Abstract
Background
In contrast to pediatric Crohn’s disease (CD), little is known in pediatric ulcerative colitis (UC) about the relationship between disease phenotype and serologic reactivity to microbial and other antigens.
Aim
The aim of this study was to examine disease phenotype and serology in a well-characterized inception cohort of children newly diagnosed with UC during the PROTECT Study (Predicting Response to Standardized Pediatric Colitis Therapy).
Methods
Patients were recruited from 29 participating centers. Demographic, clinical, laboratory, and serologic (pANCA, ASCA IgA/IgG, Anti-CBir1, and Anti-OmpC) data were obtained from children 4–17 years old with UC.
Results
Sixty-five percent of the patients had positive serology for pANCA, with 62% less than 12 years old and 66% 12 years old or older. Perinuclear anti-neutrophil cytoplasmic antibodies did not correspond to a specific phenotype though pANCA ≥100, found in 19%, was strongly associated with pancolitis (P = 0.003). Anti-CBir1 was positive in 19% and more common in younger children with 32% less than 12 years old as compared with 14% 12 years old or older (P < 0.001). No association was found in any age group between pANCA and Anti-CBir1. Relative rectal sparing was more common in +CBir1, 16% versus 7% (P = 0.02). Calprotectin was lower in Anti-CBir1+ (Median [IQR] 1495 mcg/g [973–3333] vs 2648 mcg/g [1343–4038]; P = 0.04). Vitamin D 25-OH sufficiency was associated with Anti-CBir1+ (P = 0.0009).
Conclusions
The frequency of pANCA in children was consistent with adult observations. High titer pANCA was associated with more extensive disease, supporting the idea that the magnitude of immune reactivity may reflect disease severity. Anti-CBir1+ was more common in younger ages, suggesting host-microbial interactions may differ by patient age.
Keywords: CBir1, pANCA, PROTECT, serologies, ulcerative colitis
Introduction
Ulcerative colitis (UC) denotes a phenotype of chronic inflammatory bowel disease (IBD) in which inflammation is localized to the mucosa and extends proximally from the rectum to varying extents of the large intestine. It is thought to result in genetically susceptible individuals, from an inappropriate activation of the immune system and intestinal dysbiosis at the mucosal luminal interface1. UC is remarkably heterogeneous with respect to age of onset, anatomical extent, disease course, and response to therapies2–6; in general, children have a more severe clinical phenotype than adults with more extensive disease and a greater early need for corticosteroids and biologic therapies7, 8.
The heterogeneity of IBD has prompted a search for a marker or combination of markers to distinguish IBD from non-IBD, differentiate IBD subtypes (UC versus CD), anticipate disease outcomes, and predict response to therapies. A number of studies from the early 1990s onward have evaluated various serological immune markers to fill this role9–12. Perinuclear anti-neutrophil cytoplasmic antibody (pANCA) has historically been linked to a UC phenotype, and anti-saccharomyces cerevisiae antibody (ASCA) has been associated with a classic CD behavior. Anti-flagellin (Anti-CBir1) reactivity, on the other hand, has been described to be more prevalent in younger-onset CD, characterized by a colitis phenotype13, but its prevalence in pediatric UC remains undefined.
The PROTECT Study (Predicting Response to Standardized Pediatric Colitis Therapy) was initiated in 2012 to systematically examine treatment responses of children and adolescents newly diagnosed with UC. This multicenter inception cohort was designed to examine the demographic, clinical, laboratory, endoscopic, histologic, and serologic factors that are associated with initial response to therapy. This study tests the hypothesis that serologic reactivity is related to disease phenotype, histology, and laboratory values in children with newly diagnosed UC. While this relationship has been studied thoroughly in pediatric CD, the same rigorous studies do not exist in pediatric UC, and an improved understanding of the prevalence and associations with these serologic markers would better inform diagnosis, disease classification, and, potentially, therapy.
MATERIALS AND METHODS
Patient Population
Potential study patients were recruited from the clinical practices of the 29 centers participating in PROTECT. Children between the ages of 4 and 17 years inclusive with a clinical history consistent with colonic inflammation (eg, any combination of diarrhea, bleeding, abdominal pain) were eligible for study. Complete demographic, clinical, laboratory, and serologic data were obtained along with diagnostic esophagogastroduodenoscopy and ileocolonoscopy. Eligibility required disease extent beyond the rectum, a baseline Pediatric Ulcerative Colitis Activity Index (PUCAI) 14 score of ≥10, no previous therapy for colitis, and a stool culture negative for enteric bacterial pathogens (Salmonella, Shigella, Campylobacter, E. coli 0157:H7) and Clostridium difficile toxin. Patients were all newly diagnosed with UC, and this was a clinical, endoscopic, and histologic diagnosis of UC using previously established criteria. Exclusionary criteria included any clinical, endoscopic, radiologic, or histologic evidence of CD.
Age
Age was evaluated by years in categories of 4–6, 7–10, 11–13, and 14–17 years old to assess trends in seropositivity across childhood and adolescence.
Clinical Disease Activity
Clinical activity was determined by the PUCAI (range 0–85) and endoscopic activity by the Mayo endoscopy subscore 15. PUCAI <10 denoted inactive disease/remission, 10–34 denoted mild, 35–64 denoted moderate, and ≥65 denoted severe disease. The baseline PUCAI was defined as the last PUCAI on or before the treatment start date, and the majority (>60%) occurred on the scope date itself.
