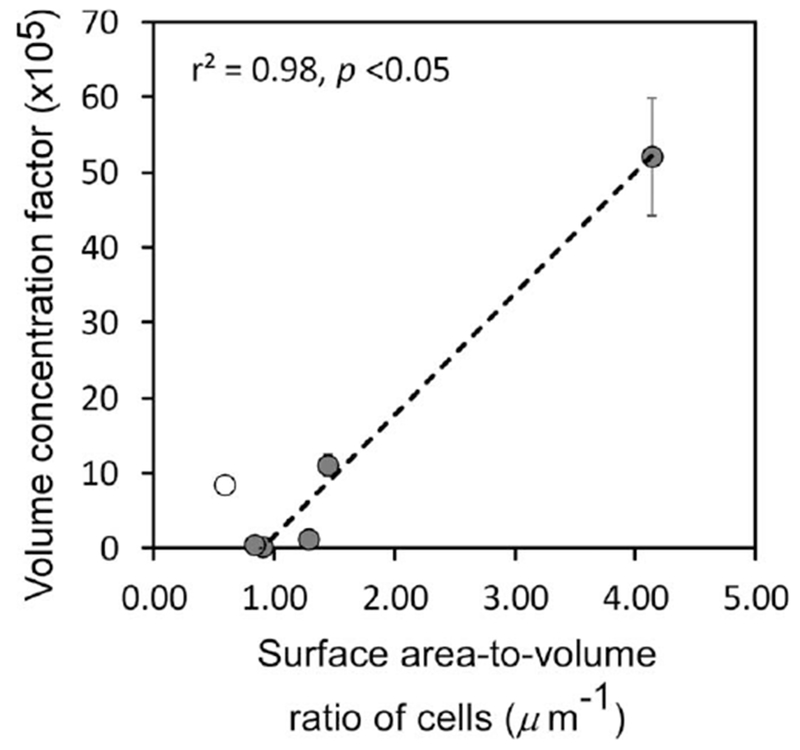
Correlation between the mean surface area-to-volume ratio of algal cells from each culture and the MeHg volume concentration factors (VCFs). Data points are the mean VCFs at 24 h (n = 3) shown with 1 SD error bars. The linear regression excludes the dinoflagellate P. minimum (open circle).
These findings are also consistent with the conclusion that passive sorption of dissolved MeHg to cell surfaces dominates the uptake pathway. Most other metals that speciate as cations in seawater behave similarly (Fisher 1986; Fisher and Reinfelder 1995). The comparable uptake rate constants of MeHg by most species at 4°C, when cell growth and metabolic activity were minimal, and at 18°C when growth rates and metabolic activity were high, is also consistent with the passive uptake of MeHg. Like temperature’s effects, the only effect of light on MeHg uptake by phytoplankton was an indirect one. Thus, uptake rate constants for short exposure periods were unaffected by light, but after several days, illuminated cells were able to grow more than cells held in constant darkness, leading to greater biomass and hence more total particulate MeHg. VCFs eventually declined in illuminated cultures held at 18°C because biodilution of cell-bound MeHg occurred when cell biomasses increased to such an extent that most of the bioavailable MeHg in the dissolved phase was exhausted. This biodilution has been observed for MeHg in cultures (Karimi et al. 2007) and natural assemblages (Pickhardt et al. 2002) of freshwater algae. It would not be expected to occur in most marine waters because plankton biomass densities are typically low enough to preclude exhausting ambient MeHg. Further, because it is retained by aquatic organisms much more effectively than most other metals (including inorganic mercury), MeHg is more prone to biodilution than other metals (Karimi et al. 2010).
