Abstract
Platelets are released from megakaryocytes. The bone marrow has been proposed to be the major site where this process occurs. Lefrançais et al. (2017) using state-of-the-art techniques including two-photon microscopy, in vivo lineage-tracing technologies, and sophisticated lung transplants reveal that the lung is also a primary site for platelet biogenesis. Strikingly, lung megakaryocytes can completely reconstitute platelet counts in the blood in mice with thrombocytopenia. This study also shows that hematopoietic progenitors, with capacity to repopulate the bone marrow after irradiation, are present in the lungs. This work brings a novel unexpected role for the lung as a niche for hematopoiesis. The emerging knowledge from this research may be important for the treatment of several disorders.
Keywords: Lung, Hematopoietic stem cells, Origin, Niche
Platelets are tiny discoid, anucleated cell fragments that have a characteristic discoid shape, range from 1 to 3 μm in diameter, and have a life span of 8 to 12 days [1, 2]. Despite of that, platelets are indispensable for processes such as hemostasis, thrombosis, wound healing, angiogenesis, immunity, and inflammation in both health and disease [3–13]. Historically, they were first visualized under a microscope by George Gulliver in the nineteenth century [14]. However, the events that lead to mature platelet production still two centuries later are not completely understood.
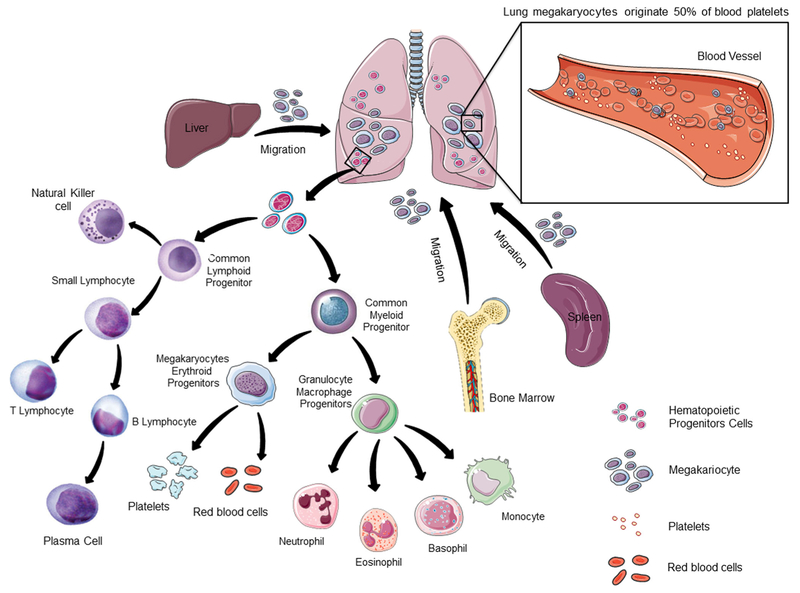
Blood platelets are formed from the cytoplasm of rare myeloid cells called megakaryocytes, which are the largest cells residing primarily within the bone marrow [15–17]. Besides the bone marrow, there has been mounting evidence suggesting that platelet release could occur in the pulmonary circulation, making the lungs a possible birthplace for platelets [18, 19]. Data supporting this includes the observation that megakaryocytes are also found in pulmonary vascular beds and a higher platelet counts in postpulmonary vessels as compared to pulmonary arteries [20, 21]. In addition, more recent study shows that lung damage reduces circulating platelets, suggesting that lungs may play an active role in the regulation of platelet formation [22]. These studies suggested the presence of a pool of newly formed platelets in the pulmonary circulation.
Understanding the mechanisms by which platelets are produced is a central question in cell biology. In a recent article in Nature, Lefrançais and colleagues demonstrated that the lung is a primary site for platelet biogenesis [23]. The authors investigated directly in real time pulmonary platelet production by intravital imaging, using two-photon microscopy and in vivo lineage-tracing technologies to track specifically megakaryocyte and platelets. These experiments revealed that the sources for blood platelets are heterogeneous. Strikingly, platelets are dynamically released from megakaryocytes located in the pulmonary circulation. Furthermore, the authors quantified the contribution of the lungs to platelet production; and it corresponded to approximately half of total platelet biogenesis [23]. Additionally, using state-of-the-art techniques including sophisticated lung transplant experiments, this study showed that lung megakaryocytes can completely reconstitute platelet counts in the blood in mice with thrombocytopenia [23].
Lefrançais and colleagues used RNA-seq to characterize lung and bone marrow megakaryocytes [23]. They described that although the total number of megakaryocytes in the lungs was comparable to that in the bone marrow, lung megakaryocytes had a shift to young immature megakaryocytes (which are more common in neonatal bone marrows) [23, 24]. Future studies should reveal which factors present in the lungs keep those megakaryocytes in the immature state.
