Abstract
The integrin leukocyte function–associated antigen-1 (LFA-1) plays a pivotal role in leukocyte adhesion and migration, but the mechanism(s) by which this integrin is regulated has remained incompletely understood. LFA-1 integrin activity requires phosphorylation of its β2-chain and interactions of its cytoplasmic tail with various cellular proteins. The α-chain is constitutively phosphorylated and necessary for cellular adhesion, but how the α-chain regulates adhesion has remained enigmatic. We now show that substitution of the α-chain phosphorylation site (S1140A) in T cells inhibits the phosphorylation of the functionally important Thr-758 in the β2-chain, binding of α-actinin and 14-3-3 protein, and expression of an integrin-activating epitope after treatment with the stromal cell–derived factor-1α. The presence of this substitution resulted in a loss of cell adhesion and directional cell migration. Moreover, LFA-1 activation through the T-cell receptor in cells expressing the S1140A LFA-1 variant resulted in less Thr-758 phosphorylation, α-actinin and talin binding, and cell adhesion. The finding that the LFA-1 α-chain regulates adhesion through the β-chain via specific phosphorylation at Ser-1140 in the α-chain has not been previously reported and emphasizes that both chains are involved in the regulation of LFA-1 integrin activity.
Keywords: integrin, phosphorylation, leukocyte, adhesion, talin, 14-3-3 protein, filamin, alpha-actinin
Introduction
Integrins are transmembrane heterodimeric receptors that communicate in two directions across the plasma membrane and mediate interactions with other cells and the extracellular environment (1). Integrins can bind ligands, resulting in outside-in signaling, whereas inside-out signaling is initiated by ligand binding to nonintegrin receptors, such as chemokine receptors or the T-cell receptor (TCR),4 which activate integrins through intracellular signaling (2, 3). The family of leukocyte-specific β2-integrins consists of four members that have a common β2-chain (CD18) and one of the α-chains (αL, CD11a; αM, CD11b; αX, CD11c; and αD, CD11d). The leukocyte function-associated antigen-1 heterodimer (LFA-1, αLβ2, CD11a/CD18) is primarily expressed on lymphocytes and binds to intercellular adhesion molecules (ICAMs). Mac-1 (macrophage 1 antigen, αMβ2, CD11b/CD18) is enriched in the myeloid lineage and is able to bind numerous ligands, among them ICAMs and complement protein iC3b. Complement receptor 4 (CR4, αXβ2, CD11c/CD18, p150,95) is expressed in monocytes, macrophages, and dendritic cells, as well as in some subsets of activated T and B cells. It is also capable of binding various ligands, including extracellular matrix molecules, cellular and soluble ligands, and denatured proteins (4, 5).
Integrins exist in at least three different conformations: closed, extended, and extended open, each of which possesses different ligand binding affinities and localization in cells. Integrins can modulate their adhesive properties within seconds. The activity of the integrins is for the main part regulated by the binding of different proteins to the cytoplasmic tails, which is mediated by integrin phosphorylation, clustering, and receptor cross-talk (6, 7).
The leukocyte integrin α-chains are constitutively phosphorylated, whereas the β2-chain becomes phosphorylated after activation through chemokines, the TCR, or phorbol esters (8–12). The integrin cytoplasmic domains are short and devoid of catalytic activity. Only a few cytoplasmic proteins have been found to specifically bind to the integrin α-chain cytoplasmic tails (13), whereas signaling, adaptor, and cytoskeletal linker proteins, including talin, kindlins, filamin, α-actinin, and 14-3-3 proteins, bind to the β2-integrin tails (14). The integrin β-chain cytoplasmic domains contain three conserved regions: the two NPX(Y/F) sequences and the serine/threonine sequence between them. Mutation of the β2-chain threonines abrogated cell adhesion, but initial experiments did not reveal phosphorylation of these (15). By using the phosphatase inhibitor okadaic acid, strong threonine phosphorylation was observed after activation (9–11). The β2-chain is phosphorylated by protein kinase C (PKC) enzymes (16). The phosphorylation of Thr-758 on β2 leads to release of bound filamin and promotes binding of 14-3-3 proteins. Talin can bind both to the Thr-758 phosphorylated and unphosphorylated chain (17). The regulation of the association between α-actinin and the β2-chain is currently poorly understood, but it has been shown that α-actinin binding is enhanced by the activation of neutrophils and T cells (18, 19).
The leukocyte β2-integrin α-chains are phosphorylated on Ser-1140 (αL), Ser-1126 (αM), and Ser-1158 (αX) (see Fig. 1A), whereas αD phosphorylation has not been studied. The α-chain phosphorylation is important for leukocyte adhesion and intracellular signaling (20–22), but the mechanism(s) has remained unknown. We have now focused on the LFA-1 integrin and show that through the phosphorylation of the α-chain, it regulates the phosphorylation, conformation, and protein binding to the β2-chain in a specific manner. When cells expressed the LFA-1 S1140A mutation, there was no phosphorylation of the β2-chain on Thr-758, but there was a loss of both α-actinin and 14-3-3 binding and the presence of the integrin activation-reporter epitope L16 upon treatment with the chemokine stromal cell–derived factor-1α (SDF-1α). Binding of filamin increased. These mutant cells displayed abrogated cell adhesion to the LFA-1 ligand ICAM-1 and impaired directional migration. When LFA-1 S1140A mutated cells were activated through the TCR, the β2 phosphorylation and the binding of α-actinin to β2 decreased as compared with WT cells, whereas the presence of the activation epitope L16 was reduced only slightly as compared with WT cells. This decreased cell adhesion to ICAM-1. The results indicate an important role for integrin α-chains in the modulation of leukocyte functions by regulating β-chain phosphorylation, which results in altered cytoplasmic protein interactions, followed by changes in adhesion and migration.
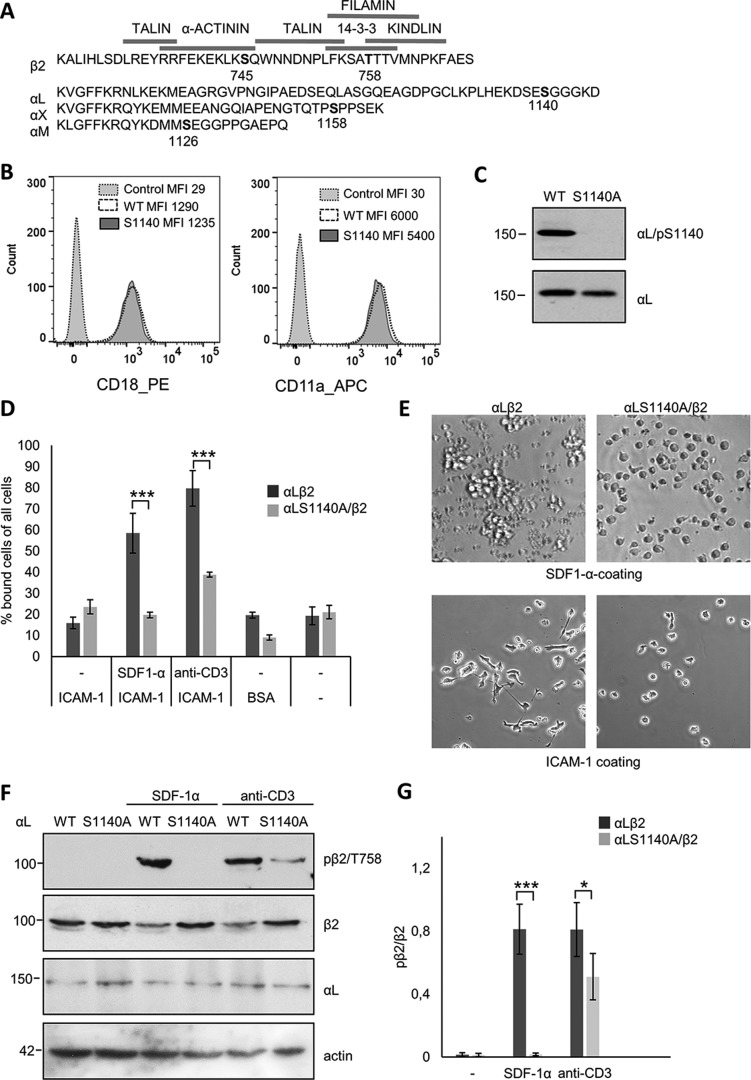
Figure 1.
