Abstract
Introduction:
Although much progress has been made in the last decade(s) toward development of effective cancer vaccines, there are still important obstacles to therapeutic successes. New generations of cancer vaccines will benefit from a combination adjuvant approach that targets multiple branches of the immune response.
Areas covered:
Herein we describe how combinatorial adjuvant strategies can help overcome important obstacles to cancer vaccine development, including antigen immunogenicity and tumor immune suppression. Tumor antigens may be both tolerogenic and may utilize active mechanisms to suppress host immunity, including downregulation of MHC molecules to evade recognition and upregulation of immune inhibitory receptors, to subvert an effective immune response. The current cancer vaccine literature was surveyed to identify advancements in the understanding of the biological mechanisms underlying poor antigen immunogenicity and tumor immune evasion, as well as adjuvant strategies designed to overcome them.
Expert commentary:
Poor immunogenicity of tumor antigens and tumor immune evasion mechanisms make the design of cancer vaccines challenging. Growing understanding of the tumor microenvironment and associated immune responses indicate the importance of augmenting not only the effector response, but also overcoming the endogenous regulatory response and tumor evasion mechanisms. Therefore, new vaccines will benefit from multi-juvanted approaches that simultaneously stimulate immunity while preventing inhibition.
1. Introduction
The development of cancer vaccines faces unique obstacles that generally do not impede development of conventional infectious disease vaccines. For example, our nascent understanding of the risk factors and early biomarkers of cancer development, as well as the heterogeneity in tumor types and progression, require targeting primarily of pre-existing tumors with therapeutic vaccines, rather than the generally more effective use of prophylactic vaccines. These realities present two fundamental problems; the problem of tumor immune suppression and the problem of antigenicity.
1.1. The immune suppression problem
First and foremost, the immune system of a cancer patient operates in a fundamentally different environment with many challenges for driving an immune response relative to that of healthy individuals. In addition to the development of immune tolerance to tumor antigens discussed below, the immune system of cancer patients is compromised both by therapy-specific and tumor-specific mechanisms.
Radiation and chemotherapeutic interventions typically target self-replicating immune cells in the process of destroying rapidly dividing neoplastic cells. In addition, tumors themselves utilize a variety of mechanisms to subvert and suppress the immune system. The chronic inflammatory environment associated with tumor progression supports development of an immunosuppressive tumor microenvironment. This environment is characterized not only by “exhaustion” of T cell and NK cell responses, but also accumulation of T regulatory cells, T helper type-2 (Th2) CD4+ T cells, tumor-associated macrophages (TAMs) and immature dendritic cells, macrophages and neutrophils (cumulatively referred to as myeloid-derived suppressor cells (MDSC)), all with suppressive phenotypes (1–3).
The addition of immune modifying vaccine adjuvants to conventional vaccines represents one of the most promising approaches to circumvent the immunosuppressive impediments to effective cancer vaccines. Not only can adjuvants jump start an immune system compromised by therapeutic interventions, but adjuvants can be tailored for specific immunomodulatory effects to target either suppressed innate or adaptive immune responses or both.
1.2. The antigen problem
Unlike infectious pathogens, tumors do not express well-defined foreign antigens that can easily be targeted, although some novel antigens or neo-antigens may arise as a result of tumor-specific mutations. In fact, two of the most widely used “cancer” vaccines today do not actually target tumor antigens, but prevent infection by the oncogenic human papillomavirus or Hepatitis B virus, the agents that cause the malignant transformations associated with cervical cancer and liver cancer respectively. However, most human cancers have not been linked to specific infectious agents with easily targeted foreign antigens, but they arise from transformations due to environmental, genetic, or lifestyle factors, thus limiting the potential for this approach.
Targeting established tumors with therapeutic vaccines is notoriously challenging. Commonly, tumors are targeted based on antigens that are over-expressed in tumor relative to normal tissue, that are typically ignored by the body as immune-privileged such as cancer-testis antigens, that are generally temporally expressed during development such as oncofetal antigens, or that arise as mutations, either oncogenic or stochastic, during tumor development (4). Such antigens may be difficult targets for reasons discussed below, or they may not be oncogenic drivers. For instance, the immune response to self-antigens may be subject to varying degrees of preexisting immunological tolerance, and mutated antigens may be patient-specific and difficult to identify for targeting. Tumor antigens may also be a variable target. Especially if not an oncogenic driver, a targeted antigen may be subject to rapid selection during tumor development, leading to antigen escape loss variants that may reduce antigen-targeted vaccine efficacy. However, these so-called tumor neo-antigens that arise naturally from selection may also be a source of new antigenic targets for strategically designed and formulated therapeutic vaccines (5;6).
2. Antigen uptake and APC activation
2.1. Antigen uptake
Three ways that adjuvants can boost tumor antigen recognition and, therefore, vaccine efficacy, are by increasing antigen uptake, cross-presentation, and determinant spreading. The first step in mounting an immune response is sampling and presentation of antigen via antigen-presenting cells (APCs) of the innate immune system (Fig. 1). Professional APCs include neutrophils, macrophages, B cells and dendritic cells (DCs). APCs sample soluble antigen through fluid phase pinocytosis or particulate antigen through receptor-mediated endocytosis. Delivery of antigen through receptor-based endocytosis offers many advantages over pinocytosis for stimulation of effective immune responses, including greater sensitivity (7) and greater selectivity of cell targets and signaling pathways (8;9) than to guide nascent innate immunity.
Figure 1: Adjuvant stimulation in cancer immunology.
