Abstract
Introduction
Cryptogenic stroke accounts for approximately 30% of all ischemic strokes. Recently, atrial cardiopathy diagnosed by the presence of one of its serum, imaging, or electrocardiogram biomarkers has been shown to be associated with ischemic stroke, particularly of embolic subtypes.
Areas covered
This paper aims to summarize data on occult atrial fibrillation and stroke, provide an overview on mechanisms, such as inflammation and fibrosis, of stroke in atrial cardiopathy, critically review data on biomarkers of atrial cardiopathy and their association with stroke, and suggest therapeutic implications, including directions for future research.
Expert commentary
Atrial cardiopathy may constitute one of the mechanisms in cryptogenic stroke, and patients with evidence of atrial cardiopathy constitute a group of patients in whom clinical trials are warranted to test anticoagulation versus antiplatelet therapy to reduce stroke recurrence risk. In addition, more studies are needed to determine the degree of overlap between these atrial cardiopathy biomarkers and which one is more useful in predicting the risk of stroke and response to anticoagulation therapy.
Keywords: Atrial Fibrillation, Stroke, Atrial disease, Atrial Cardiopathy, Atrial Dysfunction
1. Cryptogenic stroke
Several mechanisms can lead to ischemic stroke and, among these, cardioembolic stroke subtype carries the highest risk of mortality and poor functional outcome.1 Nearly 30% of ischemic strokes, however, are suspected to be related to a proximal cardio-aortic source and are referred to as stroke of unknown cause, or “cryptogenic” stroke.2 A characteristic imaging finding in cryptogenic stroke is the presence of one or more superficial infarcts on neuroimaging, a finding present in up to 60% of patients with cryptogenic stroke.3 The appearance of these infarcts, affecting the territory of major branches or distal end vessels of the cerebral arterial tree, suggests a distant embolic source. The recognition of this feature was a driving force for investigators to introduce the term Embolic Stroke of Undetermined Source (ESUS) to describe an embolic-appearing non-lacunar infarct in the absence of intracranial or extracranial stenosis of 50% or more, major cardioembolic source such as atrial fibrillation (AF), or other determined stroke mechanism.4
There are several proposed mechanisms for cryptogenic stroke, including occult paroxysmal AF, patent foramen ovale, substenotic atherosclerosis, hypercoagulability, and infectious or inflammatory cerebral vasculitis.5, 6 Since stroke prevention strategies differ among these mechanisms, determining the root cause of ischemic stroke, where possible, is more than just an academic issue.
2. Subclinical AF and ischemic stroke risk
With the increasing use of cardiac pacemakers, there has been an increased frequency of detection of asymptomatic brief runs of paroxysmal occult AF.7 Studies investigating the association between these brief asymptomatic episodes of AF and stroke risk yielded mixed results. Studies have shown an increased risk of stroke with runs of AF less than the six minutes typically considered to establish a diagnosis of AF. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study included 4060 patients with AF randomized to rate vs. rhythm control strategies. In this study, 481 patients (12%) had asymptomatic AF and the risk of stroke at 5 years was similar between patients with symptomatic vs. asymptomatic AF (3.8% vs. 4.4%, p = 0.52).7 In this study, however, nearly 60% of asymptomatic AF episodes were over 30 days in duration and only 4% were of less than 6 hour duration. Therefore, while this study showed an increased risk of stroke in patients with asymptomatic AF, it did not establish an increased stroke risk in patients with brief episodes of AF. The Asymptomatic AF and Stroke Evaluation in Pacemaker Patients and the AF Reduction Atrial Pacing Trial (ASSERT) trial enrolled 2580 patients 65 years or older with hypertension and no history of AF in whom pacemakers were implanted. During the first 3 months, 261 (10.1%) patients developed subclinical atrial tachyarrhythmias, and these were associated with increased ischemic stroke risk (HR 2.49 95% CI 1.28-4.85; mean follow up 2.5 years). Moreover, an analysis of the Stroke prevention Strategies based on Atrial Fibrillation information from implanted devices (SOS AF) project (n = 10,106 patients with cardiac devices and at least 3 months follow up) showed an association between episodes of AF lasting 5 minutes or longer and stroke risk (HR 1.76 95% CI 1.02-3.02).8 This study, like others, showed an association between AF burden and ischemic stroke risk (adjusted hazard ratio = 1.03 per hour of AF duration; 95% CI 1.00–1.05, P = 0.040).8 On the other hand, the Registry of Atrial Tachycardia and Atrial Fibrillation Episodes (RATE) enrolled 5379 patients with pacemakers (mean follow up 22.9 months). Compared to patients with no AF, patients with episodes of AF ≤ 20 seconds duration (n=12.2%) had a similar risk of stroke or TIA (HR 0.87 95% CI 0.58 – 1.31), whereas patients with episodes of AF 20 seconds or longer were more likely to have incident stroke or TIA (HR 1.51 95% CI 1.03-2.21).9 A major limitation of this study is the relatively short duration of follow-up and the small number of patients with only short runs of AF. Therefore, it remains unclear whether brief runs of subclinical AF are associated with a high enough stroke risk to warrant anticoagulation.
While anticoagulation therapy has been associated with reduced stroke risk in patients with AF10, it remains unclear whether anticoagulation therapy reduces the risk of stroke in patients with very brief or infrequent runs of subclinical AF. Ongoing clinical trials will help address this question. The Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA; NCT01938248) trial aims to enroll 4000 patients with device-detected AF of duration between 6 minutes to 24 hours and randomize them to apixaban and aspirin. The primary outcome of this study is ischemic stroke and systemic embolism at 3 years follow up.
3. AF detection after cryptogenic stroke
Prior studies have shown a strong association between AF and ischemic stroke11, particularly of embolic appearance.12 Therefore, cardiac monitoring for AF detection after a cryptogenic stroke has been an area of interest. Retrospective studies showed an AF detection rate of up to 7% on serial electrocardiograms (ECGs) or 24-48 hour Holter monitors13, up to 25% with 28-day cardiac monitors2, 14, and up to 30% at 3 years using implantable monitors.15 This research culminated in two randomized controlled trials showing that outpatient cardiac monitoring after cryptogenic stroke can increase detection of AF beyond standard monitoring approaches.
The 30-Day cardiac event monitor belt for recording paroxysmal atrial fibrillation after a cerebral ischemic event (EMBRACE) trial randomized 572 patients 55 years or older with a cryptogenic stroke or transient ischemic attack (TIA) to non-invasive ambulatory 30 day continuous ECG monitoring vs. 24-hour Holter monitor. This study showed an increased detection rate of AF episodes 30 seconds or longer using the 30 day monitor as opposed to the 24-hour Holter monitor (16.1%, vs. 3.2%, p<0.001).16 The cryptogenic stroke and underlying atrial fibrillation (CRYSTAL-AF) study randomized 441 patients 40 years or older with a cryptogenic stroke or TIA to implantable cardiac monitor (ICM) for 3 years vs. conventional follow-up. This study showed an increased detection of AF lasting 30 seconds or longer in the ICM group as compared to the conventional follow-up group at 6 months (8.9% vs. 1.4%, p<0.001) and 3 years (30.0% vs. 3.0%, p<0.001).17
In these studies, over 95% of patients with AF detected were placed on oral anticoagulation therapy for secondary stroke prevention. Although the stroke rate at 1 year was lower in patients randomized to ICM in CRYSTAL-AF as compared to conventional monitoring (7.1% vs. 9.1%, p > 0.05), CRYSTAL-AF was underpowered to determine a secondary stroke prevention benefit17 and therefore studies are needed to determine whether anticoagulation of patients with brief runs of paroxysmal occult AF detected on outpatient cardiac monitoring after a cryptogenic stroke leads to a reduction in the risk of recurrent stoke.
