Abstract
Inflammation is an important mediator of pathophysiology in bipolar disorder (BD). The omega (n)-3 and omega (n)-6 polyunsaturated fatty acid (PUFA) metabolic pathways participate in several inflammatory processes, and have been linked through epidemiological and clinical studies to BD and its response to treatment. We review the proposed role of PUFA metabolism in neuroinflammation, modulation of brain PUFA metabolism by antimanic medications in rodent models, and anti-inflammatory pharmacotherapy in BD and in major depressive disorder (MDD). Though the convergence of findings between pre-clinical and post-mortem clinical data is compelling, we investigate why human trials of PUFA as treatment are mixed. We view the biomarker and treatment study findings in light of the evidence for the hypothesis that arachidonic acid (AA) hypermetabolism contributes to BD pathophysiology, and propose that a combined high n-3 plus low n-6 diet should be tested as an adjunct to current medication in future trials.
Keywords: bipolar disorder, inflammation, arachidonic, docosahexaenoic, PUFA, n-3, diet, treatment
Bipolar disorder (BD) affects 1 – 4.4% of the population, and has an episodic, recurrent course which causes significant disability and has a complex, incompletely understood etiology.1 Effective pharmacotherapies for acute episodes and prevention of relapse include lithium salts, certain antiepileptic agents (e.g. valproic acid, carbamazepine and lamotrigine), and antipsychotic agents.2
Though mood-stabilizing medications come from several pharmaceutical classes with differing primary mechanisms of action, downregulation of brain metabolism of arachidonic acid (AA, 20:4n-6), a long chain n-6 polyunsaturated fatty acid (PUFA), has been suggested from pre-clinical studies as one common effect of mood-stabilizing medications.3–5 Linoleic acid (LA, 18:2n-6) is the dietary-essential shorter-chain n-6 PUFA precursor of AA, which is also consumed in the diet. Alterations of turnover of PUFAs in membrane lipids, including AA, and resultant alterations in cell signaling pathways in brain cell membranes have long been hypothesized to be central to perturbations of neurotransmitter systems in mood disorders.6,7
Both studies of biological concentrations of PUFAs, circulating in the plasma or incorporated into red blood cell membranes, and treatment trials using n-3 PUFAs as dietary supplements have had mixed results in BD. While heterogeneity in methods and treatment intervention are confounding factors, one additional reason may be because trials did not also involve alterations in dietary n-6 as well as n-3 PUFA intake. Pre-clinical studies suggest that neurotransmission and other brain functions depend on a balance between n-6 and n-3 PUFAs and their downstream metabolites, such as proinflammatory prostaglandins, lipoxins and thromboxanes, and antiinflammatory resolvins and neuroprotectins, respectively. We present the biological underpinnings of PUFA-related interventions in a physiological, biochemical and molecular context. To do this, we draw upon literature from pre-clinical animal work, post-mortem brain studies and parallel investigations in major depressive disorder (MDD).
We will review evidence for the proposed role of PUFA metabolism in neuroinflammation, modulation of brain PUFA metabolism by antimanic medications in rodent models, and anti-inflammatory pharmacotherapy in BD and in MDD. On the basis of the reviewed evidence, we will propose that dietary manipulation combining high n-3 PUFA with low n-6 PUFA should be tested as an adjunct to traditional mood-stabilizing medications in future clinical trials, rather than using a simple high n-3 PUFA containing diet.
Polyunsaturated fatty acids and Neuroinflammation
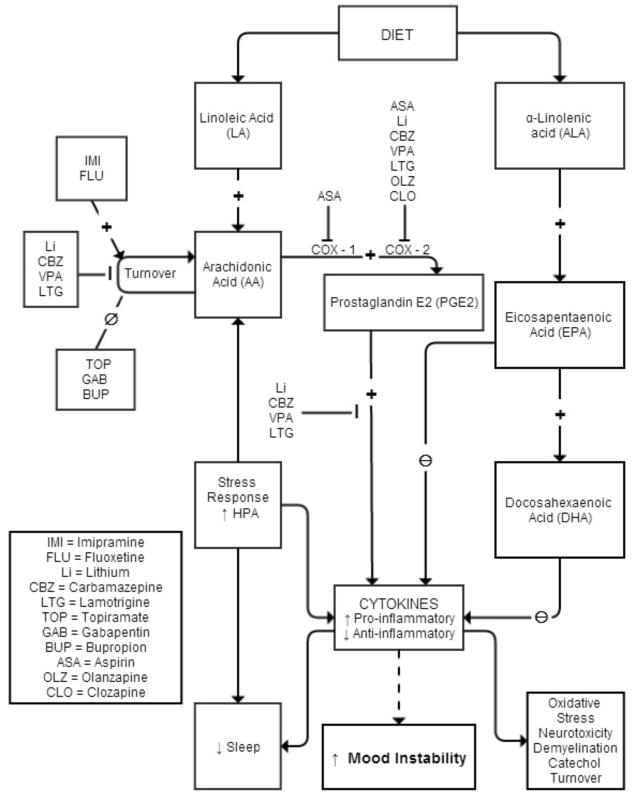
The long chain PUFAs, AA (20:4n-6) and DHA (22:6n-3), compose over 90% of PUFAs in the mammalian brain.8 AA and DHA can be either derived from the diet or be synthesized in the liver from their respective nutritionally-essential shorter-chain PUFAs, LA (18:2n-6) and ALA (18:3n-3). AA, DHA and their metabolites function as intracellular second messengers and as modulators of neuroinflammation, neurotransmission, gene transcription and other important brain process.6 Proinflammatory cytokines can stimulate release of AA from membrane phospholipids, which then is available for metabolism by cyclooxygenase (COX)-1 or COX-2 to pro-inflammatory prostaglandins (e.g. PGE2), by lipoxygenases (LOXs) to cytotoxic leukotrienes, or by cytochrome p450 epoxygenases to cytoprotective epoxyeicosatrienoic acids with the AA metabolic cascade (see Figure 1).9 PGE2 affects sleep, and may mediate pain pathways and interleukin-1 induced “sickness behavior,” which includes suppressed appetite, social withdrawal, psychomotor retardation and poor concentration, symptoms that overlap with depression.10–12 Prostaglandins also regulate the hypothalamic pituitary adrenal (HPA) axis by inducing Corticotropin Releasing Hormone (CRH) release.13,14 Acetylsalicylic acid (aspirin), a COX-1 inhibitor and COX-2 inhibitor and acetylator,15 reduces the cortisol response,16 and in a rat neuroinflammation model, chronic low-equivalent dose aspirin reduced brain levels of PGE2 and 8-isoprostane.17
