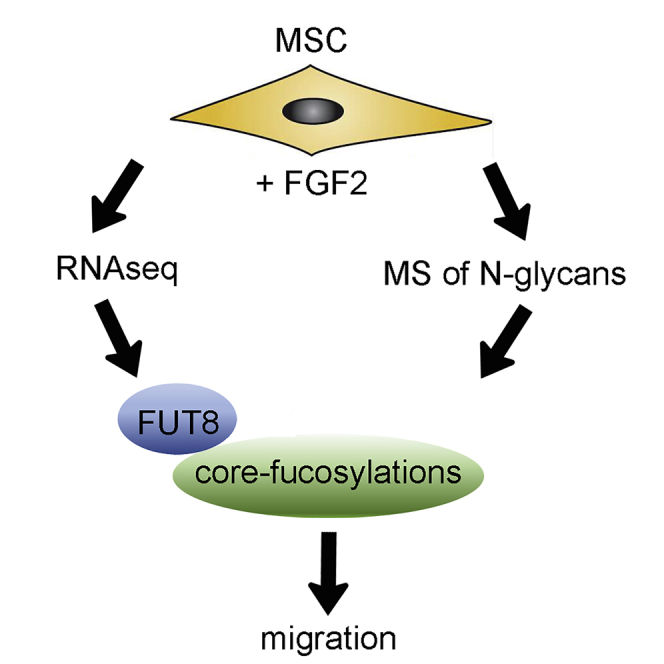
Summary
Since hundreds of clinical trials are investigating the use of multipotent stromal cells (MSCs) for therapeutic purposes, effective delivery of the cells to target tissues is critical. We have found an unexplored mechanism, by which basic fibroblast growth factor (FGF2) induces expression of fucosyltransferase 8 (FUT8) to increase core fucosylations of N-linked glycans of membrane-associated proteins, including several integrin subunits. Gain- and loss-of-function experiments show that FUT8 is both necessary and sufficient to induce migration of MSCs. Silencing FUT8 also affects migration of MSCs in zebrafish embryos and a murine bone fracture model. Finally, we use in silico modeling to show that core fucosylations restrict the degrees of freedom of glycans on the integrin’s surface, hence stabilizing glycans on a specific position. Altogether, we show a mechanism whereby FGF2 promotes migration of MSCs by modifying N-glycans. This work may help improve delivery of MSCs in therapeutic settings.
Keywords: core fucosylation, migration, FUT8, MSC, multipotent stromal cells, mesenchymal stem cells
Graphical Abstract
Highlights
-
•
FGF2 upregulates FUT8 and core fucosylations in MSCs
-
•
FUT8 is both necessary and sufficient to promote migration of MSCs
-
•
Core fucosylations stabilize the position of N-glycans on integrins
In this article, Fierro and colleagues used RNA-seq and mass spectrometry to find that FGF2 induces migration of MSCs by inducing expression of FUT8, which leads to more core fucosylations of N-linked glycans of membrane-associated proteins, including several integrin subunits. This affects cell migration in zebrafish embryos and toward bone fracture in mice.
Introduction
Despite the marked safety profile and long-established potential of multipotent stromal cells (MSCs) to promote tissue repair, their use has been limited mostly to small clinical trials, with only modest efficacy (Trounson and McDonald, 2015). A major barrier to the effective application of MSCs has been the poor ability to target these cells to tissues of interest (Karp and Leng Teo, 2009). For example, therapies aiming for bone repair require that injected cells home to the bone marrow, a process that is highly inefficient (Grayson et al., 2015, Rombouts and Ploemacher, 2003).
Glycosylation is a common post-translational modification of proteins that affects many cell functions, including adhesion, migration, and signaling (Cummings and Pierce, 2014, Moremen et al., 2012). N-glycans are sugars attached to Asparagine in Asn-X-Ser/Thr peptides, where X can be any amino acid, except for proline. It has been proposed that N-linked glycosylations (N-glycans) may affect cell migration both positively and negatively (Gu and Taniguchi, 2008), although the experimental evidence is mostly limited to selectin binding. Of note, chemical engineering of glycosylations promotes MSC homing to the bone marrow (Sackstein et al., 2008), suggesting that N-glycans could play a critical role in MSC migration.
During MSC expansion, basic fibroblast growth factor (FGF2) is often used to increase cell proliferation (Tsutsumi et al., 2001), maintain differentiation potential (Solchaga et al., 2005), and delay cellular senescence (Ito et al., 2007). It has also been shown that FGF2 promotes migration of MSCs in vitro (Schmidt et al., 2006), although no potential mechanism for FGF2-mediated migration has been described. Therefore, we aimed to determine changes at mRNA and N-glycan levels that could account for increased migration of MSCs, potentially leading to new approaches to improve MSC delivery.
