Abstract
Objectives
To evaluate circulating cytokine profiles in patients with ANCA-associated vasculitis (AAV), classified by ANCA specificity (proteinase 3 (PR3)-ANCA versus myeloperoxidase (MPO)-ANCA) or by clinical diagnosis (granulomatosis with polyangiitis (GPA) versus microscopic polyangiitis (MPA)).
Methods
A panel of 29 cytokines was tested in 186 patients with active AAV at inclusion into the Rituximab in AAV (RAVE) trial. Cytokine concentrations were compared between groups within each classification system. Multivariable analyses adjusted for age, sex, and renal insufficiency were performed; with each biomarker as dependent variable and ANCA specificity and clinical diagnosis as explanatory variables of interest.
Results
Levels of 9 circulating cytokines were significantly higher in PR3-AAV than MPO-AAV: IL-6, GM-CSF, IL-15, IL-18, CXCL8/IL-8, CCL-17/TARC, IL-18BP, sIL-2Rα, NGFβ(p<0.05); 4 were higher in MPO-AAV compared to PR3-AAV: sIL6R, sTNFRII, NGAL, sICAM-1 (p<0.05); 6 were higher in GPA than MPA: IL-6, GM-CSF, IL-15, IL-18, sIL-2Rα, NGFβ(p<0.05); 3 were higher in MPA than GPA: Osteopontin, sTNFRII, NGAL (p<0.05). For nearly all cytokines, the differences between PR3-AAV versus MPO-AAV were larger than that between GPA versus MPA. The multivariate analysis showed that 8 cytokines (IL-15, IL-8, IL-18Bp, NGF-β, sICAM-1, TARC, Osteopontin, KIM-1; p<0.05) distinguished patients with AAV better (lower P-values and larger effect sizes) when grouped by ANCA specificity than by clinical diagnosis.
Conclusions
Distinct cytokine profiles were identified for PR3-AAV versus MPO-AAV and for GPA versus MPA. Differences in these circulating immune mediators are more strongly associated with ANCA specificity than with clinical diagnosis, suggesting that the heterogeneity in the AAV subtypes extends beyond the clinical phenotypes.
Keywords: ANCA-associated vasculitis, ANCA-type, RAVE, cytokines, myeloperoxidase, proteinase-3
INTRODUCTION
Antineutrophil cytoplasmic autoantibodies (ANCA) are the primary serologic markers of ANCA-associated vasculitis (AAV), a group of primary systemic necrotizing small vessel vasculitides which includes granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) (1, 2). GPA and MPA share many of the clinical features induced by capillaritis. Yet, it remains a matter of debate whether they represent expressions of the same disease spectrum or two distinct conditions (1). GPA differs from MPA because of the presence of necrotizing granulomatous tissue inflammation, a different organ predilection, and association with different ANCA specificity (1).
Patients with GPA are more likely to have ANCA directed against proteinase 3 (PR3), whereas patients with MPA more often have ANCA against myeloperoxidase (MPO), but there is substantial overlap. Recent data suggest that ANCA specificity may associate better with genetic predisposition (3), response to therapy (4), relapse risk (5, 6) and long-term prognosis (7) than the clinical diagnoses, emphasizing the clinical utility of ANCA specificity in the classification of patients with AAV.
As for other autoimmune diseases, the role of cytokines in the pathogenesis of AAV is now emerging (8–11). Some of these circulating immune mediators have been shown to be elevated in patients with severe active AAV and to decline after treatment, distinguishing active AAV from remission better than conventional markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) (9). Even though evidence supporting the classification based on ANCA specificity is now accumulating, a specific characterization of circulating cytokine profiles associated with ANCA specificity or with clinical diagnosis has not yet been performed.
Therefore, we evaluated a panel of 29 circulating immune mediators associated with inflammation, proliferation, vascular injury, and tissue damage and repair in serum samples of patients with active AAV collected at the time of the patients’ inclusion into a large, prospective clinical trial and determined their association with ANCA specificity (PR3-AAV versus MPO-AAV) and clinical diagnosis (GPA versus MPA).