Megakaryocytes derive from hematopoietic stem cells (HSCs) that reside mainly in the bone marrow in the adulthood [25]. During early development, before the bone marrow cavities enlarged enough to support hematopoiesis, megakaryopoiesis occurs within the yolk sac, fetal liver, and spleen [26–29]. Surprisingly, Lefrançais and colleagues identified populations of megakaryocytes progenitors and HSCs in the lungs. These progenitors can migrate out of the lungs, repopulate the bone marrow, and contribute to multiple hematopoietic cells [23].
This study brings a new possible role for the lungs as a reservoir for platelets as well as for hematopoietic progenitors.
Perspectives / Future Directions
Interestingly, platelet abnormalities were reported in patients with several pulmonary diseases, such as cystic fibrosis [30, 31], asthma [32–34], pulmonary tuberculosis [35, 36], and pulmonary hypertension [37]. Whether platelets biogenesis in the lungs of those patients is affected remains unknown. Future studies will reveal whether these platelet alterations are due to changes in the pulmonary microenvironment important for platelet biogenesis and heamatopoeisis in the lungs. Also, are other organs able to compensate platelet production from the lungs in some of those diseases?
During embryonic development, blood cell production (hematopoiesis) is not limited to one site but can be found in a range of locations which vary with the developmental age [38–40]. This occurs due to the migration of hematopoietic progenitors throughout the conceptus. This migration requires the formation of supportive microenvironments, termed hematopoietic niches [41]. Hematopoietic activity can be identified in the extraembryonic yolk sac; dorsal aorta-gonad-mesonephros (AGM region); placenta; vitelline and umbilical arteries; fetal liver; spleen; and in the skeletal muscle surrounding the developing long bones [38, 39, 42–53]. Hematopoietic progenitors from the fetal liver subsequently migrate via the circulation finally to the bone marrow preceding birth, and the bone marrow becomes the predominant location for hematopoiesis throughout the adult life [54]. The presence of hematopoietic progenitors in the lungs raises questions about their ontogeny [23]. When these progenitors appear in the lungs? Are they present during early stages of the embryonic development? From which site they come to the lungs?
Extramedullary hematopoiesis refers to the presence of hematopoietic progenitors and the development of blood cells in extramedullary (outside the medullary spaces of the bone marrow) sites. It usually reflects a pathologic state, being rare in adults under physiologic circumstances. For example, extramedullary hematopoiesis occurs in the spleen and liver in hypoxia conditions due to increased erythropoietin production, and during immune responses after infections [55]. It also may happen as a result of failed bone marrow hematopoiesis. Extramedullary hematopoiesis has been also observed in other organs, less often affected, including lymph nodes [56] and kidneys [57–59]. Excessive extramedullary hematopoiesis may lead to inflammatory diseases, while inadequate extramedullary hematopoiesis leads to insufficient production of blood cells. Lefrancais and colleagues revealed the presence of hematopoietic progenitors in the lungs under physiologic conditions [23]. Whether the numbers of progenitors would increase under some pathologic conditions will be revealed in future studies. As those, from now on, will take into consideration the lungs as an organ with capacity to host extramedullary hematopoiesis (Fig. 1).
Fig. 1.
Lung as a Primary Site for Platelet Production and Hematopoiesis. It is well accepted that bone marrow is an important site for platelet biogenesis and hematopoiesis. Extramedular hematopoiesis can occur in other organs, such as liver and spleen. The study of Lefrançais and colleagues now reveales that megakaryocytes circulate through the lungs and release platelets [115]. Platelet biogenesis in the lung represents 50% of its total production. Lefrançais and colleagues also showed that the lung is a reservoir for hematopoietic progenitors, including HSCs. With the appearance of state of art technologies, future studies will reveal in detail the components of the lung hematopoietic niche
HSCs represent a functionally heterogeneous cell population in their degree of self-renewal [60, 61]. Self-renewal heterogeneity is manifested by distinct capacities of long-term (LT-HSC), intermediate-term (IT-HSC) and short-term repopulating HSCs (ST-HSC) [62], that have been distinguished by differential abilities to engraft in vivo into irradiated hosts and to maintain multilineage hematopoiesis for extended time periods and/or by serial transplantation [63]. Lefrancais and colleagues found the presence of ST-HSCs in the lungs, but not of LT-HSCs [23]. This may be due to the cellular components of the lung microenvironment. It has been shown previously that LT-HSCs require specific cells in their niches for their maintenance in a quiescent state [64]; and whenever this niches are disrupted, LT-HSCs may leave these sites [65].