Results
FGF2 Promotes Migration of MSCs by Altering Gene Expression and N-Glycans
To test the effect of FGF2 on migration of MSCs, two experimental approaches were used. In the wound/scratch assay, MSCs are seeded so that a constant gap or “wound” is left in-between the monolayer of cells. Closure of the wound over time is therefore proportional to the migration ability of the cells. To complement this assay, we used single-cell tracking, where the displacement of individual cells over time (speed) is recorded using videomicroscopy. In both in vitro assays, treatment with FGF2 significantly increased migration of MSCs (Figures 1A, 1B, and S1).
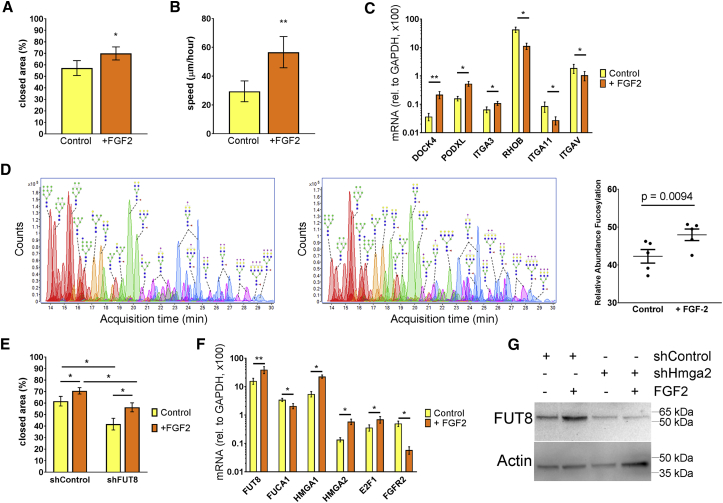
Figure 1.
FGF2 Promotes Migration by Increasing FUT8 and Core Fucosylations
(A) Wound/scratch assay, where closed area represents MSC migration after 24 hr, N = 5.
(B) Cell tracking using videomicroscopy. MSC displacement over time (speed) was recorded for 24 hr and tracked using ImageJ software. A total of 30 cells per condition (derived from two different donors) were analyzed.
(C) RT-PCR confirming differential expression of genes related to cell migration, N = 6.
(D) Representative chromatograms of N-glycans detected by MS and semi-quantification of core-fucosylation levels, N = 5.
(E) Wound/scratch assay showing the effect of silencing FUT8 on FGF2-induced migration (N = 6).
(F) RT-PCR showing differential expression of genes associated with core fucosylations and FUT8 expression, N = 6.
(G) Western blots in MSCs transduced with the indicated lentivirus and treated with or without FGF2 (N = 3).
Error bars indicate standard error of the mean (SEM). For all statistical analyses, a paired Student's t test was used, where ∗p < 0.05 and ∗∗p < 0.005, and N indicates biological replicates (MSCs derived from different donors).
To identify changes in gene expression that could account for increased migration, we performed deep-sequencing transcriptome analysis (RNA-seq) on MSCs cultured with or without FGF2 (Figure S2 and GEO repository). A large-scale gene function analysis of the 246 transcripts increased with FGF2 revealed a strong enrichment for genes related to cell-cycle progression and proliferation (Figure S2). Among the 267 downregulated transcripts, collagen-related processes were highly represented. We also confirmed several migration-related genes as regulated by FGF2, including upregulation of DOCK4, PODXL, and ITGA3 and downregulation of RHOB, ITGA11, and ITGAV (Figure 1C). These results suggest that FGF2 promotes migration of MSCs through coordinated regulation of multiple genes.
We next investigated if FGF2 could also induce post-translational changes that could account for increased migration. A major modification in membrane-associated proteins (MAPs) are N-glycans that occur in the ER and Golgi apparatus. In fact, we found multiple glycosylation-related genes differentially expressed with FGF2 (Figure S2B). Therefore, MSCs were treated with or without FGF2 and processed for extraction of MAPs and semi-quantitative analysis by mass spectrometry (MS) (Park et al., 2015). We found a decrease in high-mannose N-glycans and a mild trend to increase sialylations (Figure S1), whereas the most consistent observation was an increase in fucosylated N-glycans (Figure 1D). Most fucosylated N-glycans (92.7%) showed one fucose, which is usually attached to the first N-acetylglucosamine within the chitobiose core and are therefore called core fucosylation.