Figure 1.
Impact of psychotropic medication on metabolic pathways of the polyunsaturated fatty acids.
AA is hydrolyzed from membrane phospholipids by cytosolic or secretory phospholipase A2 (PLA2). While an early genetic linkage studies of the chromosomal region coding for sPLA2 was promising,18,19 subsequent studies failed to find significant association between PLA2 genes and BD.20–23 Serum PLA2 levels were reported to be elevated in schizophrenia, BD, MDD, post-traumatic stress disorder and substance abuse.24 While a subsequent study showed no difference between BD and control in enzyme activity of PLA2, calcium-independent PLA2 was elevated in patients with BD and a history of psychosis compared to those without psychosis.25 A recent study, however, showed lower enzyme activity of 3 PLA2 species in platelet membranes of drug-naïve BD subjects compared to control.26 Increased AA metabolism in BD has been suggested by post-mortem brain studies.27 Compared to control, frontal cortex of BD patients had increased expression of some AA metabolism enzymes, including AA-selective cytosolic cPLA2 IVA, secretory sPLA2 IIA, COX-2 and membrane prostaglandin E synthase (mPGES), while expression others (COX-1 and cytosolic PGES (cPGES)) were reduced, and of others (calcium independent iPLA2 VIA, 5-, 12-, and 15-LOX, thromboxane synthase and cytochrome p450 epoxygenase) were unchanged,27 suggesting an increase in activity in the AA cascade.18,21,22 In this regard, a rat model of excitotoxicity using chronic administration of N-methyl-D-aspartate (NMDA), a glutamate receptor agonist, showed increased cPLA2 and increased AA signaling in the frontal cortex.28,29
Data from postmortem human brain and animal models are consistent with the proposition that neuroinflammatory processes could contribute to disease progression in BD. Upregulated markers of the pro-inflammatory AA cascade, which activates pathways leading to cell dysfunction and death, have been reported in post-mortem human brain.27,30,31 Excitotoxic and pro-apoptotic factors were elevated and anti-apoptotic and synaptic markers were decreased in the BD compared to control frontal cortex.30,32 Studies of peripheral PUFA markers in BD show converging findings that abnormalities in the n-6 and n-3 metabolism pathways are present in BD. 33–40
Neuroinflammation associated with excitotoxicity and apoptosis in BD may promote future episodes and disease worsening. Although BD is an episodic illness, progressive changes in cognitive function and brain structure occur and correlate with severity and chronicity of illness. Neuroinflammation and excitotoxicity, leading to neuronal apoptosis and synaptic loss, have been hypothesized to underlie progression of BD (reviewed in Berk, et al.41). Subtle cognitive changes are reported during and between episodes, and are related to number and amount of time in manic episodes.42–44 Peripheral markers of inflammation are elevated in BD.45,46 Neuroimaging studies also reported brain atrophy and gray matter deficits in emotion regulation circuits linked to length of illness.47–49 In vivo imaging studies using fMRI have shown hemodynamic changes in the right amygdala and ventromedial prefrontal cortex and left hippocampus in euthymic BD in response to an emotion task, and activation correlated with gene expression in inflammatory pathways.50 In another study, peripheral markers of inflammation in the kyneurenine pathway were correlated with hippocampal volume.51 Additionally, a study using positron emission tomography (PET) has shown elevated markers of microglial activation in BD in the hippocampus.52
Upregulation of the AA cascade in BD also could modulate signal transduction and interfere with synaptic function,53 to promote worsening of illness and cognitive changes associated with duration of illness. Changes in the balance between the n-3 and n-6 PUFAs and their bioactive lipid autacoid derivatives (Figure 1) likely influence the inflammatory response.36
Anti-manic medications alter AA metabolism
If upregulation of the AA cascade and subsequent neuroinflammation are associated with the pathophysiology and progression of BD, effective treatments for BD might act by downregulating the brain AA cascade.3–5 Supporting this proposition, chronic administration to rats of a therapeutically relevant dose of lithium reduced AA turnover in brain phospholipids, expression of important AA metabolizing enzymes, and generation of PGE2,5 while not affecting DHA and/or palmitic acid (16:0) turnover.54 Other anti-epileptic drugs with clinically-proven anti-manic efficacy (carbamazepine, valproate, lamotrigine) also downregulated the rat brain AA metabolic cascade,55–57 while topiramate or gabapentin, anti-epileptic medications that have failed phase 3 trials, did not.4,58–60 Synthesizing these data has led to the hypothesis that therapeutic downregulation of the AA cascade can be tied specifically to effective treatment of BD. Intervention could involve drugs (see above) as well and changing the PUFA content of the diet (see below).
