Abstract
Acute hyperglycemia has been shown to augment indices of arterial stiffness in patients with insulin resistance and other co-morbidities, however, conflicting results exist in healthy young individuals. We examined if acute hyperglycemia following an oral glucose tolerance test (OGTT) increases arterial stiffness in healthy active men before, and then after reduced ambulatory physical activity to decrease insulin sensitivity. High-resolution arterial diameter tracings acquired from Doppler-ultrasound allowed the obtainment of an arterial blood pressure (BP) waveform from the diameter tracing within a cardiac cycle. In 24 subjects, this method demonstrated sufficient agreement with the traditional approach for assessing arterial compliance using applanation tonometry. In 10 men, continuous recordings of femoral and brachial artery diameter, and beat-to-beat BP (Finometer) were acquired at rest, 60, and 120 mins of an OGTT before and after 5 days of reduced activity (>10,000 to <5,000 steps/day). Compliance and beta-stiffness were quantified. Prior to reduced activity, OGTT had no effect on arterial compliance or beta-stiffness. However, following reduced activity, femoral compliance was decreased (rest= 0.10±0.03 to 120-min OGTT= 0.06±0.02 mm2/mmHg; P<0.001) and femoral beta-stiffness increased (rest= 8.7±2.7 to 120-min OGTT= 15.3±6.5 AU; P<0.001) during OGTT, while no changes occurred in brachial artery compliance (P=0.182) or stiffness (P=0.892). Insulin sensitivity (Matsuda Index) was decreased following reduced activity (P=0.002). In summary, in young healthy men, the femoral artery becomes susceptible to acute hyperglycemia following 5 days of reduced activity and the resultant decrease in insulin sensitivity, highlighting the strong influence of daily physical activity levels on vascular physiology.
Keywords: Physical Activity, Glucose Tolerance, Insulin Resistance, Vascular Stiffness
Introduction
Only 1 in 5 U.S. adults meet the current physical activity recommendations (CDC, 2016). Low habitual physical activity is linked to impaired glucose tolerance (Annuzzi et al., 1985), and even short-term reductions in ambulatory activity can lead to significant reductions in insulin sensitivity in healthy young individuals (Mikus et al., 2012; Reynolds et al., 2014). Another risk associated with inactivity is atherosclerosis, an important contributor to cardiovascular disease development. Atherosclerosis is thought to be initiated by endothelial dysfunction (Ross et al., 1984), with subsequent increases in arterial stiffness (Oliver & Webb, 2003). Importantly, recent data from our laboratory demonstrates that just 5 days of reduced ambulatory physical activity is enough to cause significant reductions in insulin sensitivity and impairment in vascular endothelial function of the legs (Boyle et al., 2013). However, the impact of reductions in insulin sensitivity via short term reductions in ambulatory activity on arterial stiffness is less clear.
Acute hyperglycemia, as occurs during an oral glucose tolerance test (OGTT), has been shown to augment indices of arterial stiffness (e.g., pulse wave velocity, cardio-ankle index, and augmentation index) in people with insulin resistance and other co-morbidities (Gordin et al., 2007; Huang et al., 2007; Gordin et al., 2016). However, conflicting evidence exists in regards to this response in healthy young individuals (Gordin et al., 2007; Huang et al., 2007; Keller et al., 2016). For example, one study reported that acute hyperglycemia increased brachial pulse wave velocity (PWV) only in young patients with type 1 diabetes, but not in healthy controls (Gordin et al., 2007). In contrast, in another report, glucose responses to an OGTT were directly correlated with increases in systemic arterial stiffness (cardio-ankle index) in healthy participants (Huang et al., 2007). More recently, decreases in insulin sensitivity mediated by a one week reduction in physical activity had no effect on central blood pressure and augmentation index at rest, or in response to sugar sweetened beverage consumption (Keller et al., 2016). Although the reason for these discrepant responses in young healthy subjects is unclear, differences in methodologies used to examine vascular stiffness may have contributed. Importantly, to date, no studies have examined any direct local measure of large artery stiffness, such as arterial compliance, during acute hyperglycemia in healthy individuals. Furthermore, it is unknown whether alterations in insulin sensitivity following an acute reduction in physical activity can negatively impact local arterial compliance at rest and/or in response to acute hyperglycemia in healthy young individuals. These are important questions to provide mechanistic insight on the effects of insulin resistance on the peripheral vascular response to glucose ingestion without the confounding influence of overt disease and other co-morbidities or factors (e.g., age).
With this background in mind, we developed a methodology to assess local arterial compliance in humans that would eliminate the need for concomitant measures of tonometry and duplex Doppler-ultrasound in bilateral arteries (Lage et al., 1993; DeVan et al., 2005; Yoshizawa et al., 2009). This was made possible by the development of a data acquisition system and custom software that allows for high resolution analysis of beat-to-beat arterial diameter and velocity (Padilla et al., 2011b; Simmons et al., 2011; Fairfax et al., 2013; Credeur et al., 2014a; Credeur et al., 2014b). Importantly, this novel approach demonstrated sufficient agreement with the traditional approach using applanation tonometry (Protocol 1). Next, we used this methodology to examine if acute hyperglycemia following an OGTT increases arterial stiffness in the arm and leg (brachial and femoral arteries, respectively) in healthy active young men before and then after 5 days of reduced ambulatory physical activity to decrease insulin sensitivity (Protocol 2). We hypothesized that arterial stiffness would increase during an OGTT in young healthy subjects only in the presence of reduced insulin sensitivity following 5 days of reduced physical activity. Furthermore, we reasoned that this response would be more pronounced in the arteries of limbs that are most affected by reductions in daily physical activity (i.e., femoral artery).
Methods
Ethical Approval
All subjects provided written informed consent prior to participating in any facet of the study. Study protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri Health Sciences (Project#: 1200428) and University of Texas at Arlington (Project#: 2016-0783) Institutional Review Boards. The results from this study were not registered in any research database as specified by clause 35 of the Declaration of Helsinki.
