Abstract
Murine leukemia virus (MuLV) is a retrovirus known causing leukemia and neurological disorders in mice, and its viral life cycle and pathogenesis have been investigated extensively over the past decades. As a natural antiviral agent, betulinic acid is a pentacyclic triterpenoid that can be found in the bark of several species of plants (particularly the white birch). One of the hurdles for betulinic acid to release its antiviral potency is its poor water solubility. In this study, we synthesized more water-soluble ionic derivatives of betulinic acid, and examined their activities against Moloney MuLV (M-MuLV). The mouse fibroblast cells stably infected with M-MuLV, 43D cells, were treated with various doses of betulinic acids and its derivatives, and the viral structural protein Gag in cells and media were detected by western blots. Two ionic derivatives containing the benzalkonium cation were found to inhibit the virus production into media and decreased Gag in cells. However, a cell proliferation assay showed that the benzalkonium cation inhibited the growth of 43D cells, suggesting that our ionic derivatives limited virus production through the inhibition of metabolism in 43D cells. Interestingly, all of these betulinic acid compounds exhibited a minimum impact on the processing and release of Gag from 43D cells, which outlines the differences of viral maturation between MuLV and human immunodeficiency virus.
Keywords: Murine leukemia virus, Betulinic acid, Viral release, Gag maturation, Human immune deficiency virus
Graphical abstract
Introduction
Retroviruses comprise a large and diverse family of enveloped RNA viruses defined by common taxonomic denominators including the structural, compositional, and replicative properties [1]. Murine leukemia viruses (MuLVs) are simple retroviruses and encode only three polyproteins that are used in the assembly of progeny virus particles; Gag (structural protein), Pol (three retroviral enzymes, i.e. protease, reverse transcriptase, and integrase), and Env [2]. MuLVs are composed of diverse strains in both endogamous and exogenous viruses, and some of them are causative agents of leukemia and neurological disorders in mice [3]. Moloney murine leukemia virus (M-MuLV) is a well-studied replication-competent oncogenic retrovirus of gammaretrovirus genus which has enabled the understandings of general phenomenon of leukemogenesis; therefore, M-MuLV has been considered as a model retrovirus [4]. The viruses bind to the host cell via interactions between envelope protein and its receptor on target cells. Subsequently, the penetrated viral cores are endocytosed and the uncoating occurs. The single stranded viral RNA genome is reverse transcribed and cDNA is integrated into the host genome by integrase. In the late phase, viral transcripts make viral proteins. At the same time, the unspliced viral RNA works as the viral genome. These viral materials are trafficked to plasma membranes and assembled, and new viruses bud from the membranes at the end.
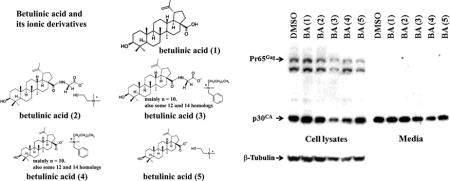
Betulinic acid (3β-hydroxyl-lup-20(29)-en-28-oic acid) is a natural pentacyclic lupine type triterpene that can be extracted from plants (e.g. birch tree bark) [5]. This natural compound has a number of medically relevant biological properties such as anti-inflammatory, anti-cancer, and anti-viral activities [5–8]. A major obstacle with the releasing of the antiviral potency of betulinic acid is due to its poor solubility in aqueous solutions and common organic solutions (such as esters, alcohols, and ethers). As a matter of fact, the solubility of betulinic acid in water is only approximately 0.02 μg/ml at room temperature [9]. In common organic solvents, the solubility of betulinic acid is also fairly low, e.g. 1% (w/v) in ethanol and 5% (w/v) in DMSO at 25°C [10]. Some derivatives of betulinic acid had shown improved water solubility, as well as enhanced biological activities when compared with betulinic acid itself [6, 11, 12]. Recently, we developed new ionic derivatives of betulinic acid with a higher water solubility and thus a stronger antiviral activity [10, 13, 14]. In the present study, we further examined the effect of betulinic acid and its derivatives (see Fig. 1) on the retroviral replication of M-MuLV.