Pharmacotherapy in BD: anti-inflammatory and n-3 PUFA agents
In addition to the proven effective mood stabilizers, other pharmaceutical agents that interfere with brain AA metabolism would be of interest to investigate clinically in BD. They might include acetylsalicylic acid (aspirin) and other non-steroidal anti-inflammatory drugs (NSAIDs) (e.g., non-selective COX inhibitors), selective COX-2 or COX-1 inhibitors. Stolk, et al. retrospectively used a Netherlands database to investigate effects of some of these agents in subjects on lithium. Long-term low-dose aspirin was associated with reduced risk for worse outcomes, while short-term use of a non-selective NSAID, or of more than one inhibitor, increased risk.61 Nery, et al. found that celecoxib (Celebrex®), a selective COX-2 inhibitor, for treatment of depression or mixed episode in BD, reduced severity of depression at one week, but did not have a sustained effect in a 6 week double-blind randomized, controlled trial as an adjunct to usual treatment.62 The effect of anti-inflammatory treatments on mood outcome in BD needs further consideration.
Studies in rodents report differing actions of antidepressants compared with mood stabilizer medication on the brain AA and cytokine-based inflammatory systems. In unanesthetized rats, chronic imipramine and fluoxetine (a selective serotonin reuptake inhibitor, SSRI), antidepressants that can increase risk for switching from depression to mania in BD patients,63 upregulated brain AA turnover and metabolism (opposite to direction of changes with mood stabilizers), but bupropion, an antidepressant causing lower switch rates, did not. 64,65 These comparisons suggest that increased brain AA metabolism may be associated with the manic phase of BD, and that stimulating AA metabolism may be associated with a switch of depression to mania with certain antidepressants. Indeed, co-administration of lithium, which depresses AA metabolism in rats, is recommended when fluoxetine is administered;66 it might dampen the untoward AA upregulation of the SSRI. In mice, SSRIs increased inflammatory markers tumor necrosis factor (TNF)-alpha, interferon (IFN)-gamma and p11 in the frontal cortex.67 Because of the complex interactions between AA metabolism and inflammation, the clinical implications of these findings remain to be elucidated. Interestingly, a study of n-3 to prevent IFN-alpha-induced depression in 162 patients treated for hepatitis C showed lower rates of IFN-alpha induced depression in EPA but not DHA treated patients, and both n-3 treatments delayed the onset of depression.68
Studies of treatment of BD with supplementation of n-3 preparations, either EPA or DHA or both, have been mixed, and several recent reviews have discussed these studies in detail.33,69 Briefly, open-label and non-randomized trials have been largely positive,70–74 while in 5/7 individual randomized clinical trials (RCTs), the intervention group did not separate from placebo for treatment of depression or mania.75–81 A meta-analysis of RCTs in BD showed a signal for treatment of BD depression, but not mania.82 Interpretation of the responses seen in RCTs is confounded by factors including differing design of trials, compliance to study drug, composition and dose of supplements, and potential publication bias.
