Abstract
Identifying and characterizing the individual contributors to bacterial cellular elongation and division will improve our understanding of their impact on cell growth and division. Here, we delineated the role of ftsQ, a terminal gene of the highly conserved division cell wall (dcw) operon, in growth, survival, and cell length maintenance in the human pathogen Mycobacterium tuberculosis (Mtb). We found that FtsQ overexpression significantly increases the cell length and number of multiseptate cells. FtsQ depletion in Mtb resulted in cells that were shorter than WT cells during the initial growth stages (4 days after FtsQ depletion) but were longer than WT cells at later stages (10 days after FtsQ depletion) and compromised the survival in vitro and in differentiated THP1 macrophages. Overexpression of N- and C-terminal FtsQ regions altered the cell length, and the C-terminal domain alone complemented the FtsQ depletion phenotype. MS analyses suggested robust FtsQ phosphorylation on Thr-24, and although phosphoablative and -mimetic mutants rescued the FtsQ depletion–associated cell viability defects, they failed to complement the cell length defects. MS and coimmunoprecipitation experiments identified 63 FtsQ-interacting partners, and we show that the interaction of FtsQ with the recently identified cell division protein SepIVA is independent of FtsQ phosphorylation and suggests a role of FtsQ in modulating cell division. FtsQ exhibited predominantly septal localization in both the presence and absence of SepIVA. Our results suggest a role for FtsQ in modulating the length, division, and survival of Mtb cells both in vitro and in the host.
Keywords: cell division, Mycobacterium tuberculosis, phosphorylation, mycobacteria, protein kinase, FtsQ, regulation, dcw operon, divisome, septation
Introduction
Cell division is fundamental to all living cells. Most of the proteins either involved or thought to be involved in this process are essential for in vitro growth of Mycobacterium tuberculosis (Mtb)2 (1). In Escherichia coli, the divisome, a macromolecular assembly arranged in a multilayered toroid shape for septation and cytokinesis, is governed by a set of ∼30 proteins that function in coordinating partition of chromosome with septa initiation and divisome stabilization followed by segregation of the mother cell into two daughter progenies (2–6). Appropriate positioning of the divisome assembly requires specialized systems such as MinCDE or the nucleoid occlusion system (SlmA in E. coli and Noc in Bacillus subtilis) (7, 8). Sequential recruitment of proteins in the divisome occurs at two distinct stages with a short delay in between (9). Proto-ring proteins FtsZ, FtsA, and ZipA are the early recruits to the divisome and ensure constriction initiation and its stabilization (10). This is followed by recruitment of FtsK, FtsQ in complex with FtsB and FtsL (FtsQBL), peptidoglycan remodelers such as FtsW (transglycosylase), FtsI (transpeptidase), FtsN, carboxypeptidases, endopeptidases, and a number of other accessory proteins, which are necessary for chromosome segregation and final cytokinesis (11–17). Recently, an FtsN-driven switch of FtsA and FtsQBL between on and off states has been proposed, implicating their functions in divisome activation (18, 19). Additionally, the recent emergence of FtsEX as a regulator of FtsA and amidases indicates multistep regulation of divisome assembly (20). However, the precise functions of individual components and mechanism of signal transfer between components remain elusive.
Tight regulation of cell division is necessary for Mtb to sustain bouts of active infection, dormancy, and reactivation in the host. The heterogeneous cell population of Mtb during growth is thought to be one of the primary reasons for prolonged treatment (21). Although the mycobacterium lacks specialized systems to ensure the accurate positioning of divisome, the combination of directional chromosome translocation and unequal bipolar growth has been suggested as a compensatory mechanism (22). Although homologs of FtsZ, FtsK, FtsB, FtsL FtsQ, FtsI, and FtsW are annotated in mycobacteria, homologs for FtsA, ZipA, and FtsN proteins found in E. coli are lacking (23, 24). The presence of these homologous proteins and a similar sequence of recruitment at the midcell suggest the partial preservation of elementary complexes and their functions in mycobacteria (24). In mycobacteria, FtsZ is the first protein to assemble at the midcell; it polymerizes and serves as an initiating site for recruitment of peptidoglycan (PG)-remodeling proteins (25). A ternary complex comprising FtsZ, FtsW (probable lipid II flippase), and FtsI (transpeptidase) is thought to stabilize the divisome assembly and regulate septal PG biosynthesis (26). A homolog of FtsK in Mycobacterium smegmatis (Msmeg) plays a role in translocation of chromosome prior to cytokinesis (22). In addition to the conserved proteins, other nonconserved proteins such as CrgA, Ssd, SepF, and SepIVA have been identified to be involved in division (24, 27, 28). CrgA has been shown to interact with various other divisome members like FtsZ, FtsI, PbpA, FtsQ, and CwsA, suggesting a complex and intertwined regulatory circuit (27). FtsE serves as a linker between FtsZ and FtsX, which in turn act as an activator of the peptidoglycan hydrolase RipC (29). LamA was recently identified as an important divisome factor that can inhibit cell wall synthesis at newly formed poles (30). Many other important enzymes/proteins involved in the cell division process require attentive inspection to comprehend the complexity of the heterogeneous cell population and their implications as drug targets.
Rv2151c (FtsQ), belonging to the polypeptide transport–associated (POTRA) domain superfamily, is an essential protein, required for in vitro growth of Mtb (31). FtsQ, a 315-aa-long protein, is highly conserved among mycobacteria and is homologous to its namesake in E. coli. Similar to its homologs, DivIBin in B. subtilis and FtsQ in E. coli, FtsQ in Mtb contains a cytosolic N-terminal domain (100 aa) connected though a single transmembrane (23 aa) to a periplasmic domain. In B. subtilis, DivIB plays a role in the engulfment stage of sporulation and is thought to function as a hydrolase as its absence led to thickening of septa (32). Moreover, DivIB has been shown to function as a chaperone for FtsL protein (33). Initiation of ftsQ transcription at the end of DNA replication period in Caulobacter crescentus suggests its function as a sensor of completion of chromosome replication (34). Structural investigation with the extracellular domain of DivIB from Geobacillus stearothermophilus has suggested further subdomain organization of the periplasmic region into α, β, and γ domains (35). Sequence alignment of FtsQ from G. stearothermophilus with its homologs showed preservation of this subdomain organization structure across bacterial kingdoms (35). These periplasmic subdomains play a role in appropriate localization and interactions with other cell division members (36, 37). Although interaction of FtsQMtb with FtsZMtb through FipAMtb has been demonstrated in mycobacterium (38), the characterization of FtsQ's role in cell division, shape maintenance, and viability has not been investigated. In this report, we investigated the functionality of FtsQ in mycobacteria by overexpressing and conditionally depleting FtsQ.
Results
Overexpression of FtsQ increases the average cell length
The ftsQ gene in mycobacteria lies in a conserved division or cell wall cluster (dcw) operon and is coexpressed along with mur genes, allowing coordinated cell wall synthesis and division (Fig. 1a). FtsQ contains an N-terminal cytosolic domain connected through a transmembrane domain to the extracytoplasmic domain (Fig. 1b). Based on the sequence alignment of FtsQMtb with its homologs from E. coli, B. subtilis, and Streptococcus pneumoniae (data not shown), the periplasmic domain of FtsQ could be further divided into α, β, and γ domains (Fig. 1b). To delineate the function of FtsQMtb, first we sought to investigate the impact of FtsQ overexpression on mycobacterial growth and survival. Msmeg was electroporated with either pNit1 (vector) or pNit-FtsQ construct wherein ftsQ is cloned under the isovaleronitrile (IVN)-inducible promoter. Western blot analysis of lysates prepared from Msmeg::pN and Msmeg::pN-ftsQ cells grown in the presence or absence of 5 μm IVN showed significant expression of FtsQ in the presence of IVN (Fig. 1c). cfu enumerated at 0, 14, and 28 h showed ∼100-fold lower survival of the strain overexpressing FtsQ as compared with the WT at 14 h, which was reduced to ∼10-fold at 28 h (Fig. 1d). These results suggest an initial delay in the growth upon overexpression that eventually seems to converge with that of the WT strain. Such growth patterns are typically observed upon overexpression of proteins critical for cell division (39). Interestingly, analysis of cell length with scanning EM (SEM) showed a significant increase in the average mean cell length upon overexpression of FtsQ from 3.2 to 4.9 μm (Fig. 1, e and f).
Figure 1.
Overexpression of FtsQ increases the average cell length. a, schematic representation of the Rv2160c-ftsQ operon. b, schematic depiction of various domains of FtsQ. N-terminal (N), transmembrane (TM), and C-terminal (C) regions and the subdomains α, β, and γ in the C-terminal region are indicated. c, fresh cultures of mc2, mc2::pNit, and mc2::pN-FtsQ were seeded at an A600 of ∼0.02 and induced with 5 μm IVN for 14 h. 30 and 10 μg of WCLs prepared from these samples were resolved, transferred to nitrocellulose membrane, and probed with α-FLAG and α-PknB antibodies, respectively. d, cultures of mc2, mc2::pNit, and mc2::pN-FtsQ were seeded at an A600 of ∼0.02 and induced with 5 μm IVN. cfu were enumerated at 0, 14, and 28 h of growth in the presence of 5 μm IVN. e, cultures of mc2, mc2::pNit, and mc2::pN-FtsQ strains were seeded at an A600 of ∼0.02 and induced with 5 μm IVN for 14 h. The samples were processed for SEM, and morphologies were observed at 15,000×. f, cell lengths for ∼200 cells/samples from e were measured using Smart Tiff software and plotted as a scattered dot plot. Mean and S.D. were calculated using GraphPad Prism6. Mean cell lengths are: mc2, 3.1 μm; mc2::pNit, 3.2 μm; mc2::pN-FtsQ, 4.9 μm. Scale bars, 1 μm. Data are representative of two biologically independent experiments. Statistical analysis was performed using two-way ANOVA. ****, p < 0.0001; ns, nonsignificant.
Next, we wanted to determine the minimum levels of FtsQ overexpression necessary and sufficient to induce an increase in the average mean cell length. Toward this, the expression of FtsQ in Msmeg harboring an episomal copy of the gene was induced with different concentrations of IVN, which led to differential levels of FtsQ expression as evaluated by real-time PCR and Western blotting (Fig. 2, a and b). With increasing concentrations of IVN (0.2, 1, to 5 μm), we observed ∼10-, 27-, and 37-fold induction of ftsQ expression, respectively, in comparison with no inducer (Fig. 2a). In agreement with this, we could detect FtsQ expression only in lysates from 1 and 5 μm IVN-induced cultures (Fig. 2b). SEM analysis showed a statistically significant increase in the cell length at all the concentrations, including in the absence of inducer (3.8 versus 3.0 μm), suggesting that the leaky overexpression from the episomal construct was sufficient to alter the average mean cell length (Fig. 2, c and d). To evaluate the impact of overexpression on septum formation, we performed transmission EM (TEM) analysis. Interestingly, we observed a multiseptum phenotype in cells overexpressing FtsQ. To quantitate the percentage of such cells, we evaluated the septation pattern for ∼60–65 cells/sample. We observed a significant increase from 4 to 14% in cells containing a bi- or multiseptum phenotype upon overexpression of FtsQ (Fig. 2, e and f). Taken together, our data indicate that overexpression of FtsQ results in elongated and multiseptate cells, implicating a crucial role of FtsQ in regulation of cell division.
Figure 2.
