Abstract
Background and aims:
Creatine is a supplement used by sportsmen to increase athletic performance by improving energy supply to muscle tissues. It is also an essential brain compound and some hypothesize that it aids cognition by improving energy supply and neuroprotection. The aim of this systematic review is to investigate the effects of oral creatine administration on cognitive function in healthy individuals.
Methods:
A search of multiple electronic databases was performed for the identification of randomized clinical trials (RCTs) examining the cognitive effects of oral creatine supplementation in healthy individuals.
Results:
Six studies (281 individuals) met our inclusion criteria. Generally, there was evidence that short term memory and intelligence/reasoning may be improved by creatine administration. Regarding other cognitive domains, such as long-term memory, spatial memory, memory scanning, attention, executive function, response inhibition, word fluency, reaction time and mental fatigue, the results were conflicting. Performance on cognitive tasks stayed unchanged in young individuals. Vegetarians responded better than meat-eaters in memory tasks but for other cognitive domains no differences were observed.
Conclusions:
Oral creatine administration may improve short-term memory and intelligence/reasoning of healthy individuals but its effect on other cognitive domains remains unclear. Findings suggest potential benefit for aging and stressed individuals. Since creatine is safe, future studies should include larger sample sizes. It is imperative that creatine should be tested on patients with dementias or cognitive impairment.
Keywords: Creatine, Memory, Cognitive function, Healthy individuals
1. Introduction
Creatine is a naturally occurring compound that is synthesized from the amino acids arginine, glycine and methionine through a two-step reaction (Andres et al., 2008). An amidino group is transferred from arginine to glycine by L-arginine-glycine amidino transferase (AGAT) leading to guanidinoacetate formation that is subsequently methylated to yield creatine by guanidinoacetate-methyltransferase (GAMT) (Wyss and Kaddurah-Daouk, 2000). A creatine transporter, SLC6A8, distributes creatine from the circulation to tissues (Wyss and Kaddurah-Daouk, 2000). Although this transporter can be utilized to import creatine in the central nervous system from the periphery through the blood brain barrier, neurons can endogenously synthesize creatine (Braissant et al., 2002). Creatine is reversibly converted to phosphocreatine by creatine kinase. The breakdown product of the creatine/phosphocreatine system, creatinine, is non-enzymatically produced at a constant rate and is excreted by the kidneys. Plasma levels of creatine that are normally about 50 mmol/L, steeply increase after creatine supplement ingestion (Deldicque et al., 2008).
Creatine’s main function is to immediately supply energy to tissues with increased energy demands, such as muscle and brain (Persky and Brazeau, 2001). This can be achieved by phosphocreatine’s high energy phosphate bonds that are available for immediate ATP replenishment in energy demanding circumstances (Persky and Brazeau, 2001). Based on these properties, it has been widely used to improve performance during anaerobic exercise (Rawson and Volek, 2003). Apart from muscle cells, neurons carry out many processes that require energy. It has been shown that exogenously administrated creatine increases energy supply to neurons in healthy adults (Persky and Brazeau, 2001; Rawson and Volek, 2003; Klivenyi et al., 1999). Supplementation of creatine can increase its brain’s levels up to a threshold, beyond which the excess is being excreted (Joncquel-Chevalier Curt et al., 2015).
An association of creatine function with cognitive performance is suggested by genetic creatine disorders (AGAT, GAMT or SLC6A8 deficiency) characterized by mental dysfunction (global developmental delay, intellectual disability) (Joncquel-Chevalier Curt et al., 2015). These effects were largely reversed following oral creatine supplementation in AGAT, GAMT but not in SLC6A8 deficiency (Braissant et al., 2011). Creatine is also thought to participate in neuronal plasticity (Jost et al., 2002). Moreover, mental training has been shown to elevate brain creatine levels, hinting to an upregulation of resting energy storage (Valenzuela et al., 2003). Higher resting creatine levels have been proven to enhance performance in cognitive tasks such as recognition memory (Ferrier et al., 2000). These data suggest putative cognitive benefits of creatine supplementation. In the present systematic review, we aimed to elucidate the effects of creatine supplementation on the cognitive performance of healthy individuals.
2. Methods
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were adopted for the conduction of the present study (Moher et al., 2009). The adherence of this established tool is associated with more complete reporting of systematic reviews (PRISMA [Internet], n.d.).
2.1. Information sources
“PubMed”, “Science Direct” and Cochrane Central Register of Controlled trials were used for the identification of studies relevant to our review. The search on the electronic databases was done up to 10/17/2017.
2.2. Eligibiity criteria
We included studies with the following characteristics: (a) double blind RCTs or double blind cross-over RCTs, (b) published in English up to 10/17/2017, (c) reporting evidence in humans only (d) subjects were healthy individuals > 12 years old (exclusion of children) (e) subjects were supplemented with either creatine or placebo (f) subjects underwent various cognitive tasks or tests before and after the administration of creatine/placebo (g) no other intervention was done on subjects.
2.3. Search
The following search query was used for the identification of studies examining the effects of creatine on cognitive function: “(creatine OR creatine monohydrate OR creatine ethyl ester) AND (cognition OR memory)”.
2.4. Study selection
Eligible studies were selected by two reviewers independently (KA, NS), based on the inclusion criteria. Disagreements were solved by the addition of a third reviewer (KB) and consensus.
2.5. Data collection process and data items
Data was extracted by two reviewers (KA, NS) and included the next fields: title, 1st author, year of publication, ID, country, design of study, total number of participants, participants assigned on creatine and placebo, basic characteristics of participants, cognitive tasks or tests, performance on these tasks/tests and adverse events.
