Abstract
Analgesic effects of ibuprofen immediate‐release/extended‐release (IR/ER) 600‐mg tablets were evaluated in 2 randomized, double‐blind, placebo‐controlled dental pain studies. Patients 16–40 years old with moderate–severe pain following third‐molar extraction received single‐dose ibuprofen 600 mg IR/ER (formulation A or B), naproxen sodium 220 mg, or placebo (2:2:2:1; study 1) or 4 doses of ibuprofen 600 mg IR/ER (formulation A) or placebo (1:1; study 2). In study 1 (n = 196), mean (standard deviation [SD]) time‐weighted sum of pain intensity difference scores for placebo, ibuprofen IR/ER A, ibuprofen IR/ER B, and naproxen, respectively, were 0.05 (9.2), 16.87 (9.4), 17.34 (10.5), and 12.66 (10.0) over 0–12 hours and ‐0.03 (4.1), 6.57 (4.4), 7.14 (5.2), and 5.14 (5.0) over 8–12 hours (all P < .001 vs placebo). In study 2 (n = 106), mean (SD) time‐weighted sum of pain relief and pain intensity difference scores were 18.2 (20.0) versus 41.5 (21.0) at 0–12 hours and 10.3 (12.0) versus 18.4 (12.1) at 8–12 hours for placebo versus ibuprofen IR/ER, respectively (P < .001 for both); efficacy was sustained over each of the four 12‐hour dosing intervals with ibuprofen. Gastrointestinal adverse events predominated with placebo both after study medication administration and after rescue medication use, if applicable. Ibuprofen 600 mg IR/ER provided safe and effective analgesia after single and multiple doses.
Keywords: ibuprofen, immediate release, extended release, dental pain, analgesia
For more than 30 years, the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen has been used safely and effectively without a prescription for analgesia and antipyresis.1 Available in more than 80 countries, ibuprofen is one of the most widely used over‐the‐counter (OTC) analgesics, and its efficacy has been extensively evaluated.1, 2 Previous studies have shown that a single 400‐mg dose of the standard formulation of ibuprofen free acid provides superior analgesic efficacy compared with acetaminophen (1000 mg) in several different clinical pain models, including third‐molar extraction, sore throat, postpartum episiotomy pain, tension‐type headache, and osteoarthritis.3, 4, 5, 6, 7 Similarly, in multiple‐dose studies, ibuprofen 1200 mg/day has been found to be at least as effective as acetaminophen 3000–4000 mg/day in relieving pain associated with osteoarthritis.7, 8, 9
Standard OTC ibuprofen is taken as a 200‐ or 400‐mg dose, which may be repeated every 4–6 hours as needed up to a maximum of 1200 mg/day.10 Ibuprofen is rapidly absorbed after single doses of regular‐release preparations, with peak plasma drug concentration occurring within 3 hours of dosing. Administration with food results in a ∼20% lowering of peak ibuprofen plasma concentrations and a delay in reaching peak concentrations compared with the fasted state. Administration of multiple doses reveals no evidence of drug accumulation or time dependence. The area under the time‐versus‐concentration curve (AUC) of ibuprofen demonstrates a nonlinear relationship from 250 to 1200 mg, possibly because of saturation of protein binding versus impaired absorption. At therapeutic concentrations, ibuprofen is >98% protein bound. Extensive enantiomeric inversion occurs on administration of ibuprofen in humans. The metabolism of ibuprofen is extensive, with the formation of 2‐(4‐[2‐hydroxy‐2‐methylpropyl]phenyl) propionic acid (2‐hydroxyibuprofen) and 2‐(3‐[2‐carboxypropyl]phenyl) propionic acid (carboxyibuprofen), 1‐hydroxyibuprofen, and 3‐hydroxyibuprofen; none of these metabolites are pharmacologically active. The cytochrome P450 2C9 is the most important catalyst for oxidative metabolism. Both drug and metabolites are excreted rapidly via urine and feces.11
In circumstances in which longer‐lasting pain is present or anticipated—such as with osteoarthritis, back pain, dysmenorrhea, or postoperative pain—analgesics that provide sustained plasma drug levels over an extended duration of time may prove advantageous. Indeed, long‐acting products may provide more consistent pain control with less breakthrough pain and may reduce the need for rescue medication (along with the potential for associated side effects).12, 13 Less frequent dosing may also be more convenient for patients.13
Given the potential clinical scenarios in which a long‐acting OTC analgesic may be preferred, Pfizer Consumer Healthcare has developed a new immediate‐release/extended‐release (IR/ER) formulation of ibuprofen containing 200 mg of IR ibuprofen and 400 mg of ER ibuprofen (total of 600 mg ibuprofen per tablet) with a proposed dosing regimen of 1 tablet every 12 hours. We report 2 clinical studies conducted to evaluate the efficacy and safety of single‐dose (study 1) and multiple‐dose (study 2) ibuprofen 600 mg IR/ER for the relief of dental pain following extraction of impacted third molars.
Methods
Protocols and informed consent forms for both studies were approved by the Sterling Institutional Review Board (Atlanta, Georgia), and both studies were conducted in accordance with International Conference on Harmonization Good Clinical Practice standards and the guiding principles of the Declaration of Helsinki (as amended in Tokyo, Venice, Hong Kong, and South Africa). All study participants provided written informed consent prior to initiation of any study procedures. Parents or legal guardians were allowed to sign the consent form for patients younger than age 18 years, in which case the patients were also required to provide assent.
Study Design
Both studies were single‐center, inpatient, randomized, double‐blind, placebo‐controlled, parallel‐group trials. Study 1 (NCT00913627) was a phase 2 trial conducted by Jean Brown Research (Salt Lake City, Utah), and study 2 (NCT01266161) was a phase 3 trial conducted by Premier Research (Austin, Texas). Participants in study 1 were randomized in a 2:2:2:1 ratio to single doses of 2 formulations of ibuprofen 600 mg IR/ER (formulations A and B), naproxen sodium 220 mg, or placebo and were evaluated for 24 hours after dosing. The 2 ibuprofen IR/ER formulations differed only in respect to manufacturing process; formulation A was subsequently chosen for further development. In study 2, participants were randomized in a 1:1 ratio to ibuprofen 600 mg IR/ER (using formulation A from study 1) or placebo administered every 12 hours for 4 doses, with assessments occurring over 48 hours.
Both studies stratified patients at study entry by sex and baseline pain severity (moderate or severe). To maintain blinding, an independent third party dispensed the study drug to blindfolded patients. No other study personnel had access to the randomization schedule or identity of study drugs.
In study 1, patients who did not achieve adequate pain relief within 1.5 hours after dosing or later were allowed rescue medication consisting of 1 or 2 tablets of acetaminophen 500 mg plus hydrocodone 5 mg. In study 2, 1 tablet of acetaminophen 500 mg plus hydrocodone 5 mg was available for those not achieving adequate pain relief within 1 hour after study drug administration; a second tablet could be provided in hour 2 or later if needed. In both studies, rescue medication use was limited to a maximum of 8 hydrocodone/acetaminophen tablets per day.