Endoscopic Assessment
Endoscopic evaluation included assessment of mucosal inflammation noting granularity, loss of vascular pattern, small superficial ulcers, mucopurulent exudate, and a line of demarcation between abnormal and normal colon in a patient whose colitis did not extend to the cecum. Note was made whether there was patchiness to the endoscopic appearance or relative rectal sparing.
Disease Extent
Disease extent was classified as proctosigmoiditis, left-sided colitis (to the splenic flexure), extensive colitis (to the hepatic flexure), and pancolitis (to the cecum). In analyses, we combined extensive colitis, pancolitis, and cases where a limited examination was performed due to severity of disease. Visual evidence of inflammation, not histology, was used to determine disease extent.
Histology
Centralized histologic examination was performed on a single rectal biopsy by one of the authors (MC) who was blinded to clinical data via a standardized scoring system that was created for PROTECT to evaluate degree of inflammation and architectural changes, and this scoring system has been previously published16. Of note, chronic features were scored as either present or absent, and these included ulcer/erosion, surface villiform changes, basal plasmacytosis, basal lymphoid aggregates, Paneth cell metaplasia, and crypt architectural abnormalities.
Laboratory Assessment
Hemoglobin (Hgb), hematocrit (Hct), white blood cell count (WBC), serum albumin, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were recorded from local site standard-of-care assessments, as available, within 4 weeks prior to initial UC treatment and not more than 2 days after initiating treatment. For some sites, the standard of care for milder disease did not include laboratory studies before a colonoscopy, and so the baseline window for participants with a mild PUCAI was extended to 8 weeks. Plasma albumin was measured at a central laboratory by ELISA per manufacturer’s instructions (Cell Biolabs, Inc., San Diego, CA) for participants with no available local serum value. We report observed values of all laboratory studies with the exception of C-reactive protein, which we report with respect to the upper limit of normal (ULN) for the local laboratory. Fecal calprotectin was determined using an ELISA (Bühlmann Laboratories AG, Schönenbuch, Switzerland) from stool samples collected before colonoscopy cleanout or at least 2 days after colonoscopy, but not more than 3 days after initial UC treatment 17, 18. Vitamin D 25-OH was performed centrally from plasma collected at baseline, and the level was defined as “sufficient” (≥30 ng/mL), “insufficient” (20-<30 ng/mL), or “deficient” (<20 ng/mL) 19.
Serology
Serologic determination of pANCA, ASCA IgG and IgA, Anti-CBir1, and anti-outer membrane C (Anti-OmpC) was performed at Cedars-Sinai Hospital, Los Angeles, California, utilizing previously published methods20. Perinuclear anti-neutrophil cytoplasmic antibody was considered high-titer at a level of ≥100 EU/mL21–23.
Statistical Analysis
Demographic, clinical, and serologic characteristics were compared across age categories: 4–6, 7–10, 11–13, and 14–17 years old. Patients were grouped as pANCA positive versus negative, pANCA titer negative versus positive, <100 versus positive and ≥100, and Anti-CBir1 positive versus negative. Groups were compared using a χ2 test or Fisher exact test for categorical assessments, a Mantel-Haenszel χ2 test for ordinal categorical assessments, a t test for normally distributed continuous assessments, and a Wilcoxon rank-sum test for continuous assessments with skewed distributions. Age-adjusted least-squares-mean titer values by disease location were obtained by analysis of covariance. All statistical tests were 2-tailed. There was no adjustment for multiple comparisons; P values are for descriptive purposes. Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Manuscript Preparation
The manuscript was written by the PROTECT Study Publication committee and subsequently approved by all authors.
ETHICAL CONSIDERATIONS
Informed consent or assent was obtained in all cases, and local investigational review boards at all investigative sites approved the study. This study was registered with clintrials.gov (NCT01536535).
RESULTS
Study Population
A total of 431 subjects were confirmed to have UC, and 399 (93%) had serology performed. Baseline demographic, clinical, and laboratory characteristics—categorized by age—of the 399 patients are shown in Table 1.