2.6. Risk of bias in individual studies
The Cochrane Collaboration’s tool was used for the assessment of risk of bias (ROB) by two reviewers independently (KA, NS) (Higgins et al., 2011). Any disagreement between authors was solved through consensus and the addition of a third reviewer (KB). Every outcome was evaluated within each study. The following domains were assessed: random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias). If one ROB domain was of high risk, the study was deemed to be of high risk. In order to be of low ROB, all domains had to be of low risk. In any cases other than the above, the study’s ROB was characterized as unclear.
For the study by Rae et al. only, which had a cross-over design, ROB assessment was based on the instructions of Cochrane Handbook Systematic Reviews of Interventions for cross-over trials (Higgins and Green, 2011).
3. Results
3.1. Search results
The search resulted in 769 potentially eligible studies. After removal of duplicated studies, 715 studies were excluded based on title and abstract. Finally, nine full – text articles were assessed for eligibility. From those, three articles were excluded because they did not meet the inclusion criteria. The flowchart in Fig. 1 provides information regarding the selection of studies.
Fig. 1.
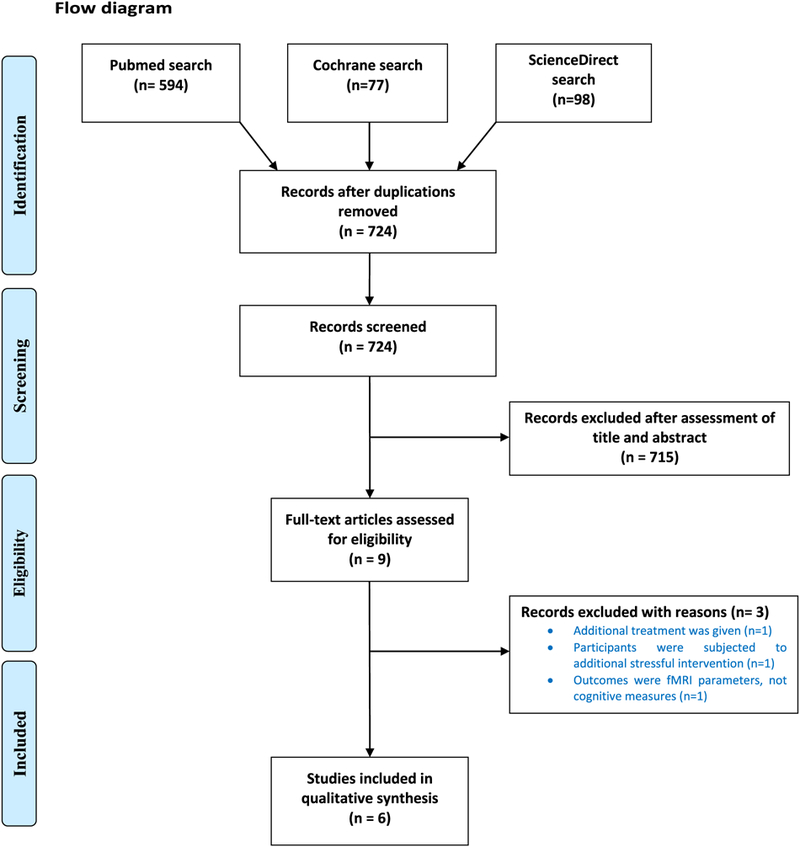
Flow diagram.
3.2. Characteristics of included studies and patients
Six studies were included for qualitative synthesis, corresponding to 281 patients. Half studies took place in UK (McMorris et al., 2007a; Ling et al., 2009; Benton and Donohoe, 2011), while the rest were conducted in different countries (Japan, Australia, USA) (Watanabe et al., 2002; Rae et al., 2003; Rawson et al., 2008). Five studies used creatine monohydrate supplementation (McMorris et al., 2007a; Benton and Donohoe, 2011; Watanabe et al., 2002; Rae et al., 2003; Rawson et al., 2008). One study used creatine in the form of creatine ethyl-ester (Ling et al., 2009). Regarding study design, five studies were RCTs (McMorris et al., 2007a; Ling et al., 2009; Benton and Donohoe, 2011; Watanabe et al., 2002; Rawson et al., 2008). One study was a “cross – over” RCT (Rae et al., 2003). The duration of treatment varied from study to study. Details regarding characteristics of studies and patients are shown in Tables 1 and 2.
Table 1.
Characteristics of included studies.
| First author and year | Country | Study type | Treatment duration (days) | Total n of patients (m/f) | Type of creatine (form) | Daily dose |
|---|---|---|---|---|---|---|
| Watanabe et al., 2002 | Japan | RCT | 5 days | 24 (19/5) | Creatine monohydrate (tbs) | 8g (in 4 doses) |
| Rae et al., 2003 | Australia | Cross-over RCT | 6 weeks | 45 (12/33) | Creatine monohydrate (liquid) | 5g |
| McMorris et al., 2007a | UK | RCT | 2 weeks | 32 (16/16) | Creatine monohydrate (liquid) | 20 g (in 4 doses) |
| Rawson et al., 2008 | USA | RCT | 6 weeks | 22 (13/9) | Creatine monohydrate (caps) | 0.03 g/kg |
| Ling et al., 2009 | UK | RCT | 2 weeks | 34 (22/12) | Creatine ethylester (liquid) | 5g |
| Benton and Donohoe, 2011 | UK | RCT | 5 days | 121a (0/121) | Creatine monohydrate (tbs) | 20 g |
RCT: randomized controlled trial, tbs.: tablets, caps: capsules.
n = 51 were meat eaters, n = 70 were vegans/vegetarians.
