Abstract
Proteases and reactive oxygen species (ROS) have long been implicated in playing key roles in host tissue injury at sites of inflammatory processes dominated by macrophage activations and/or neutrophil infiltrations. Imbalances between proteases/antiproteases and ROS/antioxidants are recognized to contribute to amplification of inflammatory-based host tissue injury. This has been especially well-documented in such respiratory tract diseases as chronic obstructive pulmonary disease, cystic fibrosis, and acute respiratory distress syndrome. Inflammation-related protease/ROS disequilibria are further confounded by recognition that proteases can increase ROS by several different mechanisms and that ROS can inactivate proteases.
The major human antiprotease, alpha-1 antitrypsin (AAT), is dramatically inactivated by ROS. AAT deficiency represents the most prevalent genetic predisposing factor leading to emphysema, a condition treated by replacement infusions of plasma-derived AAT (hAAT) at a cost of up to $200,000 per year. An updated method for production of a plant-made recombinant AAT (prAAT) engineered for enhanced oxidation resistance compared to hAAT is presented. prAAT shows comparable antintiprotease activity to hAAT, and retains full activity under oxidative conditions that would deactivate hAAT. Additionally, we show that prAAT has similar effectiveness in preventing neutrophil elastase-induced cell death in an in vitro human bronchial epithelial cell culture model. We conclude that prAAT potentially presents a viable method for the development of a “biobetter” AAT product that could be made available to subjects with a wide spectrum of inflammatory disorders characterized by overly aggressive neutrophilic infiltrations.
Keywords: Alpha-1 antitrypsin, Transient expression, Affinity purification, HBE cell culture, Oxidation resistance, Elastase
Introduction
Proteases and reactive oxygen species (ROS) have repeatedly been shown to play interacting roles in acute and chronic inflammatory processes dominated by activated neutrophils and macrophages. Deficiencies of counterbalancing antiprotease and antioxidant regulatory mechanisms may thus result in overly exuberant inflammatory host responses resulting in tissue injury [1–4]. This principal has been well documented in inflammatory lung diseases such as chronic obstructive pulmonary disease (COPD) including emphysema, cystic fibrosis, and acute respiratory distress syndrome [1, 5].
The complexity of the interactive role of proteases and ROS is well illustrated for the case of COPD, arguably the third leading cause of death in the United States [6]. In COPD the ROS released from cigarette smoke along with the ROS and proteases released from activations of host phagocyte systems combine and interact to overcome both lung antioxidant and antiprotease systems resulting in lung airway and parenchyma injury (e.g., ROS both injuring lung tissue and inactivating antiproteases while proteases injure lung tissue and further activate oxidants) [7–13].
The ROS/antioxidant protease/antiprotease paradigm is further exemplified by congenital alpha-1 antitrypsin (AAT) deficiency, which causes familial emphysema and liver disease [14, 15]. AAT, the prototypical member of the serine protease inhibitor (serpin) superfamily and the most abundant circulating protease inhibitor in human plasma, is normally present at concentrations of 130 – 200 mg/dL. Circulating AAT levels below 57 mg/dL (11 μM) represent the established threshold for AAT deficiency, leading to increased risk for development of congenital emphysema [14]. The most common mutation leading to AAT deficiency is caused by a substitution of glutamic acid to lysine at position 342 in the reactive center loop region of AAT, denoted as “Z”; the wild-type allele is denoted as “M” [16]. This mutation causes an increased tendency of the mutant AAT to polymerize and aggregate in the endoplasmic reticulum, severely decreasing its secretion and potentially compromising the function of cells synthesizing mutant AAT [17, 18]. Patients with homogeneity for the Z mutation have an increased susceptibility to lung parenchymal destruction and early-onset emphysema, strongly potentiating the effects of cigarette smoke. The current conventional understanding is that AAT deficiency-associated emphysema results from an imbalance of protease/antiprotease in lung parenchyma.
Treatment of severe AAT deficiency-associated emphysema by augmentation therapy consists of weekly infusions of 60 mg/kg body weight of human plasma-derived AAT [14]. This significantly ameliorates the progressive elastase-related lung tissue destruction [18–21]. Although AAT infusion replacement therapy is reasonably safe and of proven efficacy in severely AAT deficient emphysematous patients [22, 23], the cost of replacement therapy in the U.S. is up to $200,000 per year. There is a clear and urgent necessity to lower the product cost to keep AAT replacement therapy available and affordable to all AAT deficient patients requiring it.
Recent evidence suggests that AT may have therapeutic uses beyond the well-recognized AAT deficient protease/antiprotease emphysema-related paradigm. Numerous studies have suggested and provided both experimental and clinical evidence that AAT could be therapeutically useful in a wider spectrum of inflammatory and immune-related diseases [24–29]. These diseases include other inflammatory lung diseases such as COPD-emphysema not related to severe AAT deficiency [30, 31], cystic fibrosis [32–34], autoimmune diseases such as type I diabetes [35], and transplantation rejection [36]. Thus, there is a growing interest for alternative AAT preparations due to the protein’s apparent beneficial effect in clinical conditions other than inherited AAT deficiency-related emphysema [36]. Although numerous small molecule anti-elastase preparations [30], a wide spectrum of AAT products modified for enhanced efficacies [26, 37, 38], and many expression systems producing a wide spectrum of recombinant AAT exist [39–42], none have reached the U.S. marketplace to date.
Plant-derived recombinant human protein production represents an emerging, promising alternative technology for the production of more cost-efficient biologic therapeutic drug products [43–45] and as a rapid response to the urgent needs for therapy for emerging infectious diseases [46, 47]. As eukaryotic systems, plants are capable of being engineered for human protein production. This capability includes complex post-translational modifications of biosimilar or functionally “biobetter” therapeutic products. Plants are inherently a relatively biosafe production host as they do not propagate mammalian pathogens or contain endotoxins.
The evolution of transient expression systems provide opportunities to scale-up production processes by increasing plant production for specifically engineered protein products. These transient systems provide advantages in the capacity to go from plant gene construct to protein extraction in 2–3 weeks with potentially lower-cost upstream processing than recombinant bacterial or mammalian cell culture technologies. Our group has developed a Cucumber mosaic virus-based inducible expression system, called CMViva (for Cucucumber mosaic virus inducible viral amplicon) and engineered it for the production of functional recombinant AAT in plant systems (prAAT) [48]. Using this system, co-expression with the viral gene silencing suppressor p19 has been shown to increase the amount of prAAT product [49]. The clinical application of this plant-produced antiprotease is designed for use in an inflammatory, pro-oxidative milieu such as the respiratory tract in COPD-emphysema and cystic fibrosis [5]. It is well-recognized that oxidants, including neutrophil-related oxidants, are capable of oxidizing methionine residues in the reactive loop of AAT and thus causing loss of the protein’s antielastase activity [7, 50–52]. We have thus substituted the most critical methionine358 residue in the critical C-terminal reactive loop of our prAAT with valine, a substitution that allows for the maintenance of AAT antielastase activity but renders the prAAT less susceptible to oxidative inactivation by inflammatory oxidants [53, 54]. In this communication, we present the preliminary results of our plant-derived “biobetter” prAAT and mention future challenges for advancing the product to clinical trials. Partial results for the work presented in this paper has been previously published in abstract form [55].
