Brivaracetam was recently approved as adjunctive therapy in the treatment of focal (partial‐onset) seizures in patients 16 years of age and older with epilepsy. Brivaracetam is a selective, high‐affinity ligand for synaptic vesicle protein 2A.1, 2 In phase 2 and 3 studies,3, 4, 5, 6, 7, 8 brivaracetam has shown efficacy and good tolerability in adult patients with uncontrolled focal seizures.
Brivaracetam pharmacokinetics is dose‐proportional over a range of doses far exceeding the therapeutic doses.9, 10 It is completely absorbed orally (96.8% urinary excretion in mass balance study), shows no food effect, binds weakly to plasma proteins (17.5%), has an apparent volume of distribution corresponding to total body water, and has a plasma half‐life of approximately 9 hours.9, 10, 11 The major metabolic pathway of brivaracetam involves transformation of the amide function into a carboxylic acid by non‐CYP‐dependent enzyme amidase EC 3.5.1.4, and a second pathway involves CYP2C19‐mediated hydroxylation of the propyl side chain.11, 12, 13
An oral solution formulation of brivaracetam has been developed to provide an additional treatment option for patients who have difficulty in swallowing tablets. The objective of this study was to assess the relative bioavailability and bioequivalence of brivaracetam oral solution (10 mg/mL) and brivaracetam 50‐mg tablet (reference tablet throughout clinical development) after single administration in healthy participants.
Methods
Study Design
This was a randomized, open‐label, 2‐way crossover study that was performed at a single center (Therapharm Recherches, Caen, France). The study consisted of a screening visit (2 to 21 days prior to the first study drug administration), 2 single‐dose administrations (test [brivaracetam oral solution] and reference [brivaracetam tablet] treatments) separated by a washout period of 7 days, and a discharge visit within 7 days following a final blood sampling of the second treatment period. The study was conducted in compliance with the ethical principles originating from the Declaration of Helsinki and the Good Clinical Practice guidelines. The study protocol was reviewed and approved by an independent medical ethics committee (Comité de Protection des Personnes Nord‐Ouest I, Rouen, France). All participants provided written informed consent prior to the start of any study procedure.
Study Population
Healthy male and female participants aged 18 to 55 years with a body mass index 18.0 to 29.0 kg/m² and normal vital signs, laboratory tests, and electrocardiogram (ECG) were eligible to participate in the study. Female participants of childbearing potential had a negative pregnancy test at screening, day 1, and day 8, and used a medically accepted method of contraception during the entire study. Key exclusion criteria included pregnancy or breast‐feeding; history or presence of cardiovascular, respiratory, hepatic, renal, gastrointestinal, endocrinologic, neurologic, or psychiatric disorders; history or presence of drug addiction; excessive alcohol and caffeine consumption; smoking; and a positive test for hepatitis B or C or human immunodeficiency virus.
Study Procedures
Participants entered the center the evening before and remained until 24 hours after each administration. Each participant received a single dose of brivaracetam, administered in the morning of days 1 and 8 after an overnight fast. Test treatment was 10 mg/mL brivaracetam oral solution (5 mL), and reference treatment was brivaracetam 50‐mg oral film‐coated tablet. Randomization to 1 of 2 administration sequences (oral solution–tablet or tablet–oral solution) was balanced by gender.
Plasma samples (5 mL of blood) for brivaracetam concentration determination were taken predose and 15 minutes, 30 minutes, 45 minutes, 1 hour, 1.5 hours, 2 hours, 3 hours, 6 hours, 9 hours, 12 hours, 24 hours, and 36 hours after each administration. Adverse events (AEs) were recorded on an ongoing basis. Vital signs were measured predose and 1.5 hours and 24 hours postdose during each treatment period. A resting 12‐lead ECG, physical examination, and hematology, biochemistry, and urinalysis laboratory assessments were performed at both screening and discharge visits.
Bioanalytical Methods
Brivaracetam concentration was determined in plasma using a validated liquid chromatography electrospray ionization tandem mass spectrometry method following a previously described procedure.13 The lower limit of quantification (LLQ) was 2 ng/mL, and the quantification range was 2 to 2000 ng/mL. Briefly, plasma samples were submitted to a solid‐phase extraction procedure. Following reconstitution, the extracts were analyzed by gradient elution using 0.1% (pH 2.5) trifluoroacetic acid in acetonitrile‐water 5:95 v/v (phase A) and acetonitrile‐water 95:5 (phase B) on an Inertsil ODS‐3 column. Mean recovery and relative standard deviation (SD) on back‐calculated calibrators (n = 14 runs) ranged from 100% and 1.8% at the LLQ of 2 ng/mL to 97.6% and 5.3% at the upper limit of 2000 ng/mL, respectively. At the 3 quality control levels of 6, 75, and 1600 ng/mL (n = 28, 2 samples per level in every run), mean recovery and relative SD were 101.7% and 5.4%, 103.9% and 5.9%, and 98.1% and 6.0%, respectively.