4. AF and Stroke: association or causality
While AF has been shown to be associated with ischemic stroke, the causal association between the two remains circumstantial.18 Most importantly, while ASSERT and The Relationship Between Daily Atrial Tachyarrhythmia Burden From Implantable Device Diagnostics and Stroke (TRENDS) both show an association between subclinical AF and ischemic stroke, there was no consistent temporal association between AF and stroke. In both studies, only 25% of patients were in AF in the 30 days prior to their embolic event19, 20; this lack of temporality suggests that AF may not be the direct cause of stroke in most patients with this dysrhythmia. Instead, AF may be a marker of an underlying risk of embolic stroke associated with atrial dysfunction.
5. Atrial Cardiopathy: Rationale and Biological Mechanisms
There are many compelling reasons to argue that AF is a marker of atrial disease and that atrial dysfunction or cardiopathy may be the underlying cause of stroke in most patients with this dysrhythmia. The relationship between AF and ischemic stroke does not clearly satisfy the Bradford-Hill criteria for causality.18 Most importantly, the lack of direct evidence of a causal association, including a temporal relationship, between AF and thromboembolic events in most patients with this arrhythmia provides evidence that atrial cardiopathy may underlie most strokes in patients with AF and that AF is itself another biomarker of atrial disease.18 In addition, there is data to suggest that relying on a surface ECG to detect AF may underestimate atrial disease or dysfunction and perhaps the attributed embolic risk. There is data, for example, to suggest an electromechanical dissociation between surface ECG and echocardiographic left atrial function. A study of 41 patients with AF after elective cardioversion showed that nearly one-third of patients had non-sinus contraction of the left atrial appendage (LAA) while in normal sinus rhythm on the surface ECG.21 In fact, another study showed that 6 out of 24 patients with a history of AF and recent stroke undergoing a transesophageal echocardiogram (TEE) had a LAA flow pattern characteristic of AF despite being in sinus rhythm at the time of the TEE. These data suggest the possibility of electromechanical dissociation between the surface ECG and echocardiographic LAA function during episodes of sinus rhythm.22 Furthermore, certain genetic mutations that are associated with AF can be associated with ischemic stroke even before subjects have evidence of AF. For instance, Icelandic investigators have reported an association between two single nucleotide polymorphisms (rs2200733 and rs10033464) associated with increased risk of AF23 and ischemic stroke, especially those thought to be cardioembolic, even in those not detected to have active AF.24
Another congenital predisposition to embolic risk may be related to the morphology of the left atrial appendage. The LAA varies significantly in shape, size, and orientation to adjacent cardiac structures.25 A study of 500 postmortem hearts demonstrated that the LAA most commonly has 2 lobes (54%), followed by 3 lobes (23%), 1 lobe (20%), and 4 lobes (3%).26 In patients with AF, the number of lobes has been shown to be an independent risk factor for the presence of thrombus.27
Traditionally, TEE has been used to characterize LAA morphology beyond the number of lobes, too.28 More recently, cardiac computed tomography (CCT) and cardiac magnetic resonance (CMR) have been used to further describe LAA morphology. A retrospective study of 932 patients with AF who underwent CCT or CMR categorized the LAA into four distinct morphologies: chicken wing (48%), cactus (30%), windsock (19%), and cauliflower (3%) (Figure).29 In this study, the prevalence of ischemic stroke among different LAA morphologies was: 4% in chicken wing, 10% in windsock, 12% in cactus, and 18% in cauliflower. Compared with chicken wing morphology, there was a higher adjusted odds of stroke or TIA with the other morphologies: cactus (odds ratio [OR] 4.1, p = 0.046), windsock (OR 4.5, p = 0.038), and cauliflower (adjusted OR 8.0, p = 0.056). Other studies of patients with AF have also shown an inverse association between chicken-wing morphology and risk of ischemic stroke29, 30 and covert brain infarcts.31 Furthermore, cauliflower LAA morphology was an independent predictor of stroke risk, presumably related to extensive LAA trabeculations.32 LAA flow velocity, another inverse marker of stroke risk, was highest among patients with chicken-wing as opposed to non-chicken wing morphology,28, 33, 34 which may also explain why chicken wing morphology has the lowest risk of ischemic stroke. In addition, larger LAA orifice area has also been shown to be associated with ischemic stroke.30, 35
Figure.
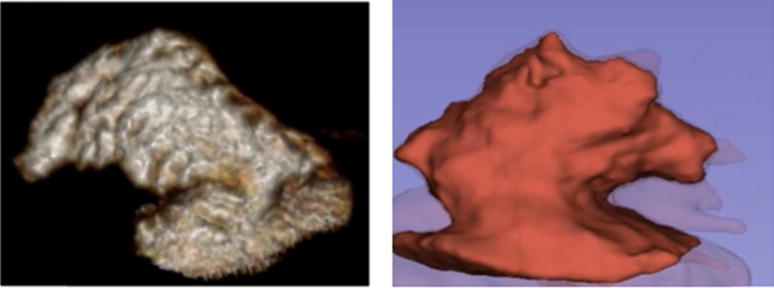
Left side showing a chicken wing left atrial appendage morphology in a 55 year old man with symptomatic carotid stenosis and right side showing the a cauliflower left atrial appendage morphology in a 61 year old man with recurrent cryptogenic strokes.
It is also possible that LAA morphology is a predictor of thrombus formation and thromboembolic risk even in the absence of AF. More studies are needed to determine the risk of thromboembolic events in patients with non-chicken wing compared to chicken wing LAA morphology in patients without AF.