Phosphorylation of Ser-1140 on αL regulates phosphorylation of β2 on Thr-758. A, amino acid sequence of the β2, αL, αM, and αX chains with the known phosphorylation sites in bold and the binding domains of talin, α-actinin, kindling, and 14-3-3 indicated. B, Jβ2.7 cells lacking αL expression (control) or expressing LFA-1 WT or S1140A were stained with β2 (CD18-PE) or αL (CD11a-APC) and analyzed by flow cytometry. Mean fluorescence intensity (MFI) is shown for each graph. C, Jβ2.7 cells expressing αL WT or S1140A were analyzed by Western blotting and immunoblotted with αL/pS1140 or αL antibodies. D, Jβ2.7 cells expressing αL WT or S1140A were activated with SDF-1α or anti-CD3 and allowed to adhere to ICAM-1, and bound cells were quantified. E, in the upper panels, Jβ2.7 cells expressing αL WT or αL S1140A were allowed to adhere to a SDF-1α–coated surface. In the lower panels, SDF-1α–activated cells were allowed to adhere to ICAM-1 and spread for 1 h. F, lysates of Jβ2.7 cells expressing αL WT or S1140A were activated with SDF-1α or anti-CD3, analyzed by SDS-PAGE, and immunoblotted with β2/Thr(P)-758, β2, αL, or actin as a loading control. G, the amount of β2/Thr(P)-758 per β2 was quantified from five separate experiments. Molecular mass markers (kDa) are shown to the left of the blots. *, p < 0.05; ***, p < 0.005.
Results
Phosphorylation of the LFA-1 α-chain regulates the phosphorylation of the β2-chain on Thr-758
Cytoplasmic protein interactions with the integrin β2-chain have been extensively studied, but the regulation of these has remained incompletely understood. We now show how the α-chain of LFA-1 regulates integrin functions by affecting the β2-chain. We used the Jβ2.7 human Jurkat T cell line expressing equal amounts of WT LFA-1 or LFA-1 containing the mutation S1140A at the known α-chain phosphorylation site (Fig. 1B). We have previously shown that this is the only phosphorylation site on the αL-chain and that a significant subset (∼40%) of αL Ser-1140 is phosphorylated in both activated and nonactivated cells (9, 20). When the phosphorylation site serine was substituted by alanine, phosphorylation could not be recognized by a Ser(P)-1140–specific antibody (Fig. 1C). Cells expressing WT LFA-1 could bind ICAM-1 after activation by chemokines such as SDF-1α or through the TCR. Cells expressing the αL S1140A mutant were unable to bind ICAM-1 after SDF-1α activation and showed much reduced binding after activation with anti-CD3 (Fig. 1D). WT cells aggregated on an SDF-1α–coated surface and SDF-1α–activated cells spread on ICAM-1, which was not seen with cells expressing the LFA-1 S1140A mutant (Fig. 1E).
Phosphorylation of the β2-chain Thr-758 is seen only after activation, e.g. by SDF-1α or through the TCR (9–11). In LFA-1 WT expressing cells, there was no phosphorylation of Thr-758 on the β2-chain without activation, but after treatment with SDF-1α or TCR, a strong label was seen. Importantly, no phosphorylation occurred on β2 Thr-758 in cells expressing the αL S1140A mutant α-chain activated with SDF-1α. In cells activated through the TCR, a significantly weaker labeling of the Thr-758 was obtained in mutant cells as compared with WT cells (Fig. 1, F and G).
Phosphorylation of Ser-1140 on the LFA-1 α-chain affects the binding of cytoskeletal proteins to the β2-chain
We further tested whether the α-chain mutation affects the interaction with proteins known to bind to the β2-chain and to be involved in cell adhesion. Fig. 2A shows immunoprecipitations from Jurkat cells under three conditions; nonactivated, SDF-1α–activated, or anti-CD3–activated cells. Western blots of supernatants showed equal amounts of proteins in WT and S1140A-expressing cells. Proteins coprecipitated with LFA-1 in the WT and mutant cell line are shown in the IP IB4 lane and quantified from three separate experiments as shown below (Fig. 2B). A significantly reduced binding of α-actinin and 14-3-3 was seen in SDF-1α–activated mutant cells compared with WT cells. Talin binding was consistently reduced, but not significantly. Filamin binding was increased. Activation of the TCR by anti-CD3 resulted in a significant reduction in talin binding. α-Actinin and 14-3-3 binding was reduced, but not significantly (Fig. 2, A and B).
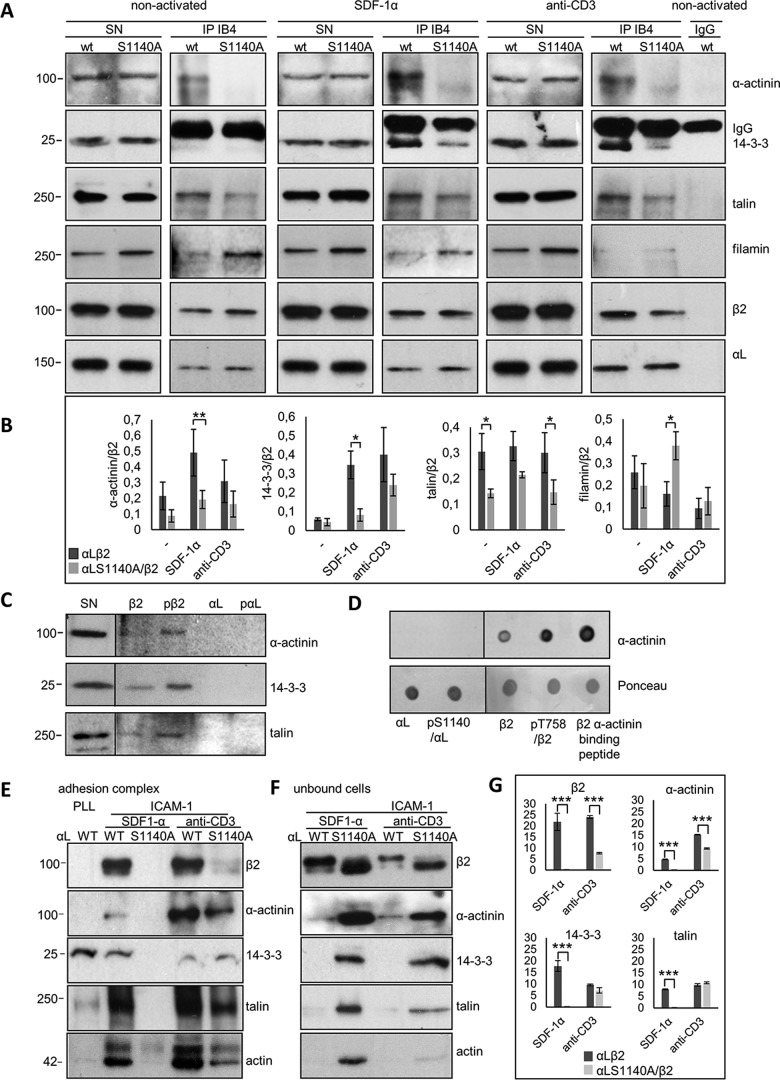
Figure 2.
α-Chain phosphorylation regulates binding of cytoplasmic interaction partners to β2 differently. A, lysates of Jβ2.7 cells expressing αL WT or S1140A were not treated or activated with SDF-1α or anti-CD3, immunoprecipitated with the LFA-1 antibody IB4, and immunoblotted for α-actinin, 14-3-3, talin, filamin, β2, or αL. B, the amount of coprecipitated proteins per immunoprecipitated β2 was quantified from three separate experiments. C, full-length cytoplasmic peptides of αL, αL/Ser(P)-1140, β2, or β2/Thr(P)-758 were bound to beads and Jurkat cell lysate added. Bound proteins were analyzed by immunoblotting with indicated antibodies. D, peptides were spotted on nitrocellulose, and purified α-actinin was added. After washing, binding was assessed with the α-actinin antibody. E and F, Jβ2.7 cells expressing αL or αL S1140A were allowed to adhere on poly-l-lysine or ICAM-1 and adhesion complexes (E) or unbound cells (F) collected, and lysates were analyzed by immunoblotting using indicated antibodies. G, the amount of protein found in the adhesion complexes was quantified from three separate experiments. SN, supernatant. Molecular mass markers (kDa) are shown to the left of the blots. Lines depict borders between two separate gels. *, p < 0.05; **, p < 0.01.
The most significant difference between αL WT and S1140A-expressing cells after SDF-1α was seen for α-actinin binding to β2. The α-actinin–binding site on the LFA-1 integrin has been mapped to the β2-chain amino acids 736–745, partly overlapping with the talin membrane-proximal binding site (23) (Fig. 1A). To verify that there is no α-actinin-binding site on αL, synthetic peptides of the cytoplasmic chains of αL, αL/Ser(P)-1140, β2, or β2/Thr(P)-758 were linked to Sepharose beads, and Jurkat cell lysates were added. Bound proteins were separated by SDS-PAGE and analyzed by immunoblotting for α-actinin, 14-3-3, and talin (Fig. 2C). In another experiment, peptides were spotted on a nitrocellulose membrane, and purified α-actinin was added. After extensive washing, protein binding was determined with specific antibodies. Experiments verified that α-actinin binds to the β2 or β2/Thr(P)-758 peptides but not to αL or αL/Ser(P)-1140 (Fig. 2D).