The pronounced loss of 203Hg from the coccolithophore cultures, but not from uninoculated medium or other algal cultures, indicates that once the Me203Hg was taken up by the coccolithophore (E. huxleyi), it was converted to a gaseous form of Hg, either Hg0 or dimethylmercury (Me2Hg). Henry’s law constants of either of these products would suggest that they would display evasive properties. The production of mercury gas in the coccolithophore cultures was probably attributable to bacterial cells that were observed to be growing in cultures of this clone. Bacteria containing the Hg resistance (mer) operon might explain the demethylation and reduction of MeHg (Barkay et al. 2003) in the coccolithophore culture. We are unaware of reports demonstrating conversion of MeHg into an evasive form of mercury, although marine microorganisms smaller than 3 μm have been shown to reduce dissolved Hg(II) to elemental Hg0, an evasive form of mercury (Mason et al. 1995).
As expected, the MeHg VCFs were positively related to the surface area-to-volume ratios of the various algal cells (inversely related to cell size) (Fig. 9). Expressed on a surface to volume ratio, the MeHg concentration factors were comparable to that found for the freshwater diatom Cyclotella meneghiniana (SA/V = 0.94 μm−1) exposed under similar conditions (Pickhardt and Fisher 2007; Luengen et al. 2012). This is broadly consistent with patterns shown for many other particle-reactive metals that sorb onto cell surfaces (Fisher and Reinfelder 1995) and is also consistent with the idea that MeHg binds passively to them. This finding would suggest that the degree of enrichment of MeHg in algal cells in natural communities could depend on the size of the predominant cells in plankton assemblages, which in turn may vary seasonally and regionally.
The unique MeHg uptake pattern in the dinoflagellate P. minimum showed a significantly higher MeHg uptake rate at 18°C than at 4°C. This suggests another pathway of MeHg acquisition in this species rather than solely passive transport. As this dinoflagellate can be mixtrophic, it may be possible that MeHg bound to organic matter or other nanoparticles was acquired via an energy-requiring process such as phagocytosis. Tranvik et al. (1993) suggested that metals bound to colloidal matter might be an important source of metals to heterotrophic flagellates. Wang and Guo (2000) found that marine colloidal materials added to cultures of a diatom and dinoflagellate affected uptake of some metals. However, there was no related study for either Hg or MeHg.
Nitrogen addition to the algal cultures increased the algal biomass in cultures after 2 d, and this led to more total particulate MeHg and eventually lower VCFs. However, the MeHg uptake rate constants over the first 4 h of exposure (during which growth differences between treatments were minimal) were unaffected by nitrogen additions. In contrast, Wang and Dei (2001a, 2001b) demonstrated significant increases in short-term Cd and Zn uptake by phytoplankton enriched with nitrogen. As with light and temperature, the principal effect of nutrient additions on MeHg uptake was an indirect one, where the greater biomass eventually resulting from higher nitrogen levels led to more total particulate MeHg in algal cells, but with each cell being less enriched in MeHg. Consequently, in eutrophic waters where algal biomasses are high, MeHg concentrations in cells may be expected to be lower, consistent with observations in lakes (Pickhardt et al. 2002) and the NW Atlantic Ocean (Hammerschmidt et al. 2013). This could be expected to result in lower MeHg concentrations in animals in eutrophic ecosystems.
The penetration of MeHg into the cytoplasm of the cells is consistent with findings for MeHg in marine and freshwater diatoms (Mason et al. 1996; Pickhardt and Fisher 2007). This has implications for the likelihood of trophic transfer of MeHg to herbivorous zooplankton, as shown for MeHg with a marine diatom (Mason et al. 1996) and for other metals for crustacean zooplankton and molluscan larvae (Reinfelder and Fisher 1991; Reinfelder and Fisher 1994; Stewart and Fisher 2003a). To our knowledge, the distribution of MeHg in other algal cell types has not been reported. Because the VCFs and uptake rate constants of MeHg were not affected by light and temperature in most algal species (Figs. 3, 4), it appears that metabolic activities did not lead to active MeHg uptake, consistent with earlier conclusions (Mason et al. 1996).
The relationship of MeHg uptake by the diatom T. pseudonana over a range of chloride concentrations coincides with observations of previous studies reported for the diatoms Thalassiosira weissflogii (Mason et al. 1996) and Ditylum brightwellii (Kim et al. 2014). The increased lipophilicity of chloro-complexed MeHg should increase the passive transport of MeHg with increasing Cl concentration and may therefore lead to greater MeHg enrichment in marine food chains than in freshwater food chains.