The concept of a “stem cell niche” was proposed by Schofield forty years ago, who hypothesized that HSCs in bone marrow are prevented to mature by influences from the surrounding cellular microenvironment [66]. Now it is known that the tissue microenvironment plays a central role in hematopoiesis. Nevertheless, most of what we currently know about the cellular and molecular interactions in the HSCs niches is based on studies done in the bone marrow.
In the adult bone marrow, a niche supporting HSCs was identified in close proximity to blood vessels and has been called perivascular niche [64, 67, 68]. The perivascular niche is heterogeneous and contains endothelial cells, smooth muscle cells, and pericytes. Pericytes have been anatomically defined by their perivascular location in the blood vessel wall in close contact with endothelial cells [69–82]. In addition to physical stabilization of blood vessels, pericytes are cells with high plasticity, and may contribute to the formation of several tissues [76]. Besides their ability to function as stem cells, recent studies show that pericytes can also regulate the function of other stem cells. For instance, pericytes are essential cellular components of the niche for HSCs in the bone marrow [65, 70]. A recent study revealed by imaging of the adult mouse bone marrow that the majority of dormant HSCs are situated close to arterioles; and genetic depletion of arteriolar pericytes results in migration of HSCs away from the arterioles, switching them into non-quiescent status [64]. Even more striking, this pivotal study led to the proposal that arteriolar pericytes form a special niche for quiescent HSCs, promoting their dormancy, essential for HSC maintenance in the bone marrow [64].
During embryonic development, perivascular niches for hematopoietic progenitors have been also described to be present in other organs such as spleen [67] and placenta [83]. Moreover, a recent report revealed that HSCs expand around fetal liver portal vessels, and pericytes from these vessels are essential for this expansion [54]. These studies suggest that blood vessels provide an adaptive niche, serving hematopoiesis at multiple developmental stages.
The lung is a highly complex organ comprised of more than 40 different cell types involved in both respiratory and nonrespiratory functions [84]. It remains completely unknown which of those cell types are important supporters of the pulmonary genesis of platelets and hematopoiesis.
Interestingly, HSCs are reportedly located in close proximity to megakaryocytes within the bone marrow [85–87]. Thus, some studies suggested a complex interaction between megakaryocytes and HSCs. Additionally, thrombopoietin was originally identified as a growth and differentiation factor for megakaryocytes [88, 89], but subsequently also proven to have an essential role in self-renewal of HSCs [90, 91]. Abnormal megakaryopoiesis in mice with mutations in the Myb or p300 genes causes reduction in HSC numbers in the bone marrow due to decrease in thrombopoietin levels combined with the impaired responsiveness of HSCs to thrombopoietin [92]. More recent findings identified a direct HSC regulation by megakaryocytes in steady state hematopoiesis in the bone marrow [85, 86, 93]. Ablation of megakaryocytes reduces HSC engraftment and proliferation [86, 94]. Thrombopoietin administration to megakaryocyte-depleted mice restores HSC function [86], suggesting thrombopoietin as one of the mechanisms for megakaryocytes regulation of HSCs. Megakaryocytes produce high levels of TGFβ, which regulate HSCs [95]. Conditional deletion of Tgfb1 in megakaryocytes increases HSC activation and proliferation [93]. In homeostatic conditions, megakaryocytes maintain HSC quiescence through TGFβ signaling; while under stress megakaryocytes promote HSC expansion via FGF-1 production [93]. Strikingly, megakaryocytes physically associate with approximately 20% of HSCs in the bone marrow [85]. Overall, these observations confirm that megakaryocytes serve as HSC-derived niche cells directly regulating HSC function. Nevertheless, it remains to be studied whether megakaryocytes are important cellular components of the pulmonary HSC niche.
A megakaryocyte-biased HSC subset has been identified through the use of high endogenous von Willebrand factor (vWF), a blood glycoprotein responsible for platelet aggregation, expression was reported in bone marrow cells [96, 97]. Whether HSCs present in the lungs are primed toward a specific lineage remains unknown.
Pulmonary pericytes were described more than forty years ago [98]. They cover the blood vessels of both the peribronchiolar and the alveolar regions of the lungs [99–101]. The ratio of pericytes to endothelial cells is approximately 1:10 in the lungs [102]. Whether pericytes have important role in the maintenance of HSCs in the lungs remains unknown. Thus, ongoing and future work will clarify what is their exact involvement of pericytes and other pulmonary cells in the lung HSC niche. Future clarification of the interactions between HSCs and their microenvironment in the lung will also contribute to exploit lung HSCs clinical potential.