Table 2.
Demographic characteristics of patients in each study.
| First author and year | Gender (m/f) | Age (years) | BMI (kg/m2) | Education (years) |
|---|---|---|---|---|
| Watanabe et al., 2002 | 19/5 | 24.4 (sd = 9.1) | NR | NR |
| Rae et al., 2003 | 12/33 |
27.5(19–37)for males 24.9(19–40) for females |
NR | NR |
| McMorris et al., 2007a | 16/16 | 76.4 (8.48) | NR | Same |
| Rawson et al., 2008 | 13/9 | 20.8 (2.2) | 25.5 (2.65) | NR |
| Ling et al., 2009 | 22/12 | 21 (1.4) | NR | NR |
| Benton and Donohoe, 2011 | 0/121 | 20.3 (SE= 2.1) | NR | NR |
Data is reported as mean (SD) in most studies.
In McMorris et al. study, data is reported as mean (SE).
In Rae et al. study, data is reported as median (range) separately for males and females.
3.3. Risk of bias
In the Rae et al. study, which was independently assessed, we found the following: i) the cross-over design was suitable, ii) there was no carry-over effect of treatment but iii) it is unknown if there was randomization of given treatments (creatine/placebo) (Higgins et al., 2011).
For the rest of the studies, overall ROB was high in one case due random “sequence generation” bias (selection bias) (McMorris et al., 2007a). For four included studies, overall ROB was unclear mostly because of unclear “allocation concealment” bias (selection bias) (Valenzuela et al., 2003; Ferrier et al., 2000; Moher et al., 2009 PRISMA [Internet], n.d.; Higgins and Green, 2011). Risk of bias for the domains “incomplete outcome data (attrition bias)” and “selective reporting (reporting bias)” was low for five studies (Valenzuela et al., 2003; Ferrier et al., 2000; Moher et al., 2009; PRISMA [Internet], n.d.; Higgins and Green, 2011). For the domains “blinding of participants and personnel” and “blinding of outcome assessment”, bias was of low risk for three studies and of unknown risk for two studies. For “random sequence generation”, risk of bias varied among studies. Finally, for “allocation concealment”, risk of bias was unclear for most studies. Details regarding ROB are presented in Figs. 2 and 3.
Fig. 2.
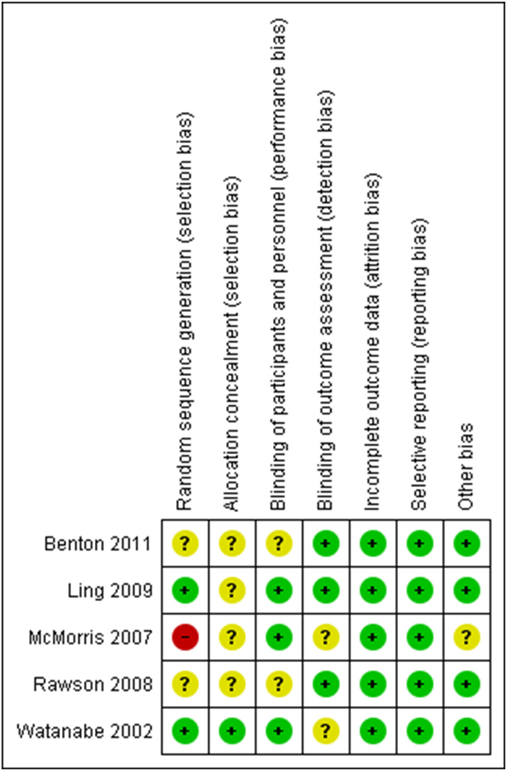
Risk of bias summary.
Fig. 3.
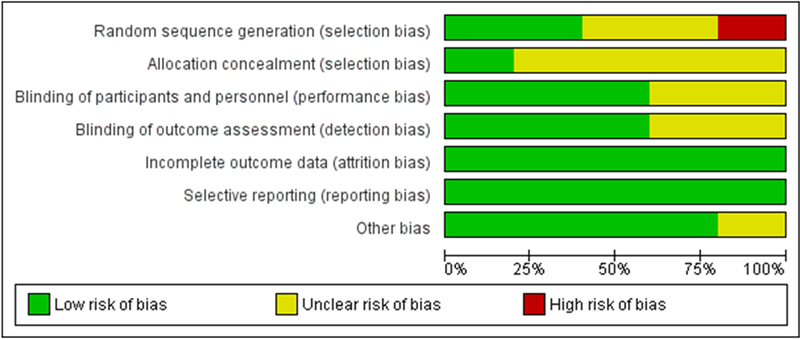
Risk of bias graph: authors’ judgements about each risk of bias item, presented as percentage across all included studies.
3.4. Results of individual studies
3.4.1. Effects of creatine on cognition
For the assessment of creatine’s effect on cognition, various cognitive tasks were used as measures. Cognitive domains that were tested included: memory, executive function, attention and reasoning. Additionally, several other complex functions such as mental fatigue, reaction time, and word fluency were assessed.