The way forward: Lessons from Migraine
We have described several ways in which altered brain AA metabolism may be important in the pathophysiology and pharmacological or dietary treatment of BD, and have highlighted the mixed results of n-3 PUFA supplementation trials. If the brain PUFA metabolism system is important in BD, what might account for the lack of consistent effects of dietary n-3 PUFA supplementation? One possibility is that n-3 PUFA supplementation without concurrent dietary reduction of n-6 PUFAs may not produce therapeutically-relevant alterations in the interactive brain n-6 and n-3 PUFA pathways.83,84 In this regard, in the past 100 years, there has been an increase in consumption of the n-6 PUFA precursor LA in the average US diet – the predictable effect of this is increasing tissue concentrations of LA, and decreasing tissue concentrations of n-3 EPA and DHA, and thus an imbalance of n-6 over n-3 PUFAs and their metabolites.85 Simple addition of an n-3 PUFA supplement without concurrent reduction in dietary n-6 LA may not alter brain PUFA metabolism to the extent required to produce clinically meaningful benefit. To gain some insight into this issue and its relevance to BD, we cite a recent dietary intervention trial in migraine headache.
Migraine headache has a clinical comorbidity with BD of around 30% for both genders when studied together,86–92 and in a recent study that investigated comorbidity by gender, 39% of women and 16% of men had migraine headache.93 The pain associated with migraine headache has been hypothesized to be caused by PGE2,94 and thus related to increased AA metabolism,95 as well as increased neuroinflammation in general.96–98 Both BD and migraine headache respond to several of the same medications, including valproic acid99,100 and lamotrigine,101 indicating a potential shared pathology.
A recent 12-week randomized clinical trial by Ramsden et al. compared clinical efficacy and biochemical effects of a high n-3 EPA + DHA plus low n-6 LA (H3-L6) diet to effects of only a low n-6 PUFA diet (L6), in 67 patients with chronic headache. Both the H3-L6 and L6 groups experienced statistically significant clinical improvement compared to the pre-intervention run-in phase, but the H3-L6 group experienced a significantly greater reduction in headache hours per day, headache days per month, headache-related quality-of-life and psychological distress.102 Clinical improvements in the H3-L6 group were accompanied by reductions in erythrocyte LA and AA, as well as bioactive oxidized LA and AA metabolites that have been linked to pain.103 The H3-L6 intervention also increased n-3 EPA, DHA and the n-3 index, as well as pathway precursors for biosynthesis of anti-inflammatory and pro-resolving EPA and DHA metabolites.95 Thus, the Ramsden et al. trial suggests that lowering dietary n-6 LA may be a key component to efficacy of n-3 PUFA supplementation in migraine treatment. Based on the clinical and neuroinflammatory links between BD and migraine, concurrent dietary n-6 lowering in BD may also be necessary for effective treatment of BD with n-3 PUFA supplementation.104
Summary & Future Directions
An extensive body of human post-mortem and animal studies implicates excessive brain AA metabolism and inadequate DHA metabolism in BD pathogenesis and progression. However, the specific molecular mechanisms linking dysfunctional AA and DHA metabolism to BD are incompletely understood. Future studies should be directed toward identifying specific signaling pathways and lipid mediators linking AA and DHA to BD pathophysiology. This line of inquiry could lead to development of novel, targeted strategies for affecting PUFA metabolism through modulation of dietary AA and DHA intake that can be tested for improvement of mood stabilization in randomized, controlled trials.
Clinical Points.
Omega-3 and omega-6 fatty acids are important for brain function and are part of inflammatory processes.
Alteration of fatty acid intake with diet may be an additional way to investigate clinical benefits of changes in the omega-3 and omega-6 pathways.
Acknowledgments
The project described was supported by the National Center for Research Resources, GrantKL2 RR033180 (EFHS), and is now at the National Center for Advancing Translational Sciences, Grant KL2 TR000126, National Institute on Aging, National Institutes of Health, Bethesda, Maryland. The contribution of S. I. Rapoport was supported entirely by the Intramural program of the National Institute on Aging, National Institutes of Health, Bethesda, Maryland. The contribution of C.E. Ramsden was supported by the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The sponsors of this research did not have direct influence over the collection, analysis or interpretation of data.
Acknowledgment of assistance: The authors would like to acknowledge Aubrey Reider, BA, for technical assistance with the figure. AR affiliation: 1Department of Psychiatry, Penn State College of Medicine and Penn State Milton S. Hershey Medical Center, Hershey, PA 17033; AR has no conflict of interest.
Footnotes
These data have not been presented elsewhere.
Financial Disclosures: EFHS has been a consultant for Projects In Knowledge, CME; CER, MSS, JD, SIR – none; AG – Pfizer Pharmaceutical (investigator-initiated grant through Penn State), Zynx (consultantship), Allergan (consultantship), Forest (consultantship), ZARS Pharma (consultantship), Healthcare Technology Systems, Inc. (major stock ownership).