Materials and Methods
Reagents
Human AAT for analytical uses was purchased from Calbiochem. Prolastin-C (Grifols, Barcelona, Spain) was provided as a gift. All other reagents were purchased from Sigma-Aldrich unless otherwise noted.
Upstream Processing
Wild-type Nicotiana benthamiana seeds were grown in a greenhouse under a controlled light cycle (16 hours light/8 hours dark) for 12–14 days in UC Sunshine Mix #1 soil, then transplanted into UC Mix soil for further growth. When plants reached 4–6 weeks in age, they were transported from the greenhouse to the lab. Plants were either selected for whole-plant agroinfiltration or infiltration of detached leaves alone. For detached leaf agroinfiltration, fully expanded leaves weighing between 0.7 g and 2.0 g were detached at the petiole using sanitized scissors.
Two strains of recombinant Agrobacterium tumefaciens strain EHA105 carrying the helper plasmid pCH32, one carrying the Tomato bushy stunt virus post-translational gene silencing suppressor p19 and one carrying a viral vector with inducible recombinant AAT expression cassette (CMViva-SPAAT), were cultured separately in LB media with appropriate antibiotics in shake flasks at 28°C and 250 rpm [56]. Seed inoculum was prepared in 25 mL of LB media with appropriate antibiotics and 10% (v/v) of each recombinant agrobacteria strain in 125 mL shake flasks for 20–24 hours. Twenty mL of Agrobacterium inoculum was added to 180 mL of LB media containing 10 mM MES pH 5.6 and 40 μM acetosyringone in 1 L shake flasks until stationary phase was reached (20–24 hours). Agrobacterium cultures were pelleted and resuspended separately in infiltration buffer consisting of 10 mM MES pH 5.6, 10 mM MgCl2, and 150 μM acetosyringone to reach a target OD600 of 0.5 for each strain and incubated for 3–5 hours in the dark at room temperature. Immediately prior to agroinfiltration, these suspensions were combined in a 1:1 volume ratio and 0.03% (v/v) Silwet L-77 was added. Detached leaves or intact plants were submerged in a well-mixed agrobacteria suspension in suitable vessel placed in a vacuum chamber, after which the chamber was sealed and vacuum was drawn to ≥20 in Hg. Vacuum pressure for infiltration was held at ≥20 in Hg for 1 minute. Agroinfiltrated detached leaves were allowed to dry in a biosafety cabinet for 1 hour before storage in the dark for 12 hours in sealed plastic boxes containing moistened Perlite. Intact plants were left at room temperature in the dark for 12 hours.
Induction solution consisted of 50 μM 17-β-estradiol and 0.03% (v/v) Silwet L-77. Detached leaves and intact plants were induced in a vacuum chamber in the same manner as agroinfiltration, but using the induction solution instead of the agroinfiltration solution 12 hours post-agroinfiltration. Leaves were stored in a controlled humidity chamber at 20°C and 90% RH in the dark in well-sealed boxes containing moistened Perlite for 6 days post-induction before collecting in sealed bags and freezing at −80°C. Intact plants were kept in a controlled environment chamber at 20°C and >80% RH for 6 days post-induction before manually detaching healthy and collecting in sealed bags and leaves and freezing at −80°C.
Downstream Processing
Frozen leaves were ground in liquid nitrogen and weighed into a prepared bottle. Extraction buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 2 mM sodium metabisulfite, pH 8.0) was added in a 4:1 extraction buffer to biomass ratio to ground tissue. The extract slurry was mixed on a nutating mixer at 4°C for 30 min to 1 hr then filtered through four layers of cheesecloth to remove the majority of cell debris and transferred to conical tubes for centrifugation at 4°C and 3,200 x g for 30 min. The supernatant was then filtered through 0.22 μm syringe filters to remove any residual cell debris then stored at −80°C for purification and analyses. Analyses were performed either immediately or within 72 hours of processing.
Alpha-1 Antitrypsin Select chromatography resin (GE Healthcare, Pittsburgh, PA) was packed into a GE Healthcare XK16/20 chromatography column with a final packed volume of 10 mL. Filtered crude extract was thawed and microfiltered using 0.22μm PVDF membrane filters (EMD Millipore, Billerica, MA) before loading directly onto the affinity resin using an AKTApure system (GE Healthcare, Pittsburgh, PA). All chromatography steps were performed with a linear flow rate of 30 cm/hr. Equilibration and resin wash were performed with 20mM Tris, 150mM NaCl, pH 7.4; elution was performed with 20mM Tris, 2M MgCl2, pH 7.4; resin stripping was performed with 70% (v/v) ethanol. All buffers used in chromatography were filtered through 0.22μm PVDF membrane filters (EMD Millipore, Billerica, MA) and degassed prior to use on the chromatography system. Eluate was collected in 1 mL fractions for analysis. Fractions containing high amounts of prAAT as identified by nonreducing SDS-PAGE analysis were pooled and buffer exchanged to PBS pH 7.4 using 10 kDa MWCO Amicon Ultra-15 Centrifugal Filter Units (EMD Millipore, Billerica, MA).
AAT Characterization
Total prAAT in both crude extracts and purified samples was measured by ELISA. Corning® 96 Well EIA/RIA Clear Flat Bottom Polystyrene High Binding Microplates (Corning Inc., Corning, NY) were coated with a 1:2857 dilution of polyclonal rabbit anti-human Alpha-1 Antitrypsin (Agilent/Dako, Santa Clara, CA) in PBS pH 7.4 overnight. Plates were blocked with a solution of 5% nonfat dry milk in PBS pH 7.4 for 1 hour at room temperature and washed with PBS pH 7.4 containing 0.05%(v/v) Tween-20 (PBST) before incubation with 50 μL of the samples of interest diluted in PBST. Plates were washed with PBST and incubated with 50 μL of a 1:3000 dilution in PBS pH 7.4 of polyclonal sheep anti-Alpha-1 Antitrypsin HRP conjugated (Abcam, San Francisco, CA) for 1 hour at room temperature. Plates were developed using 100 μL TMB Microwell Peroxidase Substrate (KPL, Milford, MA) for 7–10 minutes or until color developed and stopped with 100 μL of 1M HCl, then read on a SpectraMax 340pc Microplate Reader (Molecular Devices, Sunnyvale, CA) using a wavelength of 450 nm in endpoint mode. A standard curve was generated using a 4-parameter regression from the absorbance of samples of hAAT at known concentrations in 2x serial dilutions in PBST from 200 ng/mL to 1.56 ng/mL by SoftMax Pro v5.3 (Molecular Devices, Sunnyvale, CA) to determine the prAAT content of unknown samples.