3.5. Memory
Memory was tested in five studies (McMorris et al., 2007a; Ling et al., 2009; Benton and Donohoe, 2011; Rae et al., 2003; Rawson et al., 2008). However, different types of memory were examined in each of these studies (McMorris et al., 2007a; Ling et al., 2009; Benton and Donohoe, 2011; Rae et al., 2003; Rawson et al., 2008). Specifically, short-term memory was tested in four studies (McMorris et al., 2007a; Benton and Donohoe, 2011; Rae et al., 2003; Rawson et al., 2008). In two of these studies, there was a beneficial effect on the creatine group (McMorris et al., 2007a; Rae et al., 2003). It is important to underline that the improvements in creatine group presented in one of these two studies, are questionable due to high ROB in this study (Valenzuela et al., 2003). In another study, short term memory was improved only for vegeterians after creatine supplementation but not for meat-eaters (Benton and Donohoe, 2011). Finally, one study showed that creatine did not affect short-term memory but this study included young adults only (Rawson et al., 2008).
Except from short-term memory, Mc Morris et al. also assessed long-term memory and found it improved in the creatine group (McMorris et al., 2007a). However, this improvement in long-term memory should be cautiously assessed, as it was a high ROB study (McMorris et al., 2007a). Additional types of memories were assessed in three studies: spatial memory, memory scanning and running memory (McMorris et al., 2007a; Ling et al., 2009; Rawson et al., 2008). Improvements were observed after creatine supplementation for the first two types of memory but no effect was observed for running memory (McMorris et al., 2007a; Ling et al., 2009; Rawson et al., 2008); the caveat being that running memory was assessed in young adults only Rawson et al., 2008. In addition, it is unsure if spatial memory was truly benefited from creatine intake, as the only and one study that assessed it, was likely biased (McMorris et al., 2007a).
3.6. Executive function, attention and response inhibition
Executive function was tested in one study that showed that there was no difference after creatine supplementation (McMorris et al., 2007a). Two studies assessed sustained attention, but results for this outcome were conflicting (Ling et al., 2009; Benton and Donohoe, 2011). Response inhibition was tested in one study in which improved performance was observed for the creatine group (Ling et al., 2009).
3.7. Intelligence/reasoning
Three studies assessed aspects of intelligence (reasoning or mathematical processing) (Ling et al., 2009; Rae et al., 2003; Rawson et al., 2008). Performance was improved significantly in two studies (Rawson et al., 2008; Rae et al., 2003). However, no change was shown in the Rawson et al. study that involved young adults only (Rawson et al., 2008).
3.8. Other cognitive measures
Other cognitive tasks and measures that were used by studies, such as code substitution, mental fatigue, reaction time and word fluency, were variably affected by creatine (Benton and Donohoe, 2011; Watanabe et al., 2002; Rawson et al., 2008). The findings of each study regarding cognitive function are summarized in Table 3.
Table 3.
Cognitive measures.
| Study’s 1st author, year | Creatine group patients (males/females) | Placebo group patients (males/females) | Type of treatment | Cognitive measures | Outcome |
|---|---|---|---|---|---|
| Watanabe et al., 2002 | N = 12 (NR) | N = 12 (NR) | 8 g/d (in 4 doses) for 5 d | Uchida-Kraepelin test (UKT) | The regression coefficient of the creatine group significantly increased from 0.0115 to 0.0055 (p < 0.02, paired t-test), no significant changes in the placebo group (from 0.0078 to 0.0087, p > 0.5) |
| Rae et al., 2003 | Crossover design | Crossover design | 5 g/d for six weeks | Raven’s Advanced Progressive Matrices (RAPMs) | Oral creatine significantly increased intelligence (as measured by RAPMs done under time pressure) compared with placebo (p < 0.0001; repeated-measures ANO VA). No significant effect of treatment order (p = 0.21), although there was a significant interaction with treatment order (p = 0.0004) |
| Wechsler Auditory backward digit span (BDS) task | Oral creatine significantly affected performance on BDS (p < 0.0001), with no effect oi order (p = 0.40) | ||||
| McMorris et al., 2007a | N = 15 (8/7) | N = 17 (8/9) | 5 g creatine 4×/d for 7d | Random number generation | No difference between groups |
| Forward number recall | Creatine group was improved compared to placebo (p < 0.01) | ||||
| Backward number recall | No difference between groups | ||||
| Forward spatial recall | Creatine group was improved compared to placebo (p < 0.05) | ||||
| Backward spatial recall | Creatine group was significantly improved compared to placebo (p < 0.01) | ||||
| Long term memory test | Creatine group was significantly improved compared to placebo (p < 0.01) | ||||
| Rawson et al., 2008 | N = 11 (6/5) | N = 11 (7/4) | 0.03 g/kg per day for 6 weeks | Simple reaction time | No difference between groups |
| Immediate code substitution | No difference between groups | ||||
| Delayed code substitution | No difference between groups | ||||
| Logical reasoning | No difference between groups | ||||
| Mathematical processing | No difference between groups | ||||
| Running memory | No difference between groups | ||||
| Memory recall | No difference between groups | ||||
| Ling et al., 2009 | N = 17 (11/6) | N = 17 (11/6) | 5 g/d for 2 weeks | Memory Scanning | |
| Stimulus number in set-RT | Improvement only for cr. group (p < 0.01) | ||||
| Stimulus number in set-NI | Improvement only for cr. group (p < 0.01) | ||||
| Stimulus number not in set-RT | Improvement only for cr. group (p < 0.01) | ||||
| Stimulus number not in set-NI | Improvement only for cr. group (p < 0.05) | ||||
| Overall mean-RT | Improvement only for cr. group (p < 0.01) | ||||
| Overall NI | Improvement only for cr. group (p < 0.01) | ||||
| Number-Pair Matching | |||||
| Overall RT | Improvement only for cr. group (p < 0.01) | ||||
| Overall NI | Improvement only for cr. group (p < 0.01) | ||||
| Sustained Attention | |||||
| Number of correct responses | No change for any group | ||||
| RT correct responses | Improvements for both groups (ps < 0.05) | ||||
| Number of false positives | No change for any group | ||||
| Number of correct omissions | Improvement only for cr. group (p < 0.01) | ||||
| Number of incorrect omissions | No change for any group | ||||
| Arrow Flankers | |||||
| Overall number of false alarms | Improvement only for cr. group (p < 0.01) | ||||
| Overall RT | Improvement for both cr. group and placebo group (ps < 0.01 and < 0.05) | ||||
| Overall NI | Improvement only for cr. group (p < 0.01) | ||||
| IQ score | Improvement only for cr. group (p < 0.01) | ||||
| Benton and Donohoe, 2011 | N = 61 (0/61) | N = 60 (0/60) | 5 g/d for 5 days | Word list recall | Improvement for vegeterians of cr.group compared to meat eaters of cr.group (p < 0.001). No changes for placebo group |
| RT | No change for any group | ||||
| Vigilance | No change for any group | ||||
| Word fluency | No change for any group |
3.9. Safety
Of all included studies, one RCT reported side effects after creatine supplementation (Benton and Donohoe, 2011). These were minor and included bloating and headache (Benton and Donohoe, 2011). Compared with placebo, side effects of creatine group were not significantly different (Benton and Donohoe, 2011).