Other acquired disorders of the left atrium may also provide a milieu for thrombus formation. Left atrial enlargement (LAE), for instance, can promote stasis, endothelial dysfunction, and thrombus formation. LAE has been associated with spontaneous echocardiographic contrast and thrombus formation.36 In addition, cardiac fibrosis may provide another mechanism for cardiac embolism. Cardiac fibrosis represents the progressive accumulation of fibrotic tissue within the myocardium, a process that occurs with aging of the heart37–39 and may be particularly more common in patients with systemic inflammatory states (systemic infections and inflammatory disorders) and low-grade subclinical inflammatory states such as coronary artery disease.40 These result in myocardial infiltration by inflammatory cells such as macrophages and activation of the renin-angiotensin pathway which in turn leads to fibrotic changes and electrical and structural remodeling of the myocardium which is a milieu in which AF occurs.41 In fact, studies have shown up-regulation of various inflammatory cytokines such as interleukin-1 and 6 in AF, and these cytokines are linked to progression of AF burden and re-occurrence of AF after cardioversion.18 Therefore, while cardiac fibrosis may occur in the setting of AF-related atrial remodeling, it can occur with other factors resulting in atrial dysfunction such as advanced age37–39, valvular heart disease, hypertension, congestive heart failure, and ischemic coronary heart disease.42 Thus atrial fibrosis may precede AF, or indeed be present even in the absence of development of AF. In fact, recent literature supports the idea that AF likely occurs in the setting of cardiac scar tissue or fibrosis as evident on cardiac magnetic resonance imaging with delayed gadolinium enhancement studies.43, 44
Therefore these data combined suggest that electrical AF does not provide the sole mechanism of embolic events in patients with evidence of atrial dysfunction and that other mechanisms such as genetic factors, atrial enlargement, fibrosis, or inflammation may provide an explanation of embolic stroke even in the absence of AF.
6. Biomarkers of Atrial Cardiopathy
6.1 Echocardiographic biomarkers
a. Left Atrial Enlargement
In population-based studies, LAE has been shown to be associated with incident AF45 and incident ischemic stroke risk after adjusting for several confounders including AF.46, 47 An analysis from the Framingham study showed that LA diameter was associated with stroke risk in men (adjusted HR per 10 mm increase 2.4, 95% CI 1.6-3.7) and women (adjusted HR per 10 mm increase 1.4, 95% CI 0.9-2.1).46 An analysis from the Northern Manhattan Stroke Study (NOMASS) showed an association between left atrial index and ischemic stroke (adjusted OR 1.47 per 10 mm/1.7 m2, 95% CI 1.03-2.11).47 More recently, another analysis of NOMASS (n = 655 patients, median follow up 4 years) showed that moderate to severe LAE is an independent predictor of recurrent stroke risk, particularly related to embolism (adjusted HR 2.83 95% CI 1.03-7.81).48 Furthermore, a recent analysis from the Cardiovascular Health Study showed that LAE is associated with prevalent ischemic infarcts (adjusted RR 1.20 95% CI 1.08-1.34) and more so with non-lacunar infarcts (HR 1.28 95% CI 1.06-1.55) but not with white matter disease grade, independent of several confounders such as AF and congestive heart failure (unpublished data). These studies suggest a mechanistic relationship between LAE and ischemic stroke.
b. Spontaneous echocardiographic contrast
Spontaneous echocardiographic contrast (SEC) is rarely detected on transthoracic echocardiography but not uncommonly detected on transesophageal echocardiography. It is characterized by a dynamic smoke-like appearance on echocardiography in the left atrium or left atrial appendage and is probably a manifestation of red blood cell aggregation and hypercoagulability, particularly in an enlarged left atrium.49 This accounts for at least two of the three factors of Virchow’s triad (stasis and hypercoagulability), providing an explanation for the association between SEC and thrombus formation, embolism, and death in patients with non-valvular AF in prior studies. In patients with AF, several studies have shown the association between SEC and thrombus formation. In a post hoc-analysis of the Stroke Prevention in Atrial Fibrillation (SPAF) trials (n = 382 AF patients undergoing TEE), for example, SEC was present in nearly 63% of patients, was associated with LAA thrombus (14% vs. 3%, p< 0.001), and was the only independent predictor of future embolic events.50
c. Left atrial appendage echocardiographic markers
The left atrial appendage is thought to be the main location of left atrial thrombi in patients with AF. In a TEE study that included 317 patients with AF and a recent embolic event, about 20% had evidence of thrombus, all of which were in the left atrial appendage.51 In fact, recent trials of left atrial appendage closure showed that successful left atrial appendage closure was non-inferior to warfarin in reducing the risk of ischemic stroke, suggesting a functional and structural role of the left atrial appendage, more than the fibrillating left atrium itself, in ischemic stroke risk.52
Reduced left atrial appendage flow velocity on echocardiography can promote stasis and thrombus formation. In a cross-sectional study of patients with AF with and without stroke, patients with stroke had a lower left atrial appendage flow velocity (36 ± 19 vs 55 ± 20 cm/s, p <0.001).35 In a post-hoc analysis from the SPAF-III trial that included 721 patients who underwent TEE, a peak anterograde flow velocity of less than 20 cm/s was independently associated with thrombus formation (relative risk 2.6, p = 0.02) and risk of cardioembolism.53 A more recent cross-sectional study of 360 patients with atrial fibrillation (160 with stroke, 200 without stroke) also showed that lower left atrial appendage flow velocity was associated with ischemic stroke risk.30
While there is evidence that the LAA is the site of the majority of cardiac thrombi in patients with AF, there is limited data to suggest it is also the site of the majority of thrombi in patients with cryptogenic stroke. First, up to 20% of patients with cryptogenic stroke are found to have AF within 30 days of diagnosis.16 Second, a recent study showed an increased prevalence (65%) of biomarkers of atrial dysfunction (defined by severe left atrial enlargement on 2-D echocardiogram, p-wave terminal force in V1 ≥ 5000 μV*ms, or NT-proBNP ≥ 250 pg/mL) in patients with cryptogenic stroke.54 Third, analysis of clot histology showed that clots extracted from patients with cryptogenic stroke were similar to those with cardioembolic stroke subtype suggesting that patients with cryptogenic and cardioembolic subtypes may share the same origin and milieu of thrombus formation.55 Therefore, the LAA may also be an important site of thrombus formation in patients with cryptogenic stroke, and echocardiographic biomarkers of LAA dysfunction may provide a better estimate of the stroke recurrence risk in this patient population.
