SUMMARY
Objective:
To test the reliability, clinical and psychometric validity of the Brain Symptom and Impact Questionnaire (BASIQ) in patients with brain metastases.
Methods:
Brain metastases patients were interviewed using the BASIQ, Functional Assessment of Cancer-Brain (FACT-Br) and FACT-General (FACT-G) at baseline, with a follow-up assessment at 1 month.
Results:
Forty patients had complete one data and the median age was 64 years. Patients with higher KPS, ECOG of 2, primary breast cancer, or >3 brain metastases, scored higher on the symptom scale of the BASIQ. All subscales showed no significant change in patient symptoms from baseline to follow-up.
Conclusion:
This study supports that the reliability, clinical and psychometric validity of BASIQ to be used in brain metastases patients.
KEYWORDS : BASIQ, brain metastases, field-testing, psychometric validation, quality of life, questionnaire
Brain metastases are a significant cause of morbidity in advanced cancer patients and are a leading cause of cancer mortality [1]. Approximately 20–40% of cancer patients develop brain metastases during the course of their illness, occurring most commonly in patients with primary lung, skin, breast, kidney, or colon cancers [2,3]. With evolving screening techniques and imaging modalities, the detection and incidence of brain metastases has been on the rise [4]. Patients with newly diagnosed brain metastases often present with some degree of neurocognitive dysfunction [5]. Neurological symptoms can include behavioral changes, headaches, weakness, lack of muscle coordination, seizures and difficulty with speech [3,6].
With various complications arising from brain metastases, treatment can be a critical component in the management of brain metastases in regards to improving quality of life (QoL) and overall survival. The management of brain metastases can be categorized into therapeutic and symptomatic strategies. Symptomatic strategies include the use of anticonvulsants to prevent seizures and corticosteroids to reduce edema [4]. Therapeutic strategies include neurosurgery, stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT) and chemotherapy [4,7]. However, the treatment modality selected is often dependent on various patient factors such as age, functional status and extent of extracranial disease [4].
Employing symptomatic and/or therapeutic strategies can help maintain QoL in brain metastases patients. QoL is considered a subjective and multidimensional construct incorporating both psychosocial and physical factors [8]. Two commonly used patient reported outcome (PRO) QoL instruments measuring QoL for this particular patient population include the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Brain Neoplasm (EORTC QLQ-BN20) and the Functional Assessment of Cancer-Brain (FACT-Br). The EORTC QLQ-BN20 (often used concurrently with the EORTC QLQ-C30) and the 50-item FACT-Br (used concurrently with the FACT-General (FACT-G)) were both originally validated and intended for patients with primary brain tumors rather than patients with brain metastases [9,10]. Although patients with brain metastases often exhibit similar symptoms to patients with primary brain tumors, the prognoses and treatment regimens can be quite different. Patients with brain metastases may have different prognoses depending on the primary cancer site, extent and number of brain metastases and other sites of metastases. Furthermore, patients with primary brain tumors can often undergo surgical resection, providing quick relief of neurological symptoms [11]. Consequently, various aspects of QoL assessed on these QoL instruments could potentially be quite different in these two patient groups. These previously validated QoL instruments are also fairly lengthy questionnaires and can be burdensome for advanced cancer patients, especially considering patients with a poor performance status [12].
The Brain Metastases Symptom and Impact Questionnaire (BASIQ) version 1.0 is a patient-reported outcome (PRO) instrument measuring symptom severity and impact on daily functional activities developed with input from clinical experts and from qualitative and cognitive interviews with brain metastases patients. The 18 items of the BASIQ encompasses both symptom and impact scales relevant to patients with brain metastases. Both these scales together cover 12 domains: headaches, dizziness, nausea, numbness, energy, balance, vision, memory, cognition, vision, physical activities and self-care. With the 18-item BASIQ being considerably shorter than the 50-item combined EORTC QLQ-BN20 and QLQ-C30, and the 50-item combined FACT-G and FACT-Br, the BASIQ will ideally reduce patient burden while still maintaining coverage of the key symptoms and impact on QoL. Our previous study established content validity of the item content of BASIQ version 1.0 in patients with brain metastases and their health care professionals [13].
The purpose of this study was to test the reliability, clinical and psychometric validity of the BASIQ in patients with brain metastases through field-testing. Secondary objectives included examining the correlation between an existing QoL instrument for this patient population (FACT-Br and FACT-G) with the BASIQ, as well as examining the change in the BASIQ at 1 month after treatment.
Methods
• Patient sample
Eligible patients were over the age of 18 years with a histologically proven primary cancer, had single or multiple brain metastases and provided written informed consent. Furthermore, eligible patients were undergoing neurological resection (Group A), SRS with or without WBRT (Group B), or WBRT alone (Group C). These groups were selected since these are common treatment regimens carried out in patients with brain metastases. Patients referred for treatment of brain metastases at the Odette Cancer Centre at Sunnybrook Hospital in Toronto, Ontario, Canada were approached at the time of their clinic appointment. Patients were informed about the premise of the study and completed the consent form and questionnaire with a trained research assistant if agreeable. Patient demographic information was collected at baseline including age, gender, primary cancer site, number of brain metastases, other sites of metastases, Karnofsky Performance Score (KPS), Eastern Cooperative Oncology Group performance status (ECOG) and other variables as listed in Table 1.
Table 1. . Patient characteristics (n = 40).
