SUMMARY
DCVax® (Northwest Biotherapeutics, Inc., MD, USA) is a platform technology for delivering dendritic cell based therapeutic vaccines for a variety of cancers, including glioblastoma multiforme (GBM). DCVax®-L, one of the implementations of the DCVax platform, provides personalized active immunotherapy composed of autologous dendritic cells pulsed with autologous whole tumor lysate. Clinical trials with DCVax-L for GBM included previous Phase I/II clinical trials and an ongoing Phase III trial. Preliminary reports of patient outcomes after administration of the DCVax-L vaccine provide a promising therapeutic paradigm for patients with both initially diagnosed and recurrent GBM. Here we evaluate the current literature and clinical experience with the DCVax platform, with a particular focus on GBM treatment.
KEYWORDS : DCVax®, DCVax®-L, dendritic cell immunotherapy, dendritic cell vaccines, glioblastoma multiforme, tumor vaccine
Practice points.
DCVax® is a platform providing active immunotherapy via dendritic cell-based therapeutic vaccines for a variety of cancers.
By activating dendritic cells, DCVax helps harness an immune response that mobilizes both the innate and adaptive immune system (including cellular and humoral immune responses).
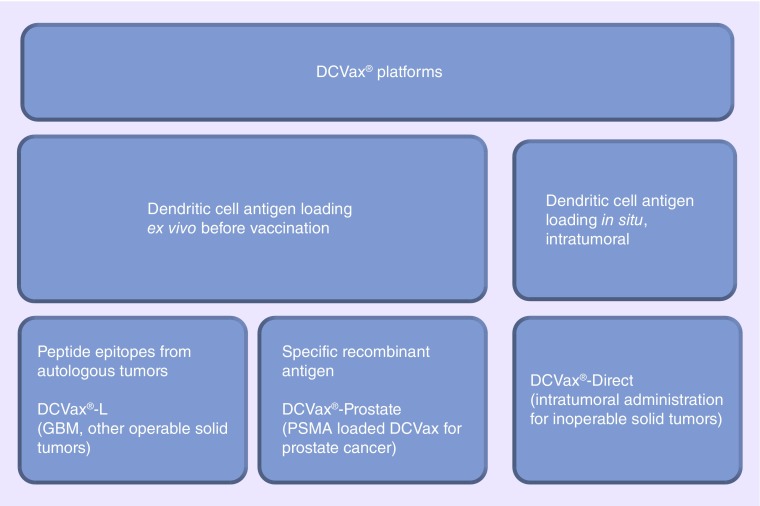
The DCVax platform can be further subdivided based on mechanism of dendritic cell antigen loading and types of antigens used for loading.
The DCVax®-L platform requires dendritic cell activation ex vivo before vaccination compared with DCVax®-Direct in which antigen loading occurs in situ intratumorally in the body. Antigens can be derived from autologous tumor lysates as in DCVax-L for glioblastoma multiforme (GBM) or specific recombinant antigenic epitopes, such as prostate-specific membrane antigen used for loading in DCVax-Prostate.
The DCVax platform has been studied in clinical trials for various cancers including prostate, ovarian and glial tumors and inoperable solid tumors.
DCVax-L for GBM has been studied in several clinical trials. Phase I and II trials completed at University of California, Los Angeles showed promising results in 39 patients with interim data showing significant survival advantage, with time to recurrence of 2 years, and median overall survival of approximately 3 years, compared with 14.6 months. Long-term survivors are also reported.
Currently DCVax for GBM is under study in a Phase III trial for patients with newly diagnosed GBM.
The promising results reported in interim results along with low toxicity and favorable safety profiles makes DCVax a very appealing therapeutic platform for GBM treatment.