6.2 Electrocardiogram markers
a. Paroxysmal supraventricular tachycardia
While paroxysmal supraventricular tachycardia (PSVT) has been considered to be a conduction disorder arising mostly in young and healthy individuals56, recent evidence suggests that as with paroxysmal AF, there are two subsets of patients with PSVT: young patients with lone PSVT and older patients with cardiovascular disease.57 Moreover, studies have shown that nearly 12% of patients with PSVT develop incident AF during a 1 year follow up.58 Therefore, while PSVT may in many patients represent a congential atrial dysrhythmia, it may also be a manifestation of atrial cardiopathy and associated increased stroke risk. In fact, an analysis of a large administrative dataset showed an association between PSVT and stroke risk that was independent of several confounding factors, including AF (adjusted HR 2.10, 95% CI 1.69-2.62).59
b. Increased P-wave terminal force in V1
The P-wave terminal force in lead V1 (PTFV1) on ECG measures the electrical conduction through the atria.60 Therefore, disorders causing atrial dysfunction such as hypertrophy, fibrosis, and elevated filling pressures may cause prolongation in the P-wave terminal force.60, 61 While studies have shown an association between PTFV1 and incident AF36, 62, recent data also show an association between PTFV1 and ischemic stroke risk independent of AF. An analysis of the Multi-Ethnic Study of Atherosclerosis (n = 6741, median follow up 8.5 years) showed that PTFV1 was associated with incident stroke (HR per SD 1.21, 95% CI 1.02-1.44).63 Another analysis from the Atherosclerosis Risk in Communities study (n = 14,542 participants, median follow up 22 years) showed an association between PTFV1 and incident ischemic stroke (HR 1.33, 95% CI 1.11-1.59) and incident non-lacunar stroke (HR 1.49, 95% CI 1.07-2.07) but not lacunar stroke (HR 0.89, 95% CI 0.57-1.40).64 An analysis of the Northern Manhattan Study (n = 1039 participants) showed an association between PTFV1 and incident ischemic stroke (adjusted HR 1.20, 95% CI 1.03-1.39) and particularly of embolic subtypes (adjusted HR 1.31, 95% CI 1.08-1.58).65 PTFV1 has also been associated with vascular brain injury. Another analysis of the Cardiovascular Health Study (n = 3129 participants) showed an association between PTFV1 and prevalent infarcts (RR per SD 1.09, 95% CI 1.04-1.16) and more so with prevalent non-lacunar infarcts (RR per SD 1.22, 95% CI 1.08-1.38) on follow-up brain MRI.66 This data suggests a mechanistic relationship between atrial cardiopathy and thromboembolic risk.
c. Others
Bayes syndrome, an arrhythmological syndrome characterized by advanced interatrial block, is another potential biomarker of atrial cardiopathy. Studies have shown that Bayes syndrome is a predictor of AF, ischemic stroke risk particularly of cardioembolic subtype, silent cerebrovascular disease, and vascular cognitive impairment.67, 68
Prolongation of the PR interval on ECG is another possible biomarker of atrial disease. Studies have shown an association between PR interval prolongation and incident AF69 and AF detection after cryptogenic stroke.70 Furthermore, a recent multicenter study showed that a prolonged PR interval (PR ≥ 200 ms) was more prevalent in cryptogenic stroke as opposed to stroke of known non-cardioembolic mechanism.71
6.3 Cardiac magnetic resonance imaging markers
a. Atrial fibrosis and delayed gadolinium enhancement
Cardiac fibrosis can be detected on MRI as a delay in uptake of gadolinium. The relationship between cardiac fibrosis on cardiac MRI and stroke risk, independent of AF, remains unclear, however, and therefore studies investigating this association are needed to determine whether and to what degree is delayed gadolinium enhancement associated with thromboembolic risk in the absence of AF.72
6.4 Serum biomarkers
a. NT-proBNP
N-Terminal pro-Brain Natriuretic Peptide (NTpro-BNP) is a serum biomarker of cardiac disease. It is released by the myocardium in response to stretch and is increased in heart failure, structural heart disease, AF, and other situations of ventricular strain.73 NT-proBNP is an independent predictor of cardiovascular events including incident AF in epidemiological and clinical populations,74–77 and NT-proBNP has been associated with subclinical cerebrovascular disease78 and risk of cardioembolic stroke.79, 80 NT-proBNP is also associated with increased likelihood of detection of paroxysmal AF in patients with ischemic stroke.81, 82
An analysis from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort (n = 1502, mean follow up 5.4 years) showed an association between serum NT pro-BNP (highest vs. lowest quartile) and stroke risk (adjusted HR 2.9, 95% CI 1.9-4.5) and this association was strongest with cardioembolic subtypes (adjusted HR 9.1, 95% CI 2.9-29.2).76 In another study from Sweden (n = 4862 participants, mean follow up 14.9 years), elevated NT-proBNP was associated with ischemic stroke, an association driven by its association with cardioembolic stroke subtype (adjusted HR for 4th vs. 1st quartile 5.64, 95% CI 1.66-19.20).79 Another analysis from the ARIC study cohort (n = 10,902 participants, mean follow up 11.3 years) showed an association between NT-proBNP level (highest vs. lowest quintile) and ischemic stroke (adjusted HR 2.61, 95% CI 1.83-3.74) and non-lacunar infarcts (adjusted HR 2.29, 95% CI 1.42-3.67), but not lacunar infarcts (adjusted HR 1.22, 95% 0.56-2.66) (23471272)
Therefore, as with other biomarkers of atrial cardiopathy, there is an association of NT pro-BNP and ischemic stroke driven by embolic stroke subtypes, arguing for a mechanistic relationship between the two.
b. HS cTnT
Cardiac troponin (cTnT) is another serum biomarker of cardiac disease. Cardiac TnT is used as a measure of cardiac injury and may be elevated in clinical and subclinical myocardial injury and structural heart disease.83 A highly sensitive (hs) cTnT assay can detect concentrations more than 10 times lower than conventional assays used for the detection of acute cardiac ischemia.83 HS cTnT has been shown to be associated with the risk of cardiovascular events in epidemiological and clinical populations.74–77 An analysis of the ARIC study (n = 10,902 participants, mean follow up 11.3 years) showed an association between hs cTnT (highest vs. lowest quintile) and ischemic stroke risk (adjusted HR 2.04, 95% CI 1.42-2.95) and non-lacunar strokes (adjusted HR 2.02, 95% CI 1.22-3.35) but not lacunar strokes (adjusted HR 1.49, 95% CI 0.65-3.39).84
Therefore HS cTnT is another serum biomarker of cardiac injury that is associated with embolic stroke subtypes. A better understanding of stroke mechanisms in patients with elevated hs cTnT may help improve stroke prevention strategies.
6.5 Serum mRNA and clot histology
Serum gene expression signatures may differ between stroke subtypes. Investigators have previously identified a set of 40 genes that help distinguish between cardioembolic and atherosclerotic subtypes.85 In a study analyzing serum mRNA on 76 patients with ischemic stroke, serum mRNA accurately predicted each of cardioembolic and large vessel disease stroke subtypes with very high accuracy (> 90%). In addition, this study found that 41% of patients with cryptogenic stroke had a serum mRNA profile similar to that of cardioembolic stroke and only 17% had a serum profile similar to that of large vessel disease, suggesting that the majority of patients with cryptogenic stroke have a cardiac source.85
In addition, histological clot analysis may help identify stroke subtype. An analysis of clot histology in patients with ischemic stroke undergoing mechanical thrombectomy showed that clots extracted from patients with cryptogenic stroke were similar to those with cardioembolic stroke subtype (high proportions of fibrin/platelets, low erythrocytes, and high leukocytes) and different from those with atherosclerotic subtypes, suggesting that patients with cryptogenic and cardioembolic subtypes may share the same origin and milieu of thrombus formation.55
7. Atrial cardiopathy and cryptogenic stroke
Atrial cardiopathy may be defined in general terms as a functional or structural disorder of the left atrium. Practically speaking, the value of the term atrial cardiopathy rests in its role as a marker of increased risk of thromboembolism, and particularly stroke. While specific criteria for atrial cardiopathy, or thresholds indicative of increased risk, are evolving, it may be provisionally diagnosed by the presence of one of the biomarkers of left atrial dysfunction discussed above, including left atrial size, P wave dispersion on the EKG, PSVT, atrial fibrosis, or elevation in serum biomarkers associated with atrial dysfunction. Some of these biomarkers may not be specific to atrial dysfunction, and the relative importance of different biomarkers has yet to be established. Thrombus formation in atrial cardiopathy is likely related to the interaction between global cardiac and atrial dysfunction described previously, along with a generalized and local pro-thrombotic and inflammatory state.