| n | 40 | |
| Mean ± SD | 61.8 ± 10.5 | |
| Median (range) | 64 (33–86) | |
| KPS | ||
| n | 36 | |
| Mean ± SD | 75.8 ± 13.4 | |
| Median (range) | 80 (40–90) | |
| KPS distribution | ||
| 40 | 1 | (2.78%) |
| 50 | 3 | (8.33%) |
| 60 | 2 | (5.56%) |
| 70 | 8 | (22.22%) |
| 80 | 12 | (33.33%) |
| 90 | 10 | (27.78%) |
| Years from primary cancer to brain cancer | ||
| n | 37 | |
| Mean ± SD | 3.7 ± 5.8 | |
| Median (range) | 2 (0–28) | |
| Patient group | ||
| A – Neurosurgery | 2 | (5.00%) |
| B – Radiosurgery w/wo WBRT | 24 | (60.00%) |
| C – WBRT alone | 14 | (35.00%) |
| ECOG | ||
| n | 36 | |
| Mean | 1 | |
| Median (range) | 1 (0–3) | |
| 0 | 10 | (27.78%) |
| 1 | 20 | (55.56%) |
| 2 | 5 | (13.89%) |
| 3 | 1 | (2.78%) |
| 4 | 0 | (0%) |
| Gender | ||
| Female | 31 | (77.50%) |
| Male | 9 | (22.50%) |
| Primary cancer site | ||
| Lung | 23 | (57.50%) |
| Breast | 9 | (22.50%) |
| Kidney | 2 | (5.00%) |
| Colon | 1 | (2.50%) |
| Melanoma | 1 | (2.50%) |
| Other | 3 | (7.50%) |
| Unknown | 1 | (2.50%) |
| Accrual | ||
| Radiotherapy clinic | 40 | (100.00%) |
| Out/in-patients | ||
| Outpatient | 40 | (100.00%) |
| Inpatient | 0 | (0%) |
| Education level | ||
| Elementary school | 1 | (2.63%) |
| High school | 19 | (50.00%) |
| College | 3 | (7.89%) |
| University | 12 | (31.58%) |
| Masters | 2 | (5.26%) |
| PhD | 1 | (2.63%) |
| Employment status | ||
| Retired | 20 | (50.00%) |
| Employed | 13 | (32.50%) |
| Unemployed | 7 | (17.50%) |
| Number of brain metastases | ||
| 1 | 20 | (51.28%) |
| 2–3 | 7 | (17.94%) |
| > 3 | 12 | (30.77%) |
| Other site of metastasis | ||
| Bone | 6 | (16.22%) |
| Lung | 4 | (10.81%) |
| Liver | 4 | (10.81%) |
| Lymph | 5 | (13.51%) |
| Other – adrenal | 1 | (2.70%) |
| None | 24 | (60.00%) |
| Previous chemotherapy | ||
| No | 11 | (28.95%) |
| Yes | 27 | (71.05%) |
| Previous hormone therapy | ||
| No | 33 | (86.84%) |
| Yes | 5 | (13.16%) |
| Previous other therapies | ||
| No | 24 | (60.00%) |
| Yes | 16 | (40.00%) |
| Baseline dexamethasone dose | ||
| 16 mg | 1 | (3.00%) |
| 12 mg | 3 | (8.00%) |
| 8 mg | 5 | (13.00%) |
| 4 mg | 3 | (8.00%) |
| 2 mg | 1 | (3.00%) |
| None | 26 | (67.00%) |
• Patient interviews
Psychometric validation of the BASIQ was conducted to test the suitability of the instrument before its general application in assessing QoL in patients with brain metastases, which included the evaluation of clinical and psychometric validity, and reliability of the instrument with different statistical methods.
Patients were interviewed using the BASIQ version 1.0 within 1 week prior to their given treatment. Patients rated each of the 18 items on a scale of 0 (“not at all”) to 10 (“extremely”) based on the past 24 h. In addition to the BASIQ, patients were also interviewed using the FACT-G and FACT-Br based on the past 24 h, as well as for the past 7 days. Each of the 50 items was rated on a scale of 0 (“not at all”) to 4 (“very much”). Interviews were conducted in person by a trained research assistant by explaining the study to the patient and asking the patient to fill out the questionnaire on their own. Patients who requested assistance were asked the questions verbally and the scores were recorded by the trained research assistant. All patients also completed a follow-up assessment approximately 1 month (4–6 weeks) following the initiation of the brain metastases specific treatment. The follow-up assessment was conducted over the telephone or in person (if the patient was booked to return to the clinic) using the BASIQ, FACT-G and FACT-Br. Patients receiving SRS with a good performance status also completed an additional follow-up assessment approximately 1 week after the initial baseline interview to assess the test–retest reproducibility of the BASIQ.
• Statistical methods
Data were analyzed using Statistical Analysis Software (SAS version 9.3 for Windows). Descriptive statistics were used to express demographic data; mean, standard deviation (SD), median and range for continuous variables and proportions for categorical variables. BASIQ scores were analyzed on an 18-item basis, as well as by symptom and impact scales. The symptom scale was calculated based on the sum of items 1–10 on the BASIQ, while the impact scale was calculated based on the sum of items 11–18. Similarly, the FACT-G and FACT-Br items were aggregated into the following subscales: physical well-being, social/family well-being, emotional well-being, functional well-being, FACT-G total score and the FACT-Br total score. The higher the total score on each subscale indicated a higher intensity of symptoms within the subscale for patients with brain metastases. Mean BASIQ scores were expressed individually, as well as by demographic covariates. Mean FACT-G and FACT-Br scores were expressed by subscale using data for the past 24 h as well as the past 7 days. Spearman correlation was calculated between each BASIQ item and demographics at baseline, between the physical well-being subscale of the FACT-G at 24 h and the BASIQ changing scores from baseline to 1 month.
Correlations of each BASIQ item score with the remaining 17 item scores were calculated at baseline and 1 month using the Pearson correlation matrix. Test–retest reliability was assessed using intraclass correlation coefficients (ICC) where a value of ≥0.75 indicates excellent agreement, 0.6–0.74 good agreement, 0.4–0.59 fair to moderate agreement and <0.4 poor agreement [14]. Convergent and divergent validity was examined by evaluating Pearson's product moment correlations between the FACT-G, FACT-Br and BASIQ scales, where r ≥ 0.40 indicates that scales are conceptually related and substantially correlated with one another [15]. The Wilcoxon signed rank test was used to assess statistical significance of differences in scores between baseline and 1-month follow-up for the BASIQ, FACT-G and FACT-Br subscales.
Results
A total of 62 patients were initially enrolled at baseline. Forty patients completed the 1-month follow-up assessment and were included in the final analyses. Of the 22 patients without the 1-month follow-up assessment, 10 patients were unable to be reached during follow-up, 6 patients deceased within the study period and 6 patients withdrew from the study. Of the 40 patients with completed 1-month follow-up data, 10 patients also participated in the 1-week follow-up assessment after the initial interview.
• Patient demographics
Patient characteristics are displayed in Table 1. The median age of patients was 64 years old. Seventy-eight percent of patients were female. Five percent of patients were categorized as Group A, 60% were Group B and 35% were Group C. The median KPS was 80 (range: 40–90) and the median ECOG was 1 (range: 0–3). The most common primary cancer sites include the lung (58%), breast (23%) and kidney (5%). All patients were accrued in an out-patient radiotherapy clinic. Fifty-one percent of patients had only one brain metastasis. Sixty percent of patients had no other sites of metastases, while the remainder had metastases to the bone (16%), lymph (14%), lung (11%), liver (11%) and/or adrenal gland (3%). In regards to prior systemic treatment, 71% of patients had prior chemotherapy, 13% had prior hormone therapy and 40% had other prior therapies such as previous radiation therapy. Sixty-seven percent of patients were not receiving dexamethasone 24 h prior to the baseline assessment and 47% of patients had a level of education higher than high school.