Protocol 1: Arterial compliance methods comparison
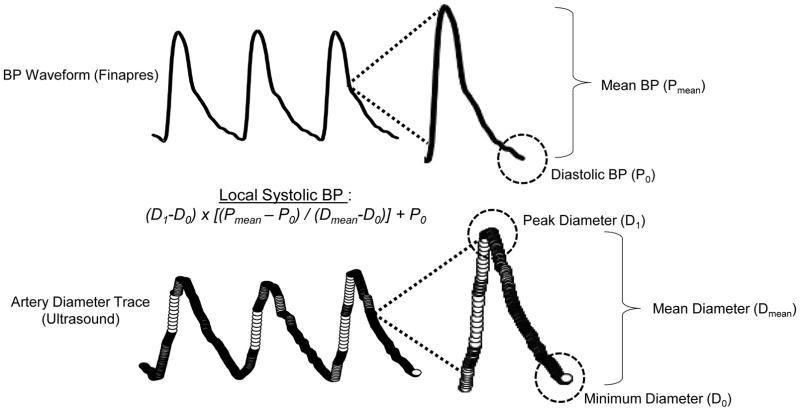
Traditionally, assessments of large artery compliance (e.g., carotid and femoral artery) utilize B-mode ultrasound to track arterial diameter changes coupled with applanation tonometry to estimate arterial blood pressure (BP) changes across the same cardiac cycles (Lage et al., 1993; DeVan et al., 2005; Yoshizawa et al., 2009). Using a two-point calibration procedure, the mean and nadir (diastolic) values from the tonometric waveforms are calibrated to a peripheral BP measure (e.g., brachial artery) to estimate local systolic BP. Arterial compliance is then calculated as the change in arterial diameter per unit change in local BP (Lage et al., 1993; DeVan et al., 2005; Yoshizawa et al., 2009). Herein, we employ a similar assessment, but remove the need for applanation tonometry in a contralateral artery, with high-resolution arterial diameter tracings from Doppler-ultrasound alone. With this approach, we are not only able to acquire local beat-to-beat arterial diameter changes, but because of the high resolution of our custom Doppler-ultrasound acquisition and software, we can also obtain an arterial BP waveform from the diameter changes within a cardiac cycle (Figure 1). This waveform is then coupled with a local arterial BP measure for calibration, similar to how the waveform obtained from applanation tonometry is calibrated. The advantage of this approach is we are assessing beat-to-beat changes in diameter and BP within the same artery simultaneously.
Figure 1.
An original record showing three cardiac cycles for arterial blood pressure (BP)—top derived from Finapres, coupled with a high-resolution ultrasound femoral artery diameter trace—below. The third cardiac cycle is expanded to show the parameters needed for estimating local systolic BP, which subsequently is used to calculate arterial compliance.
Subjects
For this comparison protocol, we purposefully studied a heterogeneous group of subjects (N=24; age=46±21 years; BMI=27±4 kg/m2; women=5), consisting of young and older, men and women. The subjects participating in this protocol were different from those studied in Protocol 2. All subjects were recruited from local Universities (University of Missouri-Columbia and University of Texas-Arlington) and surrounding areas. Each subject completed a detailed medical health history questionnaire, to exclude any uncontrolled cardiovascular, pulmonary, metabolic, or neurological disease.
Experimental measurements
Three arteries were examined for this comparison study; the common carotid (N=11) which is a more conventional vessel used to examine arterial compliance (Oliver & Webb, 2003; Stoner et al., 2015), the common femoral (N=20) and the brachial artery (N=13), large skeletal muscle arteries relevant to the experiments performed in Protocol 2. Arterial diameter recordings were obtained using a duplex Doppler-ultrasound unit (Logiq-P5; GE, Milwaukee, WI) equipped with a linear array transducer (11L; 12 MHz), in B-mode. Ultrasound data were obtained through use of a customized acquisition and analysis program (LabVIEW; National Instruments), which allowed for the generation of high-resolution beat-to-beat arterial diameter tracings (see Figure 1). The right common carotid artery was scanned 1–2 cm proximal to the bulb, the right common femoral artery was scanned 2–3 cm proximal to the superficial and profundus branches, and the right brachial was scanned 3–5 cm proximal to the antecubital fossa. High-fidelity arterial BP waveforms were simultaneously obtained on the left common carotid, common femoral and brachial arteries, respectively, using applanation tonometry (Millar Inc., Houston, TX). Heart rate (HR) was continuously monitored using a lead II surface electrocardiography—ECG (Q710; Quinton, Bothell, WA), and finger photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) was performed on the right middle finger to measure beat-to-beat arterial BP. Return-to-flow calibrations were performed before each Finometer recording to ensure that accurate measurements were obtained. Arterial BP was also measured periodically with an automated sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) to further validate absolute BP values obtained with finger photoplethysmography.
Experimental Protocol
Subjects arrived to the laboratory, at least 3 hours postprandial and having refrained from strenuous physical activity for 24 hours, and caffeine and alcohol for 12 hours. Subjects rested quietly supine for 20 minutes in a dimly-lit, and temperature controlled laboratory (21–22°C). Following rest, continuous high-resolution arterial diameter tracings (B-mode ultrasound), along with simultaneous bilateral applanation tonometry and BP (Finometer) were obtained for 60 seconds. Of the 24 subjects studied, 4 had common carotid measures performed alone on one day, 7 had carotid and femoral measures performed together on one day, and 13 had brachial and femoral measures performed together on one day. Data were then analyzed offline to calculate arterial compliance using the traditional tonometry and the ultrasound-derived methods, as described below in detail.
Data Analysis
All experimental data were acquired into a customized LabVIEW acquisition program interfaced with video output from the Doppler-ultrasound machine, as previously developed and used by our laboratory (Simmons et al., 2011; Credeur et al., 2014a; Reynolds et al., 2014). Images of the common carotid, common femoral and brachial arteries were acquired at 30-Hz, while ECG and BP waveforms (both tonometry and Finometer) were sampled simultaneously at 1-kHz, and embedded as data streams into an AVI-file containing video images from the ultrasound machine. All offline analyses were performed by the same individual (not blinded), separate from the ultrasonographer, using a customized edge-detection and wall-tracking software (LabVIEW) to determine the diameter traces (near and far wall lumen interface) over each cardiac cycle during the recording periods. These data were then processed using a second custom LabVIEW analysis program which generated synchronized beat-by-beat data of all recorded variables, including tonometry and Finometer derived BP gated by the R-wave from the ECG (Simmons et al., 2011; Credeur et al., 2014a; Reynolds et al., 2014). For the traditional arterial compliance assessment, tonometric arterial BP waveforms were calibrated by equating mean and diastolic BP from the Finometer, to the mean and diastolic BP obtained from the brachial artery (DeVan et al., 2005; Zamir et al., 2007; Yoshizawa et al., 2009). Arterial compliance was then calculated as the change in artery cross-sectional area over the change in local arterial BP (Lage et al., 1993; DeVan et al., 2005; Yoshizawa et al., 2009): Arterial Compliance (mm2 mmHg−1) = {[(D1−D0)/D0]/[2(P1−P0)]} x π x (D0)2; where D1 and D0 are the average maximal and minimum diameters across cardiac cycles, and P1 and P0 are local systolic and diastolic BP, respectively. For the ultrasound-derived method, arterial compliance was calculated the same way, except the beat-to-beat arterial diameter tracing was converted to BP waveforms (Figure 1), in place of tonometry waveforms, with Finometer BP values using a similar two-point calibration, where local systolic BP = (D1−D0) x [(Pmean – P0)/(Dmean−D0)] + P0; where Dmean and Pmean are mean diameter and BP, respectively.