Background
Despite recent therapeutic advances, the prognosis for patients with malignant gliomas – in particular glioblastoma multiforme (GBM) – remains poor. GBM is not only the most common of the primary malignant brain tumors but the most frequently diagnosed as well [1]. According to the Central Brain Tumor Registry of the USA, the incidence of gliomas in the USA is 6.02 per 100,000 of which 3.19 per 100,000 are GBMs. GBMs account for 15.6% of all primary brain tumors and 45.2% of primary malignant brain tumors [1]. Unfortunately, median survival is only 16 months and only 25% survive 2 years after initial diagnosis [1].
The current standards of treatment for newly diagnosed GBM include surgical resection with concurrent chemotherapy and radiation. Lacroix and colleagues demonstrated that resections greater than 98% of the preresection tumor volume were associated with a significant survival advantage, compared with only an 8.8-month survival for tumor resections less than 98% [2]. A more recent analysis of a larger cohort of 500 patients also showed that, for patients with newly diagnosed GBMs, more aggressive resections correlate with improved survival, and there was a noted survival benefit for resections even as low as 78% of tumor volume [3]. With concurrent temozolomide chemotherapy in addition to radiotherapy, median survival for GBM has increased from 12.1 to 14.6 months [4].
Due to the heterogeneous and highly infiltrative nature of this disease, prognosis remains dismal. Delivery of chemotherapeutics to the CNS as well as dose-limiting systemic toxicity have been impediments to successful treatment. Several treatment paradigms for novel therapeutics have been described, including local delivery of chemotherapeutics using controlled release platforms for drugs, targeted therapy, convection-enhanced delivery methods for various drugs and treatment platforms such as targeted radioimmunotherapeutic compounds as a means of local radiotherapy [5–11]. However, for the most part, success is incremental and modest at best and often accompanied by toxicity and side effects.
The failure of these experimental chemotherapeutic approaches has led to renewed interest in immunotherapy. Theoretically, immunotherapy offers greater treatment specificity, less toxicity and treatment resistance and immunologic memory to prolong therapeutic response and counteract recurrent growth [12]. Active immune therapies take advantage of therapeutic vaccine platforms to treat those who already harbor a tumor by promoting various antitumor immune responses. Dendritic cell (DC) immunotherapy promotes a wider immune response through activation of DCs, involving arms of both the innate immune system (nonspecific, mediated by natural killer cells, neutrophils, macrophages) and the more specific adaptive immune response, which includes both B-cell-mediated humoral immunity for antibody formation and T-cell-mediated cellular immunity [12–14,27]. Here we review the current DC immunotherapeutic options using the DCVax® platform for solid tumors, with a focus on the clinical experience with DCVax®-L for GBM at recurrence as well as at initial diagnosis.
Indications & usage
DCVax is a therapeutic vaccine technology platform for DC-based immunotherapy, providing active immunization for patients with various malignancies. The DCVax platform can be tailored to specific cancer treatments through either purified tumor-specific antigen or tumor cell extracts derived from patients’ tumors [13,27]. Cancer types that DCVax has been devised to treat include solid tumors such as prostate, non-small-cell lung and renal cancer, and glial tumors such as GBM. DCVax was developed and is being commercialized by Northwest Biotherapeutics, Inc. (MD, USA) and is currently in clinical trials for treatment of multiple malignancies [27].
There are three separate DCVax platforms. Two are produced by purifying autologous DC, differentiating them in vitro, and then pulsing them with either of two types of antigens. The prostate cancer vaccine pulses DC with recombinant prostate-specific membrane antigen (PSMA) to produce the DCVax-Prostate vaccine for treatment of high-stage, hormone-independent prostate cancer, which tends to be more diffuse and metastatic [13,15,27]. For patients with GBM, DCVax is likewise composed of autologous, differentiated DCs which are pulsed with autologous tumor lysate in a proprietary fashion. This approach was also studied in the context of recurrent ovarian cancer [13,16,28]. Two prior Phase I/II clinical trials conducted at University of California, Los Angeles showed encouraging results, and currently there is an ongoing Phase III trial recruiting patients with newly diagnosed GBMs for treatment with DCVax [27,30].