• BASIQ scores
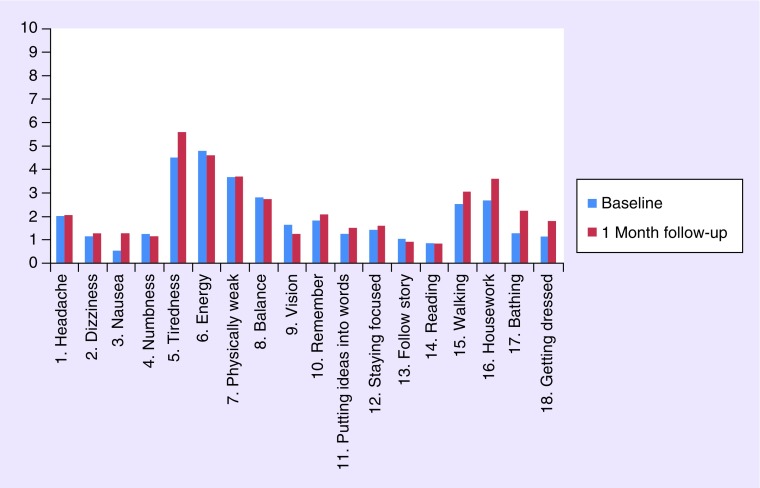
Figure 1 displays the mean BASIQ scores for patients at baseline and 1-month follow-up. Symptom and impact scales were also calculated for baseline and 1-month follow-up. Both the mean symptom scale (23.5) and mean impact scale (11.8) increased after the 1-month follow-up assessment (25.4 and 15.3, respectively). Tables 3 & 4 display the correlations of each BASIQ item score with the remaining 17-item scores at baseline and 1 month. The average correlation amongst the BASIQ subscales at baseline was 0.19 for the symptom scale and 0.56 for the impact scale (Table 3). The average correlation at 1 month was 0.23 for the symptom scale and 0.42 for the impact scale (Table 4). Overall, items on the impact scale showed significant correlations with the BASIQ items (r ≥ 40), whereas the symptom scale items did not. Mean item scores were also calculated by demographic covariates at baseline (Table 2). Patients with a higher KPS, ECOG of 2, primary breast cancer, or more than three brain metastases, generally scored higher on the symptom scale. Patients with an ECOG of 2, primary kidney cancer, or one brain metastasis, generally scored higher on the impact scale. Patients with masters or PhD level of education had lower symptom and impact scores compared with other levels of education.
Figure 1. . Mean BASIQ item scores at baseline and 1-month follow-up assessment (n = 40 patients).
Table 3. . Pearson correlation matrix for each BASIQ item score with the remaining 17 item scores at baseline.
| At baseline | BAS2 | BAS3 | BAS4 | BAS5 | BAS6 | BAS7 | BAS8 | BAS9 | BAS10 | BAS11 | BAS12 | BAS13 | BAS14 | BAS15 | BAS16 | BAS17 | BAS18 | Average r | Min. r | Max. r |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAS1 | 0.32 | -0.21 | 0.41 | 0.33 | -0.07 | 0.22 | 0.27 | 0.07 | 0.37 | 0.17 | 0.05 | -0.05 | -0.03 | 0.35 | 0.10 | 0.40 | 0.43 | 0.18 | -0.21 | 0.43 |
| BAS2 | . | 0.03 | 0.00 | 0.44 | -0.30 | 0.37 | 0.67 | 0.51 | 0.22 | 0.37 | 0.30 | 0.30 | 0.49 | 0.61 | 0.58 | 0.60 | 0.60 | 0.36 | -0.30 | 0.67 |
| BAS3 | . | . | -0.00 | 0.15 | -0.15 | 0.23 | 0.14 | -0.03 | 0.18 | 0.30 | 0.03 | 0.25 | 0.04 | -0.08 | 0.03 | 0.08 | 0.26 | 0.09 | -0.15 | 0.30 |
| BAS4 | . | . | . | 0.38 | -0.08 | 0.42 | 0.26 | 0.26 | 0.38 | 0.49 | 0.43 | 0.41 | 0.39 | 0.27 | 0.29 | 0.35 | 0.42 | 0.29 | -0.08 | 0.49 |
| BAS5 | . | . | . | . | -0.28 | 0.60 | 0.55 | 0.27 | 0.30 | 0.30 | 0.46 | 0.36 | 0.22 | 0.55 | 0.45 | 0.64 | 0.56 | 0.37 | -0.28 | 0.64 |
| BAS6 | . | . | . | . | . | -0.44 | -0.33 | 0.04 | -0.09 | -0.28 | -0.25 | -0.19 | -0.07 | -0.44 | -0.40 | -0.59 | -0.58 | -0.28 | -0.59 | 0.04 |
| BAS7 | . | . | . | . | . | . | 0.32 | 0.06 | 0.41 | 0.49 | 0.55 | 0.54 | 0.45 | 0.36 | 0.70 | 0.66 | 0.67 | 0.40 | -0.44 | 0.70 |
| BAS8 | . | . | . | . | . | . | . | 0.60 | 0.31 | 0.50 | 0.44 | 0.42 | 0.48 | 0.80 | 0.60 | 0.53 | 0.54 | 0.43 | -0.33 | 0.80 |
| BAS9 | . | . | . | . | . | . | . | . | 0.40 | 0.61 | 0.33 | 0.25 | 0.60 | 0.51 | 0.22 | 0.17 | 0.18 | 0.31 | -0.03 | 0.61 |
| BAS10 | . | . | . | . | . | . | . | . | . | 0.77 | 0.65 | 0.52 | 0.46 | 0.18 | 0.14 | 0.41 | 0.43 | 0.35 | -0.09 | 0.77 |
| BAS11 | . | . | . | . | . | . | . | . | . | . | 0.67 | 0.58 | 0.67 | 0.51 | 0.47 | 0.58 | 0.66 | 0.48 | -0.28 | 0.77 |
| BAS12 | . | . | . | . | . | . | . | . | . | . | . | 0.89 | 0.72 | 0.33 | 0.56 | 0.54 | 0.44 | 0.44 | -0.25 | 0.89 |
| BAS13 | . | . | . | . | . | . | . | . | . | . | . | . | 0.82 | 0.13 | 0.59 | 0.35 | 0.36 | 0.41 | -0.19 | 0.89 |
| BAS14 | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.28 | 0.67 | 0.26 | 0.31 | 0.42 | -0.07 | 0.82 |
| BAS15 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.63 | 0.73 | 0.68 | 0.38 | -0.44 | 0.80 |
| BAS16 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.61 | 0.62 | 0.42 | -0.40 | 0.70 |
| BAS17 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.94 | 0.43 | -0.59 | 0.94 |
| BAS18 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.44 | -0.58 | 0.94 |
| Overall | 0.33 | -0.29 | 0.68 | |||||||||||||||||
Table 4. . Pearson correlation matrix for each BASIQ item score with the remaining 17-item scores at 1-month follow-up.