Several cytokines are essential for HSC retention in the bone marrow, including stem cell factor (SCF), C-X-C motif chemokine 12 (CXCL 12), angiopoietin-1, transforming growth factor-β (TGFβ), and thrombopoietin [103–112]. Whether those molecules expressed by specific pulmonary cells are important for lung hematopoiesis remains to be elucidated. This may be addressed by using cell-specific inducible CreER drivers crossed to cytokines-specific floxed mice. In the resulting mice, specific citokynes could be deleted specifically in different cell types in the lungs and pulmonary hematopoiesis can be analysed. For instance, by similar methods, it was recently demonstrated that CXCL12 from arteriolar pericytes is essential for HSCs maintance in the bone marrow [65].
Over the last years, murine studies have contributed to our knowledge of the HSC regulation in their niches. The high homology in cells and molecules that form the bone marrow HSC niche in mice and humans highlight the importance of the mouse as a model to study the mechanisms of HSC maintenance. However, to identify whether some of the mechanisms discovered by Lefrancais and colleagues are unique to mouse lungs [23], additional studies in the human lungs must be performed.
The mechanisms of murine and human HSCs maintenance in the bone marrow are highly similar [113, 114]. Nevertheless, Lefrancais and colleagues did not explore human tissues [23], and nothing is known about blood cell production in the human lungs. Therefore, data about the murine hematopoietic niche in the lungs must be carefully translated into data about the human pulmonary hematopoietic niche. Do platelet biogenesis and hematopoiesis also occur in the lungs in human patients? Furthermore, similar to what has been shown in mice [23], are human lungs also contributing to 50% of blood platelets? What about the contribution to the total of neutrophils, B and T cells production? In this fast expanding field, more knowledge of the adult human pulmonary niche will be crucial for a better understanding of the mechanisms underlying several hematopoietic disorders.
Acknowledgements
Alexander Birbrair is supported by a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); Akiva Mintz is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD). We thank Rosa Maria Esteves Arantes for her useful comments.
Footnotes
Disclosures The authors indicate no potential conflicts of interest.
Compliance with Ethical Standards
References
- 1.Machlus KR, Thon JN, & Italiano JE Jr. (2014). Interpreting the developmental dance of the megakaryocyte: A review of the cellular and molecular processes mediating platelet formation. British Journal of Haematology, 165 (2), 227–236. [DOI] [PubMed] [Google Scholar]
- 2.He S, Ekman GJ, & Hedner U (2005). The effect of platelets on fibrin gel structure formed in the presence of recombinant factor VIIa in hemophilia plasma and in plasma from a patient with Glanzmann thrombasthenia. Journal of Thrombosis and Haemostasis : JTH, 3(2), 272–279. [DOI] [PubMed] [Google Scholar]
- 3.Ho-Tin-Noe B, Demers M, & Wagner DD (2011). How platelets safeguard vascular integrity. Journal of Thrombosis and Haemostasis : JTH, 9(Suppl 1), 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates ER, & Lau WC (2005). Controversies in antiplatelet therapy for patients with cardiovascular disease. Circulation, 111 (17), e267–e271. [DOI] [PubMed] [Google Scholar]
- 5.Laki K (1972). Our ancient heritage in blood clotting and some of its consequences. Annals ofthe New York Academy ofSciences, 202, 297–307. [DOI] [PubMed] [Google Scholar]
- 6.Osman A, Hitzler WE, & Provost P (2017). The platelets’ perspective to pathogen reduction technologies. Platelets, 1–8. [DOI] [PubMed] [Google Scholar]
- 7.Weyrich AS, & Zimmerman GA (2004). Platelets: Signaling cells in the immune continuum. Trends in Immunology, 25(9), 489–495. [DOI] [PubMed] [Google Scholar]
- 8.Tesfamariam B (2016). Involvement of platelets in tumor cell metastasis. Pharmacology & Therapeutics, 157, 112–119. [DOI] [PubMed] [Google Scholar]
- 9.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC, & Platelet Colloquium P (2009). Platelet functions beyond hemostasis. Journal of Thrombosis and Haemostasis : JTH, 7(11), 1759–1766. [DOI] [PubMed] [Google Scholar]
- 10.Semple JW, Italiano JE Jr., & Freedman J (2011). Platelets and the immune continuum. Nature Reviews Immunology, 11(4), 264–274. [DOI] [PubMed] [Google Scholar]
- 11.Davi G, & Patrono C (2007). Platelet activation and atherothrombosis. The New England Journal of Medicine, 357(24), 2482–2494. [DOI] [PubMed] [Google Scholar]
- 12.Engelmann B, & Massberg S (2013). Thrombosis as an intravascular effector of innate immunity. Nature Reviews Immunology, 13(1), 34–45. [DOI] [PubMed] [Google Scholar]