Table 1:
Demographic and Clinical Features of Study Population
| All patients N = 399 | 4–6 years N = 25 | 7–10 years N = 65 | 11–13 years N = 111 | 14–17 years N = 198 | P | |
|---|---|---|---|---|---|---|
| Age years, mean (SD) | 12.7 ± 3.3 | |||||
| Gender (% female) | 198 (50%) | 15 (60%) | 43 (66%) | 54 (49%) | 86 (43%) | 0.010 |
| Non-white race | 66/391 (17%) | 6/25 (24%) | 14/60 (23%) | 15/109 (14%) | 31/197 (16%) | 0.29b |
| Hispanic | 36/395 (9%) | 2/24 (8%) | 10/65 (15%) | 6/110 (5%) | 18/196 (9%) | 0.17b |
| Family History of IBD | 0.13b | |||||
| None | 335 (84%) | 20 (80%) | 56 (86%) | 96 (86%) | 163 (82%) | |
| UC only | 40 (10%) | 0 | 6 (9%) | 12 (11%) | 22 (11%) | |
| CD only | 13 (3%) | 3 (12%) | 2 (3%) | 2 (2%) | 6 (3%) | |
| Mixed | 11 (3%) | 2 (8%) | 1 (2%) | 1 (1%) | 7 (4%) | |
| BMI Z-score | -0.2 ± 1.3 | -0.2 ± 1.2 | -0.5 ± 1.4 | -0.2 ± 1.3 | -0.2 ± 1.3 | 0.26 |
| Proctosigmoiditis | 24 (6%) | 1 (4%) | 4 (6%) | 6 (5%) | 13 (7%) | 0.37 |
| Left-sided colitis | 41 (10%) | 2 (8%) | 5 (8%) | 8 (7%) | 26 (13%) | |
| Extensive /Pancolitisa | 334 (84%) | 22 (88%) | 56 (86%) | 97 (87%) | 159 (80%) | |
| PUCAI, mean (SD) | 49.9 ± 19.9 | 44.4 ± 21.3 | 51.8 ± 20.4 | 50.3 ± 19.1 | 49.8 ± 20.1 | 0.47 |
| PUCAI 10–30 | 95 (24%) | 9 (36%) | 13 (20%) | 26 (23%) | 47 (24%) | 0.49 |
| 35–60 | 172 (43%) | 9 (36%) | 27 (42%) | 47 (42%) | 89 (45%) | |
| ≥65 | 132 (33%) | 7 (28%) | 25 (38%) | 38 (34%) | 62 (31%) | |
| Mayo Endoscopy score | 0.32 | |||||
| Grade 1 | 53 (13%) | 5 (20%) | 9 (14%) | 13 (12%) | 26 (13%) | |
| Grade 2 | 210 (53%) | 14 (56%) | 37 (57%) | 54 (49%) | 105 (53%) | |
| Grade 3 | 136 (34%) | 6 (24%) | 19 (29%) | 44 (40%) | 67 (34%) | |
| Hospitalized at baseline | 154 (39%) | 9 (36%) | 28 (43%) | 47 (42%) | 70 (35%) | 0.54 |
aExtensive/Pancolitis/Unassessable due to severity of disease were all included in this group.
bFisher exact test comparing age groups.
Serologic Immune Markers
Perinuclear anti-neutrophil cytoplasmic antibody (pANCA)
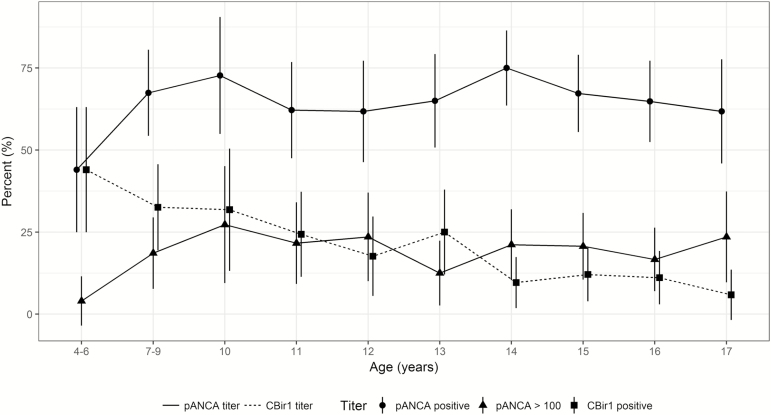
Perinuclear anti-neutrophil cytoplasmic antibody (pANCA) was positive in 65% (260 of 399) of patients. The percentage of pANCA positive was lower in ages 4–6 (44%, 11 of 25) than ages 7–17 (67%, 249/ of 34; P = 0.022) (See Fig. 1). The prevalence of pANCA was comparable across the other age groups: 7–10 (69%), 11–13 (63%), and 14–17 (68%) years old (P = 0.63). Positive pANCA did not correspond to a specific phenotype, (ie, there was no significant difference in extent of disease, PUCAI, Mayo score, rectal sparing, or basal plasmacytosis) (Table 2). However, average age-adjusted pANCA titer was somewhat higher for extensive pancolitis (least squares mean 60.0; 95% CI, 55.3–64.7) compared with proctosigmoiditis (39.2; 95% CI:,21.7–56.7) and left-sided disease (48.1; 95% CI, 34.4–61.5; P = 0.028). A pANCA titer ≥100, as observed in 19% of the patients, was strongly associated with pancolitis. All but 3 of the 76 (96%) with pANCA ≥100 had extensive colitis, as compared with 81% of the remaining 324 with pANCA <100 (P = 0.001) and 83% of the 139 who were pANCA negative (P = 0.005). However, the PUCAI and Mayo endoscopy subscores were not significantly different between groups with pANCA ≥100 and pANCA <100.
Figure 1.
Relationship of serologic reactivity and age in children newly diagnosed with UC. Bars represent 95% confidence intervals for the percent seropositive per age category.