Overall, the range of VCFs of MeHg in the six algal species (log VCF = 4.3–6.8) was comparable to findings for previous lab studies involving freshwater or marine phytoplankton (Table 4). Pickhardt and Fisher (2007) reported log VCF ranged from 5.1 to 6.2 and Miles et al. (2001) reported log VCF ranged from 5.4 to 6.9 for freshwater algae. For marine algae, Kim et al. (2014) reported log VCF values ranging from 4.9 to 6.2. Our data also agree with bioaccumulation factors of MeHg obtained from field studies (Table 4), suggesting that the experimental conditions used in this study are generally applicable to natural waters. Thus, even though the experimental MeHg concentrations exceeded typical MeHg concentrations in marine ecosystems, the degree of bioconcentration in phytoplankton is comparable. The field measurements cited in Table 4 were originally expressed on a dry weight basis, and these were converted to a volume basis by using a mean ratio of cell volume:dry weight of 5.0 (Fisher et al. 1983a). The field measurements were reported for microseston in the Northeast Atlantic (log VCF = 4.9) (Hammerschmidt et al. 2013), the subtropical North Pacific (log VCF = 5.9) (Hammerschmidt and Bowman 2012), and in the central Pacific (log VCF = 6.3) (Gosnell and Mason 2015). Relatively low VCFs were found in the North Sea (log VCF = 4.2) (Baeyens et al. 2003), and in Long Island Sound (log VCF = 4.2) (Hammerschmidt and Fitzgerald 2006a). Given the variability of VCFs noted among algal cultures in this study, the concentration factors of MeHg in natural phytoplankton assemblages in the field may be expected to vary seasonally and spatially with the phytoplankton composition and predominant cell size. This variation in MeHg VCFs among phytoplankton may be one order of magnitude or more (Fig. 9), and these differences in MeHg bioaccumulation at the base of the food web may be reflected in variations in higher trophic level animals, such as in fish. Moreover, the fraction of lithogenic materials might also influence the overall concentration factor, particularly in coastal regions, since MeHg has relatively low affinity for abiotic particles, as shown with the glass beads. VCFs in phytoplankton were about 100-fold higher than in glass beads, suggesting that MeHg binds principally to biochemical compounds (especially S and N-rich compounds such as proteins) (Vallee and Ulmer 1972; Onyido et al. 2004) associated with living cells. These findings indicate that MeHg will be more enriched in living than in abiotic particles, and consequently inversely related to the abundance of lithogenic particles (Bloom et al. 1999; Hammerschmidt et al. 2004; Hammerschmidt and Fitzgerald 2006b). Thus the relatively low VCFs seen in coastal regions such as the North Sea and Belgian coastal waters were probably due to the higher fraction of lithogenic materials collected in suspended particulate material. In contrast, high VCFs were observed in open ocean regions such as the central Pacific where biogenic particles are dominant.
Table 4.
MeHg VCFs from culture experiments (a) and field studies (b).
|
(a) Lab study | |||
| Particle type | Diameter range* (μm) | Log VCF | References |
| Marine algae | 1.0–12 (6) | 4.3–6.8 | This study |
| 5.0–32 (5) | 4.9–6.2 | Kim et al. (2014) | |
| Freshwater algae | 8.0 (1) | 4.2–5.4 | Luengen et al. (2012) |
| 2.0-8.2 (4) | 5.1–6.2 | Pickhardt and Fisher (2007) | |
| 4.0-120 (4) | 5.4–6.9 | Miles et al. (2001) | |
| Glass beads | 5.0 | 3.1 | This study |
|
(b) Field data | |||
| Sampling site | Size range (μm) | Log VCF† | References |
| North Sea | 4.2 | Baeyens et al. (2003) | |
| Belgian coast | 4.2 | Baeyens et al. (2003) | |
| Long Island Sound | >0.2 | 4.5 | Hammerschmidt and Fitzgerald (2006a) |
| Northeast Atlantic | 0.2–200 | 4.9 | Hammerschmidt et al. (2013) |
| North Pacific | 1.0–51 | 5.9 | Hammerschmidt and Bowman (2012) |
| Central Pacific | 0.2–200 | 6.3 | Gosnell and Mason (2015) |
Mean diameter of algal cells. The numbers in parentheses indicate the number of algal species tested in the study.