The third DCVax platform under evaluation is DCVax®-Direct. This platform is used in the context of unresectable solid tumors that cannot provide adequate autologous tumor for preparation of DCVax-L. While the DCVax-Direct platform starts with leukopheresis to obtain autologous monocytes which are then differentiated into DCs, these DCs are not fully differentiated in vitro but rather stimulated in vitro with bacillus Calmette-Guerin for a short time to induce DC activation, but unlike the more mature DCs, these activated or partially matured cells do not express CD83, a well-known maturation marker that was shown to provide co-stimulatory signals [17]. These activated DCVax-Direct DCs are injected unpulsed into the tumor where they are thought to take up autologous solid tumor in situ and then mature with production of IL-12 p70 and CD83 with MHC class II molecules and present these antigens to the immune system. Phase I/II clinical trials of DCVax-Direct for inoperable solid tumor cancers (including locally advanced tumors, colon, liver, melanoma, pancreatic, head and neck), are ongoing, but no interim reports are available [29].
Dosage & administration
The dose and administration regimens vary among the three platforms. For example, DCVax-L for GBM is in Phase III clinical trials, composed of three biweekly intradermal injections [13] on days 0, 10 and 20, and at weeks 8, 16, 32, 48, 72, 96 and 120 [30]. The route of administration for optimal performance of DCs has been reviewed in the literature. Advantages of intradermal delivery of DCs for treatment of tumors have been shown by some, with superior performance of DCs when delivered intradermally as opposed to intranodal delivery [18]. Yet other groups demonstrated optimal performance of DCs for tumor treatment with a combination of intradermal and intravenous delivery [19]. DCVax-Direct is currently in a Phase I/II dose-escalation vaccine composed of direct intratumoral injection of partially matured autologous DCs injected into the tumor, either directly or utilizing image guidance [20,27,29].
Clinical pharmacology
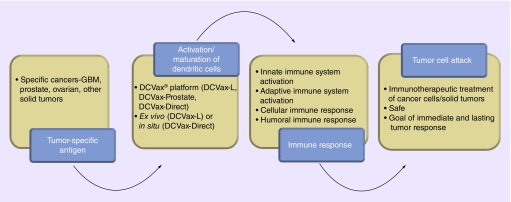
Proprietary manufacturing technology for the DCVax DC-based vaccine platform has not been disclosed by Northwest Biotherapeutics, Inc., thus the exact composition, pharmacology and biology remain undisclosed. However, a review of the general mechanisms of DC biology and cancer immunology provides insight into the probable mechanism. DCs generally reside dormant in the blood and bone marrow in an immature state. Not only does cancer usually fail to prompt DC maturation, but various tumors including GBM appear to have developed mechanisms to ‘escape’ or suppress the innate immune system [21–24]. However, once DC maturation occurs, these cells become powerful APCs that can summon multiple arms of both the innate and adaptive (cellular and humoral) immune system (Figure 1) [13,27].
Figure 1. . Schematic of immunotherapy process using the DCVax® platform.
The first step is the derivation of DCs, which involves separating a patient's autologous mononuclear cells from peripheral blood by leukopheresis and prompting maturation of these immature peripheral blood DCs using various cytokines [13]. Matured DCs are then pulsed with various antigens depending on the malignancy to be treated. DCVax-L for prostate cancer is derived by pulsing these DCs with recombinant PSMA protein to generate an antigen-specific immune response for this antigen. In contrast, for GBM in which there is less certainty regarding tumor-specific epitopes, autologous DCs are activated in vitro and pulsed with autologous whole tumor lysate in vitro prior to vaccine administration. Similar approaches are utilized for other solid tumor types (Figure 2). The manner in which this is achieved differs for different DCVax vaccines depending on tumor biology. For instance, when applying DCVax platform for GBM (DCVax-L for GBM), the autologous DCs are pulsed with proteins from tumor cells from resected GBM [13,14,27,30]. Unlike DCVax for GBM, which uses autologous tumor cell lysates for DC pulsing, for certain malignancies such as prostate cancer the DCVax platform relies on purified recombinant antigens. DCVax-Prostate vaccine takes advantage of the proprietary antigen PSMA found on late-stage, hormone-independent prostate cancer tumors, a 750 amino acid transmembrane glycoprotein expressed specifically on the endothelium of these cells [13,15]. However, it can also be found in the endothelium of other nonprostate cancers [15].