References
- 1.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 2.Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar disorders. 2009;11(6):559–595. doi: 10.1111/j.1399-5618.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport SI. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS chemical neuroscience. 2014;5(6):459–467. doi: 10.1021/cn500058v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61(2):185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry. 2002;59(7):592–596. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- 6.Hibbeln JR, Palmer JW, Davis JM. Are disturbances in lipid-protein interactions by phospholipase-A2 a predisposing factor in affective illness? Biol Psychiatry. 1989;25(7):945–961. doi: 10.1016/0006-3223(89)90274-6. [DOI] [PubMed] [Google Scholar]
- 7.Allison JH, Stewart MA. Reduced brain inositol in lithium-treated rats. Nature: New biology. 1971;233(43):267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- 8.Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. Journal of lipid research. 1968;9(5):570–579. [PubMed] [Google Scholar]
- 9.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 10.Hickie I, Lloyd A. Are cytokines associated with neuropsychiatric syndromes in humans? International journal of immunopharmacology. 1995;17(8):677–683. doi: 10.1016/0192-0561(95)00054-6. [DOI] [PubMed] [Google Scholar]
- 11.Dantzer R, Bluthe RM, Gheusi G, et al. Molecular basis of sickness behavior. Annals of the New York Academy of Sciences. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. Journal Article. [DOI] [PubMed] [Google Scholar]
- 12.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsadaniantz SM, Lebeau A, Duval P, Grimaldi B, Terlain B, Kerdelhue B. Effects of the inhibition of cyclo-oxygenase 1 or 2 or 5-lipoxygenase on the activation of the hypothalamic-pituitary-adrenal axis induced by interleukin-1beta in the male Rat. Journal of neuroendocrinology. 2000;12(8):766–773. doi: 10.1046/j.1365-2826.2000.00517.x. [DOI] [PubMed] [Google Scholar]
- 14.Navarra P, Tsagarakis S, Faria MS, Rees LH, Besser GM, Grossman AB. Interleukins-1 and -6 stimulate the release of corticotropin-releasing hormone-41 from rat hypothalamus in vitro via the eicosanoid cyclooxygenase pathway. Endocrinology. 1991;128(1):37–44. doi: 10.1210/endo-128-1-37. [DOI] [PubMed] [Google Scholar]
- 15.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39(11):1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 16.Nye EJ, Hockings GI, Grice JE, et al. Aspirin inhibits vasopressin-induced hypothalamic-pituitary-adrenal activity in normal humans. The Journal of clinical endocrinology and metabolism. 1997;82(3):812–817. doi: 10.1210/jcem.82.3.3820. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard HC, Taha AY, Rapoport SI, Yuan ZX. Low-dose aspirin (acetylsalicylate) prevents increases in brain PGE2, 15-epi-lipoxin A4 and 8-isoprostane concentrations in 9 month-old HIV-1 transgenic rats, a model for HIV-1 associated neurocognitive disorders. PLEFA. doi: 10.1016/j.plefa.2015.01.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson E, Parfitt E, Roberts Q, et al. Linkage studies of bipolar disorder in the region of the Darier’s disease gene on chromosome 12q23–24.1. Am J Med Genet. 1995;60(2):94–102. doi: 10.1002/ajmg.1320600203. [DOI] [PubMed] [Google Scholar]
- 19.Dawson E, Gill M, Curtis D, et al. Genetic association between alleles of pancreatic phospholipase A2 gene and bipolar affective disorder. Psychiatr Genet. 1995;5(4):177–180. doi: 10.1097/00041444-199524000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen N, Daniels J, Moorhead S, et al. Association study of bipolar disorder at the phospholipase A2 gene (PLA2A) in the Darier’s disease (DAR) region of chromosome 12q23-q24.1. Psychiatr Genet. 1996;6(4):195–199. doi: 10.1097/00041444-199624000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen NJ, Franks EK, Owen MJ, Craddock NJ. Mutational analysis of phospholipase A2A: a positional candidate susceptibility gene for bipolar disorder. Mol Psychiatry. 1999;4(3):274–279. doi: 10.1038/sj.mp.4000476. [DOI] [PubMed] [Google Scholar]
- 22.Meira-Lima I, Jardim D, Junqueira R, Ikenaga E, Vallada H. Allelic association study between phospholipase A2 genes and bipolar affective disorder. Bipolar Disord. 2003;5(4):295–299. doi: 10.1034/j.1399-5618.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 23.Dikeos DG, Papadimitriou GN, Souery D, et al. Lack of genetic association between the phospholipase A2 gene and bipolar mood disorder in a European multicentre case-control study. Psychiatr Genet. 2006;16(4):169–171. doi: 10.1097/01.ypg.0000218615.19892.86. [DOI] [PubMed] [Google Scholar]
- 24.Noponen M, Sanfilipo M, Samanich K, et al. Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol Psychiatry. 1993;34(9):641–649. doi: 10.1016/0006-3223(93)90157-9. [DOI] [PubMed] [Google Scholar]
- 25.Ross BM, Hughes B, Kish SJ, Warsh JJ. Serum calcium-independent phospholipase A2 activity in bipolar affective disorder. Bipolar Disord. 2006;8(3):265–270. doi: 10.1111/j.1399-5618.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 26.Ikenaga EH, Talib LL, Ferreira AS, Machado-Vieira R, Forlenza OV, Gattaz WF. Reduced activities of phospholipases A in platelets of drug-naive bipolar disorder patients. Bipolar Disord. 2014 doi: 10.1111/bdi.12229. [DOI] [PubMed] [Google Scholar]