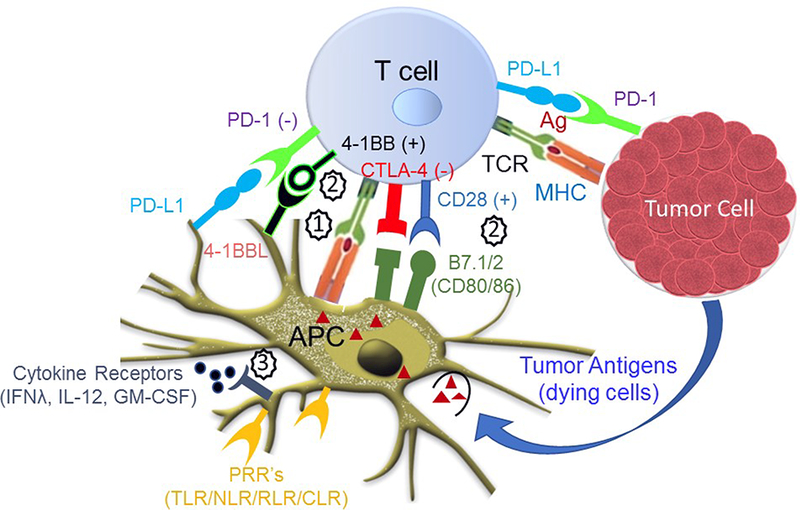
Adjuvants may work in concert with antigen (signal 1) to stimulate signals 2 and 3 for an effective immune response against tumor cells. T cell adjuvants such as soluble or engineered costimulatory molecules (4–1BBL, B7.1/2) or antibodies that block ligand interactions with inhibitory receptors (PD-1, CTLA-4) may interact with T cells as part of signal 2. Innate immune receptors such as the pathogen recognition receptors (TLRs, NLRs, RLRs, CLRs) stimulate APCs as part of signal 3, inducing inflammatory cytokine production and upregulation of costimulatory molecules (B7.1/2, 4– 1BBL).
Activation of the innate immune response is a vital early step in development of effective anti-tumor responses. Such activation relies upon activation of germ-line encoded pathogen recognition receptors (PRRs), including Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-1 (RIG)-like receptors (RLRs) and C-type lectin receptors (CLRs) by epithelial cells, neutrophils, macrophages or dendritic cells (DCs) (Fig. 1). These receptors bind pathogen-associated molecular patterns (PAMPs) from bacteria, viruses or fungi, or endogenous danger-associated molecular patterns (DAMPs), including heat-shock proteins and high mobility group box-1 (HMGB1) that are released by cells after tissue injury and bind PRRs on innate immune cells. PRR activation culminates in the release of inflammatory cytokines and chemokines, in the recruitment of innate and adaptive immune cells, and in the stimulation of antigen-presenting cells (APCs), including macrophages and DCs to phagocytose, process and present antigen to drive the adaptive immune response (10).
The DC is the primary APC to initiate antigen presentation and activation of T cells. Not surprisingly, suppression of DC maturation and activation is one of the primary mechanism for tumor immunosuppression, and tumor progression is associated with decreases in DC number, maturity and activation (11–13). Hence, effective cancer vaccine formulations couple delivery of appropriate cancer-specific antigens with immunostimulatory adjuvants that activate APC to overcome tolerance and prevent under-stimulated, abortive T cell responses.
2.2. Antigen cross-presentation
Preeminent for an effective T cells response is the ability to activate a cytotoxic CD8+ T lymphocyte response against endogenous tumor antigens (14–16). However downregulation of surface expression of major histocompatibility complex-I (MHC-I), the primary receptor for presentation of endogenous antigens, prevents recognition of endogenous tumor antigens and activation of anti-tumor effector CD8+ T cell responses. Specialized DC subsets can internalize, process and present endogenous antigens from tumor and present them to antigen-specific CD8+ T cells in a process referred to as cross-presentation. Therefore, adjuvants that stimulate DC for effective cross-presentation of tumor antigens are excellent vaccine candidates. The FDA-approved vaccine adjuvant alum have been shown to stimulate cross-presentation of tumor associated antigens (TAAs) (17), as well as several adjuvants currently in clinical development, including the TLR3 ligand I:C (18) and the saponin-based particulate adjuvant, ISCOMATRIX® (19).
3. Antigen transport
T cells recognition requires the presentation of processed peptides in association with MHC class I and class II molecules. MHC class I molecules are expressed by all cells and presents peptides derived from endogenously expressed self-proteins or intracellular pathogens. Tumor cells presents the tumor-associated epitopes (TAE) on their surface in association with MHC molecules which are further on recognized by CTLs. Numerous self-protein derived TAE have been identified which are either selectively expressed on malignant cells (20;21) or carry point mutations within the CTL epitope regions, such as p53, CDK4, B-catenin and MUM-1/L33-B (22). However, although TAE are promising targets for CTL-based immunotherapy, very few self-TAE have shown success as anti-tumor vaccines for the treatment of human cancers (23).
4. Antigen determinant spreading
Immunization with TAAs induces humoral and cellular responses. The T cell responses are epitope determinant for the TAAs. In antigen-based determinant spreading, T cells responses initiated by a single antigenic epitope evolve into multi-epitopic responses. Adjuvants may promote this process in the context of tumor antigens, but with some potential risk. For instance, administration of a blocking monoclonal antibody against CTLA-4 often used in cancer adjuvant therapy has been shown to promote antigen determinant spreading, but can accelerate the autoimmune diseases in other contexts (24). ISCOMATRIX™, a multicomponent adjuvant “immune stimulating complex” consisting of cage-like structures of phospholipid, saponin and cholesterol, has been shown to effectively promote epitope spreading and antibody affinity maturation when included in an influenza vaccine (25) and has been used previously as an adjuvant component in cancer vaccines (26). As ISCOMATRIX™ has also been shown to promote cross-presentation of tumor antigens (27), similar adjuvant strategies may provide promise for increasing the effectiveness of vaccines based on single epitopes. However, study of the role in adjuvants in this phenomenon and the importance of determinant spreading in tumor regression are still in their infancy.