METHODS
Patient classification and study design
Patients for this study were enrolled in the Rituximab in AAV (RAVE) trial, a double-blind, double-dummy-controlled trial of 197 patients with active severe GPA or MPA randomized to receive either cyclophosphamide (CYC) followed by azathioprine (AZA), or rituximab (12). A positive serum assay for PR3- or MPO-ANCA and a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) (13) of 3 or higher were required for enrollment. Details of the trial design and the main study results have been published (12, 14). Of the 197 subjects included in the RAVE trial, 187 had baseline serum available for the purpose of this study. One patient was excluded from this analysis because a clinical diagnosis of GPA versus MPA was indeterminate, thus data from 186 patients were included. Patients were classified by their ANCA specificity (PR3-AAV or MPO-AAV) and by their clinical diagnosis (GPA or MPA) provided by the RAVE trial investigators at the time of enrollment (12).
The primary aim of this study was to compare profiles of the selected serum biomarkers studied within each classification system. The secondary aim was to determine whether ANCA-based or clinical diagnosis-based classification showed more significant differences in these circulating immune mediators.
Baseline disease characteristics
Disease activity was assessed in all patients using BVAS/WG, and organ manifestations present at enrollment were recorded by the study investigators, all of whom were clinicians expert in vasculitis. We defined baseline phenotype categories (capillaritis, granulomatosis, renal involvement, and alveolar hemorrhage) based on the BVAS/WG items recorded at the time of enrollment, as previously described (6). Specific clinical features of classification grouping (ANCA specificity and clinical diagnosis) in the RAVE cohort were analyzed in a previous study (4).
Serum sample processing and cytokine assays
Serum was processed and stored at each study site, then shipped to a central repository and subsequently to Johnson laboratory at the University of Michigan (Ann Arbor, USA). All samples remained frozen at −80°C until the day the assays were performed. A panel of 29 cytokines was originally compiled for their possible role as markers of disease activity and classified roughly as molecules involved in inflammation/proliferation, chemokines, soluble receptors, markers of vascular injury, or markers of tissue damage and repair, as previously reported (9). All assays were performed using an antibody array (a set of miniaturized sandwich immunoassays) as previously described (9), except for B-cell activating factor (BAFF), which was measured by ELISA using monoclonal antibodies developed at Genentech.
Data processing and statistical analysis
Data were analyzed at the Mayo Clinic Rochester (Minnesota, USA). Two classification systems, ANCA specificity and clinical diagnosis, defined groups for comparison. Continuous variables are summarized using mean±SD, or median and interquartile range (IQR), and nominal variables are summarized using frequencies and percentages. Baseline subject characteristics were compared between groups for each classification system using the Wilcoxon rank sum test for continuous variables and the chi-square test for nominal variables. The Wilcoxon rank sum test was used to compare values of each serum cytokine between groups within each classification system. We also used parametric methods after a log10 transformation of values of each biomarker with resulting values standardized to have a mean of 0 and standard deviation of 1. We individually analyzed each cytokine, reporting individual p values and confidence intervals. Then, we evaluated the relative strength of association of biomarkers with groups defined using the two classification systems.
In order to address the question whether ANCA specificity (PR3-ANCA versus MPO-ANCA) and clinical diagnosis (GPA versus MPA) predict the level of each cytokine, we conducted a multivariable linear regression. We performed this analysis for each cytokine with the given cytokine as the dependent variable, ANCA specificity (PR3-ANCA versus MPO-ANCA), and clinical diagnosis (GPA versus MPA) as the explanatory variables of interest, and age, sex, and renal insufficiency (GFR <60mL/min/1.73m2) as covariates. Initial analyses were performed to verify the absence of ANCA-by-disease interaction effects. This model approach tries to explain the strength of the association of each biomarker with PR3-AAV or MPO-AAV and GPA or MPA. Results of the multivariable analyses were summarized by presenting the respective effect estimates (z-score) and corresponding 95% confidence intervals for ANCA specificity and clinical diagnosis.