Overexpression of FtsQ results in multiseptate phenotype. a, quantification of differential level of FtsQ expression in mc2::pN-FtsQ overexpressing strain induced with differential concentrations of IVN. -Fold change in mRNA levels of ftsQ at 0.2, 1, and 5 μm IVN was calculated with respect to the transcript levels in the absence of inducer by quantitative RT-PCR. Mean with S.D. is from three replicates. b–d, cultures of mc2, mc2::pNit, and mc2::pN-FtsQ strains were seeded at an A600 of ∼0.02 and grown in the presence of 5, 1, or 0.2 μm or no IVN for 14 h. b, 30 and 10 μg of WCLs prepared from these samples were resolved, transferred to nitrocellulose membrane, and probed with α-FLAG and α-PknB antibodies, respectively. * indicates a band due to probable cleavage of full-length FtsQ. c, samples were processed for SEM analysis, and representative images at 15,000× are shown. Scale bars, 1 μm. d, cell lengths of ∼200 cells/sample from c were measured independently using Smart Tiff software and plotted as a scattered dot plot with mean and S.D. values. Mean cell lengths obtained are: mc2, 3.1 μm; mc2::pNit, 3.3 μm; mc2::pN-FtsQ, 3.9 μm; mc2::pN-FtsQ + 0.2 μm, 3.9 μm; mc2::pN-FtsQ + 1.0 μm, 3.7 μm; mc2::pN-FtsQ + 5 μm, 4.1 μm. e, samples were processed for TEM analysis, and the representative TEM images are shown. Scale bars, 0.5 μm. f, 65–75 cells/sample from e were analyzed to calculate the percentage of nonseptate, uniseptate, and bi/multiseptate cells. 1, mc2 + 5 μm; 2, mc2::pNit + 5 μm; 3, mc2::pN-FtsQ + 5 μm; 4, mc2::pN-FtsQ + 1.0 μm; 5, mc2::pN-FtsQ + 0.2 μm; 6, mc2::pN-FtsQ. Statistical analysis was performed using two-way ANOVA. ****, p < 0.0001.
Both N- and C-terminal domains of FtsQ are critical for cell length maintenance
The N-terminal region of FtsQ including the transmembrane domain is 123 aa in length. The C-terminal domain of FtsQ in E. coli, B. subtilis, and other bacterial kingdoms contains crucial α, β, and γ domains thought to be important for its function (35, 40, 41). To evaluate the impact of overexpressing N- or C-terminal regions, we cloned the respective fragment along with the membrane-anchoring sequences into pNit1 vector. Although the full length and N-terminal domain showed significant overexpression, the C-terminal fragment displayed relatively minimal overexpression (Fig. 3a). The expression of the full length and N- and C-terminal fragments led to compromised growth (Fig. 3b), albeit to different extents. These observations were also reflected in the cellular morphology wherein their expression led to increased cell length (Fig. 3, c and d). We think the differences in the extent of growth defect or cell lengths upon N- or C-terminal fragment overexpression are most likely due to variations in the expressions of these fragments (Fig. 3a). Thus, results suggest a definitive role for both N- and C-terminal regions of FtsQ in regulating cell division.
Figure 3.
Both N- and C-terminal domains of FtsQ are critical for cell length maintenance. a, fresh cultures of mc2, mc2::pNit, mc2::pN-FtsQ, mc2::pN-FtsQ-N, and mc2::pN-FtsQ-C were seeded at an A600 of ∼0.05 and induced with 5 μm IVN for 14 h. 30 μg and 10 μg of WCLs prepared from these samples were resolved, transferred, and probed with α-FLAG and α-PknB antibodies, respectively. Bands indicated by white arrows probably arose due to cleavage of full-length FtsQ. b, cultures of mc2, mc2::pNit, mc2::pN-FtsQ, mc2::pN-FtsQ-N, and mc2::pN-FtsQ-C strains were seeded at an A600 of ∼0.05 and grown in the presence of 5 μm IVN. cfu were enumerated at 0, 14, and 28 h. Error bars represent S.E. c, samples were processed for SEM after 14 h, and morphologies were observed at 15,000×. Scale bars, 1 μm. d, cell lengths for ∼200 cells/samples from c were measured using Smart Tiff software and plotted as a scattered dot plot. Mean and S.D. were calculated using GraphPad Prism6. Mean cell lengths obtained are: mc2, 3.1 μm; mc2::pNit, 3.2 μm; mc2::pN-FtsQ, 5.7 μm; mc2::pN -FtsQ-N, 5.9 μm; mc2::pN-FtsQ-C, 4.4 μm. Data are representative of two biologically independent experiments. Statistical analysis was performed using two-way ANOVA. ***, p < 0.001; ****, p < 0.0001.
FtsQ is essential for bacterial viability in Mtb
We sought to investigate the consequence of the absence of FtsQ on bacterial growth, morphology, and survival in the host. High-throughput transposon-based mutagenesis experiments suggested ftsQ to be an essential gene for the in vitro growth of the bacteria (42). Thus, we set out to alter the expression of ftsQ at its native locus into pristinamycin-inducible expression. Toward this, we cloned −20 to 680 bp of ftsQ gene under the pristinamycin-inducible pptr promoter in pAZ vector (43) that lacks the Mtb origin of replication (suicide delivery vector) (Fig. 4a). Single homologous recombination of the construct at the native locus replaces the native ftsQ with a pristinamycin-inducible gene (Fig. 4a), which in the absence of any inducer would act as an FtsQ knockdown mutant, RvΔfQ. Recombination at the native locus was confirmed by PCR analysis using different primers sets (Fig. 4b). Streaking of Rv and RvΔfQ cultures on plates in the presence or absence of inducer clearly demonstrated the inability of RvΔfQ strain to sustain growth in the absence of inducer (Fig. 4c). cfu analysis (Fig. 4d) showed compromised survival of the mutant from day 6, eventually resulting in ∼1.5 log -fold decrease in survival on day 10 (Fig. 4d). Next we investigated the role of FtsQ in modulating the survival of pathogen in the host. Toward this, Rv or RvΔfQ grown in the presence or absence of pristinamycin was used for infecting differentiated THP1 cells. We enumerated cfu at 0, 96, and 120 h postinfection and found that at 120 h postinfection intracellular bacillary survival was compromised up to 10-fold in the FtsQ-depleted sample (Fig. 4e).
Figure 4.
FtsQ is essential for bacterial viability in Mtb. a, schematic depiction of the methodology used to generate the gene replacement mutant (RvΔfQ). Primer pairs used for PCR confirmation are indicated. b, 1% agarose gel showing PCR amplification using genomic DNA from Rv and RvΔfQ as templates. Mr denotes the l-kb ladder. The left panel shows the ftsQ (0.94-kb) gene amplicon in both Rv and RvΔfQ. The middle panel shows differential PCR amplicon of 1.1 kb obtained only in RvΔfQ. The right panel shows specific amplification of a 0.8-kb fragment in RvΔfQ. c and d, Rv and RvΔfQ grown in the presence of 1 μg/ml pristinamycin until A600 reached ∼0.8 were washed twice with PBS containing 0.05% Tween 80, and fresh cultures were seeded at an A600 of ∼0.05 in triplicates. c, 10 μl of freshly inoculated cultures was streaked on 7H11 agar plates in the presence or absence of 1 μg/ml pristinamycin. d, cfu were enumerated at 0, 2, 4, 6, 8, and 10 days. e, intramacrophage survival of RvΔfQ (+/−p) was compared with Rv (+/−p) strain after infection in phorbol 12-myristate 13-acetate–differentiated THP1 cells. Data are representative of one of the two biologically independent experiments. Statistical analysis was performed using two-way ANOVA. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, nonsignificant.
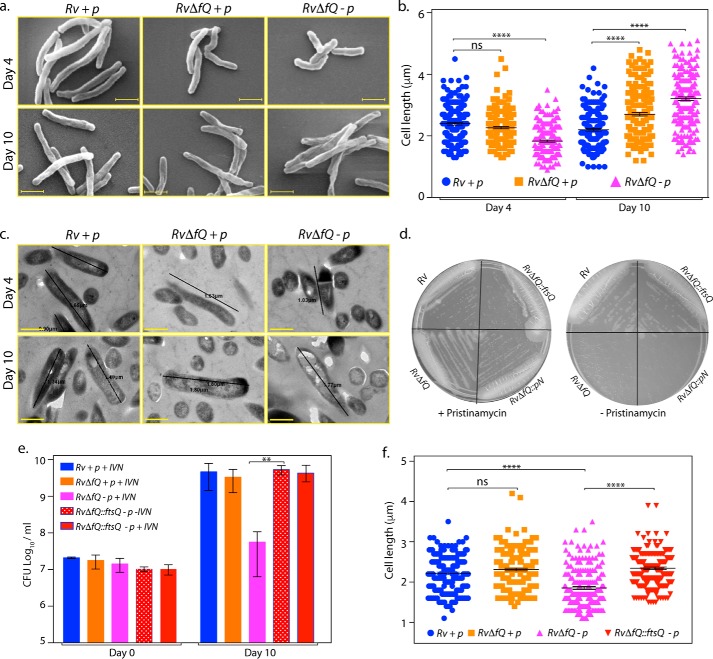
FtsQ depletion alters the mean cell length
Overexpression of FtsQ in Msmeg resulted in elongated cells, indicating a crucial role of FtsQ in regulating cell wall morphology (Fig. 1). This prompted us to investigate the effect of FtsQ depletion in Mtb on the maintenance of average mean cell length. We performed SEM and TEM analysis of samples on days 4 and 10 (early and late log-phase cultures; Fig. 5, a–c). Although the cells looked quite healthy on day 4, they looked more ruffled with indentations on day 10, and this change in morphology was much more palpable in FtsQ-depleted cells (Fig. 5a). Intriguingly, we observed two distinct cell length phenotypes with respect to time. At 4 days postdepletion, the cells appeared smaller when compared with the WT in FtsQ-depleted samples with the average cell length decreasing from 2.3 μm in WT cells to 1.8 μm in FtsQ-depleted samples (Fig. 5, a and b). Conversely, upon 10 days of depletion, the average cell length increased from 2.2 μm in H37Rv cells to 2.7 μm in RvΔfQ in the presence of pristinamycin (inducer). Importantly, the cell length increased to 3.1 μm in RvΔfQ in the absence of inducer when FtsQ was depleted (Fig. 5, a and b). We think the cell length differences observed could be due to differences in the levels of FtsQ protein in the cell. In subsequent experiments, we used 4-day-postdepletion SEM analysis of samples to assess the functionality of FtsQ. TEM analysis showed the presence of septum even in the smaller cells observed on the 4th day after depletion, suggesting that division may occur before cells reached the appropriate length. To confirm that the defects are indeed due to depletion of FtsQ, we generated complementation strain in which the protein was expressed from the episomal pNit-FtsQ construct. Although expression of vector alone could not rescue the survival defect of FtsQ mutant, complementation with vector expressing FtsQ could restore growth on plates in the absence of inducer (Fig. 5d). Furthermore, cfu analysis of WT, mutant, and complemented strains showed restoration of viability defects upon complementation (Fig. 5e). This ability of episomal FtsQ to functionally complement the phenotypic defects of FtsQ mutant was also reflected in the SEM analysis (performed 4 days after depletion) wherein the shorter cell length phenotype observed was restored to WT lengths (Fig. 5f). Based on the above data, we suggest that the presence of shorter cells in the initial and longer cells at the later stages is indicative of a regulatory role played by FtsQ in elongation and division.
Figure 5.