5. T cell priming, activation, effector phase and cross-priming
As noted, APCs play a critical role in initiation of appropriate T cell responses by providing T cells with classical activation signals that are obligatory for proper T cell education. At least two signals are essential for the activation of naïve and central memory T cells. First, naïve T cells must recognize peptide antigen bound to MHC class I or class II on DCs. This antigen recognition by T cell receptors provides an activation signal, Signal 1 (Figure 1). The Signal 1 is not sufficient for full activation, and can instead lead to T cell anergy or induction of Treg cells (28). In order for full activation to kick in, the T cell must receive a Signal 2, so-called costimulatory signals through the interaction of various costimulatory molecules (CD28, 4–1BB, OX-40, CD27) on T cells with their ligands on the surface of DCs (29). We now have considerable evidence that CD8+ T cells also requires Signal 3, cytokine signals, and that the presence or absence of Signal 3 determines whether effective activation or tolerance induction occurs in response to antigen and costimulation (30). Vaccine adjuvants are mainly designed and classified based on their functional ability to target these three critical signals of the T cell immune response. However, such classifications should be may be somewhat ambiguous, as many adjuvants, TLR4 agonists for instance, may provide more than one signal by inducing upregulation of costimulatory molecules (Signal 2) and production of production of cytokines (Signal 3).
Adjuvants facilitate Signal 1:
Adjuvants facilitating Signal 1 mainly improve antigen stability, delivery, processing and presentation to T cells. In some cases, these adjuvants also protect the antigen from degradation. Some of the examples of these adjuvants are nanoparticles, gold particles, alum, oil/water-based formulations, lipid-based vesicles and bacterial ghosts (31–33). Mineral oil-based emulsions such as Montanides (34) or nanoparticles (35) are also used to develop antigen delivery system which increases the antigen uptake, processing and presentation, thereby potentiate the Signal 1. In general, Signal 1 facilitating adjuvants are of non-microbial origin, in contrast to many Signal 2 facilitators, and for most of them, no receptor has been identified as yet. Some heat shock proteins also act as Signal 1 facilitating adjuvant and induce innate immune responses (36). HSP has also been shown to induce the maturation of APC and result in specific triggering of the acquired immune response (37).
Adjuvants facilitate Signal 2:
On the other hand, molecules and formulations that facilitate Signal 2 of the immune response represent a diverse group of adjuvants, consisting of a group of physically and chemically unrelated molecules, e.g., saponins, muramyl dipeptide, highly immunogenic immune stimulating complex (ISCOM) etc. Signal 2 adjuvants generally do not directly affect the concentration and distribution of antigen; rather these are activating ligands for specific immune receptors. A novel class of Signal 2 facilitators also includes ligands or agonistic antibodies targeting members of TNF receptors on T cell for the induction of activation, expansion, effector function and survival (38). Among this family, costimulatory 4–1BBL has shown great potential as a vaccine adjuvant due to its pleiotropic effects on the cells of innate, adaptive, and regulatory immunity. In extensive studies, SA-4–1BBL was shown to play a critical role in the generation and maintenance of CD8+ T cell responses, while having a negative impact on the frequency and suppressor function of CD4+CD25+FoxP3+ T regulatory cells that are important culprits of tumor immune evasion (39–42). As an adjuvant component of TAA-based subunit vaccines, SA-4– 1BBL showed robust therapeutic efficacy in various preclinical tumor models (43–47), establishing this molecule as an important new class of adjuvant. In a preclinical cervical cancer animal model, Sharma et al. demonstrated that HPV E7-expressing TC-1 cells can effectively be engineered to co-display an SA-chimeric form of the costimulatory molecule LIGHT (homologous to lymphotoxins, inducible expression, competes with herpesvirus glycoprotein D entry mediator, a receptor expresses on T lymphocytes) on their surface and serve as an effective vaccine with both preventive and therapeutic efficacies (48). Other Signal 2-triggering adjuvants include TLR agonists such as bacterial lipopolysaccharide (LPS) or its derivative monophosphoryl lipid-A (MPL), as well as microbial RNA or DNA or variant synthetic CpG sequence motifs (49). These agonists have been demonstrated to generate anti-tumor immunity in preclinical studies in mice by enhancing innate immunity through the activation of DCs, NK cells, monocytes, and macrophages and induction of cytokines with both direct and indirect anti-tumor activity (48).
Adjuvants facilitate Signal 3:
In order for naïve CD8 T cells to be activated to undergo clonal expansion and develop effector function they not only must receive Signal 1 and 2, but also require a Signal 3 that can be provided by IL-12 or IFN-y (50;51). Adjuvants such as alum and chitosan not only facilitate the Signal 1, but they also modulate DC activity and enhance vaccine-specific Th2 responses and their associated cytokines, IL-4, IL-5 and IL-13, inducing antibody production and influencing the quality of the adaptive immune response (Signal 3). The use of cytokines as a part of vaccine formulations is becoming more prevalent and has shown to influence both cellular and humoral immune responses, boosting the efficacy of poorly immunogenic vaccines in both pre-clinical and clinical studies (52).
While selected cytokines such as IL-4, IL-5, IL-6 enhance antibody production, IFN-γ, IL-10, TGF-β, IL-13 may induce heavy chain isotype class switching of vaccine antigen-specific antibodies. For IFN-α, IFN-γ, IL-2, IL-12, IL-15, IL-18, IL-21-based adjuvants, vaccine-induced responses have been observed (53;54). However, major challenges for successful clinical use of these cytokine-based vaccine adjuvants remain, including optimization of dosing to retain efficacy by avoiding toxicity and/or immunosuppression, as well as the addition of substantial cost to traditional formulations.