Figure 2. . Categorization of various DCVax platforms.
By contrast, DCVax-Direct is designed for all solid tumor types that are inoperable and for which no core tumor mass is available for resection [27]. Autologous monocytes are isolated and differentiated into DCs, which are then partially matured, comprising the DCVax-Direct vaccine, which is administered directly into the tumor core. They are injected either under direct visualization or under image guidance for deeper, less accessible tumors [27]. Because the DCs are still somewhat immature, they should theoretically readily ‘prime’ themselves as they take up intrinsic tumor epitopes in situ and exhibit them with MHC class II molecules after injection of the vaccine intratumorally [27]. Currently, DCVax-Direct is under study in a Phase I/II clinical trial for inoperable solid tumors (Figure 2) [29]. Preliminary reports presented at the recent annual meeting of the American Society of Clinical Oncology suggested minimal toxicity with possible efficacy [20].
Clinical evidence
The DCVax platform has been configured for treatment in several clinical trials for various cancers, including ovarian, prostate, GBM and inoperable solid tumors. However, much of the data from these trials have not yet been reported in the peer-reviewed literature, and some clinical trials are still recruiting. In December 2009, a Phase I clinical trial of autologous DC vaccination for patients with recurrent epithelial ovarian carcinoma or primary peritoneal carcinoma using DCVax-L technology in addition to bevacizumab and oral metronomic cyclophosphamide was completed [16]. For prostate cancer, DCVax-Prostate showed promising clinical benefit and immunologic response in some patients in interim analyses of Phase I/II trials conducted more than a decade ago [13,15,27]. According to Northwest Biotherapeutics, DCVax-Prostate was previously cleared by the US FDA for a Phase III trial but awaits partnering for trial commencement [27].
Currently, there are two ongoing clinical trials with DCVax – one using DCVax-L for the treatment of GBM patients with newly diagnosed tumors in a Phase III trial actively recruiting and one using the DCVax-Direct platform. The DCVax-Direct platform is currently under study in a Phase I/II clinical trial recruiting patients with tumors, including melanoma, pancreatic, colorectal, liver, locally advanced and metastatic solid tissue tumors deemed inoperable, for intratumoral administration of autologous activated DCs using the DCVax-Direct vaccine technology [29]. End points include safety and efficacy as determined by tumor response; interim data are not available [29].
For treatment of GBM, DCVax-L has been studied in completed Phase I/II studies conducted primarily at UCLA with the goal to determine safety and efficacy. From Northwest Biotherapeutics’ public reports, the Phase I/II studies have been conducted and completed at UCLA, with a Phase III study currently open [27,30]. The company reports interim data on its website, with 39 patients recruited for the Phase I/II studies of which 20 had newly diagnosed GBMs and 19 had recurrent GBM and other gliomas [27]. For those with newly diagnosed GBM who received DCVax in addition to standard of care, the median time to tumor recurrence was approximately 2 years, with median survival of 3 years [27]. Reportedly, there are substantial long-term survivors in the DCVax group, with 33% of patients exceeding 4 years and 27% reaching or exceeding 6 years; two patients have exceeded the 10-year survival mark, according to the company [27]. These results are promising and are consistent with other reported DC vaccination trial results for patients with GBMs [14]. Currently, the company is conducting a Phase III clinical trial with DCVax for newly diagnosed GBMs, with the goal to enroll 312 patients from sites across the USA, with end points of progression-free and overall survival [27,30]. Interim results are not available [27,30].