In an analysis of the Columbia University Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) dataset, nearly 65% of patients with cryptogenic stroke have atrial cardiopathy as evidenced at least one of the biomarkers described above. Allowing a 30% detection of AF, this implies that nearly 35% of patients have evidence of atrial cardiopathy without AF.86 Moreover, another study showed that biomarkers of atrial cardiopathy such as PTFV1 and LAE are weakly associated with AF detection on 30 days monitor after cryptogenic stroke suggesting that previously reported associations between these markers and stroke may not be entirely mediated by concomitant AF.87 This study, however, had several limitations including a small sample size and no outpatient cardiac monitoring was performed.
Since the concept of atrial cardiopathy is fairly novel, cut off values for its biomarkers remain unclear. In addition, the relationship between these biomarkers is uncertain. For instance, the value of each biomarker when compared to the others and degree of overlap needs to be better established. These biomarkers and their association with cerebrovascular disease are summarized in the Table.
Table.
Atrial biomarkers and stroke
| Cardiac biomarker | Association |
| Atrial Fibrillation | Associated with ischemic stroke and embolic infarcts |
| Atrial Cardiopathy Biomarkers | |
| Paroxysmal Supraventricular Tachycardia | Associated with ischemic stroke |
| Increase P-wave Terminal Force in V1 | Associated with ischemic stroke, embolic stroke subtypes, and brain infarcts |
| Left Atrial Enlargement | Associated with ischemic stroke, embolic stroke subtypes, and brain infarcts |
| N-terminal pro b-type natriuretic peptide | Associated with ischemic stroke, cardioembolic stroke subtypes, and brain infarcts |
| High sensitivity cardiac troponin T | Associated with ischemic stroke and cardioembolic stroke subtypes |
| Left atrial appendage biomarkers in atrial fibrillation | |
| Left appendage morphology | Non-chicken wing morphology associated with ischemic stroke |
| Spontaneous echocardiographic contrast | Associated with ischemic stroke risk and embolic events |
| Reduced left atrial appendage flow velocity | Associated with ischemic stroke |
8. Therapeutic implications
Patients with atrial cardiopathy and cryptogenic stroke constitute a group of patients who may benefit from anticoagulation therapy for secondary stroke prevention. Anticoagulation therapy has been shown to reduce the stroke risk in a few cardiac disorders. In patients with AF, anticoagulation with warfarin or a novel oral anticoagulant is associated with at least 50% reduction in risk of stroke or systemic embolic.88–90 In addition, warfarin reduces the risk of ischemic stroke in patients with low ejection fraction when compared to aspirin (HR 0.52, 95% CI 0.33-0.88, p = 0.0055)91 This benefit, however, was outweighed by an increased risk of intracerebral hemorrhage. Warfarin also reduces the risk of ischemic stroke in patients with mechanical heart valves,92 and in those with left ventricular thrombus after anterior myocardial infarction.93
Since most cardiac thrombi in patients with AF arise in the left atrium/left atrial appendage, and since anticoagulation is effective in reducing the risk of cardiac thrombi, it is plausible that patients with atrial cardiopathy and cryptogenic stroke constitute another group of patients who may benefit from anticoagulation therapy. In fact, a post-hoc analysis of the Warfarin Aspirin Recurrent Stroke Study (WARSS) provided evidence that in patients with the most elevated NT-proBNP (> 750 pg/ml) treatment with warfarin was associated with a reduced risk of stroke or death at 2 years when compared to treatment with aspirin (HR 0.30, 95% CI 0.12-0.84; P=0.021).94
This provides compelling evidence for conducting clinical trials testing anticoagulation therapy vs. antiplatelet therapy in patients with atrial cardiopathy and cryptogenic stroke aiming to improve secondary stroke prevention strategies. To this end, a randomized controlled trial will test the hypothesis that the anticoagulant apixaban is superior to aspirin for patients with unexplained stroke who also have one of several biomarkers indicative of left atrial cardiopathy. This trial, the National Institute of Neurological Disorders and Stroke–funded Atrial Cardiopathy and Antithrombotic Drugs in Prevention after Cryptogenic Stroke Trial (ARCADIA) will enroll 1100 patients with cryptogenic stroke at 120 US sites.
9. Conclusion
Atrial cardiopathy may constitute one of the mechanisms in cryptogenic stroke, and patients with evidence of atrial cardiopathy constitute a group of patients in whom clinical trials are warranted to test anticoagulation versus antiplatelet therapy to reduce stroke recurrence risk. In addition, more studies are needed to determine the degree of overlap between these atrial cardiopathy biomarkers and which one is more useful in predicting the risk of stroke and response to anticoagulation therapy.
10. Expert Commentary
Biomarkers of atrial dysfunction or cardiopathy have been shown to be associated with ischemic stroke risk, and particularly those related to embolism. These biomarkers may help provide a better understanding of stroke mechanisms in patients with cryptogenic stroke and therefore may constitute a therapeutic target to improve stroke prevention strategies in this patient population. It is also important to determine if the stroke risk attributed to these biomarkers is independent of co-existing atrial fibrillation.
11. Five-year view
Clinical trials are needed to study the effect of anticoagulation on reducing the risk of stroke in patients with atrial cardiopathy. In addition, more studies are needed to determine the degree of overlap between these atrial cardiopathy biomarkers, and to establish which biomarkers are most useful in predicting the risk of stroke and response to anticoagulation therapy.
12. Key issues
Atrial cardiopathy may be defined in general terms as a functional or structural disorder of the left atrium.
Biomarkers of atrial dysfunction or cardiopathy have been associated with ischemic stroke risk, and particularly those related to embolism
Nearly 65% of patients with cryptogenic stroke have atrial cardiopathy as evidenced by at least one of the biomarkers
Patients with atrial cardiopathy and cryptogenic stroke constitute a group of patients who may benefit from anticoagulation therapy for secondary stroke prevention, and ongoing trials are underway.
Acknowledgments
Funding
This paper was not funded.