Pharmacokinetic and Statistical Calculations
Pharmacokinetic parameters, calculated using standard noncompartmental methods, were maximum plasma concentration (Cmax), median time to maximum plasma concentration (tmax), area under the plasma concentration‐vs‐time curve from time 0 to the last quantifiable data point (AUCt), area under the plasma concentration‐vs‐time curve from time zero to infinity (AUC∞), plasma half‐life (t½), and plasma clearance (CL/F).
Bioequivalence of the formulations was evaluated from the ratio (test oral solution vs reference 50‐mg tablet) of back‐transformed geometric LSMs obtained by analysis of variance (ANOVA) for Cmax, AUCt, and AUC∞ along with the respective 90%CIs. Bioequivalence was concluded if 90%CIs were fully contained within the 0.80 to 1.25 range. For tmax, a distribution‐free 90%CI (Hodges‐Lehmann's method)14 was calculated for the median difference between test and reference formulations.
Using estimates for the residual coefficient of variation (CVres) of 8% for AUC and 23% for Cmax, a type‐I error of 0.05, a true ratio of 1 between treatments, and acceptance range at 0.80 to 1.25, a sample size of 24 participants was estimated to have 90% statistical power for showing bioequivalence between test and reference treatments.
Calculations were performed using WinNonlin Professional version 5.2 (Pharsight Corporation, Mountain View, California) and SAS version 9.1.3 (SAS Institute, Cary, North Carolina).
Results
Participants
Twenty‐four participants were randomized and completed both study treatments. Equal numbers of males and females (n = 12) were randomized with gender balance in treatment sequences. Median (range) age was 40.7 (19.1 to 54.9) years, median (range) body weight was 64 (49 to 88) kg, and median (range) body mass index was 23.2 (18.7 to 26.7) kg/m2. All participants were white.
Pharmacokinetics
The mean plasma concentration‐vs‐time profiles were similar for both oral solution and tablet (Figure 1A); peak concentration was reached rapidly. Median tmax for the oral solution was slightly earlier than for the tablet (0.63 hours vs 1.00 hour), and the mean Cmax was similar (1.42 μg/mL oral solution, 1.34 μg/mL tablet) (Table 1). Mean AUC∞ was 15.9 and 16.3 μg·h/mL, respectively, for oral solution and tablet (Table 1). CIs for the Cmax and AUC ratios were entirely included within the bioequivalence limits (0.80 to 1.25) (Table 1). Residual variability was low (coefficient of variation of 13% for Cmax and < 5% for AUCt and AUC∞). Plasma half‐life and clearance were approximately 9 hours and 3.4 L/h, respectively (Table 1). Scatter plots of individual values for AUC∞ and Cmax are shown in Figures 1B and 1C. In most participants, Cmax and AUC∞ were similar for both formulations.
Figure 1.
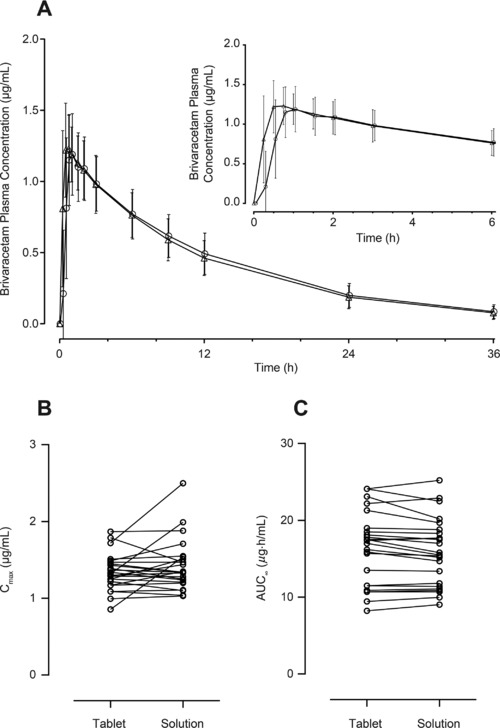
Arithmetic mean (standard deviation) plasma concentration (μg/mL)‐vs‐time profiles following single‐dose brivaracetam 50‐mg oral solution (Δ) or brivaracetam 50‐mg tablet (○) (A) (n = 24). Scatter plots of individual values of Cmax (B) and AUC∞ (C) following single‐dose brivaracetam 50‐mg oral solution and brivaracetam 50‐mg tablet (n = 24). AUC∞, area under the plasma concentration‐vs‐time curve from time 0 to infinity; Cmax, maximum plasma concentration.