- 27.Kim HW, Rapoport SI, Rao JS. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry. 2011;16(4):419–428. doi: 10.1038/mp.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J Lipid Res. 2008;49(1):162–168. doi: 10.1194/jlr.M700406-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Rao JS, Ertley RN, Rapoport SI, Bazinet RP, Lee HJ. Chronic NMDA administration to rats up-regulates frontal cortex cytosolic phospholipase A2 and its transcription factor, activator protein-2. J Neurochem. 2007;102(6):1918–1927. doi: 10.1111/j.1471-4159.2007.04648.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37(3):596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brock TG. Arachidonic acid binds 14-3-3zeta, releases 14-3-3zeta from phosphorylated BAD and induces aggregation of 14-3-3zeta. Neurochem Res. 2008;33(5):801–807. doi: 10.1007/s11064-007-9498-3. [DOI] [PubMed] [Google Scholar]
- 32.Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15(4):384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders EFH, Ramsden CE, Sherazy MS, Gelenberg AJ, Davis JM, Rapoport SI. Omega-3 and Omega-6 Polyunsaturated Fatty Acids in Bipolar Disorder: A review of Biomarker and Treatment Studies. The Journal of clinical psychiatry. 2016 doi: 10.4088/JCP.15r09925. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunders EF, Reider A, Singh G, Gelenberg AJ, Rapoport SI. Low unesterified:esterified eicosapentaenoic acid (EPA) plasma concentration ratio is associated with bipolar disorder episodes, and omega-3 plasma concentrations are altered by treatment. Bipolar Disord. 2015;17(7):729–742. doi: 10.1111/bdi.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu CC, Huang SY, Su KP, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13(2):99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 36.Sublette ME, Bosetti F, DeMar JC, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9(7):759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43(11):1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- 38.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126(1–2):303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans SJ, Kamali M, Prossin AR, et al. Association of plasma omega-3 and omega-6 lipids with burden of disease measures in bipolar subjects. J Psychiatr Res. 2012;46(11):1435–1441. doi: 10.1016/j.jpsychires.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pomponi M, Janiri L, La Torre G, et al. Plasma levels of n-3 fatty acids in bipolar patients: deficit restricted to DHA. J Psychiatr Res. 2013;47(3):337–342. doi: 10.1016/j.jpsychires.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience and biobehavioral reviews. 2011;35(3):804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Vederman AC, Weisenbach SL, Rapport LJ, et al. Modality-specific alterations in the perception of emotional stimuli in Bipolar Disorder compared to Healthy Controls and Major Depressive Disorder. Cortex. 2012;48(8):1027–1034. doi: 10.1016/j.cortex.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan KA, Vederman AC, McFadden EM, et al. Differential executive functioning performance by phase of bipolar disorder. Bipolar Disord. 2012;14(5):527–536. doi: 10.1111/j.1399-5618.2012.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langenecker SA, Saunders EF, Kade AM, Ransom MT, McInnis MG. Intermediate: cognitive phenotypes in bipolar disorder. J Affect Disord. 2010;122(3):285–293. doi: 10.1016/j.jad.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haarman BC, Riemersma-Van der Lek RF, Burger H, et al. Relationship between clinical features and inflammation-related monocyte gene expression in bipolar disorder - towards a better understanding of psychoimmunological interactions. Bipolar Disord. 2014;16(2):137–150. doi: 10.1111/bdi.12142. [DOI] [PubMed] [Google Scholar]
- 46.Becking K, Boschloo L, Vogelzangs N, et al. The association between immune activation and manic symptoms in patients with a depressive disorder. Translational psychiatry. 2013;3:e314. doi: 10.1038/tp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bora E, Yucel M, Pantelis C, Berk M. Meta-analytic review of neurocognition in bipolar II disorder. Acta Psychiatrica Scandinavica. 2011;123(3):165–174. doi: 10.1111/j.1600-0447.2010.01638.x. [DOI] [PubMed] [Google Scholar]
- 48.DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology. 1999;21(1):63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 49.Lyoo IK, Sung YH, Dager SR, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar disorders. 2006;8(1):65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 50.Savitz J, Frank MB, Victor T, et al. Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain Behav Immun. 2013;31:161–171. doi: 10.1016/j.bbi.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savitz J, Dantzer R, Wurfel BE, et al. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology. 2015;52:200–211. doi: 10.1016/j.psyneuen.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haarman BC, Burger H, Doorduin J, et al. Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder - A combined magnetic resonance imaging and positron emission tomography study. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Garrido R, Springer JE, Hennig B, Toborek M. Apoptosis of spinal cord neurons by preventing depletion nicotine attenuates arachidonic acid-induced of neurotrophic factors. Journal of neurotrauma. 2003;20(11):1201–1213. doi: 10.1089/089771503322584628. [DOI] [PubMed] [Google Scholar]