Protocol 2: Arterial compliance during an OGTT before and after reduced physical activity
Given the limited and equivocal data available regarding changes in large artery stiffness in response to acute hyperglycemia in healthy young individuals (Gordin et al., 2007; Huang et al., 2007), we applied our ultrasound-derived arterial compliance assessment at rest and during an OGTT. These measures were performed before and after 5 days of reduced ambulatory physical activity (<5,000 steps/day) to decrease insulin sensitivity (Boyle et al., 2013; Reynolds et al., 2014).
Subjects
Ten healthy young men (age, 25±4 yrs; BMI, 25±2 kg/m2; VO2 max, 50±7 mL/kg/min) were studied. The rationale for studying only men stems from evidence that changes in menstrual cycle status could impact arterial stiffening, independent of changes in physical activity (Williams et al., 2001; Hayashi et al., 2006; Gavin et al., 2009; Adkisson et al., 2010). All subjects were recreationally active, defined as completing at least 90 min of primarily lower-body aerobic exercise ≥3 days per week (self-reported) and taking >10,000 steps/day, but were not training for competitive endurance events. Prior to the reduced physical activity intervention, pedometers (Walk 4 Life Duo, Plainfield, IL) were worn and daily energy expenditure above 3 METs (kilojoules) was determined via a physical activity monitor (Body Media), for three consecutive days. The subjects for Protocol 2 represent a subset of data examining the effects of 5 days of reduced physical activity on vascular endothelial function and insulin stimulated blood flow which were recently published by our laboratory (Boyle et al., 2013; Reynolds et al., 2014; Holwerda et al., 2015).
Experimental Measurements
Arterial compliance was assessed using the same ultrasound-derived method as described in Protocol 1. Simultaneous images of the right common femoral artery and left brachial artery were obtained using two separate Doppler-ultrasound units (Logiq-P5 for the femoral, and Logiq-7 for brachial artery; GE, Milwaukee, WI) equipped with linear array transducers (10–12 MHz). Probe position was marked on the skin and stabilized using customized clamps to ensure consistency in Doppler-ultrasound recordings between study time points. Similar to Protocol 1, the brachial artery was scanned 3–5 cm proximal to the antecubital fossa, the common femoral artery was scanned 2–3 cm proximal to the superficial and profundus branches, HR was continuously monitored using ECG, and the Finometer was used to measure beat-to-beat arterial BP.
Pre-Intervention Procedures
Prior to the reduced activity intervention, subjects underwent a graded maximal treadmill exercise test to determine maximal oxygen uptake (VO2 max). Two days prior to baseline testing, subjects consumed standardized meals (57% carbohydrate, 28% fat, and 15% protein) and continued this diet throughout the study intervention, as previously described (Boyle et al., 2013; Reynolds et al., 2014; Holwerda et al., 2015). A 45-minute supervised treadmill exercise session at a moderate intensity, i.e., 60% of heart rate reserve (Swain et al., 1998), was also performed 24 hours prior to baseline testing to control for the amount of exercise, and time of day by which each subject received their last bout of structured physical activity (Boyle et al., 2013). Subjects then began the 5 days of reduced physical activity (<5,000 steps/day) immediately following baseline testing. This volume of steps was chosen because it closely resembles the daily step-count of most physically inactive individuals (Bassett et al., 2010). To achieve the desired reduction in steps, subjects monitored their daily steps via pedometers, refrained from any structured physical activity, were encouraged to take the elevator instead of stairs, park closer to door entrances, and in certain situations, were transported around campus in a wheelchair by study personnel. In order to verify daily steps and energy expenditure, subjects continued wearing pedometers and physical activity monitors throughout the 5-day intervention (Boyle et al., 2013; Reynolds et al., 2014; Holwerda et al., 2015). Due to technical reasons, physical activity monitor data were not able to be collected in two subjects, however, pedometers were worn and step counts were obtained from these individuals.
Experimental Protocol
Subjects were fasted 12 hours prior to testing. All study visits were performed in the morning hours (7:00–10:00am) in a temperature controlled room (21–22°C). Subjects were positioned supine, and a catheter was inserted into the left antecubital vein. After 15 minutes of rest, baseline blood samples were obtained for plasma glucose and insulin, followed by baseline arterial compliance measurements. Following completion of these measures, the OGTT was performed (Mikus et al., 2012; Reynolds et al., 2014) in which subjects consumed a 75-gram glucose drink within 1 minute. Blood samples were drawn every 30 minutes for the following 2 hours, and femoral and brachial artery compliance measures were made at 60 and 120 minutes following start of the OGTT. This protocol was repeated following 5 days of reduced physical activity.
Data Analysis
All experimental data were acquired and analyzed using the same procedures outlined in Protocol 1. Ultrasound-derived arterial compliance was calculated by converting beat-to-beat arterial diameter tracings to a BP waveform from the Finometer waveform using a two-point calibration procedure, and subsequently, factored into the following equation: Arterial Compliance (mm2 mmHg−1) = {[(D1−D0)/D0]/[2(P1−P0)]} x π x (D0)2. In addition, the beta-stiffness index was calculated to provide an index of arterial compliance that is relatively independent of changes in distending pressure and diameter (Lage et al., 1993; DeVan et al., 2005; Yoshizawa et al., 2009): Beta-Stiffness (AU) = [ln (P1/P0)]/[(D1−D0)/D0)].
Plasma glucose and insulin during the OGTT
Plasma blood glucose measures were performed using the glucose oxidase method (Sigma, St. Louis, MO), and insulin was measured via enzyme-linked immunosorbent assays (Immulite 1000 Analyzer, Siemens, Deerfield, IL). Plasma glucose and insulin responses were examined from rest, to 60 and 120 minutes following start of the OGTT. Both glucose, and insulin area under the curve (AUC) during the OGTT were calculated using the trapezoidal method. The homeostatic model assessment for insulin resistance (HOMA-IR) was performed to assess fasting insulin resistance (Matthews et al., 1985; Mikus et al., 2012), and insulin sensitivity during the OGTT was assessed using the Matsuda index, as performed previously (Matsuda & DeFronzo, 1999; Mikus et al., 2011).
Statistical Analysis
For Protocol 1, the reliability between arterial compliance measures [traditional approach (applanation tonometry) vs. high-resolution ultrasound] were examined using intra-class correlations (ICC). The ICC estimates and their 95% confidence intervals were calculated using SPSS statistical package version 23 (SPSS Inc, Chicago, IL) based on average measures (k=2), absolute agreement, and a two-way mixed-effects model (Mcgraw, 1996). Bland-Altman plots were generated to examine agreement between methods (Bland & Altman, 1999) and created using MedCalc Statistical Software® (Version 17.7.2). For Protocol 2, the effects of reduced physical activity on study outcomes were examined using separate two-way repeated measure ANOVAs (Study Visit x OGTT Time) performed using Sigma-Stat (Jandel Scientific Software; Chicago, IL). Bonferroni corrections were used for post-hoc analyses when appropriate. Paired t-tests were used to compare baseline differences. All compliance and beta-stiffness data are expressed in absolute terms, and as percent deltas (%Δ) from rest and presented as mean ± SD, unless otherwise specified. Statistical significance was set a priori at P<0.05.