Table 2:
Relationship of pANCA to Clinical and Laboratory Features in Children Newly Diagnosed with UC
| pANCA- | pANCA+ | P | pANCA + and < 100 | pANCA ≥ 100 | P comparing pANCA-, <100, ≥100 | |
|---|---|---|---|---|---|---|
| N = 139 | N = 260 | N = 184 | N = 76 | |||
| Age, mean (SD) | 12.5 ± 3.6 | 12.8 ± 3.1 | 0.29 | 12.7 ± 3.2 | 13.1 ± 2.8 | 0.41 |
| pANCA (%) stratified by age categories | — | — | 0.22 | — | — | 0.26 |
| 4–6 | 14/25 (56%) | 11/25 (44%) | 0.022 | 10/25 (40%) | 1/25 (4%) | 0.03b |
| 7–10 | 20/65 (31%) | 45/65 (69%) | — | 31/65 (48%) | 14/65 (22%) | — |
| 11–13 | 41/111 (37%) | 70/111 (63%) | — | 49/111 (44%) | 21/111 (19%) | — |
| 14–17 | 64/198 (32%) | 134/198 (68%) | — | 94/198 (47%) | 40/198 (20%) | — |
| Disease extent (%) | — | — | 0.60 | — | — | 0.004 |
| Proctosigmoiditis | 12 (9%) | 12 (5%) | — | 11 (6%) | 1 (1%) | — |
| Left-sided colitis | 12 (9%) | 29 (11%) | — | 27 (15%) | 2 (3%) | — |
| Extensive /Pancolitisa | 115 (83%) | 219 (84%) | — | 146 (79%) | 73 (96%) | — |
| PUCAI, mean (SD) | 48.3 ± 20.6 | 50.8 ± 19.6 | 0.23 | 50.6 ± 20.1 | 51.4 ± 18.3 | 0.45 |
| PUCAI score (%) | — | — | 0.24 | — | — | 0.47 |
| 10–30 | 41 (29%) | 54 (21%) | — | 39 (21%) | 15 (20%) | — |
| 35–60 | 53 (38%) | 119 (46%) | — | 81 (44%) | 38 (50%) | — |
| ≥65 | 45 (32%) | 87 (33%) | — | 64 (35%) | 23 (30%) | — |
| Mayo Endoscopic Sub-score (%) | — | — | 0.50 | — | — | 0.55 |
| Grade 1 | 23 (17%) | 30 (12%) | — | 21 (11%) | 9 (12%) | — |
| Grade 2 | 69 (50%) | 141 (54%) | — | 104 (57%) | 37 (49%) | — |
| Grade 3 | 47 (34%) | 89 (34%) | — | 59 (32%) | 30 (39%) | — |
| Relative rectal sparing (%) | 13/139 (9%) | 23/259 (9%) | 0.88 | 13/183 (7%) | 10/76 (13%) | 0.30 |
| Basal plasmacytosis (%) | 56/111 (50%) | 112/210 (53%) | 0.62 | 74/151 (49%) | 38/59 (64%) | 0.12 |
| Hgb (g/dL) | N = 130 | N = 246 | 0.59 | N = 176 | N = 70 | 0.53 |
| Mean (SD) | 11.5 ± 2.4 | 11.4 ± 2.1 | — | 11.5 ± 2.1 | 11.2 ± 2.1 | — |
| ESR (mm/hr) | N = 126 | N = 236 | 0.043 | N = 170 | N = 66 | — |
| Median (IQR) | 22 (11, 35) | 26 (12, 47) | — | 23 (11,41) | 34 (22,55) | <0.0001 |
| Albumin (g/dL) | N = 139 | N = 258 | 0.39 | N = 182 | N = 76 | |
| Mean (SD) | 3.7 ± 0.7 | 3.6 ± 0.7 | 3.7 ± 0.7 | 3.6 ± 0.7 | 0.43 | |
| Calprotectin (mcg/g) | N = 82 | N = 146 | 0.20 | N = 108 | N = 38 | — |
| Median (IQR) | 2023 (1083,3975) | 2676 (1378,3928) | — | 2932 (1414,4036) | 1754 (1088,3544) | 0.17 |
| CRP or hsCRP (%) | N = 103 | N = 192 | — | N = 142 | N = 50 | — |
| >ULN | 40 (39%) | 92 (48%) | 0.13 | 63 (44%) | 29 (58%) | 0.08 |
| >2xULN | 27 (26%) | 64 (33%) | 0.21 | 45 (32%) | 19 (38%) | 0.32 |
| CBir1 positive (%) | 33 (24%) | 44 (17%) | 0.10 | 28 (15%) | 16 (21%) | 0.14 |
aExtensive/Pancolitis /Unassessable due to severity of disease were all included in this group. b Comparison of pANCA for age <7 vs age ≥7.
Anti-flagellin (Anti-CBir1)
Anti-CBir1 was positive in 19% (77 of 399) of patients and more commonly positive in younger children. The percentage of Anti-CBir1 positive decreased steadily with age (Spearman rank correlation = −0.25, P < 0.001), with the highest in ages 4–6 (44%) and lowest in ages 14–17 (10%) (Fig. 1). Table 3 characterizes the associations of Anti-CBir1 with phenotype and baseline laboratory studies. More limited disease (proctosigmoiditis or left-sided colitis) was more common in Anti-CBir1-negative patients (18% vs 9%, P = 0.041). However, relative rectal sparing was more common in Anti-CBir1-positive patients (16% vs 7 %, P = 0.023), and basal plasmacytosis on rectal biopsy was less common (38% vs 56% P = 0.016). There was no association between pANCA and Anti-CBir1 in any age group.