Differences were considered significant when p<0.05. Multiple-comparisons correction methods were not applied in parametric, non-parametric and multivariate analyses since each cytokine was analyzed separately and without considering a set of statistical inferences simultaneously. The two-tailed p-value for each analysis is presented in Supplementary Material. All analyses were performed using JMP and SAS software (SAS Institute, Cary, NC).
RESULTS
Demographics and clinical features of patients at enrollment
The 186 subjects included 92 male and 94 female patients with a median age of 52 years (IQR 44–66; range 15–92), all of whom had severe disease: median BVAS/WG 8 (IQR 5–10, range 3–23). Among these patients, 124 had PR3-ANCA and 62 MPO-ANCA, whereas 140 were diagnosed with GPA and 46 with MPA. Baseline characteristics of PR3-ANCA versus MPO-ANCA and of GPA versus MPA are summarized in Table 1. There was substantial overlap in clinical disease manifestations between patients classified by ANCA specificity or clinical diagnosis. Yet, within each classification group (ANCA specificity and clinical diagnosis), demographics, creatinine clearance, and all phenotype categories except alveolar hemorrhage were significantly different (p<0.05 for all comparisons) (Table 1). BVAS/WG scores were not differently distributed across the subsets.
Table 1.
Baseline characteristics of the 186 patients with AAV according to serological and clinical classifications
| Patient Features | ANCA Specificity | AAV Clinical Diagnosis | ||||
|---|---|---|---|---|---|---|
| PR3-AAV (124) | MPO-AAV (62) | p value | GPA (140) | MPA (46) | p value | |
| Age at diagnosis, median (IQR) | 51 (40; 60) | 59 (47;71) | <0.001* | 51 (43; 60) | 66.5 (45; 72.3) | <0.001* |
| Male sex, number (%) | 70 (56.5%) | 22 (35.4) | 0.004* | 75 (53.6%) | 16 (34.8%) | 0.047* |
| Any capillaritis’ manifestation, | 98 (79.0%) | 60 (96.8%) | 0.002* | 112 (80.0%) | 45 (97.8%) | 0.016* |
| Any granulomatous manifestation, number (%) | 93 (75.0%) | 23 (37.1%) | <0.001* | 106 (75.7%) | 10 (21.7%) | <0.001* |
| Alveolar hemorrhage, number (%) | 32 (25.8%) | 13 (21.0%) | 0.413 | 34 (24.3%) | 11 (23.9%) | 0.849 |
| Any renal involvement | 71 (57.3%) | 50 (80.6%) | 0.003* | 83 (59.3%) | 38 (82.6%) | 0.007* |
| Baseline creatinine clearance (mL/min), median (IQR) | 92.1 (64.0; 121.5) | 50.26 (30.2; 73.71) | <0.001* | 91.1 (58.7; 121.4) | 46.6 (29.9; 71.6) | <0.001* |
| Steroid and/or immunosuppressive treatment at screening | 64 (51.6%) | 34 (54.8%) | 0.802 | 70 (50.0%) | 28 (60.9%) | 0.266 |
| Baseline BVAS/WG score, median (IQR) | 8 (5; 10) | 8 (6;10) | 0.867 | 8 (5; 10) | 7 (5; 9) | 0.141 |
AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; PR3, proteinase 3; MPO, myeloperoxidase; GPA, granulomatous polyangiitis; MPA, microscopic polyangiitis; BVAS/WG, Birmingham Vasculitis Activity Score for Wegener's Granulomatosis; IQR, inter-quartile range; SD, standard deviation. See the Methods section for the definition of the clinical phenotype categories
Capillaritis was defined as the presence of one or more of the following BVAS/WG items: cutaneous purpura, scleritis, retinal hemorrhage or exudate, sensorineural deafness, hematuria, red blood cell casts on urinalysis or glomerulonephritis, increase in creatinine level, alveolar hemorrhage, mesenteric ischemia, sensory peripheral neuropathy, or motor mononeuritis multiplex. In contrast, BVAS/WG items reflecting underlying necrotizing granulomatous inflammation included mouth ulcers, retro-orbital mass/proptosis, bloody nasal discharge, sinus involvement, salivary gland enlargement, subglottic inflammation, conductive deafness, other major or minor ear/nose/throat involvement, pulmonary nodule/cavity, endobronchial involvement, meningitis, and cord lesion. Patients were considered to have renal disease if any renal item on the BVAS/WG (hematuria, red blood cell casts or glomerulonephritis, increase in creatinine level, or “other”) was scored. A patient was categorized as having alveolar hemorrhage only if that item was scored on the BVAS/WG. All other BVAS/WG items cannot be clearly attributed to either necrotizing granulomatous inflammation or capillaritis and were, therefore, not considered to categorize the patient one way or another.