To study the differences in LFA-1-mediated adhesion complex formation in cells adhering to the ligand, the complexes from Jurkat cells binding to coated ICAM-1 or poly-l-lysine were purified, and their protein content was analyzed. Proteins found in the adhesion sites of LFA-1 WT-expressing cells and bound to ICAM-1 included the integrin β2 and αL chains, α-actinin, 14-3-3, and talin, after both SDF-1α and anti-CD3 activation. In contrast, ICAM-1 adhesion complexes from LFA-1 S1140A-expressing cells contained none of the proteins found in WT LFA-1–expressing cells activated with SDF-1α, indicating that stable adhesion complexes were not formed (Fig. 2E). Instead, these proteins were detected in the Western blotting of unbound cells (Fig. 2F). After anti-CD3 activation of S1140A-expressing cells, the same proteins were present in the ICAM-1 adhesion complexes, although less than in WT LFA-1–expressing cells. The proteins in the adhesion complexes were quantified from three separate experiments (Fig. 2G). This result indicates that Jβ2.7 LFA-1 S1140A-expressing cells can be at least partly activated to bind ligand by anti-CD3, which is not the case for SDF-1α activation (Figs. 1, D and E, and 2E). Jβ2.7 Jurkat cells lacking LFA-1 expression did not bind to ICAM-1, which indicates that binding takes place solely through LFA-1 (not shown).
A negative charge on both the α- and β2-chain phosphorylation sites increases binding of α-actinin to LFA-1
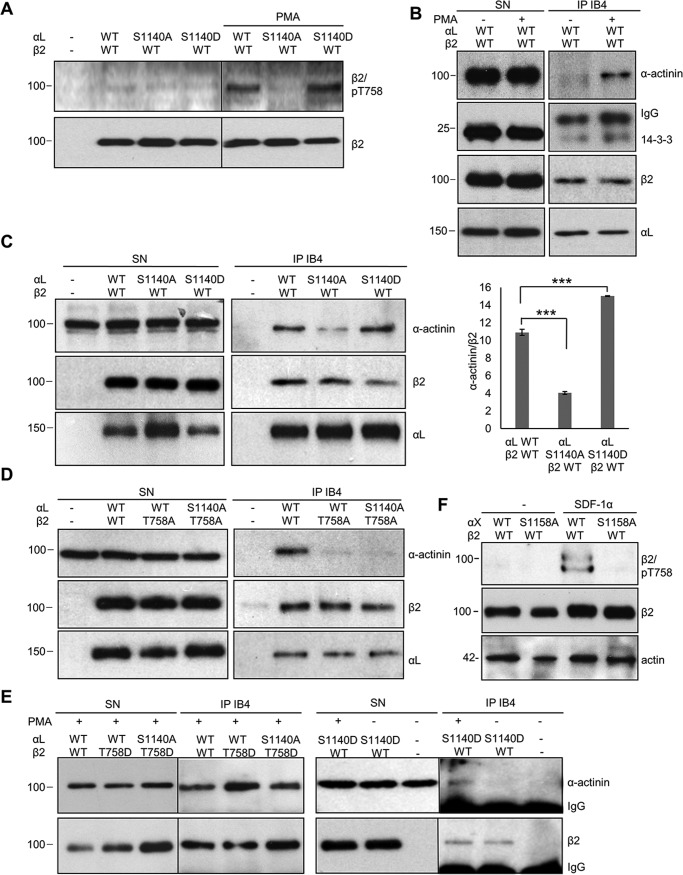
We were not able to transfect Jβ2.7 cells with the αL S1140D mutant and therefore used COS7 cells, which have been shown to express functional heterodimeric integrins on their cell surface after transfection with β2-integrins (24). The αL-chain is phosphorylated in COS7 cells on Ser-1140 (20). COS7 cells do not contain the SDF-1α receptor or TCR, but integrins can be activated by phorbol esters. PMA activation of COS7 cells transfected with WT LFA-1 led to increased Thr-758 phosphorylation on β2 (Fig. 3A) and increased α-actinin and 14-3-3 binding (Fig. 3B). COS7 cells were then transfected with WT αL, the nonphosphorylatable S1140A or the S1140D mutant that mimics phosphorylated Ser-1140. β2 Thr-758 was phosphorylated in cells expressing the S1140D mutant but not in cells expressing S1140A (Fig. 3A). Lysates from PMA-activated cells were immunoprecipitated with the LFA-1 antibody IB4, and the precipitates were immunoblotted for α-actinin, β2, and αL. Binding of α-actinin to LFA-1 was reduced in LFA-1 S1140A-expressing cells in accordance with the results from Jβ2.7 cells. The LFA-1 S1140D mutation resembled that of WT LFA-1 phosphorylated on Ser-1140, supporting the model that the negative charge on the α-chain is important for β2 Thr-758 phosphorylation, enabling stronger α-actinin binding (Fig. 3C). Mutation of Thr-758 to alanine resulted in decreased binding of α-actinin (Fig. 3D). Cells expressing β2 T758D and the S1140A αL-chain were able to bind α-actinin; however, binding was further increased when a negative charge was present on both chains, represented by cells expressing αL WT Ser-1140 and β2 T758D. αL S1140D-expressing cells did not bind α-actinin without PMA activation (Fig. 3E). This verifies that the negative charge on the αL-chain Ser-1140 is important for β2 Thr-758 phosphorylation and for increased α-actinin binding, which requires the phosphorylated β2 Thr-758.
Figure 3.
Phosphorylations (negative charges) on both the α- and the β2-chains regulate α-actinin binding to LFA-1. A, COS7 cells were transfected with WT αL, αL/S1140A, or αL/S1140D together with WT β2 and cells activated with PMA or left untreated. Lysates were immunoblotted for β2/Thr(P)-758 or β2, showing that PMA activation increases β2 Thr-758 phosphorylation in WT αL and αL/S1140D-expressing cells. B, lysates of PMA-activated cells expressing WT LFA-1 were immunoprecipitated with the LFA-1 antibody IB4, and precipitates were immunoblotted for α-actinin, 14-3-3, β2, or αL. C, COS7 cells were transfected with the αL WT, or the S1140A or S1140D mutant, and β2 and lysates were immunoprecipitated with the LFA-1 antibody IB4, and precipitates were immunoblotted for α-actinin, β2, or αL. The amount of coprecipitated α-actinin per immunoprecipitated β2 was quantified from three separate experiments. D, COS7 cells were transfected with LFA-1 WT or the phosphorylation mutants αL/S1140A or β2/T758A; lysates were immunoprecipitated with IB4; and precipitates were immunoblotted for α-actinin, β2, or αL. E, COS7 cells were transfected with the WT αL, αL/S1140A, or αL/S1140D and WT β2 or β2/T758D; activated with PMA; or left untreated and analyzed as above. F, K562 cells were transfected with αX WT or the phosphorylation mutant S1158A and β2, and lysates were immunoblotted with β2/Thr(P)-758 or β2. The amount of β2/Thr(P)-758 per β2 was quantified from three separate experiments. SN, supernatant. Molecular mass markers (kDa) are shown to the left of the blots. ***, p < 0.005.
We then extended the study to another α-chain, αXβ2. This integrin is expressed in myeloid cells. We therefore stably expressed αXβ2 in the myeloid/erythroleukemic cell line K562. Activation of these cells with SDF-1α resulted in phosphorylation of β2 Thr-758 in WT αXβ2-expressing cells, whereas no phosphorylation of β2 Thr-758 was detected in cells expressing the α-chain phosphorylation mutant αX S1158A (Fig. 3F). This result further emphasizes the role of α-chain phosphorylation for β2-chain phosphorylation. The double band could mean that some β2-chains are phosphorylated on additional sites (16).