| At month 1 | BAS2 | BAS3 | BAS4 | BAS5 | BAS6 | BAS7 | BAS8 | BAS9 | BAS10 | BAS11 | BAS12 | BAS13 | BAS14 | BAS15 | BAS16 | BAS17 | BAS18 | Average r | Min. r | Max. r |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAS1 | 0.28 | 0.14 | 0.17 | 0.33 | -0.35 | 0.11 | 0.43 | 0.74 | 0.28 | 0.54 | 0.38 | 0.36 | 0.22 | 0.00 | -0.08 | -0.05 | 0.19 | 0.22 | -0.35 | 0.74 |
| BAS2 | . | 0.40 | 0.63 | 0.08 | 0.14 | 0.31 | 0.57 | 0.26 | 0.34 | 0.44 | 0.49 | 0.17 | 0.37 | 0.30 | 0.18 | 0.42 | 0.49 | 0.35 | 0.08 | 0.63 |
| BAS3 | . | . | 0.19 | 0.37 | -0.12 | 0.33 | 0.23 | 0.14 | 0.12 | 0.07 | 0.09 | 0.13 | 0.31 | 0.01 | 0.34 | 0.04 | 0.11 | 0.17 | -0.12 | 0.40 |
| BAS4 | . | . | . | 0.30 | 0.01 | 0.45 | 0.51 | 0.23 | 0.52 | 0.53 | 0.53 | 0.32 | 0.19 | 0.43 | 0.28 | 0.49 | 0.66 | 0.39 | 0.01 | 0.66 |
| BAS5 | . | . | . | . | -0.38 | 0.69 | 0.47 | 0.28 | 0.22 | 0.27 | 0.18 | 0.09 | 0.07 | 0.32 | 0.48 | 0.16 | 0.30 | 0.24 | -0.38 | 0.69 |
| BAS6 | . | . | . | . | . | -0.07 | -0.34 | -0.52 | -0.17 | -0.18 | -0.07 | -0.16 | -0.39 | -0.18 | -0.21 | -0.14 | -0.15 | -0.18 | -0.52 | 0.14 |
| BAS7 | . | . | . | . | . | . | 0.38 | 0.16 | 0.41 | 0.38 | 0.35 | 0.31 | 0.05 | 0.42 | 0.52 | 0.26 | 0.49 | 0.34 | -0.07 | 0.69 |
| BAS8 | . | . | . | . | . | . | . | 0.43 | 0.24 | 0.43 | 0.35 | 0.14 | 0.41 | 0.45 | 0.23 | 0.49 | 0.47 | 0.34 | -0.34 | 0.57 |
| BAS9 | . | . | . | . | . | . | . | . | 0.23 | 0.28 | 0.19 | 0.29 | 0.40 | 0.11 | -0.01 | 0.23 | 0.18 | 0.18 | -0.52 | 0.43 |
| BAS10 | . | . | . | . | . | . | . | . | . | 0.82 | 0.90 | 0.81 | 0.02 | 0.47 | 0.49 | 0.42 | 0.85 | 0.42 | -0.17 | 0.90 |
| BAS11 | . | . | . | . | . | . | . | . | . | . | 0.84 | 0.72 | 0.02 | 0.36 | 0.34 | 0.39 | 0.78 | 0.40 | -0.18 | 0.84 |
| BAS12 | . | . | . | . | . | . | . | . | . | . | . | 0.75 | 0.11 | 0.48 | 0.33 | 0.39 | 0.82 | 0.42 | -0.07 | 0.90 |
| BAS13 | . | . | . | . | . | . | . | . | . | . | . | . | 0.17 | 0.31 | 0.24 | 0.23 | 0.63 | 0.32 | -0.16 | 0.81 |
| BAS14 | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.26 | 0.11 | 0.06 | 0.07 | 0.14 | -0.39 | 0.41 |
| BAS15 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.72 | 0.48 | 0.57 | 0.34 | -0.18 | 0.72 |
| BAS16 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.41 | 0.55 | 0.31 | -0.21 | 0.72 |
| BAS17 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.70 | 0.31 | -0.14 | 0.70 |
| BAS18 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0.47 | -0.15 | 0.85 |
| Overall | 0.29 | -0.21 | 0.66 | |||||||||||||||||
Table 2. . Mean BASIQ item scores by demographic covariates and Spearman correlation (r) between BASIQ items and demographics at baseline.
| BASIQ item | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Symptom | Impact | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KPS | ≤70 n = 14 | 2.56 | 0.79 | 0.07 | 0.86 | 5 | 3.86 | 4.5 | 3.07 | 0.14 | 2 | 1.21 | 1.57 | 0.62 | 0 | 3.57 | 3.69 | 2.38 | 2.07 | 21.93 | 14.64 |
| >70 n = 22 | 1.38 | 1.18 | 0.59 | 1.73 | 4.32 | 5.36 | 3.05 | 2.68 | 2.55 | 1.68 | 1.14 | 1.23 | 1.41 | 1.45 | 1.82 | 2.27 | 0.77 | 0.73 | 24.14 | 10.68 | |
| r | -0.42† | 0.16 | 0.18 | -0.21 | -0.14 | 0.24 | -0.23 | 0.07 | 0.46† | 0.22 | 0.03 | 0.2 | 0.3 | 0.39 | -0.21 | -0.12 | -0.28 | -0.32 | -0.09 | -0.11 | |
| ECOG | 0 n = 10 | 1.89 | 0.7 | 0 | 1.9 | 3.5 | 4.9 | 2.5 | 2.1 | 1.7 | 2.1 | 1.2 | 1.2 | 1.2 | 1.22 | 1.7 | 1.7 | 1 | 1.1 | 21.1 | 10.2 |
| 1 n = 20 | 0.92 | 0.95 | 0.7 | 1.3 | 4.9 | 5.05 | 3.7 | 2.55 | 2.05 | 1.55 | 1.2 | 1.45 | 1.32 | 0.95 | 2.3 | 3.15 | 0.74 | 0.55 | 23.3 | 11.5 | |
| 2 n = 5 | 5.33 | 2 | 0 | 1 | 5.4 | 3.8 | 6 | 5.4 | 0 | 2 | 0.6 | 0.8 | 0 | 0 | 4.8 | 3.6 | 4.2 | 4 | 28.8 | 18 | |
| 3 n = 1 | 1 | 1 | 0 | 0 | 5 | 3 | 1 | 3 | 0 | 3 | 3 | 4 | 2 | 0 | 3 | NA | 3 | 3 | 17 | 18 | |
| r | 0.17 | 0.25 | 0.21 | 0.11 | 0.44† | -0.12 | 0.37 | 0.23 | -0.19 | -0.14 | 0.03 | -0.02 | -0.08 | -0.05 | 0.17 | 0.17 | 0.31 | 0.13 | 0.44* | 0.29 | |
| Primary cancer site | Breast n = 9 | 1 | 2.33 | 0 | 2.56 | 3.89 | 4.89 | 4 | 5.11 | 4.22 | 2.56 | 2.11 | 1.67 | 1 | 2.43 | 4.67 | 3.89 | 2.22 | 2 | 30.11 | 19.44 |