Functional AAT content in both crude and purified samples was measured by a residual elastase activity assay. Briefly, a 1:1 volume ratio of 3 μg/mL PPE to AAT samples were incubated in a water bath at 37°C for 15 minutes before loading to a 96 well plate containing 100 μL of elastase assay buffer (20 mM Tris, 150 mM NaCl, 0.01% Tween-80, pH 8.1) in each well. Standards consisted of PPE in concentrations of 0 to 1.75 μg/mL in 0.25 μg/mL increments. Immediately prior to measuring the change in absorbance using a SpectraMax 340pc Microplate Reader, 50 μL of the elastase substrate, 4 mM n-Succinyl-Ala-Ala-Ala-p-Nitroanilide, was added to each well. The change on OD405 was monitored in SoftMax Pro v5.3 software in kinetic mode for 10 minutes with readings taken every 22 seconds. SoftMax Pro v5.3 software was used to generate a standard curve relating initial reaction rate (mOD/min) to functional elastase concentration (μg/mL) and determine the AAT content in each sample based on the assumption that 1 molecule of functional AAT inhibits 1 molecule of PPE [57, 58]. R2 values for data presented in this article were greater than 0.99.
Hydrogen peroxide and sodium hypochlorite were used to measure the impact of oxidation conditions on the activity of prAAT and Prolastin-C®. For oxidation by hydrogen peroxide, samples of prAAT and Prolastin-C® were incubated in 48.9 mM H2O2 for 1 hour at room temperature before desalting and buffer exchange to assay buffer using disposable PD-10 desalting columns (GE Healthcare, Pittsburgh, PA) prepacked with 2.5 mL Sephadex G-25 media and assaying using the residual elastase activity assay described above. For oxidation by sodium hypochlorite, samples of prAAT and Prolastin-C® were incubated 100 μM sodium hypochlorite on a plate shaker at room temperature for 15 minutes before desalting and buffer exchange to assay buffer using disposable PD MiniTrap G-25 desalting columns (GE Healthcare, Pittsburgh, PA) prepacked with 0.5 mL Sephadex G-25 media and determination of inhibitory activity. Control samples in both cases consisted of an aliquot of AAT treated with an equivalent amount of elastase assay buffer without oxidizer and handled in an identical manner. The inhibitory activity of oxidized AAT was compared directly to the activity of its equivalent control (oxidized prAAT compared to control prAAT; oxidized Prolastin-C® compared to control Prolastin-C®).
Protein identification by LC-MS/MS was performed using purified prAAT, which was buffer exchanged to 50 mM ammonium bicarbonate in centrifugal filter units as described previously.
HBE Cell Culture
Normal human bronchial epithelial cell line (HBE) was a gift from JR Yankaskas, University of North Carolina provided to Dr. Reen Wu, University of California at Davis. Cells were grown in DMEM/F-12 (Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12; Life Technologies Inc., Carlsbad, CA) supplemented with insulin (2 mg/mL), transferrin (2.5 mg/mL), epidermal growth factor (10 μg/mL), dexamethasone (0.05 mM), cholera toxin (10 μg/mL) and bovine hypothalamus extract (15 μg/mL). Experiments were conducted with cells at passages 4–6 that were maintained at 37°C in 5% CO2 and >95% RH air incubator. Prior to experimental treatments, cells were pre-equilibrated in fresh medium for 1 h.
Cell Viability Assay
HBE cells were seeded onto 96-well plates at density of 5 10×103 cells per well for 2–3 days to 80% confluency. They were then treated with added amounts of Prolastin-C or prAAT at the indicated concentration and time intervals. Cell viability was evaluated using the (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) tetrazolium reduction (MTT) assay (Chemicon, Temecula, CA). After 48 h of treatment, 20 μL of the MTT solution (10 mg/ml) was added into each well and incubated for 3–4 hours at 37°C. The quantity of formazan (presumably directly proportional to the number of viable cells) is measured by recording changes in absorbance at 570 nm using a plate reading spectrophotometer.
Cell Protection from Elastase Assay
Again cells were seeded onto 96-well plates at density of 5 10×103 cells per well and grown to 80% confluence and pre-incubated with 0, 31.25, 62.5,125, 250, 500, 1000 or 2000 mg/dL with either Prolastin-C or with prAAT for 2h. Cells were then treated with 0, 100 or 500 nM of neutrophil elastase for 48 h and subsequently assessed for cell viability using the MTT assay described above. Data was analyzed by independent t-test comparing control cells against treated cells.
Results
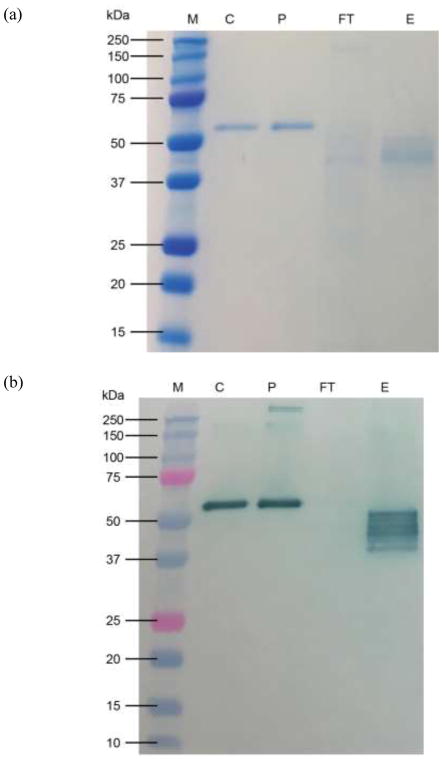
prAAT was produced in both intact plants and detached leaves. Total AAT yields were measured by ELISA as 783±11 mg total AAT/kg FW in detached leaves and 285±34 mg total AAT/kg FW in intact plants. Functional AAT yields were measured by residual elastase activity assay as 363±22 mg functional AAT/kg FW in detached leaves and 56.3±5.6 mg functional AAT/kg FW in intact plants. The single-step affinity chromatography yielded highly pure prAAT from detached leaves as shown by SDS-PAGE and companion immunoblotting (Figure 1, below) with a yield of 71±3% from overall downstream processing. Several bands appear on the immunoblot representing prAAT; we have yet to fully characterize these bands but suspect they are due to differences in extent of glycosylation and may potentially include degradation products from digestion by plant proteases.
Figure 1.
SDS-PAGE (a) and Western immunoblot (b) of Alpha-1 Antitrypsin Select (GE Healthcare, Pittsburgh, PA) chromatography for plant recombinant AAT samples. M – molecular weight marker; C – Analytical standard human AAT (Calbiochem,EMD Millipore, Billerica, MA ); P – Prolastin-C® (Grifols, Barcelona, Spain); FT – Unbound protein from collected flowthrough; E – eluted protein. The band for prAAT appears at a lower molecular weight than both bands for human AAT due to differing glycosylation patterns.