- 13.Danielli JF (1940). Capillary permeability and oedema in the perfused frog. The Journal of Physiology, 98(1), 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robb-Smith AH (1967). Why the platelets were discovered. British Journal of Haematology, 13(4), 618–637. [DOI] [PubMed] [Google Scholar]
- 15.Pease DC (1956). An electron microscopic study of red bone marrow. Blood, 11(6), 501–526. [PubMed] [Google Scholar]
- 16.Italiano JE Jr., Lecine P, Shivdasani RA, & Hartwig JH (1999). Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. The Journal of Cell Biology, 147(6), 1299–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakeff A, & Maat B (1974). Separation of megakaryocytes from mouse bone marrow by velocity sedimentation. Blood, 43(4), 591–595. [PubMed] [Google Scholar]
- 18.Weyrich AS, & Zimmerman GA (2013). Platelets in lung biology. Annual Review of Physiology, 75, 569–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geddis AE, & Kaushansky K (2007). Immunology. The root of platelet production. Science, 317(5845), 1689–1691. [DOI] [PubMed] [Google Scholar]
- 20.Howell WH, & Donahue DD (1937). The production ofblood platelets in the lungs. The Journal of Experimental Medicine, 65(2), 177–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallinikos-Maniatis A (1969). Megakaryocytes and platelets in central venous and arterial blood. Acta Haematologica, 42(6), 330–335. [DOI] [PubMed] [Google Scholar]
- 22.Xiao da W, Yang M, Yang J, Hon KL, & Fok FT (2006). Lung damage may induce thrombocytopenia. Platelets, 17(5), 347–349. [DOI] [PubMed] [Google Scholar]
- 23.Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, et al. (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature, 544(7648), 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sola-Visner MC, Christensen RD, Hutson AD, & Rimsza LM (2007). Megakaryocyte size and concentration in the bone marrow of thrombocytopenic and nonthrombocytopenic neonates. Pediatric Research, 61(4), 479–484. [DOI] [PubMed] [Google Scholar]
- 25.Pang L, Weiss MJ, & Poncz M (2005). Megakaryocyte biology and related disorders. The Journal of Clinical Investigation, 115(12), 3332–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long MW, Williams N, & Ebbe S (1982). Immature megakaryocytes in the mouse: Physical characteristics, cell cycle status, and in vitro responsiveness to thrombopoietic stimulatory factor. Blood, 59(3), 569–575. [PubMed] [Google Scholar]
- 27.Gordon MY, Bearpark AD, Clarke D, & Dowding CR (1990). Haemopoietic stem cell subpopulations in mouse and man: Discrimination by differential adherence and marrow repopulating ability. Bone Marrow Transplantation, 5(Suppl 1), 6–8. [PubMed] [Google Scholar]
- 28.Ogawa M (1993). Differentiation and proliferation ofhematopoietic stem cells. Blood, 81(11), 2844–2853. [PubMed] [Google Scholar]
- 29.Morita Y, Iseki A, Okamura S, Suzuki S, Nakauchi H, & Ema H (2011). Functional characterization of hematopoietic stem cells in the spleen. Experimental Hematology, 39(3), 351–359 e353. [DOI] [PubMed] [Google Scholar]
- 30.Gross S, & Luckey C (1969). The oxygen tension-platelet relationship in cystic fibrosis. The American Review ofRespiratory Disease, 100(4), 513–517. [DOI] [PubMed] [Google Scholar]
- 31.O’Sullivan BP, & Michelson AD (2006). The inflammatory role of platelets in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine, 173(5), 483–490. [DOI] [PubMed] [Google Scholar]
- 32.Kemona-Chetnik I, Bodzenta-Lukaszyk A, Butkiewicz A, & Dymnicka-Piekarska V (2007). Kemona H: [Thrombocytopoesis in allergic asthma]. Polskie Archiwum Medycyny Wewnętrznej, 117(1–2), 9–13. [PubMed] [Google Scholar]
- 33.Kornerup KN, & Page CP (2007). The role of platelets in the pathophysiology of asthma. Platelets, 18(5), 319–328. [DOI] [PubMed] [Google Scholar]
- 34.Stoll P, & Lommatzsch M (2014). Platelets in asthma: Does size matter? Respiration; International Review of Thoracic Diseases, 88(1), 22–23. [DOI] [PubMed] [Google Scholar]
- 35.Tozkoparan E, Deniz O, Ucar E, Bilgic H, & Ekiz K (2007). Changes in platelet count and indices in pulmonary tuberculosis. Clinical Chemistry and Laboratory Medicine, 45(8), 1009–1013. [DOI] [PubMed] [Google Scholar]
- 36.Gunluoglu G, Yazar EE, Veske NS, Seyhan EC, & Altin S (2014). Mean platelet volume as an inflammation marker in active pulmonary tuberculosis. Multidisciplinary Respiratory Medicine, 9(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroll MH, & Afshar-Kharghan V (2012). Platelets in pulmonary vascular physiology and pathology. Pulmonary Circulation, 2(3), 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Drees MA, Yeo JH, Boumelhem BB, Antas VI, Brigden KW, Colonne CK, & Fraser ST (2015). Making blood: The Haematopoietic niche throughout ontogeny. Stem Cells International 2015, 571893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palis J, Robertson S, Kennedy M, Wall C, & Keller G (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development, 126(22), 5073–5084. [DOI] [PubMed] [Google Scholar]