Patient Populations
The 2 studies had nearly identical inclusion criteria. Eligible patients were male or female between 16 and 40 years of age who were in generally good health. Study inclusion required moderate or severe baseline pain following surgical extraction of third molars (1 or 2 in study 1; ≥2 in study 2), at least 1 of which was required to involve a partial or full bony mandibular impaction. Moderate or severe baseline postoperative pain was defined as a score of 2 or 3 on a 4‐point categorical pain severity rating scale (from 0 = none to 3 = severe), confirmed by a score of at least 50 mm on a 100‐mm visual analog scale. In study 1, preoperative medications and anesthetics included short‐acting local anesthetics (mepivacaine or lidocaine), with or without a vasoconstrictor, nitrous oxide, and/or midazolam; study 2 allowed the same preoperative medications as in study 1 and in addition allowed topical benzocaine and/or fentanyl use. Patients required clearance for participation by the attending dentist or physician investigator and had to be capable of comprehending the informed consent form and reliably recording the requested information on the analgesic questionnaire.
Potential participants were excluded for any significant medical disorder that might place them at increased risk (eg, poorly controlled hypertension, diabetes, hyper‐ or hypothyroidism, or significantly impaired cardiac, renal, or hepatic function) or if they had a bleeding disorder or peptic ulcer disease currently or within the past 2 years. Women were excluded if they were pregnant, breast‐feeding, or of childbearing potential and not using medically approved contraception. Additional exclusion criteria included acute localized dental/alveolar infection at the time of surgery, a history of alcoholism or substance abuse within the past year (study 1) or past 2 years (study 2), or a history of allergic reaction to any NSAID, codeine, hydrocodone, or acetaminophen. Patients were required to abstain from intake of caffeine, chocolate, or alcohol within 4 hours of taking the first dose of study medication (study 1) or after midnight on the evening preceding surgery and during the subsequent 48‐hour evaluation period (study 2). Patients were ineligible if they had used prescription or OTC first‐generation antihistamines within 24 hours of taking study medication (study 2 only), a bisphosphonate any time in the 5 years prior to enrollment, or, aside from preanesthetic medication/anesthesia for the surgery, any analgesic (studies 1 and 2) or glucocorticoids (study 2 only) within 5 times the drug's half‐life prior to administration of the first dose of study medication. Routine use of oral analgesics ≥ 5 times per week (habituation) was also grounds for exclusion. Study 1 excluded patients taking any medication contraindicated for use with NSAIDs or who were taking psychotropic drugs (generally referring to antidepressant therapy) unless the dose was stable for ≥2 months. Study 2 excluded patients taking monoamine oxidase inhibitors within 2 months of screening, or antianxiety, antipsychotic, or neuroleptic agents within 14 days of surgery. Patients taking antidepressants were allowed to participate if the dose was stable for ≥30 days and did not vary throughout the study period. Patients using an investigational product or who participated in an investigational trial within the prior 30 days were excluded. Study site staff, sponsor employees, and relatives of study site personnel or (in study 2) the sponsor were all ineligible.
Participant Assessments
Pain status was assessed using validated scales that are well established and typical for these types of studies. Pain intensity was assessed with the question “How much pain do you have at this time?” and was rated on a 4‐point categorical pain intensity rating scale (with responses of 0 = none, 1 = mild, 2 = moderate, and 3 = severe) at baseline and 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 16, and 24 hours after study drug administration in study 1 and at baseline and 0.5, 1, 1.5, 2, 4, 6, 8, 10, 12, 16, 20, 24, 28, 32, 36, 40, 44, and 48 hours after the first dose in study 2. Pain intensity also was assessed immediately before any rescue medication was given in both studies.
Pain relief was assessed with the question “How much relief do you have from your starting pain?” and was rated on a 5‐point categorical pain relief rating scale to indicate how much relief patients had compared with their starting pain (with responses of 0 = none, 1 = a little, 2 = some, 3 = a lot, and 4 = complete). In study 1, pain relief was assessed at the same posttreatment times as the pain intensity rating scale; in study 2, pain relief was assessed through 12 hours after the first dose.
The double stopwatch method was used to evaluate times to first perceptible relief and meaningful pain relief. At dosing (time 0), the study coordinator started 2 stopwatches with covered faces. Patients were given the first stopwatch and instructed to stop it when they first experienced any pain relief. If and when patients stopped the first watch, they were given the second stopwatch and instructed to stop it when they experienced pain relief they considered meaningful. Stopwatches remained active until stopped by the patient, rescue medication was administered, 6 hours had elapsed (study 1 only), or the next dose of study medication was administered (ie, 12 hours [study 2 only]).
Efficacy Outcome Measures
Coprimary efficacy measures in study 1 included the time‐weighted sum of pain intensity difference scores from 0 to 12 hours (SPID 0–12) and from 8 to 12 hours (SPID 8–12). Pain intensity difference scores (PIDs) were calculated by subtracting the score at each postdosing assessment point from the baseline score; a higher PID indicated greater improvement. In study 2, coprimary efficacy measures were the time‐weighted sum of pain relief and pain intensity difference scores from 0 to 12 hours (SPRID 0–12) and from 8 to 12 hours (SPRID 8–12).
Secondary efficacy parameters in study 1 included time to confirmed first perceptible relief, time to meaningful pain relief, time to treatment failure, and percentage of treatment failures at 8, 9, 10, 11, and 12 hours. Time to confirmed first perceptible relief was defined as the time the patient stopped the first stopwatch, provided they confirmed it by also pressing the second stopwatch, indicating meaningful relief. In study 1, patients who never stopped the second stopwatch were censored at 6 hours for both time to confirmed first perceptible relief and time to meaningful relief. In study 2, those who failed to achieve first perceptible and/or meaningful relief were censored at the time of the second dose, whereas those who did not achieve confirmation of first perceptible relief by also pressing the second stopwatch were censored at the time they pressed the first stopwatch. Time to treatment failure was defined as time to first use of rescue medication or to study discontinuation because of lack of efficacy. Secondary efficacy measures in study 2 included time to meaningful pain relief; SPID 0–12, 8–12, 12–24, 20–24, 0–24, 24–36, 32–36, 36–48, 44–48, and 24–48; time to first rescue medication use during the first dosing interval; and percentage of patients taking rescue medication during the first dosing interval and overall.
Safety Measurements
Patients were monitored during surgery and inpatient follow‐up for adverse events (AEs), which were recorded when reported or observed. AEs were summarized using Medical Dictionary for Regulatory Activities, versions 9.0 (study 1) and 13.0 (study 2).
Statistical Analyses
For study 1, a sample size of at least 196 patients (56 in each active treatment group and 28 in the placebo group) was estimated to provide ≥80% power to detect a treatment difference of 8.75 units for SPID 0–12 at a 2‐sided .05 significance threshold. This determination assumed a variability estimate of 10.3 units, based on a previously completed randomized, double‐blind, placebo‐controlled study that compared OTC ibuprofen versus celecoxib in dental pain.14 This sample size was also estimated to provide ≥87% power to detect a treatment difference of 3 units for SPID 8–12, assuming a variability estimate of 4.13 units.