Results
Protocol 1: Arterial compliance methods comparison
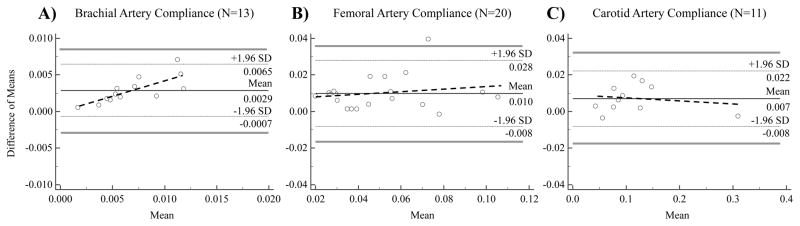
Intra-class correlation coefficients revealed a high degree of reliability between methods for the brachial (high-resolution ultrasound= 0.008±0.001 mm2/mmHg vs. tonometry+ultrasound= 0.005±0.01 mm2/mmHg; ICC: 0.920 (0.738, 0.976), P=5.5E−4), common femoral (high-resolution ultrasound= 0.06±0.01 mm2/mmHg vs. tonometry+ultrasound= 0.05±0.01 mm2/mmHg; ICC: 0.964 (0.908, 0.986), P=5.56E−10), and common carotid artery derived compliance measures (high-resolution ultrasound= 0.12±0.02 mm2/mmHg vs. tonometry+ultrasound= 0.11±0.02 mm2/mmHg; ICC: 0.997 (0.989, 0.999), P=2.3E−11). In addition, we examined the linear relationship between paired compliance derived measures from the two methods. The R-squared values (Figure 2) from the estimated linear regression models ranged from (0.87, 0.98), showing possible strong linear correlations between paired measures, although this does not substantiate method agreement. Accordingly, results from Bland-Altman plots indicate that the limits of agreement (LoA) were (−0.00068, 0.0065) for brachial artery, (−0.008, 0.028) for femoral artery, and (−0.008, 0.02) for carotid artery (Figure 3). We expected 95% of differences to fall within these limits. Because the differences within these limits were not deemed clinically important, i.e., they did not exceed the maximum allowed clinical difference (standard error of measurement) as reported by other studies (DeVan et al., 2005; Yoshizawa et al., 2009), the two methods (high-resolution ultrasound versus tonometry+ultrasound) of measuring arterial compliance were considered to agree sufficiently for this novel method to be used in place of the traditional one.
Figure 2.
Individual data points highlighting the relationship between the traditional (tonometry+ultrasound) and ultrasound-only (high-resolution ultrasound) derived arterial compliance assessments for the brachial (Panel A; N=13), femoral (Panel B; N=20), and carotid arteries (Panel C; N=11).
Figure 3.
Bland-Altman plots comparing the tonometry+ultrasound and high-resolution ultrasound derived arterial compliance assessments for the brachial (Panel A; N=13), femoral (Panel B; N=20), and carotid arteries (Panel C; N=11). Horizontal lines are positioned at mean difference between measurements (black solid line), ±1.96 standard deviations around mean difference, i.e., upper and lower limits of agreement (light dotted lines), and over maximum allowed difference in methods (gray solid lines), i.e., outer 95% confidence interval of upper and lower limits of agreement, respectively. Regression lines (darker dotted lines) are used to examine the presence of a proportional bias.
There appeared to be some potential proportional bias in Figure 3(A) in that the differences appeared to be greater as the mean increased. This issue was investigated by fitting a regression line to the Bland-Altman plots in Figure 3(A–C). The slope was 0.434 (0.194, 0.674) with P=0.002 for the brachial artery (Figure 3A), suggesting a proportional bias may be present. The slopes, confidence intervals, and P values for femoral and carotid artery compliance were: slope=0.067 (−0.119, 0.253), P=0.459 and slope=−0.013 (−0.093, 0.067), P=0.722, respectively. In the presence of the proportional bias, Bland and Altman recommend a log transformation because the back-transformed differences are easy to interpret (Bland & Altman, 1999). After transforming the raw values from the two methods, an additional Bland-Altman plot was generated. The plot (not shown) indicated some improvement. The regression line fitted to this plot yielded a slope=0.063 (−0.113, 0.239) with P=0.446. The mean difference was <0.0001 on the log scale and the LoA were (0.127, 0.702). The confidence intervals for the LoA were (−0.029, 0.282) for the lower limits and (0.547, 0.858) for the upper limit.
Our ICC estimates and Bland-Altman plots (for assessing reliability) were obtained without adjusting for the effects of covariates such as age and sex. Theoretically, it is feasible to incorporate such adjustment into these two methods, although most statistical software is limited to the existing methods without the covariate adjustment. Mixed-effects models may be an alternative approach to calculating ICC while adjusting for covariates, with the method of measuring arterial tonometry and covariates being fixed effects and the subject effect being random. The resulting ICC is estimated as the ratio of the random effect variance to the total variance. Using this approach we found the adjusted ICC values were only slightly smaller than the unadjusted ones. For example, the adjusted ICC was 0.86 as compared to the unadjusted ICC 0.92 for brachial artery, 0.92 vs 0.96 for femoral artery, and 0.995 vs 0.997 for carotid artery. Such discrepancy may be due to the adjustment of covariates as well as the difference in estimation techniques used by the mixed-modeling approach and the ANOVA approach.
Protocol 2: Arterial compliance during an OGTT before and after reduced physical activity
As anticipated, average daily step count (pre-reduced activity: 12,368±1,060 vs. 5 days reduced activity 3,731±723 steps, P=0.11E-7), and kilocalories expended above 3 METs per day (pre-reduced activity: 5,474±1,801 vs. 5 days reduced activity: 2,143±1,401 Kcal/day >3 METs, P=0.003) were significantly reduced following the 5 days of reduced activity.