- 40.Tavian M, & Peault B (2005). Embryonic development of the human hematopoietic system. The International Journal of Developmental Biology, 49(2–3), 243–250. [DOI] [PubMed] [Google Scholar]
- 41.Bowman TV, & Zon LI (2009). Lessons from the niche for generation and expansion of hematopoietic stem cells. Drug Discovery Today Therapeutic Strategies, 6(4), 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swain A, Inoue T, Tan KS, Nakanishi Y, & Sugiyama D (2014). Intrinsic and extrinsic regulation of mammalian hematopoiesis in the fetal liver. Histology and Histopathology, 29(9), 1077–1082. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Y, Inoue-Yokoo T, Kulkeaw K, Yanagi-Mizuochi C, Shirasawa S, Nakanishi Y, & Sugiyama D (2015). Embryonic hematopoietic progenitor cells reside in muscle before bone marrow hematopoiesis. PloS One, 10(9), e0138621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medvinsky AL, Samoylina NL, Muller AM, & Dzierzak EA (1993). An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature, 364(6432), 64–67. [DOI] [PubMed] [Google Scholar]
- 45.Medvinsky A, Rybtsov S, & Taoudi S (2011). Embryonic origin of the adult hematopoietic system: Advances and questions. Development, 138 (6), 1017–1031. [DOI] [PubMed] [Google Scholar]
- 46.Baron MH (2005). Early patterning of the mouse embryo: Implications for hematopoietic commitment and differentiation. Experimental Hematology, 33(9), 1015–1020. [DOI] [PubMed] [Google Scholar]
- 47.Baron MH, Isern J, & Fraser ST (2012). The embryonic origins of erythropoiesis in mammals. Blood, 119(21), 4828–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barminko J, Reinholt B, & Baron MH (2016). Development and differentiation of the erythroid lineage in mammals. Developmental and Comparative Immunology, 58, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, & Medvinsky A (2002). Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): Role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development, 129(21), 4891–4899. [DOI] [PubMed] [Google Scholar]
- 50.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, & Dzierzak E (1994). Development of hematopoietic stem cell activity in the mouse embryo. Immunity, 1(4), 291–301. [DOI] [PubMed] [Google Scholar]
- 51.Medvinsky A, & Dzierzak E (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell, 86(6), 897–906. [DOI] [PubMed] [Google Scholar]
- 52.Sugiyama D, & Tsuji K (2006). Definitive hematopoiesis from endothelial cells in the mouse embryo; a simple guide. Trends in Cardiovascular Medicine, 16(2), 45–49. [DOI] [PubMed] [Google Scholar]
- 53.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, & Yoder MC (2008). All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood, 111(7), 3435–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, et al. (2016). Fetal liver hematopoietic stem cell niches associate with portal vessels. Science, 351(6269), 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bozzini CE, Barrio Rendo ME, Devoto FC, & Epper CE (1970). Studies on medullary and extramedullary erythropoiesis in the adult mouse. The American Journal of Physiology, 219(3), 724–728. [DOI] [PubMed] [Google Scholar]
- 56.Bowen JM, Perry AM, Quist E, & Akhtari M (2015). Extramedullary hematopoiesis in a sentinel lymph node as an early sign of chronic myelomonocytic leukemia. Case Reports in Pathology, 2015, 594970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnuelle P, Waldherr R, Lehmann KJ, Woenckhaus J, Back W, Niemir Z, & van der Woude FJ (1999). Idiopathic myelofibrosis with extramedullary hematopoiesis in the kidneys. Clinical Nephrology, 52(4), 256–262. [PubMed] [Google Scholar]
- 58.Woodward N, Ancliffe P, Griffiths MH, & Cohen S (2000). Renal myelofibrosis: An unusual cause of renal impairment. Nephrology, Dialysis, Transplantation, 15(2), 257–258. [DOI] [PubMed] [Google Scholar]
- 59.Lewis DJ, Moul JW, Williams SC, Sesterhenn IA, & Colon E (1994). Perirenal liposarcoma containing extramedullary hematopoiesis associated with renal cell carcinoma. Urology, 43(1), 106–109. [DOI] [PubMed] [Google Scholar]
- 60.Guenechea G, Gan OI, Dorrell C, & Dick JE (2001). Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nature Immunology, 2(1), 75–82. [DOI] [PubMed] [Google Scholar]
- 61.Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, & Nakauchi H (2005). Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Developmental Cell, 8(6), 907–914. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M Rudolph KL, Ema H, & Nakauchi H (2013). Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell, 154(5), 1112–1126. [DOI] [PubMed] [Google Scholar]
- 63.Purton LE, & Scadden DT (2007). Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell, 1(3), 263–270. [DOI] [PubMed] [Google Scholar]