Phosphorylation of αL affects the cellular localization of α-actinin and the activation epitope of LFA-1
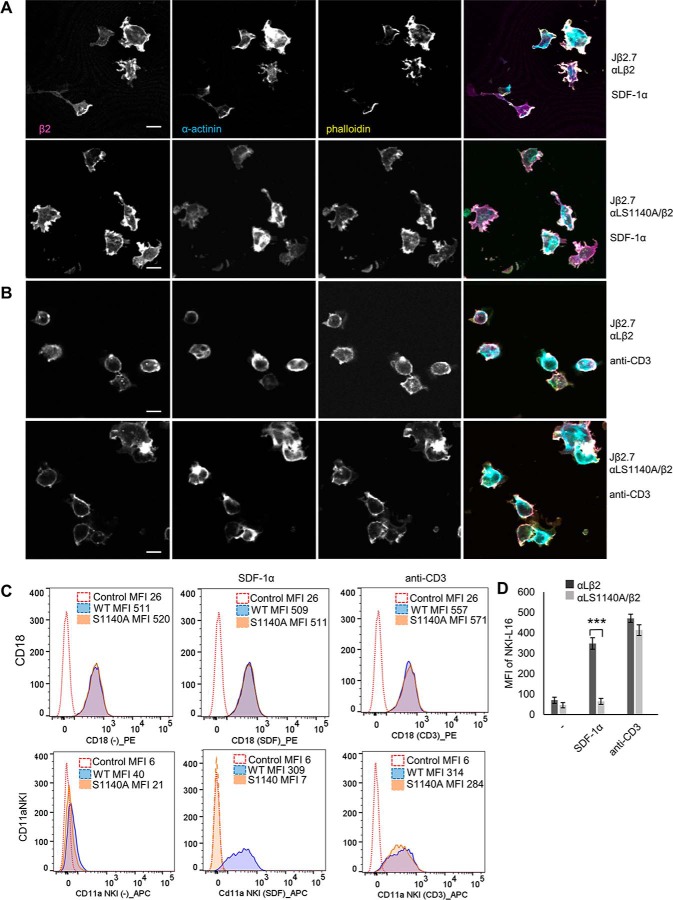
We next studied whether there is a difference in the localization of α-actinin in cells expressing LFA-1 WT or S1140A. The cells were activated with SDF-1α or anti-CD3 and allowed to adhere to ICAM-1, fixed, and stained for β2, for α-actinin, or by phalloidin (Fig. 4, A, and B). In cells expressing WT LFA-1, α-actinin localized to the membrane and ruffling edges and colocalized with β2 and phalloidin both in SDF-1α and anti-CD3 activated cells (Pearson's correlation coefficient for β2 and α-actinin at the membrane was 0.78 for SDF-1α and 0.70 for anti-CD3 activation). Colocalization was also seen in anti-CD3–activated cells expressing the S1140A mutant, but less in mutant cells activated with SDF-1α where α-actinin staining was more diffused (Pearson's correlation coefficient for β2 and α-actinin at the membrane was 0.47 for SDF-1α and 0.69 for anti-CD3 activation).
Figure 4.
Phosphorylation of αL affects α-actinin localization. Jβ2.7 cells expressing αL WT or S1140A were activated with SDF-1α (A) or anti-CD3 (B), allowed to adhere to ICAM-1, fixed, and stained for β2, α-actinin, and phalloidin. The scale bar represents 10 μm (C). Jβ2.7 cells lacking αL expression (control) or cells expressing αL WT or S1140A were left untreated or activated with SDF-1α or anti-CD3. The cells were stained for β2 (CD18-PE) or NKI-L16–APC recognizing the activated form of the integrin and analyzed by flow cytometry. Mean fluorescence intensity (MFI) is shown for each graph and quantification of mean fluorescence intensity from three separate experiments (D). ***, p < 0.005.
The effect of αL S1140A phosphorylation on LFA-1 activation was next identified by labeling cells with the NKI-L16 antibody recognizing the fully active epitope L16 of LFA-1 (25). Cells expressing the WT LFA-1 activated with SDF-1α were strongly positive for the L16 epitope, which was not detected in the S1140A-expressing cells. In contrast, L16 positivity was detected after anti-CD3 activation of S1140A-expressing cells (Fig. 4, C and D).
Phosphorylation of the LFA-1 α- and β-chains and binding of α-actinin are needed for chemotaxis
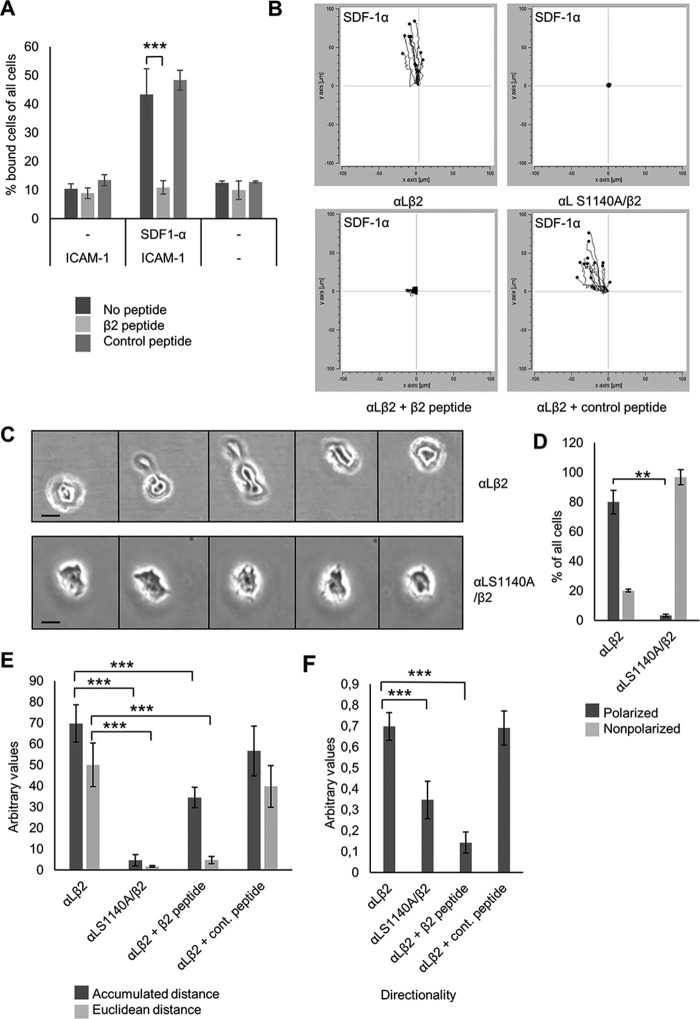
The linking of integrins to the actin cytoskeleton by α-actinin is important for cell adhesion and migration (26). Cell adhesion was studied by transfecting Jurkat cells expressing LFA-1 with a β2-peptide containing the α-actinin–binding site of β2, which blocks the interaction between α-actinin and the β2-chain but does not affect talin binding (19). Static adhesion to ICAM-1 was inhibited by β2-peptide transfection into cells activated by SDF-1α, but not by the control peptide (Fig. 5A). To analyze the role of the α-actinin/integrin interaction in migration, Jurkat cells expressing WT LFA-1 or LFA-1 S1140A were allowed to migrate over an ICAM-1–coated surface toward SDF-1α. In the mutant cells, with reduced interaction between the integrin and α-actinin, directional migration was impaired (Fig. 5B and videos S1 and S2). Cells transfected with the β2 peptide, but not the control peptide, were also unable to migrate (Fig. 5B). Mutant cells were not able to strongly adhere to ICAM-1 and could be washed off by pipetting, which was not the case for WT cells. The mutant cells formed extensions but did not spread or become polarized to the same extent as WT cells and were not able to move toward the chemokine gradient. Instead they ruffled in different directions, but the cell body remained stationary (Fig. 5, C and D). Both the accumulated and euclidean distances, as well as directionality, were reduced in cells with impaired α-actinin–integrin binding (Fig. 5E).
Figure 5.
Phosphorylation of αL and α-actinin binding affect cell migration toward the chemokine SDF-1α. A, Jβ2.7 cells expressing αL WT or S1140A with or without SDF-1α activation transfected with the β2-peptide or control peptide were allowed adhere on ICAM-1, and bound cells were quantified. B, Jβ2.7 cells expressing αL WT or S1140A were allowed to migrate over an ICAM-1 covered surface toward the chemokine SDF-1α. Tracks of 15 migrating cells of one representative experiment are shown. C, bright-field images of migrating Jβ2.7 cells expressing αL WT or S1140A at five different time points (pictures taken at 30-min intervals). The scale bar represents 10 μm. D, quantification of polarized and nonpolarized cells from 20 screens. E and F, accumulated and euclidean distance (E) and directionality of migration (F) were quantified by a chemotaxis and migration tool for 50 cells from three experiments. **, p < 0.01; ***, p < 0.005.
Discussion
Integrin activity has to be tightly regulated to allow rapid and precise changes between different activation states. The roles of the β-chain cytoplasmic tails have been extensively studied and shown to be essential for integrin function. Chemokines, such as SDF-1α, bind to their receptors resulting in phospholipase C activation followed by signaling through CalDag, Rap1, and RapL (27). The proximal signaling events from the TCR are different and include the Lck tyrosine kinase (28), which then phosphorylates the ZAP-70 kinase, and phospholipase Cγ (27). PKC enzymes then become activated in both pathways and phosphorylate Thr-758 on β2. Phosphorylation of the β2-chain is known to regulate the affinity for different binding proteins (7). Filamin binds primarily to the unphosphorylated β2-chain, whereas β2 phosphorylated on Thr-758 binds 14-3-3 proteins and initiates intracellular signaling through Tiam1–Rac1 and inhibitory signaling to α4β1 (12, 17, 29, 30).