FtsQ depletion alters the mean cell length. a–c, fresh cultures of Rv and RvΔfQ were seeded at an A600 of ∼0.05 and grown in the presence (+p) or absence (−p) of inducer. Samples were harvested after days 4 and 10. a, the samples were processed for SEM, and morphologies were observed at 15,000×. Representative images are shown. Scale bars, 1 μm. b, cell lengths for ∼200 cells/samples from a were measured and plotted. Mean cell lengths obtained at day 4 are: Rv +p, 2.3 μm; RvΔfQ +p, 2.2 μm; RvΔfQ −p, 1.8 μm. Mean cell lengths obtained at day 10 are: Rv +p, 2.1 μm; RvΔfQ +p, 2.7 μm; RvΔfQ −p, 3.2 μm. c, representative TEM images for Rv +p, RvΔfQ +p, and RvΔfQ −p at days 4 and 10. Scale bars, 0.5 μm. d, RvΔfQ strain was transformed with pNit and pNit-FtsQ constructs to generate RvΔfQ::pN and RvΔfQ::ftsQ, respectively. Cultures were seeded at an A600 of ∼0.05, and 10 μl was streaked on 7H11 agar in the absence or presence of 1 μg/ml pristinamycin. e, cultures were seeded at an A600 of ∼ 0.05 in the presence or absence of 1 μg/ml pristinamycin or 0.2 μm IVN as indicated. cfu were enumerated at 0 and 10 days. Error bars represent S.E. f, experiment performed as described above except that cells were harvested at day 4 and processed for SEM. Cell lengths for ∼200 cells/sample were measured and plotted. Mean cell length values are: Rv +p, 2.2 μm; RvΔfQ::pN +p, 2.3 μm; RvΔfQ::pN −p, 1.8 μm; RvΔfQ::ftsQ −p, 2.2 μm. Data are representative of two biologically independent experiments. Statistical analysis was performed using two-way ANOVA. **, p < 0.01; ****, p < 0.0001; ns, nonsignificant.
C-terminal domains are critical for the functionality of FtsQ
Overexpression of either N- or C-terminal fragments of FtsQ increased the cell length of Msmeg (Fig. 3). Thus, we wanted to decipher whether the phenotypes observed upon depletion of FtsQ in RvΔfQ strain could be complemented by the expression of either N- or C-terminal fragments. RvΔfQ strain was electroporated with pNit, pNit-FtsQ, pNit-FtsQ-N, or pNit-FtsQ-C constructs to generate different complementation strains. We assessed the survival of these complementation strains on 7H11 plates with or without pristinamycin (Fig. 6a). Although complementation with the full length and C-terminal fragments resulted in growth in the absence of pristinamycin, neither the vector nor the N-terminal fragment could rescue the growth defects (Fig. 6a). Next we determined cfu 10 days postdepletion in these samples. In concurrence with the above data, although the C-terminal fragment successfully rescued the ∼2.5 log -fold decrease in survival upon FtsQ depletion, the N-terminal fragment failed to do so (Fig. 6b). SEM analysis of 4-day-depleted sample also suggested that episomal expression of both full-length FtsQ and the C-terminal fragment could restore the cell length to the WT level (Fig. 6c). It is apparent from the data that the C-terminal fragment is necessary and sufficient to rescue the observed FtsQ depletion phenotypes. Because the C-terminal region contains α, β, and γ domains, we further evaluated the impact of deleting either γ or both β–γ domains. Analysis of growth on 7H11 plates with or without pristinamycin suggested that the expression of either deletion mutants could not rescue the growth in the absence of inducer. The results obtained were in concurrence with the above conclusion when we analyzed cfu 10 days postdepletion or the cell lengths 4 days postdepletion (SEM) (Fig. 6, e and f). Together these results suggest that the C-terminal domain and all the subdomains within are essential for the functionality of FtsQ.
Figure 6.
C-terminal domains are critical for the functionality of FtsQ. a–c, RvΔfQ was electroporated with pNit, pNit-FtsQ, pNit-FtsQ-N, and pNit-FtsQ-C to generate RvΔfQ::pN, RvΔfQ::ftsQ, RvΔfQ::ftsQ-N, and RvΔfQ::ftsQ-C. Cultures were seeded at an A600 of ∼0.05 in the presence of 0.2 μm IVN and in the presence (+p) or absence (−p) of pristinamycin. a, 10 μl of freshly inoculated cultures was streaked on 7H11 agar in the absence or presence of 1 μg/ml pristinamycin. b, cfu were enumerated at 0 and 10 days. Error bars represent S.E. c, cells were harvested at day 4 and processed for SEM. Cell lengths for ∼200 cells/sample were measured and plotted. Mean cell lengths obtained at day 4 are: Rv +p, 2.5 μm; RvΔfQ::pN +p, 2.6 μm; RvΔfQ::pN −p, 1.8 μm; RvΔfQ::ftsQ −p, 2.5 μm; RvΔfQ::ftsQ-N −p, 1.6 μm; RvΔfQ::ftsQ-C −p, 2.5 μm. d, RvΔfQ was electroporated with pNit-FtsQ, pNit-FtsQ-C, pNit-FtsQ-Cα, and pNit-FtsQ-Cβ to generate RvΔfQ::ftsQ, RvΔfQ::ftsQ-C, RvΔfQ::ftsQ-Cα, and RvΔfQ::ftsQ-Cβ. 10 μl of freshly inoculated cultures was streaked on 7H11 agar in the absence or presence of 1 μg/ml pristinamycin. e, cfu of Rv + p, RvΔfQ::pN +p, RvΔfQ::pN −p, RvΔfQ::ftsQ −p, RvΔfQ::ftsQ-Cα −p, and RvΔfQ::ftsQ-Cβ −p were enumerated at 0 and 10 days. Error bars represent S.E. f, cells were harvested at day 4 and processed for SEM. Cell lengths for ∼200 cells/sample were measured and plotted. Mean cell lengths obtained at day 4 are: RvΔfQ::ftsQ −p, 2.1 μm; RvΔfQ::ftsQ-Cα −p, 1.9 μm; RvΔfQ::ftsQ-Cβ −p, 1.8 μm. Data are representative of two biologically independent experiments. Statistical analysis was performed using two-way ANOVA. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, nonsignificant.
FtsQ is phosphorylated at Thr-24 residue, and phosphorylation influences cell division
We performed high-throughput phosphoproteomics to identify novel targets of protein kinases in Mtb (data not shown). FtsQ is among the substrates that we identified consistently in every biological replicate. Moreover, FtsQ has also been identified as a target in other phosphoproteomic studies (44–46). We obtained one phosphopeptide corresponding to a precursor mass of 2140.92, a doubly charged peptide from residues 13 to 32. MS/MS analysis of the precursor clearly identified Thr-24 to be the phosphorylation site on FtsQ (Fig. 7a). To determine the stoichiometry of phosphorylation, Mtb was electroporated with pNit-FtsQ, which contains an N-terminal FLAG tag, and FLAG-FtsQ was immunoprecipitated and processed for LC-MS analysis. The sum of all isotopic peaks at the MS1 level for any peptide is indicative of its quantity. We calculated the area of peaks for the phospho- and the corresponding unphosphorylated peptide using the precursor ion area detector node. The ratio of the area for the total phosphorylated peptide with respect to the corresponding area for the total unphosphorylated peptide provides the stoichiometry. Based on these calculations from two biologically independent experiments, the stoichiometry of Thr-24 phosphorylation was observed to be ∼28–30% (Fig. 7b). To understand the role of phosphorylation, if any, in modulating the function of FtsQ, we generated phosphoablative (T24A) and phosphomimetic (T24E) mutants and electroporated RvΔfQ strain with pNit constructs expressing the above mutants. To evaluate the ability of mutants to complement growth, we analyzed three different aspects: growth on 7H11 plates in the absence of pristinamycin (Fig. 7c), growth in liquid media upon depletion (Fig. 7d), and cell length 4 days postdepletion (Fig. 7e). Both the phosphoablative and phosphomimetic mutants complemented the growth on 7H11 (Fig. 7c) as well as in the liquid media (Fig. 7e). Although the phosphomimetic mutant could partially complement cell length defects observed 4 days postdepletion, the phosphoablative mutant completely failed to do so (Fig. 7e). Based on the above data, we speculate that the cell viability and cell length defects associated with FtsQ depletion are independent traits and are regulated at different levels. Phosphorylation seems to play a role in modulating the cell length defects but has no apparent role in cell viability.
Figure 7.
FtsQ is phosphorylated at Thr-24 residue, and phosphorylation influences cell division. a and b, RvΔfQ::ftsQ strain was inoculated at an A600 of ∼0.1, induced with 5 μm IVN, and grown until A600 reached ∼0.8. FLAG-tagged FtsQ was immunoprecipitated, fragmented using trypsin, and subjected to LC-MS as described previously (83). a, MS/MS spectrum of precursor m/z 1070.45949 (+2) and MH+ 2140.91899 Da of the semitryptic phosphopeptide VADDAAEEAVpTEPLATESK where pT is phosphothreonine. The unambiguous location of the intact phosphate group on Thr-24 was determined by the presence of the b and y ion series containing b6–11, b13, b15–17, y4, and y7–14. b, tabular representation of identified stoichiometry of phosphorylation for the Thr-24 site. c–e, RvΔfQ was electroporated with pNit, pNit-FtsQ, pNit-FtsQ-T24E, and pNit-FtsQ-T24A to generate RvΔfQ::pN, RvΔfQ::ftsQ, RvΔfQ::ftsQ-T24E, and RvΔfQ::ftsQ-T24A. Cultures were seeded at an A600 of ∼0.05 in the presence of 0.2 μm IVN and in the presence (+p) or absence (−p) of pristinamycin. c, 10 μl of freshly inoculated cultures was streaked on 7H11 agar in the absence or presence of 1 μg/ml pristinamycin. d, cfu were enumerated at 0 and 10 days. Error bars represent S.E. e, cells were harvested at day 4 and processed for SEM. Cell lengths for ∼200 cells/sample were measured and plotted. Mean cell lengths obtained at day 4 are: Rv +p, 2.3 μm; RvΔfQ::pN +p, 2.2 μm; RvΔfQ::pN −p, 1.7 μm; RvΔfQ::ftsQ −p, 2.2 μm; RvΔfQ::ftsQ-T24E −p, 1.9 μm; RvΔfQ::ftsQ-T24A −p, 1.7 μm. Data are representative of two biologically independent experiments. Statistical analysis was performed using two-way ANOVA. *, p < 0.1; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, nonsignificant.