Figure 1.
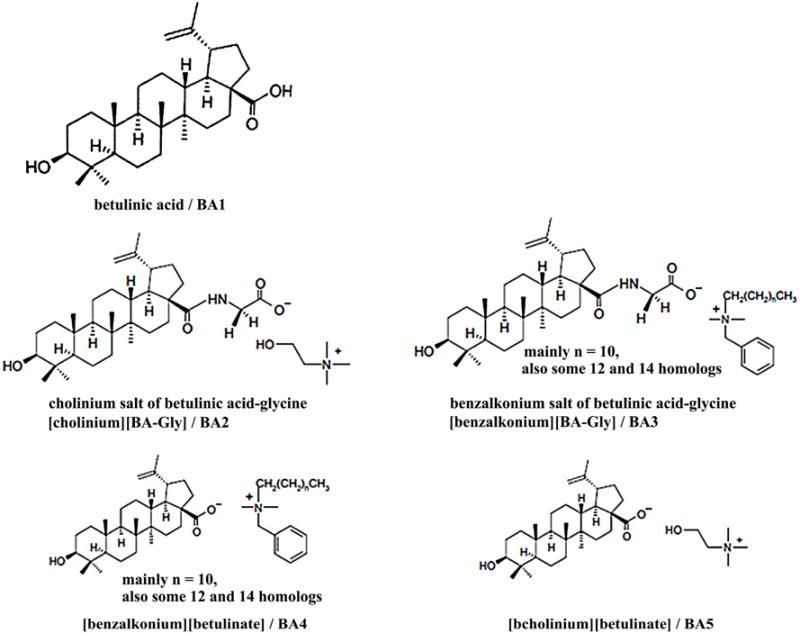
Structures of betulinic acid (BA1) and ionic derivatives (BA2–BA5).
Materials and Methods
Synthesis of ionic salts of betulinic acid conjugated with glycine
The betulinic acid, glycine methyl ester hydrochloride, N, N′-dicyclohexylcarbodiimide (DCC), 4-(dimethylamino)pyridine (DMAP), choline chloride, benzalkonium chloride, and Amberlyst A26 hydroxide form (Sigma-Aldrich, St. Louis, MO) were used to prepare ionic salts of betulinic acid conjugated with glycine (Fig. 1). The procedures for preparing ionic derivatives of betulinic acid are described previously [15, 16]. In brief, betulinic acid (BA1, 204.4 mg), glycine methyl ester (104.0 mg), and triethylamine (8 mg) were dissolved in 50 ml of anhydrous tetrahydrofuran (THF) at room temperature. Into the reaction mixture, DCC (107.9 mg) and DMAP (30.3 mg) were added and the mixture was stirred at room temperature under argon for a duration of 48 hrs. After the reaction was completed, the precipitate (dicyclohexylurea as byproduct) was filtered off and the filtrate was further evaporated under vacuum to remove THF. The crude product was then dissolved in 100 ml of ether/ethyl acetate (2:1, v/v) and extracted with 100 ml of water to remove the excess water-soluble carbodiimide reagent and any remaining dicyclohexylurea. The organic layer was further extracted with 1.0 N HCl (2 × 100 ml) to remove DMAP [17] saturated NaHCO3 solution (1 × 100 ml), and lastly with water (1 × 100 ml) [18, 19]. The purified organic layer was then dried over Na2SO4. After filtration, ethyl acetate was evaporated under vacuum to yield the glycine methyl ester conjugate of betulinic acid. The methyl ester (298 mg) was dissolved in 100 ml of THF/H2O (4:1, v/v) solution, followed by the addition of LiOH (67.5 mg). The reaction mixture was then stirred at room temperature for 4 hrs under argon. Once the reaction completed, THF was evaporated under vacuum and the product obtained was dissolved in 200 ml of ethyl acetate, followed by sequential washing with water, 0.1 M HCl, and water once again. The organic layer was then dried with Na2SO4, and after filtration the solvent was removed under vacuum to yield glycine conjugate of betulinic acid (231.4 mg). The choline hydroxide was prepared from choline chloride following an anion-exchange column approach used previously [20]. The glycinylated betulinic acid, BA4, was dissolved in 100 ml of THF/H2O (4:1, v/v), followed by the addition of a five-fold molar excess of choline hydroxide (0.55 g). The reaction mixture was stirred at room temperature for a duration of 24 hrs. THF was removed by rotary evaporation. The crude product was extracted into 100 ml of ethyl acetate, and washed with 100 ml of distilled water to remove the excess choline hydroxide. The organic layer was dried and ethyl acetate was removed under vacuum to give the cholinium salt of betulinic acid-glycine([Choline][BA-Gly], (BA2, 171 mg) (Fig. 1). Using the above reaction strategies, we prepared the benzalkonium salt of glycinylated betulinic acid ([Bzk][BA-Gly], BA3, and cholinium betulinate ([Choline][BA], BA5) starting with betulinic acid. These compounds were used to treat mouse fibroblast cells.