Table 1.
Pharmacokinetic Parameters of Single‐Dose Brivaracetam 50‐mg Oral Solution (5 mL, 10 mg/mL) and Brivaracetam 50‐mg Tablet (n = 24)
| Parameter (Units)a | Brivaracetam Tablet, n = 24 | Brivaracetam Oral Solution, n = 24 | CVres (%)c | Point Estimate (90%CI)f |
|---|---|---|---|---|
| Cmax (μg/mL)b | 1.34 (0.238) | 1.42 (0.331) | 13.0 | 1.06 (0.99, 1.13) |
| tmax (hours)d | 1 (0.25‐3) | 0.63 (0.25‐2) | NA | −0.25 (−0.50, −0.13)e |
| AUCt (μg·h/mL)b | 15.1 (3.91) | 14.8 (3.62) | 4.1 | 0.99 (0.97, 1.01) |
| AUC∞ (μg·h/mL)b | 16.3 (4.70) | 15.9 (4.37) | 4.0 | 0.98 (0.96, 1.00) |
| t½ (hours)b | 9.13 (1.75) | 8.90 (1.71) | NA | NA |
| CL/F (L/h)b | 3.35 (1.10) | 3.37 (0.96) | NA | NA |
ANOVA, analysis of variance; AUC∞, area under the plasma concentration‐versus‐time curve from time 0 to infinity; AUCt, area under the plasma concentration‐versus‐time curve from time 0 to the last quantifiable data point; CI, confidence interval; CL/F, plasma clearance; Cmax, maximum plasma concentration; CVres, residual coefficient of variation; NA, not applicable; tmax, time to maximum plasma concentration; t½, plasma half‐life.
Arithmetic mean (standard deviation).
ANOVA residual error representing intraindividual variability.
Median (range).
Median point estimate (90% nonparametric confidence interval of the difference between oral solution and tablet).
Point estimate and 90%CI for the geometric least‐squares means ratio (oral solution/tablet).
Tolerability
Overall, 20/24 participants (83.3%) reported ≥1 treatment‐emergent adverse event (TEAE), and all but 3 were considered to be related to the treatment. Most TEAEs were reported during the first 3 hours following drug administration, and all had resolved by study completion. The incidence of TEAEs was 79.2% (19/24) for the oral solution and 70.8% (17/24) for the tablet. The most frequently reported TEAEs were fatigue (n = 10 oral solution, n = 9 tablet), somnolence (n = 5 oral solution, n = 6 tablet), dizziness (n = 9 oral solution, n = 4 tablet), feeling drunk (n = 4 oral solution, n = 1 tablet), nausea (n = 1 oral solution, n = 2 tablet), and blurred vision (n = 1 oral solution, n = 1 tablet). Most of the TEAEs were mild in severity; no severe AEs and no serious AEs were reported. There were no clinically relevant changes in hematology, chemistry, urinalysis, or vital signs. No physical abnormalities and no clinically relevant ECG abnormalities were reported.
Discussion
In this phase 1, randomized, open‐label, 2‐way crossover study, it was demonstrated that brivaracetam 50‐mg oral solution (5 mL, 10 mg/mL) was bioequivalent to brivaracetam 50‐mg oral tablet after single administration in 24 healthy participants, when tested under fasting conditions. This conclusion was based on the finding that the 90%CI of the geometric means ratio of the 2 formulations fell within the bioequivalence range (0.80 to 1.25) for Cmax, AUCt, and AUC∞. The mean brivaracetam plasma concentration‐vs‐time profiles were similar for both oral solution and tablet. Brivaracetam was rapidly absorbed following both formulations. As expected, absorption with the oral solution was slightly more rapid than that with the tablet, as evidenced by median tmax values of 0.63 hours and 1.00 hour, respectively, with a median difference of 15 minutes. The small difference was consistent with the fast in vitro dissolution of the tablet (98% in 15 minutes; UCB data on file). Cmax and AUC∞ were very similar between individual participants, and on average. Inter‐ and intraindividual variabilities were low for both formulations. Because brivaracetam oral solution and tablet have closely similar plasma concentration‐vs‐time profiles, switching between the 2 formulations would not be expected to result in pharmacokinetic, tolerability, or safety issues in patients. Good tolerability of brivaracetam oral solution was observed in a phase 2a study in pediatric patients with epilepsy.15 Brivaracetam oral solution offers an additional treatment option for patients who have difficulty in swallowing tablets, potentially increasing the chances of adherence to therapy.