4. Discussion
4.1. Effects of creatine supplementation on cognition of healthy indviduals
In the present systematic review, there is evidence that creatine supplementation may improve short term memory and intelligence/reasoning. Regarding long term memory, spatial memory, executive function and attention, the effect was not clear. Young adults did not show changes in any task, after creatine administration. This is in accordance to studies showing that the elderly require additional energy in comparison with young individuals for completing cognitive tasks (Toescu, 2005). Thus, this additional required energy for the elderly may be provided through creatine administration, whereas younger individuals do not have such needs. There is a tendency for increasing creatine levels with aging, but this change does not counteract the cognitive decline due to aging (Pfefferbaum et al., 1999). It is possible that creatine administration improves cognition of diseased, aged or stressed individuals whereas for younger, unstressed individuals there is no such a benefit (Mcmorris et al., 2007b; Fernandez-Espejo, 2004; Turner et al., 2015; Bender et al., 2005).
In the studies reviewed here, some individuals showed improved performance for some tasks while no improvements for other. In the McMorris et al. study, performance on the backward number recall task was not affected, but performance on the forward number recall task was affected positively (McMorris et al., 2007a). This may partially be explained by the fact that backward number recall is more difficult than forward number recall and the two tasks may activate different brain areas with different energetic needs (Sun et al., 2005; Brugger, 1997). Furthermore, there is evidence that brain effects of may be regionally specific (Dechent et al., 1999). Thus, both the difficulty of the task and the brain area activated during the task may determine the response of healthy individuals to creatine administration. However, these factors alone cannot explain all the results. It is possible that in a demanding test, individuals could benefit from creatine supplementation up to a threshold but when difficulty exceeds that threshold, creatine supplementation may no longer impact any benefits. However, extra caution should be taken, regarding McMorris et al. study due to increased likelihood of high ROB (McMorris et al., 2007a).
Another factor that may account for different responses in each task is creatine dosing (daily dose x duration of treatment). Oral creatine intake is known to increase brain’s creatine concentration (Dechent et al., 1999). However, accumulation in tissues does not depend only on daily dosing and supplementation duration (Pan and Takahashi, 2007a; Wilkinson et al., 2006). According to the literature, one of the greatest determinants of creatine accumulation in brain after supplementation of creatine is basal creatine level (Pan and Takahashi, 2007b) and the potential for increase in brain’s creatine levels after supplementation is inversely related to its baseline brain levels (Pan and Takahashi, 2007b; Rawson et al., 2002). It is also possible that creatine uptake in the brain may be affected by insulin levels or a recent meal, as is the case with muscles’ creatine uptake (Steenge et al., 1998; Preen et al., 2003; Green et al., 1996a; Green et al., 1996b; Steenge et al., 2000). Also, general diet habits may affect the response to exogenous creatine since this nutrient is mostly found in meat and fish. One would expect that vegans/vegetarians may have greater improvements post creatine supplementation due to absence of creatine in their diet. Of interest, in the Benton et al. study, the results were same for both meat-eaters and vegan/vegetarians for most cognitive tasks (Benton and Donohoe, 2011). The above data suggests that creatine accumulation and thus performance in various cognitive tasks is not only a matter of dosing but may be affected by factors such as basal creatine levels, insulin levels, previous meals and general diet habits.
4.2. Creatine’s link to cognitive function
The importance of creatine in brain function is suggested by the presence of creatine kinase (CK) isoforms in multiple brain areas including the cerebellum, hippocampus, pontine reticular formation, red nucleus, cerebral cortex and choroid plexus (Kaldis et al., 1996; Friedman and Roberts, 1994) (see also the brain distribution of CKMT1A (Anon, 2018a); and the Allen Human Brain Atlas (Anon, 2018b)). On the other hand, inborn errors of metabolism that result in deficiency of creatine transporter or creatine itself are characterized by cognitive and neurodevelopmental defects (Stöckler et al., 1996; Salomons et al., 2003), which may be reversed by creatine supplementation (Stöckler et al., 1996). In addition, experimental deletion of CK in rodents results in learning problems (Jost et al., 2002). All these facts underline the importance of creatine in cognitive function.