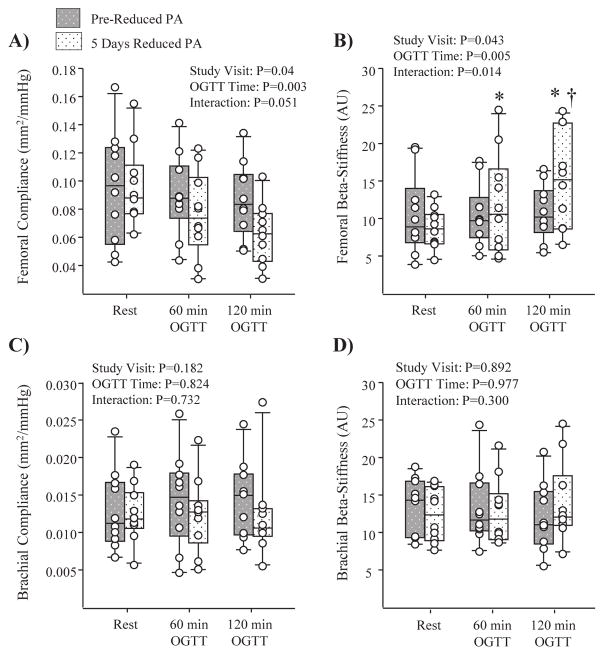
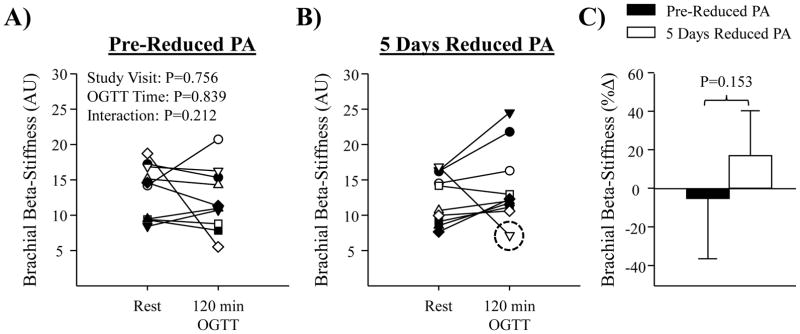
Artery diameter, compliance and beta-stiffness responses to the OGTT at pre-reduced activity and following the 5 days of reduced physical activity are summarized in Table 1. There was no effect of the reduced physical activity intervention on resting arterial compliance or beta-stiffness, prior to the OGTT. However, resting brachial artery diameter exhibited a significant decrease following 5 days of reduced physical activity (Table 1, P=0.0007). In response to the OGTT, no change in brachial or femoral artery diameter was observed, on either study visit. Although there was no effect of the OGTT on femoral arterial compliance or beta-stiffness at pre-reduced activity; following 5 days of reduced physical activity there was a significant reduction in femoral artery compliance, and an increase in femoral artery beta-stiffness during the OGTT, most notable at the 120 minute time-point (Figure 4A and B). Indeed, a significant reduction in femoral arterial compliance was observed (%-change at 120 mins: pre-reduced activity= −3±21% vs. 5 days reduced physical activity= −34±19%; P=0.006) along with an increase in femoral beta-stiffness (Figure 4) during the OGTT. As shown by individual responses in Figure 5A and B, this finding was consistent among subjects. For brachial artery measurements, there was no significant effect of the OGTT on arterial compliance (Figure 4C and D), or beta-stiffness (Figure 6) at pre-reduced activity, or following 5 days of reduced physical activity.
Table 1.
Artery Diameter, Compliance and Beta-Stiffness between Study Visits
| N=10 | Pre-Reduced PA | 5 Days Reduced PA | P-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Rest | 60 min OGTT | 120 min OGTT | Rest | 60 min OGTT | 120 min OGTT | Study Visit | OGTT Time | Interaction | |
| Femoral Diameter (cm) | 1.04±0.06 | 1.04±0.07 | 1.05±0.08 | 1.01±0.08 | 1.02±0.09 | 1.02±0.07 | 0.024 | 0.176 | 0.820 |
| Brachial Diameter (cm) | 0.45±0.05 | 0.45±0.05 | 0.44±0.05 | 0.42±0.04 | 0.42±0.05 | 0.43±0.05 | 0.002 | 0.298 | 0.050 |
| Femoral Compliance (mm2 mmHg−1) | 0.10±0.04 | 0.09±0.03 | 0.09±0.03 | 0.10±0.03 | 0.08±0.03 | 0.06±0.02 | 0.040 | 0.003 | 0.051 |
| Brachial Compliance (mm2 mmHg−1) | 0.013±0.005 | 0.014±0.006 | 0.015±0.005 | 0.012±0.004 | 0.012±0.005 | 0.012±0.006 | 0.182 | 0.824 | 0.732 |
| Femoral Beta-Stiffness (AU) | 10.5±5.4 | 10.6±4.1 | 10.8±3.7 | 8.7±2.7 | 11.9±6.7 | 15.3±6.5 | 0.043 | 0.005 | 0.014 |
| Brachial Beta-Stiffness (AU) | 13.3±3.8 | 13.3±4.9 | 12.2±4.5 | 12.4±3.6 | 12.8±4.3 | 14.0±5.3 | 0.892 | 0.977 | 0.300 |
Physical Activity: PA
Figure 4.
Box-and-whisker plots depicting the median, 10th, 25th, 75th, and 90th percentiles (N=10). Panels A and B show summary data for femoral artery compliance and beta-stiffness, while Panels C and D depict summary data for brachial artery compliance and beta-stiffness, at rest, 60 and 120 mins of an oral glucose tolerance test (OGTT), administered pre-reduced physical activity (PA) (grey patterned bars) and following 5 days of reduced PA (white patterned bars). *P < 0.05 vs. rest; † P < 0.05 vs. 120 min at pre-reduced PA.
Figure 5.
Panels A and B show individual data for femoral beta-stiffness at rest and 120 mins of the OGTT, at pre-reduced physical activity (PA), and following 5 days of reduced PA (N=10); *P < 0.05 vs. rest, † P < 0.05 vs. 120 min at pre-reduced PA. Each data point is represented by a unique symbol to facilitate individual comparisons between Panels A and B. Panel C shows mean summary data for %Δ in femoral beta-stiffness from rest to 120-mins of the OGTT, administered pre-reduced PA (black bar) and following 5 days reduced PA (white bar). Values are means ± SD. #P < 0.05 vs. pre-reduced PA.
Figure 6.
Panels A and B show individual data for brachial beta-stiffness at rest and 120 mins of the OGTT, administered pre-reduced physical activity (PA), and following 5 days of reduced PA (N=10). Panel C shows mean summary data for %Δ in brachial beta-stiffness from rest to 120-mins of the OGTT, pre-reduced PA (black bar) and following 5 days reduced PA (white bar). Values are means ± SD. A sub-analysis was performed by removing the observed outlier (dotted circle; right, Panel B) and revealed a trend (P=0.056) for an increase in Brachial Beta-Stiffness during the OGTT following 5 days of reduced PA.