Declaration of Conflicting Interests
Christian Otoul, Shikiko Watanabe, Suzanne McCabe, and Armel Stockis are employees of UCB Pharma. Niki Panagiotaki, PhD (QXV Communications, an Ashfield business, part of UDG Healthcare plc), provided editorial support, which was funded by UCB Pharma. This study was sponsored by UCB Pharma. UCB Pharma was involved in the design and conduct of the study, and collection, management, and analysis of the data.
Acknowledgments
The authors thank Evelyn Guénolé, MD (Therapharm Recherche, Caen, France), for her role as clinical investigator.
References
- 1. Kenda BM, Matagne AC, Talaga PE, et al. Discovery of 4‐substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47(3):530–549. [DOI] [PubMed] [Google Scholar]
- 2. Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti‐convulsant properties. Eur J Pharmacol. 2011;664(1–3):36–44. [DOI] [PubMed] [Google Scholar]
- 3. French JA, Costantini C, Brodsky A, von Rosenstiel P. Adjunctive brivaracetam for refractory partial‐onset seizures. A randomized, controlled trial. Neurology. 2010;75(6):519–525. [DOI] [PubMed] [Google Scholar]
- 4. Van Paesschen W, Hirsch E, Johnson M, Falter U, von Rosenstiel P. Efficacy and tolerability of adjunctive brivaracetam in adults with uncontrolled partial‐onset seizures: a phase IIb, randomized, controlled trial. Epilepsia. 2013;54(1):89–97. [DOI] [PubMed] [Google Scholar]
- 5. Biton V, Berkovic SF, Abou‐Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double‐blind, placebo‐controlled trial. Epilepsia. 2014;55(1):57–66. [DOI] [PubMed] [Google Scholar]
- 6. Kwan P, Trinka E, van Paesschen W, Rektor I, Johnson ME, Lu S. Adjunctive brivaracetam for uncontrolled focal and generalized epilepsies: results of a phase III, double‐blind, randomized, placebo‐controlled, flexible‐dose trial. Epilepsia. 2014;55(1):38–46. [DOI] [PubMed] [Google Scholar]
- 7. Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double‐blind, randomized, placebo‐controlled trial. Epilepsia. 2014;55(1):47–56. [DOI] [PubMed] [Google Scholar]
- 8. Klein P, Schiemann J, Sperling MR, et al. A randomized, double‐blind, placebo‐controlled, multicenter, parallel‐group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial‐onset seizures. Epilepsia. 2015;56(12):1890–1898. [DOI] [PubMed] [Google Scholar]
- 9. Sargentini‐Maier ML, Rolan P, Connell J, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol. 2007;63(6):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rolan P, Sargentini‐Maier ML, Pigeolet E, Stockis A. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after multiple increasing oral doses in healthy males. Br J Clin Pharmacol. 2008;66(1):71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sargentini‐Maier ML, Espie P, Coquette A, Stockis A. Pharmacokinetics and metabolism of 14C‐brivaracetam, a novel SV2A ligand, in healthy subjects. Drug Metab Dispos. 2008;36(1):36–45. [DOI] [PubMed] [Google Scholar]
- 12. Stockis A, Watanabe S, Rouits E, Matsuguma K, Irie S. Brivaracetam single and multiple rising oral dose study in healthy Japanese participants: influence of CYP2C19 genotype. Drug Metab Pharmacokinet. 2014;29(5):394–399. [DOI] [PubMed] [Google Scholar]
- 13. Stockis A, Watanabe S, Scheen AJ, et al. Effect of rifampin on the disposition of brivaracetam in human subjects: further insights into brivaracetam hydrolysis. Drug Metab Dispos. 2016;44(6):792–799. [DOI] [PubMed] [Google Scholar]
- 14. Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Statist. 1963;34(2):598–611. [Google Scholar]
- 15. Liu E, Hepner A, Dilley D, Stockis A, Daniels A. Safety and tolerability of adjunctive brivaracetam administered as oral solution in pediatric patients aged ≥1 month to 16 years with epilepsy. Epilepsy Curr. 2014;14(Suppl 1):390–391. [Google Scholar]