For study 2, variability estimates of 21.1 and 10.7 units were used for SPRID 0–12 and SPRID 8–12, respectively, based on results from study 1. Using those assumptions, a sample size of 100 patients (50 per treatment group) was estimated to provide ≥90% power (α = .05, 2 sided) to detect differences of 15 and 7.5 units on the respective coprimary end points.
In study 1, the worst observation was carried forward for pain intensity and pain relief assessments following any rescue medication use. A similar rule was applied to study 2, but only for scores assessed within 4 hours of rescue use because this was a multiple‐dose study. Summary scores of SPID and SPRID for periods of interest, as well as pain relief and PID scores at each postdosing point, were analyzed by analysis of variance with treatment, sex, and categorical baseline pain severity rating in the model. Times to confirmed first perceptible relief, meaningful relief, and treatment failure in study 1 and time to first rescue medication in study 2 were analyzed using the proportional hazards regression model with terms for treatment, sex, and baseline pain severity. The Cochran‐Mantel‐Haenszel test, controlling for sex and baseline pain severity, was used in study 1 to evaluate proportion of treatment failures and in study 2 to evaluate proportion of patients taking rescue medication in the first dosing interval and overall. All tests were done at a 2‐sided 5% significance level. All statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina).
To protect from type I error from multiple comparisons, the 2 primary parameters in each study were tested sequentially. In study 1, for SPID 0–12, if the overall test showed that at least 1 ibuprofen IR/ER formulation was significantly better than placebo, then each ibuprofen IR/ER–versus–placebo comparison was eligible for statistical significance if P ≤ .05. The SPID 8–12 was not eligible for statistical significance unless both pairwise comparisons for SPID 0–12 were also significant. Because the naproxen treatment arm was only included to establish the assay sensitivity of the study and to gain an assessment of relative efficacy of the 2 ibuprofen test prototypes, the naproxen‐versus‐placebo comparison was only tested as a secondary hypothesis. Similarly in study 2, the SPRID 8–12 could not be considered significant unless SPRID 0–12 was significant.
The protocol for study 1 called for statistical comparisons only for active treatments versus placebo; however, statistical comparisons between ibuprofen IR/ER formulations (pooled) and naproxen were also performed as a post hoc analysis using the same statistical methods outlined above.
Efficacy analyses were based on the intent‐to‐treat (ITT) population, which included all randomized patients who received study drug and provided a baseline pain assessment. The safety population consisted of all patients who received study drug and contributed follow‐up data.
Results
Patient Disposition and Baseline Characteristics
In both studies, all randomized patients (study 1, n = 196; study 2, n = 106) received study medication and were included in the ITT and safety populations. No patients withdrew from study 1. Five patients discontinued participating in study 2: 1 in the ibuprofen IR/ER group discontinued during the first dosing interval because of lack of efficacy, 1 in the placebo group was ineligible because of recent use of intravenous dexamethasone, and 3 others in the placebo group were discontinued because of vomiting within 4 hours of dosing. Treatment groups in each respective study were comparable in terms of demographic and baseline characteristics (Table 1).
Table 1.
Baseline Demographic, Surgical, and Pain Characteristics of Participants in Single‐Dose (Study 1) and Multiple‐Dose (Study 2) Investigations of Ibuprofen 600 mg IR/ER
| Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 29) | Ibuprofen 600 mg IR/ER Formulation A (n = 53) | Ibuprofen 600 mg IR/ER Formulation B (n = 56) | Naproxen 220 mg (n = 58) | Ibuprofen 600 mg IR/ER (n = 54) | Placebo (n = 52) | |
| Sex, n (%) | ||||||
| Male | 17 (58.6) | 31 (58.5) | 33 (58.9) | 34 (58.6) | 18 (33.3) | 19 (36.5) |
| Female | 12 (41.4) | 22 (41.5) | 23 (41.1) | 24 (41.4) | 36 (66.7) | 33 (63.5) |
| Race, n (%) | ||||||
| White | 28 (96.6) | 46 (86.8) | 51 (91.1) | 55 (94.8) | 47 (87.0) | 42 (80.8) |
| Black | 1 (3.4) | 1 (1.9) | 0 | 0 | 3 (5.6) | 6 (11.5) |
| Asian | 0 | 2 (3.8) | 1 (1.8) | 1 (1.7) | 2 (3.7) | 2 (3.8) |
| Othera | 0 | 4 (7.6) | 4 (7.2) | 2 (3.4) | 2 (3.8) | 2 (3.8) |
| Age, years | ||||||
| Mean (SD) | 18.1 (2.1) | 18.6 (2.4) | 18.7 (2.7) | 18.3 (2.6) | 21.3 (3.6) | 21.1 (3.3) |
| Range | 16–27 | 16–25 | 16–29 | 16–28 | 16–34 | 16–29 |
| Duration of procedure, min | ||||||
| Mean (SD) | 8.8 (5.5) | 7.1 (2.5) | 7.6 (2.9) | 8.7 (6.3) | 9.0 (2.8) | 10.1 (3.7) |
| Range | 4.0–28.0 | 2.0–15.0 | 4.0–15.0 | 3.0–35.0 | 5.0–20.0 | 6.0–25.0 |
| Number of teeth extracted, n (%) | ||||||
| 1 | 0 | 1 (1.9) | 0 | 2 (3.4) | 0 | 0 |
| 2 | 29 (100.0) | 52 (98.1) | 56 (100.0) | 56 (96.6) | 54 (100.0) | 52 (100.0) |
| Trauma rating, n (%) | ||||||
| Mild | 0 | 1 (1.9) | 0 | 0 | 0 | 0 |
| Moderate | 27 (93.1) | 50 (94.3) | 54 (96.4) | 52 (89.7) | 45 (83.3) | 36 (69.2) |
| Severe | 2 (6.9) | 2 (3.8) | 2 (3.6) | 6 (10.3) | 9 (16.7) | 16 (30.8) |
| Time from end of surgery to study medication, h | ||||||
| Mean (SD) | 3.1 (0.7) | 3.3 (0.9) | 3.3 (0.9) | 3.2 (0.9) | 2.8 (0.9) | 2.6 (0.8) |
| Range | 1.8–4.5 | 0.9–5.2 | 1.1–5.5 | 1.4–5.1 | 1.4–4.8 | 1.3–4.5 |
| Baseline VAS pain intensity, mm | ||||||
| Mean (SD) | 73.9 (11.6) | 77.1 (13.2) | 74.8 (12.4) | 75.1 (12.3) | 75.7 (12.2) | 73.8 (12.3) |
| Range | 53–96 | 50–100 | 56–100 | 51–99 | 54–100 | 53–100 |
| Baseline categorical pain severity, n (%) | ||||||
| Moderate | 18 (62.1) | 29 (54.7) | 33 (58.9) | 34 (58.6) | 39 (72.2) | 37 (71.2) |
| Severe | 11 (37.9) | 24 (45.3) | 23 (41.1) | 24 (41.4) | 15 (27.8) | 15 (28.8) |
ER, extended release; IR, immediate release; SD, standard deviation; VAS, visual analog scale.
aOther, American Indian, Alaskan native, native Hawaiian, other Pacific Islander.