Literature values were originally on a dry wt basis; they were converted to VCFs by using a mean cell volume:dry wt ratio of 5.0, based on measured values from 6 marine phytoplankton cells (Fisher et al. 1983a)
The VCFs of MeHg reported here are higher than for most other metals, even particle-reactive metals such as Zn, Cd, Ag, Pb, Po, and inorganic Hg (Fisher et al. 1984; Fisher et al. 1987; Stewart and Fisher 2003b), and about the same as the transuranic elements Pu and Am (Fisher et al. 1983a). Importantly, MeHg penetrates into the cytoplasm of algal cells to a much greater than the other metals (particularly true for Pb, Pu, Am, and inorganic Hg), which largely remain sorbed on the surface of cells. Consequently, the MeHg is assimilated by herbivores that feed on these phytoplankton (and subsequently passed up food chains) to a much greater extent than occurs with other metals (Reinfelder and Fisher 1991).
Acknowledgments
We appreciate helpful discussions with R. Mason and J. Reinfelder and helpful comments from two anonymous reviewers. Research reported in this publication was supported by NSF Award PLR 1260345, NIEHS Award P42ES007373, and NYSERDA Award 34357
References
- Baeyens W, and others. 2003. Bioconcentration and biomagnification of mercury and methylmercury in North Sea and Scheldt estuary fish. Arch. Environ. Contam. Toxicol 45: 498–508. doi: 10.1007/s00244-003-2136-4 [DOI] [PubMed] [Google Scholar]
- Barkay T, Miller SM, and Summers AO. 2003. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev 27: 355–384. doi: 10.1016/S0168-6445(03)00046-9 [DOI] [PubMed] [Google Scholar]
- Bloom NS, and others. 1999. Speciation and cycling of mercury in Lavaca Bay, Texas, sediments. Environ. Sci. Technol 33: 7–13. doi: 10.1021/es980379d [DOI] [Google Scholar]
- Bowman KL, Hammerschmidt CR, Lamborg CH, and Swarr G. 2015. Mercury in the North Atlantic Ocean: The U.S. GEOTRACES zonal and meridional sections. Deep-Sea Res. Part II 116: 251–261. doi: 10.1016/j.dsr2.2014.07.004 [DOI] [Google Scholar]
- Fisher NS 1986. On the reactivity of metals for marine phytoplankton. Limnol. Oceanogr 31: 443–449. doi: 10.4319/lo.1986.31.2.0443 [DOI] [Google Scholar]
- Fisher NS 2002. Advantages and problems in the application of radiotracers for determining the bioaccumulation of contaminants in aquatic organisms. In Borretzen P, Jolle T, and Strand P [eds.], Proc. Intl. Conf. Radioactivity in the Environment, 1–5 Sep 2002, Monaco. Norwegian Radiation Protection Authority, Østerås, p 573–576. [Google Scholar]
- Fisher NS, Bjerregaard P, and Fowler SW. 1983a. Interactions of marine plankton with transuranic elements. 1. Biokinetics of neptunium, plutonium, americium, and californium in phytoplankton. Limnol. Oceanogr 28: 432–447. doi: 10.4319/lo.1983.28.3.0432 [DOI] [Google Scholar]
- Fisher NS, Burns KA, Cherry RD, and Heyraud M. 1983b. Accumulation and cellular distribution of 241Am, 210Po, and 210Pb in two marine algae. Mar. Ecol. Prog. Ser 11: 233–237. doi: 10.3354/meps011233 [DOI] [Google Scholar]
- Fisher NS, Bohe M, and Teyssie JL. 1984. Accumulation and toxicity of Cd, Zn, Ag, and Hg in four marine phytoplankters. Mar. Ecol. Prog. Ser 18: 201–213. doi: 10.3354/meps018201 [DOI] [Google Scholar]