Table 3:
Relationship of CBir1 to clinical and laboratory features of children newly diagnosed with UC
| CBir1+ N = 77 | CBir1- N = 322 | P | |
|---|---|---|---|
| Age, mean (SD) | 10.9 ± 3.6 | 13.1 ± 3.1 | <0.0001 |
| CBIR (%) stratified by age categories | <0.0001 | ||
| 4–6 | 11/25 (44%) | 14/25 (56%) | — |
| 7–10 | 21/65 (32%) | 44/65 (68%) | — |
| 11–13 | 25/111 (23%) | 86/111 (77%) | — |
| 14–17 | 20/198 (10%) | 178/198 (90%) | — |
| Disease extent (%) | 0.041 | ||
| Proctosigmoiditis | 0 (0%) | 24 (7%) | — |
| Left-sided colitis | 7 (9%) | 34 (11%) | — |
| Extensive /Pancolitisa | 70 (91%) | 264 (82%) | — |
| PUCAI, mean (SD) | 45.7 ± 21.4 | 51.0 ± 19.4 | 0.052 |
| PUCAI score (%) | 0.095 | ||
| 10–30 | 25 (32%) | 70 (22%) | — |
| 35–60 | 30 (39%) | 142 (44%) | — |
| ≥65 | 22 (29%) | 110 (34%) | — |
| Mayo Endoscopic Sub-score (%) | |||
| Grade 1 | 13 (17%) | 40 (12%) | 0.18 |
| Grade 2 | 42 (55%) | 168 (52%) | — |
| Grade 3 | 22 (29%) | 114 (35%) | — |
| Relative rectal sparing (%) | 12/76 (16%) | 24/322 (7%) | 0.023 |
| Basal plasmacytosis (%) | 22/58 (38%) | 146/263 (56%) | 0.015 |
| Hgb (g/dL) | 11.0 ± 2.2 | 11.5 ± 2.2 | 0.063 |
| Mean (SD) | N = 75 | N = 301 | — |
| ESR (mm/hr) | 25 (16, 49) | 24 (12, 40) | 0.33 |
| Median (IQR) | N = 70 | N = 292 | — |
| Albumin (g/dL) | 3.7 ± 0.8 | 3.6 ± 0.7 | 0.26 |
| Mean (SD) | N = 77 | N = 320 | |
| Calprotectin (mcg/g) | 1495 (971, 3333) | 2634 (1324, 4022) | 0.046 |
| Median (IQR) | N = 35 | N = 193 | — |
| CRP or hsCRP (%) | |||
| >ULN | 24 (44%) | 108 (45%) | 0.85 |
| >2xULN | 17 (31%) | 74 (31%) | 0.99 |
| N = 55 | N = 240 | — | |
| 25-OH Vitamin D (ng/mL) | 30.9 (27.2,38.0) | 27.6 (23.0,33.4) | 0.0002 |
| Median (IQR) | N = 75 | N = 317 | — |
| 25-OH Vitamin D (%) | — | — | 0.0009 |
| <20 Deficient | 5 (7%) | 37 (12%) | — |
| 20-< 30 Insufficient | 25 (33%) | 159 (50%) | — |
| ≥30 Sufficient | 45 (60%) | 121 (38%) | 0.005b |
| pANCA positive (%) | 44 (57%) | 216 (67%) | 0.10 |
aExtensive/Pancolitis /Unassessable due to severity of disease were all included in this group.
b P value for association of CBir1 with 25-OH vitamin D ≥30 stratified by age-group based on a Mantel-Haenszel χ2 test.
Fecal calprotectin was higher in the Anti-CBir1-negative group (median: 2634 mcg/g, interquartile range [IQR]: 1324–4022]) compared with the Anti-CBir1-positive group (median: 1495 mcg/g [IQR: 973–3333], P = 0.046), despite similar Mayo endoscopy subscores, ESR, and CRP. Additionally, both the median 25-OH vitamin D level (P = 0.0002) and the percentage of patients with sufficient levels of 25-OH vitamin D (P = 0.0009) were strikingly higher in the Anti-CBir1-positive group. This relationship between vitamin D sufficiency and Anti-CBir1 positivity remained significant when the patients were stratified by age group (P = 0.005) (Table 3).
Anti-outer membrane C (Anti-OmpC) and Anti-saccharomyces cerevisiae antibody (ASCA)
Anti-OmpC and ASCA IgA/IgG were positive in only a small number of our pediatric UC patients, 5% and 2%, respectively.
Discussion
In our large, rigorously phenotyped inception cohort of children with UC, we have shown that 65% of children newly diagnosed with UC have a positive pANCA titer. This is similar to the rate of pANCA positivity in adults, which has been shown to be 60%–65%10–12, 24–28. We also demonstrated in our pediatric population that there is an association between high titer pANCA (≥100) and more extensive disease; yet, interestingly, we found that the PUCAI and Mayo endoscopic subscores were not significantly different between high and low titer pANCA groups. Previous studies have shown that pANCA titers can be used to risk stratify patients into immunologically distinct subgroups20. In a pediatric study from 1998, Ruemmele et al observed differences in the titer of pANCA within subgroups of IBD, with equivalently high titers in UC-like CD with pancolitis (median of 68.2 EU/mL) and UC (median of 57.7 EU/mL)9. This stratification by pANCA titer has been further explored in adult UC, with Fleshner et al showing that a pre-operative pANCA >100 titer was a risk factor for the development of chronic pouchitis after ileal pouch-anal anastomosis23 and White et al reporting an association between high titer pANCA and both pancolitis and backwash ileitis29. Our observed association of higher pANCA titer with more extensive endoscopic disease activity corroborates this relationship between magnitude of immune reactivity and extent of disease activity.
Beyond phenotypic predictions, pANCA titers have been linked to response to therapy. Importantly, Sandborn et al showed in a 1996 retrospective study of 56 UC patients with left-sided disease that those who were pANCA-positive were more likely to have treatment-resistant disease (90% vs 62%)30. And in a larger 2007 study of 100 IBD patients starting infliximab (IFX) therapy, it was confirmed that patients who were pANCA+/ASCA− had lower early clinical response—55% vs 76% 31. In children, Dubinsky et al in 2010 reported that pANCA positivity is independently associated with primary nonresponse to anti-TNFα therapy in both CD and UC (odds ratio [OR]: 5.4; P = 0.01) 32. Subsequent long-term analysis of the PROTECT cohort will be required to determine if a relationship exists between pANCA titer and response to various therapies and ultimate outcomes.