Previous work indicates a regulatory role for the membrane-proximal part of α-chain cytoplasmic domains in integrin regulation. The proximal portions of both α and β cytoplasmic domains are α-helical and associate with each other (31). The GFFKR motif in the α-chain is well conserved and is important in keeping the integrins in a nonadhesive state by interacting by ionic bonds with the β-chain. When this motif is deleted or mutated, integrins become active (32–37). Binding of talin can perturb the membrane-proximal association, disrupting the intersubunit interactions, which results in activation (38, 39). In fact, most of the known negative regulators of integrins bind to the conserved membrane-proximal site (40–42).
The α-chain membrane distal regions vary in lengths and sequences, and their roles in integrin activation have been less defined. Deletion of the membrane distal regions of some α-chains affects cell adhesion and spreading, but similar deletions in other α-chains have no effect on adhesion (15, 43–49). This shows that there are different regulatory roles of the membrane distal regions among different integrins. Interestingly, deletion of the αL membrane distal part diminishes talin- and kindlin-induced integrin conformational change and ligand binding (50). We have previously shown that β2-integrin α-chain phosphorylation is needed for adhesion after activation induced by chemokine, ligand, or active Rap1 (20–22). The regulatory mechanisms have not been known. We now show that phosphorylation of the α-chain of LFA-1 is needed for β2-chain phosphorylation and its protein interactions.
A significant part of the LFA-1 α-chain is phosphorylated on the single site Ser-1140 (9, 20), and mutation of the serine residue to alanine inhibited or reduced phosphorylation of Thr-758 in the β2-chain. Treatment with the phosphatase inhibitor okadaic acid increased α-chain phosphorylation (9), indicating that there is a cycle of phosphorylation and dephosphorylation events of the α-chain. This could, in turn, regulate the phosphorylation of Thr-758 of the β2-chain, enabling rapid cell adhesion and deadhesion for example during movement. We extended the study to include another α-chain, αX, which is known to be phosphorylated on Ser-1158 (22). Phosphorylation of β2 Thr-758 was impaired also in myeloid/erythroid K562 cells expressing αX S1158A.
Although activation of cells with both SDF-1α and through the TCR results in phosphorylation of Thr-758 on β2, differences were seen between the LFA-1 WT and S1140A-expressing cells. LFA-1 β2 could not be phosphorylated on Thr-758, nor bind to ICAM-1 in mutant cells activated with the chemokine, but showed only a reduced phosphorylation compared with WT after anti-CD3 activation. This reflects the differences in signaling downstream of the TCR and chemokine receptor and indicates that additional factors play a role in the regulation of β2 phosphorylation. In WT expressing cells, Thr-758 on β2 is readily phosphorylated upon SDF-1α treatment, and S1140D is functionally similar evidently because of the negative charge at position Ser-1140.
The S1140A α-chain phosphorylation mutation markedly decreased α-actinin binding to the β2-chain and impaired cell migration toward the SDF-1α chemokine. Decreased binding was also seen with 14-3-3 and an increase in binding of filamin, which is most likely a cause of decreased Thr-758 phosphorylation in the mutant cell line. There was also a decrease in talin binding in the mutant cells activated with anti-CD3. Because the most prominent difference in binding was seen with α-actinin, we investigated this further. α-Actinin is essential for cell migration (26). Phosphorylation of both αL and β2 regulated the binding between α-actinin and LFA-1. The strongest binding occurred when both αL Ser-1140 and β2 Thr-758 were phosphorylated.
A negative charge on the αL-chain, either by Ser-1140 phosphorylation or by expressing the S1140D mutant, was needed to enable phosphorylation of the β2 Thr-758. α-Actinin was able to bind the β2-chain also without α-chain phosphorylation only if there was a negative charge on Thr-758, as in the β2 T758D mutant, which could override the S1140A mutation and bind α-actinin. Binding was, however, stronger with a negative charge on both chains, indicating that the α-chain also directly regulates α-actinin binding. The αL cytoplasmic tail forms a triple-helical structure. Helix 3 makes contacts with both helices 1 and 2, and helices 1 and 3 are in contact with the β2-tail. Interestingly, Ser-1140 is located in helix 3 at the negatively charged surface of the αL tail, and its phosphorylation can enhance the negative charge of this surface (51). We conclude that αL Ser-1140 phosphorylation is required for β2 Thr-758 phosphorylation, possibly by making the site accessible to PKC enzymes, and as the chains move apart allowing new cytoskeletal interactions, as shown by increased α-actinin binding. This, in turn, would have an effect on cytoskeleton-mediated processes in cells.
We show that LFA-1 S1140A-expressing cells with impaired α-actinin binding exhibited impaired directional migration toward the SDF-1α chemokine. Cells expressing the LFA-1 S1140A mutant had a rounded and less polarized morphology compared with WT expressing cells. The same could be seen with cells where the β2-α-actinin interaction was disrupted by a peptide corresponding to the α-actinin–binding site on β2. This peptide has shown selectivity in binding α-actinin and not talin, although it contains part of the talin membrane-proximal site (19). Impaired migration in S1140A-expressing cells appears to be a result of the lack of α-actinin linking β2 to the cytoskeleton, which is known to be important for cell migration. A conserved region (residues 733–742) in the β2 cytoplasmic domain is critical for its cytoskeletal association (34). This region overlaps with the mapped α-actinin site (residues 736–746). Furthermore, a regulatory domain in β2 between residues 748–762 inhibits the constitutive association of the β2 tail with α-actinin (23). This sequence contains both the Thr-758 phosphorylation site and binding sites for talin and 14-3-3. Phosphorylation of Ser-1140 on αL and Thr-758 on β2 may release the inhibitory structure of the β2-chain, resulting in α-actinin binding, activation of the integrin binding site as shown with the NKI-L16 antibody, and migration of cells. We cannot, however, rule out the possibility that the reduced migration of LFA-1 S1140A-expressing cells is due to impaired binding of other proteins to the α-chain. Cells lacking α-actinin have a decreased ability to translocate but adhere more strongly to ICAM-1 (19). In our migration experiment, the LFA-1 S1140A-expressing cells were able to weakly adhere and make membrane projections but did not migrate toward the chemokine. This indicates that α-actinin may link the active integrin to the cytoskeleton to mediate directional migration.
At the leading edge, adhesions are constantly turned over, and the cell membrane attaches and then detaches from ICAM-1 when making new ligand contacts. Phosphorylation–dephosphorylation of αL Ser-1140 and αX Ser-1158 could provide a fast way of regulating integrin–α-actinin cytoskeleton linkage, dynamic adhesion, and migration. Our data establish an essential role for the α-chain cytoplasmic domain phosphorylation in the regulation of the β-chain and thus integrin activity.
Experimental procedures
Reagents and antibodies
Human ICAM-1–Fc and SDF-1α were from R&D Systems (Minneapolis, MN). The following antibodies were used: MHM24 (Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA), anti-CD3 (Immunotools, Friesoythe, Germany), anti-talin 8d4 (Sigma–Aldrich), mouse anti–α-actinin clone AT6/172 and anti-filamin mAb 1678 (Merck Millipore), rabbit anti-α-actinin EP2527Y (Abcam, Cambridge, UK), pan anti-14-3-3 (Santa Cruz Biotechnology, Dallas, TX); a polyclonal rabbit antiserum against the β2-chain phosphorylated on Thr-758 was produced by GenicBio Ltd. (Shanghai, China), and NKI-L16 was from Acris Antibodies. The R2E7B antibody against human β2 was previously described (52). IB4, which recognizes the heterodimeric forms of β2-integrins, was a gift from M. Arnaout (Massachusetts General Hospital, Boston, MA). Okadaic acid (CAS 78111-17-8) was from Santa Cruz Biotechnology, and horseradish peroxidase–linked antibodies against mouse and rabbit IgG were from Cell Signaling (Danvers, MA). APC-conjugated mouse secondary antibody was from Immunotools. CD11a-APC– and CD18-PE–conjugated antibodies were from BD Biosciences.