6. Tumor suppression mechanisms
Apart from muted antitumor activity by the immune system, immune evasion mechanism such as suppression, escape, and subversion also contribute towards the rapid growth of tumors. These mechanisms include avoidance of immune surveillance, downregulation of MHC class I or co-stimulatory molecules, enhancement of inhibitory molecules (CTLA-4, PD-1), recruitment of regulatory cells (Treg, MDSC, type 2 macrophages) and production of immunosuppressive cytokines, IL-10 and TGF-β. The choice of the adjuvants for vaccine development strategies that not only directly enhance anti-tumor immunity but also block checkpoints or suppressor networks will have potential to eliminate the tumor.
TLR agonists such as MPL, while acting as potent adjuvants in some cases, also promote Tregs (55)). Some of the targeted monoclonal antibodies as Signal 2 facilitators block the co-inhibitory molecules on T cells, which interfere with the signals delivered by costimulatory molecules, resulting into compromised T cell activation and function. For example, CTLA-4-induced co-inhibitory signals on activated T cells trigger a natural inhibitory response which dampens excessive T cell effector functions. However, blockade of this inhibitory response by monoclonal antibodies, such as ipilimumab or tremelimumab, prevents this con-inhibitory signal and provides an opportunity for costimulatory molecules such as CD28, 4–1BB, OX40 etc. to deliver unhindered Signal 2, resulting in a significantly enhanced T-cell activation and proliferation (56). Blockade of PD-1, another checkpoint inhibitory pathway for T cells showed similar out come and stimulates immune effector triggering (57).
7. Combination adjuvants for cancer vaccines
7.1. Rationale
Adjuvants are critical components of many vaccines and enhance the magnitude, breadth, quality and longevity of the immune response to the antigens. The majority of existing vaccines with adjuvant components contain only single adjuvants; however, many of these promising formulations have been sidelined owing to a number of limitations, including induction of immune responses of low potency or of inappropriate nature (i.e Th2 versus Th1 response). Consequently, investigators are exploring many novel adjuvant technologies for future vaccine candidates, including adjuvant combinations. Vaccinations using adjuvant combinations can result in complimentary and even synergistic enhancement of immune responses by stimulating and activating a variety of cells and immune mechanisms, including dendritic-cell maturation, T-cell expansion and relief of tumor-associated immune suppression. This approach has shown great potential and presents a unique opportunity for investigators to tailor immune responses to specific vaccines.
Adjuvant combinations are especially important for the development of cancer vaccines where there is a limited choice of clinically approved adjuvants. The rationale for the use of multi-adjuvanted therapy can be seen in the combination of vaccines and checkpoint inhibitors due to with complimentary modes of action on immune cells. Perhaps one of the most promising examples is the use of the GM-CSF-secreting vaccine (GVAX), which activates immune cells, with ipilimumab, a CTLA-4 blocking antibody designed to increase T cell activation and function (58). Additionally, the combination of anti-CTLA-4 and anti-PD-1 antibodies has shown evidence of synergy with cancer vaccines in preclinical (59), and clinical studies (60).
7.2. Combination strategies
The limitations with single adjuvant vaccines are driving investigators more and more to explore combination adjuvants. This is especially important as cancer antigens are usually self-or modified self-antigens in origin, which means cancer vaccines are required to overcome immunological tolerance as well. Currently, several combinations of various classes of adjuvants are being explored to steer the desired vaccine efficacy. For example, the use of alum adjuvant alone, which was until recently the only adjuvant used clinically in humans, has shown very modest efficacy in an HPV vaccine. However, when combined with MPL, a derivative of LPS, these two adjuvants induce a potent immune response (61). Consistent with this report, a novel TLR4 agonist, CIA06, in combination with aluminum salts, provides similar protection (62). Another example is the use of a combination of Poly IC and CpG in B16-F10 tumor model. This combination synergistically induced cytokine responses that resulted in potent anti-tumor efficacy (63). Further, the injection of a mixture of TLR9 and TLR7/8 agonists directly into established solid tumors in mice led to increased recruitment of tumor infiltrating CTL and NK cells, reduced the numbers of immunosuppressive MDSC and significantly reduced the tumor burden while providing long-term protection against regrowth (Zhao G et al (2014) PMC4075973).
More recently, Srivastava et al. demonstrated the use of a multi-adjuvanted approach by combining two different classes of adjuvants, SA-4–1BBL and MPL, with their distinct immune cell targets, signaling pathways, and significant roles as individual agents for the activation and maintenance of innate and cellular immune responses. This study demonstrated that combination of these adjuvants targeted the innate, adaptive and regulatory arms of the immune system, resulting in eradication of tumors in various preclinical models without detectable toxicity (64). Most importantly, therapeutic efficacy of the SA4–1BBL/MPL combined adjuvants therapy was associated with significant infiltration of CD8+ T cells into the tumor and marked reduction of CD4+CD25+FoxP3+ T regulatory cells with a favorable intra-tumoral T effector:T regulatory cell ratio, an indicator of potential clinical relevance. In another study, the combination of MF59 with the TLR9 agonist CpG demonstrated enhanced prophylactic efficacy by inducing a potent Th1 anti-tumor response in a preclinical melanoma tumor model (65). Further, a novel mucosal adjuvant formulation being developed, nanoscale emulsion (NE), improved the Th1 associated immune response, the IL-17 response and the regulatory T cell response following intranasal immunization with alum adjuvanted Hepatitis B surface antigens (NE-HBs) (66).
The mechanisms of synergy in vivo between components of adjuvant combinations can be difficult to tease out. For example, signaling by TLR4 and TLR7/8 adjuvants through different pathways in the same or disparate innate immune cell types may have synergistic or antagonistic effects, either through signal amplification (67;68) or through paracrine signaling (69;70). Alternatively, simultaneously targeting adjuvant receptors on innate immune cells and costimulatory/inhibitory receptors on adaptive immune cells may remarkably enhance or suppress effector and memory T cell responses. Therefore, the choice of the appropriate adjuvant combination will require comprehensive knowledge of both the signaling pathways and and cell expression patterns of targeted receptors.