FtsQ interacts with SepIVA protein in a phosphorylation-independent manner
In E. coli, FtsQ is known to be part of a macromolecular divisome assembly. We were interested in identifying interacting partners of Mtb FtsQ to gain possible insights into its involvement in cell division. We electroporated Rv with either pNit1, pNit-FtsQ, pNit-FtsQ-T24A, or pNit-FtsQ-T24E constructs to generate Rv::pN, Rv::pN-FtsQ, Rv::pN-FtsQ-T24A, and Rv::pN-FtsQ-T24E strains wherein the expression of FLAG-FtsQ could be induced with IVN. The whole-cell lysates prepared from three biological replicates were independently immunoprecipitated with FLAG-M2 beads. Western blot analysis of the samples clearly established efficient IP (Fig. 8a). The immunoprecipitated samples were loaded for SDS-PAGE, a short while after the samples entered the resolving gel the run was terminated, and the gel pieces were sliced out. The gel slices were trypsinized, and the peptides were identified with LC-MS/MS. Each sample was run twice through the mass spectrometer, and only those proteins identified in both the runs were used for analysis. The proteins found in the vector-transformed Mtb were subtracted from those found in the corresponding FLAG-FtsQ IPs. We identified 117, 122, and 111 interacting partners in three independent replicates of FLAG-FtsQ IP wherein 63 proteins were common to all three experiments (Fig. 8b and Table S1). Similarly, three replicates of FLAG-FtsQ-T24A and FLAG-FtsQ-T24E IPs were analyzed to investigate phosphorylation-dependent interactions (Fig. 8c and Table S1). We found six and 24 interacting proteins common in all three biological replicates of FLAG-FtsQ-T24A and FLAG-FtsQ-T24E samples, respectively. After applying these stringent criteria, we identified four interacting partners in all nine samples from 18 MS runs (Fig. 8d and Table S1). The presence of these four proteins in both phosphoablative and phosphomimetic mutant immunoprecipitated samples indicates that interaction of these proteins with FtsQ is phosphorylation-independent. We also identified one protein that specifically associated with FtsQ-T24E but not with FtsQ or FtsQ-T24A. We identified 38 interacting partners that were not found either with T24A or with T24E. We do not yet know the implications of these findings. One of the four interacting partners that was consistently found is a recently characterized cell division protein, SepIVA (24). Similar to Wag31, SepIVA has a characteristic DivIVA domain, found in cell division proteins across the bacterial kingdom (47). We sought to validate the interactome data by probing FtsQ, FtsQ-T24A, and FtsQ-T24E IP samples with anti-SepIVA antibodies (Fig. 8e). It is apparent from the data that SepIVA interacted with both WT and phosphomutants of FtsQ, validating the interactome data. Taken together, we have identified cell division protein SepIVA to be a novel phosphorylation-independent interacting partner of FtsQ.
Figure 8.
FtsQ interacts with SepIVA protein in a phosphorylation-independent manner. a, 30 and 10 μg of WCLs prepared from Rv::pN, Rv::pN-ftsQ, Rv::pN-ftsQ-T24A, and Rv::pN-ftsQ-T24E strains in the presence of 5 μm IVN were resolved by SDS-PAGE, transferred, and probed with α-FLAG and α-PknB antibodies, respectively. 1 mg of WCL was immunoprecipitated, and one-tenth of the IP was probed with α-FLAG. b, Venn diagram showing 63 interacting partners found in common in three biological replicates of FLAG-FtsQ, each containing 117, 122, and 111 unique proteins. Unique proteins were obtained after subtracting the proteins identified in the corresponding control IP. c, 63, 6, and 24 interacting proteins were identified in all three biological sets of FLAG-FtsQ, FLAG-FtsQ-T24A, and FLAG-FtsQ-T24E after subtracting the proteins identified in the corresponding control IP. The Venn diagram shows four interacting partners found in common among FLAG-FtsQ, FLAG-FtsQ-T24A, and FLAG-FtsQ-T24E. d, table showing a list of the four interacting partners. e, 30 μg of WCLs prepared from Rv::pN, Rv::pN-ftsQ, Rv::pN-ftsQ-T24A, and Rv::pN-ftsQ-T24E strains in the presence of 5 μm IVN was resolved by SDS-PAGE, transferred, and probed with α-FLAG and α-SepIVA antibodies, respectively. 1 mg of WCL from each sample was immunoprecipitated, one-tenth of the IP was probed with α-FLAG antibody, and nine-tenths of the IP were probed with α-SepIVA antibody. * indicates a band due to probable cleavage of full-length FtsQ.
FtsQ shows predominantly septal localization in both the presence and absence of SepIVA protein
As a constituent of the divisome assembly, localization of FtsQ protein at the septal region has been demonstrated in rod-shaped bacteria (48). However, in polar growing bacteria like mycobacteria where divisome and elongasome components overlap at times, it is important to investigate the localization of proteins for insight into their involvement. Localization of Wag31 and FtsZ proteins at subpoles and septum is thought to be suggestive of their roles in elongasome and divisome complexes, respectively (30). To investigate the localization of FtsQ, we electroporated pN-GFP-FtsQ into mc2 to generate mc2::gfQ strain. We observed that FtsQ localizes at septal, subpolar, and polar regions of cells (Fig. 9a). However, when we quantitated the localization of FtsQ in 150 independent cells, we observed that in ∼40% of the population it is localized to the septal region, which is indicative of its probable role in divisome assembly (Fig. 9b).
Figure 9.
FtsQ shows predominantly septal localization both in the presence and absence of SepIVA protein. a and b, fresh cultures of mc2::gfQ were seeded at an A600 of ∼0.1 and grown for 14 h at 30 °C. a, cells were harvested, fixed with 4% paraformaldehyde, and washed with 1× PBS. Cells were imaged with a Zeiss Imager.M1 microscope. Scale bars, 5 μm. b, bar graph representing the percent distribution of GFP-FtsQ in different localization patterns. ∼150 cells were counted to calculate the percent distribution. c–g, generation and characterization of mc2Δsep deletion strain in mc2. c, schematic depiction of the methodology used to generate the gene replacement mutant (mc2Δsep). Primer pairs used for PCR confirmation are indicated. d, mc2 cultures were electroporated with pST-KirT-sep to generate merodiploid strain mc2::sep. Cultures of the merodiploid strain was seeded at an A600 of ∼0.1 in the presence and absence of ATc. 30 and 10 μg of lysates were prepared, resolved, transferred, and probed with α-FLAG and α-PknB antibodies, respectively. e, genomic DNA was prepared from mc2 and mc2Δsep mutant, and PCR amplifications were performed with the primers sets as indicated. The left panel shows the amplification with primers specific for rodAMsm gene, which was used as a control. The PCR products were resolved on a 1% agarose gel; M denotes the l-kb ladder. The second and third panels show the PCR amplification with F1–R1 and F2–R2 primers, respectively. 1.1- and 1.2-kb PCR products are expected only in the mc2Δsep mutant, not in mc2. The right panel shows PCR amplification using sepIVAMsm gene forward and reverse primers. Both mc2 and mc2Δsep amplify ∼0.7 kb of sepIVAMsm gene due to the presence of native and integrated copies of the gene, respectively. However, in the case of mc2Δsep, we expect a 1.7-kb additional band due to the presence of hygromycin at the native locus. f, mc2Δsep mutant strain was seeded at an A600 of ∼0.1 in the presence or absence of ATc (200 ng/ml). 30 and 10 μg of WCLs from cultures grown in the absence or presence of ATc for 6 and 12 h were resolved, transferred, and probed with α-FLAG and α-GroEL antibodies, respectively. g, photographs of mc2Δsep cultures grown in the absence or presence of ATc for 18 h. h, fresh cultures of mc2Δsep::gfQ were seeded at an A600 of ∼0.25 in the presence or absence of ATc (200 ng/ml) at 37 °C for 12 h. These cultures were subcultured at an A600 of ∼0.25 at 30 °C for 10 h in the presence or absence of ATc (25 ng/ml), and GFP-FtsQ expression was induced with 0.2 μm IVN. Cells were harvested, fixed, and imaged using a Zeiss LSM 510 Meta confocal microscope. Scale bars, 5 μm. i, bar graph representing the percent distribution of GFP-FtsQ in different localization patterns. ∼100 cells were counted to calculate the percent distribution.
We have consistently identified interaction between FtsQ and SepIVA, a recently identified cell division protein that contains a DivIVA domain (24). DivIVA domain–containing proteins are membrane curvature–sensitive proteins (49). We sought to investigate whether the interaction between SepIVA and FtsQ is important for septal localization of FtsQ. Based on high-throughput transposon mutagenesis studies, SepIVA has been identified to be essential for the in vitro growth of the pathogen (42). Thus, we first integrated an anhydrotetracycline (ATc)-regulatable copy (Tet-Off) of sepIVAMsm into the L5 site to generate a merodiploid mc2::sep strain (Fig. 9c). We first determined the ability of ATc to turn off the expression by performing Western blotting of the lysates prepared in the presence or absence of ATc from two independent colonies of mc2::sep strain (Fig. 9d). Both mc2::sep colonies showed efficient depletion of integrated FLAG-SepIVAMsm in the presence of ATc (Fig. 9d). Next we replaced the native copy of sepIVAMsm with hygr, and the replacement of sepIVAMsm at the native locus was confirmed by performing multiple PCRs (Fig. 9e). Western blot analysis of lysates prepared from mc2Δsep strain at different time points post-ATc addition clearly showed efficient depletion of FLAG-SepIVAMsm by 12 h (Fig. 9f). At 18 h post-ATc addition, we observed significantly compromised growth (Fig. 9g), confirming the essentiality of SepIVA for the in vitro growth. To assess the localization of FtsQ in the presence and absence of SepIVA, we electroporated pN-GFP-FtsQ into mc2Δsep strain to generate mc2Δsep::gfQ. Localization of GFP-FtsQ was determined in the presence and absence of ATc. We quantitated ∼100 independent cells in the presence and absence of SepIVA (Fig. 9i). We did not observe any statistically significant changes in the localization of GFP-FtsQ upon depletion of SepIVA (Fig. 9, h and i). These results suggest that recruitment of FtsQ during cell division precedes recruitment of SepIVA. Taken together, our data suggest a definitive role for FtsQ in modulating cell length, cell division, and eventual survival during in vitro growth.
Discussion
The ability of an organism to maintain its cell length necessitates stringent regulation of cellular elongation and division, which in turn are reliant on the coordinated involvement of macromolecular elongasome and divisome assemblies, respectively. Identification and characterization of individual contributors to these complexes are necessary to understand the sequence of recruitment and their impact on cell growth and division. Divisome assembly–driven septation and cytokinesis mark the decisive last step of the cell cycle and are well-regulated for precise cell division. The divisomal components are known to maintain a constant stoichiometry by exhibiting intrinsic regulation. Elevated levels of divisomal components like FtsA, ZipA, ZapC (stabilizer of FtsZ bundling), and FtsQ in E. coli (FtsQEc) and FtsZ, CrgA, and Ssd (Rv3660c) in mycobacterium have been shown to cause filamentation, suggesting concentration to be a major determinant for optimal cell division (25, 27, 28, 50–52). Interestingly, it was observed that overexpression of FtsZ alleviates the cell division blockage induced by overexpression of FtsA, suggesting that appropriate ratios of cell division proteins are necessary in determining the rate and timing of cell division (53–55). In accordance with these observations, results showed that overexpression as well as the depletion of FtsQ led to changes in the average mean cell length (Figs. 1 and 4). Interestingly, the perturbation in the levels of FtsQ need not to be very drastic as we observed that subtle changes in the level of FtsQ resulted in altered mean cell length (Figs. 2 and 4).
The observed elongated phenotype upon FtsQ overexpression (Fig. 1) could be due to either its role in peripheral PG biosynthesis (elongation) or inadequacy in undergoing septation (division). Inadequacy in undergoing septation could be the result of either incompetent initiation or completion of the septation process. Overexpression of ZipA in E. coli causes formation of smooth filaments, characterized by the absence of any visible invagination, suggesting that block is at the early stage of cell division (51). In contrast, overexpression of FtsQ in E. coli leads to a multiseptate pattern (52), suggesting block at completion of the septation process. In our studies, we have observed an increased proportion of biseptate filamentous cells upon FtsQ overexpression, indicating that cell division was likely blocked at later stages of cell division and that cells are able to initiate formation of second septa before completion of the first, implying altered coordination among cell division members (Fig. 2).