Adverse reactions
In general, there have been few significant reported side effects from DC vaccines created from tumor lysates [14]. Side effects after such vaccinations have included mild fevers, rashes and irritation at the injection sites [14]. Trial results for DCVax are largely unpublished in the peer-reviewed literature, but the sponsoring company reports that DCVax-L is nontoxic and that, to date, in clinical trials that have included more than 1000 treatments, no significant serious adverse events have been noted [27], similar to other reported GBM vaccine trials in which vaccines have had very limited toxicity [14,27], although autoimmune encephalitis has been reported in primate models [25,26].
Drug interactions
DCVax is currently still under investigation in clinical trials. No significant drug interactions have been reported.
Use in specific populations
DCVax-L for prostate and DCVax direct for solid tumors can be administered to any patient with prostate or other solid tumor who meets eligibility criterion, respectively.
Conclusion
DCVax is a platform technology for delivery of active dendritic-cell-based immunotherapy vaccines for various cancers including GBM. The DCVax-L platform requires DC loading ex vivo before vaccination, while the DCVax-Direct platform allows for in situ antigen loading of DCs intratumoraly. Phase I and II trials with DCVax for GBM show promising results, with interim data reporting of significant survival advantage, time to recurrence of 2 years, and overall survival of approximately 3 years. Currently, DCVax for GBM is in a Phase III study for patients with newly diagnosed GBM. Both the promising interim results and low toxicity profile make DCVax a favorable therapeutic for GBM.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncol. 2013;15:1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides epidemiologic data for primary brain and CNS tumors.
- 2.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]; • It establishes the importance of extent of resection in the treatment of glioblastoma multiforme.
- 3.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Eng. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]; • Establishes the efficacy of concurrent chemotherapy (temozolomide) and radiation for glioblastoma multiforme treatment as standard of care.
- 5.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat. Rev. Dr. Disc. 2004;3:499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 6.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules into the brain. Proc. Natl Acad. Sci. USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]; • It describes the process of convection enhanced delivery of therapeutics for treatment of CNS conditions.
- 7.Hdeib A, Sloan A. Targeted radioimmunotherapy: the role of 131I-chTNT-1/B mAb (Cotara) for treatment of high grade gliomas. Future Oncol. 2012;8(6):659–669. doi: 10.2217/fon.12.58. [DOI] [PubMed] [Google Scholar]
- 8.Hdeib A, Sloan AE. Convection-enhanced delivery of 131I-chTNT-1/B mAB for treatment of high-grade adult gliomas. Exp. Opin. Biol. Ther. 2011;11(6):799–806. doi: 10.1517/14712598.2011.579097. [DOI] [PubMed] [Google Scholar]
- 9.Mehta A, Chol BD, Ajay D, et al. Convection enhanced delivery of macromolecules for brain tumors. Curr. Dr. Disc. Technol. 2012;9(4):305–310. doi: 10.2174/157016312803305951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow up of a multicenter controlled trial. Acta. Neurochir. (Wien.) 2006;148(3):269–275. doi: 10.1007/s00701-005-0707-z. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Deng H, Peng H, Wang Q. Functional nanoparticles in targeting glioma diagnosis and therapies. J. Nanosci. Nanotech. 2014;14(1):415–432. doi: 10.1166/jnn.2014.8757. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler CJ, Black KL. Vaccines for glioblastoma and high-grade glioma. Exp. Rev. Vacc. 2011;10(6):875–886. doi: 10.1586/erv.11.71. [DOI] [PubMed] [Google Scholar]