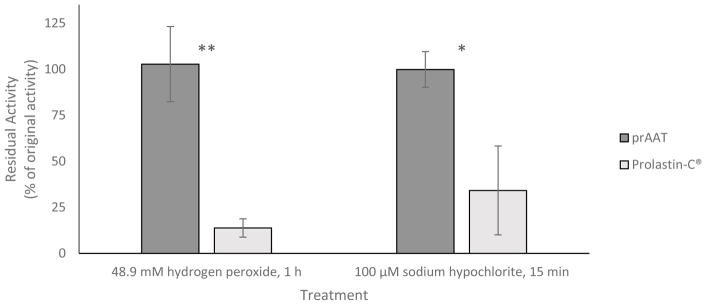
After oxidation with 48.9 mM H2O2 for 1 hour at room temperature, Prolastin-C® was found to retain (13.8±5.0)% of its inhibitory activity against PPE; prAAT retained (103±20)% of its inhibitory activity against PPE under equivalent conditions. Both samples were compared to an untreated control as described above. Similar results were found under oxidation with 100 μM NaOCl, with Prolastin-C® retaining (29±22)% and prAAT retaining (100±10)% of the equivalent control sample’s inhibitory activity against PPE, respectively (Figure 2).
Figure 2.
prAAT is significantly more resistant to oxidation than Prolastin-C®. After incubation in 48.9 mM hydrogen peroxide for 1 h at room temperature or 100 μM NaOCl at room temperature for 15 min, Prolastin-C® was significantly less able to inhibit porcine pancreatic elastase while prAAT retained its inhibitory activity. * indicates p < 0.05, ** indicates p < 0.01. Error bars represent one standard deviation of triplicate assays.
Mass spectrometry analysis confirmed that our prAAT had fully intact N- and C-termini as shown in Figure 3. The mature, 394 amino acid sequence was identified with 95% coverage. Both correct cleavage of the RAmy3D secretion signal peptide and the amino acid substitution from methionine358 to valine358 were confirmed.
Figure 3.
Mass spectrometry analysis of plant recombinant alpha-1 antitrypsin. The mature protein sequence was found to be intact, with 95% sequence coverage observed. This analysis confirmed the presence of the M to V substitution introduced into our plant recombinant AAT at residue 358 of the human AAT sequence.
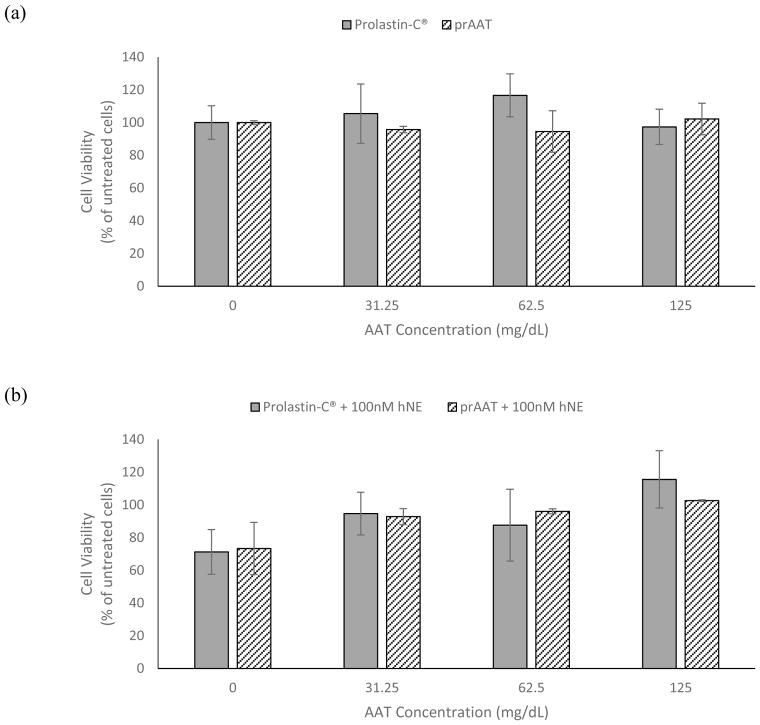
As shown in Figure 4, Prolastin-C® and prAAT provided no significant difference in their protection of human bronchial epithelial cells from neutrophil elastase-induced cell death. For concentrations up to 125 mg/dL, neither prAAT nor Prolastin-C® alone affected cell viability. When exposed to 100 nM human neutrophil elastase, 31.25 mg/dL of either Prolastin-C® or prAAT was required to prevent significant cell death. At least 62.5 mg/dL of either Prolastin-C® or prAAT was required to prevent significant cell death when cells were treated with 500nM human neutrophil elastase.
Figure 4.
HBE cells were treated with either Prolastin-C® or prAAT at concentrations ranging from 0 to 125 mg/dL before exposure to human neutrophil elastase. For untreated cells, cells treated with 100 nM hNE, and cells treated with 500 nM hNE, there was no significant difference in cell viability after 48 hours of incubation between cells treated with Prolastin-C® and cells treated with prAAT.
Discussion
Major findings of the current studies demonstrate that prAAT can be produced and purified with high yields using a transient, plant-based expression system in less than a week; this prAAT is biologically active against elastase as analyzed in vitro; and that the protein can be modified to confer resistance to neutrophilic oxidative metabolites.
Comparisons to prior reports of plant-made recombinant AAT
Prior reports of plant-made AAT have reported successful production in transient expression systems [49, 59, 60] and purification from crude extracts [49, 59]. The only prior report of oxidation-resistant plant-made AAT used transgenic tomato plants for production [61] and did not discuss oxidation by sodium hypochlorite. To our knowledge, no prior report has been made describing the oxidative resistance of plant-made AAT expressed in a transient system. While we did not observe the 40 kDa fragment identified by Castilho et al., we suspect that our prAAT is similarly susceptible to degradation by or interaction with plant proteases. Several other factors contribute to the difference in observed results, namely the use of different genetic constructs for the production of AAT, the difference in protein modifications, and the methods used to recover recombinant proteins from agroinfiltrated tissues. Castilho et al. used the magnICON vector system to produce strep-tagged AAT, while we used the CMViva system to produce untagged AAT with a single amino acid substitution; these systems are under the control of different promoters and use different signal peptides. The work reported in this article was performed using Agrobacterium tumefaciens strain EHA105 while the work reported by Castilho et al. was performed using strains UIA143 and GV3101 pMP90. Our primary aims also differed; Castilho et al. sought to characterize plant glycosylation of proteins and cleavage in the apoplast fluid, and used intercellular fluid recovery as a primary method of extraction while we performed total tissue grinds and sought to specifically characterize the inhibitory activity and oxidation resistance of our prAAT.