Treatment of 43D cells with betulinic acid and its derivatives
Establishment of 43D mouse fibroblast cell line stably infected with the wild-type M-MuLV was described previously [21]. The 43D cells were cultured with DMEM media containing 10% Hyclone calf serum (GE Healthcare Life Sciences, PA), 100 IU/ml of Penicillin and 100ug/ml of Streptomycin (Corning, VA) at 37°C with 5% CO2. Cells were cultivated in 60 mm and 100 mm BioLite cell culture treated dishes (Thermo Scientific, MA, USA). The 43D cells were treated with betulinic acid, its derivatives, or benzalkonium chloride (BKC) for 24 hrs, and then the media were replaced. The cells and viruses were gathered 24 hrs further incubation after the second treatment of the chemicals.
Detection of MuLV and beta-Tubulin
The sodium dodecyl sulfate (SDS) samples were prepared from the cells and the viruses released into media. The proteins were extracted from the cells with radioimmunoprecipitation assay (RIPA) buffer which is a common buffer used to lyse cultured mammalian cells. This buffer was composed of 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, and 1 × AMRESCO Protease Inhibitors (AMRESCO LLC, Solon OH, USA). In order to obtain SDS samples, 43D cells were incubated with 120 μL of RIPA on ice. The cell debris was span down by centrifugation at 13,000 rpm for 15 minutes at 4°C. The supernatant was transferred into new micro-centrifuge tubes, and 4 × SDS buffer was added and boiled for 3 minutes. Separately, the viruses in media were concentrated by ultracentrifugation at 25,000 rpm with a Beckman SW28 ultracentrifuge rotor for 1 hr at 4°C [22]. All media were removed, and the viruses were suspended in 160 μL of 1 × SDS sample buffer (62.5 mM Tris-HCl (pH 6.8), 2.5% SDS, 0.002% bromophenol blue, 0.7135 M β-mercaptoethanol, 10% glycerol). The viral proteins were detected by SDS-PAGE and western blots with the rabbit serum for M-MuLV p30CA [23], anti-rabbit IgG conjugated with horseradish peroxidase. The images were acquired by the SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) and Gel Doc™ XR+ Gel Documentation System (Bio-Rad, CA, USA). To quantify the viral release efficiency, each Pr65Gag and p30CA (a precursor and a cleaved MuLV Gag proteins) band in the cells and media was quantified with the densitometry software AlphaEaseFC (Alpha Innotech, CA, USA), and the percentage of released p30CA divided by the total Gag proteins (Pr65Gag and p30CA) in cells and media was calculated [21]. Different exposures of the blots were analyzed to ensure that densitometry was in the linear range. Beta-Tubulin was detected by SDS-PAGE and western blots with anti-beta-Tubulin (Cell Signaling Technology, MA) and goat anti-mouse IgG conjugated with horseradish peroxidase (ImmunoReagents, NC).