Unlike pANCA, the microbial antibodies, ASCA, Anti-OmpC, and Anti-CBir1 are more common in CD than UC with prevalence in CD of 34%–76%, 17%–24%, and 55%–56%, respectively13, 25, 33, 34. Our data confirmed that antibodies to ASCA and Anti-OmpC are uncommon in pediatric UC. Importantly, although Anti-CBir1 reactivity is negatively associated with UC in adults35, 36, 19% of our population was Anti-CBir1-positive. This might raise suspicion about the diagnosis of UC in our study population; however, observation of the study cohort for 1 to 3 years has resulted in no change in diagnosis of any of the study patients, suggesting that Anti-CBir is more common in pediatric UC than previously appreciated. In part, this might be due to the fact that Anti-CBir1 reactivity is more common with younger age, as demonstrated now in our pediatric UC cohort and previously in pediatric CD13.
From a phenotypic perspective, Anti-CBir1 reactivity was associated with rectal sparing, more limited disease extent, lower frequency of basal plasmacytosis on rectal biopsy, and lower fecal calprotectin. Spiliadis et al in 1987 were the first group to report 12 UC patients with relative endoscopic rectal sparing from their 27-year experience37. Glickman et al in 2004 described that histologically patchy disease and relative rectal sparing occurred in 21% and 26%, respectively, of 73 pediatric UC patients; interestingly, they also had a comparison group of adults that had neither rectal sparing nor patchy disease. In this same group, another 16% had only very subtle histologic findings of chronic inflammation38, supporting a prior study by Washington et al, who showed no evidence of crypt architectural distortion in 34% of biopsies in new-onset UC39. Rajwal et al reported that, in general, relative rectal sparing is more common in those younger than 10 years old40. This is compatible with our observations that Anti-CBir1 was associated with younger age, as well as relative-rectal sparing. It is unclear as to whether there are prognostic implications of rectal sparing as it relates to therapeutic response or surgical outcomes. Additionally, the CBir1 antigen has a high degree of homology to bacterial flagellins, resembling those from enteric microbiota including Butyrivibrio, Roseburia, Thermotoga, and Clostridium species20. Further examination of Anti-CBir1, in conjunction with microbiome data, is warranted.
Lower levels of the damage-associated molecular pattern proteins S100A8/S100A9, collectively called fecal calprotectin, in the Anti-CBir1-positive group might be explained by the relationship between the innate and adaptive immune system. Fecal calprotectin mediates a number of different antimicrobial functions, and since some patients are low fecal calprotectin expressers, this could theoretically lead to mitigation of the microbiota adherent to the mucosa and altered triggering of Anti-CBir1 antibodies17, 25, 34.
The relationship between higher, more often sufficient levels of 25-OH vitamin D and Anti-CBir1-positive serologies is intriguing. It is recognized that vitamin D is an important component of both the innate and adaptive immune systems. Immune cells produce both the vitamin D receptor (VDR) and the enzymes to convert vitamin D3 into its active 1,25(OH)2D3 form, and this active form can modulate numerous cell types, including activated T- and B-cells, macrophages, and dendritic cells, leading to an observed antimicrobial effect41, 42. Additionally, vitamin D affects intestinal barrier function, and VDR-/- mice have been observed to have disruptions in their tight junctions, leading to increased susceptibility to DSS injury43. It is possible that vitamin D mediates the immune response in such a way that alters the adaptive anti-flagellin response, leading to production of Anti-CBir1.
Our study has several limitations. Due to its multicenter nature, there may have been site-specific differences in clinical scoring despite prespecified guidelines in the PROTECT study protocol. Mayo endoscopy su-scores were guided at each site by standardized photographs. Histologic examination was done by a central reader (MHC). There was also a larger acceptable window for attaining baseline labs, which included the serological assessment, before treatment in those with mild disease (8 weeks vs 4 weeks); however, it is unlikely that there would have been large shifts in titers in this mild population over this short interval. Given the exploratory nature of our study, unadjusted P values were used.
In summary, our study greatly extends the knowledge base of serologic expression in children with UC. Future work will be required to examine the relationship of serologic reactivity to colonic community microbial profiling as well as rectal gene expression. These relationships may provide a platform to better understand disease pathogenesis and perhaps response to therapy.
Acknowledgements
We are grateful to PROTECT patients/families for their enthusiastic cooperation with the study, and research coordinators at all the PROTECT sites for their time and dedication. We would like to thank the following PROTECT Study Group Principal Investigators for their contributions to the study: Keith Benkov, MD, Jonathan Evans, MD, Stephen Guthery, MD, Michael Kappelman, MD, Dedrick Moulton, MD, Jennifer Strople, MD, Boris Sudel MD, Prateek Wali, MD, and David Ziring, MD, as well as Vin Tangpricha, MD, for performing the 25-OH vitamin D analyses.
Glossary
Abbreviations:
- IBD
inflammatory bowel disease
- UC
ulcerative colitis
- CD
Crohn’s disease
- pANCA
perinuclear anti-neutrophil cytoplasmic
- ASCA
anti-saccharomyces cerevisiae antibody
- Anti-CBir1
anti-flagellin
- Anti-OmpC
anti-outer membrane C
- PUCAI
pediatric ulcerative colitis activity index
- HPF
high-power field
- Hgb
hemoglobin
- Hct
hematocrit
- WBC
white blood cell count
- ESR
erythrocyte sedimentation rate
- CRP
C-reactive protein
- ULN
upper limit of normal
- VDR
vitamin D receptor.