Although it is a subtle point, both the prior works referenced above [60, 61] modified the amino acid sequence of AAT to include tags for subcellular localization (the KDEL sequence used by Jha et al.) or ease of identification (the Strep tag used by Castilho et al.). The prAAT produced in this report is unmodified with the exception of the single amino acid substitution M358V. This is the first report of production and purification of untagged AAT in a plant system of which we are aware.
This paper represents the first report of a direct comparison of a plant-made, recombinant human AAT to a commercially available therapeutic formulation, and shows that our prAAT is comparable to Prolastin-C® for protection against elastase-induced cell death.
prAAT Production Strategies
To produce functional plant recombinant AAT, we investigated different incubation conditions post-agroinfiltration. Based on our yields and activity of AAT produced, we selected detached leaf infiltration as optimal for the CMViva system. During the 6-day incubation period, monitoring of the humidity within the incubation chamber was critical to obtain the highest yields of AAT per gram leaf biomass. Typical yields ranged from 200 mg functional AAT/kg biomass to 700 mg functional AAT/kg biomass measured at the time of extraction with liquid nitrogen. We successfully achieved purification from clarified protein extracts using a two-step process of microfiltration and affinity chromatography. Further downstream processing was achieved using 10 kDa MWCO Amicon Ultra-15 Centrifugal Filter Units (EMD Millipore, Billerica, MA) for diafiltration and concentration of small volumes (up to approximately 50 mL) of purified prAAT. Despite the inclusion of 2 mM sodium metabisulfite, a polyphenol oxidase inhibitor, in the extraction buffer, crude plant extracts retained a high amount of phenolic compounds. This gave the extracts a dark brown color and permanently discolored the chromatography resin, as well as led to significant fouling of the ultrafiltration membranes at small scale. We suspect that these issues would be largely mitigated by scale-up of the upstream process, allowing the use of tangential flow filtration for concentration and diafiltration.
We observed that detached leaf tissues produced a significantly higher amount of prAAT per gram FW than intact, healthy plants. We currently suspect that, as the leaf is detached from the intact plant and begins dying upon detaching from the stem, the leaf retains all resources within the detached tissue and is able to use them for recombinant protein production instead of plant growth. Our research group has noticed similar trends with other molecules in other expression systems (data not shown).
Anti-elastase activity of prAAT compared to hAAT
We evaluated the specific activity of purified prAAT by comparing our activity determined by residual elastase activity and total AAT content in the sample determined by ELISA. Specific activity rarely exceeded 0.4 mg functional AAT per mg total AAT, typically falling within the range of 0.3–0.35 mg functional AAT per mg total AAT. This was approximately half the specific activity of Prolastin-C®, which was found to be 0.72±0.06 mg functional AAT per mg total AAT by the same methods. We suspect that cleavage by apoplastic proteases is causing a loss in inhibitory activity compared to hAAT, as we have not observed any loss in inhibitory activity throughout the downstream process. Degradation fragments can be seen in the immunoblot in Figure 1b.
prAAT is more resistant to neutrophil-related oxidation than hAAT
The prAAT produced in this report has had a single amino acid substitution to confer greater resistance to oxidation (M358V). We found that under strongly oxidizing conditions in an aqueous solution of either hydrogen peroxide or sodium hypochlorite, our prAAT retained greater than 95% of its original inhibitory activity against PPE. While prior reports have suggested that oxidation of either methionine 351 or methionine 358 can lead to loss of inhibitory activity [53, 54] we observed that protection of methionine 358 by substitution with valine was sufficient to retain activity under oxidative levels sufficient to deactivate hAAT.
prAAT protects cells from elastase-induced cytotoxicity
At AAT concentrations up to 125 mg/dL, we observed that prAAT and Prolastin-C® provide the same degree of protection against neutrophil elastase-induced cell death. This suggests that prAAT could be suitable for treatment of AATD as the required threshold level of protection targeted by AATD treatment, 11 μM, corresponds to approximately 57 mg/dL. We were unable to study the effects of prAAT at higher concentrations than 125 mg/dL due to difficulties in the process of concentrating the purified protein; additionally, at concentrations above 250 mg/dL of Prolastin-C®, we observed cytotoxic effects. We suspect these effects were due to osmotic conditions in the culture medium and not due to the properties of AAT.
Limitations and challenges
Although the present report provides solid data that prAAT inhibits elastase as effectively as hAAT and is less susceptible to oxidative inactivation, it is unlikely that protease inhibition accounts for the full spectrum of AAT biologic activities. Many reports have highlighted a plethora of anti-inflammatory and immune-modulating attributes of hAAT independent of its antiprotease activity [24, 27, 62–66]. Unpublished studies in our group have shown that extensive variations in AAT glycan structures do not seem to significantly modulate intrinsic AAT anti-elastase activities, a finding consistent with our prAAT. It is well known that plants produce different mature glycan structures than mammalian cells [67], and as such our prAAT has different glycan structures than hAAT at each of the molecule’s three N-linked glycosylation sites. Although it could be expected that these plant glycoforms could initiate immune system responses in humans, repeated infusions of plant-made glucocerebrosidase have not been shown to elicit immune responses in patients with Gaucher’s disease patients [68]. However, studies have shown that glycans can modify plasma pharmacokinetics [64, 69] and a number of neutrophil functions such as chemotaxis [70], NADPH activity [71], and NET formation [72]. Studies specifically designed to more exclusively examine the effects of prAAT glycosylation on non-antiprotease AAT modulations of inflammatory and immune processes are important for future characterization work.
Conclusion
We have successfully produced and purified recombinant AAT in N. benthamiana. We have confirmed that a single amino acid substitution from methionine to valine in the active site loop is sufficient to provide resistance to oxidation by oxidants including sodium hypochlorite without negating the ability of the protein to inhibit elastase activity. Additionally, we have shown that prAAT and commercially available hAAT provide similar protection to human respiratory tract epithelial cells when challenged with cytotoxic concentrations of neutrophil elastase. Further studies are being directed toward greater optimization of prAAT activity, yields and extraction efficiencies; characterization of the impact of prAAT N-linked oligosaccharides on bioactivity as compared to hAAT with a focus on non-antiprotease inflammatory and immune activities; and initiations of in vivo pharmacokinetics and therapeutic effectiveness.
Highlights.
Proteases and oxidants play key roles in inflammatory processes
AAT is the most prevalent human antiprotease but is sensitive to oxidation
Recombinant AAT can be produced in plant species
Plant-based production can present a cheaper alternative to expensive AAT therapy
A “biobetter” oxidation-resistant AAT can be efficiently produced in N. benthamiana
Acknowledgments
We are grateful to the services of the UC Davis Proteomics Core for providing full sequence analysis of prAAT.
The project described was supported by the Alpha-1 Foundation and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award TL1 TR001861. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Alpha-1 Foundation or the NIH.