FtsN, a late cell recruiter protein of E. coli, contains a cytoplasmic N-terminal region and a C-terminal region containing a sporulation-related repeat (SPOR) domain. The N-terminal region of FtsN plays a role in interaction with FtsA (early divisome protein), and the C-terminal SPOR domain is responsible for sensing PG and mediating midcell localization (56–58). FtsQ, a bitopic protein, encompasses the N-terminal cytoplasmic region connected through a transmembrane domain to the C-terminal periplasmic region (Fig. 1). FtsQEc localizes to the midcell and is involved in recruitment of interacting proteins necessary for division (37). Although the periplasmic region of FtsQ was found to be essential for its interaction with FtsB/FtsL in E. coli and PBP2B in B. subtilis, the functionality of the N-terminal cytosolic region has not yet been elucidated (41, 59). We observed that overexpression of both N- and C-terminal domains of mycobacterial FtsQ led to elongation of cells (Fig. 3), suggesting that both the domains are independently important for its functionality. However, it remains elusive whether both the N- and C-terminal domains interact with a similar or different set of proteins. We noticed that the number of bands detected in the Western blot for FtsQ varied from one to three (Figs. 1–3 and 8). The appearance of these additional bands depends on the amount of protein loaded, resolution of the gel, and the enhanced chemiluminescence exposure times. FtsQ is a single transmembrane–containing protein, and we speculate that it may undergo spontaneous cleavage during storage at susceptible sites, which is likely responsible for the multiple bands.
Deletion of many Fts proteins in E. coli is known to cause filamentation, and hence they are categorized as divisome proteins (60, 61). In contrast, a point mutation in FtsL (E88K) of E. coli results in smaller cells and an enhanced cell division rate, subsequently leading to cell lysis. We observed that depletion of FtsQ in Mtb for 4 and 10 days, respectively, gave two distinct phenotypes. Although we observed shorter cells with distinct septa on day 4, the cells were longer at 10 days postdepletion, eventually leading to cell death (Figs. 4 and 5). The elongation phenotype observed at day 10 is consistent with a recent report wherein depletion of FtsQ in Msmeg was shown to result in elongated and branched cells (24). We speculate that distinct morphologies could be due to differences in the protein levels of FtsQ.
Depletion of FipA and PonA1/PonA2 is known to reduce bacterial replication in macrophages and murine infection models, respectively (38, 62, 63). The recent finding that a C-terminal fragment of and full-length Wag31 induced production of T cell cytokines such as IL-10 and IL-17 suggests a crucial role of cell division proteins in maintenance of bacillus survival during infection (64). The observed reduction in bacillus growth during macrophage infection with the FtsQ depletion strain indicates that FtsQ plays an important role in maintenance of cell division and hence persistent survival of the pathogen during host infection (Fig. 4). However, we observed marginal changes, if any, in the cell length upon infection in the presence or absence of FtsQ (data not shown).
We observed that overexpression of both N- and C-terminal regions impacted cell morphology (Fig. 3). Although both the full length and C-terminal domain could complement FtsQ functionality, the N-terminal domain failed to do so (Figs. 5 and 6). Similar observations were noted in both E. coli and B. subtilis wherein the cytoplasmic domain was found to be dispensable for the function of FtsQ and DivIB, respectively (65, 66). Interestingly, a point mutation in the C-terminal α domain (V92D) of FtsQEc made the cytoplasmic N-terminal domain essential for its functionality, suggesting possible cross-talk between domains (67). Thus, although the N-terminal region of FtsQ fails to complement (Fig. 6), its possible role in the optimal functionality of FtsQ cannot be overruled. Two E. coli subdomains of FtsQ, aa 1–135 and 136–276, complemented the mutant, suggesting that many of the interactions may have been mediated by both domains (68). However, in Mtb, the loss of viability as well as cell length defects upon FtsQ depletion could not be complemented even in the absence of the C-terminal γ domain, indicating that all the C-terminal subdomains are independently essential for its functionality (Fig. 6).
Phosphorylation of cell division proteins such as FtsZ and Wag31 has been shown to be important for modulating their functionality (69, 70). Essential mycobacterial serine/threonine protein kinases PknA and PknB and the sole phosphatase PstP play an important role in regulating cell division and cell wall synthesis processes (71–74). Phosphorylation of FtsZ by PknA was reported to regulate its GTPase activity (69). Furthermore, PknA-mediated phosphorylation of FtsZ on Thr-343 and FipA on Thr-77 is necessary for cell division under oxidative stress conditions (38). Phosphorylation of Wag31, a regulator of cell shape and cell wall synthesis, alters the growth rate as well as its interaction with its kinase PknA (70, 75). In addition to FtsZ, FipA, and Wag31, which have been biochemically shown to be the targets of PknA, high-throughput phosphoproteomic studies have identified phosphorylation sites on other cell division proteins such as FtsI, FtsK, and FtsQ (44–46). However, the functional implications of phosphorylation have not been elucidated. Our studies revealed that FtsQ is phosphorylated on Thr-24 in the N-terminal domain with ∼30% stoichiometry (Fig. 7). Although there were no significant changes in viability upon complementation with the phosphomimetic and ablative mutants, we observed that the phosphoablative (T24A) mutant failed to complement the cell length defects observed (Fig. 7). We hypothesize that the phosphorylation at Thr-24 in FtsQ may be important in the context of recruitment of one or more proteins necessary for regulating cell length.
Previous studies have demonstrated a pairwise interaction between a numbers of cell division members such as LamA-PonA1, FtsZ-FtsW, FtsW-FtsI, FtsI-Wag31, Wag31-CwsA, CwsA-CrgA, CrgA-PBPA, CrgA-FtsQ, SepF-FtsZ, and FtsZ-FipA-FtsQ, suggesting a complex and multiple interactions among the members of the divisome in mycobacteria (26, 27, 30, 38, 76–78). In this report, using MS, we have identified novel interacting partners for FtsQ (Fig. 8). Because MS-based identification is very sensitive, we have applied highly stringent criteria for the analysis. We have only considered those proteins that were identified in all three biological replicates but not in the corresponding control IPs. We have identified 63 interacting proteins in all three biological replicates with WT FtsQ. Significant numbers of these interacting partners were ribosomal proteins (Table S1), which may be present because of their relative abundance. Interestingly, we did not find Wag31 or FtsZ in even one of these runs. However, one cannot rule out the possibility of transient interaction between FtsQ and the other cell division proteins at the pole or septum at specific stages of cell division.
While this manuscript was under consideration, Wu et al. (24) reported the characterization of novel septal factors in Msmeg. They performed pulldown experiments with cross-linked FtsQ-Strep and identified 48 Msmeg proteins among which 41 proteins had homologs in Mtb. When we compared our list with that of Wu et al. (Ref. 24), we found only two proteins, namely SepIVA and FhaA, to be common (Table S1). The limited overlap may be due to differences in the pulldown protocol (vis-à-vis cross-linking) and/or the species used for the experiment (Mtb versus Msmeg). We have identified four interacting proteins, which were found with WT as well as phosphosite mutants. In addition to the newly identified cell division protein SepIVA (24), we have identified transketolase, a metabolic enzyme; a single-stranded binding protein involved in DNA replication; and a conserved hypothetical protein whose function is unknown. Furthermore, we have identified Rv3140 only in the FtsQ-T24E sample. Rv3140 is annotated as a probable acyl-CoA dehydrogenase but has not yet been characterized. With the exception of SepIVA, we have neither validated or explored the implications of these interactions any further.
Localization of FtsQ at the septal region in E. coli indicates its predominant function during division (48). We have also detected predominant septal localization of FtsQ in mycobacteria, indicating its possible function during division (Fig. 9). These results are in agreement with a recent report in which FtsI, FtsQ, FtsL, and FtsB proteins were found to be at the septum (24). With the exception of SepIVA, we have not identified any other protein involved in the cell division process as an interacting partner (Table S1). However, we cannot exclude the possibility of transient spatiotemporal interactions between FtsQ and other cell division proteins. SepIVA protein is known to possess a conserved DivIVA domain and to be localized to the septum, and its depletion results in elongated cells, thus indicating its involvement in the cell division process (24). Because both FtsQ and SepIVA are localized to the septum and they interact with each other, we investigated the localization of FtsQ in the presence or absence of SepIVA. We did not find significant differences in the localization of FtsQ in the absence of SepIVA (Fig. 9). These results suggest that SepIVA is recruited to the septum after FtsQ. The domains of FtsQ and SepIVA involved in their interaction and the biological impact of abrogating their interaction on cell division will be investigated in the future.
Materials and methods
Reagents, bacterial strains, and growth conditions
Bacterial strains used in the study are listed in Table 1. Restriction/modification enzymes were procured from New England Biolabs and MBI-Fermentas (Thermo Scientific). Oligonucleotide primers were procured from Sigma-Aldrich. Analytical grade chemicals were purchased from Sigma-Aldrich, Amresco, Merck, or Biobasic Canada. Pristinamycin 1A was purchased from Molcon Corp., Canada. pENTR/Directional TOPO Cloning kit was purchased from Invitrogen. pNit1 vector (79) was a kind gift from Christopher M. Sassetti. Anti-FLAG mAb was purchased from Sigma. EM chemicals were obtained from Electron Microscopy Sciences, and growth medium components were acquired from BD Biosciences. E. coli DH5α strain (Invitrogen) was used for cloning and was grown in LB broth at 200 rpm or LB agar in the presence of either kanamycin (50 μg/ml) or hygromycin (150 μg/ml). Msmeg mc2155 or Mtb H37Rv strains were grown at 100 rpm at 37 °C in Middlebrook 7H9 broth (BD Biosciences) supplemented with 10% ADC (5% BSA fraction V, 2% dextrose, 0.85% NaCl, catalase 0.004%) and 0.05% Tween 80 in the absence or presence of kanamycin (25 μg/ml) or hygromycin (100 μg/ml). Msmeg mc2155 or Mtb strains were plated on Middlebrook 7H10 plates supplemented with OADC (ADC + 0.06% oleic acid).
Table 1.