- 64.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. (2013). Arteriolar niches maintain haematopoietic stem cell quiescence. Nature, 502(7473), 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asada N, Kunisaki Y, Pierce H, Wang Z, Fernande z N.F., Birbrair A, Ma’ayan A, Frenette PS (2017). Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nature cell biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schofield R (1978). The relationship between the spleen colonyforming cell and the haemopoietic stem cell. Blood Cells, 4(1–2), 7–25. [PubMed] [Google Scholar]
- 67.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, & Morrison SJ (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell, 121(7), 1109–1121. [DOI] [PubMed] [Google Scholar]
- 68.Sena I, Prazeres P, Santos G, Borges I, Azevedo P, Andreotti J, Almeida V, Paiva A, Guerra D, Lousado L, Souto L, Mintz A, & Birbrair A (2017). Identity of Gli1+ cells in the bone marrow. Experimental Hematology. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Birbrair A, & Delbono O (2015). Pericytes are essential for skeletal muscle formation. Stem Cell Reviews, 11(4), 547–548. [DOI] [PubMed] [Google Scholar]
- 70.Birbrair A, Frenette PS (2016). Niche heterogeneity in the bone marrow. Annals of the new York Academy of Sciences, 1370, 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, & Delbono O (2014). Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Research & Therapy, 5(6), 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, & Delbono O (2013). Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Research, 10(1), 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, & Delbono O (2013). Role ofpericytes in skeletal muscle regeneration and fat accumulation. Stem Cells and Development, 22(16), 2298–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, & Delbono O (2013). Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. American Journal of Physiology. Cell Physiology, 305(11), C1098–C1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, & Delbono O (2014). Pericytes: Multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Frontiers in Aging Neuroscience, 6, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, & Delbono O (2015). Pericytes at the intersection between tissue regeneration and pathology. Clinical Science (London, England), 128(2), 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, & Delbono O (2014). Type-2 pericytes participate in normal and tumoral angiogenesis. American Journal of Physiology. Cell Physiology, 307(1), C25–C38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro dos Santos GS, Mintz A et al. (2017) Pericytes are heterogeneous in their origin within the same tissue. Developmental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O (2017) How plastic are pericytes? Stem cells and development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, & Delbono O (2013). Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Experimental Cell Research, 319(1), 45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O, & Rota M (2011) Nestin-GFP Transgene Reveals Neural Precursor Cells in Adult Skeletal Muscle. PLoS ONE, 6(2), e16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista B, Nguyen VT et al. (2017) Novel Peripherally Derived Neural-Like Stem Cells as Therapeutic Carriers for Treating Glioblastomas. STEM CELLS Translational Medicine, 6(2), 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, & Mikkola HK (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell, 2(3), 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crapo JD, Barry BE, Gehr P, Bachofen M, & Weibel ER (1982). Cell number and cell characteristics of the normal human lung. The American Review of Respiratory Disease, 126(2), 332–337. [DOI] [PubMed] [Google Scholar]
- 85.Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, et al. (2014). Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nature Medicine, 20(11), 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamura-Ishizu A, Takubo K, Fujioka M, & Suda T (2014). Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochemical and Biophysical Research Communications, 454(2), 353–357. [DOI] [PubMed] [Google Scholar]
- 87.Heazlewood SY, Neaves RJ, Williams B, Haylock DN, Adams TE, & Nilsson SK (2013). Megakaryocytes colocalise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Research, 11(2), 782–792. [DOI] [PubMed] [Google Scholar]
- 88.Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, Forstrom JW, Buddle MM, Oort PJ, Hagen FS, et al. (1994). Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature, 369(6481), 568–571. [DOI] [PubMed] [Google Scholar]
- 89.Kimura S, Roberts AW, Metcalf D, & Alexander WS (1998). Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bersenev A, Wu C, Balcerek J, & Tong W (2008). Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. The Journal of Clinical Investigation, 118(8), 2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, et al. (2007). Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell, 1(6), 685–697. [DOI] [PubMed] [Google Scholar]