Notably, the MS analysis was in accordance with the RNA-seq data, because core fucosylations are catalyzed by α-1,6-fucosyltransferase (FUT8) (Yang and Wang, 2016), and FUT8 was significantly upregulated by FGF2 (Figure 1F). Hydrolysis of core fucosylations is catalyzed by both α-L-fucosidase 1 (FUCA1) and FUCA2. Expression of FUCA2 was not affected by FGF2, while FUCA1 was downregulated by FGF2, although this reduction (0.2-fold) was not as pronounced as the increase in FUT8 (2.0-fold). Since fibroblasts derived from FUT8−/− mice show impaired migration (Zhao et al., 2006), we tested if FUT8 would affect migration of human MSCs. Indeed, the increased migration induced by FGF2 was strongly reduced in MSCs transduced with a lentivirus to silence FUT8 (shFUT8; Figure 1E), suggesting that core fucosylations are necessary for migration of MSCs.
We previously showed that FGF2 quickly increases High-Mobility Group 2A (HMGA2) mRNA and protein levels in MSCs (Kalomoiris et al., 2016). In addition to HMGA2, we also found the homolog HMGA1 strongly increased by FGF2 (Figure 1F). To test if the increase of FUT8 is regulated by HMGA2, we transduced MSCs with either a control short hairpin RNA (shControl) or a shRNA to silence HMGA2 (shHMGA2) and then incubated the cells with or without FGF2. In MSCs transduced with shControl, FGF2 markedly increased FUT8 protein expression. However, in MSCs transduced with shHMGA2, FUT8 expression was very low, even in the presence of FGF2, suggesting that HMGA2 acts downstream of FGF2 to regulate FUT8 expression (Figure 1G). To determine potential transcription factors that could induce FUT8 expression, we found that the FUT8 promoter shows multiple E2F binding sites, including at least three for E2F1. Interestingly, E2F1 expression was increased with FGF2 (Figure 1F), while others have shown that HMGA2 increases E2F1 activity (Fedele et al., 2006). Overall, these results suggest that FGF2 increases core fucosylations through a signaling pathway involving HMGA2, leading to transcriptional increase of FUT8.
Fucosylation of Integrins Are Essential for Migration of MSCs
To determine which specific MAPs were core-fucosylated, we used a liquid chromatography-tandem MS (LC-MS/MS)-based multiple reaction monitoring (MRM) approach for glycoprotein analysis (Hong et al., 2013). We consistently detected a total of 241 peptides corresponding to 118 different MAPs, treated with or without FGF2 (Table S1). Most proteins showed 1–10 different types of N-glycans (average 6.5; Figure 2A) and no correlation was found between relative mRNA levels (determined by RNA-seq) and number of types of N-glycans (measured by MRM). However, among integrins, mRNA levels strongly correlated with the variety of N-glycans found (Figure 2B). Integrin β1 stands out for exhibiting on average 70 different types of N-glycans, 56% of them fucosylated. Also, integrin subunits α3, α5, α11, and αV showed fucosylated N-glycans, although at low levels, as compared with β1 (Figure 2C). These results show that various integrins, and especially subunit β1, are core-fucosylated.
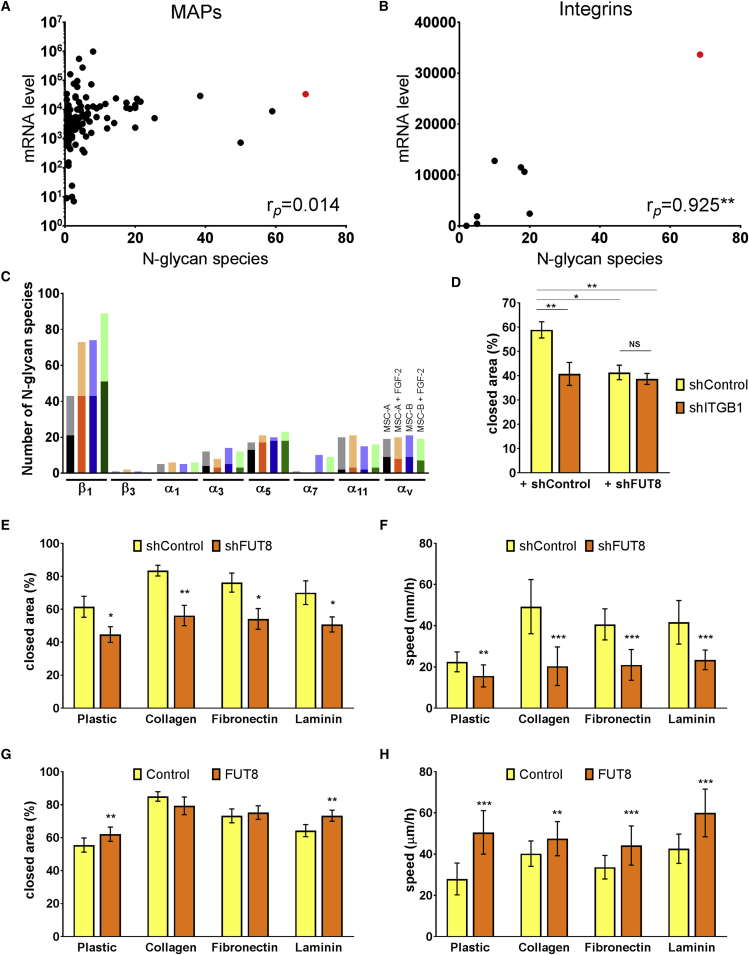
Figure 2.