Study 1: Single‐Dose Efficacy
Ibuprofen IR/ER formulation A was associated with significant (P < .05) improvements in PID and pain relief scores compared with placebo beginning 15 minutes after dosing (the first on‐treatment assessment), with benefits persisting throughout the 24‐hour assessment period (Figure 1 and Supplemental Figure S1). With ibuprofen IR/ER formulation B and naproxen, the pain relief rating score first differed significantly from placebo at 30 minutes; significant differences persisted throughout the 24‐hour study period. Each of the 3 active treatments provided improvements in SPID 0–12 and SPID 8–12 scores, which were significantly better (P < .001) than with placebo (Figure 2). SPID 0–4 and SPID 4–8 results were generally consistent with those of SPID 0–12 and 8–12, with all 3 active treatments statistically superior to placebo.
Figure 1.
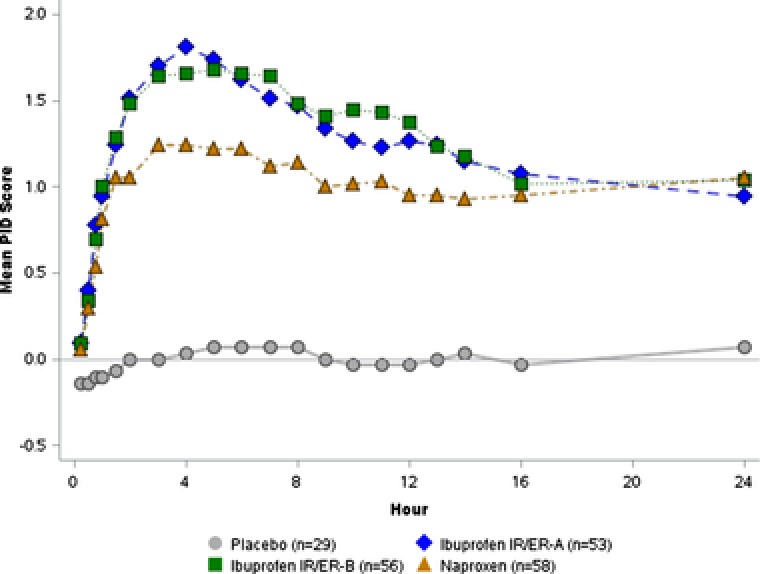
Study 1 — single‐dose efficacy of 2 formulations of ibuprofen 600 mg IR/ER compared with placebo and naproxen, mean pain intensity difference (PID) scores. A higher score indicates a greater reduction in pain intensity. P < .05 versus placebo for all active treatments at all times starting at first assessment (15 minutes). ER, extended release; IR, immediate release.
Figure 2.
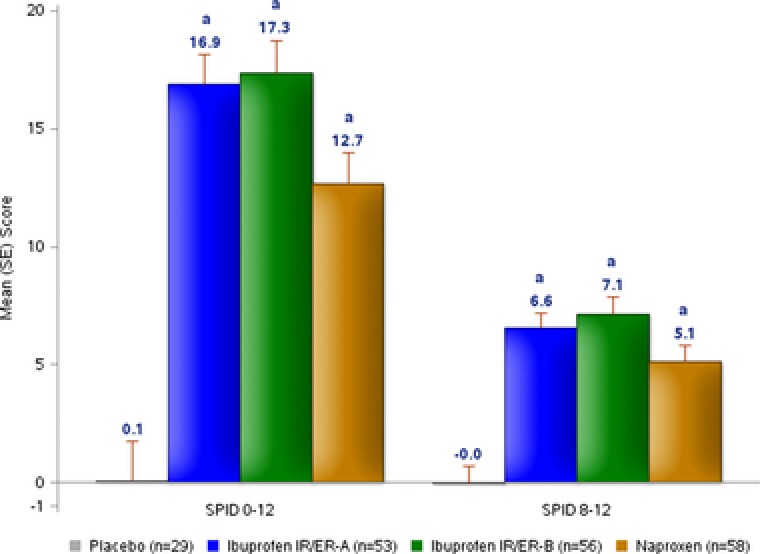
Study 1 — single‐dose efficacy of 2 formulations of ibuprofen 600 mg IR/ER compared with placebo and naproxen, mean (SE) SPID 0–12 and SPID 8–12 (coprimary outcomes). ER, extended release; IR, immediate release; SE, standard error; SPID, time‐weighted sum of pain intensity difference. a P < .001 versus placebo.
In the post hoc analysis, the pooled ibuprofen group was associated with significant improvements in both SPID 0–12 (P = .003) and SPID 8–12 (P = .024) scores compared with naproxen sodium (Table 2). PID and pain relief scores were significantly higher, indicating improvement, in the pooled ibuprofen group compared with naproxen at 45 minutes (pain relief only) and at 2, 3, 4, 5, 6, 7, 8, 9, 10, and 12 hours (for PID and pain relief); there were no significant differences at points > 12 hours.
Table 2.
Post Hoc Analysis: Pooled Ibuprofen IR/ER Versus Naproxen Sodium (Study 1)
| Parameter | IBU 600 mg IR/ER Pooled (n = 109) vs Placebo (n = 29) | Naproxen Sodium 220 mg (n = 58) vs Placebo (n = 29) | IBU 600 mg IR/ER Pooled (n = 109) vs Naproxen Sodium 220 mg (n = 58) |
|---|---|---|---|
| SPID 0–12, treatment | 16.6 (12.9–20.2) | 12.3 (8.3–16.3) | 4.3 (1.5–7.1) |
| difference (95%CI)a | P < .001 | P < .001 | P = .003 |
| SPID 8–12, treatment | 6.7 (4.9–8.6) | 5.1 (3.0–7.1) | 1.7 (0.2–3.1) |
| difference (95%CI)a | P < .001 | P < .001 | P = .024 |
| TFPR (confirmed), hazard | 13.6 (5.0–37.0) | 8.9 (3.2–25.0) | 1.5 (1.1–2.2) |
| ratio (95%CI)b | P < .001 | P < .001 | P = .026 |
| TMR, hazard ratio | 14.1 (5.2–38.5) | 8.9 (3.2–24.8) | 1.6 (1.1–2.3) |
| (95%CI)b | P < .001 | P < .001 | P = .014 |
CI, confidence interval; ER, extended release; IBU, ibuprofen; IR, immediate release; SPID, time‐weighted sum of pain intensity difference; TFPR, time to first perceptible relief (confirmed by meaningful relief); TMR, time to meaningful relief.
Treatment difference and corresponding 95%CI were calculated based on least‐squares means from the analysis of variance model.
Hazard ratio and corresponding 95%CI were calculated based on the Wald statistic from the proportional hazard model.
Confirmed first perceptible relief occurred significantly faster with ibuprofen IR/ER than with placebo; median time to confirmed first perceptible relief was 29.4 minutes for both formulations of ibuprofen IR/ER, 32.1 minutes for naproxen, and >6 hours with placebo (Figure 3A). Furthermore, time to confirmed first perceptible relief was significantly faster with pooled ibuprofen IR/ER compared with naproxen, (P = .026; Table 2). Time to meaningful pain relief occurred significantly sooner with each active treatment compared with placebo (P < .001); median times were >6 hours for placebo, 59.7 minutes for IR/ER formulation A, 76.7 minutes for IR/ER formulation B, and 84.1 minutes for naproxen. Similar to what was observed in the post hoc analysis of time to confirmed first perceptible relief, time to meaningful relief was significantly faster for the pooled ibuprofen groups relative to naproxen (P = .014; Table 2).