Blood cytokine profiles between disease categories
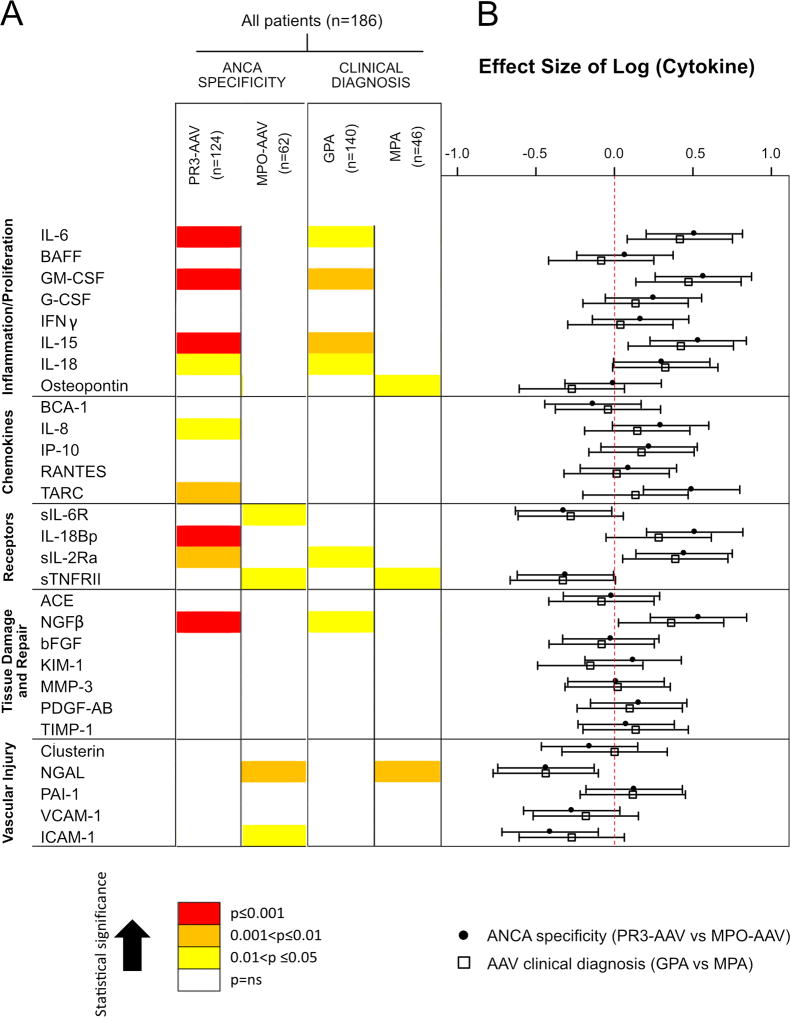
As previously reported, each of the cytokines tested at baseline in the RAVE cohort was significantly increased compared to healthy controls (HC) except RANTES, ACE, bFGF and VCAM-1 (9). Circulating cytokine profiles differed significantly between patients with PR3-ANCA versus MPO-ANCA (Figure 1A, Supplementary Table 1). Levels of 9 proteins were higher in PR3-AAV compared to MPO-AAV (IL-6, GM-CSF, IL-15, IL-18, CXCL8/IL-8, CCL17/TARC, IL-18BP, sIL-2Rα, NGFβ), whereas 4 biomarkers were higher in MPO-AAV compared to PR3-AAV (sIL6R, sTNFRII, NGAL, sICAM-1). In contrast, the same biomarkers were less often associated with either GPA or MPA when patients were classified according to the clinical diagnosis (Figure 1A, Supplementary Table 1): serum levels of 6 markers were higher in GPA compared to MPA (IL-6, GM-CSF, IL-15, IL-18, sIL-2Ra, NGFβ), and 3 were higher in MPA compared to GPA (Osteopontin, sTNFRII, NGAL). Thus, more cytokines were associated with either PR3-AAV or MPO-AAV than with either GPA or MPA. The difference between biomarker concentrations was also greater for PR3-ANCA versus MPO-ANCA than for GPA versus MPA in 9 cases (Figure 1A–B). PR3-AAV was the category with the highest number of significantly associated serum biomarkers, most of them with a stronger association when compared to other subsets (Figure 1, red boxes).