Clinical studies have begun to evaluate the impact of therapeutic vaccines against various cancers have strategically focused on multi-adjuvanted vaccines. PROSTVAC®-VF (also called PSA-TRICOM), a combination vaccine comprising T-lymphocyte activation antigen CD80 (B7), intracellular adhesion molecule 1 (ICAM-1), and lymphocyte function associated antigen 3 (LFA-3), has showed great potential in clinical studies. PROSTVAC-VF immunotherapy showed 44% reduction in the death rate and an 8.5-month improvement in median overall survival in men with metastatic prostate cancer (71). In addition, an improved clinical response was observed in a phase III clinical study with a formulation containing gp100:209–217 peptide plus incomplete Freund’s adjuvant (Montanide ISA-51) in combination with IL-2 in patients with locally- advanced stage III and stage IV melanoma (72), while a combination of PD-1/PD-L1-blocking mAbs and ipilimumab has also exhibited promising clinical response (73).
7.3. Combination of adjuvants and immununomodulatory antibodies
The rationale for the use of multi-adjuvanted therapy can also be seen in the combination of vaccines that combine adjuvants that target and immununomodulatory antibodies targeting lymphocytes due to their complimentary modes of action on immune cells. An interesting example of this strategy is the use of the cell-based, GM-CSF-secreting vaccine (GVAX), which activates immune cells, combined with ipilimumab, an antibody that blocks the inhibitory receptor CTLA-4 on T cells, activation and function (58). When combined with a low-dose chemotherapy regimen, this combination induced a significant increase in infiltrating CD8 T cells with increased tumor cell lysis activity and increased survival in a metastatic prostate cancer model. Additionally, the combination of anti-CTLA-4 and anti-PD-1 antibodies has shown evidence of synergy with cancer vaccines in clinical melanoma studies (60).
Beside CTLA-4 and PD-1 inhibitors, additional immune checkpoint inhibitors, including inhibitors of T cell immunoglobulin-3 (TIM-3), lymphocyte activation gene-3 (LAG-3) and T cell immunoglobulin and ITIM domain (TIGIT), have shown promising efficacy when used in combination cancer immunotherapy strategies. TIM-3, a specific marker for fully differentiated, IFN-producing CD4 T cells that is also highly expressed on tumor infiltrating dendritic cells, binds HMGB1 and weakens the innate immune response to the tumor tissue (70). Blockade with a combination of antibodies against TIM-3 and PD-1 prolonged survival in acute myelogenous leukemia mouse, and the combination is in early phase clinical trial (71). LAG-3 and PD-1 were found to be co-expressed on the tumor-infiltrating lymphocytes (TILs) of ovarian cancer patients, and combined anti-LAG-3/anti PD-1 treatment was successful in a mouse model of fibrosarcoma and adenocarcinoma tumors (72). TIGIT, an inhibitory receptor of the IgG superfamily, suppresses anti-tumor immunity by Treg cells and, in combination with anti-PDL-1, showed complete tumor protection in colorectal carcinoma mouse model (73).
Although formulating antigen with combinations of adjuvants or combinations of adjuvants with immunomodulators such as immune checkpoint blockers may seem to be a virtual panacea for development of effective cancer vaccines, this approach should be utilized with some caution. Initial strategies should continue to focus on combinations of adjuvants or immunomodulators with well-defined mechanisms of action, including the cell types and signaling pathways targeted. In addition, close attention should be paid to data for adjuvants previously used in combination in vaccine settings. In this way, developers have a better chance of avoiding combinations that may have antagonistic, rather than additive or synergistic effects on immune responses (74). Similarly, the possible effects of combinations that synergistically induce remarkably high levels of inflammatory mediators in preclinical models, such as TNFα, IL-lβ, IL-12 and IFNγ (75;76), and may pose a potential risk for local and/or systemic inflammation.
8. Expert commentary
Recent advancements have allowed researchers to have better understanding of tumor microenvironment and its associated immune response that mediate and regulate antitumor immunity, thereby provided a foundation for the rational design of vaccines. This is especially critical in the case of cancer vaccines, where multifaceted evasion strategies set in place by malignant cells can limit the efficacy of the vaccines. While vaccines that target the effector arm of the immune system generate some promising antitumor responses, many of them fail to block the regulatory responses associated with the tumor. Conversely, vaccines that are designed to globally suppress regulatory immunity increase the risks for the patient of developing autoimmune diseases.
Therefore, it is critical to know which type and strength of immune responses are required when designing a vaccine against cancer. In this context, single adjuvanted vaccines are unlikely to result in clinically relevant anti-tumor responses due to the inability to induce potent and appropriate and/or relevant immune responses. As such, designing multi-adjuvanted vaccines that not only activate the effector antitumor response that target tumor but also block inhibitory pathways in the immunosuppressive tumor microenvironment, although challenging, may be the way forward. Recent clinical response of the combination of FDA approved checkpoint inhibitors, anti-CTLA-4 (ipilimumab) and anti-PD-1 (pembrolizumab) against metastatic melanoma has established the advantages of multi-adjuvanted therapy. Information gathered from these and ongoing clinical studies, together with a more comprehensive understanding of adjuvant mechanisms, may provide great opportunities to further accelerate the development new class of multi-adjuvated vaccines with more effective and less-toxic outcomes.