Core Fucosylations on Integrins Regulate Migration of MSCs
(A) Correlation analysis of membrane associated proteins mRNA (as determined by RNA-seq; N = 4) and number of N-glycans types found by MRM/MS (N = 2, each with and without FGF2). Integrin β1 is highlighted in red.
(B) Correlation analysis of integrins, ∗∗p = 0.0011, as determined using a Pearson correlation analysis.
(C) Number of N-glycans found in integrins expressed in MSCs. Dark section of each column denotes fucosylated N-glycans, light section is non-fucosylated N-glycans.
(D) Wound/scratch assay on uncoated plates of MSCs transduced twice, with either shControl or shITGB1 (to silence integrin β1), and shControl or shFUT8. N = 6.
(E and G) Wound/scratch assays of MSCs cultured over different substrates; N = 6 for both experiments.
(F and H) Videomicroscopy over 12 hr of MSCs cultured over different substrates, transduced to either silence (E) or over-express (G) FUT8. N = 30 cells, derived from three different donors.
Error bars indicate standard error of the mean (SEM). Statistical differences were determined using two-way ANOVA with post hoc Student's t test (paired for D, E, and G and non-paired for F and H), where ∗p < 0.05 and ∗∗p < 0.005. ∗∗∗p < 10−5, and N indicates biological replicates, except when otherwise indicated.
The role of core fucosylation on integrin β1 was experimentally supported by a wound/scratch assay. Here, silencing FUT8 shows the same inhibitory effect as silencing integrin β1, while silencing both FUT8 and integrin β1, leads to no additional inhibition (Figure 2D).
To further assess the functional effect of core fucosylations, we performed migration assays over different extracellular matrix, known to be specific integrin ligands. Based on integrin's expression levels and fucosylations, we used collagen I to assess α11β1, fibronectin to assess α5β1 and αVβ1, and laminin to assess for α3β1 activity. Using the wound/scratch assay and single-cell tracking, we found that silencing FUT8 inhibited migration on all substrates (Figures 2D and 2E). In the wound/scratch assay, over-expression of FUT8 increased MSC migration over polystyrene (uncoated plates) and laminin (Figure 2F), while over collagen and fibronectin, MSCs virtually closed the wounds within 24 hr, hence no additional increase in migration could be detected. However, in single-cell tracking assays, over-expression of FUT8 increased migration of MSCs on all surfaces tested (Figure 2G). These results suggest that core fucosylations on integrin β1 are both necessary and sufficient to promote MSC migration.
FUT8 Is Necessary for Migration of MSCs In Vivo
Zebrafish embryos have been used as platforms for assaying mammalian cancer cell behavior, as xenotransplanted cells develop tumors and metastasize (Marques et al., 2009). The transparency of embryos and absence of a functional innate immune system until 2 days post fertilization (dpf), allow imaging of fluorescently labeled transplanted cells over time. To study the role of FUT8 on migration of MSCs in vivo, we introduced green or red fluorescently labeled MSCs into wild-type zebrafish blastulae (4 hr postfertilization [hpf]). One day after transplantation, control MSCs distributed mostly in the anterior embryo (brain, eye, heart, gill arches) and, to a lesser extent, in the trunk and tail (Figures 3A and 3B). In contrast, shFUT8 transduced MSCs accumulate almost exclusively in the lateral trunk, often directly under the skin (posterior to the hindbrain and anterior to the yolk extension; Figure 3C), and they tended to clump together. We quantified the position of MSCs along the anterior-posterior axis and found a significant difference between control and FUT8 mRNA-depleted cells (Figure 3D).
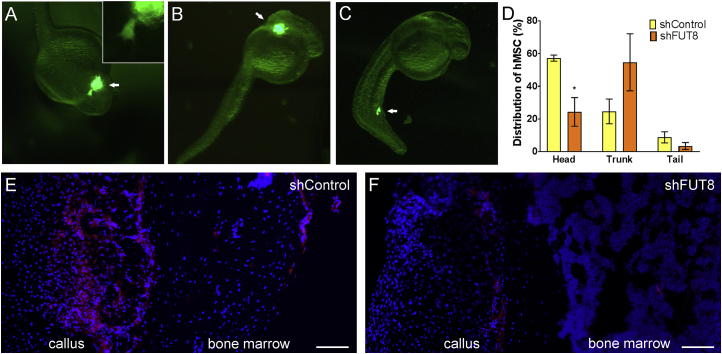
Figure 3.