Cardiovascular and metabolic parameter responses to OGTT
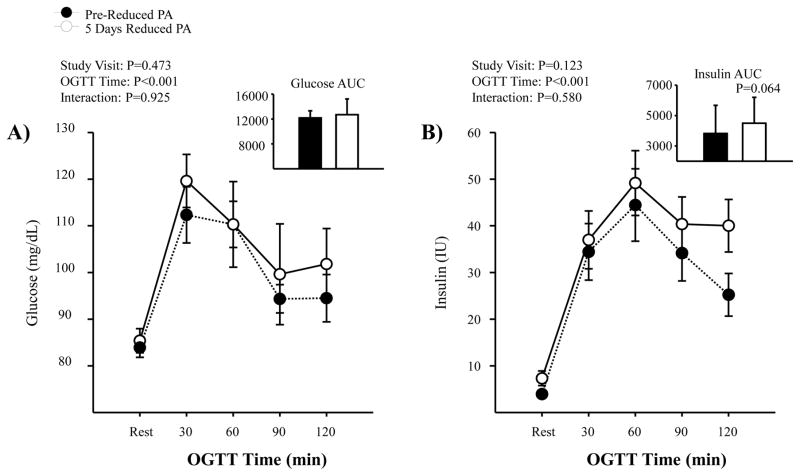
During the OGTT, HR increased from rest to 120 minutes post OGTT during both study visits (P<0.001); and, reduced physical activity did not lead to a greater HR response (HR change at 120 mins: pre-reduced activity= +6±5 bpm vs. 5 days reduced physical activity= +10±8 bpm; P=0.18). For MAP, no difference was noted for changes during the OGTT (rest to 120 mins), at pre-reduced activity or following 5 days of reduced physical activity (MAP change at 120 mins: pre-reduced activity= −0.3±6 mmHg vs. 5 days reduced physical activity= +2±6 mmHg; P=0.52). HOMA-IR increased following reduced physical activity (pre-reduced activity=0.8±0.5 AU, vs. 5 days reduced physical activity=1.5±1 AU; P=0.017). Although both glucose and insulin were increased in response to the OGTT (Figure 7), responses were not significantly different before and after 5 days of reduced physical activity. However, the insulin response appeared to have increased up to 15 IU higher at 120 mins following the 5 days of reduced physical activity. Insulin sensitivity, calculated by the Matsuda Index, was reduced following 5 days of reduced physical activity (pre-reduced activity=12.1±5.8 AU, vs. 5 days reduced physical activity=8.1±3.7 AU; P=0.002).
Figure 7.
Summary data for plasma glucose (Panel A) and insulin (Panel B) during the OGTT, administered pre-reduced physical activity (PA) (black) and following 5 days of reduced PA (white) (N=10). Values are means ± SD.
Discussion
Herein, we have developed a novel ultrasound-derived approach for assessing arterial compliance, and compared it with a traditional technique using applanation tonometry in terms of absolute agreement. This approach allows for the simultaneous and continuous acquisition of both artery diameter and local BP changes within the same vessel. We then used this approach to examine if decreases in physical activity and concomitant reductions in insulin sensitivity can augment regional large artery stiffness at rest or in response to acute hyperglycemia in healthy young individuals. The novel findings of our study are: 1) No changes in femoral or brachial arterial compliance were observed at rest following 5 days of reduced activity, 2) Femoral artery compliance, but not brachial artery compliance, decreased during a 2-hour OGTT following 5 days of reduced physical activity, and 3) No changes in arterial compliance were found during the OGTT when subjects were normally active (pre-reduced activity intervention). The result that only the femoral artery exhibited a reduction in compliance following the 5 days of reduced physical activity suggests it is the large arteries of limbs primarily undergoing the reduction in physical activity that are affected. Collectively, these data suggest that acute reductions in daily physical activity do not affect resting arterial compliance in healthy young men; however, the femoral artery becomes more susceptible to changes in arterial stiffness during acute hyperglycemia following 5 days of reduced physical activity and the resultant decreases in insulin sensitivity.
Although increases in plasma glucose, as occur during an OGTT, have been shown to acutely augment indices of arterial stiffness in at risk populations for CVD (Gordin et al., 2007; Huang et al., 2007), conflicting results exist regarding this response in healthy young individuals (Gordin et al., 2007; Huang et al., 2007; Keller et al., 2016). We found that acute hyperglycemia had no impact on arterial compliance in healthy active young men. However, following 5 days of reduced physical activity, and the presence of reduced insulin sensitivity, femoral artery compliance decreased during the OGTT. In contrast, brachial artery compliance was unaffected. These findings suggest that the vasculature most influenced by the reduced activity intervention was primarily affected. This is in line with our previous work reporting reductions in endothelial function in the leg but not the arm following 5 days of reduced physical activity (Boyle et al., 2013). Our data suggest that one’s level of activity and degree of insulin sensitivity impacts arterial stiffness responses to acute hyperglycemia. These outcomes have important implications for consideration when examining vascular responses to glucose ingestion and may lend insight into the mixed results previously reported on vascular stiffness responses during an OGTT in healthy young subjects (Gordin et al., 2007; Huang et al., 2007). It is unclear if it is the change in activity, change in insulin sensitivity, or both factors that are responsible for altering arterial stiffness responsiveness. However, the discovery that increases in arterial stiffness in response to glucose occurred in the femoral artery only suggests that alterations in physical activity may be playing a primary role. Indeed, despite the decrease in insulin sensitivity following the reduced physical activity, brachial artery stiffness during the OGTT was unaffected.
The mechanism(s) by which acute hyperglycemia selectively decreases femoral artery compliance following 5 days of reduced physical activity remain unclear. Hyperglycemia-induced increases in oxidative stress have been shown to directly impact arterial stiffness through effects on nitric oxide production and bio-availability (Cosentino et al., 1997; Jackson et al., 1998; Mullan et al., 2004; Srinivasan et al., 2004). However, it is unclear why this would impact the femoral artery but not the brachial artery following inactivity. Likewise, acute increases in insulin as occur during an OGTT or mixed meal can raise muscle sympathetic nerve activity in healthy active individuals (Young et al., 2010). Importantly, sympatho-excitation can also transiently reduce arterial compliance and endothelial function (Dyson et al., 2006; Zamir et al., 2007). Thus, a greater insulin response during the OGTT following reduced physical activity (see Figure 7B) could have transiently increased femoral artery stiffening through a sympathetically-mediated mechanism (i.e., alpha-adrenergic vasoconstriction). Previous work demonstrating greater alpha-adrenergic responsiveness in the leg compared to the arm may explain why only the femoral artery was affected (Pawelczyk & Levine, 2002). Nevertheless, additional studies are needed to discern the mechanism(s) responsible for the heightened vascular stiffness responses of the femoral artery to glucose following acute reductions in daily physical activity.
Even though the arms remain relatively active during the reduced physical activity intervention, some would argue that a reduction in leg activity may impact the vasculature of the arms as well (Green et al., 2008; Thijssen et al., 2008; Padilla et al., 2011a). However, as noted above, no change was noted for brachial artery compliance or beta-stiffness during the OGTT following the 5 days of reduced physical activity. This finding is not entirely surprising, given our previous work demonstrating that endothelial function decreased only in legs following a 5 day reduction in physical activity (Boyle et al., 2013). Notably, the brachial artery did exhibit a significant reduction in resting diameter following reduced activity. It is unclear if this reflects inward remodeling or increased vasoconstrictor tone per se. Future research is warranted to better understand the diameter changes following acute reductions in physical activity in young healthy men. Future work will also be needed to explore whether short-term reductions in physical activity can affect arterial compliance in other important vessels, such as the carotid artery.