9. Five-year view
One of the major obstacles to effective cancer vaccine development is targeting of tumor antigens that may have low immunogenicity in the tumor environment or may mutate to evade the immune response. Combination adjuvant approaches are currently being developed and will be optimized as a promising means toward overcoming self-tolerance and tumor evasion mechanisms to induce a potent anti-tumor response. Simultaneously, emerging techniques in automated epitope prediction and massively parallel sequencing (MPS) of individual tumors are enabling efficient identification of dominant antigenic epitopes and neo-antigens for specific patients (77). In the coming years, the potential to couple potent immunostimulatory adjuvant combinations with identification of effective antigenic targets for individual patients may well provide a revolution in development of more effective, “personalized” therapeutic cancer vaccines. Similarly new developments in adjuvant discovery as well as identification of dominant neo-antigens for a given tumor type may allow the development of effective preventative vaccines for high risk individuals.
9. Key issues:
The cancer patient’s immune response is compromised not only by existing tolerance to tumor antigens, but also by therapy-specific and tumor-specific suppression.
Adjuvants can boost tumor antigen recognition and; therefore, vaccine efficacy, by augmenting antigen uptake, cross-presentation, and determinant spreading through stimulation of APC
The effect of adjuvants on T cell responses depend on their effects on priming, activation, effector phase and cross-priming through Signals 1–3.
Tumor suppression mechanism often include expression of inhibitory receptors that suppress or subvert recruited immune cells. Such suppression mechanisms may be prevented with the use of antibodies to both stimulate costimulatory receptors and block inhibitory receptor engagement.
The use of novel adjuvant combinations can induce complimentary and even synergistic enhancement of immune responses by stimulating and activating a variety of cells and immune mechanisms
Although adjuvant combinations show great promise for development of effective vaccines, strategies should continue to focus on combinations of adjuvants or immunomodulators with well-defined mechanisms of action and synergies to reduce the risks of unexpected clinical complications.
Acknowledgments
Funding
The mansucript was funded by grants from the National Institutes of Health, USA.
Footnotes
Declaration of interest
H Shirwan is a recipient of grants from NIH as well as CEO of FasCure Therapeutics, LLC that has an option to license SA-4–1BBL adjuvant cited in this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Fernandez A, Oliver L, Alvarez R, Fernandez LE, Lee KP, Mesa C. Adjuvants and myeloid-derived suppressor cells: enemies or allies in therapeutic cancer vaccination. Hum.Vaccin.Immunother 2014;10(11):3251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.**.Flores-Borja F, Irshad S, Gordon P, Wong F, Sheriff I, Tutt A, Ng T. Crosstalk between Innate Lymphoid Cells and Other Immune Cells in the Tumor Microenvironment. J.Immunol.Res 2016;2016:7803091.In-depth review of tolerance and tumor immune evasion strategies
- 3.Taylor JG, Gribben JG. Microenvironment abnormalities and lymphomagenesis: Immunological aspects. Semin.Cancer Biol 2015. October;34:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterfield LH. Cancer vaccines. BMJ 2015. April 22;350:h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.*.Bobisse S, Foukas PG, Coukos G, Harari A. Neoantigen-based cancer immunotherapy. Ann.Transl.Med 2016. July;4(14):262 Excellent nsight into applications for explointing neoantigens for cancer therapeutics.Excellent nsight into applications for explointing neoantigens for cancer therapeutics.
- 6.Overwijk WW, Wang E, Marincola FM, Rammensee HG, Restifo NP. Mining the mutanome: developing highly personalized Immunotherapies based on mutational analysis of tumors. J.Immunother. Cancer 2013;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer DF, Linderman JJ. The relationship between antigen concentration, antigen internalization, and antigenic complexes: modeling insights into antigen processing and presentation. J.Cell Biol 1990. July; 111(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 2003. March 6;422(6927):37–44 [DOI] [PubMed] [Google Scholar]
- 9.Xiao G, Gan LS. Receptor-mediated endocytosis and brain delivery of therapeutic biologics. Int.J.Cell Biol 2013;2013:703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubensky TW, Jr. Reed SG. Adjuvants for cancer vaccines. Semin.Immunol 2010. June;22(3):155–61 [DOI] [PubMed] [Google Scholar]
- 11.Farren MR, Carlson LM, Lee KP. Tumor-mediated inhibition of dendritic cell differentiation is mediated by down regulation of protein kinase C beta II expression. Immunol.Res 2010. March;46(1–3):165–76 [DOI] [PubMed] [Google Scholar]
- 12.Pandey VK, Amin PJ, Shankar BS. COX-2 inhibitor prevents tumor induced down regulation of classical DC lineage specific transcription factor Zbtb46 resulting in immunocompetent DC and decreased tumor burden. Immunol.Lett 2017. April;184:23–33 [DOI] [PubMed] [Google Scholar]
- 13.van den Hout MFCM, Koster BD, Sluijter BJR, Molenkamp BG, van d V, van den Eertwegh AJM, Scheper RJ, van Leeuwen PAM, van den Tol MP, de Gruijl TD. Melanoma Sequentially Suppresses Different DC Subsets in the Sentinel Lymph Node, Affecting Disease Spread and Recurrence. Cancer Immunol.Res 2017. November;5(11):969–77 [DOI] [PubMed] [Google Scholar]
- 14.Comber JD, Philip R. MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. Ther.Adv.Vaccines 2014. May;2(3):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunol.Rev 2014. January;257(1):264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medler TR, Cotechini T, Coussens LM. Immune response to cancer therapy: mounting an effective antitumor response and mechanisms of resistance. Trends Cancer 2015. September 1;1(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLeod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, Marrack P. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc.Natl.Acad.Sci.U.S.A 2011. May 10;108(19):7914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azuma M, Ebihara T, Oshiumi H, Matsumoto M, Seya T. Cross-priming for antitumor CTL induced by soluble Ag + polyI:C depends on the TICAM-1 pathway in mouse CD11c(+)/CD8alpha(+) dendritic cells. Oncoimmunology. 2012. August 1;1(5):581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson NS, Yang B, Morelli AB, Koernig S, Yang A, Loeser S, Airey D, Provan L, Hass P, Braley H, et al. ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol.Cell Biol 2012. May;90(5):540–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De PE, Lethe B, Brasseur F, Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J.Exp.Med 1994. March 1;179(3):921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De PE, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991. December 13;254(5038):1643–7 [DOI] [PubMed] [Google Scholar]
- 22.Robbins PF, Kawakami Y. Human tumor antigens recognized by T cells. Curr.Opin.Immunol 1996. October;8(5):628–36 [DOI] [PubMed] [Google Scholar]
- 23.Khanna R Tumour surveillance: missing peptides and MHC molecules. Immunol. Cell Biol. 1998. February;76(1):20–6 [DOI] [PubMed] [Google Scholar]
- 24.Wang HB, Shi FD, Li H, Chambers BJ, Link H, Ljunggren HG. Anti-CTLA-4 antibody treatment triggers determinant spreading and enhances murine myasthenia gravis. J.Immunol. 2001. May 15;166(10):6430–6 [DOI] [PubMed] [Google Scholar]
- 25.*.Chung KY, Coyle EM, Jani D, King LR, Bhardwaj R, Fries L, Smith G, Glenn G, Golding H, Khurana S. ISCOMATRIX adjuvant promotes epitope spreading and antibody affinity maturation of influenza A H7N9 virus like particle vaccine that correlate with virus neutralization in humans. Vaccine 2015. July 31;33(32):3953–62Highlights possible use of adjuvants in promoting epitope spreading to increase vaccine potency.