Figure 3.
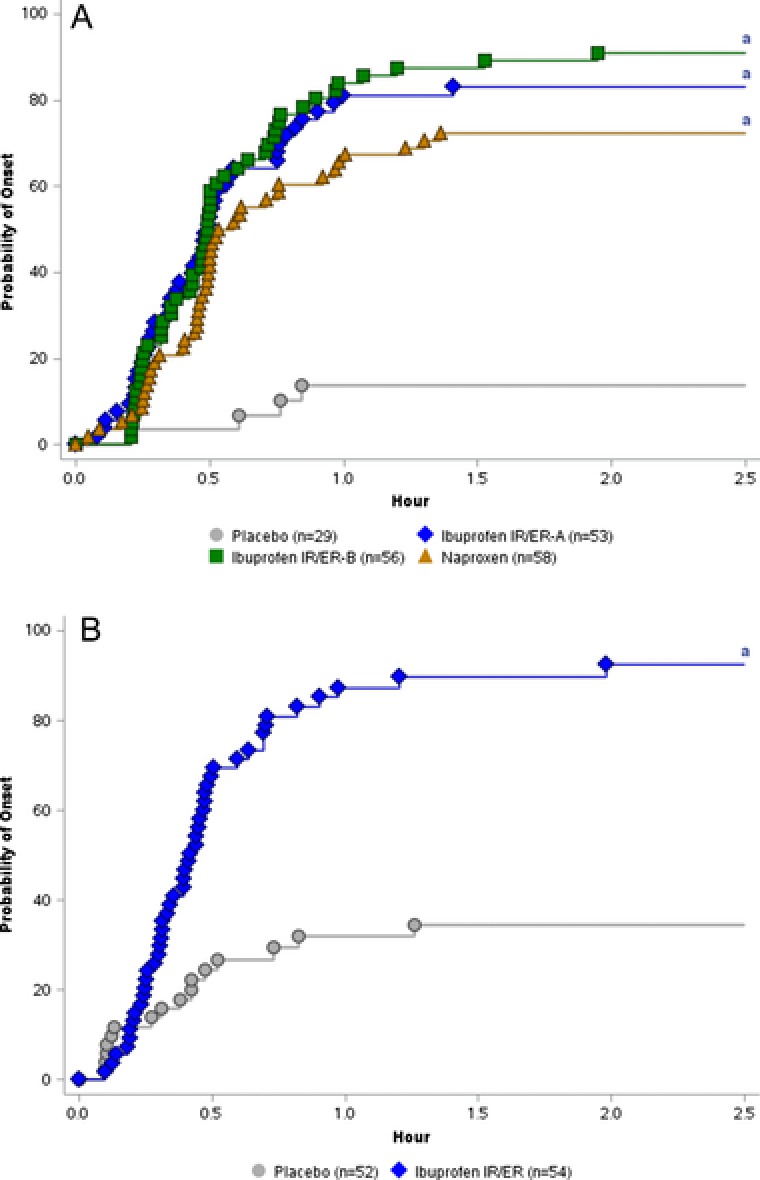
Kaplan‐Meier curve for time to confirmed first perceptible relief in (A) study 1 (single‐dose study) and (B) study 2 (multiple‐dose study). Note: X‐axis is truncated to 2.5 hours. No events occurred later than 2.5 hours. ER, extended release; IR, immediate release. a P < .001.
Median time to treatment failure, which was related to rescue medication use in all cases, was greater than 24 hours for all active treatments and was significantly (P < .001) longer than with placebo (1.7 hours). At 12 hours, treatment failure had occurred in 20.8% of those taking ibuprofen IR/ER formulation A, 17.9% of those taking ibuprofen IR/ER formulation B, 32.8% of those taking naproxen, and 82.8% of those taking placebo; at 24 hours, the rates of treatment failure were 41.5%, 41.1%, 37.9%, and 86.2%, respectively. The percentage of treatment failures from hours 8 to 12 was significantly lower for each active treatment compared with placebo (P < .001). On post hoc analysis, a significantly higher percentage of patients failed treatment in the naproxen sodium group versus in the pooled percentage of ibuprofen patients at hours 8, 9, 10, and 12 following study medication administration (P < .05 for all).
Study 2: Multiple‐Dose Efficacy
In study 2, ibuprofen 600 mg IR/ER demonstrated statistically significant superiority over placebo on both coprimary efficacy parameters, mean SPRID 0–12 (difference of 23.76) and SPRID 8–12 (difference of 8.42) following the first dose (P < .001 for both; Figure 4).
Figure 4.
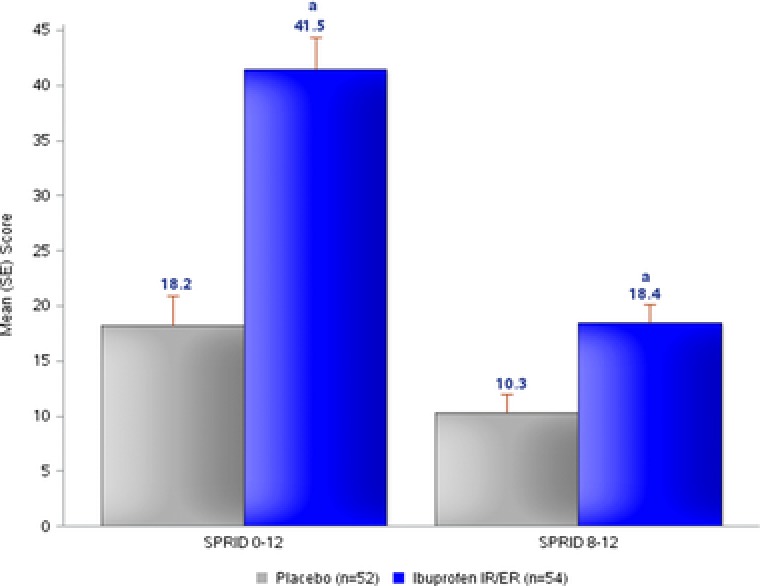
Study 2 — multiple‐dose efficacy of ibuprofen 600 mg IR/ER every 12 hours compared with placebo, mean (SE) sum of pain relief and pain intensity difference (SPRID) scores at 0–12 and 8–12 hours (primary efficacy analyses). ER, extended release; IR, immediate release; SE, standard error. a P < .001.
Ibuprofen 600 mg IR/ER significantly (P < .05) reduced pain intensity compared with placebo at all assessments except for the final assessment at the end of each dosing interval (ie, at 12, 24, 36, and 48 hours; Figure 5). Pain relief during the first dosing interval was significantly (P < .001) greater with ibuprofen IR/ER than with placebo at all times until hour 12 (Supplemental Figure S2).
Figure 5.