Strains used in the study
| Strains | Description | Source |
|---|---|---|
| mc2155 | Wildtype M. smegmatis mc2155 strain | ATCC, 700084 |
| mc2::pNit | mc2 strain electroporated with ectopic, IVN-inducible pNit vector; Kanr | This study |
| mc2::pN-FtsQ | mc2 strain electroporated with pNit-FtsQ (aa 1–315) construct | This study |
| mc2::pN-FtsQ-N | mc2 strain electroporated with pNit-FtsQ-N (aa 1–123) construct | This study |
| mc2::pN-FtsQ-C | mc2 strain electroporated with pNit-FtsQ-C (aa 101–315) construct | This study |
| mc2::gfQ | mc2 strain electroporated with pN-GFP-FtsQ construct | This study |
| mc2::sep | mc2 strain electroporated with pST-KirT-SepIVA construct | This study |
| mc2Δsep | mc2::sep electroporated with linearized AES | This study |
| mc2Δsep::gfQ | mc2Δsep strain electroporated with pN-GFP-FtsQ | This study |
| Rv | Wildtype M. tuberculosis H37Rv strain | ATCC |
| Rv::pN | Rv electroporated with pNit vector | This study |
| Rv::pN-ftsQ | Rv electroporated with pNit-FtsQ construct | This study |
| Rv::pN-ftsQ-T24A | Rv electroporated with pNit-FtsQ-T24A construct | This study |
| Rv::pN-ftsQ-T24E | Rv electroporated with pNit-FtsQ-T24E construct | This study |
| RvΔfQ | Rv ftsQ conditional mutant; ftsQ gene expression is under the regulation of pristinamycin-inducible pptr promoter | This study |
| RvΔfQ::pN | RvΔfQ electroporated with pNit vector | This study |
| RvΔfQ::ftsQ | RvΔfQ electroporated with pNit-FtsQ construct | This study |
| RvΔfQ::ftsQ-N | RvΔfQ electroporated with pNit-FtsQ-N construct | This study |
| RvΔfQ::ftsQ-C | RvΔfQ electroporated with pNit-FtsQ-C construct | This study |
| RvΔfQ::ftsQ-Cα | RvΔfQ electroporated with pNit-FtsQ-Cα construct | This study |
| RvΔfQ::ftsQ-Cβ | RvΔfQ electroporated with pNit-FtsQ-Cβ construct | This study |
| RvΔfQ::ftsQ-T24E | RvΔfQ electroporated with pNit-FtsQ-T24E construct | This study |
| RvΔfQ::ftsQ-T24A | RvΔfQ electroporated with pNit-FtsQ-T24A construct | This study |
Generation of ftsQ expression constructs and RvΔfQ gene replacement mutant
Full-length Mtb H37Rv ftsQ was amplified from H37Rv genomic DNA using gene-specific primers and Phusion DNA polymerase (New England Biolabs), and the amplicons were cloned into the NdeI-HindIII sites in pNit1 vector. pNit-FtsQ-N (1–369 bp), pNit-FtsQ-C (300–945 bp), pNit-FtsQ-Cα (1–579 bp), and pNit-FtsQ-Cβ (1–783 bp) were generated by amplifying the respective regions using specific forward and reverse primers and cloning the amplicons into the NdeI-HindIII sites in pNit1 vector. All point mutations were generated by overlapping PCR using appropriate mutagenic primers, and the mutations were confirmed by DNA sequencing. To generate the gene replacement mutant in Mtb, the −20 to 680 bp region of ftsQ was PCR-amplified, and the amplicons were cloned into the NcoI-SphI sites under the pristinamycin-inducible promoter in vector pAZI9479 (43) to generate pAZ-ftsQ. The suicide delivery vector pAZ-ftsQ was electroporated into H37Rv, and colonies were selected on 7H10 agar containing hygromycin and pristinamycin (2 μg/ml). Gene replacement at the native locus was confirmed with specific PCRs.
Growth kinetics, SEM, and TEM experiments
To analyze the growth pattern of WT and recombinant strains of Msmeg, cultures were grown until A600 reached ∼0.8, washed once with PBS containing 0.05% Tween 80, and seeded at an initial A600 of 0.02 or 0.05 in the absence or presence of varied concentrations of IVN (0.2–5 μm). The growth was monitored every 3 h for 30 h. Rv, RvΔfQ mutant, or RvΔfQ complemented strains were grown until A600 reached ∼0.8 in the presence of the inducer pristinamycin 1A and then seeded at an A600 of 0.05 in the presence or absence of pristinamycin 1A or 0.2 μm IVN. The growth was monitored every 24 h for 10 days. To evaluate the bacteriostatic or bactericidal effects of FtsQ, Rv, RvΔfQ mutant, or RvΔfQ complemented strains were withdrawn on 0 and 10 days, and different dilutions were spotted on plates containing pristinamycin. SEM and TEM experiments were performed as described previously (80, 81). For SEM, cells were visualized under the microscope at 10,000× or 15,000× magnifications after coating with gold particles (Carl Zeiss, EVO LS SEM). For TEM, cells were sliced and stained followed by examination under a Tecnai G2 20 twin (FEI) transmission electron microscope.
THP1 macrophage infection experiments
Bacterial cultures of Rv and RvΔfQ grown in the presence of pristinamycin were seeded in the presence or absence of pristinamycin in 7H9 at an A600 of ∼0.1. THP1 cells were cultured, maintained in RPMI 1640 medium + 10% FBS (heat-inactivated) + 1% penicillin-streptomycin. For cfu enumeration, 2 × 105 cells/24-well plate were seeded in triplicates for each sample. The cells were differentiated with phorbol 12-myristate 13-acetate and infected as described previously (80) at a multiplicity of infection of 1:5 with Rv and RvΔfQ in the presence or absence of pristinamycin. Cells were lysed after 96 and 120 h, and cfu were enumerated.
Identification of interacting partners and phosphorylation sites
To identify interacting partners, cultures of Rv::pN, Rv::pN-FtsQ, Rv::pN-FtsQ-T24A, and Rv::pN-FtsQ-T24E were seeded at an A600 of ∼0.1 in the presence of 5 μm IVN. Western blotting was performed to evaluate expression; 1 mg of lysate was immunoprecipitated using FLAG-M2 beads (Sigma). The bound FtsQ and interacting partners were eluted by adding 2× SDS sample dye to the beads. Samples were resolved on a 10% polyacrylamide gel until the dye front was ∼1.5 cm into the resolving gel. Gel pieces were sliced and processed for trypsinization as described earlier (82). MS and MS/MS analyses were performed as described previously to determine the stoichiometry of phosphorylation and interacting partners (83).
Validation of SepIVA–FtsQ interaction
The coding sequence of sepIVA was PCR-amplified using H37Rv genomic DNA as the template. The amplicon was digested with NdeI-HindIII enzymes and cloned into the corresponding sites in pQE2 vector (Qiagen). The construct was transformed into E. coli BL21-DE3 CodonPlus strain, and expression was induced with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside at 22 °C for 12–16 h. His-tagged protein was purified as described earlier (84). To prepare antisera against SepIVA, four BALB/c mice were immunized subcutaneously with a mixture containing 50 μg of protein and an equal volume of Freund's incomplete adjuvant (Sigma). The immunization procedure was repeated twice (2 weeks apart). Two weeks after the last immunization, mice were euthanized, and sera were collected. To validate the interaction of FtsQ with SepIVA, 1 mg of whole-cell lysates (WCLs) from strains expressing FLAG-FtsQ WT and mutants was immunoprecipitated. One-tenth of the IP was probed with α-FLAG antibody, and nine-tenths of the IP were probed with α-SepIVA antibody (1:2000 dilution).
Generation of mc2Δsep mutant
To generate the integrating construct, sepIVA was amplified from mc2 genomic DNA using gene-specific primers, and the amplicon was cloned into the NdeI-HindIII sites in pST-KirT vector (80). The construct was electroporated in mc2 to generate an mc2::sep merodiploid strain. Two independent mc2::sep merodiploid colonies were assessed for depletion of FLAG-SepIVA upon ATc addition (200 ng/ml) by probing WCLs with anti-FLAG antibody. 5′ and 3′ genomic flank sequences of sepIVAMsm (∼800 bp each) were amplified, and allelic exchange substrate (AES) was generated as described earlier (85, 86). Linearization of AES, electroelution, and mutant generation and confirmation were performed as described previously (87).
Localization of GFP-FtsQ in Msmeg and mc2Δsep deletion strains
The genes gfpm2+ and ftsQ were amplified from plasmids pMN437 (88) and pNit-FtsQ, respectively. The gfpm2+ and ftsQ amplicons were digested with NdeI-SapI and SapI-HindIII, respectively, and cloned into the NdeI-HindIII sites in pNit1 to generate the pN-GFP-FtsQ construct. The construct was electroporated into Msmeg and mc2Δsep strains to generate mc2::gfQ and mc2Δsep::gfQ strains. Culture of mc2::gfQ were seeded at an A600 of ∼0.1 at 30 °C for 14 h to express GFP-fused FtsQ protein. mc2Δsep::gfQ strain was seeded at an A600 of ∼0.25 in the presence and absence of 200 ng/ml ATc overnight at 37 °C. These cultures were subcultured at an A600 of ∼0.25 at 30 °C for 10 h in the presence or absence of ATc (25 ng/ml), and GFP-FtsQ expression was induced with 0.2 μm IVN. Cells were harvested, fixed with 4% paraformaldehyde, and imaged using a Zeiss LSM 510 Meta confocal microscope as described in previous reports (72).
Statistical analysis
Two-way ANOVA was used to analyze the significance of results unless otherwise specified. GraphPad Prism version 5.0 was used for plotting the results, and modifications were made using Adobe Illustrator CS5.1.
Author contributions
P. J. and V. K. N. conceptualization; P. J., B. M., and M. Z. K. formal analysis; P. J. validation; P. J., B. M., M. Z. K., S. L., and A. S. investigation; P. J., B. M., M. Z. K., S. L., and A. S. methodology; P. J. and V. K. N. writing-original draft; B. M. data curation; B. M. and M. Z. K. writing-review and editing; S. L. and A. S. visualization; V. K. N. supervision; V. K. N. funding acquisition; V. K. N. project administration; B. M. performed mass spectrometry experiment and data analysis; M. Z. K. generation and characterization of Msmeg SepIVA mutant; S. L. performed microscopy experiments along with P. J.; A. S. performed TEM experiment along with P. J.
Supplementary Material
Acknowledgments
We thank Prof. Christopher M. Sassetti and Dr. Francesca Forti for the kind gifts of pNit1 and pAZI9479 vectors. We thank the Scanning Electron Microscopy, Confocal Microscopy, and Mass Spectrometry Facilities at the National Institute of Immunology and Rekha Rani and Shanta Sen for support in managing these facilities. We also thank the Transmission Electron Microscopy Facility at the Institute of Genomics and Integrative Biology.
This work was supported by Department of Science and Technology, Government of India Grant EMR/2014/000877 (to V. K. N.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Table S1.
- Mtb
- M. tuberculosis
- PG
- peptidoglycan
- Msmeg
- M. smegmatis
- aa
- amino acid(s)
- IVN
- isovaleronitrile
- SEM
- scanning EM
- TEM
- transmission EM
- ATc
- anhydrotetracycline
- Ec
- E. coli
- SPOR
- sporulation-related repeat
- WCL
- whole-cell lysate
- AES
- allelic exchange substrate
- ANOVA
- analysis of variance.