AlamarBlue Cell Viability Assay
Cell viability was assessed using the AlamarBlue Cell Viability Assay [24] from Bio-Rad following the manufacturer’s protocols. Briefly, 5 × 103 43D cells were placed on 96-well plates (100 μl of media/well). After overnight incubation, the cells were treated with DMSO, 10 μM, 30 μM and 100 μM of betulinic acid, its derivatives, or BKC for 48 hrs respectively, then 10 μL of AlamarBlue solution was added to the wells. Following two-hour incubation, the reaction was stopped by 50 μL of 3% SDS. Absorbance of 570 nm/600 nm was measured by NanoDrop 2000 UV-Vis Spectrophotometer (Thermo Scientific) to determine reduction of resazurin dye. The DMSO treated cells were set to 100% and then the viability readings from the treated wells were calculated as a percentage of the control.
Statistical analysis
Students T-test was used to evaluate viral release efficiency from 43D cells treated with betulinic acid or its derivatives.
Results
To determine the inhibition of betulinic acid and its ionic derivatives against M-MuLV release, 43D cells were treated with 30 μM of these compounds for 48 hrs. Viral Gag protein is translated as poly-Gag, Pr65Gag which is further cleaved into some smaller Gag proteins such as p30CA through the maturation of viruses [2]. Since the cleavage of Gag proteins to yield p30CA occurs during budding/viral release, the majority of Gag proteins in secreted viruses is p30CA (Fig. 2A). Poly-Gag proteins Pr65Gag in cells and p30CA in cells and viruses (media) were detected by SDS-PAGE and western blots with rabbit polyclonal anti-p30CA antibodies [23]. Compounds BA1, BA2, and BA5 did not show any significant impact on the intracellular Gag production and its processing, but compounds BA3 and BA4 decreased the intracellular Gag proteins. The signals for beta-Tubulin in the cells treated with compounds BA3 and BA4 exhibited a small decrease. Compounds BA3 and BA4 decreased the amount of p30CA in media, but the virus release efficiency was not affected by any of the compounds BA1-BA5 (Fig. 2B). Treatment of 10 μM of betulinic acids and its derivatives did not change Gag in cells and in viruses as detected by western blots (data not shown).
Figure 2.
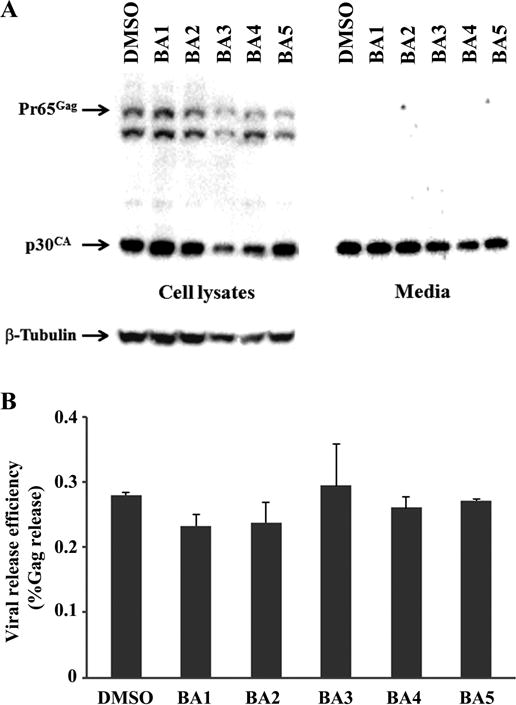
Effect of betulinic acid and its ionic derivatives on MuLV replication. (A) 43D cells were treated with 30 μM of the compounds for 48 hrs, and Gag proteins in cells and media were detected by western blots using the rabbit serum for anti-p30CA. (B) The intensity of Gag signals detected by western blots was quantified by immunodensitometry and the viral release efficiency was calculated. The figure shows the relative virus release efficiency (% Gag release) with standard deviation.