Brain cells use the phosphorylated adenine nucleotide ATP for energy production (Erecińska and Silver, 1989). The phosphate creatine/creatine (PCr/Cr) system is linked to adenine nucleotides through the creatine phosphokinase reaction (Erecińska and Silver, 1989). In general, creatine is implicated in cellular energy homeostasis (Saks et al., 2000; Wallimann et al., 1992). There is also evidence that creatine contributes to neuroprotection by reversing mitochondrial dysfunction in neurodegenerative diseases. In a study of Huntington’s disease patients, creatine decreased glutamate availability (the primary mediator of excitotoxicity) (Bender et al., 2005). On the other hand, and despite early promising results of creatine supplementation in Parkinson’s disease, a multicenter, double-blind, placebo-controlled, randomized efficacy trial of creatine for at least five years was terminated early for futility (Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators et al., 2015). According to another theory, creatine may have an impact on cognitive function by regulating glucose levels, in an insulin independent way (Benton and Owens, 1993; Donohoe and Benton, n.d.; Rooney et al., 2003).
4.3. Limitations and strengths
One of the major limitations of this systematic review is the heterogeneity of the included studies. There is heterogeneity in respect to the baseline characteristics of the individuals such as age and sex. Additionally, in one study the majority of the patients were vegans/vegetarians (Benton and Donohoe, 2011). Also, creatine supplementation protocols differed from study to study. Moreover, each study assessed different domains of cognition and made use of different cognitive tasks. For these reasons, a quantitative analysis (meta-analysis) was not feasible.
The review included 281 patients which is a relatively small sample size. Studies were conducted in various world regions such as Japan, USA, Australia and UK. This may be a strength point of the review as it allows for easier generalizability of the findings.
5. Conclusion
The present systematic review examined the effects of oral creatine administration on cognitive function in healthy individuals. Creatine supplementation has been proven safe and is usually used by athletes to increase their athletic performance. In addition, creatine is an essential compound for the brain and may aid various brain regions in terms of energy supply and neuroprotection. The studies included in this review provide evidence that oral creatine intake may improve performance on memory and intelligence tasks. For other aspects of cognition, such as attention, executive function, response inhibition, word fluency, reaction time and mental fatigue, the results were inconclusive. Interestingly, young adults were not affected by supplementation. Creatine supplementation may have a selective positive effect on individuals subjected to various kinds of stress and aging. Since creatine is safe and shows some benefits on cognition of healthy individuals, it is imperative that studies including patients with dementias or cognitive impairment should be conducted in future.
References
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR, 2008. July 1 Functions and effects of creatine in the central nervous system. Brain Res Bull [Internet] 76 (4), 329–343. [cited 2017 Oct 29]. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0361923008001007. [DOI] [PubMed] [Google Scholar]
- Anon, 2018a. Microarray Data :: Allen Brain Atlas: Human Brain [Internet] [cited 2018 Jan 4]. Available from: http://human.brain-map.org/microarray/search/show? exact_match=false&search_term=CKMT1A&search_type=gene&page_num=0.
- Anon, 2018b. Tissue Expression of CKMT1A - Summary - The Human Protein Atlas [Internet] [cited 2018 Jan 4]. Available from: https://www.proteinatlas.org/ENSG00000223572-CKMT1A/tissue.
- Bender A, Auer DP, Merl T, Reilmann R, Saemann P, Yassouridis A, et al. , 2005. January Creatine supplementation lowers brain glutamate levels in Huntington’s disease. J Neurol [Internet] 252 (1), 36–41. [cited 2017 Oct 29]. Available from: http://link.springer.com/10.1007/s00415-005-0595-4. [DOI] [PubMed] [Google Scholar]
- Benton D, Donohoe R, 2011. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br J Nutr [Internet] 105 (7), 1100–1105. Available from: http://www.journals.cambridge.org/abstract_S0007114510004733. [DOI] [PubMed] [Google Scholar]
- Benton D, Owens DS, 1993. Blood glucose and human memory. Psychopharmacology (Berl) [Internet] 113 (1), 83–88. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/7862833. [DOI] [PubMed] [Google Scholar]
- Braissant O, Henry H, Villard A-M, Zurich M-G, Loup M, Eilers B, et al. , 2002. November 15 Ammonium-induced impairment of axonal growth is prevented through glial creatine. J Neurosci [Internet] 22 (22), 9810–9820. [cited 2018 Apr 4]. Available from http://www.ncbi.nlm.nih.gov/pubmed/12427837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Henry H, Béard E, Uldry J, 2011. May 10 Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids [Internet] 40 (5), 1315–1324. [cited 2018 Apr 4]. Available from: http://link.springer.com/10.1007/s00726-011-0852-z. [DOI] [PubMed] [Google Scholar]
- Brugger P, 1997. April 4 Variables that influence the generation of random sequences: an update. Percept Mot Skills [Internet] 84 (2), 627–661. [cited 2017 Oct 29]. Available from: http://journals.sagepub.com/doi/10.2466/pms.1997.84.2.627. [DOI] [PubMed] [Google Scholar]
- Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J, 1999. September Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am J Physiol [Internet] 277 (3 Pt 2), R698–704. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/10484486. [DOI] [PubMed] [Google Scholar]