| Kidney n = 2 | 1 | 0.5 | 0 | 0 | 6.5 | 1.5 | 3 | 1.5 | 1 | 5 | 4.5 | 4.5 | 3.5 | 0 | 5 | 7 | 1.5 | 1.5 | 19.5 | 24 | |
| Lung n = 23 | 2 | 0.61 | 0.61 | 1.17 | 4.7 | 4.96 | 3.65 | 2.04 | 0.61 | 1.22 | 0.35 | 0.78 | 0.91 | 0.48 | 1.39 | 2.13 | 1.09 | 1 | 21.04 | 8.04 | |
| Other n = 6 | 3.5 | 1.67 | 1.17 | 0 | 3.83 | 5 | 3.33 | 2.83 | 1.83 | 2 | 2.33 | 2.5 | 0.5 | 0.6 | 2.83 | 2.2 | 0.2 | 0.2 | 24 | 10.67 | |
| r | -0.1 | 0.32 | -0.19 | -0.28 | -0.22 | 0.23 | 0.01 | 0.21 | 0.43† | 0.15 | 0.40† | 0.17 | 0.12 | 0.46† | 0.3 | 0.26 | 0.05 | -0.02 | 0.12 | 0.18 | |
| Education | ≤ High school n = 20 | 2.71 | 1.25 | 1 | 1.25 | 4.75 | 4.85 | 3.8 | 3.6 | 0.85 | 1.7 | 1 | 1.6 | 1.17 | 0.6 | 2.6 | 2.44 | 1.58 | 1.47 | 24.95 | 11.8 |
| College/University n = 15 | 1.45 | 1.4 | 0 | 1.4 | 3.73 | 5.47 | 3 | 2.47 | 3 | 2.13 | 1.33 | 1.07 | 0.73 | 1.27 | 2.2 | 2.53 | 0.71 | 0.73 | 23.67 | 10.53 | |
| Masters/PhD n = 3 | 0 | 0 | 0.33 | 0.33 | 3.67 | 3.67 | 3.33 | 0.33 | 0.33 | 0 | 0.67 | 0.33 | 0.33 | 0.67 | 0.33 | 1.67 | 0.33 | 0.33 | 12 | 4.67 | |
| r | -0.24 | 0.01 | -0.33 | -0.25 | -0.45† | -0.04 | -0.34 | -0.22 | 0.27 | -0.15 | 0.29 | -0.15 | -0.05 | 0.27 | 0.01 | 0.01 | -0.07 | 0.05 | -0.4 | -0.15 | |
| Number of brain metastases | 1 n = 20 | 2.07 | 1.15 | 0.15 | 1.3 | 3.95 | 4.7 | 2.75 | 2.8 | 1.65 | 1.7 | 1.25 | 1.65 | 1.53 | 1.17 | 2.55 | 2.79 | 1.16 | 1.1 | 21.7 | 12.8 |
| 2–3 n = 7 | 0 | 0.29 | 1.43 | 1.71 | 5.29 | 5.71 | 3.57 | 1.71 | 1.71 | 1.71 | 1 | 0.71 | 0.71 | 0.5 | 1.57 | 2.71 | 0.43 | 0.71 | 23.14 | 8.29 | |
| >3 n = 12 | 2.88 | 1.75 | 0.67 | 1 | 5 | 4.5 | 5.08 | 3.08 | 1.67 | 1.75 | 1.33 | 1.25 | 0.45 | 0.55 | 2.75 | 2.73 | 1.36 | 0.91 | 26.42 | 10.83 | |
| r | -0.14 | -0.09 | -0.06 | -0.03 | 0.17 | 0.08 | 0.25 | -0.27 | -0.24 | 0.26 | 0.06 | -0.1 | -0.2 | -0.25 | -0.2 | -0.05 | 0.03 | 0.05 | 0 | -0.07 | |
| Other metastases | Lymph n = 5 | 1.5 | 1.2 | 0 | 2 | 6.6 | 5 | 4.6 | 3 | 1.8 | 2.6 | 2 | 3.2 | 2.6 | 2 | 3.2 | 3.2 | 3.6 | 2.6 | 28 | 22 |
| r | -0.06 | 0.04 | -0.16 | 0.1 | 0.44† | -0.13 | 0.23 | 0.09 | -0.09 | 0.19 | 0.2 | 0.48† | 0.33 | -0.01 | 0.15 | 0.08 | 0.43† | 0.21 | 0.09 | 0.26 | |
| Lung n = 4 | 3 | 1.25 | 0 | 5.25 | 5.25 | 4 | 5.5 | 2.25 | 4.5 | 3.75 | 2.75 | 2.75 | 2 | 2.25 | 3.5 | 4 | 2 | 2.25 | 33.25 | 21.5 | |
| r | 0.23 | 0.11 | -0.11 | 0.50† | 0.1 | -0.26 | 0.25 | 0.1 | 0.35 | 0.18 | 0.18 | 0.2 | 0.15 | 0.2 | 0.09 | 0.19 | 0.16 | 0.41† | 0.16 | 0.16 | |
| Bone n = 6 | 0.25 | 0.83 | 0.17 | 2.67 | 5.5 | 4.67 | 6.5 | 1.67 | 2.5 | 1.33 | 1 | 1.67 | 1.67 | 1.83 | 2.67 | 5.33 | 1.17 | 0.83 | 26 | 16.17 | |
| r | -0.33 | 0.09 | -0.17 | -0.05 | 0.07 | -0.13 | 0.37 | -0.06 | -0.14 | -0.22 | 0.1 | 0.22 | 0.14 | 0.23 | -0.03 | 0.37 | -0.11 | -0.12 | -0.08 | 0.22 | |
| Liver n = 4 | 2.5 | 1.25 | 0.25 | 4.25 | 5.5 | 4.5 | 6.75 | 4.25 | 4.25 | 5.25 | 3 | 3.5 | 2 | 2.67 | 5 | 4.25 | 4 | 4 | 37.5 | 27.75 | |
| r | 0.28 | 0.34 | -0.08 | 0.40 † | 0.24 | -0.24 | 0.3 | 0.39 | 0.39 | 0.43† | 0.43† | 0.44† | 0.38 | 0.44† | 0.36 | 0.31 | 0.41† | 0.41† | 0.38 | 0.38 | |
| None n = 24 | 2.31 | 1.08 | 0.54 | 0.71 | 4.17 | 4.92 | 2.88 | 3 | 1.38 | 1.58 | 1.08 | 0.92 | 0.74 | 0.43 | 2.5 | 2.48 | 0.96 | 0.96 | 21.79 | 9.88 | |
| r | 0.21 | -0.1 | 0.25 | -0.11 | -0.18 | 0.12 | -0.34 | 0.08 | 0.04 | 0.05 | -0.06 | -0.32 | -0.21 | -0.18 | 0.14 | -0.23 | -0.03 | -0.02 | 0.06 | -0.17 | |
| Dex (mg/day) | 0.1 to 9.9 n = 9 | 1.29 | 1.89 | 1.11 | 1.56 | 3.67 | 4.44 | 3.22 | 3.22 | 2.67 | 1.44 | 1.78 | 0.78 | 1 | 1.5 | 2 | 2 | 1.11 | 1.22 | 24.22 | 11 |
| 10 to 39 n = 4 | 1.67 | 1.25 | 0 | 1.75 | 4.25 | 5 | 4 | 4.75 | 2.25 | 4.75 | 2 | 2.5 | 2 | 2.67 | 3.25 | 3.5 | 2.67 | 2.25 | 29.25 | 19.5 | |
| None n = 26 | 2.35 | 0.92 | 0.42 | 1.12 | 4.81 | 4.88 | 3.77 | 2.5 | 1.23 | 1.58 | 0.96 | 1.5 | 0.92 | 0.39 | 2.69 | 2.84 | 1.2 | 1 | 22.77 | 11.19 | |
| r | -0.14 | 0.33 | 0.11 | 0.2 | 0.03 | -0.14 | 0.28 | 0.3 | 0.49† | 0.35 | 0.67† | 0.11 | 0.24 | 0.58† | 0.33 | 0.31 | 0.37 | 0.33 | 0.35 | 0.37 | |
†Strong correlation (r ≥ 0.40).