It was reported that betulinic acid suppressed some signaling pathways involved in the cellular metabolism and could induce cell death [25]. To assess the relationship between the inhibition of M-MuLV Gag production and the general cellular metabolism in 43D cells treated with these compounds, cell growth was measured by AlmarBlue cell proliferation assay which monitored the reducing environment of living cells [24]. The cells were treated with different concentrations of betulinic acids and its derivatives for 48 hrs. Compounds BA3 and BA4 reduced the cellular activities by 35% at 30 μM and by 95% at 100 μM, but other compounds did not show a strong inhibitory effect at 30 μM (Fig. 3), which was correlated with the beta-Tubulin in the 43D cells treated with 30 μM and 100 μM of compounds BA3 and BA4 for 48 hrs (Fig. 2A). These data suggest that compounds BA3 and BA4 could decrease the M-MuLV Gag production by inhibiting the cellular metabolisms. Since both compounds contain the benzalkonium cation, we tested the inhibitory effect of benzalkonium cation on cell growth. The 43D cells treated with BKC impaired metabolism in a dose-dependent manner, and BA2 did not show synergistic effects with BKC (Fig. 4A). BKC decreased the signals for Gag and beta-Tubulin in cells and p30CA in media (Fig. 4B), which suggested that the benzalkonium cation inhibited viral production by decreasing the metabolism in 43D cells, and compounds BA3, BA4 and BKC seem not to affect intracellular Gag production and its processing.
Figure 3.
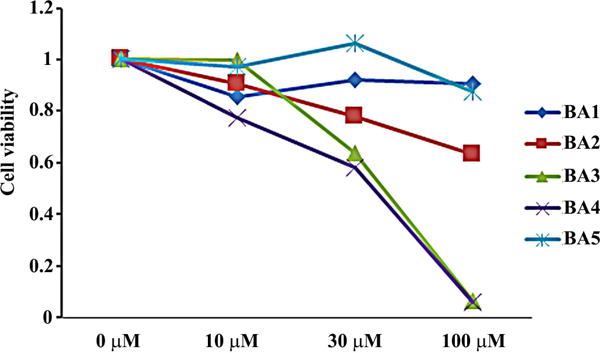
Cytotoxicity of betulinic acid and its ionic derivatives in 43D cells. (A) 43D cells were treated with different doses of betulinic acid and its ionic derivatives for 48 hrs. The effect of these compounds on cell growth was measured by the AlmarBlue cell proliferation assay. The figure illustrates the relative cell proliferation to the control. The averages of two experiments are shown.
Figure 4.
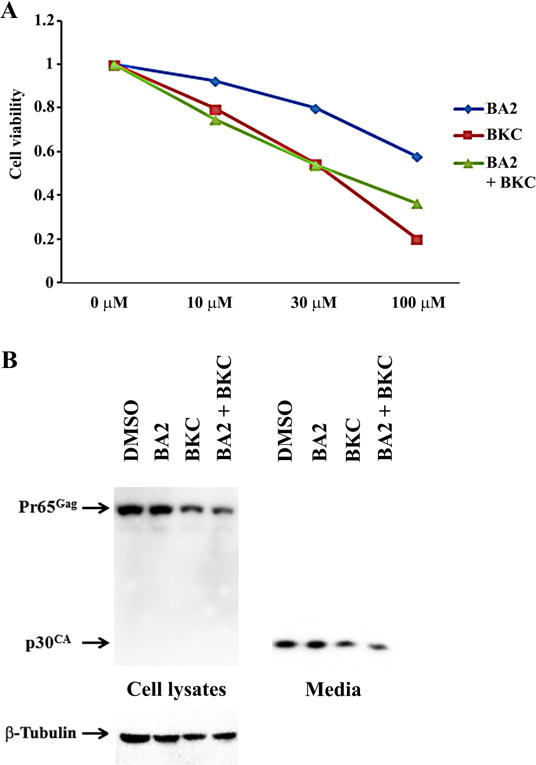
Effects of benzalkonium chloride on 43D growth and MuLV replication. (A) 43D cells were treated with different doses of benzalkonium chloride (BKC) and the cholinium salt of betulinic acid-glycine, BA2 for 48 hrs. The effect of the compounds on cell growth was measured by the AlmarBlue cell proliferation assay. The figure illustrates the relative cell proliferation to the control. The averages of two experiments are shown. (B) 43D cells were treated with 30 μM of the compounds for 48 hrs, and Gag proteins in cells and media were detected by western blots using the rabbit serum for anti-p30CA.