Conflicts of interest: ES has no conflicts to declare. SD is on the independent data monitoring committee at Lycera Corporation. DM is the owner of and has shares in Biotagenics. BB has no conflicts to declare. AM receives research support from and is a consultant for Abbvie. AM is also a consultant for Janssen, Merck, and Takeda and is a speaker for Abbvie and Janssen. NL is a consultant for Abbvie. CG is a consultant for Abbvie. DK has no conflicts to declare. JM is a consultant for Janssen, UCB and Lilly. SB has no conflicts to declare. JR received grant funding from Janssen and Abbvie, in addition to being a consultant for Abbvie, Janssen, Luitpold and UCB. RB has no conflicts to declare. MOH has research grants from Abbvie Immunology and Janssen and does consulting for Hoffman Laroche. MP has no conflicts to declare. AO is on the advisory board of Janssen and Abbvie and receives research support from Abbvie, Janssen, Shire and Astellas. MH has received research grants from Genentech, AbbVie, Sucampo and Janssen. JN has no conflicts to declare. AP is on the speaker’s bureau for Abbvie. PR is a consultant for Shire and Leutpold and a speaker for Abbvie and receives research support from TechLab. MAM and TW have no conflicts to declare. MC is a consultant for Shire, Regeneron, Adare and Receptos and has received research grants from Shire, Regeneron and Receptos. SK is a consultant for Janssen and UCB. LD has received grant support from Abbvie and Janssen. JH is on the advisory board for Janssen and a consultant for Abbvie, Takeda, Lilly, Boerhinger-Ingelheim, Allergan, Pfizer and Astra Zeneca. MD is a consultant for Abbvie, Boehringer-Ingelheim, Celgene, Genetech, Janssen, Pfizer, Prometheus Labs, Salix, Shire, Takeda and UCB.
Author Contributions: ES, SD, TW, MC, SK, LD, JH and MD conceived and designed the study. DM, BB, AG, NL, CS, DK, JM, SB, JR, RB, MOH, MP, AO, MH, JN, AS, PR, TW, SK, LD, JH, MD and the PROTECT Study Group acquired data. All authors except the PROTECT Study Group analyzed and interpreted data. ES, SD, AG, NL, JM, TW, SK, LD, JH, and MD drafted the manuscript. All authors except the PROTECT Study Group critically revised the manuscript for important intellectual content. Davis and Marquis performed statistical analysis.
Supported by NIDDK 5U01DK095745.
Contributor Information
PROTECT Study Group:
Keith Benkov, Jonathan Evans, Stephen Guthery, Michael Kappelman, Dedrick Moulton, Jennifer Strople, Boris Sudel, Prateek Wali, David Ziring, and Vin Tangpricha
References
- 1. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. [DOI] [PubMed] [Google Scholar]
- 2. Hyams J, Davis P, Lerer T, et al. . Clinical outcome of ulcerative proctitis in children. J Pediatr Gastroenterol Nutr. 1997;25:149–152. [DOI] [PubMed] [Google Scholar]
- 3. Gower-Rousseau C, Dauchet L, Vernier-Massouille G, et al. . The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 2009;104:2080–2088. [DOI] [PubMed] [Google Scholar]
- 4. Turner D, Mack D, Leleiko N, et al. . Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282–2291. [DOI] [PubMed] [Google Scholar]
- 5. Solberg IC, Lygren I, Jahnsen J, et al. ; IBSEN Study Group Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN study). Scand J Gastroenterol. 2009;44:431–440. [DOI] [PubMed] [Google Scholar]
- 6. Höie O, Schouten LJ, Wolters FL, et al. ; European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD) Ulcerative colitis: no rise in mortality in a european-wide population based cohort 10 years after diagnosis. Gut. 2007;56:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Limbergen J, Russell RK, Drummond HE, et al. . Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–1122. [DOI] [PubMed] [Google Scholar]
- 8. Hyams J, Markowitz J, Lerer T, et al. ; Pediatric Inflammatory Bowel Disease Collaborative Research Group The natural history of corticosteroid therapy for ulcerative colitis in children. Clin Gastroenterol Hepatol. 2006;4:1118–1123. [DOI] [PubMed] [Google Scholar]
- 9. Ruemmele FM, Targan SR, Levy G, et al. . Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–829. [DOI] [PubMed] [Google Scholar]
- 10. Quinton JF, Sendid B, Reumaux D, et al. . Anti-saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubinsky MC. What is the role of serological markers in the diagnosis of IBD?Inflamm Bowel Dis. 2008;14:S185–S186. [DOI] [PubMed] [Google Scholar]
- 12. Mitsuyama K, Niwa M, Takedatsu H, et al. . Antibody markers in the diagnosis of inflammatory bowel disease. World J Gastroenterol. 2016;22:1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Markowitz J, Kugathasan S, Dubinsky M, et al. . Age of diagnosis influences serologic responses in children with Crohn’s disease: a possible clue to etiology?Inflamm Bowel Dis. 2009;15:714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turner D, Otley AR, Mack D, et al. . Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–432. [DOI] [PubMed] [Google Scholar]
- 15. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. [DOI] [PubMed] [Google Scholar]