Footnotes
Declaration of interest M.S.V. Elkind receives compensation for providing consultative services for Abbott, Biotelemetry/Cardionet, Boehringer-Ingelheim, and Sanofi-Regeneron Partnership; receives study medication in kind but no personal compensation from the BMS-Pfizer Alliance for a clinical trial of atrial cardiopathy; and receives royalties from UpToDate for chapters related to stroke; and serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. M.S.V. Elkind’s institution, Columbia University, receives compensation through a service agreement with Medtronic for M.S.V. Elkind’s effort on clinical trials related to cardiac monitoring. H. Kamel was on a speaker’s bureau for Genentech, regarding alteplase for acute ischemic stroke; is an unpaid consultant for Medtronic and iRhythm, regarding heart-rhythm monitoring after stroke; and is a JAMA Neurology deputy editor. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Arboix A, Alio J. Acute cardioembolic stroke: An update. Expert Rev Cardiovasc Ther. 2011;9:367–379. doi: 10.1586/erc.10.192. [DOI] [PubMed] [Google Scholar]
- 2.Yaghi S, Elkind MS. Cryptogenic stroke: A diagnostic challenge. Neurol Clin Pract. 2014;4:386–393. doi: 10.1212/CPJ.0000000000000086. This paper highlights possible mechanisms in cryptogenic stroke and a simple diagnostic algorithm for these patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: The pfo-asa study. Atrial septal aneurysm. Stroke. 2002;33:706–711. doi: 10.1161/hs0302.104543. [DOI] [PubMed] [Google Scholar]
- 4.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. This paper highlights a new definition for cryptogenic stroke which emphasizes the importance of a full diagnostic evaluation for all stroke patients. [DOI] [PubMed] [Google Scholar]
- 5.Yaghi S, Bernstein RA, Passman R, Okin PM, Furie KL. Cryptogenic stroke: Research and practice. Circ Res. 2017;120:527–540. doi: 10.1161/CIRCRESAHA.116.308447. [DOI] [PubMed] [Google Scholar]
- 6.Arboix A, Marti-Vilalta JL. New concepts in lacunar stroke etiology: The constellation of small-vessel arterial disease. Cerebrovasc Dis. 2004;17(Suppl 1):58–62. doi: 10.1159/000074796. [DOI] [PubMed] [Google Scholar]
- 7.Flaker GC, Belew K, Beckman K, Vidaillet H, Kron J, Safford R, et al. Asymptomatic atrial fibrillation: Demographic features and prognostic information from the atrial fibrillation followup investigation of rhythm management (affirm) study. Am Heart J. 2005;149:657–663. doi: 10.1016/j.ahj.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, et al. Device-detected atrial fibrillation and risk for stroke: An analysis of >10,000 patients from the sos af project (stroke prevention strategies based on atrial fibrillation information from implanted devices) Eur Heart J. 2014;35:508–516. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swiryn S, Orlov MV, Benditt DG, DiMarco JP, Lloyd-Jones DM, Karst E, et al. Clinical implications of brief device-detected atrial tachyarrhythmias in a cardiac rhythm management device population: Results from the registry of atrial tachycardia and atrial fibrillation episodes. Circulation. 2016;134:1130–1140. doi: 10.1161/CIRCULATIONAHA.115.020252. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 11.Wolf PA, Dawber TR, Thomas HE, Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The framingham study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 12.Lodder J, Bamford JM, Sandercock PA, Jones LN, Warlow CP. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke. 1990;21:375–381. doi: 10.1161/01.str.21.3.375. [DOI] [PubMed] [Google Scholar]
- 13.Rizos T, Guntner J, Jenetzky E, Marquardt L, Reichardt C, Becker R, et al. Continuous stroke unit electrocardiographic monitoring versus 24-hour holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694. doi: 10.1161/STROKEAHA.112.654954. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt A, Majid A, Razak A, Kassab M, Hussain S, Safdar A. Predictors of occult paroxysmal atrial fibrillation in cryptogenic strokes detected by long-term noninvasive cardiac monitoring. Stroke research and treatment. 2011;2011:172074. doi: 10.4061/2011/172074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojo-Martinez E, Sandin-Fuentes M, Calleja-Sanz AI, Cortijo-Garcia E, Garcia-Bermejo P, Ruiz-Pinero M, et al. High performance of an implantable holter monitor in the detection of concealed paroxysmal atrial fibrillation in patients with cryptogenic stroke and a suspected embolic mechanism. Revista de neurologia. 2013;57:251–257. [PubMed] [Google Scholar]
- 16.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 17.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 18.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: Time for a new model. Stroke. 2016;47:895–900. doi: 10.1161/STROKEAHA.115.012004. This paper provides an overview on the rationale for the concept of atrial cardiopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 20.Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, et al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: A subgroup analysis of trends. Heart Rhythm. 2011;8:1416–1423. doi: 10.1016/j.hrthm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Bellotti P, Spirito P, Lupi G, Vecchio C. Left atrial appendage function assessed by transesophageal echocardiography before and on the day after elective cardioversion for nonvalvular atrial fibrillation. Am J Cardiol. 1998;81:1199–1202. doi: 10.1016/s0002-9149(98)00089-7. [DOI] [PubMed] [Google Scholar]
- 22.Warraich HJ, Gandhavadi M, Manning WJ. Mechanical discordance of the left atrium and appendage: A novel mechanism of stroke in paroxysmal atrial fibrillation. Stroke. 2014;45:1481–1484. doi: 10.1161/STROKEAHA.114.004800. [DOI] [PubMed] [Google Scholar]
- 23.Henningsen KM, Olesen MS, Haunsoe S, Svendsen JH. Association of rs2200733 at 4q25 with early onset of lone atrial fibrillation in young patients. Scandinavian cardiovascular journal: SCJ. 2011;45:324–326. doi: 10.3109/14017431.2011.594081. [DOI] [PubMed] [Google Scholar]
- 24.Gretarsdottir S, Thorleifsson G, Manolescu A, Styrkarsdottir U, Helgadottir A, Gschwendtner A, et al. Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann Neurol. 2008;64:402–409. doi: 10.1002/ana.21480. [DOI] [PubMed] [Google Scholar]
- 25.Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: Anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7:1251–1265. doi: 10.1016/j.jcmg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Veinot JP, Harrity PJ, Gentile F, Khandheria BK, Bailey KR, Eickholt JT, et al. Anatomy of the normal left atrial appendage: A quantitative study of age-related changes in 500 autopsy hearts: Implications for echocardiographic examination. Circulation. 1997;96:3112–3115. doi: 10.1161/01.cir.96.9.3112. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M, Seo Y, Kawamatsu N, Sato K, Sugano A, Machino-Ohtsuka T, et al. Complex left atrial appendage morphology and left atrial appendage thrombus formation in patients with atrial fibrillation. Circ Cardiovasc Imaging. 2014;7:337–343. doi: 10.1161/CIRCIMAGING.113.001317. [DOI] [PubMed] [Google Scholar]
- 28.Petersen M, Roehrich A, Balzer J, Shin DI, Meyer C, Kelm M, et al. Left atrial appendage morphology is closely associated with specific echocardiographic flow pattern in patients with atrial fibrillation. Europace. 2015;17:539–545. doi: 10.1093/europace/euu347. [DOI] [PubMed] [Google Scholar]
- 29.Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Lee JM, Seo J, Uhm JS, Kim YJ, Lee HJ, Kim JY, et al. Why is left atrial appendage morphology related to strokes? An analysis of the flow velocity and orifice size of the left atrial appendage. J Cardiovasc Electrophysiol. 2015 doi: 10.1111/jce.12710. [DOI] [PubMed] [Google Scholar]