Cell cultures, immunoprecipitation, and immunoblotting
COS7 cells were cultured in Dulbecco's modified Eagle's medium and the human T-cell lymphoma cell line Jβ2.7, which lacks the αL-chain (53), was grown in RPMI 1640 supplemented with 10% FBS, 2 mm l-glutamine, and 100 units/ml penicillin/streptomycin. Jβ2.7 cells expressing WT αL or S1140A αL together with β2 have been described (7). K562 cells stably expressing WT αX or αX S1158A together with β2 have been previously described (22). K562 cells were cultured in RPMI medium supplemented with 0.5 mg/ml G418 (Calbiochem/Merck Millipore), 10% FBS, 2 mm l-glutamine, and 100 units/ml penicillin-streptomycin. The cells were treated with SDF-1α (50 ng/ml) for 20 min, anti-CD3 (10 μg/ml) for 30 min or left untreated. For immunoblotting with the Thr(P)-758/β2 antibody, cells were treated with 1 μg/ml of okadaic acid for 5 min. Cells were washed once with cold PBS, lysed on ice in 2% immunoprecipitation assay buffer (50 mm Tris-HCl, pH 7.8, 150 mm NaCl, 1% Triton X-100, 1% Nonidet P-40, 15 mm MgCl2, and 5 mm EDTA) with protease and phosphatase inhibitors (Roche Applied Science) for 30 min. Lysates were centrifuged at 20,000 × g for 1 h at 4 °C. Prewashed protein G-Sepharose beads were added for 2 h at 4 °C. The unbound fraction was mixed with IB4 or α-actinin antibody overnight at 4 °C, and protein G-Sepharose beads for another 2 h at 4 °C. The beads were washed three times with 1% immunoprecipitation assay buffer (50 mm Tris-HCl, pH 7.8, 150 mm NaCl, 0.5% Triton X-100, 0.5% Nonidet P-40, 15 mm MgCl2, and 5 mm EDTA), and bound proteins were eluted with Laemmli sample buffer, separated on SDS-PAGE, immunoblotted, and detected using ECL (Pierce). The figures show one representative picture of at least three experiments. The amount of coprecipitated proteins per immunoprecipitated β2 was quantified from three separate experiments by ImageJ. For the calculation of p values, one-way analysis of variance (Bonferroni post hoc) or unpaired Student's t test was used. The mean standard deviations are given in the figures.
Adhesion complex isolation
Integrin adhesion complexes were isolated as previously described (54). Briefly, the Jurkat Jβ2.7 cells (5 × 106/2.5 ml) activated with SDF-1α for 15 min or anti-CD3 for 30 min were allowed to adhere on ICAM-1 (3 μg/ml) or poly-l-lysine–coated (0.01%) 6-cm plates for 1 h. The cells were washed once with prewarmed RPMI 1640 and 6 mm dimethyl 3,3′-dithiobispropionimidate cross-linker (Fisher Scientific) was added for 15 min in the incubator. 3,3′-Dithiobispropionimidate was quenched with 1 m Tris-HCl, pH 8, for 5 min at room temperature. The cells were carefully washed once with ice-cold PBS and once with modified radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 5 mm disodium EDTA, pH 8,0, 5% SDS, 1% Triton X-100, 1% sodium deoxy cholate). 100 μl of adhesion recovery solution (125 mm Tris-HCl, pH 6.8, 1% SDS, 150 mm DTT) was added, and adhesion complexes were scraped off and collected. Four volumes of −20 °C acetone were added to each sample, which were stored at −80 °C. Precipitated proteins were centrifuged for 20 min at 160,00 × g, and the pellets were resuspended in reducing Laemmli sample buffer and heated at 85 °C for 5 min.
Cell transfections
αL was subcloned into the pcDNA3.1 vector (Thermo Scientific). β2 in the pcDNA3.1 vector was from Addgene (Cambridge, MA) (plasmid 8640 (55); the αX WT and αX S1158A mutant in the pcDM8 vector have been described (22). The mutations S1140A and S1140D in αL were introduced by site-directed mutagenesis (56). All constructs were checked by sequencing. COS7 cells were transiently cotransfected using FuGENE HD transfection reagent according to the manufacturer's instructions (Promega, Madison, WI) and harvested 48 h after the transfection.
The α-actinin–binding site-containing the β2 peptide HLSDLREYRRFEKEKLKSC and the β2 cytoplasmic chain peptides with and without phosphate on Thr-758 C-NNDNPLFKSA(pT)-TTVMNPK and C-NNDNPLFKSATTTVMNPK were obtained from GenicBio Ltd. (Shanghai, China). As a control, the P621 peptide VDVDSDGSTDLVIGA was used. The β2 peptide containing the α-actinin–binding site or the control peptide P621 was transfected into Jβ2.7 expressing LFA-1 WT using Pro-Ject protein transfection reagent kit according to the manufacturer's instructions (Thermo Scientific). Briefly, 5 μg of peptide in 50 μl of PBS was added to 10 μl of the dried Pierce reagent tube, incubated for 5 min at room temperature, and added to 500,000 cells/ml in RPMI 1640 without serum in a six-well plate. After 4 h, the cells were washed twice with PBS and stored in RPMI 1640.
Immunofluorescence
Jurkat Jβ2.7 cells were activated with SDF-1α (50 ng/ml) for 20 min or anti-CD3 (10 μg/ml) for 30 min and allowed to adhere on ICAM-1 (6 μg/ml) coated 8-well Ibidi chambers (μ-Slide 8-well, ibiTreat) for 30 min and fixed in 4% formaldehyde for 20 min at 37 °C. The plates were washed twice with PBS. The cells were stained with primary antibodies against β2 (R2E7B) and α-actinin (EP2527Y) and Alexa Fluor 488 or 633 secondary antibodies or TRITC-phalloidin (Invitrogen), washed, and mounted with Prolong Gold antifade reagent (Thermo Scientific). The Images were acquired using a Leica TCS SP8 STED 3X CW 3D confocal microscope (Wetzlar, Germany) with the HC PL Apo 20×/0.75 IMM CORR CS2 (glycerol) objective at room temperature and analyzed with Leica Application Suite and Fiji (National Institutes of Health). For colocalization studies, confocal images were automatically analyzed, and individual cells were manually selected as region of interest.
Flow cytometry
Jurkat Jβ2.7 cells were activated with SDF-1α (100 ng/ml) or anti-CD3 (10 μg/ml) or left untreated. 1 × 106 cells per treatment were incubated with NKI-L16 (1:100) or CD18-PE in Hepes buffer on ice for an hour. APC-conjugated goat anti-mouse was used as the secondary antibody. Flow data were acquired using an LSR II flow cytometer (Becton Dickinson) and analyzed using BD FACSDiva software.
Peptide affinity chromatography and dot blot
Sepharose-conjugated lyophilized peptides αL: C-KVGFFKRNLKEKMEAGRGVPNGIPAEDSELASGQEAGDPGCLKPLHEKDSESGGGKD; phospho-αL: C-KVGFFKRNLKEKMEAGRGVPNGIPAEDSEQLASGQEAGDPGCLKPLHEKDSEpSGGGKD; β2: C-KALIHLSDLREYRRFE-KEKLKSQWNNDNPLFKSATTTVMNPKFAES; and phospho-β2: C-KALIHLSDLREYRRF-EKEKLKSQWNNDNPLFKSApTTTVMNPKFAES (GenicBio Ltd., Shanghai, China) were dissolved in PBS with 0.01% Triton X-100. 150 million Jurkat JE6.1 cells were lysed with 2% immunoprecipitation buffer and added to peptide-conjugated beads for 8–12 h on a rotator at 4 °C. The samples were washed four times with 1% immunoprecipitation buffer, and bound proteins were detected by immunoblotting. For dot-blot analyses, 5 μg of free peptides were spotted on a nitrocellulose membrane; filters were blocked with 5% milk for 1 h. 5 μg of human α-actinin protein (Sigma–Aldrich, A9776) was added on the peptide spots. The membranes were incubated for 2 h on a shaker in coupling buffer (50 mm Tris, 5 mm EDTA, pH 8.3), washed twice with TBS, and bound α-actinin was detected with the EP2527Y antibody.
Cell adhesion and migration assays
For static adhesion assays, soluble ICAM-1 (6 μg/ml) was coated on flat-bottomed 96-well microtiter plates (NUNC MaxiSorp, Thermo Scientific) overnight at 4 °C. The plates were then washed twice with PBS and blocked with 1% heat-denatured BSA for 1 h. 200,000 Jβ2.7 cells expressing LFA-1 WT or αL S1140A/β2 with or without activation with SDF-1α (50 ng/ml) for 20 min or anti-CD3 (10 μg/ml) for 30 min were allowed to adhere for 30 min. Unbound cells were removed by washing three times in PBS, and the bound cells were lysed and detected using p-nitrophenyl phosphate substrate tablets (Sigma–Aldrich, S0942) dissolved in buffer (50 mm NaCH3COO, pH 5, 1% Triton X-100) at a concentration of 3 mg/ml. 100 μl of substrate solution was added to each well containing lysate and incubated for 45 min at 37 °C. The reaction was stopped with 1 m NaOH, and the reactivity was detected by measuring absorbance at 405 nm.