• FACT-G & FACT-Br scores
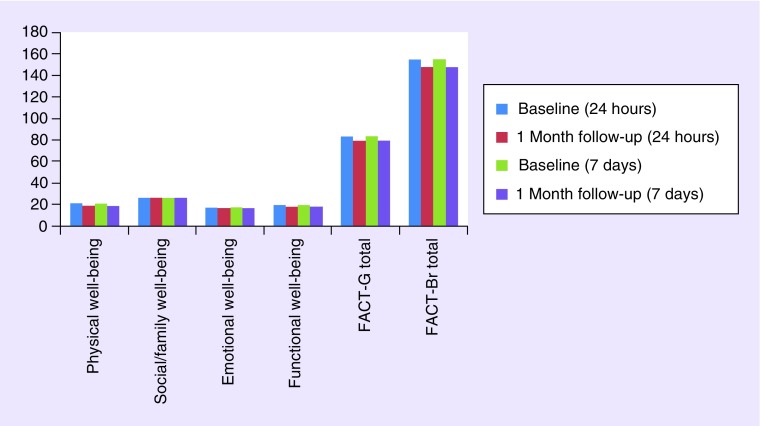
Figure 2 displays the mean FACT-G and FACT-Br subscale scores for patients at baseline and 1-month follow-up based on the assessment of the past 7 days as well as past 24 h.
Figure 2. . Mean FACT-G and FACT-Br subscale scores for the past 24 h and past 7 days at baseline and 1-month follow-up (n = 40 patients).
• Test–retest reliability
Test–retest reliability was analyzed using data from the 10 patients with 1 week follow-up data from their initial interview. The symptom scale revealed good reliability (ICC = 0.72; 95% CI: 0.42–0.92) and the impact scale revealed excellent reliability (ICC = 0.79; 95% CI: 0.57–0.94).
• Clinical validity
Clinical validity refers to the comparison of the clinical aspect of the BASIQ to the FACT-G and FACT-Br. These analyses revealed that the BASIQ impact scale was conceptually correlated with all subscales of the FACT-G and FACT-Br for both the assessment of the past 24 h and past 7 days at baseline and 1-month follow-up. However, the BASIQ symptom scale was not conceptually correlated with the social/family well-being subscale and the emotional well-being subscale of the FACT-G for both assessments in the past 24 h and past 7 days (r < 0.40). In regards to the validity of the changed scales over time (i.e., from baseline to 1 month), the BASIQ symptom scale was not correlated with the social/family well-being subscale, emotional well-being subscale and the functional well-being subscale of the FACT-G for both the assessment of the past 24 h and past 7 days. Furthermore, the BASIQ impact scale was correlated with all subscales except the social/family well-being subscale and the functional well-being subscale for both the past 24 h and past 7 days assessments.
• Change in scores over time
The Wilcoxon signed rank test revealed no significant changes in the BASIQ symptom and impact scales over time from baseline to 1-month follow-up. The test also revealed no significant changes in the FACT-G and FACT-Br subscales except for in the physical well-being subscale of the FACT-G. Further investigation of which BASIQ items changing values were highly correlated to the physical well-being changing values of the FACT-G is displayed in Table 5. The analysis revealed that BASIQ items including energy, balance, staying focused, walking, housework, bathing and getting dressed were highly correlated to the physical well-being scale of the FACT-G.
Table 5. . Spearman correlation between the physical well-being subscale of the FACT-G at 24 h and the BASIQ changing scores from baseline to 1 month.
| BASIQ item (month 1 – baseline) | Physical well-being (24 h) changing from baseline | |
|---|---|---|
| Correlation coefficient | p-value | |
| 1 | 0.01† | 0.996 |
| 2 | -0.23 | 0.37 |
| 3 | -0.29 | 0.25 |
| 4 | -0.11† | 0.68 |
| 5 | -0.16† | 0.54 |
| 6 | 0.45‡ | 0.067 |
| 7 | -0.29 | 0.26 |
| 8 | -0.50‡ | 0.041 |
| 9 | 0.16† | 0.54 |
| 10 | -0.31 | 0.23 |
| 11 | -0.23 | 0.38 |
| 12 | -0.41‡ | 0.10 |
| 13 | -0.13† | 0.63 |
| 14 | 0.17† | 0.52 |
| 15 | -0.69‡ | 0.0024 |
| 16 | -0.57‡ | 0.016 |
| 17 | -0.52‡ | 0.032 |
| 18 | -0.52‡ | 0.033 |
†Very weak correlation.
‡Strong correlation (≥0.40).
Discussion
This is the first study to conduct the psychometric validation of the BASIQ version 1.0. Furthermore, the BASIQ is the first patient-reported questionnaire originally developed specifically to measure symptom severity and impact on activities of daily living and QoL for patients with brain metastases, rather than patients with primary brain tumors as in the FACT-Br and the EORTC QLQ-BN20. The time taken to complete a lengthy QoL questionnaire can often be burdensome for patients, especially in patients with a poor performance status [16]. Compared to these instruments, the 18-item BASIQ is considerably briefer and less burdensome for the patient to complete, but still adequately assesses the key symptoms and functional activities affected by brain metastases.