- 13.Knutson KL. Technology evaluation: DCVax, Northwest Biotherapeutics. Curr. Opin. Mol. Therap. 2002;4(4):403–407. [PubMed] [Google Scholar]
- 14.Wheeler CJ, Black KL. DCVax®-Brain and DC vaccines in the treatment of GBM. Exp. Opin. Invest. Dr. 2009;18(4):509–559. doi: 10.1517/13543780902841951. [DOI] [PubMed] [Google Scholar]
- 15.Fishman M. A changing world for DCVax: a PSMA loaded autologous dendritic cell vaccine for prostate cancer. Exp. Opin. Biol. Ther. 2009;9(12):1565–1575. doi: 10.1517/14712590903446921. [DOI] [PubMed] [Google Scholar]
- 16.Kandalaft LE, Powell DJ, Jr, Chiang CL, et al. Autologus lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmnology. 2013;2(1):e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aerts-Toegaert C, Heirman C, Tuyaerts S, et al. CD83 expression on dendritic cells and T cells: correlation with effective immune responses. Eur. J. Immunol. 2007;37(3):686–695. doi: 10.1002/eji.200636535. [DOI] [PubMed] [Google Scholar]
- 18.Lesterhuis WJ, de Vries IJ, Schreibelt G, et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin. Cancer Res. 2011;17(17):5725–5735. doi: 10.1158/1078-0432.CCR-11-1261. [DOI] [PubMed] [Google Scholar]
- 19.Wilgenhof S, Van Nuffel AM, Benteyn D, et al. A Phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann. Oncol. 24(10):2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 20.Subbiah C, Murthy R, Kayaleh O, Bosch ML. Local and systemic antitumor effects of activated autologous dendritic cells for intratumoral injection: a Phase I/II trial. Abstract presented at the American Society of Clinical Oncology (ASCO), Chicago, IL, 30 May–3 June 2014. J. Clin. Oncol. 2014;32(5) [Google Scholar]
- 21.Avril T, Vauleon E, Tanguy-Royer S, Mosser J, Quillien V. Mechanisms of immunomodulation in human glioblastoma. Immunother. 2011;3(4 Suppl. 1):42–44. doi: 10.2217/imt.11.39. [DOI] [PubMed] [Google Scholar]
- 22.Weber J. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with ipilimumab (MDX-010) Oncologist. 2008;13(Suppl. 4):16–25. doi: 10.1634/theoncologist.13-S4-16. [DOI] [PubMed] [Google Scholar]
- 23.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12(7):864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 24.Topalian SL, Hodi S, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Eng. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bigner DD, Pitts CM, Wikstrand CJ. Induction of lethal experimental allergic encephalomyelitis in nonhuman primates and guinea pigs with human glioblastoma tissue. J. Neurosurg. 1981;55(1):32–42. doi: 10.3171/jns.1981.55.1.0032. [DOI] [PubMed] [Google Scholar]
- 26.Ajay D, Sanchez-Perez L, Choi BD, De Leon G, Sampson JH. Immunotherapy with tumor vaccines for the treatment of malignant gliomas. Curr. Dr. Disc. Technol. 2012;9(4):237–255. doi: 10.2174/157016312803305933. [DOI] [PubMed] [Google Scholar]
- 27.Northwest Biotherapeutics: DCVax® . http://nwbio.com; • Northwest Biotherapeutics website, the company that produces and commercializes the DCVax® platform technology, providing information regarding DCVax and clinical trials information.
- 28.ClinicalTrials.gov. A Phase I clinical trial of autologous dendritic cell vaccine for recurrent ovarian or primary peritoneal cancer. http://clinicaltrials.gov/ct2/show/NCT00683241?term=DCVax&rank=6
- 29.ClinicalTrials.gov. Safety and efficacy study of DCVax-Direct in solid tumors. http://clinicaltrials.gov/ct2/show/NCT01882946?term=DCVax&rank=4
- 30.ClinicalTrials.gov. Safety of a drug [DCVax®-L] to treat newly diagnosed GBM brain cancer. http://www.clinicaltrials.gov/ct2/show/study/NCT00045968?term=dcvax&rank=1&show_locs=Y#locn