- 31.Anselmino M, Scaglione M, Di Biase L, Gili S, Santangeli P, Corsinovi L, et al. Left atrial appendage morphology and silent cerebral ischemia in patients with atrial fibrillation. Heart Rhythm. 2014;11:2–7. doi: 10.1016/j.hrthm.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Khurram IM, Dewire J, Mager M, Maqbool F, Zimmerman SL, Zipunnikov V, et al. Relationship between left atrial appendage morphology and stroke in patients with atrial fibrillation. Heart Rhythm. 2013;10:1843–1849. doi: 10.1016/j.hrthm.2013.09.065. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima K, Fukushima N, Kato K, Ejima K, Sato H, Fukushima K, et al. Correlation between left atrial appendage morphology and flow velocity in patients with paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2015 doi: 10.1093/ehjci/jev117. [DOI] [PubMed] [Google Scholar]
- 34.Kishima H, Mine T, Ashida K, Sugahara M, Kodani T, Masuyama T. Does left atrial appendage morphology influence left atrial appendage flow velocity? Circ J. 2015 doi: 10.1253/circj.CJ-14-1380. [DOI] [PubMed] [Google Scholar]
- 35.Lee JM, Shim J, Uhm JS, Kim YJ, Lee HJ, Pak HN, et al. Impact of increased orifice size and decreased flow velocity of left atrial appendage on stroke in nonvalvular atrial fibrillation. Am J Cardiol. 2014;113:963–969. doi: 10.1016/j.amjcard.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 36.Eranti A, Aro AL, Kerola T, Anttonen O, Rissanen HA, Tikkanen JT, et al. Prevalence and prognostic significance of abnormal p terminal force in lead v1 of the ecg in the general population. Circulation Arrhythmia and electrophysiology. 2014;7:1116–1121. doi: 10.1161/CIRCEP.114.001557. [DOI] [PubMed] [Google Scholar]
- 37.Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, et al. Myocardial extracellular volume fraction from t1 measurements in healthy volunteers and mice: Relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6:672–683. doi: 10.1016/j.jcmg.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Liu X, Wei J. Ageing related periostin expression increase from cardiac fibroblasts promotes cardiomyocytes senescent. Biochemical and biophysical research communications. 2014;452:497–502. doi: 10.1016/j.bbrc.2014.08.109. [DOI] [PubMed] [Google Scholar]
- 39.Wu J, Xia S, Kalionis B, Wan W, Sun T. The role of oxidative stress and inflammation in cardiovascular aging. BioMed research international. 2014;2014:615312. doi: 10.1155/2014/615312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 41.Jalife J. Mechanisms of persistent atrial fibrillation. Current opinion in cardiology. 2014;29:20–27. doi: 10.1097/HCO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 42.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, et al. Electrical remodeling of the atria in congestive heart failure: Electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 43.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: Mechanisms and clinical implications. J Am Coll Cardiol. 2015;66:943–959. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 44.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, et al. Evaluating the atrial myopathy underlying atrial fibrillation: Identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132:278–291. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wozakowska-Kaplon B. Changes in left atrial size in patients with persistent atrial fibrillation: A prospective echocardiographic study with a 5-year follow-up period. Int J Cardiol. 2005;101:47–52. doi: 10.1016/j.ijcard.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The framingham heart study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 47.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 48.Yaghi S, Moon YP, Mora-McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, et al. Left atrial enlargement and stroke recurrence: The northern manhattan stroke study. Stroke. 2015;46:1488–1493. doi: 10.1161/STROKEAHA.115.008711. This study shows an association between left atrial enlargement and recurrent stroke of embolic subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Black IW. Spontaneous echo contrast: Where there’s smoke there’s fire. Echocardiography (Mount Kisco, NY ) 2000;17:373–382. doi: 10.1111/j.1540-8175.2000.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 50.Leung DY, Black IW, Cranney GB, Hopkins AP, Walsh WF. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1994;24:755–762. doi: 10.1016/0735-1097(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 51.Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: A transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–459. doi: 10.1016/0735-1097(94)00396-8. [DOI] [PubMed] [Google Scholar]
- 52.Holmes DR, Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The prevail trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 53.Goldman ME, Pearce LA, Hart RG, Zabalgoitia M, Asinger RW, Safford R, et al. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (the stroke prevention in atrial fibrillation [spaf-iii] study) J Am Soc Echocardiogr. 1999;12:1080–1087. doi: 10.1016/s0894-7317(99)70105-7. [DOI] [PubMed] [Google Scholar]
- 54.Yaghi S, Boehme AK, Hazan R, Hod EA, Canaan A, Andrews HF, et al. Atrial cardiopathy and cryptogenic stroke: A cross-sectional pilot study. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2015 doi: 10.1016/j.jstrokecerebrovasdis.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boeckh-Behrens T, Kleine JF, Zimmer C, Neff F, Scheipl F, Pelisek J, et al. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke. 2016;47:1864–1871. doi: 10.1161/STROKEAHA.116.013105. [DOI] [PubMed] [Google Scholar]
- 56.Ganz LI, Friedman PL. Supraventricular tachycardia. N Engl J Med. 1995;332:162–173. doi: 10.1056/NEJM199501193320307. [DOI] [PubMed] [Google Scholar]
- 57.Orejarena LA, Vidaillet H, Jr, DeStefano F, Nordstrom DL, Vierkant RA, Smith PN, et al. Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol. 1998;31:150–157. doi: 10.1016/s0735-1097(97)00422-1. [DOI] [PubMed] [Google Scholar]
- 58.Hamer ME, Wilkinson WE, Clair WK, Page RL, McCarthy EA, Pritchett EL. Incidence of symptomatic atrial fibrillation in patients with paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 1995;25:984–988. doi: 10.1016/0735-1097(94)00512-o. [DOI] [PubMed] [Google Scholar]
- 59.Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke; a journal of cerebral circulation. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, et al. Aha/accf/hrs recommendations for the standardization and interpretation of the electrocardiogram: Part v: Electrocardiogram changes associated with cardiac chamber hypertrophy: A scientific statement from the american heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the american college of cardiology foundation; and the heart rhythm society: Endorsed by the international society for computerized electrocardiology. Circulation. 2009;119:e251–261. doi: 10.1161/CIRCULATIONAHA.108.191097. [DOI] [PubMed] [Google Scholar]