Through the process of validating the BASIQ in patients with brain metastases, the BASIQ was administered with an existing QoL questionnaire for this patient population, the FACT-Br with the FACT-G. When examining the convergent and divergent validity of the BASIQ with the FACT-G and FACT-Br, the analyses revealed that the symptom scale of the BASIQ was not correlated with the social/family well-being scale and the emotional well-being scale of the FACT-G. However, this can be expected as the BASIQ symptom scale solely focuses on physical symptoms such as headaches and numbness, rather than social or emotional factors. Considering these two subscales should not correlate with the BASIQ, the validity analyses show that the FACT-Br subscales that should be correlated with the BASIQ symptom and impact scales are correlated, which ultimately validates the BASIQ scales.
Additional analysis involving the correlation of each BASIQ item score with the remaining 17-item scores revealed that only items on the impact scale showed significant correlation. This could possibly be due to higher variation in responses among the items on the symptom scale (items 1–10), as seen in Figure 1. A study conducted by Wong et al. examined QoL in patients with brain metastases following WBRT at our center. The study revealed that most commonly experienced symptoms by patients at baseline included headaches, problems with balance, weakness and fatigue [17]. All of these items are included in the symptom scale of the BASIQ.
QoL is expected to change over time as a patient's condition changes, especially when the disease progresses and causes deterioration in neurocognitive function [18]. In order to assess whether the BASIQ accurately assesses change in patient QoL over the span of 1 month, the BASIQ was cross-validated with the FACT-G and the FACT-Br. Since the BASIQ did not reveal any significant change in patients from their baseline to 1-month assessment, the FACT-G and FACT-Br were used to confirm that QoL of patients did not significantly change over time. Consequently, the FACT-G and FACT-Br also did not reveal significant change in QoL over 1 month, except for the physical well-being scale of the FACT-G. Further analysis revealed that the BASIQ items that were not strongly correlated with the physical well-being scale include headache, numbness, tiredness, vision, ability to follow a story and reading. These results can be expected because these items are not included on the physical well-being scale of the FACT-G, which is likely why the physical well-being scale showed significant change over time in comparison to the BASIQ.
Since the BASIQ showed no significant change in QoL of patients over time, which coincides with the FACT-G and FACT-Br, it can be established that the BASIQ can be used to adequately assess QoL in patients with brain metastases over a period of 1 month. To further verify this point, similar results were found by Wong et al. using the Spitzer Quality of Life Index questionnaire, which is another instrument that has been employed in patients with brain metastases. The results of their study revealed that 47% of patients had stable or improved neurological function overtime and although certain QoL domains improved, all other domains did not significantly change over time at 1 month follow up. The patient sample involved in the study had a median age of 64 years and median KPS of 70, which is almost identical to our patient sample [17]. Consequently, this makes it more convincing that patients within our study sample indeed did not experience a significant change in QoL after 1 month when considering that other QoL instruments in patients with brain metastases revealed the same results.
The fact that the BASIQ symptom and impact scales showed no significant change in patients after 1 month could also be attributed to the baseline performance status of our patient sample. The median Karnofsky performance status (KPS) of patients was 80 and 67% of patients were not receiving dexamethasone at baseline. A KPS of 80 suggests the patient shows some signs of disease or symptoms and can carry out normal activity with effort [19]. Furthermore, patients not being prescribed dexamethasone at baseline could possibly be explained by their good overall condition, lack of neurological symptoms, or minimal cerebral edema [20]. Consequently, the good performance status of the majority of patients in our patient sample could explain the reason why the BASIQ symptom and impact scales showed no significant change over time at 1-month follow-up.
Despite showing no significant change in the statistical analyses, the BASIQ scores still generally increased from baseline to 1 month. This can be expected since patients with brain metastases often have progressive disease resulting in deterioration and decline in overall performance status. A study conducted by Bezjak et al. looked at patient symptoms and overall performance status over time in patients after receiving WBRT for their brain metastases. The study revealed that 23% had stable disease, and 55% of patients had progressed or deceased after 1 month. Furthermore, patient-rated symptoms generally increased from baseline to 1 month [21]. These results generally reflect the results of our study especially considering that some patients originally accrued at baseline were deceased or unable to complete a follow-up at 1 month.
Overall, the BASIQ was well-received by patients, especially when comparing the length of the BASIQ to the combined FACT-G and FACT-Br. One potential problem with the BASIQ is that it was difficult to distinguish between a severity of 0 to 10 in comparison to a checkbox for whether the patient experienced a particular symptom. This issue only applies to items 1 (headache) and 14 (reading). For instance, since item 3 (nausea) does not have this option, a patient who did not experience nausea would rate this item as 0, whereas a patient who did not read would check the box “did not read”. To address this discrepancy, our current ongoing research is examining the validity of removing the checkbox option. Another limitation was that our patient sample consisted of outpatients with relatively good performance status, which could have resulted in different BASIQ scores compared with patients with poorer performance status, who are cognitively impaired, or are inpatients. Patients in unstable conditions such as these should be tested to verify whether a decrease in QoL is analogous between the BASIQ, FACT-G and FACT-Br, and if there is a difference in sensitivity among these scales. Another limitation was that our study only included 1-month follow-up.
Conclusion
Maintaining QoL in patients is an important aspect of palliative care, especially when considering patients with brain metastases. There are currently no questionnaires that were originally designed for patients with brain metastases. The 18-item BASIQ version 1.0 adequately assesses key symptoms and impact on QoL from a brain metastases patient's perspective, without being burdensome in length as seen in previous PRO questionnaires QoL used for this patient population which assess multiple dimensions of QoL but are significantly longer. Our study supports the successful psychometric validation of the BASIQ by confirming the reliability, validity and suitability of the instrument to be used in patients with brain metastases.
Future perspective
The BASIQ can be used in future clinical trials involving patients with brain metastases when assessing QoL over the span of up to 1 month. Future studies will examine the validation of the BASIQ to assess changes in QoL over a longer period of time.
EXECUTIVE SUMMARY.
The Brain Metastases Symptom and Impact Questionnaire (BASIQ) version 1.0 is a patient-reported outcome (PRO) instrument measuring symptom severity and impact on daily functional activities developed with input from clinical experts and from qualitative and cognitive interviews with brain metastases patients.
The 18 items of the BASIQ encompass both symptom and impact scales relevant to patients with brain metastases. Both these scales together cover 12 domains: headaches, dizziness, nausea, numbness, energy, balance, vision, memory, cognition, vision, physical activities and self-care.
The purpose of the study was to test the reliability, clinical and psychometric validity of the BASIQ in patients with brain metastases.
Patients with brain metastases were interviewed using the BASIQ 1 week prior to their treatment, along with the Functional Assessment of Cancer-Brain (FACT-Br) and FACT-General (FACT-G).