- 16. Boyle B, Collins MH, Wang Z, et al. . Histologic correlates of clinical and endoscopic severity in children newly diagnosed with ulcerative colitis. In press; 2017 [DOI] [PMC free article] [PubMed]
- 17. Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524–534. [DOI] [PubMed] [Google Scholar]
- 18. Burri E, Manz M, Rothen C, et al. . Monoclonal antibody testing for fecal calprotectin is superior to polyclonal testing of fecal calprotectin and lactoferrin to identify organic intestinal disease in patients with abdominal discomfort. Clin Chim Acta. 2013;416:41–47. [DOI] [PubMed] [Google Scholar]
- 19. Lee MJ, Kearns MD, Smith EM, et al. . Free 25-hydroxyvitamin D concentrations in cystic fibrosis. Am J Med Sci. 2015;350:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dubinsky MC, Kugathasan S, Mei L, et al. ; Western Regional Pediatric IBD Research Alliance; Pediatric IBD Collaborative Research Group; Wisconsin Pediatric IBD Alliance Increased immune reactivity predicts aggressive complicating Crohn’s disease in children. Clin Gastroenterol Hepatol. 2008;6:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohavy O, Bruckner D, Gordon LK, et al. . Colonic bacteria express an ulcerative colitis panca-related protein epitope. Infect Immun. 2000;68:1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vasiliauskas EA, Kam LY, Karp LC, et al. . Marker antibody expression stratifies Crohn’s disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleshner PR, Vasiliauskas EA, Kam LY, et al. . High level perinuclear antineutrophil cytoplasmic antibody (panca) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birimberg-Schwartz L, Wilson DC, Kolho KL, et al. ; paediatric IBD Porto group of ESPGHAN Panca and ASCA in children with IBD-unclassified, Crohn’s colitis, and ulcerative colitis-A longitudinal report from the IBD porto group of ESPGHAN. Inflamm Bowel Dis. 2016;22:1908–1914. [DOI] [PubMed] [Google Scholar]
- 25. Cioffi M, Rosa AD, Serao R, et al. . Laboratory markers in ulcerative colitis: current insights and future advances. World J Gastrointest Pathophysiol. 2015;6:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reese GE, Constantinides VA, Simillis C, et al. . Diagnostic precision of anti-saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–2422. [DOI] [PubMed] [Google Scholar]
- 27. Vasiliauskas EA, Plevy SE, Landers CJ, et al. . Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn’s disease define a clinical subgroup. Gastroenterology. 1996;110:1810–1819. [DOI] [PubMed] [Google Scholar]
- 28. Peyrin-Biroulet L, Standaert-Vitse A, Branche J, et al. . IBD serological panels: facts and perspectives. Inflamm Bowel Dis. 2007;13:1561–1566. [DOI] [PubMed] [Google Scholar]
- 29. White E, Melmed GY, Vasiliauskas EA, et al. . A prospective analysis of clinical variables, serologic factors, and outcome of ileal pouch-anal anastomosis in patients with backwash ileitis. Dis Colon Rectum. 2010;53:987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandborn WJ, Landers CJ, Tremaine WJ, et al. . Association of antineutrophil cytoplasmic antibodies with resistance to treatment of left-sided ulcerative colitis: results of a pilot study. Mayo Clin Proc. 1996;71:431–436. [DOI] [PubMed] [Google Scholar]
- 31. Ferrante M, Vermeire S, Katsanos KH, et al. . Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13:123–128. [DOI] [PubMed] [Google Scholar]
- 32. Dubinsky MC, Mei L, Friedman M, et al. . Genome wide association (GWA) predictors of anti-tnfalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- 34. Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149:1275–1285.e2. [DOI] [PubMed] [Google Scholar]
- 35. Targan SR, Landers CJ, Yang H, et al. . Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020–2028. [DOI] [PubMed] [Google Scholar]
- 36. Papadakis KA, Yang H, Ippoliti A, et al. . Anti-flagellin (CBir1) phenotypic and genetic Crohn’s disease associations. Inflamm Bowel Dis. 2007;13:524–530. [DOI] [PubMed] [Google Scholar]
- 37. Spiliadis CA, Spiliadis CA, Lennard-Jones JE. Ulcerative colitis with relative sparing of the rectum. Clinical features, histology, and prognosis. Dis Colon Rectum. 1987;30:334–336. [DOI] [PubMed] [Google Scholar]
- 38. Glickman JN, Bousvaros A, Farraye FA, et al. . Pediatric patients with untreated ulcerative colitis may present initially with unusual morphologic findings. Am J Surg Pathol. 2004;28:190–197. [DOI] [PubMed] [Google Scholar]
- 39. Washington K, Greenson JK, Montgomery E, et al. . Histopathology of ulcerative colitis in initial rectal biopsy in children. Am J Surg Pathol. 2002;26:1441–1449. [DOI] [PubMed] [Google Scholar]
- 40. Rajwal SR, Puntis JW, McClean P, et al. . Endoscopic rectal sparing in children with untreated ulcerative colitis. J Pediatr Gastroenterol Nutr. 2004;38:66–69. [DOI] [PubMed] [Google Scholar]
- 41. Reich KM, Fedorak RN, Madsen K, et al. . Vitamin D improves inflammatory bowel disease outcomes: basic science and clinical review. World J Gastroenterol. 2014;20:4934–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kong J, Zhang Z, Musch MW, et al. . Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–G216. [DOI] [PubMed] [Google Scholar]