- 61.Tiffany Win T, Ambale Venkatesh B, Volpe GJ, Mewton N, Rizzi P, Sharma RK, et al. Associations of electrocardiographic p-wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: The primeri study. Heart Rhythm. 2015;12:155–162. doi: 10.1016/j.hrthm.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ecg predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the atherosclerosis risk in communities (aric) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Jr, Nazarian S, et al. P-wave morphology and the risk of incident ischemic stroke in the multi-ethnic study of atherosclerosis. Stroke. 2014;45:2786–2788. doi: 10.1161/STROKEAHA.114.006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamel H, O’Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the atherosclerosis risk in communities study. Ann Neurol. 2015;78:670–678. doi: 10.1002/ana.24482. This paper proves an association between electrocardiographic left atrial abnormality and embolic stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamel H, Hunter M, Moon YP, Yaghi S, Cheung K, Di Tullio MR, et al. Electrocardiographic left atrial abnormality and risk of stroke: Northern manhattan study. Stroke. 2015;46:3208–3212. doi: 10.1161/STROKEAHA.115.009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamel H, Bartz TM, Longstreth WT, Jr, Okin PM, Thacker EL, Patton KK, et al. Association between left atrial abnormality on ecg and vascular brain injury on mri in the cardiovascular health study. Stroke. 2015;46:711–716. doi: 10.1161/STROKEAHA.114.007762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arboix A, Marti L, Dorison S, Sanchez MJ. Bayes syndrome and acute cardioembolic ischemic stroke. World journal of clinical cases. 2017;5:93–101. doi: 10.12998/wjcc.v5.i3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Selles M, Masso-van Roessel A, Alvarez-Garcia J, Garcia de la Villa B, Cruz-Jentoft AJ, Vidan MT, et al. Interatrial block and atrial arrhythmias in centenarians: Prevalence, associations, and clinical implications. Heart Rhythm. 2016;13:645–651. doi: 10.1016/j.hrthm.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 69.Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy D, et al. Long-term outcomes in individuals with prolonged pr interval or first-degree atrioventricular block. JAMA. 2009;301:2571–2577. doi: 10.1001/jama.2009.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thijs VN, Brachmann J, Morillo CA, Passman RS, Sanna T, Bernstein RA, et al. Predictors for atrial fibrillation detection after cryptogenic stroke: Results from crystal af. Neurology. 2016;86:261–269. doi: 10.1212/WNL.0000000000002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montalvo M, Tadi P, Merkler A, Gialdini G, Martin-Schild S, Navalkele D, et al. Pr interval prolongation and cryptogenic stroke: A multicenter retrospective study. J Stroke Cerebrovasc Dis. 2017 doi: 10.1016/j.jstrokecerebrovasdis.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 72.Yaghi S, Liberman AL, Atalay M, Song C, Furie KL, Kamel H, et al. Cardiac magnetic resonance imaging: A new tool to identify cardioaortic sources in ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2017;88:31–37. doi: 10.1136/jnnp-2016-314023. [DOI] [PubMed] [Google Scholar]
- 73.Rutten JH, Mattace-Raso FU, Steyerberg EW, Lindemans J, Hofman A, Wieberdink RG, et al. Amino-terminal pro-b-type natriuretic peptide improves cardiovascular and cerebrovascular risk prediction in the population: The rotterdam study. Hypertension. 2010;55:785–791. doi: 10.1161/HYPERTENSIONAHA.109.143313. [DOI] [PubMed] [Google Scholar]
- 74.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin t and elevated n-terminal pro-b-type natriuretic peptide predict mortality in older adults: Results from the rancho bernardo study. Journal of the American College of Cardiology. 2008;52:450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, c-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. Jama. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 76.Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, et al. N-terminal pro-b-type natriuretic peptide and stroke risk: The reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45:1646–1650. doi: 10.1161/STROKEAHA.114.004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin t measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dadu RT, Fornage M, Virani SS, Nambi V, Hoogeveen RC, Boerwinkle E, et al. Cardiovascular biomarkers and subclinical brain disease in the atherosclerosis risk in communities study. Stroke; a journal of cerebral circulation. 2013;44:1803–1808. doi: 10.1161/STROKEAHA.113.001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berntsson J, Zia E, Borne Y, Melander O, Hedblad B, Engstrom G. Plasma natriuretic peptides and incidence of subtypes of ischemic stroke. Cerebrovasc Dis. 2014;37:444–450. doi: 10.1159/000363279. [DOI] [PubMed] [Google Scholar]
- 80.Llombart V, Antolin-Fontes A, Bustamante A, Giralt D, Rost NS, Furie K, et al. B-type natriuretic peptides help in cardioembolic stroke diagnosis: Pooled data meta-analysis. Stroke; a journal of cerebral circulation. 2015;46:1187–1195. doi: 10.1161/STROKEAHA.114.008311. [DOI] [PubMed] [Google Scholar]
- 81.Scheitz JF, Erdur H, Haeusler KG, Audebert HJ, Roser M, Laufs U, et al. Insular cortex lesions, cardiac troponin, and detection of previously unknown atrial fibrillation in acute ischemic stroke: Insights from the troponin elevation in acute ischemic stroke study. Stroke; a journal of cerebral circulation. 2015;46:1196–1201. doi: 10.1161/STROKEAHA.115.008681. [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez-Yanez M, Arias-Rivas S, Santamaria-Cadavid M, Sobrino T, Castillo J, Blanco M. High pro-bnp levels predict the occurrence of atrial fibrillation after cryptogenic stroke. Neurology. 2013;81:444–447. doi: 10.1212/WNL.0b013e31829d8773. [DOI] [PubMed] [Google Scholar]
- 83.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin t detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. Jama. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, et al. Troponin t, n-terminal pro-b-type natriuretic peptide, and incidence of stroke: The atherosclerosis risk in communities study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173. This paper proves an association between serum cardiac biomarkers and embolic stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jickling GC, Xu H, Stamova B, Ander BP, Zhan X, Tian Y, et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann Neurol. 2010;68:681–692. doi: 10.1002/ana.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yaghi S, Boehme AK, Hazan R, Hod EA, Canaan A, Andrews HF, et al. Atrial cardiopathy and cryptogenic stroke: A cross-sectional pilot study. J Stroke Cerebrovasc Dis. 2016;25:110–114. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.001. This paper provides data on the prevalance of cardiac biomarkers in patients with cryptogenic stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sebasigari D, Merkler A, Guo Y, Gialdini G, Kummer B, Hemendinger M, et al. Biomarkers of atrial cardiopathy and atrial fibrillation detection on mobile outpatient continuous telemetry after embolic stroke of undetermined source. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2017 doi: 10.1016/j.jstrokecerebrovasdis.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 88.van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: An individual patient meta-analysis. Jama. 2002;288:2441–2448. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 89.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 90.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 91.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–641. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 93.Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: A meta-analysis. J Am Coll Cardiol. 1993;22:1004–1009. doi: 10.1016/0735-1097(93)90409-t. [DOI] [PubMed] [Google Scholar]
- 94.Longstreth WT, Jr, Kronmal RA, Thompson JL, Christenson RH, Levine SR, Gross R, et al. Amino terminal pro-b-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44:714–719. doi: 10.1161/STROKEAHA.112.675942. This study shows reduction in the 2 year recurrent stroke or death risk with warfarin, compared to aspirin, in patients with elevated NT-proBNP, which is one of atrial cardiopathy biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]