Abbreviations
- AAT
alpha-1 antitrypsin
- AATD
alpha-1 antitrypsin deficiency
- COPD
chronic obstructive pulmonary disease
- hAAT
human alpha-1 antitrypsin
- prAAT
plant recombinant alpha-1 antitrypsin
- SDS-PAGE
sodium dodecyl sulfide-polyacrylamide gel electrophoresis
- ELISA
enzyme-linked immunosorbent assay
- PPE
porcine pancreatic assay
- HBE
human bronchial epithelial
- hNE
human neutrophil elastase
- N-Suc-(Ala)3-pNa
N-succinyl-Ala-Ala-Ala-p-nitroanilide
- FW
fresh weight
- H2O2
hydrogen peroxide
- NaOCl
sodium hypochlorite
- RH
relative humidity
- ROS
reactive oxygen species
- serpin
serine protease inhibitor
Footnotes
Conflict of Interest
Karen McDonald is the Scientific Advisor and Co-Founder of Inserogen Inc., which conducts research to produce plant recombinant Alpha-1 Antitrypsin for therapeutic use.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 5. Oxford: Oxford University Press; 2015. [Google Scholar]
- 2.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 3.Meyer-Hoffert U, Wiedow O. Neutrophil serine proteases: mediators of innate immune responses. Curr Opin Hematol. 2011;18:19–24. doi: 10.1097/MOH.0b013e32834115d1. [DOI] [PubMed] [Google Scholar]
- 4.Soualmia F, El Amri C. Serine protease inhibitors to treat inflammation: a patent review (2011–2016) Expert Opin Ther Pat. 2018;28:93–110. doi: 10.1080/13543776.2018.1406478. [DOI] [PubMed] [Google Scholar]
- 5.Cross CE, van der Vliet A, O'Neill CA, Eiserich JP. Reactive oxygen species and the lung. The Lancet. 1994;344:930–933. doi: 10.1016/s0140-6736(94)92275-6. [DOI] [PubMed] [Google Scholar]
- 6.National Center for Health S. Health, United States. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville (MD): National Center for Health Statistics (US); 2016. [PubMed] [Google Scholar]
- 7.Evans MD, Pryor WA. Cigarette smoking, emphysema, and damage to alpha 1-proteinase inhibitor. Am J Physiol. 1994;266:L593–611. doi: 10.1152/ajplung.1994.266.6.L593. [DOI] [PubMed] [Google Scholar]
- 8.Brown GM, Donaldson K. Degradation of connective tissue components by lung derived leucocytes in vitro: role of proteases and oxidants. Thorax. 1988;43:132–139. doi: 10.1136/thx.43.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoshiba K, Yasuda K, Yasui S, Tamaoki J, Nagai A. Serine proteases increase oxidative stress in lung cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L556–564. doi: 10.1152/ajplung.2001.281.3.L556. [DOI] [PubMed] [Google Scholar]
- 10.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 11.Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–421. doi: 10.2147/COPD.S10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer BM, Voynow JA, Ghio AJ. COPD: balancing oxidants and antioxidants. Int J Chron Obstruct Pulmon Dis. 2015;10:261–276. doi: 10.2147/COPD.S42414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuto T, Kamei S, Nohara H, Fujikawa H, Tasaki Y, Sugahara T, Ono T, Matsumoto C, Sakaguchi Y, Maruta K, et al. Pharmacological and genetic reappraisals of protease and oxidative stress pathways in a mouse model of obstructive lung diseases. Sci Rep. 2016;6:39305. doi: 10.1038/srep39305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- 15.Greene CM, Marciniak SJ, Teckman J, Ferrarotti I, Brantly ML, Lomas DA, Stoller JK, McElvaney NG. alpha 1-Antitrypsin deficiency. Nature Reviews Disease Primers. 2016:2. doi: 10.1038/nrdp.2016.51. [DOI] [PubMed] [Google Scholar]
- 16.Krotova K, Marek GW, Wang RL, Aslanidi G, Hoffman BE, Khodayari N, Rouhani FN, Brantly ML. Alpha-1 Antitrypsin-Deficient Macrophages Have Increased Matriptase-Mediated Proteolytic Activity. American Journal of Respiratory Cell and Molecular Biology. 2017;57:238–247. doi: 10.1165/rcmb.2016-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roussel BD, Irving JA, Ekeowa UI, Belorgey D, Haq I, Ordóñez A, Kruppa AJ, Duvoix A, Rashid ST, Crowther DC, et al. Unravelling the twists and turns of the serpinopathies. FEBS Journal. 2011;278:3859–3867. doi: 10.1111/j.1742-4658.2011.08201.x. [DOI] [PubMed] [Google Scholar]
- 18.Group TA--ADRS. Survival and FEV1Decline in Individuals with Severe Deficiency of α1-Antitrypsin. American Journal of Respiratory and Critical Care Medicine. 1998;158:49–59. doi: 10.1164/ajrccm.158.1.9712017. [DOI] [PubMed] [Google Scholar]
- 19.Kueppers F. The role of augmentation therapy in alpha-1 antitrypsin deficiency. Current Medical Research and Opinion. 2011;27:579–588. doi: 10.1185/03007995.2010.548750. [DOI] [PubMed] [Google Scholar]
- 20.Chapman KR, Burdon JGW, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, Stoel BC, Huang L, Yao Z, Edelman JM, McElvaney NG. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. The Lancet. 2015;386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 21.McElvaney NG, Burdon J, Holmes M, Glanville A, Wark PAB, Thompson PJ, Hernandez P, Chlumsky J, Teschler H, Ficker JH, et al. Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE) The Lancet Respiratory Medicine. 2017;5:51–60. doi: 10.1016/S2213-2600(16)30430-1. [DOI] [PubMed] [Google Scholar]
- 22.Stockley RA, Turner AM. α-1-Antitrypsin deficiency: clinical variability, assessment, and treatment. Trends in Molecular Medicine. 2014;20:105–115. doi: 10.1016/j.molmed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Edgar R, Patel M, Bayliss S, Crossley D, Sapey E, Turner A. Treatment of lung disease in alpha-1 antitrypsin deficiency: a systematic review. International Journal of Chronic Obstructive Pulmonary Disease. 2017;12:1295–1308. doi: 10.2147/COPD.S130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonigk D, Al-Omari M, Maegel L, Mueller M, Izykowski N, Hong J, Hong K, Kim S-H, Dorsch M, Mahadeva R, et al. Anti-inflammatory and immunomodulatory properties of alpha 1-antitrypsin without inhibition of elastase. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15007–15012. doi: 10.1073/pnas.1309648110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe K-H, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, et al. A Novel Antiapoptotic Role for α1-Antitrypsin in the Prevention of Pulmonary Emphysema. American Journal of Respiratory and Critical Care Medicine. 2006;173:1222–1228. doi: 10.1164/rccm.200512-1842OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brantly M. α1-Antitrypsin: Not Just an Antiprotease. American Journal of Respiratory Cell and Molecular Biology. 2002;27:652–654. doi: 10.1165/rcmb.F250. [DOI] [PubMed] [Google Scholar]