- Fisher NS, Teyssie JL, Krishnaswami S, and Baskaran M. 1987. Accumulation of Th, Pb, U, and Ra in marine phytoplankton and its geochemical significance. Limnol. Oceanogr 32: 131–142. doi: 10.4319/lo.1987.32.1.0131 [DOI] [Google Scholar]
- Fisher NS, and Reinfelder JR. 1995. The trophic transfer of metals in marine systems, p. 363–406. In Tessier A and Turner DR [eds.], Metal speciation and bioavailability in aquatic systems. John Wiley & Sons. [Google Scholar]
- Fitzgerald WF, Lamborg CH, and Hammerschmidt CR. 2007. Marine biogeochemical cycling of mercury. Chem. Rev 107: 641–662. doi: 10.1021/cr050353m [DOI] [PubMed] [Google Scholar]
- Gobler CJ, Buck NJ, Sieracki ME, and Sañudo-Wilhelmy SA. 2006. Nitrogen and silicon limitation of phytoplankton communities across an urban estuary: The East River-Long Island Sound system. Estuar. Coast. Shelf Sci 68: 127–138. doi: 10.1016/j.ecss.2006.02.001 [DOI] [Google Scholar]
- Gorski PR, Armstrong DE, Hurley JP, and Shafer MM. 2006. Speciation of aqueous methylmercury influences uptake by a freshwater alga (Selenastrum capricornutum). Environ. Toxicol. Chem 25: 534–540. doi: 10.1897/04-530R.1 [DOI] [PubMed] [Google Scholar]
- Gosnell KJ, and Mason RP. 2015. Mercury and methylmercury incidence and bioaccumulation in plankton from the central Pacific Ocean. Mar. Chem 177: 772–780. doi: 10.1016/j.marchem.2015.07.005 [DOI] [Google Scholar]
- Grandjean P, Satoh H, Murata K, and Eto K. 2010. Adverse effects of methylmercury: Environmental health research implications. Environ. Health Perspect 118: 1137–1145. doi: 10.1289/ehp.0901757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard RRL 1975. Culture of phytoplankton for feeding marine invertebrates, p. 29–60. In Smith WL and Chanley MH [eds.], Culture of marine invertebrate animals. Plenum Press. [Google Scholar]
- Guillard RRL, and Ryther JH. 1962. Studies of marine planktonic diatoms I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol 8: 229–239. doi: 10.1139/m62-029 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, and Visscher PT. 2004. Biogeochemistry of methylmercury in sediments of Long Island Sound. Mar. Chem 90: 31–52. doi: 10.1016/j.marchem.2004.02.024 [DOI] [Google Scholar]
- Hammerschmidt CR, and Fitzgerald WF. 2006a. Bioaccumulation and trophic transfer of methylmercury in Long Island Sound. Arch. Environ. Contam. Toxicol 51: 416–424. doi: 10.1007/s00244-005-0265-7 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, and Fitzgerald WF. 2006b. Methylmercury cycling in sediments on the continental shelf of southern New England. Geochim. Cosmochim. Acta 70: 918–930. doi: 10.1016/j.gca.2005.10.020 [DOI] [Google Scholar]
- Hammerschmidt CR, and Bowman KL. 2012. Vertical methylmercury distribution in the subtropical North Pacific Ocean. Mar. Chem 132: 77–82. doi: 10.1016/j.marchem.2012.02.005 [DOI] [Google Scholar]
- Hammerschmidt CR, Finiguerra MB, Weller RL, and Fitzgerald WF. 2013. Methylmercury accumulation in plankton on the continental margin of the Northwest Atlantic Ocean. Environ. Sci. Technol 47: 3671–3677. doi: 10.1021/es3048619 [DOI] [PubMed] [Google Scholar]