Figure 1. Association of circulating cytokines with ANCA type and AAV clinical diagnosis subgroups (PR3-AAV versus MPO-AAV and GPA versus MPA.
A Graphical representation of circulating cytokine profiles. The strengths of associations are represented by colors (yellow, 0.01<p ≤0.05; orange, 0.001<p≤0.01; red, p≤0.001) in each classification system. B Parametric analyses of the biomarkers (effect size) (see Methods for further information).
Footnotes: AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; GPA, granulomatous polyangiitis; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PR3, proteinase 3; IL, interleukin; BAFF, B-Cell Activating Factor; GM-CSF, granulocyte–monocyte colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; IFNγ, interferon gamma; BCA-1, CXCL13; IL-8, CXCL8; IP-10, CXCL10; RANTES, CCL5 (also known as Regulated on Activation, Normal T Cell Expressed and Secreted); TARC, CCL17 (also known as thymus and activation regulated chemokine); sIL-6R, soluble IL 6 receptor; IL-18Bp, interleukin 18 binding protein; sIL-2R, soluble IL 2 receptor; sTNF-RII, soluble TNF receptor II; ACE, Angiotensin-converting enzyme; NGFβ, nerve growth factor β; bFGF, basic fibroblast growth factor; KIM-1, kidney injury molecule-1; MMP-3, matrix metalloproteinase-3; PDGF-AB, platelet-derived growth factor, A and B subunits; TIMP-1, tissue inhibitor of metalloproteinases-1; NGAL, neutrophil gelatinase-associated lipocalin; PAI-1, plasminogen activator inhibitor-1; ICAM1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
We also compared cytokine levels among subgroups of subjects with AAV by combining ANCA specificity and clinical phenotype, namely PR3-ANCA-GPA (n=121), PR3-ANCA-MPA (n=3), MPO-ANCA-GPA (n=19), and MPO-ANCA-MPA (n=43). The serum levels of 10 cytokines out of 29 were significantly different between PR3-ANCA-GPA and MPO-ANCA-GPA, 2 cytokines between MPO-ANCA-GPA and MPO-ANCA-MPA (i.e. KIM-1 and Osteopontin), and 12 cytokines between PR3-ANCA-GPA and MPO-ANCA-MPA (Supplementary Table 2).
Separation of cytokines by ANCA specificity and clinical diagnosis
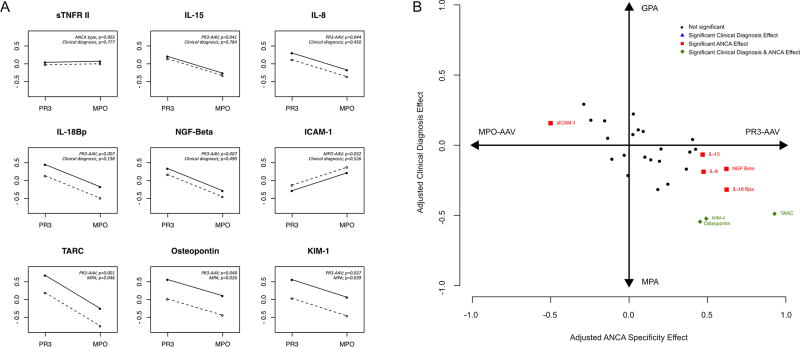
To evaluate how strongly each cytokine is associated with either ANCA specificity or clinical diagnosis, a multivariable analysis directly comparing each classification system was performed. From this analysis 8 biomarkers were found to have significant independent multivariable associations with ANCA specificity and/or clinical diagnosis (Figure 2A, Supplementary Table 3, and Supplementary Figure 1). For 5 biomarkers there was an independent association only with ANCA specificity (IL-18BP, NGF, IL-8 and IL-15 with PR3-AAV; sICAM-1 with MPO-AAV), and for 3 biomarkers there was a significant association with both ANCA specificity and clinical diagnosis (Osteopontin, KIM-1, TARC, all with PR3-AAV and MPA). No biomarker was found to have a significant association with the clinical diagnosis alone. Figure 2B illustrates the relative capacity of the two classification systems to explain the values of all the tested biomarkers simultaneously.