Silencing FUT8 Affects Migration of MSC in Zebrafish and Mice
(A) Representative image of GFP-expressing human MSCs around the head of 1-dpf zebrafish. Insert shows high magnification, where protrusions from an individual cell are visible.
(B) MSCs distributed around the trunk region.
(C) MSCs distributed in the tail. Distribution of transplanted cells is highlighted by arrows.
(D) Quantification of cell distribution, where ∗p < 0.05 as assessed using a paired Student's t test (N = 5 independent experiments performed with biological replicates).
(E) Seven days after bone fracture and intramuscular injection of cells, mice were analyzed for presence of tdTomato-expressing mouse MSCs. Control cells distribute abundantly in callus and bone marrow of the fractured femur.
(F) MSCs with shFUT8 only minimally reach the new callus and bone marrow on fracture site.
Error bars indicate standard error of the mean (SEM). Scale bars, 100 μm.
We recently showed that over-expression of FGF2 in MSCs accelerates bone repair in mice, which we hypothesized is driven by increased migration, since with FGF2, more MSCs were found at the fracture site at early time points (Zhang et al., 2017). We therefore tested if inhibition of FUT8 would alter the incorporation of MSCs into the fractured femur. Immediately following a femoral fracture in the right hindlimb, FGF2-treated cells (transduced with either shControl or shFUT8) were injected into both left and right thighs close to the fracture site. Cells were then allowed to migrate toward the fracture site for 7 days. As previously reported (Zhang et al., 2017), only a very few cells incorporated into the left, unfractured, femur (not shown). On the fractured site, control MSCs were abundantly found incorporated into the callus and some cells were observed in the bone marrow. In contrast, MSCs transduced with shFUT8 were barely detectable at the fracture site. Since these cells were still present in the muscle (around the injection site), we conclude that shFUT8 did not affect cell retention, but rather inhibited cell migration toward the fracture site. These results further support that FUT8 is essential for migration of MSCs in vivo.
Core Fucosylation May Limit the Movement of N-Glycans
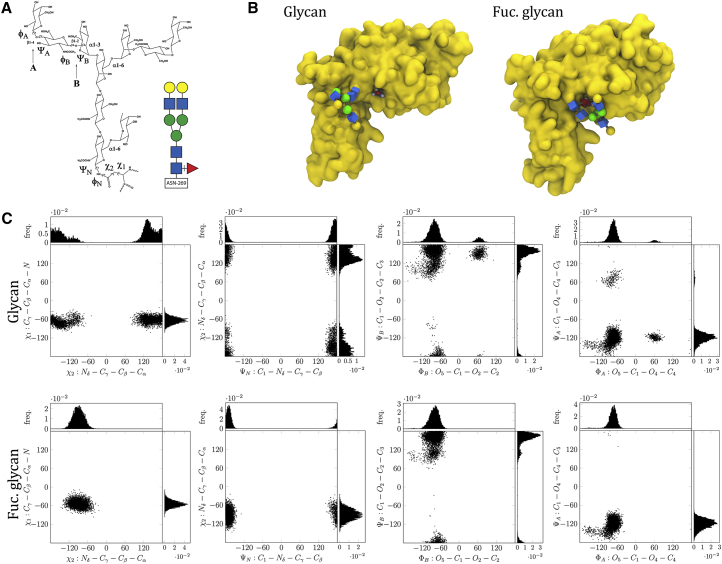
To study how core fucosylations affect protein function, we used molecular dynamics. We detected six glycosylated residues on integrin β1, all in the extracellular domain, facing the surface of the protein (Video S1). We performed minimization and equilibration calculations to attach in silico one glycan (Figure 4A) to Asn265 as actually found in our MRM analysis in both forms, with and without core fucose. After extensive equilibration, we performed simulations and found that the fucosylated glycan shows more restricted movement, as compared with the non-fucosylated glycan (Videos S2 and S3). This was at least partially due to hydrogen bonds built in-between the fucose and Asn329 (Figure 4B). The restricted movements of the fucosylated glycan were also noticeable in dihedral angles. Interestingly, we found the strongest effects on the bond in-between the glycan and Asn265 (torsions of the acetamido and hydroxymethyl groups) and on the α1,3 antenna (torsion angles A and B) (Figure 4C). In contrast, dihedral angles along the center portion (chitobiose core) of the glycan and the α1,6 antenna were only minimally altered (Figure S4). Altogether, molecular dynamics suggest that core fucosylations may reduce total glycan ensembles, possibly favoring the interaction of the glycan with other molecules.
Figure 4.
Molecular Modeling Suggests that Core Fucosylations Alter the Orientation of N-Glycans on Integrin β1
(A) Schematic view of N-glycan analyzed, depicting dihedral angles; colored insert shows a cartoon version, where blue squares are N-acetylglucosamine, green circles are mannose, yellow circles are galactose, and red triangle is fucose.