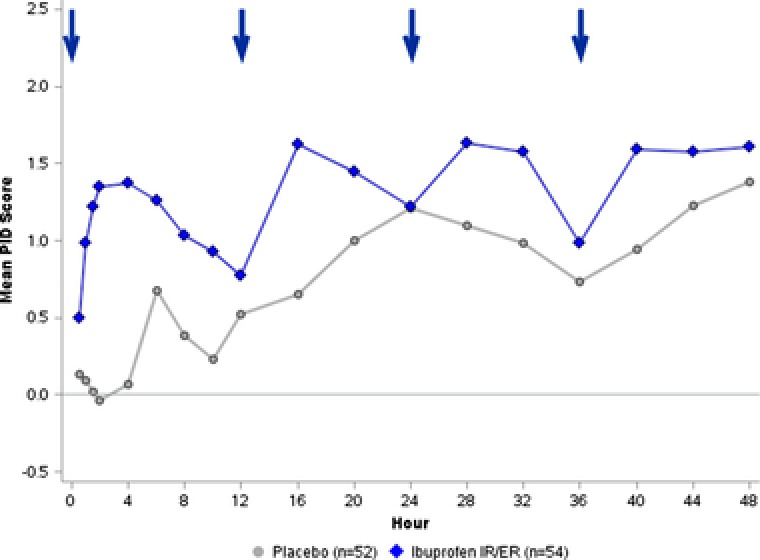
Study 2 — multiple‐dose efficacy of ibuprofen 600 mg IR/ER every 12 hours compared with placebo, mean pain intensity difference (PID) scores. A higher PID score indicates greater reduction in pain intensity. PID scores were significantly (P < .05) greater for ibuprofen IR/ER than for placebo at all assessments except hours 12, 24, 36, and 48 (ie, the end of each dosing interval). Arrows denote dosage administration. ER, extended release; IR, immediate release.
Time to confirmed first perceptible relief was significantly shorter with ibuprofen IR/ER versus placebo, with median times of 25.0 minutes versus >12 hours, respectively (P < .001; Figure 3B). Likewise, time to meaningful pain relief was significantly shorter (median, 54.2 minutes) with ibuprofen IR/ER compared with placebo (median, >12 hours; P < .001). SPID scores were significantly (P < .05) greater with ibuprofen IR/ER versus placebo at all specified time intervals except for 20–24 hours (Supplemental Figure S3). Ibuprofen 600 mg IR/ER was associated with significantly less rescue medication usage overall across the entire study (0.6 vs 2.5 pills; P < .001), as well as during each of the 12‐hour dosing intervals (interval 1, 0.4 vs 1.3; P < .001; interval 2, 0.1 vs 0.4; P < .001; interval 3, 0.1 vs 0.5; P = .002; and interval 4, 0.1 vs 0.3; P = .021) versus placebo, respectively. Ibuprofen IR/ER was associated with a significantly longer time to first rescue medication use during the first 12 hours compared with placebo (median, >12 vs 1.6 hours, respectively; P < .001). A significantly (P < .001) lower percentage of participants treated with ibuprofen IR/ER took rescue medications during the first dosing interval compared with placebo (29.6% vs 76.9%, respectively) and over the entire 48‐hour study period (31.5% vs 78.8%, respectively).
Safety Evaluation
Ibuprofen IR/ER was well tolerated in both studies. In study 1, 46 treatment‐emergent adverse events (TEAEs) were reported by 25 patients, including 8 taking placebo (27.6%), 3 taking ibuprofen IR/ER formulation A (5.7%), 8 taking ibuprofen IR/ER formulation B (14.3%), and 6 taking naproxen (10.3%); see Table 3. The overall AE incidence rate (P = .045), the rate of gastrointestinal system organ class AEs (P = .027), and the rates of nausea (P = .012) and vomiting (P = .003) were significantly different among treatment groups because of the higher event rates seen with placebo compared with active treatments. In study 2, 44 TEAEs were reported by 31 patients, including 23 patients taking placebo (44.2%) and 8 taking ibuprofen IR/ER (14.8%). Similar to study 1, the incidence of AEs seen within the placebo group in study 2 was significantly greater than that noted in the ibuprofen 600 mg IR/ER group for both the overall incidence of AEs (P = .001) and the incidence of gastrointestinal disorders (P < .001). The AE rates were not significantly different between placebo and ibuprofen IR/ER for any other system organ class. Interestingly, 14 subjects taking placebo (26.9%) and 3 taking ibuprofen 600 mg IR/ER (5.6%) reported AEs after taking rescue medication (acetaminophen/hydrocodone HCl 500/5 mg).
Table 3.
Adverse Events Occurring in ≥3% of Any Treatment Group in the Single‐Dose and Multiple‐Dose Studies of Ibuprofen IR/ER
| Study 1: Single‐Dose Study | Study 2: Multiple‐Dose Study | |||||
|---|---|---|---|---|---|---|
| AE, n (%) | Placebo (n = 29) | Ibuprofen IR/ER Formulation A (n = 53) | Ibuprofen IR/ER Formulation B (n = 56) | Naproxen (n = 58) | Placebo (n = 52) | Ibuprofen IR/ER (n = 54) |
| Patients with any AE | 8 (27.6) | 3 (5.7) | 8 (14.3) | 6 (10.3) | 23 (44.2) | 8 (14.8) |
| Nausea | 6 (20.7) | 1 (1.9) | 5 (8.9) | 2 (3.4) | 10 (19.2) | 1 (1.9) |
| Vomiting | 5 (17.2) | 0 | 3 (5.4) | 1 (1.7) | 6 (11.5) | 0 |
| Headache | 1 (3.4) | 2 (3.8) | 1 (1.8) | 1 (1.7) | 9 (17.3) | 4 (7.4) |
| Dizziness | 1 (3.4) | 0 | 2 (3.6) | 2 (3.4) | 1 (1.9) | 0 |
| Flushing | 0 | 0 | 0 | 0 | 2 (3.8) | 0 |
| Pruritus | 0 | 0 | 1 (1.8) | 0 | 0 | 2 (3.7) |
AE, adverse event; ER, extended release; IR, immediate release.
In study 1, 1 AE in each of the 3 active treatment groups and 7 AEs in the placebo group were deemed related to study treatment by the investigator. In study 2, 30 AEs in the placebo group (16 cases of nausea/vomiting, 7 cases of headache, 2 cases of flushing, and 1 each of chills, feeling hot, dizziness, rash, anxiety) and 5 AEs in the ibuprofen IR/ER group (3 cases of headache, 1 case of nausea/vomiting, and 1 case of pruritus) were considered treatment related. In study 2, AEs followed the ingestion of rescue medication in 14 patients (26.9%) in the placebo group and 3 (5.6%) in the ibuprofen IR/ER group.
In both studies, most AEs were mild or moderate in intensity. In study 1, 1 AE was considered severe (vomiting by a patient in the naproxen group), and in study 2, 5 AEs were considered severe (1 case each of nausea and headache with placebo; 1 case each of burning skin sensation, pruritus, and skin pain with ibuprofen IR/ER). No deaths or serious AEs occurred in either study. No patients in study 1 discontinued because of AEs; in study 2, 3 patients in the placebo group discontinued because of vomiting, 2 of whom had received rescue medication within 90 minutes prior to the vomiting episode.