Perspectives
Our results have important implications regarding cardiovascular and metabolic health. The skeletal muscle vasculature is important for a metabolic response to increases in glucose and insulin. Indeed, skeletal muscle is a primary site for glucose disposal (Thiebaud et al., 1982; DeFronzo & Tripathy, 2009). With our ultrasound-derived arterial compliance assessment, we identified a reduction in femoral artery compliance but not brachial artery compliance during acute hyperglycemia. Importantly this was only found when subjects were inactive for 5 days (<5,000 steps/day) and not when they were achieving higher levels of physical activity (>10,000 steps/day). The direct impact of a decrease in femoral artery compliance on skeletal muscle glucose uptake during a glucose challenge remains unclear. However, combined with our previous work demonstrating a reduction in endothelial function following 5 days of reduced physical activity (Boyle et al., 2013), our collective results provide clear evidence for the negative impact of acute reductions in daily physical activity on the peripheral vasculature. Considering that as much as 40% of insulin-mediated increases in glucose uptake can be attributed to insulin-stimulated vasodilation (Laakso et al., 1990; Bjornholm & Zierath, 2005), it is plausible that the negative consequence of inactivity on the vasculature are contributing to changes in insulin sensitivity, which would further support a link between cardiovascular and metabolic health. Indeed, these young healthy subjects exhibited a reduction in insulin sensitivity following the 5 days of reduced physical activity. Overall, our data highlight the importance of maintaining adequate amounts of ambulatory physical activity (i.e., >10,000 steps/day), as it relates to the susceptibility of the peripheral vasculature to acute hyperglycemia. Nevertheless, it is important to note that the current findings are only applicable to healthy young men, as future reduced physical activity studies that include healthy young women are needed.
Limitations
For protocol 1, we acknowledge the small sample size used. Our intent was not to validate the Doppler-ultrasound method but rather to investigate how close the Doppler-ultrasound method came to estimating arterial compliance with the more conventional arterial tonometry method. To fully validate this methodology with 80% power for the Bland Altman analysis, an N=83 would be needed (Lu, 2016), which was not feasible for this small scale study. Nevertheless, with the 24 subjects in which Doppler-ultrasound method was compared to arterial tonometry, we had reasonable agreement to use the Doppler-ultrasound method to assess arterial compliance during the OGTT before and after 5 days of reduced physical activity
Conclusion
In summary, our results demonstrate for the first time that short-term reductions in ambulatory physical activity in young healthy men can lead to a decrease in large artery compliance in response to acute hyperglycemia, specifically within the region exposed to the greatest change in skeletal muscle use (i.e., femoral artery).
New Findings.
What is the central question of this research?
To better understand the effects of acute hyperglycemia on arterial stiffness in healthy young individuals, we assessed arterial stiffness in physically-active men before, and then after reduced ambulatory physical activity to decrease insulin sensitivity.
What is the main finding and its importance?
During an oral glucose tolerance test, we identified an increase in leg arterial stiffness (i.e., reduced femoral artery compliance), only when subjects were inactive for 5 days (<5,000 steps/day) and not when they were engaging in regular physical activity (>10,000 steps/day). These results demonstrate the deleterious consequence of acute reductions in daily physical activity on the peripheral vasculature response to acute hyperglycemia.
Acknowledgments
The authors would like to thank Charla Jay (University of Missouri) for her technical assistance, as well as, the time and effort put in by all volunteer subjects.
Grants
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant RO1-HL 093167 (P. J. Fadel). D. P. Credeur and S.W. Holwerda were supported by National Institutes of Health (NIH) Grant T32-5T32AR048523-09. L. J. Reynolds was supported by American Heart Association (AHA) Grant 12Pre-12080242.
Footnotes
Disclosures
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. No other competing interests, financial or otherwise, are declared by the author(s).
Author Contributions
D.P.C., L.J.R., S.W.H, J.P.T and P.J.F conception and design of research; D.P.C., L.J.R., S.W.H, J.R.V., and B.E.Y. performed experiments; D.P.C., L.J.R., S.W.H, J.R.V, B.E.Y., and J.W. analyzed data; J.W. provided statistical consultation for all analyses, D.P.C., L.J.R., S.W.H, J.R.V, B.E.Y., J.P.T and P.J.F interpreted results of experiment; D.P.C. prepared figures; D.P.C. drafted manuscript; D.P.C., L.J.R., S.W.H, J.R.V, B.E.Y., J.W., J.P.T and P.J.F edited and revised manuscript; and D.P.C., L.J.R., S.W.H, J.R.V, B.E.Y., J.W., J.P.T and P.J.F approved final version of manuscript.