(B) Extracellular domain of integrin β1 with N-glycan and fucosylated N-glycan. Amino acid Asp329 is highlighted; during modeling, the fucosylated N-glycan often establishes hydrogen bonds with it.
(C) Torsion dihedral angles over simulation showing more restricted N-glycan orientation with core fucosylation.
A still image of Video S1 is included in this pdf
A still image of Video S1 is included in this pdf
A still image of Video S1 is included in this pdf
Discussion
Our gain- and loss-of function experiments demonstrate that FUT8 affects migration of MSCs. Core fucosylations on N-glycans attached to integrin β1 seemed particularly relevant, because integrin β1 showed over ten times more types of N-glycans as compared with the average for MAPs. Also, silencing integrin β1 or FUT8 inhibited migration similarly, with no additive effect when silencing both genes together. Of note, in fibroblasts, regulation of integrins is associated with the different α subunits, while β1 integrin is considered to be synthesized in excess (Heino et al., 1989). We now propose that β1 integrin is primarily regulated at the post-translational level. Integrin β1 is the predominant adhesion receptor subfamily in human MSCs (Gronthos et al., 2001), where ligand binding, dimerization, or clustering (Guo et al., 2002) and interaction with tetraspanins (Goschnick et al., 2006) are all likely dependent on changes on glycosylation.
Our study in mice with bone fracture further supports a critical role of FUT8 on cell migration, but how reduced migration affects the therapeutic effect of MSCs remains unknown. Bone repair is assessed 3–8 weeks after fracture, while we determined incorporation of cells into the fracture 1 week after injection. Also, future work is necessary to determine if pre-incubation with FGF2 or direct increase of core fucosylations on integrin β1 can improve MSC homing.
Xenotransplantation of human MSCs in zebrafish is a promising tool to assess cell migration in vivo, even at the single-cell level. Future work is necessary to understand how in this model, cell migration is dictated, since it possibly involves chemotaxis, adhesion, and cell motility.
Since core fucosylations associate with various types of metastatic tumors (Geng et al., 2004, Taniguchi and Kizuka, 2015, Wang et al., 2014), it will be important to determine if our findings are confirmed in other cell types, including cancer cells.
In accordance with the mitogenic effect of FGF2 on MSCs, we found that most of the upregulated genes with FGF2 relate to cell-cycle progression and proliferation. Arguably, the wound/scratch assays with FGF2 could be biased by increased cell numbers. However, after 24 hr, MSCs treated with FGF2 are only 10% more than control cells (not shown), indicating that most of the wound closure is due to an effect on cell migration, rather than proliferation. Similarly, we observed that immediately after cell division, MSCs exert the highest motility. We therefore avoided tracking cells that had undergone recent (within 1 hr) cell division. Of note, FGF2 reduces cell adhesion to uncoated plates, a process that was not affected by either silencing or over-expressing FUT8 (not shown).
Many genes are regulated by both HMGA1 and HMGA2, but some genes are specifically regulated by only one of them (Federico et al., 2014). Since HMGA2 strongly increases E2F1 activity (Fedele et al., 2006), but we found both HMGA1 and HMGA2 increased by FGF2, it will be important to test if also silencing HMGA1 could affect FGF2 -mediated FUT8 expression. It will be also relevant to verify the FGF2/HMGA2/FUT8 pathway in other types of stem cells, because in many of them HMGA2 is an essential regulator of self-renewal.
In addition to increased core fucosylations with FGF2, we also found a decrease in high-mannose N-glycans. This is consistent with the notion that high-mannose N-glycans need to be first trimmed in the ER membrane, prior to being core-fucosylated in the late Golgi cisternae (Kornfeld and Kornfeld, 1985). The role of most N-glycan modifications in MSCs remains unknown and deserves future investigation. In addition to migration, N-glycans may affect receptor function or the interaction with other cells and substrates.
Finally, altering integrin function affects migration, but also adhesion and survival, and can even define cell fate under differentiation cues (Dalby et al., 2014). Although technically challenging, changes in N-glycans of specific proteins or even specific residues hold the potential to modulate and alter protein function. Our results suggest that FGF2 triggers transcriptional changes, which lead to post-translational modifications, hence affecting cellular migration in a highly complex, but coordinated way.
Experimental Procedures
Cell Culture
Human and mouse MSCs were isolated from bone marrow (Fierro et al., 2011). Each experiment repetition (N) was performed with MSCs derived from a different donor. All experiments with FGF2 use 24-hr incubation, 10 ng/mL FGF2.
In Vitro Migration Assays
For the wound/scratch assay, MSCs were seeded into plates with Cytoselect inserts. The next day, inserts were removed, leaving a “wound” (open area), and pictures were taken immediately and 24 hr after, unless otherwise described. For videomicroscopy, cell displacement was recorded using in a BioStation microscope.