- Deldicque L, Décombaz J, Zbinden Foncea H, Vuichoud J, Poortmans JR, Francaux M, 2008. January 15 Kinetics of creatine ingested as a food ingredient. Eur J Appl Physiol [Internet] 102 (2), 133–143. [cited 2018 Apr 4]. Available from: http://link.springer.com/10.1007/s00421-007-0558-9. [DOI] [PubMed] [Google Scholar]
- Donohoe RT, Benton D. Glucose tolerance predicts performance on tests of memory and cognition. Physiol Behav [Internet]. n.d. [cited 2017 Oct 29]; 71(3–4):395–401. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11150572. [DOI] [PubMed] [Google Scholar]
- Donohoe RT, Benton D, 2000. Glucose tolerance predicts performance on tests of memory and cognition. Physiol. Behav. 71 (3–4), 395–401. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11150572. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo E, 2004. February Pathogenesis of Parkinson’s disease: prospects of neuroprotective and restorative therapies. Mol Neurobiol [Internet] 29 (1), 15–30. [cited 2017 Oct 29]. Available from: http://link.springer.com/10.1385/MN:29:1:15. [DOI] [PubMed] [Google Scholar]
- Ferrier CH, Alarcón G, Glover A, Koutroumanidis M, Morris RG, Simmons A, et al. , 2000. December 26 N-Acetylaspartate and creatine levels measured by (1)H MRS relate to recognition memory. Neurology [Internet] 55 (12), 1874–1883. [cited 2018 Apr 4]. Available from http://www.ncbi.nlm.nih.gov/pubmed/11134388. [DOI] [PubMed] [Google Scholar]
- Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL, 1996. Nova. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am J Physiol [Internet] 271 (5 Pt 1), E821–6. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/8944667. [DOI] [PubMed] [Google Scholar]
- Green AL, Simpson EJ, Littlewood JJ, Macdonald IA, Greenhaff PL, 1996. Octb. Carbohydrate ingestion augments creatine retention during creatine feeding in humans. Acta Physiol Scand [Internet] 158 (2), 195–202. [cited 2017 Oct 29]. Available from: http://doi.wiley.com/10.1046/j.1365-201X.1996.528300000.x. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, 2011. In: Green S (Ed.), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, (Available from http://handbook.cochrane.org [Internet]. [cited 2018 Jan 5]. Available from: http://training.cochrane.org/handbook).
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. , 2011. October 18 The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ [Internet] 343, d5928 [cited 2017 Oct 25]. Available from http://www.ncbi.nlm.nih.gov/pubmed/22008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncquel-Chevalier Curt M, Voicu P-M, Fontaine M, Dessein A-F, Porchet N, Mention-Mulliez K, et al. , 2015. December Creatine biosynthesis and transport in health and disease. Biochimie [Internet] 119, 146–165. [cited 2017 Oct 25]. Available from http://www.ncbi.nlm.nih.gov/pubmed/26542286. [DOI] [PubMed] [Google Scholar]
- Jost CR, Van Der Zee CEEM, In’t Zandt HJA, Oerlemans F, Verheij M, Streijger F, et al. , 2002. May Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci [Internet] 15 (10), 1692–1706. [cited 2017 Oct 24]. Available from http://www.ncbi.nlm.nih.gov/pubmed/12059977. [DOI] [PubMed] [Google Scholar]
- Kaldis P, Hemmer W, Zanolla E, Holtzman D, Wallimann T, 1996. “Hot spots” of creatine kinase localization in brain: cerebellum, hippocampus and choroid plexus. Dev Neurosci [Internet] 18 (5–6), 542–554. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/8940630. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, et al. , 1999. March Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med [Internet] 5 (3), 347–350. [cited 2017 Oct 24]. Available from http://www.ncbi.nlm.nih.gov/pubmed/10086395. [DOI] [PubMed] [Google Scholar]
- Ling J, Kritikos M, Tiplady B, 2009. Cognitive effects of creatine ethyl ester supplementation. Behav. Pharmacol. 20 (8), 673–679. [DOI] [PubMed] [Google Scholar]
- McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A, 2007a. Creatine supplementation and cognitive performance in elderly individuals. Aging Neuropsychol. Cogn. 14 (5), 517–528. [DOI] [PubMed] [Google Scholar]
- Mcmorris T, Harris RC, Howard AN, Langridge G, Hall B, Corbett J, et al. , 2007b. Creatine Supplementation, Sleep Deprivation, Cortisol, Melatonin and Behavior. 90 pp. 21–28. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group TP, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med [Internet] 3 (3), e123–30. [cited 2017 Oct 25]. Available from http://www.ncbi.nlm.nih.gov/pubmed/21603045. [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Takahashi K, 2007. April 21a Cerebral energetic effects of creatine supplementation in humans. Am J Physiol Regul Integr Comp Physiol [Internet] 292 (4), R1745–50. [cited 2017 Oct 29]. Available from: http://ajpregu.physiology.org/cgi/doi/10.1152/ajpregu.00717.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Takahashi K, 2007. April 21b Cerebral energetic effects of creatine supplementation in humans. Am J Physiol Regul Integr Comp Physiol [Internet] 292 (4), R1745–50. [cited 2017 Oct 24]. Available from: http://ajpregu.physiology.org/cgi/doi/10.1152/ajpregu.00717.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky AM, Brazeau GA, 2001. June Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev [Internet] 53 (2), 161–176. [cited 2017 Oct 24]. Available from http://www.ncbi.nlm.nih.gov/pubmed/11356982. [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO, 1999. February In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med [Internet] 41 (2), 276–284. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/10080274. [DOI] [PubMed] [Google Scholar]
- Preen D, Dawson B, Goodman C, Beilby J, Ching S, 2003. March Creatine supplementation: a comparison of loading and maintenance protocols on creatine uptake by human skeletal muscle. Int J Sport Nutr Exerc Metab [Internet] 13 (1), 97–111. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/12660409. [DOI] [PubMed] [Google Scholar]
- PRISMA [Internet] [cited 2018 Apr 4]. Available from: http://www.prisma-statement.org/Endorsement/EndorsePRISMA.aspx.