- 26.McKenzie A, Watt M, Gittleson C. ISCOMATRIX() vaccines: Safety in human clinical studies. Hum.Vaccin 2010. March 30;6(3) [DOI] [PubMed] [Google Scholar]
- 27.Schnurr M, Orban M, Robson NC, Shin A, Braley H, Airey D, Cebon J, Maraskovsky E, Endres S. ISCOMATRIX adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II. J.Immunol 2009. February 1;182(3):1253–9 [DOI] [PubMed] [Google Scholar]
- 28.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr.Opin.Immunol 2010. October;22(5):552–9. PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stockl J, Herndler-Brandstetter D, Grubeck-Loebenstein B, Reipert BM, Pickl WF, et al. The capacity of the TNF family members 4–1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur.J.Immunol 2008. October;38(10):2678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr.Opin.Immunol 2010. June;22(3):333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrovsky N Novel human polysaccharide adjuvants with dual Th1 and Th2 potentiating activity. Vaccine 2006. April 12;24 Suppl 2:S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tissot AC, Renhofa R, Schmitz N, Cielens I, Meijerink E, Ose V, Jennings GT, Saudan P, Pumpens P, Bachmann MF. Versatile virus-like particle carrier for epitope based vaccines. PLoS One. 2010. March 23;5(3):e9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muhammad A, Champeimont J, Mayr UB, Lubitz W, Kudela P. Bacterial ghosts as carriers of protein subunit and DNA-encoded antigens for vaccine applications. Expert.Rev.Vaccines 2012. January;11(1):97–116 [DOI] [PubMed] [Google Scholar]
- 34.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N.Engl.J.Med 2011. June 2;364(22):2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva JM, Videira M, Gaspar R, Preat V, Florindo HF. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J.Control Release 2013. June 10;168(2):179–99 [DOI] [PubMed] [Google Scholar]
- 36.Nishikawa M, Takemoto S, Takakura Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Int.J.Pharm 2008. April 16;354(1–2):23–7 [DOI] [PubMed] [Google Scholar]
- 37.Walker KB, Keeble J, Colaco C. Mycobacterial heat shock proteins as vaccines - a model of facilitated antigen presentation. Curr.Mol.Med 2007. June;7(4):339–50 [DOI] [PubMed] [Google Scholar]
- 38.Shirwan H, Sharma RK, Srivastava AK, Yolcu ES. Co-stimulatory tumor necrosis factor ligands as adjuvants for the development of subunit-based anticancer vaccines. Oncoimmunology. 2013. April 1;2(4):e23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma RK, Yolcu ES, Shirwan H. SA-4–1BBL as a novel adjuvant for the development of therapeutic cancer vaccines. Expert.Rev.Vaccines 2014. March;13(3):387–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madireddi S, Schabowsky RH, Srivastava AK, Sharma RK, Yolcu ES, Shirwan H. SA-4– 1BBL costimulation inhibits conversion of conventional CD4+ T cells into CD4+ FoxP3+ T regulatory cells by production of IFN-gamma. PLoS One 2012;7(8):e42459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin.Invest 2005. December;115(12):3623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schabowsky RH, Madireddi S, Sharma R, Yolcu ES, Shirwan H. Targeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapy. Curr Opin Investig.Drugs 2007. December;8(12):1002–8 [PubMed] [Google Scholar]
- 43.Srivastava AK, Dinc G, Sharma RK, Yolcu ES, Zhao H, Shirwan H. SA-4–1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res. 2014. November 15;74(22):6441–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma RK, Yolcu ES, Srivastava AK, Shirwan H. CD4(+) T cells play a critical role in the generation of primary and memory antitumor immune responses elicited by SA-4– 1BBL and TAA-based vaccines in mouse tumor models. PLoS One 2013;8(9):e73145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srivastava AK, Sharma RK, Yolcu ES, Ulker V, MacLeod K, Dinc G, Shirwan H. Prime-boost vaccination with SA-4–1BBL costimulatory molecule and survivin eradicates lung carcinoma in CD8+ T and NK cell dependent manner. PLoS One 2012;7(11):e48463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma RK, Schabowsky RH, Srivastava A, Elpek KG, Madireddi S, Zhao H, Zhong Z, Miller RW, MacLeod KJ, Yolcu ES, et al. 4–1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res. 2010;70(10):3945–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Bandura-Morgan L, Shirwan H. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel from of 4–1BBL eradicates established tumors. Cancer Res 2009;69(10):4319–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schabowsky RH, Sharma RK, Madireddi S, Srivastava A, Yolcu ES, Shirwan H. ProtEx technology for the generation of novel therapeutic cancer vaccines. Exp.Mol.Pathol 2009. June;86(3):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janeway CA Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb.Symp.Quant.Biol 1989;54 Pt 1:1–13 [DOI] [PubMed] [Google Scholar]
- 50.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J.Immunol 2002. June 1;168(11):5521–9 [DOI] [PubMed] [Google Scholar]
- 51.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J.Immunol 2005. April 15;174(8):4465–9 [DOI] [PubMed] [Google Scholar]