References
- 1. DeJesus M. A., Gerrick E. R., Xu W., Park S. W., Long J. E., Boutte C. C., Rubin E. J., Schnappinger D., Ehrt S., Fortune S. M., Sassetti C. M., and Ioerger T. R. (2017) Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 8, e02133–16 10.1128/mBio.02133-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Söderstrom B., Skoog K., Blom H., Weiss D. S., von Heijne G., and Daley D. O. (2014) Disassembly of the divisome in Escherichia coli: evidence that FtsZ dissociates before compartmentalization. Mol. Microbiol. 92, 1–9 10.1111/mmi.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu G., Huang T., Buss J., Coltharp C., Hensel Z., and Xiao J. (2010) In vivo structure of the E. coli FtsZ-ring revealed by photoactivated localization microscopy (PALM). PLoS One 5, e12682 10.1371/journal.pone.0012682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haeusser D. P., and Margolin W. (2016) Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat. Rev. Microbiol. 14, 305–319 10.1038/nrmicro.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lutkenhaus J., Pichoff S., and Du S. (2012) Bacterial cytokinesis: from Z ring to divisome. Cytoskeleton 69, 778–790 10.1002/cm.21054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du S., and Lutkenhaus J. (2017) Assembly and activation of the Escherichia coli divisome. Mol. Microbiol. 105, 177–187 10.1111/mmi.13696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernhardt T. G., and de Boer P. A. (2005) SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol. Cell 18, 555–564 10.1016/j.molcel.2005.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu L. J., and Errington J. (2004) Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell 117, 915–925 10.1016/j.cell.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 9. Aarsman M. E., Piette A., Fraipont C., Vinkenvleugel T. M., Nguyen-Distèche M., and den Blaauwen T. (2005) Maturation of the Escherichia coli divisome occurs in two steps. Mol. Microbiol. 55, 1631–1645 10.1111/j.1365-2958.2005.04502.x [DOI] [PubMed] [Google Scholar]
- 10. Rico A. I., Krupka M., and Vicente M. (2013) In the beginning, Escherichia coli assembled the proto-ring: an initial phase of division. J. Biol. Chem. 288, 20830–20836 10.1074/jbc.R113.479519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Männik J., Bailey M. W., O'Neill J. C., and Männik J. (2017) Kinetics of large-scale chromosomal movement during asymmetric cell division in Escherichia coli. PLoS Genet. 13, e1006638 10.1371/journal.pgen.1006638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glas M., van den Berg van Saparoea H. B., McLaughlin S. H., Roseboom W., Liu F., Koningstein G. M., Fish A., den Blaauwen T., Heck A. J., de Jong L., Bitter W., de Esch I. J., and Luirink J. (2015) The soluble periplasmic domains of Escherichia coli cell division proteins FtsQ/FtsB/FtsL form a trimeric complex with submicromolar affinity. J. Biol. Chem. 290, 21498–21509 10.1074/jbc.M115.654756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Botta G. A., and Park J. T. (1981) Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J. Bacteriol. 145, 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho H., Wivagg C. N., Kapoor M., Barry Z., Rohs P. D., Suh H., Marto J. A., Garner E. C., and Bernhardt T. G. (2016) Bacterial cell wall biogenesis is mediated by SEDS and PBP polymerase families functioning semi-autonomously. Nat. Microbiol. 1, 16172 10.1038/nmicrobiol.2016.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pichoff S., Du S., and Lutkenhaus J. (2015) The bypass of ZipA by overexpression of FtsN requires a previously unknown conserved FtsN motif essential for FtsA-FtsN interaction supporting a model in which FtsA monomers recruit late cell division proteins to the Z ring. Mol. Microbiol. 95, 971–987 10.1111/mmi.12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goehring N. W., and Beckwith J. (2005) Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr. Biol. 15, R514–R526 10.1016/j.cub.2005.06.038 [DOI] [PubMed] [Google Scholar]
- 17. Priyadarshini R., Popham D. L., and Young K. D. (2006) Daughter cell separation by penicillin-binding proteins and peptidoglycan amidases in Escherichia coli. J. Bacteriol. 188, 5345–5355 10.1128/JB.00476-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu B., Persons L., Lee L., and de Boer P. A. (2015) Roles for both FtsA and the FtsBLQ subcomplex in FtsN-stimulated cell constriction in Escherichia coli. Mol. Microbiol. 95, 945–970 10.1111/mmi.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsang M. J., and Bernhardt T. G. (2015) A role for the FtsQLB complex in cytokinetic ring activation revealed by an ftsL allele that accelerates division. Mol. Microbiol. 95, 925–944 10.1111/mmi.12905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du S., Pichoff S., and Lutkenhaus J. (2016) FtsEX acts on FtsA to regulate divisome assembly and activity. Proc. Natl. Acad. Sci. U.S.A. 113, E5052–E5061 10.1073/pnas.1606656113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aldridge B. B., Fernandez-Suarez M., Heller D., Ambravaneswaran V., Irimia D., Toner M., and Fortune S. M. (2012) Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science 335, 100–104 10.1126/science.1216166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh B., Nitharwal R. G., Ramesh M., Pettersson B. M., Kirsebom L. A., and Dasgupta S. (2013) Asymmetric growth and division in Mycobacterium spp.: compensatory mechanisms for non-medial septa. Mol. Microbiol. 88, 64–76 10.1111/mmi.12169 [DOI] [PubMed] [Google Scholar]
- 23. Hett E. C., and Rubin E. J. (2008) Bacterial growth and cell division: a mycobacterial perspective. Microbiol. Mol. Biol. Rev. 72, 126–156 10.1128/MMBR.00028-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu K. J., Zhang J., Baranowski C., Leung V., Rego E. H., Morita Y. S., Rubin E. J., and Boutte C. C. (2018) Characterization of conserved and novel septal factors in Mycobacterium smegmatis. J. Bacteriol. 200, e00649–17 10.1128/JB.00649-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dziadek J., Madiraju M. V., Rutherford S. A., Atkinson M. A., and Rajagopalan M. (2002) Physiological consequences associated with overproduction of Mycobacterium tuberculosis FtsZ in mycobacterial hosts. Microbiology 148, 961–971 10.1099/00221287-148-4-961 [DOI] [PubMed] [Google Scholar]
- 26. Datta P., Dasgupta A., Singh A. K., Mukherjee P., Kundu M., and Basu J. (2006) Interaction between FtsW and penicillin-binding protein 3 (PBP3) directs PBP3 to mid-cell, controls cell septation and mediates the formation of a trimeric complex involving FtsZ, FtsW and PBP3 in mycobacteria. Mol. Microbiol. 62, 1655–1673 10.1111/j.1365-2958.2006.05491.x [DOI] [PubMed] [Google Scholar]
- 27. Plocinski P., Ziolkiewicz M., Kiran M., Vadrevu S. I., Nguyen H. B., Hugonnet J., Veckerle C., Arthur M., Dziadek J., Cross T. A., Madiraju M., and Rajagopalan M. (2011) Characterization of CrgA, a new partner of the Mycobacterium tuberculosis peptidoglycan polymerization complexes. J. Bacteriol. 193, 3246–3256 10.1128/JB.00188-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. England K., Crew R., and Slayden R. A. (2011) Mycobacterium tuberculosis septum site determining protein, Ssd encoded by rv3660c, promotes filamentation and elicits an alternative metabolic and dormancy stress response. BMC Microbiol. 11, 79 10.1186/1471-2180-11-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mavrici D., Marakalala M. J., Holton J. M., Prigozhin D. M., Gee C. L., Zhang Y. J., Rubin E. J., and Alber T. (2014) Mycobacterium tuberculosis FtsX extracellular domain activates the peptidoglycan hydrolase, RipC. Proc. Natl. Acad. Sci. U.S.A. 111, 8037–8042 10.1073/pnas.1321812111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rego E. H., Audette R. E., and Rubin E. J. (2017) Deletion of a mycobacterial divisome factor collapses single-cell phenotypic heterogeneity. Nature 546, 153–157 10.1038/nature22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Griffin J. E., Pandey A. K., Gilmore S. A., Mizrahi V., McKinney J. D., Bertozzi C. R., and Sassetti C. M. (2012) Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem. Biol. 19, 218–227 10.1016/j.chembiol.2011.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson L. S., Beech P. L., Real G., Henriques A. O., and Harry E. J. (2006) Requirement for the cell division protein DivIB in polar cell division and engulfment during sporulation in Bacillus subtilis. J. Bacteriol. 188, 7677–7685 10.1128/JB.01072-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daniel R. A., and Errington J. (2000) Intrinsic instability of the essential cell division protein FtsL of Bacillus subtilis and a role for DivIB protein in FtsL turnover. Mol. Microbiol. 36, 278–289 10.1046/j.1365-2958.2000.01857.x [DOI] [PubMed] [Google Scholar]
- 34. Sackett M. J., Kelly A. J., and Brun Y. V. (1998) Ordered expression of ftsQA and ftsZ during the Caulobacter crescentus cell cycle. Mol. Microbiol. 28, 421–434 10.1046/j.1365-2958.1998.00753.x [DOI] [PubMed] [Google Scholar]
- 35. Robson S. A., and King G. F. (2006) Domain architecture and structure of the bacterial cell division protein DivIB. Proc. Natl. Acad. Sci. U.S.A. 103, 6700–6705 10.1073/pnas.0601397103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sánchez-Pulido L., Devos D., Genevrois S., Vicente M., and Valencia A. (2003) POTRA: a conserved domain in the FtsQ family and a class of β-barrel outer membrane proteins. Trends Biochem. Sci. 28, 523–526 10.1016/j.tibs.2003.08.003 [DOI] [PubMed] [Google Scholar]
- 37. van den Ent F., Vinkenvleugel T. M., Ind A., West P., Veprintsev D., Nanninga N., den Blaauwen T., and Löwe J. (2008) Structural and mutational analysis of the cell division protein FtsQ. Mol. Microbiol. 68, 110–123 10.1111/j.1365-2958.2008.06141.x [DOI] [PubMed] [Google Scholar]
- 38. Sureka K., Hossain T., Mukherjee P., Chatterjee P., Datta P., Kundu M., and Basu J. (2010) Novel role of phosphorylation-dependent interaction between FtsZ and FipA in mycobacterial cell division. PLoS One 5, e8590 10.1371/journal.pone.0008590 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Ramos A., Honrubia M. P., Valbuena N., Vaquera J., Mateos L. M., and Gil J. A. (2003) Involvement of DivIVA in the morphology of the rod-shaped actinomycete Brevibacterium lactofermentum. Microbiology 149, 3531–3542 10.1099/mic.0.26653-0 [DOI] [PubMed] [Google Scholar]
- 40. Noirclerc-Savoye M., Le Gouëllec A., Morlot C., Dideberg O., Vernet T., and Zapun A. (2005) In vitro reconstitution of a trimeric complex of DivIB, DivIC and FtsL, and their transient co-localization at the division site in Streptococcus pneumoniae. Mol. Microbiol. 55, 413–424 [DOI] [PubMed] [Google Scholar]
- 41. Masson S., Kern T., Le Gouëllec A., Giustini C., Simorre J. P., Callow P., Vernet T., Gabel F., and Zapun A. (2009) Central domain of DivIB caps the C-terminal regions of the FtsL/DivIC coiled-coil rod. J. Biol. Chem. 284, 27687–27700 10.1074/jbc.M109.019471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Griffin J. E., Gawronski J. D., Dejesus M. A., Ioerger T. R., Akerley B. J., and Sassetti C. M. (2011) High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 7, e1002251 10.1371/journal.ppat.1002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forti F., Crosta A., and Ghisotti D. (2009) Pristinamycin-inducible gene regulation in mycobacteria. J. Biotechnol. 140, 270–277 10.1016/j.jbiotec.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 44. Prisic S., Dankwa S., Schwartz D., Chou M. F., Locasale J. W., Kang C. M., Bemis G., Church G. M., Steen H., and Husson R. N. (2010) Extensive phosphorylation with overlapping specificity by Mycobacterium tuberculosis serine/threonine protein kinases. Proc. Natl. Acad. Sci. U.S.A. 107, 7521–7526 10.1073/pnas.0913482107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fortuin S., Tomazella G. G., Nagaraj N., Sampson S. L., Gey van Pittius N. C., Soares N. C., Wiker H. G., de Souza G. A., and Warren R. M. (2015) Phosphoproteomics analysis of a clinical Mycobacterium tuberculosis Beijing isolate: expanding the mycobacterial phosphoproteome catalog. Front. Microbiol. 6, 6 10.3389/fmicb.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verma R., Pinto S. M., Patil A. H., Advani J., Subba P., Kumar M., Sharma J., Dey G., Ravikumar R., Buggi S., Satishchandra P., Sharma K., Suar M., Tripathy S. P., Chauhan D. S., et al. (2017) Quantitative proteomic and phosphoproteomic analysis of H37Ra and H37Rv strains of Mycobacterium tuberculosis. J. Proteome Res. 16, 1632–1645 10.1021/acs.jproteome.6b00983 [DOI] [PubMed] [Google Scholar]