- Karimi R, Chen CY, Pickhardt PC, Fisher NS, and Folt CL. 2007. Stoichiometric controls of mercury dilution by growth. Proc. Natl. Acad. Sci. U. S. A 104: 7477–7482. doi: 10.1073/pnas.0611261104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi R, Fisher NS, and Folt CL. 2010. Multielement stoichiometry in aquatic invertebrates: When growth dilution matters. Am. Nat 176: 699–709. doi: 10.1086/657046 [DOI] [PubMed] [Google Scholar]
- Karimi R, Fitzgerald TP, and Fisher NS. 2012. A quantitative synthesis of mercury in commercial seafood and implications for exposure in the United States. Environ. Health Perspect 120: 1512–1519. doi: 10.1289/ehp.1205122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester DR, Duedall IW, Connors DN, and Pytkowicz RM. 1967. Preparation of artificial seawater. Limnol. Oceanogr 12: 176–179. doi: 10.4319/lo.1967.12.10176 [DOI] [Google Scholar]
- Kim H, Duong H, Kim E, Lee BG, and Han S. 2014. Effects of phytoplankton cell size and chloride concentration on the bioaccumulation of methylmercury in marine phytoplankton. Environ. Toxicol 29: 936–941. doi: 10.1002/tox.21821 [DOI] [PubMed] [Google Scholar]
- Lamborg CH, Bowman K, Hammerschmidt C, Gilmour C, Munson K, Selin N, and Tseng C-M. 2014. Mercury in the anthropocene ocean. Oceanography 27: 76–87. doi: 10.5670/oceanog.2014.11 [DOI] [Google Scholar]
- Lawson NM, and Mason RP. 1998. Accumulation of mercury in estuarine food chains. Biogeochemistry 40: 235–247. doi: 10.1023/a:1005959211768 [DOI] [Google Scholar]
- Luengen AC, Fisher NS, and Bergamaschi BA. 2012. Dissolved organic matter reduces algal accumulation of methylmercury. Environ. Toxicol. Chem 31: 1712–1719. doi: 10.1002/etc.1885 [DOI] [PubMed] [Google Scholar]
- Mason R, Morel FA, and Hemond H. 1995. The role of microorganisms in elemental mercury formation in natural waters. Water Air Soil Pollut. 80: 775–787. doi: 10.1007/BF01189729 [DOI] [Google Scholar]
- Mason RP, Reinfelder JR, and Morel FMM. 1996. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ. Sci. Technol 30: 1835–1845. doi: 10.1021/es950373d [DOI] [Google Scholar]
- Mason RP, Choi AL, Fitzgerald WF, Hammerschmidt CR, Lamborg CH, Soerensen AL, and Sunderland EM. 2012. Mercury biogeochemical cycling in the ocean and policy implications. Environ. Res 119: 101–117. doi: 10.1016/j.envres.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles CJ, Moye HA, Phlips EJ, and Sargent B. 2001. Partitioning of monomethylmercury between freshwater algae and water. Environ. Sci. Technol 35: 4277–4282. doi: 10.1021/es010792c [DOI] [PubMed] [Google Scholar]
- Morel FMM, Kraepiel AML, and Amyot M. 1998. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst 29: 543–566. doi: 10.1146/annurev.ecolsys.29.1.543 [DOI] [Google Scholar]
- Onyido I, Norris AR, and Buncel E. 2004. Biomolecule-mercury interactions: Modalities of DNA base-mercury binding mechanisms. Remediation strategies. Chem. Rev 104: 5911–5930. doi: 10.1021/cr030443w [DOI] [PubMed] [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, and Blum JD. 2002. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc. Natl. Acad. Sci. U. S. A 99: 4419–4423. doi: 10.1073/pnas.072531099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, and Blum JD. 2005. Impacts of zooplankton composition and algal enrichment on the accumulation of mercury in an experimental freshwater food web. Sci. Total Environ 339: 89–101. doi: 10.1016/j.scitotenv.2004.07.025 [DOI] [PubMed] [Google Scholar]