At the end of both study 1 and study 2, blood pressure readings were lower in the active treatment groups compared with those taking placebo, likely a reflection of pain reduction and corresponding decreases in sympathetic activity.
Discussion
The 2 studies reported here are among the first to evaluate the clinical efficacy of a new long‐acting formulation of ibuprofen 600‐mg IR/ER tablets that is administered orally every 12 hours. This formulation was developed for OTC use to provide consumers with longer, more consistent pain relief in addition to greater dosing convenience (ie, fewer doses per day). Both a single‐dose and a multiple‐dose pharmacokinetic study supporting product development have shown that ibuprofen 600 mg IR/ER is bioequivalent to standard ibuprofen 200 mg administered every 4 hours × 3 in terms of the overall extent (AUC from time 0 to 12 hours, to last measurable concentration, and to infinity) and rate (maximum concentration, Cmax) of ibuprofen absorption; however, the Cmax for ibuprofen 600 mg IR/ER after the first dose was higher than after the first dose of standard IR ibuprofen.15
In the current studies, ibuprofen 600 mg IR/ER provided rapid relief from postoperative dental pain, beginning at the first postdose assessment in both studies (15 minutes in study 1; 30 minutes in study 2). The time to confirmed first perceptible relief (median, 29.4 minutes in study 1 and 25.0 minutes in study 2) and time to meaningful pain relief (median, 59.7 minutes in study 1 [for the formulation ultimately chosen for clinical development] and 54.2 minutes in study 2) with ibuprofen IR/ER were statistically superior to placebo and illustrate a rapid onset of action with this new ibuprofen formulation, an important attribute for any acute analgesic medication. In addition, time to meaningful pain relief occurred ∼25 minutes earlier with ibuprofen IR/ER than with naproxen sodium; on post hoc analysis, this difference was statistically significant in favor of ibuprofen 600 mg IR/ER. Furthermore, post hoc analyses revealed significant differences favoring ibuprofen 600 mg IR/ER over naproxen sodium 220 mg on both coprimary efficacy end points and many secondary outcomes.
The analgesic effect of ibuprofen IR/ER was maintained for at least 12 hours after dosing. In study 1, the percentage of patients taking ibuprofen IR/ER formulation A or B who required rescue medication (20.8% and 17.9%, respectively) was significantly lower than with placebo (82.8%). In addition, both ibuprofen formulations were superior to placebo for PID, pain relief, and pain relief combined with pain intensity difference at each hourly point from 8 through 12 hours, as well as for the composite assessments SPID 8–12, time‐weighted sum of pain relief scores from 8 to 12 hours, and SPRID 8–12, clearly demonstrating a 12‐hour duration of effect. Similarly, in study 2, efficacy over the 12‐hour period was significantly superior with ibuprofen IR/ER versus placebo for SPRID 0–12 and SPRID 8–12 in the first dosing interval. These findings were supported by observations over the 3 subsequent 12‐hour dosing intervals, although PID scores were not significantly different from ibuprofen IR/ER versus placebo (P > .05) at the end of each dosing interval (hours 12, 24, 36, and 48), which may be reflective of the effect of rescue medication use in the placebo group. Furthermore, the percentage of patients who used rescue medication was significantly lower for ibuprofen IR/ER over both 12 hours (29.6%) and 48 hours (31.5%) versus placebo (76.9% and 78.8%, respectively). We were able to locate 1 other study, reported in abstract only, in which analgesic efficacy of an ibuprofen 600‐mg IR/ER tablet was demonstrated in the third‐molar dental extraction pain model.16
The third‐molar extraction model of dental pain is considered the gold standard of acute pain models and has been used to evaluate the efficacy and safety of a number of analgesics.3, 17, 18, 19, 20 Third‐molar extraction procedures are fairly uniform, require minimal anesthesia, and result in a well‐characterized, consistent intense pain lasting more than 12 hours and gradually declining over the next 1–2 days.21 This model has good sensitivity in distinguishing active treatment from placebo (as in the current studies) because third‐molar extraction pain is associated with a very low placebo response rate.21, 22 This model has also been found to be highly sensitive in single‐dose studies3, 17, 20, 23 and is adequately sensitive in multiple‐dose studies to permit detection of statistically significant differences between active treatments.24, 25 In addition, the third‐molar extraction model is able to demonstrate efficacy across a broad range of analgesics and has predicted treatment regimens of experimental analgesics that were eventually approved by regulatory bodies.21 Furthermore, a recent comparison of pain models based on standard effect sizes found that this model (and the bunionectomy model) had a higher assay sensitivity than 2 other postsurgical pain models.26
Previous healthy‐volunteer pharmacokinetic studies with this formulation of ibuprofen IR/ER have shown that it is bioequivalent to standard ibuprofen 200 mg IR tablets administered as 1 tablet 3 times daily every 4 hours with regard to both the extent and rate of absorption after both single and multiple doses.15 The current findings indicate that ibuprofen 600 mg IR/ER provides both rapid and sustained analgesic efficacy over 12 hours.
One potential limitation of this analysis is that generalizability cannot be confirmed. Patients undergoing third‐molar extraction tend to be young adults with relatively few concurrent medical conditions, 21, 24 which is advantageous for analysis but also may limit generalizability of the findings to older patients with comorbid illnesses. However, it should be noted that standard ibuprofen IR has a long history of safe and effective OTC use across a multitude of pain state etiologies in adults of all ages. Thus, it is likely that the efficacy of the longer‐acting formulation of ibuprofen demonstrated here would extend to other types of persistent pain (eg, osteoarthritis, headache, backache, dysmenorrhea), including those in older persons.
Conclusions
Ibuprofen 600 mg IR/ER demonstrated analgesic efficacy superior to placebo in the third‐molar dental pain model after single and multiple doses. Ibuprofen IR/ER provided both a rapid onset of analgesia and a 12‐hour duration of effect and was safe and well tolerated, with dosing lasting up to 2 days. A post hoc analysis suggested that ibuprofen provides faster onset of analgesia and a superior duration of effect compared with a single dose of naproxen sodium 220 mg. Thus, ibuprofen IR/ER provides a long‐acting formulation of ibuprofen that offers the benefits of fast and sustained pain relief plus the convenience of less frequent dosing for patients with longer‐lasting pain.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplemental Figure S1. Study 1 — single‐dose efficacy of 2 formulations of ibuprofen 600 mg IR/ER compared with placebo and naproxen. Mean pain relief rating scores over time.
Supplemental Figure S2. Study 2 — multiple‐dose efficacy of ibuprofen 600 mg IR/ER every 12 hours compared with placebo, mean (SE) pain relief rating scores.
Supplemental Figure S3. Study 2 — multiple‐dose efficacy of ibuprofen 600 mg IR/ER every 12 hours compared with placebo, mean (SE) time‐weighted sum of pain intensity difference (SPID) scores at specified intervals (key secondary end point).
Declaration of Conflicting Interests
Dr. Christensen has no conflicts to disclose. Dr. Daniels was an employee of Premier Research at the time the study was performed. Dr. Paluch and Dr. Meeves are employees of Pfizer Consumer Healthcare. Dr. Jayawardena is a former employee of Pfizer Consumer Healthcare.