- Rae C, Digney AL, Mcewan SR, Bates TC, August 2003. Oral Creatine Monohydrate Supplementation Improves Brain Performance: A Double-Blind. 2006. pp. 2147–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson ES, Volek JS, 2003. November Effects of creatine supplementation and resistance training on muscle strength and weightlifting performance. J Strength Cond Res [Internet] 17 (4), 822–831. [cited 2017 Oct 24]. Available from http://www.ncbi.nlm.nih.gov/pubmed/14636102. [DOI] [PubMed] [Google Scholar]
- Rawson ES, Clarkson PM, Price TB, Miles MP, 2002. January Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiol Scand [Internet] 174 (1), 57–65. [cited 2017 Oct 29]. Available from: http://doi.wiley.com/10.1046/j.1365-201x.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- Rawson ES, Lieberman HR, Walsh TM, Zuber SM, Harhart JM, Matthews TC, 2008. Creatine supplementation does not improve cognitive function in young adults. Physiol. Behav. 95 (1), 130–134. [DOI] [PubMed] [Google Scholar]
- Friedman DL, Roberts R, 1994. May 15 Compartmentation of brain-type creatine kinase and ubiquitous mitochondrial creatine kinase in neurons: evidence for a creatine phosphate energy shuttle in adult rat brain. J Comp Neurol [Internet] 343 (3), 500–511. [cited 2017 Oct 29]. Available from: http://doi.wiley.com/10.1002/cne.903430311. [DOI] [PubMed] [Google Scholar]
- Rooney KB, Bryson JM, Digney AL, Rae CD, Thompson CH, 2003. Creatine supplementation affects glucose homeostasis but not insulin secretion in humans. Ann Nutr Metab [Internet] 47 (1), 11–15. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/12624482. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kongas O, Vendelin M, Kay L, 2000. April Role of the creatine/phosphocreatine system in the regulation of mitochondrial respiration. Acta Physiol Scand [Internet] 168 (4), 635–641. [cited 2017 Oct 29]. Available from: http://doi.wiley.com/10.1046/j.1365-201x.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- Salomons GS, van Dooren SJM, Verhoeven NM, Marsden D, Schwartz C, Cecil KM, et al. , 2003. X-linked creatine transporter defect: an overview. J Inherit Metab Dis [Internet] 26 (2–3), 309–318. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/12889669. [DOI] [PubMed] [Google Scholar]
- Steenge GR, Lambourne J, Casey A, Macdonald IA, Greenhaff PL, 1998. December Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am J Physiol [Internet] 275 (6 Pt 1), E974–9. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/9843739. [DOI] [PubMed] [Google Scholar]
- Steenge GR, Simpson EJ, Greenhaff PL, 2000. September Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J Appl Physiol [Internet] 89 (3), 1165–1171. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/10956365. [DOI] [PubMed] [Google Scholar]
- Stöckler S, Hanefeld F, Frahm J, 1996. September 21 Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet (London, England) [Internet] 348 (9030), 789–790. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/8813986. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang X, Chen X, Zhang P, Bao M, Zhang D, et al. , 2005. May 15 Age-dependent brain activation during forward and backward digit recall revealed by fMRI. Neuroimage [Internet] 26 (1), 36–47. [cited 2017 Oct 29]. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1053811905000558. [DOI] [PubMed] [Google Scholar]
- Toescu EC, 2005. December 29 Normal brain ageing: models and mechanisms. Philos Trans R Soc Lond B Biol Sci [Internet] 360 (1464), 2347–2354. [cited 2017 Oct 29]. Available from: http://rstb.royalsocietypublishing.org/cgi/doi/10.1098/rstb.2005.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, Byblow WD, Gant N, 2015. January 28 Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. J Neurosci [Internet] 35 (4), 1773–1780. [cited 2017 Oct 29]. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3113-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela MJ, Jones M, Wen W, Rae C, Graham S, Shnier R, et al. , 2003. July 18 Memory training alters hippocampal neurochemistry in healthy elderly. Neuroreport [Internet] 14 (10), 1333–1337. [cited 2017 Oct 24]. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00001756-200307180-00010. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM, 1992. January 1 Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the “phosphocreatine circuit” for cellular energy homeostasis. Biochem J [Internet] 281 (Pt 1), 21–40. [cited 2017 Oct 29]. Available from http://www.ncbi.nlm.nih.gov/pubmed/1731757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Kato N, Kato T, 2002. Effects of Creatine on Mental Fatigue and Cerebral Hemoglobin Oxygenation. 42. [DOI] [PubMed] [Google Scholar]
- Wilkinson ID, Mitchel N, Breivik S, Greenwood P, Griffiths PD, Winter EM, et al. , 2006. January Effects of creatine supplementation on cerebral white matter in competitive sportsmen. Clin J Sport Med [Internet] 16 (1), 63–67. [cited 2017 Oct 29]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16377978. [DOI] [PubMed] [Google Scholar]
- Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators, Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, et al. , 2015. February 10 Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA [Internet] 313 (6), 584–593. [cited 2018 Jan 4]. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R, 2000. Jul. Creatine and creatinine metabolism. Physiol Rev [Internet] 80 (3), 1107–1213. [cited 2017 Oct 24]. Available from http://www.ncbi.nlm.nih.gov/pubmed/10893433. [DOI] [PubMed] [Google Scholar]