Figure 2. Multivariate analysis comparing the two classification systems (PR3-AAV versus MPO-AAV and GPA versus MPA) for each cytokine.
A The panel displays the 8 soluble mediators with a significant association with ANCA type and/or clinical diagnosis classifications and an explanatory figure of a molecule not associated with either ANCA specificity or clinical diagnosis is shown (sTNFR II; upper left). For each biomarker, the association with both ANCA specificity and clinical diagnosis is represented. The magnitude of the difference between GPA and MPA is visually depicted by the distance between 2 lines, each one representing a clinical diagnosis (dotted line for GPA and the solid line for MPA), whereas the difference between PR3-AAV versus MPO-AAV is visually represented by the slope of the lines. The statistical model forces them to be parallel. The direction and the grade of inclination represent the type and the strength of the association with ANCA-type classification, respectively. B Scatter plot representing all the serum cytokines studied, comparing the effect of ANCA type (x-axis) and of clinical diagnosis (y-axis). The more a biomarker is skewed to the left or to the right, the stronger it discriminates patients by ANCA type classification, the more a biomarker is skewed to the bottom or to the top, the stronger it discriminates patients by clinical diagnosis classification.
DISCUSSION
The results of this exploratory analysis conducted in patients with severe active AAV suggest that circulating serum cytokines better reflect ANCA specificity than clinical diagnosis. Using a panel of 29 circulating cytokines which individually have already been shown to be associated with disease activity of AAV or implicated in its pathogenesis (8, 9), we demonstrated that these molecules are more strongly related to ANCA specificity (PR3-AAV versus MPO-AAV) than to clinical diagnosis (GPA versus MPA).
This study identified distinct cytokine profiles for PR3-AAV versus MPO-AAV and for GPA versus MPA, with a higher number of cytokines associated and a larger effect size in favor of PR3-AAV than MPO-AAV, GPA or MPA (Figure 1, red boxes). These findings indirectly suggest that certain combinations of pathways might be more involved in PR3-AAV than in GPA, MPA and MPO-AAV. For instance, signaling cascades critical for proliferation or survival of PR3-ANCA producing B cells may drive or potentially be impacted by this cytokine network (11, 15).
Since different subsets of patients showed distinctive cytokine profiles, our results suggest that different targeted treatment approaches could be evaluated separately in clinical trials for the different subsets of AAV, similar to other autoimmune diseases in which different cytokine profiles correspond to different disease activity and treatment responsiveness (16). Our study had low power to detect differences in subgroups obtained by dividing all the subjects defined by the combination of ANCA specificity and clinical phenotype, and we were unable to compare cytokine levels of the PR3-ANCA-MPA subgroup because this subgroup comprised only 3 patients. Nevertheless, the differences observed between the PR3-ANCA versus MPO-ANCA subgroups were also seen when PR3-ANCA-GPA was compared to MPO-ANCA-GPA, consistent with the main observation that cytokine profiles are more closely related to ANCA-specificity than disease phenotype. Further functional studies are needed to elucidate the interrelationships between these circulating molecules and pathophysiologic or protective mechanisms in which they participate.