RNA-Seq
Total RNA from four different donors treated with or without FGF2 sequencing was performed in two SE50 lanes using the Hiseq2000 platform (Illumina).
Mass Spectrometry
For N-glycan analysis, cells were processed for NanoLC/ESI-QTOF-MS, as previously described (Park et al., 2015). For LC-MS/MS analysis, enriched glycopeptides were identified using a Q Exactive Plus mass spectrometer.
Migration Assay toward Bone Fracture in Mice
Studies in mice were performed strictly following protocols approved by the Institutional Animal Care and Use Committee (IACUC) at UC Davis. Closed transverse diaphysis fractures of the right femur were generated as previously described (Zhang et al., 2017). Immediately after, mouse MSCs (transduced with either shControl or shFUT8, and pre-incubated FGF2) were injected intramuscularly (five mice per group), near the fracture site. After 7 days, mice were euthanized and samples fixed and embedded to visualize the injected cells based on tdTomato.
Modeling Analysis
The crystal structure of β1 integrin was taken from the α5β1 integrin headpiece in complex with an arginylglycylaspartic acid (RGD) peptide (PDB ID: 3VI4) (Nagae et al., 2012). After minimization and equilibration steps, four independent simulations of 70 ns were collected. To compare glycans with or without core fucose, torsion angles were collected from each simulation with a temporal resolution of 50 ps.
Statistical Analysis
Results were presented as mean ± SEM. Depending on the number of compared conditions, one-way ANOVA, two-way ANOVA, or two-tailed Student's t test were conducted using GraphPad Prism Software; p values < 0.05 were considered statistically significant.
Author Contributions
B.A., D.T., C.S., A.M., A.R., and F.A.F. performed all experiments and analyzed results, with the following exceptions: G.X., D.P., and C.B.L. did the studies related to N-glycan analysis, W.Y. did the studies in mice, L.J. and M.L.A. did the studies in zebrafish, S.K. did the RNA-seq, and A.J. did the in silico modeling. F.A.F. and C.B.L. designed the overall project and interpreted results. F.A.F. wrote the manuscript.
Acknowledgments
We would like to thank Dr. Steven Frese for support in the analysis of RNA-seq data and Lee M. Cheung for technical help on the zebrafish experiments.
Published: July 5, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, one table, and three videos and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.06.007.
Accession Numbers
RNA-seq data are available at GEO under accession number: GEO: GSE115240.
Supporting Citations
The following references appear in the Supplemental Information: Horwitz et al., 2005, Kirschner et al., 2008, Maier et al., 2015, Sasaki et al., 2013.
Supplemental Information
References
- Cummings R.D., Pierce J.M. The challenge and promise of glycomics. Chem. Biol. 2014;21:1–15. doi: 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby M.J., Gadegaard N., Oreffo R.O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014;13:558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- Fedele M., Visone R., De Martino I., Troncone G., Palmieri D., Battista S., Ciarmiello A., Pallante P., Arra C., Melillo R.M. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell. 2006;9:459–471. doi: 10.1016/j.ccr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Federico A., Forzati F., Esposito F., Arra C., Palma G., Barbieri A., Palmieri D., Fedele M., Pierantoni G.M., De Martino I. Hmga1/Hmga2 double knock-out mice display a “superpygmy” phenotype. Biol. Open. 2014;3:372–378. doi: 10.1242/bio.20146759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro F.A., Kalomoiris S., Sondergaard C.S., Nolta J.A. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011;29:1727–1737. doi: 10.1002/stem.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F., Shi B.Z., Yuan Y.F., Wu X.Z. The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: prognostic implications. Cell Res. 2004;14:423–433. doi: 10.1038/sj.cr.7290243. [DOI] [PubMed] [Google Scholar]
- Goschnick M.W., Lau L.M., Wee J.L., Liu Y.S., Hogarth P.M., Robb L.M., Hickey M.J., Wright M.D., Jackson D.E. Impaired “outside-in” integrin alphaIIbbeta3 signaling and thrombus stability in TSSC6-deficient mice. Blood. 2006;108:1911–1918. doi: 10.1182/blood-2006-02-004267. [DOI] [PubMed] [Google Scholar]
- Grayson W.L., Bunnell B.A., Martin E., Frazier T., Hung B.P., Gimble J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015;11:140–150. doi: 10.1038/nrendo.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S., Simmons P.J., Graves S.E., Robey P.G. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28:174–181. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- Gu J., Taniguchi N. Potential of N-glycan in cell adhesion and migration as either a positive or negative regulator. Cell Adh. Migr. 2008;2:243–245. doi: 10.4161/cam.2.4.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H.B., Lee I., Kamar M., Akiyama S.K., Pierce M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Res. 2002;62:6837–6845. [PubMed] [Google Scholar]