Discussion
Our results demonstrated that two ionic derivatives of betulinic acid (compounds BA3 and BA4) reduced the cellular metabolisms, and decreased the MuLV production from 43D cells without alteration of the virus release efficiency or the maturation of viral particles. It was reported that both betulinic acid and platanic acid isolated from the leaves of Syzigium claviforum inhibited human immunodeficiency virus (HIV) replication in H9 lymphocyte cells [7]. A subsequent study [8] demonstrated that the major inhibitory steps in HIV-1 replication by betulinic acid and its derivatives are membrane fusion, viral assembly, and maturation. One betulinic acid derivative with alteration at C3, 3-O-(3,3-dimethylsuccinyl) betulinic acid/PA-457/DSB blocks a late step of virus replication through inhibiting the conversion of p25CA-SP1 to p24 [26]. Cleavages of the precursor form of Gag including p24 and SP1 Gag spacer peptide is crucial to the structural alterations necessary for the formation of mature HIV-1 particles. The study using lentiviruses and their mutants responsible for DSB resistance indicated that Valine and Leucine immediately flanking the p25CA–SP1 cleavage site determined the viral sensitivity to DSB [8, 26, 27]. We confirmed that betulinic acid inhibited the cleavage of p25CA–SP1, and increased the amount of p25CA-SP1 by western blots (data not shown), but did not observe the inhibition of Gag cleavage in MuLV with betulinic acid and its ionic derivatives (Fig. 2A) although our ionic derivatives of betulinic acid displayed improved water solubilities, and exhibited a stronger antiviral activity against herpes simplex virus type-2 (HSV-2) than betulinic acid [28]. MuLV Gag is cleaved during virus maturation into MA, p12, p30CA, and NC [2], while amino acids flanking the MA-p12 are Leucine and Tyrosine, amino acids flanking the p12-p30CA are Alanine and Phenylalanine, and amino acids flanking the p30CA-NC are Leucine and Leucine. Since DSB failed to show any significant inhibition of HIV-1 PR in vitro using a synthetic peptide and recombinant Gag [26, 29], this compound seems to target unique sequences in Gag to prevent viral maturation through unknown mechanisms. Our results are in agreement with the previous data indicating that DSB targeted the specific amino acid sequence flanking to the cleavage site, and suggest that amino acid sequence found in MuLV Gag and MuLV protease are not targeted by betulinic acid and its derivatives.
As shown in Fig. 3, compounds BA3 and BA4 reduced the cellular metabolism, and inhibited the growth of 43D cells, which was largely due to the amount of beta-Tubulin in cells treated with the compounds (Fig. 2). Compounds BA3 and BA4 are quaternary ammonium salts that contain the benzalkonium cation, and our experiments using BKC suggested that the benzalkonium cation in BA3 and BA4 showed cytotoxic effects (Fig. 4). Therefore, our observations are consistent with our data showing that cytotoxic effects to BA3 and BKC are comparable in a couple of prostate cancer cells (unpublished data). BKC has been reported to show antimicrobial activities against bacteria [30], HIV-1 [31] and fungi [32] through a variety of cellular changes such as cell membrane damage, adenosine triphosphate depletion, generation of oxidative stress, and cell apoptosis. Our data imply that such effects induced by BKC seem not affect maturation and release of MuLV particles (Fig. 4).
In summary, our current experiments using betulinic acid and its derivatives outline the differences of viral maturation between MuLV and HIV-1 through evaluating the MuLV production in cells dosed with betulinic acid compounds.