The multivariable analysis directly comparing each classification system identified 8 cytokines separating PR3-AAV from MPO-AAV, 3 of them (KIM-1, TARC, Osteopontin) also associated with clinical diagnosis and specifically with MPA. Intriguingly, no cytokines were associated only with clinical diagnosis (either GPA or MPA), thus suggesting that these circulating immune mediators are better distinguished by ANCA specificity rather than by clinical diagnosis. In clinical practice, establishing an unequivocal diagnosis of GPA or MPA is often challenging for a variety of reasons including incomplete disease manifestations at time of first diagnosis and disagreements between experts about the application of different definition schemes during the diagnostic evaluation of individual patients. In contrast, the identification of ANCA specificity is readily available, and usually does not change during patient follow-up (1). Our findings provide a molecular basis supporting the concept of an ANCA-based classification of AAV, which has already been shown to convey useful information about clinical outcomes and prognoses (1, 4, 5). The difference, if existing, between a PR3-ANCA- versus MPO-ANCA-mediated inflammation is not yet entirely characterized, unlike for GPA versus MPA inflammation. Histopathological differences in inflammation between GPA and MPA are striking, and would have suggested that cytokines correlate with phenotype rather than ANCA specificity since serum cytokines may be considered bona fide surrogates of the immunopathological events occurring in AAV. Therefore, our findings represent the first formal demonstration of combinations of cytokine pathways differently activated in distinct AAV subsets, and particularly in ANCA-based subsets. This study has several strengths. First, the study cohort was recruited though a stringent clinical trial protocol at centers expert in the study of AAV. Second, the specimens were collected, handled, and studied using strict protocols and the assays performed in a single laboratory with laboratory personnel blinded to the group assignment.
This study also has limitations to be considered when interpreting the findings and implications of the work. First, we acknowledge that the absence of detailed data on recent dosing of glucocorticoids is a limitation. The use of glucocorticoids initiated as first treatment for the active disease before blood sampling at the time of screening may thus have influenced our results in individual patients. However, recent treatment was not significantly different in each subgroup at the screening visit (Table 1). Moreover, we had previously shown that glucocorticoid treatment prior to obtaining the blood sample had no significant detectable effect on the levels of the majority of the cytokines (9), allowing us to conclude that the differences we observed in our study among distinct AAV subsets were not driven by the effect of glucocorticoid. Second, patients with AAV without ANCA and those with non-severe disease activity were excluded from the RAVE trial and our findings cannot therefore be generalized to all patients with GPA or MPA. However, recent evidence supports that patients with AAV who are consistently ANCA-negative may represent different subsets of disease with different pathogenesis (1). Third, the comparison of GPA to MPA had lower statistical power to detect differences than did the comparison of PR3-ANCA to MPO-ANCA, due to the greater imbalance in the number of patients in the clinical diagnosis groups. However, the comparison of PR3-AAV and MPO-AAV had not only lower p-values, but also larger estimated effect sizes than the comparison by clinical diagnosis, highlighting the strength of our findings. Finally, given the non-comprehensive and relatively limited number of cytokines studied, we were not able to comprehensively investigate possible mutual interactions or effects of the different cytokine pathways, thus not providing a pathophysiologic explanation of our results. The biomarkers tested were not specifically selected to represent pathophysiologic processes considered likely to differentiate subsets of AAV, and a replication of these results in an independent cohort of patients with AAV is recommended. The understanding of the reciprocal influence of these mediators is beyond the scope of our study.
In conclusion, this analysis supports the concept of an ANCA-based classification of AAV by showing that a set of selected serum biomarkers associate more strongly with either PR3-AAV or MPO-AAV, than with GPA or MPA. Distinct cytokine profiles were identified for PR3-AAV versus MPO-AAV and for GPA versus MPA. Differences in these circulating immune mediators are more strongly associated with ANCA specificity than with clinical diagnosis, suggesting that the heterogeneity in the AAV subtypes extends beyond the clinical phenotypes identified by the conventional clinical classification (GPA versus MPA). Our results provide additional support for stratification of patients by ANCA specificity for treatment trials.