The median age of patients was 64 years and the median KPS was 80. Patients with higher KPS, ECOG of 2, primary breast cancer, or more than three brain metastases, scored higher on the symptom scale of the BASIQ. Patients with an ECOG of 2, primary kidney cancer, or one brain metastasis, scored higher on the impact scale.
In regards to validity, the symptom scale of the BASIQ was not correlated with the social/family well-being scale and the emotional well-being scale of the FACT-G.
The BASIQ, FACT-G and FACT-Br subscales showed no significant change in patient symptoms from baseline to 1-month follow-up except for the physical well-being subscale on the FACT-G.
Overall, the BASIQ was well-received by patients, especially when comparing the length of the BASIQ to the combined FACT-G and FACT-Br.
Since the BASIQ showed no significant change in QoL of patients over time, which coincides with the FACT-G and FACT-Br, it can be established that the BASIQ can be used to adequately assess QoL in patients with brain metastases over a period of 1 month.
The good performance status of the majority of patients in our patient sample could explain the reason why the BASIQ symptom and impact scales showed no significant change over time at 1-month follow-up.
Despite showing no significant change in the statistical analyses, the BASIQ scores still generally increased from baseline to 1 month. This can be expected since patients with brain metastases often have progressive disease resulting in deterioration and decline in overall performance status.
The 18-item BASIQ has successfully undergone psychometric validation. The BASIQ adequately assesses QoL in patients with brain metastases up to the span of 1 month and can be used in future clinical trials involving this patient population.
Future studies will involve validating the BASIQ to assess QoL past 1 month.
Acknowledgements
The authors thank the sponsor of Abbvie and the survey participants.
Appendix I. . • Brain Metastases Symptom & Impact Questionnaire (BASIQ).
Instructions: Please circle the number that best describes the symptoms you experienced in the past 24 h because of the cancer that has spread to your brain.
Symptoms (Part 1)
Headache
Dizziness
Nauseous
Numbness
Tired
Energy
Physically weak
Balance
Vision
Memory
Interferences (Part 2)
Putting ideas into words
Staying focused
Following a story
Reading
Walking
Doing things around the house
Bathing
Getting dressed
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Serres S, Soto MS, Hamilton A, et al. Molecular MRI enables early and sensitive detection of brain metastases. Proc. Natl Acad. Sci. USA. 2012;109(17):6674–6679. doi: 10.1073/pnas.1117412109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle M, Bradley NM, Li K, et al. Quality of life in patients with brain metastases treated with a palliative course of whole-brain radiotherapy. J. Palliat. Med. 2007;10(2):367–374. doi: 10.1089/jpm.2006.0202. [DOI] [PubMed] [Google Scholar]
- 3.Thomas SS, Dunbar EM. Modern multidisciplinary management of brain metastases. Curr. Oncol. Rep. 2010;12(1):34–40. doi: 10.1007/s11912-009-0073-8. [DOI] [PubMed] [Google Scholar]; • Comprehensive review of current treatment options available for patients with brain metastases.
- 4.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12(7):884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Maor MH, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery. 2007;60(2):277–283. doi: 10.1227/01.NEU.0000249272.64439.B1. [DOI] [PubMed] [Google Scholar]
- 6.Posner JB. Management of central nervous system metastases. Semin. Oncol. 1977;1:81–91. [PubMed] [Google Scholar]
- 7.Tsao MN, Lloyd N, Wong R, Chow E, Rakovitch E, Laperriere N. Whole brain radiotherapy for the treatment of multiple brain metastases. Cochrane Database Syst. Rev. 2006;(3):CD003869. doi: 10.1002/14651858.CD003869.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Movsas B. Quality of life in oncology trials: a clinical guide. Semin. Radiat. Oncol. 2003;13(3):235–247. doi: 10.1016/S1053-4296(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 9.Caissie A, Nguyen J, Chen E, et al. Quality of life in patients with brain metastases using the EORTC QLQ-BN20+2 and QLQ-C15-PAL. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(4):1238–1245. doi: 10.1016/j.ijrobp.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Chen E, Cella D, Zeng L, et al. Content validation of the FACT-Br with patients and health-care professionals to assess quality of life in patients with brain metastases. J. Radiat. Oncol. 2012;3(1):105–113. [Google Scholar]; •• Study of similar nature to the present study, which validated the use of the FACT-Br in patients with brain metastases.
- 11.Campos S, Davey P, Hird A, et al. Brain metastasis from an unknown primary, or primary brain tumour? A diagnostic dilemma. Curr. Oncol. 2009;16(1):62–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Caissie A, Culleton S, Nguyen J, et al. EORTC QLQ-C15-PAL quality of life scores in patients with advanced cancer referred for palliative radiotherapy. Support. Care Cancer. 2012;20(4):841–848. doi: 10.1007/s00520-011-1160-6. [DOI] [PubMed] [Google Scholar]
- 13.Bedard G, Ray S, Zhang L, et al. Content validation of the brain symptom and impact questionnaire (BASIQ) in patients and health-care professionals to assess quality of life in patients with brain metastases. Support. Care Cancer. 2013;21(Suppl. 1):S132. [Google Scholar]; •• First phase of the present study, which involved the content validation for the Brain Metastases Symptom and Impact Questionnaire (BASIQ) questionnaire.
- 14.Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am. J. Ment. Defic. 1981;86(2):127–137. [PubMed] [Google Scholar]
- 15.Nunnally JC, Bernstein IH. Psychometric Theory (3rd Edition) Mc Graw-Hill; NY, USA: 1994. [Google Scholar]
- 16.Johnson C, Fitzsimmons D, Gilbert J, et al. Development of the European Organisation for Research and Treatment of Cancer quality of life questionnaire module for older people with cancer: the EORTC QLQ-ELD15. Eur. J. Cancer. 2010;46(12):2242–2252. doi: 10.1016/j.ejca.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(4):1125–1131. doi: 10.1016/j.ijrobp.2008.12.013. [DOI] [PubMed] [Google Scholar]; • Examines quality of life and symptoms in patients with brain metastases follow palliative radiotherapy, which is a similar patient population to the present study.
- 18.Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2008;71(1):64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 19.Friendlander AH, Ettinger RL. Karnofsky performance status scale. Spec. Care Dentist. 2009;29(4):147–148. doi: 10.1111/j.1754-4505.2009.00088.x. [DOI] [PubMed] [Google Scholar]
- 20.Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects? Support. Care Cancer. 2002;10(4):322–328. doi: 10.1007/s00520-001-0333-0. [DOI] [PubMed] [Google Scholar]
- 21.Bezjak A, Adam J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur. J. Cancer. 2002;38(4):487–496. doi: 10.1016/s0959-8049(01)00150-2. [DOI] [PubMed] [Google Scholar]