References
- Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010;235:111–118. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annuzzi G, Vaccaro O, Caprio S, Di Bonito P, Caso P, Riccardi G, Rivellese A. Association between low habitual physical activity and impaired glucose tolerance. Clin Physiol. 1985;5:63–70. doi: 10.1111/j.1475-097x.1985.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Bassett DR, Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc. 2010;42:1819–1825. doi: 10.1249/MSS.0b013e3181dc2e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornholm M, Zierath JR. Insulin signal transduction in human skeletal muscle: identifying the defects in Type II diabetes. Biochem Soc Trans. 2005;33:354–357. doi: 10.1042/BST0330354. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol (1985) 2013;115:1519–1525. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Center for Disease Control and Prevention: Facts about Physical Activity. Vol. 2016. Centers for Disease Control and Prevention; 2016. [Google Scholar]
- Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96:25–28. doi: 10.1161/01.cir.96.1.25. [DOI] [PubMed] [Google Scholar]
- Credeur DP, Holwerda SW, Boyle LJ, Vianna LC, Jensen AK, Fadel PJ. Effect of aging on carotid baroreflex control of blood pressure and leg vascular conductance in women. Am J Physiol Heart Circ Physiol. 2014a;306:H1417–1425. doi: 10.1152/ajpheart.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credeur DP, Holwerda SW, Restaino RM, King PM, Crutcher KL, Laughlin MH, Padilla J, Fadel PJ. Characterizing Rapid Onset Vasodilation to Single Muscle Contractions in the Human Leg. J Appl Physiol (1985) 2014b;118:455–464. doi: 10.1152/japplphysiol.00785.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVan AE, Anton MM, Cook JN, Neidre DB, Cortez-Cooper MY, Tanaka H. Acute effects of resistance exercise on arterial compliance. J Appl Physiol (1985) 2005;98:2287–2291. doi: 10.1152/japplphysiol.00002.2005. [DOI] [PubMed] [Google Scholar]
- Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol. 2006;290:H1446–1453. doi: 10.1152/ajpheart.00771.2005. [DOI] [PubMed] [Google Scholar]
- Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. Am J Physiol Heart Circ Physiol. 2013;305:H867–874. doi: 10.1152/ajpheart.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94:3513–3520. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin D, Ronnback M, Forsblom C, Heikkila O, Saraheimo M, Groop PH. Acute hyperglycaemia rapidly increases arterial stiffness in young patients with type 1 diabetes. Diabetologia. 2007;50:1808–1814. doi: 10.1007/s00125-007-0730-0. [DOI] [PubMed] [Google Scholar]
- Gordin D, Saraheimo M, Tuomikangas J, Soro-Paavonen A, Forsblom C, Paavonen K, Steckel-Hamann B, Vandenhende F, Nicolaou L, Pavo I, Koivisto V, Groop PH. Influence of Postprandial Hyperglycemic Conditions on Arterial Stiffness in Patients With Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:1134–1143. doi: 10.1210/jc.2015-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Maiorana AJ, Cable NT. Point: exercise training does induce vascular adaptations beyond the active muscle beds. J Appl Physiol (1985) 2008;105:1002–1004. doi: 10.1152/japplphysiol.90570.2008. discussion 1007. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Miyachi M, Seno N, Takahashi K, Yamazaki K, Sugawara J, Yokoi T, Onodera S, Mesaki N. Variations in carotid arterial compliance during the menstrual cycle in young women. Exp Physiol. 2006;91:465–472. doi: 10.1113/expphysiol.2005.032011. [DOI] [PubMed] [Google Scholar]
- Holwerda SW, Reynolds LJ, Restaino RM, Credeur DP, Leidy HJ, Thyfault JP, Fadel PJ. The influence of reduced insulin sensitivity via short-term reductions in physical activity on cardiac baroreflex sensitivity during acute hyperglycemia. J Appl Physiol (1985) 2015;119:1383–1392. doi: 10.1152/japplphysiol.00584.2015. [DOI] [PubMed] [Google Scholar]
- Huang CL, Chen MF, Jeng JS, Lin LY, Wang WL, Feng MH, Liau CS, Hwang BS, Lee YT, Su TC. Postchallenge hyperglycaemic spike associate with arterial stiffness. Int J Clin Pract. 2007;61:397–402. doi: 10.1111/j.1742-1241.2006.01227.x. [DOI] [PubMed] [Google Scholar]
- Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- Keller J, Kahlhofer J, Peter A, Bosy-Westphal A. Effects of Low versus High Glycemic Index Sugar-Sweetened Beverages on Postprandial Vasodilatation and Inactivity-Induced Impairment of Glucose Metabolism in Healthy Men. Nutrients. 2016:8. doi: 10.3390/nu8120802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage SG, Polak JF, O’Leary DH, Creager MA. Relationship of arterial compliance to baroreflex function in hypertensive patients. Am J Physiol. 1993;265:H232–237. doi: 10.1152/ajpheart.1993.265.1.H232. [DOI] [PubMed] [Google Scholar]
- Lu MJ, Zhong WH, Liu YX, Miao HZ, Li YC, Ji MH. Sample Size for Assessing Agreement between Two Methods of Measurement by Bland-Altman Method. Int J Biostat. 2016;12:215–0039. doi: 10.1515/ijb-2015-0039. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mcgraw KO, Wong SP. Forming Inferences about Some Intraclass Correlation Coefficients. Psychological Methods. 1996;1:30–46. [Google Scholar]
- Mikus CR, Fairfax ST, Libla JL, Boyle LJ, Vianna LC, Oberlin DJ, Uptergrove GM, Deo SH, Kim A, Kanaley JA, Fadel PJ, Thyfault JP. Seven days of aerobic exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes. J Appl Physiol (1985) 2011;111:657–664. doi: 10.1152/japplphysiol.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc. 2012;44:225–231. doi: 10.1249/MSS.0b013e31822ac0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan BA, Ennis CN, Fee HJ, Young IS, McCance DR. Protective effects of ascorbic acid on arterial hemodynamics during acute hyperglycemia. Am J Physiol Heart Circ Physiol. 2004;287:H1262–1268. doi: 10.1152/ajpheart.00153.2003. [DOI] [PubMed] [Google Scholar]
- Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–566. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011a;26:132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol. 2011b;96:1019–1027. doi: 10.1113/expphysiol.2011.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol (1985) 2002;92:2105–2113. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- Reynolds LJ, Credeur DP, Holwerda SW, Leidy HJ, Fadel PJ, Thyfault JP. Acute Inactivity Impairs Glycemic Control but Not Blood Flow to Glucose Ingestion. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Faggiotto A, Bowen-Pope D, Raines E. The role of endothelial injury and platelet and macrophage interactions in atherosclerosis. Circulation. 1984;70:III77–82. [PubMed] [Google Scholar]
- Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol (1985) 2011;110:389–397. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Hatley ME, Bolick DT, Palmer LA, Edelstein D, Brownlee M, Hedrick CC. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia. 2004;47:1727–1734. doi: 10.1007/s00125-004-1525-1. [DOI] [PubMed] [Google Scholar]
- Stoner L, Credeur D, Dolbow DR, Gater DR. Vascular health toolbox for spinal cord injury: Recommendations for clinical practice. Atherosclerosis. 2015;243:373–382. doi: 10.1016/j.atherosclerosis.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Swain DP, Leutholtz BC, King ME, Haas LA, Branch JD. Relationship between % heart rate reserve and % VO2 reserve in treadmill exercise. Med Sci Sports Exerc. 1998;30:318–321. doi: 10.1097/00005768-199802000-00022. [DOI] [PubMed] [Google Scholar]
- Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E, Felber JP. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31:957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Green DJ, Maiorana A, Cable NT, Hopman MT. Last word on point: counterpoint: exercise training does/does not induce vascular adaptations beyond the active muscle beds. J Appl Physiol (1985) 2008;105:1011. doi: 10.1152/japplphysiol.90931.2008. [DOI] [PubMed] [Google Scholar]
- Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–5395. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides and aerobic exercise on arterial compliance in postmenopausal women. Am J Physiol Heart Circ Physiol. 2009;297:H1899–1903. doi: 10.1152/ajpheart.00433.2009. [DOI] [PubMed] [Google Scholar]
- Young CN, Deo SH, Kim A, Horiuchi M, Mikus CR, Uptergrove GM, Thyfault JP, Fadel PJ. Influence of endurance training on central sympathetic outflow to skeletal muscle in response to a mixed meal. J Appl Physiol (1985) 2010;108:882–890. doi: 10.1152/japplphysiol.01174.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir M, Goswami R, Salzer D, Shoemaker JK. Role of vascular bed compliance in vasomotor control in human skeletal muscle. Exp Physiol. 2007;92:841–848. doi: 10.1113/expphysiol.2007.037937. [DOI] [PubMed] [Google Scholar]