Supplementary Material
Acknowledgments
Funding: This work was sponsored by the Vasculitis Clinical Research Consortium which has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319, RC1 AR058303 and P60 AR047785), the National Center for Research Resources (U54 RR019497), the National Institute of Neurological Disorders and Stroke (NS064808), and the Office of Rare Diseases Research. The RAVE Trial was performed with the support of the Immune Tolerance Network (NIH Contract N01 AI15416), an international clinical research consortium supported by the National Institute of Allergy and Infectious Diseases and the Juvenile Diabetes Research Foundation (see online appendix for the list of all members of the RAVE-ITN Research Group). Genentech and Biogen Idec provided the study medications and partial funding. At the Mayo Clinic and Foundation, the trial was supported by a Clinical and Translational Science Award from the National Center for Research Resources (NCRR) (RR024150-01), at Johns Hopkins University, by grants from the NCRR (RR025005) and career development awards (K24 AR049185 to JHS, and K23 AR052820 to PS), and at Boston University, by a Clinical and Translational Science Award (RR 025771), grants from the National Institutes of Health (M01 RR00533) and a career development award (K24 AR02224 to Dr. Peter A Merkel). Dr. Paul A Monach was supported by an Arthritis Investigator Award from the Arthritis Foundation.
References
- 1.Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nature reviews Rheumatology. 2016;12(10):570–9. doi: 10.1038/nrrheum.2016.123. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis and rheumatism. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 3.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. The New England journal of medicine. 2012;367(3):214–23. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unizony S, Villarreal M, Miloslavsky EM, Lu N, Merkel PA, Spiera R, et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Annals of the rheumatic diseases. 2016;75(6):1166–9. doi: 10.1136/annrheumdis-2015-208073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Annals of internal medicine. 2005;143(9):621–31. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Fussner LA, Hummel AM, Schroeder DR, Silva F, Cartin-Ceba R, Snyder MR, et al. Factors Determining the Clinical Utility of Serial Measurements of Antineutrophil Cytoplasmic Antibodies Targeting Proteinase 3. Arthritis & rheumatology (Hoboken, NJ) 2016;68(7):1700–10. doi: 10.1002/art.39637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanna A, Guarino L, Tam FW, Rodriquez-Cubillo B, Levy JB, Cairns TD, et al. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(7):1185–92. doi: 10.1093/ndt/gfu237. [DOI] [PubMed] [Google Scholar]
- 8.Monach PA, Tomasson G, Specks U, Stone JH, Cuthbertson D, Krischer J, et al. Circulating markers of vascular injury and angiogenesis in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis and rheumatism. 2011;63(12):3988–97. doi: 10.1002/art.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monach PA, Warner RL, Tomasson G, Specks U, Stone JH, Ding L, et al. Serum proteins reflecting inflammation, injury and repair as biomarkers of disease activity in ANCA-associated vasculitis. Annals of the rheumatic diseases. 2013;72(8):1342–50. doi: 10.1136/annrheumdis-2012-201981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berti A, Cavalli G, Campochiaro C, Guglielmi B, Baldissera E, Cappio S, et al. Interleukin-6 in ANCA-associated vasculitis: Rationale for successful treatment with tocilizumab. Semin Arthritis Rheum. 2015;45(1):48–54. doi: 10.1016/j.semarthrit.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Lepse N, Land J, Rutgers A, Kallenberg CG, Stegeman CA, Abdulahad WH, et al. Toll-like receptor 9 activation enhances B cell activating factor and interleukin-21 induced anti-proteinase 3 autoantibody production in vitro. Rheumatology (Oxford, England) 2016;55(1):162–72. doi: 10.1093/rheumatology/kev293. [DOI] [PubMed] [Google Scholar]
- 12.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. The New England journal of medicine. 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener's granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis and rheumatism. 2001;44(4):912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. The New England journal of medicine. 2013;369(5):417–27. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornec D, Berti A, Hummel A, Peikert T, Pers JO, Specks U. Identification and phenotyping of circulating autoreactive proteinase 3-specific B cells in patients with PR3-ANCA associated vasculitis and healthy controls. Journal of autoimmunity. 2017 doi: 10.1016/j.jaut.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Ronnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Current opinion in rheumatology. 2013;25(2):248–53. doi: 10.1097/BOR.0b013e32835c7e32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.