For the chemotactic cell migration assay, μ-slide chemotaxis chambers (Ibidi, Germany) were used. The assays were performed according to the manufacturer's instructions. Briefly, the μ-Slide chemotaxis slides were coated with 6 μg/ml ICAM-1 at 37 °C for 1 h and washed with PBS. 6 μl of cells at a concentration of 3 × 106 cells/ml were seeded in the center chamber of a μ-slide. Two reservoirs were filled with 60 μl of serum-free RPMI 1640. One of the filling ports was filled with 10 μl of chemoattractant SDF-1α (1 μg/ml) solution by removing 10 μl of medium from the other port on the same side of the device. Cell migration was recorded by mounting the μ-Slide on the stage of an inverted microscope in a cell incubator. For the trajectory analysis, pictures were taken every 4 min for 4 h using the Cell-IQ high content screening microscope (×10 lens) (Chip-Man Technologies Ltd., Tampere, Finland). The cells were tracked with the Fiji ImageJ manual tracking program (National Institutes of Health), tracks of 15 cells from one representative experiment are shown in the figure, and tracks of 50 cells each from three separate experiments were analyzed with the Chemotaxis and Migration Tools software (Ibidi for ImageJ). Statistical analyses were performed using one-way analysis of variance (Bonferroni post hoc) or unpaired Student's t test. Mean standard deviations are included in figures.
Author contributions
F. J., S. M., M. G., and C. G. G. conceptualization; F. J., S. M., T. R., L. V., M. G., and C. G. G. data curation; F. J., S. M., T. R., L. V., M. G., and C. G. G. formal analysis; F. J., S. M., T. R., L. V., M. G., and C. G. G. methodology; F. J., M. G., and C. G. G. writing-original draft; F. J., S. M., T. R., M. G., and C. G. G. writing-review and editing; M. G. and C. G. G. supervision; M. G. and C. G. G. funding acquisition; M. G. and C. G. G. investigation; M. G. and C. G. G. project administration; C. G. G. resources.
Supplementary Material
This work was supported by the Academy of Finland, the Sigrid Jusélius Foundation, the Medicinska Understödsföreningen Liv och Hälsa, the Finska Läkaresällskapet, the Wilhelm and Else Stockmann Foundation, the Ruth och Nils-Erik Stenbäck Foundation, and the Magnus Ehrnrooth Foundation. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Videos S1 and S2.
- TCR
- T-cell receptor
- LFA-1
- leukocyte function-associated antigen-1
- ICAM
- intercellular adhesion molecule
- PKC
- protein kinase C
- SDF
- stromal cell–derived factor
- PMA
- phorbol 12-myristate 13-acetate
- APC
- allophycocyanin.
References
- 1. Hynes R. O. (2002) Integrins: Bidirectional, allosteric signaling machines. Cell 110, 673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- 2. Gahmberg C. G., Fagerholm S. C., Nurmi S. M., Chavakis T., Marchesan S., and Grönholm M. (2009) Regulation of integrin activity and signalling. Biochim. Biophys. Acta 1790, 431–444 10.1016/j.bbagen.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hogg N., Patzak I., and Willenbrock F. (2011) The insider's guide to leukocyte integrin signalling and function. Nat. Rev. Immunol. 11, 416–426 10.1038/nri2986 [DOI] [PubMed] [Google Scholar]
- 4. Gahmberg C. G., Tolvanen M., and Kotovuori P. (1997) Leukocyte adhesion: structure and function of human leukocyte β2-integrins and their cellular ligands. Eur. J. Biochem. 245, 215–232 10.1111/j.1432-1033.1997.00215.x [DOI] [PubMed] [Google Scholar]
- 5. Tan S. (2012) The leucocyte β2 (CD18) integrins: the structure, functional regulation and signalling properties. Biosci. Rep. 32, 241–269 10.1042/BSR20110101 [DOI] [PubMed] [Google Scholar]
- 6. Luo B.-H., Carman C. V., and Springer T. A. (2007) Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 10.1146/annurev.immunol.25.022106.141618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gahmberg C. G., Grönholm M., and Uotila L. M. (2014) Regulation of integrin activity by phosphorylation. Adv. Exp. Med. Biol. 819, 85–96 10.1007/978-94-017-9153-3_6 [DOI] [PubMed] [Google Scholar]
- 8. Chatila T. A., Geha R. S., and Arnaout M. A. (1989) Constitutive and stimulus-induced phosphorylation of CD11/CD18 leukocyte adhesion molecules. J. Cell Biol. 109, 3435–3444 10.1083/jcb.109.6.3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valmu L., and Gahmberg C. G. (1995) Treatment with okadaic acid reveals strong threonine phosphorylation of CD18 after activation of CD11/CD18 leukocyte integrins with phorbol esters or CD3 antibodies. J. Immunol. 155, 1175–1183 [PubMed] [Google Scholar]
- 10. Valmu L., Hilden T. J., van Willigen G., and Gahmberg C. G. (1999) Characterization of β2 (CD18) integrin phosphorylation in phorbol ester-activated T lymphocytes. Biochem. J. 339, 119–125 10.1042/0264-6021:3390119,10.1042/bj3390119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hilden T. J., Valmu L., Kärkkäinen S., and Gahmberg C. G. (2003) Threonine phosphorylation sites in the β2 and β7 leukocyte integrin polypeptides. J. Immunol. 170, 4170–4177 10.4049/jimmunol.170.8.4170 [DOI] [PubMed] [Google Scholar]
- 12. Uotila L. M., Jahan F., Soto Hinojosa L., Melandri E., Grönholm M., and Gahmberg C. G. (2014) Specific phosphorylations transmit signals from leukocyte β2 to β1 integrins and regulate adhesion. J. Biol. Chem. 289, 32230–32242 10.1074/jbc.M114.588111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abram C. L., and Lowell C. A. (2009) The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 27, 339–362 10.1146/annurev.immunol.021908.132554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Legate K. R., and Fässler R. (2009) Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J. Cell Sci. 122, 187–198 10.1242/jcs.041624 [DOI] [PubMed] [Google Scholar]
- 15. Hibbs M. L., Jakes S., Stacker S. A., Wallace R. W., and Springer T. A. (1991) The cytoplasmic domain of the integrin lymphocyte function-associated antigen 1 β subunit: sites required for binding to intercellular adhesion molecule 1 and the phorbol ester-stimulated phosphorylation site. J. Exp. Med. 174, 1227–1238 10.1084/jem.174.5.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fagerholm S., Morrice N., Gahmberg C. G., and Cohen P. (2002) Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. J. Biol. Chem. 277, 1728–1738 10.1074/jbc.M106856200 [DOI] [PubMed] [Google Scholar]
- 17. Takala H., Nurminen E., Nurmi S. M., Aatonen M., Strandin T., Takatalo M., Kiema T., Gahmberg C. G., Ylänne J., and Fagerholm S. C. (2008) β2 integrin phosphorylation on Thr758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood 112, 1853–1862 10.1182/blood-2007-12-127795 [DOI] [PubMed] [Google Scholar]
- 18. Pavalko F. M., and LaRoche S. M. (1993) Activation of human neutrophils induces an interaction between the integrin β2-subunit (CD18) and the actin binding protein α-actinin. J. Immunol. 151, 3795–3807 [PubMed] [Google Scholar]
- 19. Stanley P., Smith A., McDowall A., Nicol A., Zicha D., and Hogg N. (2008) Intermediate-affinity LFA-1 binds α-actinin-1 to control migration at the leading edge of the T cell. EMBO J. 27, 62–75 10.1038/sj.emboj.7601959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fagerholm S. C., Hilden T. J., Nurmi S. M., and Gahmberg C. G. (2005) Specific integrin α and β chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J. Cell Biol. 171, 705–715 10.1083/jcb.200504016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fagerholm S. C., Varis M., Stefanidakis M., Hilden T. J., and Gahmberg C. G. (2006) α-Chain phosphorylation of the human leukocyte CD11b/CD18 (Mac-1) integrin is pivotal for integrin activation to bind ICAMs and leukocyte extravasation. Blood 108, 3379–3386 10.1182/blood-2006-03-013557 [DOI] [PubMed] [Google Scholar]