- 27.Baraldo S, Balestro E, Bazzan E, Tine ME, Biondini D, Turato G, Cosio MG, Saetta M. Alpha-1 Antitrypsin Deficiency Today: New Insights in the Immunological Pathways. Respiration. 2016;91:380–385. doi: 10.1159/000445692. [DOI] [PubMed] [Google Scholar]
- 28.Hurley K, Reeves EP, Carroll TP, McElvaney NG. Tumor necrosis factor-alpha driven inflammation in alpha-1 antitrypsin deficiency: a new model of pathogenesis and treatment. Expert review of respiratory medicine. 2016;10:207–222. doi: 10.1586/17476348.2016.1127759. [DOI] [PubMed] [Google Scholar]
- 29.Bergin DA, Reeves EP, Hurley K, Wolfe R, Jameel R, Fitzgerald S, McElvaney NG. The Circulating Proteinase Inhibitor alpha-1 Antitrypsin Regulates Neutrophil Degranulation and Autoimmunity. Science Translational Medicine. 2014:6. doi: 10.1126/scitranslmed.3007116. [DOI] [PubMed] [Google Scholar]
- 30.Lakshmi S, Reddy A, Reddy R. Emerging pharmaceutical therapies for COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2017;12:2141–2156. doi: 10.2147/COPD.S121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lomas DA. Does Protease-Antiprotease Imbalance Explain Chronic Obstructive Pulmonary Disease? Ann Am Thorac Soc. 2016;13(Suppl 2):S130–137. doi: 10.1513/AnnalsATS.201504-196KV. [DOI] [PubMed] [Google Scholar]
- 32.Martin SL, Downey D, Bilton D, Keogan MT, Edgar J, Elborn JS, Team ACS. Safety and efficacy of recombinant alpha(1)-antitrypsin therapy in cystic fibrosis. Pediatric Pulmonology. 2006;41:177–183. doi: 10.1002/ppul.20345. [DOI] [PubMed] [Google Scholar]
- 33.Twigg MS, Brockbank S, Lowry P, FitzGerald SP, Taggart C, Weldon S. The Role of Serine Proteases and Antiproteases in the Cystic Fibrosis Lung. Mediators of Inflammation. 2015;2015:1–10. doi: 10.1155/2015/293053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaggar A, Chen J, Chmiel JF, Dorkin HL, Flume PA, Griffin R, Nichols D, Donaldson SH. Inhaled alpha(1)-proteinase inhibitor therapy in patients with cystic fibrosis. Journal of Cystic Fibrosis. 2016;15:227–233. doi: 10.1016/j.jcf.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rachmiel M, Strauss P, Dror N, Benzaquen H, Horesh O, Tov N, Weintrob N, Landau Z, Ben-Ami M, Haim A, et al. Alpha-1 antitrypsin therapy is safe and well tolerated in children and adolescents with recent onset type 1 diabetes mellitus. Pediatric Diabetes. 2015;17:351–359. doi: 10.1111/pedi.12283. [DOI] [PubMed] [Google Scholar]
- 36.Kandregula CAB, Aseervatham GSB, Bentley GT, Kandasamy R. Alpha-1 antitrypsin: Associated diseases and therapeutic uses. Clinica Chimica Acta. 2016;459:109–116. doi: 10.1016/j.cca.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 37.Luisetti M, Travis J. Bioengineering: alpha(1)-proteinase inhibitor site-specific mutagenesis - The prospect for improving the inhibitor. Chest. 1996;110:S278–S283. doi: 10.1378/chest.110.6_supplement.278s. [DOI] [PubMed] [Google Scholar]
- 38.Toldo S, Mauro AG, Marchetti C, Rose SW, Mezzaroma E, Van Tassell BW, Kim S, Dinarello CA, Abbate A. Recombinant Human Alpha-1 Antitrypsin-Fc Fusion Protein Reduces Mouse Myocardial Inflammatory Injury After Ischemia-Reperfusion Independent of Elastase Inhibition. Journal of Cardiovascular Pharmacology. 2016;68:27–32. doi: 10.1097/FJC.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 39.Huang JM, Sutliff TD, Wu LY, Nandi S, Benge K, Terashima M, Ralston AH, Drohan W, Huang N, Rodriguez RL. Expression and purification of functional human alpha-1-antitrypsin from cultured plant cells. Biotechnology Progress. 2001;17:126–133. doi: 10.1021/bp0001516. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal S, Singh R, Sanyal I, Amla DV. Expression of modified gene encoding functional human alpha-1-antitrypsin protein in transgenic tomato plants. Transgenic Res. 2008;17:881–896. doi: 10.1007/s11248-008-9173-8. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Hilder TL, van der Drift K, Sloan J, Wee K. Structural characterization of recombinant alpha-1-antitrypsin expressed in a human cell line. Analytical Biochemistry. 2013;437:20–28. doi: 10.1016/j.ab.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Jaberie H, Naghibalhossaini F. Recombinant production of native human alpha-1-antitrypsin protein in the liver HepG2 cells. Biotechnology Letters. 2016;38:1683–1690. doi: 10.1007/s10529-016-2150-z. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K, Raboanatahiry N, Zhu B, Li M. Progress in Genome Editing Technology and Its Application in Plants. Frontiers in Plant Science. 2017:8. doi: 10.3389/fpls.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollak C. An evidence-based review of the potential benefits of taliglucerase alfa in the treatment of patients with Gaucher disease. Core Evidence. 2012:15. doi: 10.2147/CE.S20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kytidou K, Beenakker TJM, Westerhof LB, Hokke CH, Moolenaar GF, Goosen N, Mirzaian M, Ferraz MJ, de Geus M, Kallemeijn WW, et al. Human Alpha Galactosidases Transiently Produced in Nicotiana benthamiana Leaves: New Insights in Substrate Specificities with Relevance for Fabry Disease. Frontiers in Plant Science. 2017:8. doi: 10.3389/fpls.2017.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheshukova EV, Komarova TV, Dorokhov YL. Plant factories for the production of monoclonal antibodies. Biochemistry-Moscow. 2016;81:1118–1135. doi: 10.1134/S0006297916100102. [DOI] [PubMed] [Google Scholar]
- 47.Nandi S, Kwong AT, Holtz BR, Erwin RL, Marcel S, McDonald KA. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. mAbs. 2016;8:1456–1466. doi: 10.1080/19420862.2016.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudarshana MR, Plesha MA, Uratsu SL, Falk BW, Dandekar AM, Huang TK, McDonald KA. A chemically inducible cucumber mosaic virus amplicon system for expression of heterologous proteins in plant tissues. Plant Biotechnol J. 2006;4:551–559. doi: 10.1111/j.1467-7652.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 49.Plesha MA, Huang T-K, Dandekar AM, Falk BW, McDonald KA. Optimization of the Bioprocessing Conditions for Scale-Up of Transient Production of a Heterologous Protein in Plants Using a Chemically Inducible Viral Amplicon Expression System. Biotechnology Progress. 2009;25:722–734. doi: 10.1002/btpr.149. [DOI] [PubMed] [Google Scholar]