Supplementary Material
Acknowledgments
The authors appreciate Dr. Hung Fan (University of California, Irvine) for his rabbit serum (anti-p30CA) and helpful comments. We acknowledge the assistance of the undergraduate program coordinator, Ms. Devi Chellu, and research associate, Mr. Harshavardhan Kenche, and faculty members at Savannah State University. The authors also thank Drs. Melissa Visalli and Robert Visalli at Mercer University, School of Medicine, and Yoko Nitta for their technical assistance and helpful discussion. The research was supported in part by the NIH RISE program (1R25GM096956) and the Savannah State University mini-grant to TN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spencer TE, Palmarini M. Application of next generation sequencing in mammalian embryogenomics: lessons learned from endogenous betaretroviruses of sheep. Animal reproduction science. 2012;134:95–103. doi: 10.1016/j.anireprosci.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rein A. Murine leukemia viruses: objects and organisms. Advances in virology. 2011;2011:403419. doi: 10.1155/2011/403419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor (New York): Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 4.Fan H. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends in microbiology. 1997;5:74–82. doi: 10.1016/S0966-842X(96)10076-7. [DOI] [PubMed] [Google Scholar]
- 5.Cichewicz RH, Kouzi SA. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev. 2004;24:90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- 6.Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Current medicinal chemistry. 2005;12:657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- 7.Fujioka T, Kashiwada Y, Kilkuskie RE, Cosentino LM, Ballas LM, Jiang JB, Janzen WP, Chen IS, Lee KH. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod. 1994;57:243–247. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 8.Aiken C, Chen CH. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol Med. 2005;11:31–36. doi: 10.1016/j.molmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Jäger S, Winkler K, Pfüller U, Scheffler A. Solubility studies of oleanolic acid and betulinic acid in aqueous solutions and plant extracts of Viscum album L. Planta medica. 2007;73:157–162. doi: 10.1055/s-2007-967106. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Jones CL, Cowins JV. Lipase dissolution and stabilization in ether-functionalized ionic liquids. Green Chemistry. 2009;11:1128–1138. [Google Scholar]
- 11.Baglin I, Mitaine-Offer AC, Nour M, Tan K, Cave C, Lacaille-Dubois M-A. A review of natural and modified betulinic, ursolic and echinocystic acid derivatives as potential antitumor and anti-HIV agents. Mini reviews in medicinal chemistry. 2003;3:525–539. doi: 10.2174/1389557033487917. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee R, Kumar V, Srivastava SK, Agarwal SK, Burman AC. Betulinic acid derivatives as anticancer agents: structure activity relationship. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2006;6:271–279. doi: 10.2174/187152006776930846. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Baker GA, Song Z, Olubajo O, Crittle T, Peters D. Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green chemistry. 2008;10:696–705. [Google Scholar]
- 14.Visalli RJ, Ziobrowski H, Badri KR, He JJ, Zhang X, Arumugam SR, Zhao H. Ionic derivatives of betulinic acid exhibit antiviral activity against herpes simplex virus type-2 (HSV-2), but not HIV-1 reverse transcriptase. Bioorganic & medicinal chemistry letters. 2015;25:3168–3171. doi: 10.1016/j.bmcl.2015.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Holmes SS, Baker GA, Challa S, Bose HS, Song Z. Ionic derivatives of betulinic acid as novel HIV-1 protease inhibitors, J. Enzyme Inhib Med Chem. 2012;27:715–721. doi: 10.3109/14756366.2011.611134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suresh C, Zhao H, Gumbs A, Chetty CS, Bose HS. New ionic derivatives of betulinic acid as highly potent anti-cancer agents. Bioorganic & medicinal chemistry letters. 2012;22:1734–1738. doi: 10.1016/j.bmcl.2011.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho PT, Ngu KY. An effective synthesis of N-(9-fluorenylmethyloxycarbonyl). alpha.-amino aldehydes from S-benzyl thioesters. The Journal of Organic Chemistry. 1993;58:2313–2316. [Google Scholar]