- 22. Uotila L. M., Aatonen M., and Gahmberg C. G. (2013) Integrin CD11c/CD18 α-chain phosphorylation is functionally important. J. Biol. Chem. 288, 33494–33499 10.1074/jbc.C113.497446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sampath R., Gallagher P. J., and Pavalko F. M. (1998) Cytoskeletal interactions with the leukocyte integrin β2 cytoplasmic tail: activation-dependent regulation of associations with talin and α-actinin. J. Biol. Chem. 273, 33588–33594 10.1074/jbc.273.50.33588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larson R. S., Hibbs M. L., and Springer T. A. (1990) The leukocyte integrin LFA-1 reconstituted by cDNA transfection in a nonhematopoietic cell line is functionally active and not transiently regulated. Cell Regul. 1, 359–367 10.1091/mbc.1.4.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Kooyk Y., Weder P., Hogervorst F., Verhoeven A. J., van Seventer G., te Velde A. A., Borst J., Keizer G. D., and Figdor C. G. (1991) Activation of LFA-1 through a Ca2+-dependent epitope stimulates lymphocyte adhesion. J. Cell Biol. 112, 345–354 10.1083/jcb.112.2.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Otey C. A., and Carpen O. (2004) α-actinin revisited: a fresh look at an old player. Cell Motil. Cytoskeleton 58, 104–111 10.1002/cm.20007 [DOI] [PubMed] [Google Scholar]
- 27. Kinashi T. (2005) Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 5, 546–559 10.1038/nri1646 [DOI] [PubMed] [Google Scholar]
- 28. Fagerholm S., Hilden T. J., and Gahmberg C. G. (2002) Lck tyrosine kinase important for activation of the CD11a/CD18-integrins in human T lymphocytes. Eur. J. Immunol. 32, 1670–1678 10.1002/1521-4141(200206)32:6%3C1670::AID-IMMU1670%3E3.0.CO%3B2-M [DOI] [PubMed] [Google Scholar]
- 29. Grönholm M., Jahan F., Marchesan S., Karvonen U., Aatonen M., Narumanchi S., and Gahmberg C. G. (2011) TCR-induced activation of LFA-1 involves signaling through Tiam1. J. Immunol. 187, 3613–3619 10.4049/jimmunol.1100704 [DOI] [PubMed] [Google Scholar]
- 30. Grönholm M., Jahan F., Bryushkova E. A., Madhavan S., Aglialoro F., Soto Hinojosa L., Uotila L. M., and Gahmberg C. G. (2016) LFA-1 integrin antibodies inhibit leukocyte α4β1-mediated adhesion by intracellular signaling. Blood 128, 1270–1281 10.1182/blood-2016-03-705160 [DOI] [PubMed] [Google Scholar]
- 31. Vinogradova O., Velyvis A., Velyviene A., Hu B., Haas T., Plow E., and Qin J. (2002) A structural mechanism of integrin αIIβ3 “inside-out” activation as regulated by its cytoplasmic face. Cell 110, 587–597 10.1016/S0092-8674(02)00906-6 [DOI] [PubMed] [Google Scholar]
- 32. O'Toole T. E., Mandelman D., Forsyth J., Shattil S. J., Plow E. F., and Ginsberg M. H. (1991) Modulation of the affinity of integrin αIIbβ3 (GPIIb-IIIa) by the cytoplasmic domain of αIIb. Science 254, 845–847 10.1126/science.1948065 [DOI] [PubMed] [Google Scholar]
- 33. O'Toole T. E., Katagiri Y., Faull R. J., Peter K., Tamura R., Quaranta V., Loftus J. C., Shattil S. J., and Ginsberg M. H. (1994) Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 124, 1047–1059 10.1083/jcb.124.6.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pardi R., Bossi G., Inverardi L., Rovida E., and Bender J. R. (1995) Conserved regions in the cytoplasmic domains of the leukocyte integrin αLβ2 are involved in endoplasmic reticulum retention, dimerization, and cytoskeletal association. J. Immunol. 155, 1252–1263 [PubMed] [Google Scholar]
- 35. Hughes P. E., O'Toole T. E., Ylänne J., Shattil S. J., and Ginsberg M. H. (1995) The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J. Biol. Chem. 270, 12411–12417 10.1074/jbc.270.21.12411 [DOI] [PubMed] [Google Scholar]
- 36. Hughes P. E., Diaz-Gonzalez F., Leong L., Wu C., McDonald J. A., Shattil S. J., and Ginsberg M. H. (1996) Breaking the integrin hinge: A defined structural constraint regulates integrin signaling. J. Biol. Chem. 271, 6571–6574 10.1074/jbc.271.12.6571 [DOI] [PubMed] [Google Scholar]
- 37. Lu C. F., and Springer T. A. (1997) The α subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J. Immunol. 159, 268–278 [PubMed] [Google Scholar]
- 38. Vinogradova O., Vaynberg J., Kong X., Haas T. A., Plow E. F., and Qin J. (2004) Membrane-mediated structural transitions at the cytoplasmic face during integrin activation. Proc. Natl. Acad. Sci. U.S.A. 101, 4094–4099 10.1073/pnas.0400742101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y. F., Tang R. H., Puan K. J., Law S. K., and Tan S. M. (2007) The cytosolic protein talin induces an intermediate affinity integrin αLβ2. J. Biol. Chem. 282, 24310–24319 10.1074/jbc.M701860200 [DOI] [PubMed] [Google Scholar]
- 40. Pouwels J., Nevo J., Pellinen T., Ylänne J., and Ivaska J. (2012) Negative regulators of integrin activity. J. Cell Sci. 125, 3271–3280 10.1242/jcs.093641 [DOI] [PubMed] [Google Scholar]
- 41. Bouvard D., Pouwels J., De Franceschi N., and Ivaska J. (2013) Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat. Rev. Mol. Cell Biol. 14, 430–442 10.1038/nrm3599 [DOI] [PubMed] [Google Scholar]
- 42. Morse E. M., Brahme N. N., and Calderwood D. A. (2014) Integrin cytoplasmic tail interactions. Biochemistry 53, 810–820 10.1021/bi401596q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kassner P. D., and Hemler M. E. (1993) Interchangeable α chain cytoplasmic domains play a positive role in control of cell adhesion mediated by VLA-4, a β1 integrin. J. Exp. Med. 178, 649–660 10.1084/jem.178.2.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaw L. M., and Mercurio A. M. (1993) Regulation of α6β1 integrin laminin receptor function by the cytoplasmic domain of the α6 subunit. J. Cell Biol. 123, 1017–1025 10.1083/jcb.123.4.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Filardo E. J., and Cheresh D. A. (1994) A β turn in the cytoplasmic tail of the integrin αv subunit influences conformation and ligand binding of αvβ3. J. Biol. Chem. 269, 4641–4647 [PubMed] [Google Scholar]
- 46. Kassner P. D., Alon R., Springer T. A., and Hemler M. E. (1995) Specialized functional properties of the integrin α4 cytoplasmic domain. Mol. Biol. Cell. 6, 661–674 10.1091/mbc.6.6.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ylänne J., Huuskonen J., O'Toole T. E., Ginsberg M. H., Virtanen I., and Gahmberg C. G. (1995) Mutation of the cytoplasmic domain of the integrin β3 subunit: differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J. Biol. Chem. 270, 9550–9557 10.1074/jbc.270.16.9550 [DOI] [PubMed] [Google Scholar]
- 48. Yauch R. L., Felsenfeld D. P., Kraeft S. K., Chen L. B., Sheetz M. P., and Hemler M. E. (1997) Mutational evidence for control of cell adhesion through integrin diffusion/clustering, independent of ligand binding. J. Exp. Med. 186, 1347–1355 10.1084/jem.186.8.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abair T. D., Bulus N., Borza C., Sundaramoorthy M., Zent R., and Pozzi A. (2008) Functional analysis of the cytoplasmic domain of the integrin α1 subunit in endothelial cells. Blood 112, 3242–3254 10.1182/blood-2007-12-126433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu J., Wang Z., Thinn A. M., Ma Y. Q., and Zhu J. (2015) The dual structural roles of the membrane distal region of the α-integrin cytoplasmic tail during integrin inside-out activation. J. Cell Sci. 128, 1718–1731 10.1242/jcs.160663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bhunia A., Tang X.-Y., Mohanram H., Tan S.-M., and Bhattacharjya S. (2009) NMR solution conformations and interactions of integrin αLβ2 cytoplasmic tails. J. Biol. Chem. 284, 3873–3884 10.1074/jbc.M807236200 [DOI] [PubMed] [Google Scholar]
- 52. Nortamo P., Patarroyo M., Kantor C., Suopanki J., and Gahmberg C. G. (1988) Immunological mapping of the human leucocyte adhesion glycoprotein gp90 (CD18) by monoclonal antibodies. Scand. J. Immunol. 28, 537–546 10.1111/j.1365-3083.1988.tb01485.x [DOI] [PubMed] [Google Scholar]
- 53. Weber K. S., York M. R., Springer T. A., and Klickstein L. B. (1997) Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: the αL and β2 subunits are interdependent for cell surface expression. J. Immunol. 158, 273–279 [PubMed] [Google Scholar]
- 54. Jones M. C., Humphries J. D., Byron A., Millon-Frémillon A., Robertson J., Paul N. R., Ng D. H. J., Askari J. A., and Humphries M. J. (2015) Isolation of integrin-based adhesion complexes. Curr. Protoc. Cell Biol. 66, 9.8.1–9.8.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., and Springer T. A. (1987) Cloning of the β subunit of the leukocyte adhesion proteins: Homology to an extracellular matrix receptor defines a novel supergene family. Cell 48, 681–690 10.1016/0092-8674(87)90246-7 [DOI] [PubMed] [Google Scholar]
- 56. Weiner M. P., Costa G. L., Schoettlin W., Cline J., Mathur E., and Bauer J. C. (1994) Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene 151, 119–123 10.1016/0378-1119(94)90641-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.