- 50.Clark RA, Stone PJ, Hag AE, Calore JD, Franzblau C. Myeloperoxidase-catalyzed Inactivation of Alpha-1-Protease Inhibitor by Human Neutrophils. Journal of Biological Chemistry. 1981;256:3348–3353. [PubMed] [Google Scholar]
- 51.Johnson D, Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979;254:4022–4026. [PubMed] [Google Scholar]
- 52.Wasil M, Halliwell B, Hutchison DCS, Baum H. The Antioxidant Action of Human Extracellular Fluids - Effect of Human Serum and its Protein Components on the Inactivation of Alpha1-Antiproteinase by Hypochlorous Acid and by Hydrogen Peroxide. Biochemical Journal. 1987;243:219–223. doi: 10.1042/bj2430219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taggart C, Cervantes-Laurean D, Kim G, McElvaney NG, Wehr N, Moss J, Levine RL. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000;275:27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- 54.Griffiths SW, Cooney CL. Relationship between protein structure and methionine oxidation in recombinant human alpha 1-antitrypsin. Biochemistry. 2002;41:6245–6252. doi: 10.1021/bi025599p. [DOI] [PubMed] [Google Scholar]
- 55.Silberstein DZ, Karuppanan K, Cross CE, Eiserich JP, McDonald KA. Plant-Based Production, Purification, and Characterization of Oxidation-Resistant Alpha-1 Antitrypsin (abstract) Free Radic Biol Med. 2016;100:S59–60. [Google Scholar]
- 56.Rattanaporn K. Chemical Engineering. University of California; Davis: 2013. Quantitative RNA Analysis and Roles of Viral RNA Silencing Suppressors in Transient Theraputic Protein Production in Plant Tissues using a Viral Amplicon-Based System. [Google Scholar]
- 57.Shin JS, Yu MH. Kinetic dissection of alpha(1)-antitrypsin inhibition mechanism. Journal of Biological Chemistry. 2002;277:11629–11635. doi: 10.1074/jbc.M111168200. [DOI] [PubMed] [Google Scholar]
- 58.Gooptu B, Dickens JA, Lomas DA. The molecular and cellular pathology of α1-antitrypsin deficiency. Trends in Molecular Medicine. 2014;20:116–127. doi: 10.1016/j.molmed.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Hawkins CL, Davies MJ. Inactivation of protease inhibitors and lysozyme by hypochlorous acid: role of side-chain oxidation and protein unfolding in loss of biological function. Chem Res Toxicol. 2005;18:1600–1610. doi: 10.1021/tx050207b. [DOI] [PubMed] [Google Scholar]
- 60.Castilho A, Windwarder M, Gattinger P, Mach L, Strasser R, Altmann F, Steinkellner H. Proteolytic and N-Glycan Processing of Human alpha1-Antitrypsin Expressed in Nicotiana benthamiana. Plant Physiol. 2014;166:1839–1851. doi: 10.1104/pp.114.250720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jha S, Sanyal I, Amla DV. Single amino acid substitutions in recombinant plant-derived human alpha(1)-proteinase inhibitor confer enhanced stability and functional efficacy. Biochimica Et Biophysica Acta-General Subjects. 2014;1840:416–427. doi: 10.1016/j.bbagen.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 62.Bergin DA, Hurley K, McElvaney NG, Reeves EP. Alpha-1 antitrypsin: a potent anti-inflammatory and potential novel therapeutic agent. Arch Immunol Ther Exp (Warsz) 2012;60:81–97. doi: 10.1007/s00005-012-0162-5. [DOI] [PubMed] [Google Scholar]
- 63.Dunlea DM, McElvaney OJ, Lacey N, White MM, McCarthy C, Hawkins P, Reeves EP, McElvaney NG. The Impact of Altered Glycosylation on the Anti-Inflammatory Properties of Alpha-1 Antitrypsin. Irish Journal of Medical Science. 2016;185:S501–S501. [Google Scholar]
- 64.McCarthy C, Saldova R, Wormald MR, Rudd PM, McElvaney NG, Reeves EP. The role and importance of glycosylation of acute phase proteins with focus on alpha-1 antitrypsin in acute and chronic inflammatory conditions. J Proteome Res. 2014;13:3131–3143. doi: 10.1021/pr500146y. [DOI] [PubMed] [Google Scholar]
- 65.Reeves EP, Cosgrove S, Bergin DA, Greene CM, McElvaney NG. New Strategies in Drug Development Focusing on the Anti-Protease-Protease Balance in Alpha-1 Antitrypsin Deficiency. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry. 2010;9:314–329. [Google Scholar]
- 66.McCarthy C, Dunlea DM, Saldova R, Henry M, Meleady P, McElvaney OJ, Marsh B, Rudd PM, Reeves EP, McElvaney NG. Glycosylation Repurposes Alpha-1 Antitrypsin for Resolution of Community-acquired-pneumonia. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201709-1954LE. [DOI] [PubMed] [Google Scholar]
- 67.Limkul J, Iizuka S, Sato Y, Misaki R, Ohashi T, Ohashi T, Fujiyama K. The production of human glucocerebrosidase in glyco-engineered Nicotiana benthamiana plants. Plant Biotechnology Journal. 2016;14:1682–1694. doi: 10.1111/pbi.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaaltiel Y, Tekoah Y. Plant specific N-glycans do not have proven adverse effects in humans. Nat Biotech. 2016;34:706–708. doi: 10.1038/nbt.3556. [DOI] [PubMed] [Google Scholar]
- 69.Lusch A, Kaup M, Marx U, Tauber R, Blanchard V, Berger M. Development and Analysis of Alpha 1-Antitrypsin Neoglycoproteins: The Impact of Additional N-Glycosylation Sites on Serum Half-Life. Molecular Pharmaceutics. 2013;10:2616–2629. doi: 10.1021/mp400043r. [DOI] [PubMed] [Google Scholar]
- 70.Bergin DA, Reeves EP, Meleady P, Henry M, McElvaney OJ, Carroll TP, Condron C, Chotirmall SH, Clynes M, O’Neill SJ, McElvaney NG. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. Journal of Clinical Investigation. 2010;120:4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bucurenci N, Blake DR, Chidwick K, Winyard PG. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS Lett. 1992;300:21–24. doi: 10.1016/0014-5793(92)80156-b. [DOI] [PubMed] [Google Scholar]
- 72.Yost CC, Schwertz H, Cody MJ, Wallace JA, Campbell RA, Vieira-de-Abreu A, Araujo CV, Schubert S, Harris ES, Rowley JW, et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J Clin Invest. 2016;126:3783–3798. doi: 10.1172/JCI83873. [DOI] [PMC free article] [PubMed] [Google Scholar]