- 92.de Graaf CA, Kauppi M, Baldwin T, Hyland CD, Metcalf D, Willson TA, Carpinelli MR, Smyth GK, Alexander WS, & Hilton DJ (2010). Regulation of hematopoietic stem cells by their mature progeny. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21689–21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, & Li L (2014). Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nature Medicine, 20(11), 1321–1326. [DOI] [PubMed] [Google Scholar]
- 94.Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P, Dominici M, & Horwitz EM (2013). Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood, 121(26), 5238–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soderberg SS, Karlsson G, & Karlsson S (2009). Complex and context dependent regulation of hematopoiesis by TGF-beta superfamily signaling. Annals of the New York Academy of Sciences, 1176, 55–69. [DOI] [PubMed] [Google Scholar]
- 96.Kent DG, Copley MR, Benz C, Wohrer S, Dykstra BJ, Ma E, Cheyne J, Zhao Y, Bowie MB, Zhao Y, et al. (2009). Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood, 113(25), 6342–6350. [DOI] [PubMed] [Google Scholar]
- 97.Sanjuan-Pla A, Macaulay IC, Jensen CT, Woll PS, Luis TC, Mead A, Moore S, Carella C, Matsuoka S, Bouriez Jones T, et al. (2013). Platelet-biased stem cells reside at the apex ofthe haematopoietic stem-cell hierarchy. Nature, 502(7470), 232–236. [DOI] [PubMed] [Google Scholar]
- 98.Weibel ER (1974). On pericytes, particularly their existence on lung capillaries. Microvascular Research, 8(2), 218–235. [DOI] [PubMed] [Google Scholar]
- 99.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, & Duffield JS (2013). Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine, 188(7), 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, & Hogan BL (2011). Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proceedings of the National Academy of Sciences of the United States of America, 108(52), E1475–E1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, et al. (2014). Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation, 129(15), 1586–1597. [DOI] [PubMed] [Google Scholar]
- 102.Shepro D, & Morel NM (1993). Pericyte physiology. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 7(11), 1031–1038. [DOI] [PubMed] [Google Scholar]
- 103.Folman CC, Linthorst GE, van Mourik J, van Willigen G, de Jonge E, Levi M, de Haas M, & von dem Borne AE (2000). Platelets release thrombopoietin (Tpo) upon activation: Another regulatory loop in thrombocytopoiesis? Thrombosis and Haemostasis, 83(6), 923–930. [PubMed] [Google Scholar]
- 104.Levesque JP, Hendy J, Winkler IG, Takamatsu Y, & Simmons PJ (2003). Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Experimental Hematology, 31(2), 109–117. [DOI] [PubMed] [Google Scholar]
- 105.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. (2002). Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell, 109(5), 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, et al. (2002). G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunology, 3(7), 687–694. [DOI] [PubMed] [Google Scholar]
- 107.Valenzuela-Fernandez A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, Delaunay T, Lazarini F, Virelizier JL, Chignard M, et al. (2002). Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. The Journal of Biological Chemistry, 277(18), 15677–15689. [DOI] [PubMed] [Google Scholar]
- 108.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, & Simmons PJ (2001). Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood, 98(5), 1289–1297. [DOI] [PubMed] [Google Scholar]
- 109.Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, Pinho S, Leboeuf M, Noizat C, van Rooijen N, et al. (2013). CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature Medicine, 19(4), 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meyer A, Wang W, Qu J, Croft L, Degen JL, Coller BS, & Ahamed J (2012). Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood, 119(4), 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Labelle M, Begum S, & Hynes RO (2011). Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell, 20(5), 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, & Frenette PS (2013). PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. The Journal of Experimental Medicine, 210(7), 1351–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, & Dick JE (2011). Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science, 333(6039), 218–221. [DOI] [PubMed] [Google Scholar]
- 114.Guezguez B, Campbell CJ, Boyd AL, Karanu F, Casado FL, Di Cresce C, Collins TJ, Shapovalova Z, Xenocostas A, & Bhatia M (2013). Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell, 13(2), 175–189. [DOI] [PubMed] [Google Scholar]
- 115.Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR et al. (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature, 544(7648), 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]