- 54.Chang MC, Bell JM, Purdon AD, Chikhale EG, Grange E. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochemical research. 1999;24(3):399–406. doi: 10.1023/a:1020989701330. [DOI] [PubMed] [Google Scholar]
- 55.Bazinet RP, Weis MT, Rapoport SI, Rosenberger TA. Valproic acid selectively inhibits conversion of arachidonic acid to arachidonoyl-CoA by brain microsomal long-chain fatty acyl-CoA synthetases: relevance to bipolar disorder. Psychopharmacology. 2006;184(1):122–129. doi: 10.1007/s00213-005-0272-4. [DOI] [PubMed] [Google Scholar]
- 56.Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry. 2006;59(5):401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Lee HJ, Ertley RN, Rapoport SI, Bazinet RP, Rao JS. Chronic administration of lamotrigine downregulates COX-2 mRNA and protein in rat frontal cortex. Neurochem Res. 2008;33(5):861–866. doi: 10.1007/s11064-007-9526-3. [DOI] [PubMed] [Google Scholar]
- 58.Lee HJ, Ghelardoni S, Chang L, Bosetti F, Rapoport SI, Bazinet RP. Topiramate does not alter the kinetics of arachidonic or docosahexaenoic acid in brain phospholipids of the unanesthetized rat. Neurochem Res. 2005;30(5):677–683. doi: 10.1007/s11064-005-2756-3. [DOI] [PubMed] [Google Scholar]
- 59.Ghelardoni S, Bazinet RP, Rapoport SI, Bosetti F. Topiramate does not alter expression in rat brain of enzymes of arachidonic acid metabolism. Psychopharmacology. 2005;180(3):523–529. doi: 10.1007/s00213-005-2189-3. [DOI] [PubMed] [Google Scholar]
- 60.Reese EA, Cheon Y, Ramadan E, et al. Gabapentin’s minimal action on markers of rat brain arachidonic acid metabolism agrees with its inefficacy against bipolar disorder. Prostaglandins Leukot Essent Fatty Acids. 2012;87(2–3):71–77. doi: 10.1016/j.plefa.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stolk P, Souverein PC, Wilting I, et al. Is aspirin useful in patients on lithium? A pharmacoepidemiological study related to bipolar disorder. Prostaglandins Leukot Essent Fatty Acids. 2010;82(1):9–14. doi: 10.1016/j.plefa.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nery FG, Monkul ES, Hatch JP, et al. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psychopharmacol. 2008;23(2):87–94. doi: 10.1002/hup.912. [DOI] [PubMed] [Google Scholar]
- 63.Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249–1262. doi: 10.1176/appi.ajp.2013.13020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee HJ, Rao JS, Chang L, Rapoport SI, Kim HW. Chronic imipramine but not bupropion increases arachidonic acid signaling in rat brain: is this related to ‘switching’ in bipolar disorder? Mol Psychiatry. 2010;15(6):602–614. doi: 10.1038/mp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao JS, Ertley RN, Lee HJ, Rapoport SI, Bazinet RP. Chronic fluoxetine upregulates activity, protein and mRNA levels of cytosolic phospholipase A2 in rat frontal cortex. The pharmacogenomics journal. 2006;6(6):413–420. doi: 10.1038/sj.tpj.6500391. [DOI] [PubMed] [Google Scholar]
- 66.Edwards SJ, Hamilton V, Nherera L, Trevor N. Lithium or an atypical antipsychotic drug in the management of treatment-resistant depression: a systematic review and economic evaluation. Health Technol Assess. 2013;17(54):1–190. doi: 10.3310/hta17540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(22):9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su KP, Lai HC, Yang HT, et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry. 2014;76(7):559–566. doi: 10.1016/j.biopsych.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. The Journal of clinical psychiatry. 2011 doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 70.Hirashima F, Parow AM, Stoll AL, et al. Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am J Psychiatry. 2004;161(10):1922–1924. doi: 10.1176/ajp.161.10.1922. [DOI] [PubMed] [Google Scholar]
- 71.Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentaenoic acid in bipolar depression: report of a small open-label study. J Clin Psychiatry. 2005;66(6):726–729. doi: 10.4088/jcp.v66n0608. [DOI] [PubMed] [Google Scholar]
- 72.Sagduyu K, Dokucu ME, Eddy BA, Craigen G, Baldassano CF, Yildiz A. Omega-3 fatty acids decreased irritability of patients with bipolar disorder in an add-on, open label study. Nutrition journal. 2005;4:6. doi: 10.1186/1475-2891-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wozniak J, Biederman J, Mick E, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17(6–7):440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63(8):1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 75.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 76.Chiu CC, Huang SY, Chen CC, Su KP. Omega-3 fatty acids are more beneficial in the depressive phase than in the manic phase in patients with bipolar I disorder. J Clin Psychiatry. 2005;66(12):1613–1614. doi: 10.4088/jcp.v66n1219b. [DOI] [PubMed] [Google Scholar]