- 52.Schijns VE, Degen WG. Vaccine immunopotentiators of the future. Clin.Pharmacol.Ther 2007. December;82(6):750–5 [DOI] [PubMed] [Google Scholar]
- 53.Morrow MP, Yan J, Pankhong P, Ferraro B, Lewis MG, Khan AS, Sardesai NY, Weiner DB. Unique Th1/Th2 phenotypes induced during priming and memory phases by use of interleukin-12 (IL-12) or IL-28B vaccine adjuvants in rhesus macaques. Clin.Vaccine Immunol 2010. October;17(10):1493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johansen P, Mohanan D, Martinez-Gomez JM, Kundig TM, Gander B. Lympho-geographical concepts in vaccine delivery. J.Control Release 2010. November 20;148(1):56–62 [DOI] [PubMed] [Google Scholar]
- 55.Srivastava AK, Dinc G, Sharma RK, Yolcu ES, Zhao H, Shirwan H. SA-4–1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res. 2014. November 15;74(22):6441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ascierto PA, Marincola FM, Ribas A. Anti-CTLA4 monoclonal antibodies: the past and the future in clinical application. J.Transl.Med 2011. November 13;9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Badoual C, Hans S, Merillon N, Van RC, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013. January 1;73(1):128–38 [DOI] [PubMed] [Google Scholar]
- 58.Wada S, Jackson CM, Yoshimura K, Yen HR, Getnet D, Harris TJ, Goldberg MV, Bruno TC, Grosso JF, Durham N, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J.Transl.Med 2013. April 4; 11:89 PMCID: PMC3666941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc.Natl.Acad.Sci.U.S.A 1998. August 18;95(17):10067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, Xu Y, Adams S, Bhardwaj N, Busam K, et al. CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol.Immunother 2011. August;60(8):1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J.Immunol 2009. November 15;183(10):6186–97 [DOI] [PubMed] [Google Scholar]
- 62.Han JE, Wui SR, Park SA, Lee NG, Kim KS, Cho YJ, Kim HJ, Kim HJ. Comparison of the immune responses to the CIA06-adjuvanted human papillomavirus L1 VLP vaccine with those against the licensed HPV vaccine Cervarix in mice. Vaccine 2012. June 13;30(28):4127–34 [DOI] [PubMed] [Google Scholar]
- 63.Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BR. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004. August 15;64(16):5850–60 [DOI] [PubMed] [Google Scholar]
- 64.**.Srivastava AK, Dinc G, Sharma RK, Yolcu ES, Zhao H, Shirwan H. SA-4–1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res. 2014. November 15;74(22):6441–51.Higlights recent work done in the Shirwan lab on combination of innate immune and T cell adjuvants.
- 65.Yang M, Yan Y, Fang M, Wan M, Wu X, Zhang X, Zhao T, Wei H, Song D, Wang L, et al. MF59 formulated with CpG ODN as a potent adjuvant of recombinant HSP65-MUC1 for inducing anti-MUC1+ tumor immunity in mice. Int.Immunopharmacol 2012. August;13(4):408–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bielinska AU, O’Konek JJ, Janczak KW, Baker JR Jr., Immunomodulation of TH2 biased immunity with mucosal administration of nanoemulsion adjuvant. Vaccine 2016. July 25;34(34):4017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol. 2007. June;247(2):72–84 [DOI] [PubMed] [Google Scholar]
- 68.Fischetti L, Zhong Z, Pinder CL, Tregoning JS, Shattock RJ. The synergistic effects of combining TLR ligand based adjuvants on the cytokine response are dependent upon p38/JNK signalling. Cytokine 2017. November;99:287–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coccia M, Collignon C, Herve C, Chalon A, Welsby I, Detienne S, Helden MJV, Dutta S, Genito CJ, Waters NC, et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNgamma response promoting vaccine immunogenicity. NPJ.Vaccines 2017;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kreutz M, Bakdash G, Dolen Y, Skold AE, van Hout-Kuijer MA, de Vries IJ, Figdor CG. Type I IFN-mediated synergistic activation of mouse and human DC subsets by TLR agonists. Eur.J.Immunol 2015. October;45(10):2798–809 [DOI] [PubMed] [Google Scholar]
- 71.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J.Clin.Oncol 2010. March 1;28(7):1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N.Engl.J.Med 2011. June 2;364(22):2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N.Engl.J.Med 2013. July 11;369(2):122–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKay PF, King DF, Mann JF, Barinaga G, Carter D, Shattock RJ. TLR4 and TLR7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs when Administered via the ID or IN Routes. PLoS.One. 2016;11(2):e0148984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat.Immunol 2005. August;6(8):769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petricevic B, Wessner B, Sachet M, Vrbanec D, Spittler A, Bergmann M. CL097, a TLR7/8 ligand, inhibits TLR-4--dependent activation of IRAK-M and BCL-3 expression. Shock 2009. November;32(5):484–90 [DOI] [PubMed] [Google Scholar]
- 77.**.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014. May 9;344(6184):641–5Ilustrates exciting new breakthroughs in identifying and exploiting effective patient-specific antigen targets combining automated epitope prediction and parallel sequencing.