- Heino J., Ignotz R.A., Hemler M.E., Crouse C., Massague J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J. Biol. Chem. 1989;264:380–388. [PubMed] [Google Scholar]
- Hong Q., Lebrilla C.B., Miyamoto S., Ruhaak L.R. Absolute quantitation of immunoglobulin G and its glycoforms using multiple reaction monitoring. Anal. Chem. 2013;85:8585–8593. doi: 10.1021/ac4009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., Deans R.J., Krause D.S., Keating A., International Society for Cellular Theraphy Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Ito T., Sawada R., Fujiwara Y., Seyama Y., Tsuchiya T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem. Biophys. Res. Commun. 2007;359:108–114. doi: 10.1016/j.bbrc.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Kalomoiris S., Cicchetto A.C., Lakatos K., Nolta J.A., Fierro F.A. Fibroblast growth factor 2 regulates high mobility group A2 expression in human bone marrow-derived mesenchymal stem cells. J.Cell. Biochem. 2016;117:2128–2137. doi: 10.1002/jcb.25519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp J.M., Leng Teo G.S. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Kirschner K.N., Yongye A.B., Tschampel S.M., Gonzalez-Outeiriño J., Daniels C.R., Foley B.L., Woods R.J. GLYCAM06: a generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008;29:622–655. doi: 10.1002/jcc.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Maier J.A., Martinez C., Kasavajhala K., Wickstrom L., Hauser K.E., Simmerling C. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theor. Comput. 2015;11:3696–3713. doi: 10.1021/acs.jctc.5b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques I.J., Weiss F.U., Vlecken D.H., Nitsche C., Bakkers J., Lagendijk A.K., Partecke L.I., Heidecke C.D., Lerch M.M., Bagowski C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128. doi: 10.1186/1471-2407-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae M., Re S., Mihara E., Nogi T., Sugita Y., Takagi J. Crystal structure of α5β1 integrin ectodomain: atomic details of the fibronectin receptor. J. Cell Biol. 2012;197:131–140. doi: 10.1083/jcb.201111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D., Brune K.A., Mitra A., Marusina A.I., Maverakis E., Lebrilla C.B. Characteristic changes in cell surface glycosylation accompany intestinal epithelial cell (IEC) differentiation: high mannose structures dominate the cell surface glycome of undifferentiated enterocytes. Mol. Cell. Proteomics. 2015;14:2910–2921. doi: 10.1074/mcp.M115.053983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts W.J., Ploemacher R.E. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- Sackstein R., Merzaban J.S., Cain D.W., Dagia N.M., Spencer J.A., Lin C.P., Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Toda T., Furukawa T., Mawatari Y., Takaesu R., Shimizu M., Wada R., Kato D., Utsugi T., Ohtsu M. alpha-1,6-Fucosyltransferase (FUT8) inhibits hemoglobin production during differentiation of murine and K562 human erythroleukemia cells. J. Biol. Chem. 2013;288:16839–16847. doi: 10.1074/jbc.M113.459594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Ladage D., Schinkothe T., Klausmann U., Ulrichs C., Klinz F.J., Brixius K., Arnhold S., Desai B., Mehlhorn U. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750–1758. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- Solchaga L.A., Penick K., Porter J.D., Goldberg V.M., Caplan A.I., Welter J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- Taniguchi N., Kizuka Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 2015;126:11–51. doi: 10.1016/bs.acr.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Trounson A., McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S., Shimazu A., Miyazaki K., Pan H., Koike C., Yoshida E., Takagishi K., Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem. Biophys. Res. Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Wang X., Chen J., Li Q.K., Peskoe S.B., Zhang B., Choi C., Platz E.A., Zhang H. Overexpression of alpha (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 2014;24:935–944. doi: 10.1093/glycob/cwu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Wang L.X. Mammalian alpha1,6-fucosyltransferase (FUT8) is the sole enzyme responsible for the N-acetylglucosaminyltransferase I-independent core fucosylation of high-mannose N-glycans. J. Biol. Chem. 2016;291:11064–11071. doi: 10.1074/jbc.M116.720789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kot A., Lay Y.E., Fierro F.A., Chen H., Lane N.E., Yao W. Acceleration of fracture healing by overexpression of basic fibroblast growth factor in the mesenchymal stromal cells. Stem Cells Transl. Med. 2017;6:1880–1893. doi: 10.1002/sctm.17-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Itoh S., Wang X., Isaji T., Miyoshi E., Kariya Y., Miyazaki K., Kawasaki N., Taniguchi N., Gu J. Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. J. Biol. Chem. 2006;281:38343–38350. doi: 10.1074/jbc.M608764200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A still image of Video S1 is included in this pdf
A still image of Video S1 is included in this pdf
A still image of Video S1 is included in this pdf