- 77.Keck PE, Jr, Mintz J, McElroy SL, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60(9):1020–1022. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 78.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 79.Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21(4):435–439. doi: 10.1177/0269881106067787. [DOI] [PubMed] [Google Scholar]
- 80.Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord. 2010;12(2):142–154. doi: 10.1111/j.1399-5618.2010.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy BL, Stoll AL, Harris PQ, et al. Omega-3 fatty acid treatment, with or without cytidine, fails to show therapeutic properties in bipolar disorder: a double-blind, randomized add-on clinical trial. J Clin Psychopharmacol. 2012;32(5):699–703. doi: 10.1097/JCP.0b013e318266854c. [DOI] [PubMed] [Google Scholar]
- 82.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81–86. doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 83.Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins, leukotrienes, and essential fatty acids. 2008;79(3–5):153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan VH, Primiani CT, Rao JS, Ahn K, Rapoport SI, Blanchard H. Coordination of gene expression of arachidonic and docosahexaenoic acid cascade enzymes during human brain development and aging. PloS One. 2014;9(6):e100858. doi: 10.1371/journal.pone.0100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baptista T, Uzcategui E, Arape Y, et al. Migraine life-time prevalence in mental disorders: concurrent comparisons with first-degree relatives and the general population. Investigacion clinica. 2012;53(1):38–51. [PubMed] [Google Scholar]
- 87.Ortiz A, Cervantes P, Zlotnik G, et al. Cross-prevalence of migraine and bipolar disorder. Bipolar Disord. 2010;12(4):397–403. doi: 10.1111/j.1399-5618.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 88.Dilsaver SC, Benazzi F, Oedegaard KJ, Fasmer OB, Akiskal KK, Akiskal HS. Migraine headache in affectively ill latino adults of mexican american origin is associated with bipolarity. Prim Care Companion J Clin Psychiatry. 2009;11(6):302–306. doi: 10.4088/PCC.08m00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McIntyre RS, Konarski JZ, Soczynska JK, et al. Medical comorbidity in bipolar disorder: implications for functional outcomes and health service utilization. Psychiatr Serv. 2006;57(8):1140–1144. doi: 10.1176/ps.2006.57.8.1140. [DOI] [PubMed] [Google Scholar]
- 90.McIntyre RS, Konarski JZ, Wilkins K, Bouffard B, Soczynska JK, Kennedy SH. The prevalence and impact of migraine headache in bipolar disorder: results from the Canadian Community Health Survey. Headache. 2006;46(6):973–982. doi: 10.1111/j.1526-4610.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 91.Fasmer OB. The prevalence of migraine in patients with bipolar and unipolar affective disorders. Cephalalgia. 2001;21(9):894–899. doi: 10.1046/j.1468-2982.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 92.Fasmer OB, Oedegaard KJ. Clinical characteristics of patients with major affective disorders and comorbid migraine. World J Biol Psychiatry. 2001;2(3):149–155. doi: 10.3109/15622970109026801. [DOI] [PubMed] [Google Scholar]
- 93.Saunders EF, Nazir R, Kamali M, et al. Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with comorbid migraine. J Clin Psychiatry. 2014;75(5):512–519. doi: 10.4088/JCP.13m08623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia. 2012;32(11):822–833. doi: 10.1177/0333102412451360. [DOI] [PubMed] [Google Scholar]
- 95.Ramsden CE, Mann JD, Faurot KR, et al. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials. 2011;12:97. doi: 10.1186/1745-6215-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moskowitz MA, Buzzi MG. Migraine general aspects. Handbook of clinical neurology. 2010;97:253–266. doi: 10.1016/S0072-9752(10)97021-8. [DOI] [PubMed] [Google Scholar]
- 97.Buzzi MG, Moskowitz MA. The pathophysiology of migraine: year 2005. The journal of headache and pain. 2005;6(3):105–111. doi: 10.1007/s10194-005-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buzzi MG, Moskowitz MA. The trigemino-vascular system and migraine. Pathologie-biologie. 1992;40(4):313–317. [PubMed] [Google Scholar]
- 99.investigators B. Geddes JR, Goodwin GM, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385–395. doi: 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- 100.Linde M, Mulleners WM, Chronicle EP, McCrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;6:CD010611. doi: 10.1002/14651858.CD010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lampl C, Katsarava Z, Diener HC, Limmroth V. Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura. J Neurol Neurosurg Psychiatry. 2005;76(12):1730–1732. doi: 10.1136/jnnp.2005.063750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramsden CE, Faurot KR, Zamora D, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013;154(11):2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramsden CE, Ringel A, Feldstein AE, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87(4–5):135–141. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.MacIntosh BA, Ramsden CE, Faurot KR, et al. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. The British journal of nutrition. 2013;110(3):559–568. doi: 10.1017/S0007114512005181. [DOI] [PMC free article] [PubMed] [Google Scholar]