- 47. Kaval K. G., and Halbedel S. (2012) Architecturally the same, but playing a different game: the diverse species-specific roles of DivIVA proteins. Virulence 3, 406–407 10.4161/viru.20747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buddelmeijer N., Aarsman M. E., Kolk A. H., Vicente M., and Nanninga N. (1998) Localization of cell division protein FtsQ by immunofluorescence microscopy in dividing and nondividing cells of Escherichia coli. J. Bacteriol. 180, 6107–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lenarcic R., Halbedel S., Visser L., Shaw M., Wu L. J., Errington J., Marenduzzo D., and Hamoen L. W. (2009) Localisation of DivIVA by targeting to negatively curved membranes. EMBO J. 28, 2272–2282 10.1038/emboj.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ortiz C., Casanova M., Palacios P., and Vicente M. (2017) The hypermorph FtsA* protein has an in vivo role in relieving the Escherichia coli proto-ring block caused by excess ZapC. PLoS One 12, e0184184 10.1371/journal.pone.0184184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hale C. A., and de Boer P. A. (1997) Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 88, 175–185 10.1016/S0092-8674(00)81838-3 [DOI] [PubMed] [Google Scholar]
- 52. Carson M. J., Barondess J., and Beckwith J. (1991) The FtsQ protein of Escherichia coli: membrane topology, abundance, and cell division phenotypes due to overproduction and insertion mutations. J. Bacteriol. 173, 2187–2195 10.1128/jb.173.7.2187-2195.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dai K., and Lutkenhaus J. (1992) The proper ratio of FtsZ to FtsA is required for cell division to occur in Escherichia coli. J. Bacteriol. 174, 6145–6151 10.1128/jb.174.19.6145-6151.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dewar S. J., Begg K. J., and Donachie W. D. (1992) Inhibition of cell division initiation by an imbalance in the ratio of FtsA to FtsZ. J. Bacteriol. 174, 6314–6316 10.1128/jb.174.19.6314-6316.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rueda S., Vicente M., and Mingorance J. (2003) Concentration and assembly of the division ring proteins FtsZ, FtsA, and ZipA during the Escherichia coli cell cycle. J. Bacteriol. 185, 3344–3351 10.1128/JB.185.11.3344-3351.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Busiek K. K., and Margolin W. (2014) A role for FtsA in SPOR-independent localization of the essential Escherichia coli cell division protein FtsN. Mol. Microbiol. 92, 1212–1226 10.1111/mmi.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ursinus A., van den Ent F., Brechtel S., de Pedro M., Höltje J. V., Löwe J., and Vollmer W. (2004) Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli. J. Bacteriol. 186, 6728–6737 10.1128/JB.186.20.6728-6737.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gerding M. A., Liu B., Bendezú F. O., Hale C. A., Bernhardt T. G., and de Boer P. A. (2009) Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J. Bacteriol. 191, 7383–7401 10.1128/JB.00811-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rowland S. L., Wadsworth K. D., Robson S. A., Robichon C., Beckwith J., and King G. F. (2010) Evidence from artificial septal targeting and site-directed mutagenesis that residues in the extracytoplasmic β domain of DivIB mediate its interaction with the divisomal transpeptidase PBP 2B. J. Bacteriol. 192, 6116–6125 10.1128/JB.00783-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spratt B. G. (1977) Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J. Bacteriol. 131, 293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ricard M., and Hirota Y. (1973) Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J. Bacteriol. 116, 314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kieser K. J., Boutte C. C., Kester J. C., Baer C. E., Barczak A. K., Meniche X., Chao M. C., Rego E. H., Sassetti C. M., Fortune S. M., and Rubin E. J. (2015) Phosphorylation of the peptidoglycan synthase PonA1 governs the rate of polar elongation in mycobacteria. PLoS Pathog. 11, e1005010 10.1371/journal.ppat.1005010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Y. J., Reddy M. C., Ioerger T. R., Rothchild A. C., Dartois V., Schuster B. M., Trauner A., Wallis D., Galaviz S., Huttenhower C., Sacchettini J. C., Behar S. M., and Rubin E. J. (2013) Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell 155, 1296–1308 10.1016/j.cell.2013.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Samten B., Fannin S., Sarva K., Yi N., Madiraju M., and Rajagopalan M. (2016) Modulation of human T cell cytokines by the Mycobacterium tuberculosis-secreted protein Wag31. Tuberculosis 101S, S99–S104 10.1016/j.tube.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 65. Dai K., Xu Y., and Lutkenhaus J. (1996) Topological characterization of the essential Escherichia coli cell division protein FtsN. J. Bacteriol. 178, 1328–1334 10.1128/jb.178.5.1328-1334.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Katis V. L., and Wake R. G. (1999) Membrane-bound division proteins DivIB and DivIC of Bacillus subtilis function solely through their external domains in both vegetative and sporulation division. J. Bacteriol. 181, 2710–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goehring N. W., Petrovska I., Boyd D., and Beckwith J. (2007) Mutants, suppressors, and wrinkled colonies: mutant alleles of the cell division gene ftsQ point to functional domains in FtsQ and a role for domain 1C of FtsA in divisome assembly. J. Bacteriol. 189, 633–645 10.1128/JB.00991-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. D'Ulisse V., Fagioli M., Ghelardini P., and Paolozzi L. (2007) Three functional subdomains of the Escherichia coli FtsQ protein are involved in its interaction with the other division proteins. Microbiology 153, 124–138 10.1099/mic.0.2006/000265-0 [DOI] [PubMed] [Google Scholar]
- 69. Thakur M., and Chakraborti P. K. (2006) GTPase activity of mycobacterial FtsZ is impaired due to its transphosphorylation by the eukaryotic-type Ser/Thr kinase, PknA. J. Biol. Chem. 281, 40107–40113 10.1074/jbc.M607216200 [DOI] [PubMed] [Google Scholar]
- 70. Kang C. M., Nyayapathy S., Lee J. Y., Suh J. W., and Husson R. N. (2008) Wag31, a homologue of the cell division protein DivIVA, regulates growth, morphology and polar cell wall synthesis in mycobacteria. Microbiology 154, 725–735 10.1099/mic.0.2007/014076-0 [DOI] [PubMed] [Google Scholar]
- 71. Chawla Y., Upadhyay S., Khan S., Nagarajan S. N., Forti F., and Nandicoori V. K. (2014) Protein kinase B (PknB) of Mycobacterium tuberculosis is essential for growth of the pathogen in vitro as well as for survival within the host. J. Biol. Chem. 289, 13858–13875 10.1074/jbc.M114.563536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nagarajan S. N., Upadhyay S., Chawla Y., Khan S., Naz S., Subramanian J., Gandotra S., and Nandicoori V. K. (2015) Protein kinase A (PknA) of Mycobacterium tuberculosis is independently activated and is critical for growth in vitro and survival of the pathogen in the host. J. Biol. Chem. 290, 9626–9645 10.1074/jbc.M114.611822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sharma A. K., Arora D., Singh L. K., Gangwal A., Sajid A., Molle V., Singh Y., and Nandicoori V. K. (2016) Serine/threonine protein phosphatase PstP of Mycobacterium tuberculosis is necessary for accurate cell division and survival of pathogen. J. Biol. Chem. 291, 24215–24230 10.1074/jbc.M116.754531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kang C. M., Abbott D. W., Park S. T., Dascher C. C., Cantley L. C., and Husson R. N. (2005) The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev. 19, 1692–1704 10.1101/gad.1311105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee J. J., Kan C. M., Lee J. H., Park K. S., Jeon J. H., and Lee S. H. (2014) Phosphorylation-dependent interaction between a serine/threonine kinase PknA and a putative cell division protein Wag31 in Mycobacterium tuberculosis. New Microbiol. 37, 525–533 [PubMed] [Google Scholar]
- 76. Datta P., Dasgupta A., Bhakta S., and Basu J. (2002) Interaction between FtsZ and FtsW of Mycobacterium tuberculosis. J. Biol. Chem. 277, 24983–24987 10.1074/jbc.M203847200 [DOI] [PubMed] [Google Scholar]
- 77. Plocinski P., Arora N., Sarva K., Blaszczyk E., Qin H., Das N., Plocinska R., Ziolkiewicz M., Dziadek J., Kiran M., Gorla P., Cross T. A., Madiraju M., and Rajagopalan M. (2012) Mycobacterium tuberculosis CwsA interacts with CrgA and Wag31, and the CrgA-CwsA complex is involved in peptidoglycan synthesis and cell shape determination. J. Bacteriol. 194, 6398–6409 10.1128/JB.01005-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gupta S., Banerjee S. K., Chatterjee A., Sharma A. K., Kundu M., and Basu J. (2015) Essential protein SepF of mycobacteria interacts with FtsZ and MurG to regulate cell growth and division. Microbiology 161, 1627–1638 10.1099/mic.0.000108 [DOI] [PubMed] [Google Scholar]
- 79. Pandey A. K., Raman S., Proff R., Joshi S., Kang C. M., Rubin E. J., Husson R. N., and Sassetti C. M. (2009) Nitrile-inducible gene expression in mycobacteria. Tuberculosis 89, 12–16 10.1016/j.tube.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Soni V., Upadhayay S., Suryadevara P., Samla G., Singh A., Yogeeswari P., Sriram D., and Nandicoori V. K. (2015) Depletion of M. tuberculosis GlmU from infected murine lungs effects the clearance of the pathogen. PLoS Pathog. 11, e1005235 10.1371/journal.ppat.1005235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ghosh J., Larsson P., Singh B., Pettersson B. M., Islam N. M., Sarkar S. N., Dasgupta S., and Kirsebom L. A. (2009) Sporulation in mycobacteria. Proc. Natl. Acad. Sci. U.S.A. 106, 10781–10786 10.1073/pnas.0904104106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shevchenko A., Tomas H., Havlis J., Olsen J. V., and Mann M. (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 10.1038/nprot.2006.468 [DOI] [PubMed] [Google Scholar]
- 83. Rajanala K., Sarkar A., Jhingan G. D., Priyadarshini R., Jalan M., Sengupta S., and Nandicoori V. K. (2014) Phosphorylation of nucleoporin Tpr governs its differential localization and is required for its mitotic function. J. Cell Sci. 127, 3505–3520 10.1242/jcs.149112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tiwari D., Singh R. K., Goswami K., Verma S. K., Prakash B., and Nandicoori V. K. (2009) Key residues in Mycobacterium tuberculosis protein kinase G play a role in regulating kinase activity and survival in the host. J. Biol. Chem. 284, 27467–27479 10.1074/jbc.M109.036095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jain P., Hsu T., Arai M., Biermann K., Thaler D. S., Nguyen A., González P. A., Tufariello J. M., Kriakov J., Chen B., Larsen M. H., and Jacobs W. R. Jr. (2014) Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. MBio 5, e01245–14 10.1128/mBio.01245-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Khan M. Z., Bhaskar A., Upadhyay S., Kumari P., Rajmani R. S., Jain P., Singh A., Kumar D., Bhavesh N. S., and Nandicoori V. K. (2017) Protein kinase G confers survival advantage to Mycobacterium tuberculosis during latency-like conditions. J. Biol. Chem. 292, 16093–16108 10.1074/jbc.M117.797563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arora D., Chawla Y., Malakar B., Singh A., and Nandicoori V. K. (2018) The transpeptidase PbpA and noncanonical transglycosylase RodA of Mycobacterium tuberculosis play important roles in regulating bacterial cell lengths. J. Biol. Chem. 293, 6497–6516 10.1074/jbc.M117.811190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Song H., Sandie R., Wang Y., Andrade-Navarro M. A., and Niederweis M. (2008) Identification of outer membrane proteins of Mycobacterium tuberculosis. Tuberculosis 88, 526–544 10.1016/j.tube.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.