- 18.Rodrigues PC, Roth T, Fiebig HH, Unger C, Mülhaupt R, Kratz F. Correlation of the acid-sensitivity of polyethylene glycol daunorubicin conjugates with their in vitro antiproliferative activity. Bioorganic & medicinal chemistry. 2006;14:4110–4117. doi: 10.1016/j.bmc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Ammazzalorso A, Amoroso R, Bettoni G, De Filippis B, Giampietro L, Pierini M, Tricca ML. Asymmetric synthesis of (S)-ibuprofen by esterification with amides of (S)-lactic acid as chiral auxiliaries: experimental and theoretical results. Tetrahedron letters. 2002;43:4325–4328. [Google Scholar]
- 20.Zhao H, Holmes SS, Baker GA, Challa S, Bose HS, Song Z. Ionic derivatives of betulinic acid as novel HIV-1 protease inhibitors. J Enzyme Inhib Med Chem. 2012;27:715–721. doi: 10.3109/14756366.2011.611134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitta T, Kuznetsov Y, McPherson A, Fan H. Murine leukemia virus glycosylated Gag (gPr80gag) facilitates interferon-sensitive virus release through lipid rafts. Proc Natl Acad Sci U S A. 2010;107:1190–1195. doi: 10.1073/pnas.0908660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitta T, Hofacre A, Hull S, Fan H. Identification and mutational analysis of a Rej response element in Jaagsiekte sheep retrovirus RNA. J Virol. 2009;83:12499–12511. doi: 10.1128/JVI.01754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller-Lantzsch N, Fan H. Monospecific immunoprecipitation of murine leukemia virus polyribosomes: identification of p30 protein-specific messenger RNA. Cell. 1976;9:579–588. doi: 10.1016/0092-8674(76)90040-4. [DOI] [PubMed] [Google Scholar]
- 24.Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu T, Pang Q, Zhou D, Zhang A, Luo S, Wang Y, Yan X. Proteomic investigation into betulinic acid-induced apoptosis of human cervical cancer HeLa cells. PLoS ONE. 2014;9:e105768. doi: 10.1371/journal.pone.0105768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li F, Goila-Gaur R, Salzwedel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Martin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13555–13560. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Chen CH, Aiken C. The sequence of the CA-SP1 junction accounts for the differential sensitivity of HIV-1 and SIV to the small molecule maturation inhibitor 3-O-{3′,3′-dimethylsuccinyl}-betulinic acid. Retrovirology [electronic resource] 2004;1:15. doi: 10.1186/1742-4690-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visalli RJ, Ziobrowski H, Badri KR, He JJ, Zhang X, Arumugam SR, Zhao H. Ionic derivatives of betulinic acid exhibit antiviral activity against herpes simplex virus type-2 (HSV-2), but not HIV-1 reverse transcriptase, Bioorganic & Medicinal Chemistry Letters. Bioorg Med Chem Lett. 2015;25:3168–3171. doi: 10.1016/j.bmcl.2015.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanamoto T, Kashiwada Y, Kanbara K, Gotoh K, Yoshimori M, Goto T, Sano K, Nakashima H. Anti-human immunodeficiency virus activity of YK-FH312 (a betulinic acid derivative), a novel compound blocking viral maturation. Antimicrob Agents Chemother. 2001;45:1225–1230. doi: 10.1128/AAC.45.4.1225-1230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houari A, Di Martino P. Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Lett Appl Microbiol. 2007;45:652–656. doi: 10.1111/j.1472-765X.2007.02249.x. [DOI] [PubMed] [Google Scholar]
- 31.Wainberg MA, Spira B, Bleau G, Thomas R. Inactivation of human immunodeficiency virus type 1 in tissue culture fluid and in genital secretions by the spermicide benzalkonium chloride. J Clin Microbiol. 1990;28:156–158. doi: 10.1128/jcm.28.1.156-158.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, He Y, Li X, Gao C, Zhou L, Sun S, Pang G. Antifungal effect of ophthalmic preservatives phenylmercuric nitrate and benzalkonium chloride on ocular pathogenic filamentous fungi. Diagn Microbiol Infect Dis. 2013;75:64–67. doi: 10.1016/j.diagmicrobio.2012.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