Funding
This study was sponsored by Pfizer Consumer Healthcare. Medical writing support was provided by Lauren Cerruto and John H. Simmons, MD, of Peloton Advantage, LLC, and was funded by Pfizer.
Acknowledgments
This work was previously presented in part at the American Pain Society's 34th Annual Scientific Meeting, May 13–16, 2015, Palm Springs, California.
References
- 1. Rainsford KD. Fifty years of ibuprofen: advancing pain and fever management. Int J Clin Pract Suppl. 2013(178):1–2. [DOI] [PubMed] [Google Scholar]
- 2. Rainsford KD. Ibuprofen: from invention to an OTC therapeutic mainstay. Int J Clin Pract Suppl. 2013(178):9–20. [DOI] [PubMed] [Google Scholar]
- 3. Cooper SA, Schachtel BP, Goldman E, Gelb S, Cohn P. Ibuprofen and acetaminophen in the relief of acute pain: a randomized, double‐blind, placebo‐controlled study. J Clin Pharmacol. 1989;29(11):1026–1030. [DOI] [PubMed] [Google Scholar]
- 4. Schachtel BP, Fillingim JM, Thoden WR, Lane AC, Baybutt RI. Sore throat pain in the evaluation of mild analgesics. Clin Pharmacol Ther. 1988;44(6):704–711. [DOI] [PubMed] [Google Scholar]
- 5. Schachtel BP, Thoden WR, Baybutt RI. Ibuprofen and acetaminophen in the relief of postpartum episiotomy pain. J Clin Pharmacol. 1989;29(6):550–553. [DOI] [PubMed] [Google Scholar]
- 6. Schachtel BP, Furey SA, Thoden WR. Nonprescription ibuprofen and acetaminophen in the treatment of tension‐type headache. J Clin Pharmacol. 1996;36(12):1120–1125. [DOI] [PubMed] [Google Scholar]
- 7. Boureau F, Schneid H, Zeghari N, Wall R, Bourgeois P. The IPSO study: ibuprofen, paracetamol study in osteoarthritis. A randomised comparative clinical study comparing the efficacy and safety of ibuprofen and paracetamol analgesic treatment of osteoarthritis of the knee or hip. Ann Rheum Dis. 2004;63(9):1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. N Engl J Med. 1991;325(2):87–91. [DOI] [PubMed] [Google Scholar]
- 9. Altman RD. Ibuprofen, acetaminophen and placebo in osteoarthritis of the knee: a six‐day double‐blind study [abstract 1995]. Arthritis Rheum. 1999;42(suppl):S403. [Google Scholar]
- 10. Rainsford KD, Roberts SC, Brown S. Ibuprofen and paracetamol: relative safety in non‐prescription dosages. J Pharm Pharmacol. 1997;49(4):345–376. [DOI] [PubMed] [Google Scholar]
- 11. Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34(2):101–154. [DOI] [PubMed] [Google Scholar]
- 12. McCarberg B. Tramadol extended‐release in the management of chronic pain. Ther Clin Risk Manag. 2007;3(3):401–410. [PMC free article] [PubMed] [Google Scholar]
- 13. Nicholson B. Benefits of extended‐release opioid analgesic formulations in the treatment of chronic pain. Pain Pract. 2009;9(1):71–81. [DOI] [PubMed] [Google Scholar]
- 14. Doyle G, Jayawardena S, Ashraf E, Cooper SA. Efficacy and tolerability of nonprescription ibuprofen versus celecoxib for dental pain. J Clin Pharmacol. 2002;42(8):912–919. [DOI] [PubMed] [Google Scholar]
- 15. Legg T, Paluch E, Jayawardena S. Single‐ and multiple‐dose pharmacokinetics of immediate‐release/extended‐release ibuprofen tablets [published online ahead of print 2016]. Clin Pharmacol Drug Dev. doi: 10.1002/cpdd.288 [DOI] [PubMed]
- 16. Turner S, Cooper S, Christensen S, et al. NSAIDs and acetaminophen. J Pain. 2010;11(4 suppl):S43. [Google Scholar]
- 17. Cooper SA, Needle SE, Kruger GO. Comparative analgesic potency of aspirin and ibuprofen. J Oral Surg. 1977;35(11):898–903. [PubMed] [Google Scholar]
- 18. Cooper SA, Quinn PD, MacAfee K, Hersh EV, Sullivan D, Lamp C. Ibuprofen controlled‐release formulation. A clinical trial in dental impaction pain. Oral Surg Oral Med Oral Pathol. 1993;75(6):677–683. [DOI] [PubMed] [Google Scholar]
- 19. Desjardins PJ, Milles M, Frey V, Gubitosa L, Mardirossian G, Schneider R. Controlled release ibuprofen vs. multiple dose ibuprofen in dental impaction pain [abstract PIII‐32]. Clin Pharmacol Ther. 1991;49(2):182. [Google Scholar]
- 20. Forbes JA, Moore EM, Allen HW, Beaver WT. Evaluation of an ibuprofen controlled‐release tablet and placebo in postoperative oral surgery pain. Pharmacotherapy. 1991;11(3):242–248. [PubMed] [Google Scholar]
- 21. Cooper SA, Desjardins PJ. The value of the dental impaction pain model in drug development. Methods Mol Biol. 2010;617:175–190. [DOI] [PubMed] [Google Scholar]
- 22. Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity In: Max MB, Portenoy RK, Laska EM, eds. The Design of Analgesic Clinical Trials (Advance in Pain Research and Therapy). 18th ed New York, NY: Raven Press; 1991:117–125. [Google Scholar]
- 23. Hersh EV, Cooper S, Betts N, et al. Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery. Oral Surg Oral Med Oral Pathol. 1993;76(6):680–687. [DOI] [PubMed] [Google Scholar]
- 24. Cooper SA, Beaver WT. A model to evaluate mild analgesics in oral surgery outpatients. Clin Pharmacol Ther. 1976;20(2):241–250. [DOI] [PubMed] [Google Scholar]
- 25. McQuay HJ, Carroll D, Guest PG, Robson S, Wiffen PJ, Juniper RP. A multiple dose comparison of ibuprofen and dihydrocodeine after third molar surgery. Br J Oral Maxillofac Surg. 1993;31(2):95–100. [DOI] [PubMed] [Google Scholar]
- 26. Singla NK, Desjardins PJ, Chang PD. A comparison of the clinical and experimental characteristics of four acute surgical pain models: dental extraction, bunionectomy, joint replacement, and soft tissue surgery. Pain. 2014;155(3):441–456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplemental Figure S1. Study 1 — single‐dose efficacy of 2 formulations of ibuprofen 600 mg IR/ER compared with placebo and naproxen. Mean pain relief rating scores over time.
Supplemental Figure S2. Study 2 — multiple‐dose efficacy of ibuprofen 600 mg IR/ER every 12 hours compared with placebo, mean (SE) pain relief rating scores.
Supplemental Figure S3. Study 2 — multiple‐dose efficacy of ibuprofen 600 mg IR/ER every 12 hours compared with placebo, mean (SE) time‐weighted sum of pain intensity difference (SPID) scores at specified intervals (key secondary end point).