- Pickhardt PC, and Fisher NS. 2007. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environ. Sci. Technol 41: 125–131. doi: 10.1021/es060966w [DOI] [PubMed] [Google Scholar]
- Reinfelder JR, and Fisher NS. 1991. The assimilation of elements ingested by marine copepods. Science 251: 794–796. doi: 10.1126/science.251.4995.794 [DOI] [PubMed] [Google Scholar]
- Reinfelder JR, and Fisher NS. 1994. The assimilation of elements ingested by marine planktonic bivalve larvae. Limnol. Oceanogr 39: 12–20. doi: 10.4319/lo.1994.39.1.0012 [DOI] [Google Scholar]
- Rouleau C, and Block M. 1997. Fast and high-yield synthesis of radioactive CH3203Hg(II). Appl. Organomet. Chem 11: 751–753. doi: [DOI] [Google Scholar]
- Selin NE 2009. Global biogeochemical cycling of mercury: A review. Annu. Rev. Environ. Resour 34: 43. doi: 10.1146/annurev.environ.051308.084314 [DOI] [Google Scholar]
- Stewart GM, and Fisher NS. 2003a. Bioaccumulation of polonium-210 in marine copepods. Limnol. Oceanogr 48: 2011–2019. doi: 10.4319/lo.2003.48.3.1193 [DOI] [Google Scholar]
- Stewart GM, and Fisher NS. 2003b. Experimental studies on the accumulation of polonium-210 by marine phytoplankton. Limnol. Oceanogr 48: 1193–1201. doi: 10.4319/lo.2003.48.3.1193 [DOI] [Google Scholar]
- Sunderland EM 2007. Mercury exposure from domestic and imported estuarine and marine fish in the US seafood market. Environ. Health Perspect 115: 235–242. doi: 10.1289/ehp.9377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranvik L, Sherr EB, and Sherr BF. 1993. Uptake and utilization of ‘colloidal DOM’ by heterotrophic flagellates in seawater. Mar. Ecol. Prog. Ser 92: 301–309. doi: 10.3354/meps092301 [DOI] [Google Scholar]
- Vallee BL, and Ulmer DD. 1972. Biochemical effects of mercury, cadmium, and lead. Annu. Rev. Biochem 41: 91–128. doi: 10.1146/annurev.bi.41.070172.000515 [DOI] [PubMed] [Google Scholar]
- Wang W-X, and Guo L. 2000. Bioavailability of colloid-bound Cd, Cr, and Zn to marine plankton. Mar. Ecol. Prog. Ser 202: 41–49. doi: 10.3354/meps202041 [DOI] [Google Scholar]
- Wang W-X, and Dei RC. 2001a. Effects of major nutrient additions on metal uptake in phytoplankton. Environ. Pollut 111: 233–240. doi: 10.1016/S0269-7491(00)00071-3 [DOI] [PubMed] [Google Scholar]
- Wang W-X, and Dei RC. 2001b. Metal uptake in a coastal diatom influenced by major nutrients (N, P, and Si). Water Res 35: 315–321. doi: 10.1016/S0043-1354(00)00256-6 [DOI] [PubMed] [Google Scholar]
- Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, and Wente SP. 1998. Bioaccumulation of mercury in pelagic freshwater food webs. Sci. Total Environ 219: 183–208. doi: 10.1016/S0048-9697(98)00228-9 [DOI] [PubMed] [Google Scholar]
- Wiener JG, Krabbenhoft DP, Heinz GH, and Scheuhammer AM. 2003. Ecotoxicology of mercury, p. 409–463. In Hoffman DJ, Rattner BA, Burton GA, and Cairns J [eds.], Handbook of ecotoxicology. CRC Press. [Google Scholar]
- Zhong H, and Wang W-X. 2009. Controls of dissolved organic matter and chloride on mercury uptake by a marine diatom. Environ. Sci. Technol 43: 8998–9003. doi: 10.1021/es901646k [DOI] [PubMed] [Google Scholar]


